Abstract
Objective:
To review our multicenter experience with cyclophosphamide in the treatment of children with multiple sclerosis (MS).
Methods:
Retrospective chart review of children with MS treated with cyclophosphamide. Demographic, clinical, treatment, and MRI parameters were collected.
Results:
We identified 17 children with MS treated with cyclophosphamide. All but one had worsening of Expanded Disability Status Scale scores or multiple relapses prior to treatment initiation. Children were treated with one of three regimens: 1) induction therapy alone; 2) induction therapy with pulse maintenance therapy; or 3) pulse maintenance therapy alone. Treatment resulted in a reduction in relapse rate and stabilization of disability scores assessed 1 year after treatment initiation in the majority of patients. Longer follow-up was available for most cases. Cyclophosphamide was well tolerated in most patients. However, side effects included vomiting, transient alopecia, osteoporosis, and amenorrhea. One patient developed bladder carcinoma that was successfully treated.
Conclusions:
Cyclophosphamide is an option for the treatment of children with aggressive multiple sclerosis refractory to first-line therapies. Recommendations regarding patient selection, treatment administration, and monitoring are discussed.
GLOSSARY
- ARR
= annualized relapse rates;
- EDSS
= Expanded Disability Status Scale;
- IVIg
= IV immunoglobulin;
- MS
= multiple sclerosis;
- PLEX
= plasmapheresis;
- RRMS
= relapsing remitting multiple sclerosis;
- SPMS
= secondary progressive multiple sclerosis.
Intravenous cyclophosphamide (Cytoxan™; BMS Oncology, Princeton, NJ) is an alkylating agent that is used mainly as a second-line treatment in multiple sclerosis (MS). It is generally administered as monthly maintenance therapy, in some cases preceded by an induction course. Cyclophosphamide has been shown to be effective in relapse rate reduction1,2 and in control of MRI lesion accrual1,3; however, effects in delaying disease progression have been variable.4–7 Several studies have suggested that cyclophosphamide treatment may be most beneficial in younger adult patients,7–9 and in patients with early secondary progressive MS.6,7,10
MS onset before the age of 18 years is estimated at 2.7%–10.5%11–13 of all patients. Children experience more frequent relapses than adults, suggesting a highly inflammatory disease.14 Several studies have demonstrated that on average, patients with pediatric-onset MS have a longer disease duration from first attack prior to the onset of progressive disability, although the age at which this outcome is achieved is younger than is seen in adult-onset MS.11,13,15 A subset of patients experience an aggressive form of disease resulting in early neurologic and cognitive disability.16
Acute relapse-related care for pediatric MS typically involves IV corticosteroids.17 IV immunoglobulin (IVIg) and plasma exchange are employed in refractory cases.18,19 Beta-interferon and glatiramer acetate have been used as prophylactic therapy with reasonable success in children20–22; however, some children fail first-line treatment, as evidenced by ongoing relapses, MRI activity, and disability accrual, requiring second-line therapy.
We present our multicenter experience with the use of cyclophosphamide for children with severe relapses or MS disease activity unresponsive to conventional disease-modifying therapies.
METHODS
A retrospective analysis of all children with relapsing CNS demyelination, categorized as relapsing remitting multiple sclerosis (RRMS) or secondary progressive multiple sclerosis (SPMS) as per recent international guidelines,23 treated with one or more doses of cyclophosphamide was performed. SPMS was defined by the accrual of sustained physical disability (present for 6 months or longer) in the absence of clinical relapses.
All children were cared for at Brigham and Women's Hospital, Boston, MA; Childrens' Hospital of Boston, MA; Massachusetts General Hospital, Boston; or The Hospital for Sick Children, Toronto, Canada, between January 1990 and April 2008. Clinical data were retrieved through review of the established clinical databases at each center and by chart review.
Demographic data collected included age, sex, and ethnicity. The dosing regimen and total dose of cyclophosphamide administered to patients was recorded. Expanded Disability Status Scale (EDSS) scores; annual relapse rates before, during, and after treatment with cyclophosphamide; and all reported side effects were determined. All available laboratory results were recorded.
For each patient, all brain MRI scans obtained from 12 months before initiation of cyclophosphamide and up to 12 months after treatment were reviewed. Scans were scored at each institution using a predefined standardized MRI scoring protocol.
The internal review board of each involved hospital approved this study.
RESULTS
Patients.
