SUMMARY
Testosterone and estrogen are essential for male behaviors in vertebrates. How these two signaling pathways interact to control masculinization of the brain and behavior remains to be established. Circulating testosterone activates the androgen receptor (AR) and also serves as the source of estrogen in the brain. We have used a genetic strategy to delete AR specifically in the mouse nervous system. This approach permits us to determine the function of AR in sexually dimorphic behaviors in males while maintaining circulating testosterone levels within the normal range. We find that AR mutant males exhibit masculine sexual and territorial displays, but they have striking deficits in specific components of these behaviors. Taken together with the surprisingly limited expression of AR in the developing brain, our findings indicate that testosterone acts as a precursor to estrogen to masculinize the brain and behavior, and signals via AR to control the levels of male behavioral displays.
INTRODUCTION
All sexually reproducing animals exhibit gender dimorphisms in behaviors that are characteristic of the species. Such sex differences in behaviors can be observed in many displays, including in mating, territorial defense, and parental care. Gonadal steroid hormones play a critical role in the neural circuits that mediate sexually dimorphic behaviors: they organize the differentiation of these circuits in the developing animal, and activate these neural pathways to influence sex specific behaviors in the mature organism. Such an “organizational” effect is thought to lead to irreversible modifications in subsequent behavior, whereas the “activational” function of the hormones results in acute changes in the behavioral repertoire (Arnold et al., 2003; Goy and McEwen, 1980; Morris et al., 2004; Phoenix et al., 1959). Male typical patterns of behavior are controlled by both testosterone and estrogen in many vertebrates, including mammals. However, the relative contribution of these two hormone signaling pathways to the masculine differentiation of brain and behavior remains to be determined.
The requirement of estrogen for male behaviors appears counter-intuitive as this circulating ovarian hormone is essentially undetectable in the males of most species. Testosterone, or a related androgen, is an obligate precursor of estrogen, and circulating testosterone in males can be metabolized into estrogen in the brain by the enzyme aromatase (Balthazart and Ball, 1998; MacLusky and Naftolin, 1981; Naftolin and Ryan, 1975). It is this target derived estrogen that controls male behaviors, and male mice null for aromatase display profound deficits in male typical mating and aggression (Honda et al., 1998; Toda et al., 2001a; Toda et al., 2001b). The precursor-product relation between testosterone and estrogen raises the possibility that the sole function of testosterone in the neural control of male behaviors is to serve as a circulating prohormone for estrogen (Figure 1A). Alternatively, testosterone may act not only as a precursor for estrogen, but it may also signal via AR in neurons to drive male behaviors (Figure 1B). Consistent with the latter scenario, male mice constitutively mutant for AR do not mate or fight, and pharmacological studies also indicate a role for this receptor in controlling these behaviors (Finney and Erpino, 1976; Ohno et al., 1974; Sato et al., 2004; Wallis and Luttge, 1975). Importantly, these studies do not necessarily distinguish between a peripheral versus a neural-specific function of AR in regulating male behaviors. Moreover, such pharmacological studies often utilized dihydrotestosterone (DHT) to examine AR function, as this steroid is a non-aromatizable androgen. Recent evidence indicates, however, that 3βAdiol, a DHT metabolite found in vivo, is an estrogenic steroid capable of signaling via nuclear estrogen receptors (Ishikawa et al., 2006; Pak et al., 2005; Sikora et al., 2009; Wahlgren et al., 2008; Weihua et al., 2001). This makes it difficult to unambiguously define a role for AR in controlling male behaviors using DHT. The use of constitutive AR mutants is also not definitive in defining a role for AR in male behaviors as these animals have low, often undetectable, levels of circulating testosterone (Sato et al., 2004) (SAJ, unpublished observations) resulting from postnatal testicular atrophy. Consequently, the behavioral deficits in constitutive AR mutant males could result solely from inadequate estrogen synthesis and signaling in the brain due to the low levels of circulating testosterone.
Figure 1. Models for the role of testosterone in masculinizing the brain and behavior.
Schematics illustrating possible mechanisms whereby testosterone controls male typical behaviors.
(A) Testosterone acts as a circulating prohormone for estrogen synthesis via the action of aromatase in the brain. In this scenario, it is locally derived estrogen that masculinizes the brain and behavior.
(B) Testosterone is not only a prohormone for estrogen, but it also activates its cognate receptor, androgen receptor (AR), to influence directly the neural circuits that control male behaviors.
In contrast to the uncertainty regarding the role of AR in the neural circuits that control male mating and territoriality, the contribution of estrogen signaling to these behaviors is firmly established. Male mice doubly mutant for the nuclear estrogen receptors ERα, β display a complete abrogation of these masculine behaviors despite normal levels of circulating testosterone, indicating that this hormone cannot elicit male typical sexual and aggressive behaviors solely by signaling via AR (Dupont et al., 2000; Ogawa et al., 2000; Ogawa et al., 1997; Ohno et al., 1974; Sato et al., 2004; Wersinger et al., 1997) (MVW, unpublished observations). Both testosterone and estrogen, however, appear critical during development and adult life in males for the display of dimorphic behaviors such as inter-male aggression (Bakker et al., 2006; Finney and Erpino, 1976; Motelica-Heino et al., 1993; Wu et al., 2009). Consistent with the apparent non-redundant requirement for these hormones in male mating and fighting, their cognate receptors are expressed in overlapping, but not identical, sexually dimorphic patterns in neuronal populations critical for these behaviors (Perez et al., 2003; Shah et al., 2004; Simerly et al., 1990). Thus, AR and ERα, β are widely expressed in inter-connected limbic regions such as the medial amygdala, the bed nucleus of the stria terminalis, and the preoptic hypothalamus. Despite numerous studies documenting a role for testosterone in male specific patterning of gene expression and behavior, the extent to which this hormone signals through AR for masculinizing the brain and behavior remains unclear (Juntti et al., 2008).