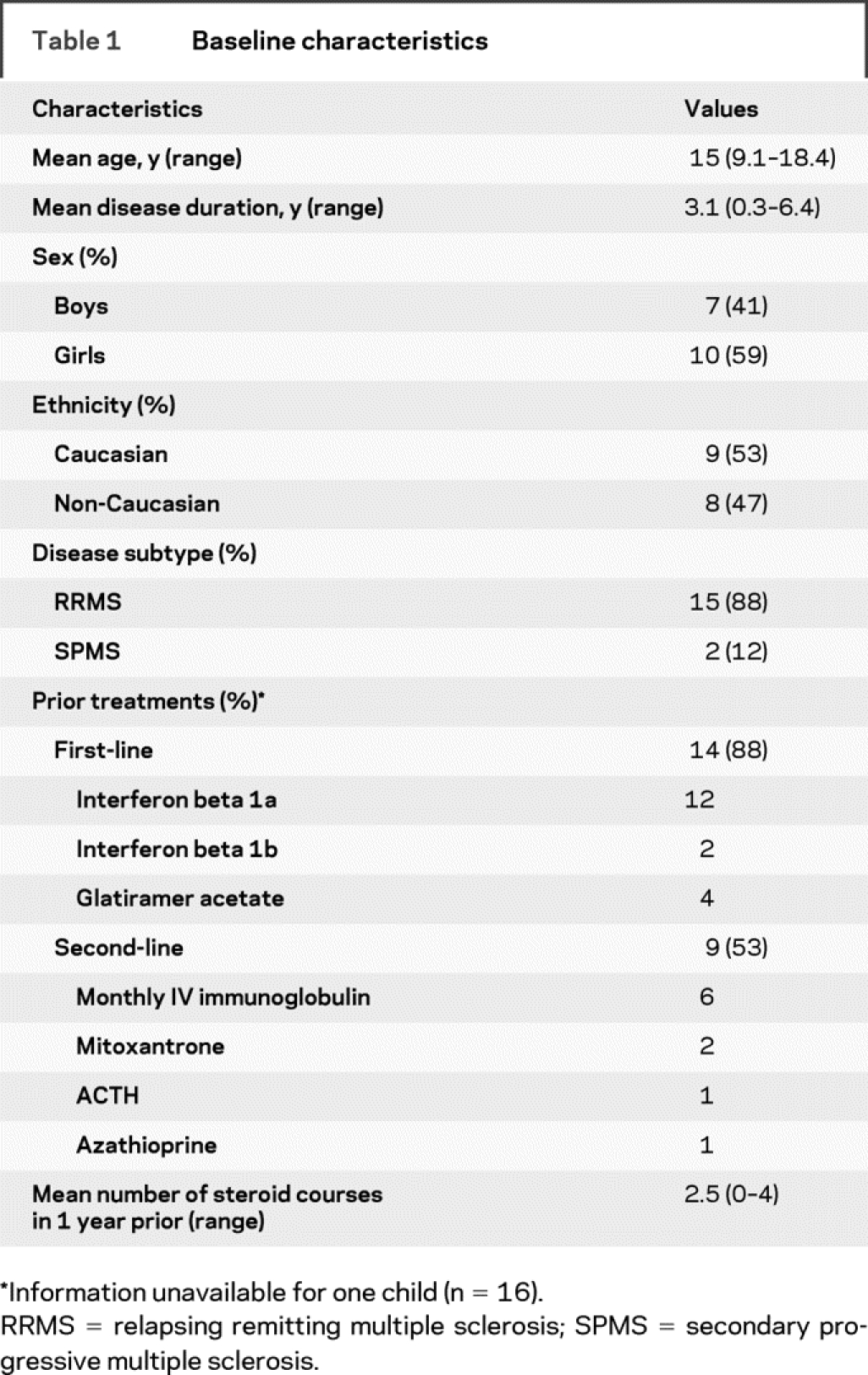
Baseline patient characteristics are summarized in table 1. All 17 children were initially diagnosed with RRMS, and two had entered SPMS at the time of cyclophosphamide therapy. The 17 children had a mean age at first cyclophosphamide use of 15.0 years (range 9.1 to 18.4 years) and mean disease duration of 3.1 years (range 0.3 to 6.4 years). Ten children (59%) were girls and 7 (41%) were boys. Nine children (53%) were Caucasian and 8 (47%) were non-Caucasian. EDSS scores at treatment initiation were available for 16 children and mean EDSS was 3.7. All children had worsening of EDSS scores or multiple relapses in the year before therapy. Of the 16 children with available information, there were a total of 51 relapses in the year before the initiation of cyclophosphamide. Four children had an EDSS of 6.0 or higher. Fourteen of 16 children (88%) had previously been treated with interferon-beta or glatiramer acetate; nine children were treated with more than one disease-modifying therapy before the cyclophosphamide initiation, and of these, two were treated with mitoxantrone.
Table 1 Baseline characteristics
Cyclophosphamide administration.
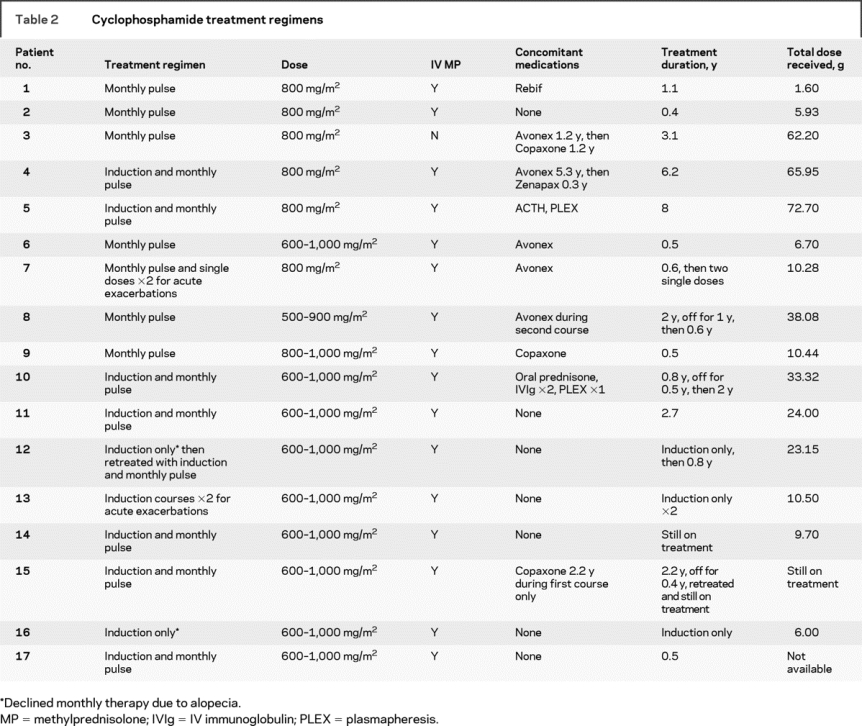
Three regimens for cyclophosphamide administration were employed at the discretion of the responsible physicians (table 2): 1) an induction regimen of five doses provided over 8 days (dosing predicated by white blood cell counts) followed by monthly pulse treatments; 2) a single induction course of five doses over 8 days; or 3) monthly cyclophosphamide administration without induction. In general, cyclophosphamide was administered at 600 to 1,000 mg/m2 per dose and the minimum dose required to achieve lymphopenia (nadir total white blood cell count less than 3,000/mm3 or between 1,500 and 2,000/mm3, depending on the institution) was used to guide subsequent maintenance treatments. Table 2 outlines the treatments provided to all 17 children. Fifteen children (88%) received monthly pulse therapy and 8 of the 15 (53%) received an induction course of cyclophosphamide prior to monthly maintenance treatment. Two children received induction courses or single doses only without maintenance treatments at times of acute exacerbations. Two families declined monthly maintenance therapy due to significant alopecia postinduction. One of these children later underwent retreatment with induction and monthly therapy. Eight children (53%) who received monthly cyclophosphamide treatments received concomitant therapy with at least one other disease-modifying agent. This was usually initiated near the end of the treatment course in order to facilitate transition back to standard therapy.
Table 2 Cyclophosphamide treatment regimens
Response to treatment.
Fourteen children received monthly maintenance cyclophosphamide therapy with or without an induction course (table 2). Clinical response to treatment was measured by relapse rates and changes in EDSS scores. MRI disease activity was measured by the presence of new T2 lesions and the presence of lesions that enhanced with gadolinium. Patient 5 was excluded from the analysis of treatment effect due to lack of clinical information. Patients 13 and 16 received induction courses only and will be discussed separately.
Before cyclophosphamide treatment, the 14 children had a mean of 3.8 relapses per year. In the first year of cyclophosphamide therapy, seven children remained relapse-free and mean annual relapse rate was 1.1 (figure, A). One year after treatment completion, three children remained relapse-free, and annualized relapse rate calculated from 12 children was 1.6. Of 12 children with available data, 10 (83%) showed stabilization or improvement in EDSS over the course of cyclophosphamide treatment and mean change in EDSS was a reduction of 1.3 points (figure, B). Only one child had an EDSS 6 or higher at the end of treatment.
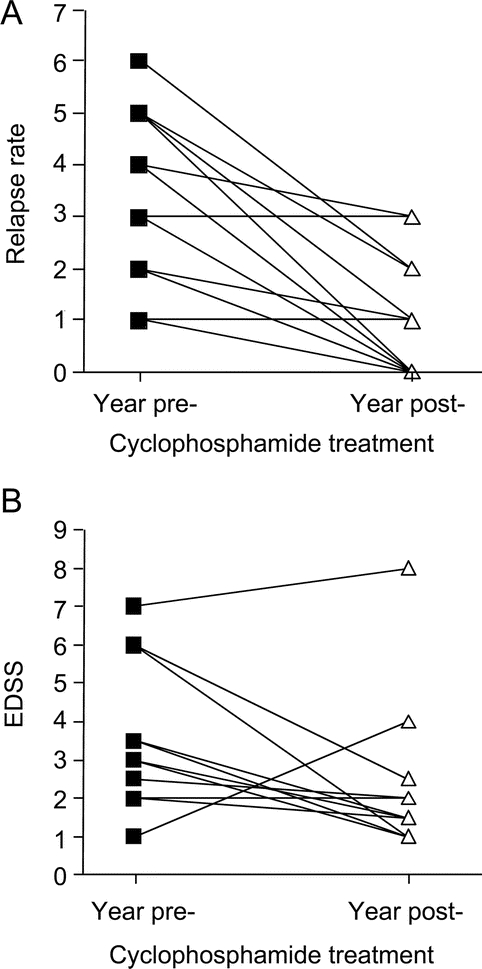
Figure Clinical scores precyclophosphamide and postcyclophosphamide treatment
Relapse rates in 14 patients (A) and Expanded Disability Status Scale scores in 12 patients (B) in the year before and 1 year after cyclophosphamide initiation. Patients included were treated with maintenance pulse cyclophosphamide therapy and had recorded values at both time points.
Before cyclophosphamide treatment, all 14 children had new T2-weighted lesions on brain MRI scans and 10 of 11 children (91%) who received gadolinium had enhancing lesions. In the first year of cyclophosphamide therapy, 12 children had brain MRIs performed. Nine of these children (75%) continued to display new T2 lesions. Gadolinium was administered to 9 children and 5 (67%) had new enhancing lesions while on treatment.
Patient 13 received two cyclophosphamide induction courses during acute exacerbations, but did not receive any monthly therapy. While he continued to have new T2 lesions on subsequent MRI scans, after each induction course he remained relapse-free for over 1 year on monotherapy with glatiramer acetate.
Patient 16 received an induction course, but declined monthly therapy due to severe alopecia. She had had two clinical relapses and worsened EDSS in the year prior to treatment initiation. One year following her induction course, her EDSS improved from 3.5 to 1.5 and she had one further clinical relapse with new T2 lesions on MRI. She continues on interferon-beta treatment.
Children were followed for a mean of 2.7 years following cyclophosphamide treatment (range 0–14 years). Of the 17 children, 7 (41%) remained treated with a single standard first-line therapy. One child was not on any disease-modifying agents 1 year after cyclophosphamide. The remaining 9 children (53%) required combination therapy or treatment with a second-line therapeutic agent.
Cyclophosphamide tolerability.
Short-term and long-term treatment-related side effects are summarized in table 3. Fifteen children (88%) experienced some nausea or vomiting that in one case was prolonged (beyond 5 days post-treatment). All children achieved lymphopenia, consistent with therapy goals. Anemia was present in 10 children and thrombocytopenia was present in 3 children, one of whom was diagnosed with idiopathic thrombocytopenic purpura. One child had bloodwork performed out of country and so laboratory values for this child were unavailable for review. Ten children reported reversible alopecia or hair thinning and in two cases this was severe enough to discontinue therapy. Three infections requiring antibiotic therapy were reported: two central line infections and one urinary tract infection. Other reported side effects are listed in table 3.
Table 3 Treatment-related side effects
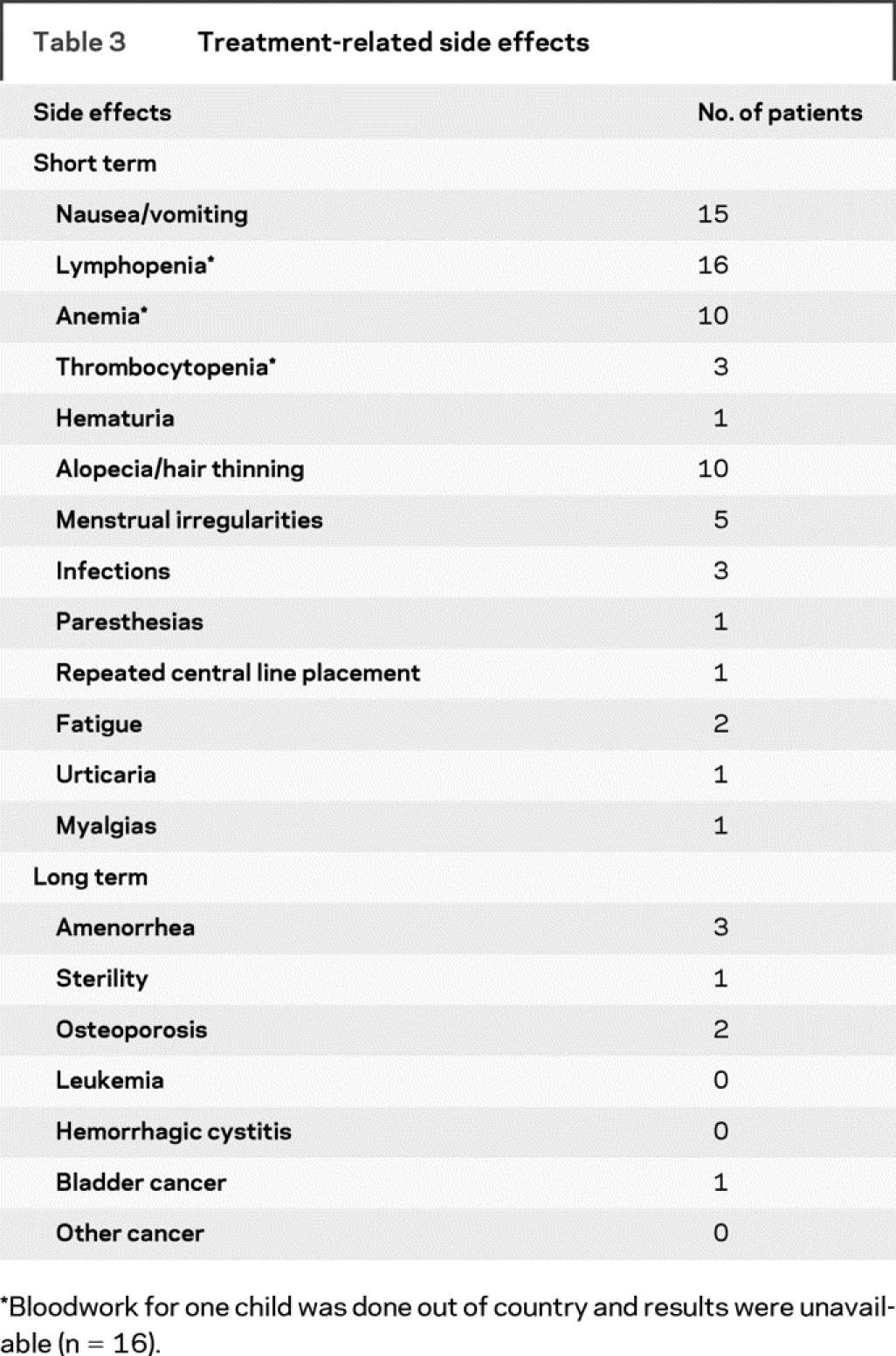
Evaluation of long-term complications revealed that three girls reported amenorrhea and one developed sterility as determined by fertility testing. Fertility was not formally assessed in the majority of patients, and the current young age of many patients necessitates ongoing observation to determine if fertility has been impacted. Two children had osteoporosis documented by bone density scans; however, this may be related to concomitant administration of steroids with cyclophosphamide and the use of multiple steroid courses for relapses. There were no reported fractures. One adolescent who had received a total of 72.7 g of cyclophosphamide over a period of 8 years developed hematuria, transitional cell carcinoma of the bladder, and a papillary tumor, which was successfully treated with intravesicular BCG. No other malignancies were reported.
DISCUSSION
We have described our retrospective multicenter experience with cyclophosphamide in the management of children with severe MS. Cyclophosphamide therapy was associated with improvement in relapse-related neurologic deficits when administered acutely and with marked reduction in relapses and stabilization of EDSS scores in the majority of children receiving induction or maintenance therapy.
Childhood-onset MS is relatively rare and available literature on therapy is limited to retrospective studies of first-line disease-modifying treatments.20–22 Although no standardized approach to the definition of treatment success, or failure, exists, there is a cohort of children who will continue to worsen on first-line treatment. Indeed, defining and identifying treatment failure has been a difficult task in the adult MS population, and a multitude of definitions have been used. The presence of continued relapses and formation of new MRI lesions despite therapy are generally considered indicators of refractory disease. Other important indicators of refractory disease, particularly for the consideration of cyclophosphamide treatment, are the presence of poor recovery from relapses and disease progression in the absence of relapses. In the majority of the cases we described, children experienced either poor recovery from relapses or progressive disease, as evidenced by increased EDSS scores in the year prior to cyclophosphamide initiation.
In our cohort, cyclophosphamide treatment reduced relapse rate and stabilized disability scores in the majority of patients. New gadolinium-enhancing and T2 MRI lesions were present even in the first year following cyclophosphamide initiation, suggesting that the onset of therapeutic effect may be delayed and incomplete. Although beyond the scope of this study, quantitative lesion analyses are required to determine the extent of cyclophosphamide effects on MRI parameters in children. We observed that over 50% of patients with over 1 year of follow-up after the cessation of therapy continued to experience frequent relapses and required additional second-line treatments, suggesting that cyclophosphamide treatment did not induce a permanent suppression of the inflammatory process in this cohort of pediatric MS patients. Our results may differ from observations in adults1 because of the selection of a particularly active pediatric MS population, or may be due to key differences in the immunologic response in children compared to adults, a hypothesis which requires further exploration.
Forty-one percent of children treated with cyclophosphamide were non-Caucasian in ethnicity. Several studies examining the effect of race on MS-associated disability in the adult population have demonstrated increased levels of disability in African American patients.24–26 We have reported on the more diverse ancestry of pediatric MS cohorts, relative to adult-onset MS populations,14,27 raising the possibility that ethnicity may influence disease severity, either defined by the early onset of MS, or by an aggressive disease course. Larger, multinational studies are required to validate this observation.
Cyclophosphamide therapy was associated with several adverse events in our cohort, the most significant being the development of bladder carcinoma. Risk of bladder carcinoma has been linked to cumulative cyclophosphamide dosage of 100 g or more.28 Long-term follow-up of patients treated with cyclophosphamide demonstrated an increased risk of bladder carcinoma as long as 17 years post-treatment,29 necessitating lifelong surveillance in exposed patients. Use of Mesna during the treatment period may help to reduce the incidence of hemorrhagic cystitis and bladder cancer. The risk of secondary lymphoma or leukemia and other malignancies are also a concern for children exposed to cyclophosphamide, and these risks may be partially dependent on the total cumulative dose.29 Concomitant steroid administration improves tolerability, but increases the risk of osteoporosis, which was a significant finding in our cohort.
Risk of infertility is an important consideration, and must be balanced with potential benefits of treatment. A study in childhood cancer survivors found that cyclophosphamide exposure between the ages of 13 and 20 years was an independent risk factor for acute ovarian failure.30 In females, postpubertal exposure seems to pose a higher risk to fertility than prepubertal exposure.31,32 Analyses of cyclophosphamide use in adults suggest that female infertility may be associated with cumulative dose received and patient age.33,34 GnRH agonists such as leuprolide acetate have been shown to reduce the risk of premature ovarian failure,35 and should be considered at the time of treatment initiation. Studies from the pediatric oncology literature suggest that cyclophosphamide administered both before and after puberty pose risks to male fertility in later life.36,37 Sperm cryopreservation should be considered in postpubertal males.
Although our retrospective study provides important information regarding the safety profile of cyclophosphamide in pediatric MS, we recommend that a prospective, multicenter study of cyclophosphamide in patients with refractory disease be considered to better define treatment outcomes. In our study, 15 of 17 patients experienced two or more relapses in the year prior to cyclophosphamide initiation, and therefore, these criteria may be used as a guideline to define refractory disease. Of the two cases that did not fit this definition, patient 3 had sequential MRIs with new lesions, and patient 17 progressed by 0.5 EDSS points to a pretreatment EDSS of 7 in the 9 months prior to cyclophosphamide initiation, and also demonstrated new lesion activity on MRI. Future clinical trials may consider a treatment length of 6–12 months, and response should be evaluated at 6-month intervals, clinically and by MRI, to follow potential clinically silent lesion accrual. Cumulative lifetime dose should be limited to 80 g to minimize side effects. Our results suggest that for patients undergoing a severe relapse unresponsive to corticosteroids, IVIg, or plasma exchange, an induction course of cyclophosphamide may be considered; however, prospective studies are required to definitively determine efficacy.
Our study did not examine use of cyclophosphamide in monophasic pediatric demyelinating diseases, and we cannot comment on a role for cyclophosphamide in children with disorders such as acute disseminated encephalomyelitis. Efficacy of cyclophosphamide treatment has been reported in children with severe transverse myelitis.38
The recent availability or imminent availability of novel immunomodulatory and immunosuppressive agents, such as natalizumab, rituximab, daclizumab, alemtuzumab, and cladribine provide potential future avenues for children requiring more intensive MS therapy. However, the risk of progressive multifocal leukoencephalopathy, severe infections, melanoma, and other malignancies is a major concern, particularly in the context of an immature immune system or in a population of patients experiencing primary exposures to viral infections, such as the JC virus. These risks must be compared to those experienced by children and adults exposed to cyclophosphamide, particularly the risks of bladder cancer and secondary malignancies as well as infertility.
While cyclophosphamide remains a second-line agent in the care of children with MS, it may have an important role in the treatment of some of the most severely affected children. Further studies will be needed to determine its efficacy and safety profile compared to emerging second- and third-line therapies. Multinational collaboration to define safety, treatment response, and failure of both conventional and second-line agents, as well as to develop standardized treatment algorithms for children with MS, would be invaluable.
ACKNOWLEDGMENT
The authors thank Howard L. Weiner, MD, for critical review of this manuscript.
Address correspondence and reprint requests to Dr. Tanuja Chitnis, Partners Pediatric Multiple Sclerosis Center, Massachusetts General Hospital, ACC-708, 55 Fruit Street, Boston, MA 02114 tchitnis@partners.org.
Editorial, page 2064.
e-Pub ahead of print on May 13, 2009, at www.neurology.org.
Supported by the Pediatric Multiple Sclerosis Centers of Excellence Grant from the National Multiple Sclerosis Society, USA (T.C.), and by the Multiple Sclerosis Scientific Research Foundation of Canada (B.L.B.). M.P.G. is supported by a National Multiple Sclerosis Society (Central New England Chapter) Clinical Fellowship.
Disclosure: The authors report no disclosures.
Medications: Cyclophosphamide (Cytoxan™; BMS Oncology, Princeton, NJ).
Received October 16, 2008. Accepted in final form February 9, 2009.
REFERENCES
- 1.Smith DR, Weinstock-Guttman B, Cohen JA, et al. A randomized blinded trial of combination therapy with cyclophosphamide in patients-with active multiple sclerosis on interferon beta. Mult Scler 2005;11:573–582. [DOI] [PubMed] [Google Scholar]
- 2.Reggio E, Nicoletti A, Fiorilla T, Politi G, Reggio A, Patti F. The combination of cyclophosphamide plus interferon beta as rescue therapy could be used to treat relapsing-remitting multiple sclerosis patients: twenty-four months follow-up. J Neurol 2005;252:1255–1261. [DOI] [PubMed] [Google Scholar]
- 3.Zipoli V, Portaccio E, Hakiki B, Siracusa G, Sorbi S, Amato MP. Intravenous mitoxantrone and cyclophosphamide as second-line therapy in multiple sclerosis: an open-label comparative study of efficacy and safety. J Neurol Sci 2008;266:25–30. [DOI] [PubMed] [Google Scholar]
- 4.The Canadian Cooperative Multiple Sclerosis Study Group. The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet 1991;337:441–446. [PubMed] [Google Scholar]
- 5.Hauser SL, Dawson DM, Lehrich JR, et al. Intensive immunosuppression in progressive multiple sclerosis: a randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med 1983;308:173–180. [DOI] [PubMed] [Google Scholar]
- 6.Hohol MJ, Olek MJ, Orav EJ, et al. Treatment of progressive multiple sclerosis with pulse cyclophosphamide/methylprednisolone: response to therapy is linked to the duration of progressive disease. Mult Scler 1999;5:403–409. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL, Mackin GA, Orav EJ, et al. Intermittent cyclophosphamide pulse therapy in progressive multiple sclerosis: final report of the Northeast Cooperative Multiple Sclerosis Treatment Group. Neurology 1993;43:910–918. [DOI] [PubMed] [Google Scholar]
- 8.Hommers OR, Lamers KJ, Reekers P. Effect of intensive immunosuppression on the course of chronic progressive multiple sclerosis. J Neurol 1980;223:177–190. [DOI] [PubMed] [Google Scholar]
- 9.Gonsette RE, Demonty L, Delmotte P. Intensive immunosuppression with cyclophosphamide in multiple sclerosis: follow up of 110 patients for 2–6 years. J Neurol 1977;214:173–181. [DOI] [PubMed] [Google Scholar]
- 10.Perini P, Calabrese M, Tiberio M, Ranzato F, Battistin L, Gallo P. Mitoxantrone versus cyclophosphamide in secondary-progressive multiple sclerosis: a comparative study. J Neurol 2006;253:1034–1040. [DOI] [PubMed] [Google Scholar]
- 11.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D. Early onset multiple sclerosis: a longitudinal study. Neurology 2002;59:1006–1010. [DOI] [PubMed] [Google Scholar]
- 12.Duquette P, Murray TJ, Pleines J, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. J Pediatr 1987;111:359–363. [DOI] [PubMed] [Google Scholar]
- 13.Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology 2002;59:1922–1928. [DOI] [PubMed] [Google Scholar]
- 14.Gorman M, Healy B, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared to adult-onset multiple sclerosis. Arch Neurol 2009;66:54–59. [DOI] [PubMed] [Google Scholar]
- 15.Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007;356:2603–2613. [DOI] [PubMed] [Google Scholar]
- 16.Ness JM, Chabas D, Sadovnick AD, Pohl D, Banwell B, Weinstock-Guttman B. Clinical features of children and adolescents with multiple sclerosis. Neurology 2007;68:S37–S45. [DOI] [PubMed] [Google Scholar]
- 17.Banwell B. Treatment of children and adolescents with multiple sclerosis. Expert Rev Neurother 2005;5:391–401. [DOI] [PubMed] [Google Scholar]
- 18.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 2002;58:143–146. [DOI] [PubMed] [Google Scholar]
- 19.Khurana DS, Melvin JJ, Kothare SV, et al. Acute disseminated encephalomyelitis in children: discordant neurologic and neuroimaging abnormalities and response to plasmapheresis. Pediatrics 2005;116:431–436. [DOI] [PubMed] [Google Scholar]
- 20.Banwell B, Reder AT, Krupp L, et al. Safety and tolerability of interferon beta-1b in pediatric multiple sclerosis. Neurology 2006;66:472–476. [DOI] [PubMed] [Google Scholar]
- 21.Pohl D, Rostasy K, Gartner J, Hanefeld F. Treatment of early onset multiple sclerosis with subcutaneous interferon beta-1a. Neurology 2005;64:888–890. [DOI] [PubMed] [Google Scholar]
- 22.Ghezzi A. Immunomodulatory treatment of early onset multiple sclerosis: results of an Italian Co-operative Study. Neurol Sci 2005;26 suppl 4:S183–186. [DOI] [PubMed] [Google Scholar]
- 23.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007;68:S7–S12. [DOI] [PubMed] [Google Scholar]
- 24.Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult Scler 2003;9:293–298. [DOI] [PubMed] [Google Scholar]
- 25.Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 2004;63:2039–2045. [DOI] [PubMed] [Google Scholar]
- 26.Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Does multiple sclerosis-associated disability differ between races? Neurology 2006;66:1235–1240. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy J, O'Connor P, Sadovnick AD, Perara M, Yee I, Banwell B. Age at onset of multiple sclerosis may be influenced by place of residence during childhood rather than ancestry. Neuroepidemiology 2006;26:162–167. [DOI] [PubMed] [Google Scholar]
- 28.Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996;124:477–484. [DOI] [PubMed] [Google Scholar]
- 29.Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis: a 20-year followup study. Arthritis Rheum 1995;38:1120–1127. [DOI] [PubMed] [Google Scholar]
- 30.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006;91:1723–1728. [DOI] [PubMed] [Google Scholar]
- 31.Ortin TT, Shostak CA, Donaldson SS. Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys 1990;19:873–880. [DOI] [PubMed] [Google Scholar]
- 32.Wallace WH, Shalet SM, Tetlow LJ, Morris-Jones PH. Ovarian function following the treatment of childhood acute lymphoblastic leukaemia. Med Pediatr Oncol 1993;21:333–339. [DOI] [PubMed] [Google Scholar]
- 33.Portaccio E, Zipoli V, Siracusa G, Piacentini S, Sorbi S, Amato MP. Safety and tolerability of cyclophosphamide ‘pulses’ in multiple sclerosis: a prospective study in a clinical cohort. Mult Scler 2003;9:446–450. [DOI] [PubMed] [Google Scholar]
- 34.Boumpas DT, Austin HA 3rd, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med 1993;119:366–369. [DOI] [PubMed] [Google Scholar]
- 35.Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod 1996;11:1620–1626. [DOI] [PubMed] [Google Scholar]
- 36.van Casteren NJ, van der Linden GH, Hakvoort-Cammel FG, Hahlen K, Dohle GR, van den Heuvel-Eibrink MM. Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer 2009;52:108–112. [DOI] [PubMed] [Google Scholar]
- 37.Ben Arush MW, Solt I, Lightman A, Linn S, Kuten A. Male gonadal function in survivors of childhood Hodgkin and non-Hodgkin lymphoma. Pediatr Hematol Oncol 2000;17:239–245. [DOI] [PubMed] [Google Scholar]
- 38.Pidcock FS, Krishnan C, Crawford TO, Salorio CF, Trovato M, Kerr DA. Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurology 2007;68:1474–1480. [DOI] [PubMed] [Google Scholar]