Our findings indicate that AR is unlikely to play a major role in the differentiation of the neural circuits that control male typical behaviors. We find a surprisingly sparse expression of AR in the developing brain in areas such as the bed nucleus of the stria terminalis and preoptic hypothalamus that are thought to be important for dimorphic behaviors. By comparison, the estrogen receptors and aromatase are expressed by many neurons in these regions at birth, and we show that estrogen signaling is necessary and sufficient for the sexual differentiation of AR within these populations. In addition, we find that male mice bearing a nervous system restricted deletion of AR exhibit a masculine repertoire of sexual and territorial behaviors with diminutions in specific components of these displays. Adult male mice mutant for AR in the brain have normal levels of circulating testosterone, and these behavioral deficits therefore reflect a requirement for AR in male behaviors rather than inadequate circulating testosterone for estrogen synthesis in the brain. Taken together, our results indicate that testosterone signaling via AR does not control masculine differentiation of the brain and behavior. Rather, AR signaling regulates the extent of male typical behavioral displays.
RESULTS
Sparse expression of AR in the developing brain
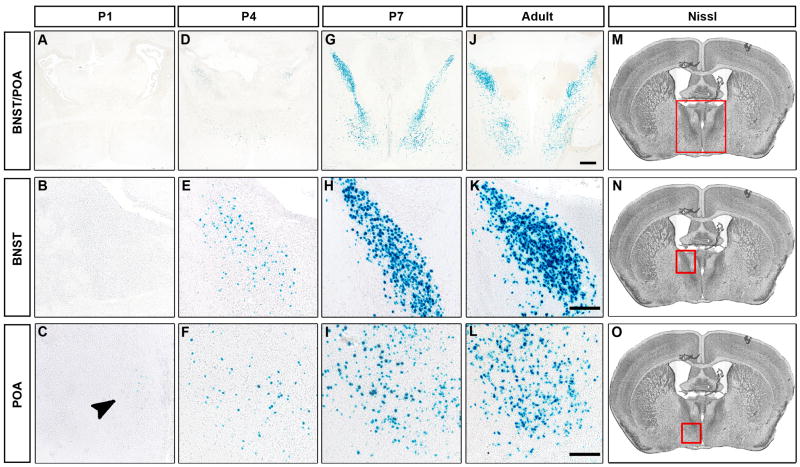
There is a male specific transient spike in serum testosterone at birth (postnatal day 1, P1) followed by a sharp drop in circulating titer within 36 hours, and these low baseline levels of testosterone persist until puberty. By contrast, the ovaries are quiescent during the neonatal period, and there is little circulating estrogen (or testosterone) in female pups (McCarthy, 2008; Motelica-Heino et al., 1988). The male specific surge of testosterone and its subsequent conversion to estrogen is thought to be critical for the sexual differentiation of the neural circuits that control many dimorphic behaviors (Motelica-Heino et al., 1993; Peters et al., 1972; Phoenix et al., 1959; Whalen and Nadler, 1963; Wu et al., 2009). We wished to identify the neurons that respond to this critical testosterone surge via AR at birth. We analyzed AR expression in mice bearing a previously described knock-in AR allele (AR-IPIN allele) that permits co-expression of the sensitive, genetically encoded reporter, nuclear β-galactosidase (βgal), in all cells that express AR (Shah et al., 2004). Previous work has implicated AR expressing regions such as the medial amygdala (MeA), posterior medial component of the medial subdivision of the bed nucleus of the stria terminalis (BNST), and the preoptic hypothalamus (POA) as being critical for the display of male typical mating, aggression, and territorial marking (Commins and Yahr, 1984; Kondo et al., 1998; Liu et al., 1997; Meisel and Sachs, 1994). Surprisingly, we only detected occasional, faintly AR positive cells in these regions at P1 (Figures 2A–C and S1D). There were >15–90 fold fewer AR expressing cells in these regions at birth (BNST 12.1 ± 5.7; MeA 16.4 ± 10.3; POA 59.8 ± 20.3; n = 6) than in older animals (Figure 3) (Shah et al., 2004; Wu et al., 2009). Similar results were obtained by directly immunolabeling for AR (data not shown). We could detect more AR positive cells in the MeA, BNST, and POA at P4, a timepoint by which the testosterone surge has already subsided (Figures 2D–F and S1E), but even at this age there appeared to be significantly fewer AR expressing cells than observed in adults. In contrast to this sparse and faint AR labeling in the BNST, POA, and MeA, AR expression could be reliably detected in a small pool of neurons in the vicinity of the arcuate (ArcN) and ventromedial (VMH) nuclei of the hypothalamus at P1 and P4 (Figure S1A, B). The expression pattern of AR in the BNST, POA, and MeA resolved into widespread, intense labeling in these areas at P7, resembling the pattern observed in the adult brain (Figures 2G–L, S1, and data not shown).
Figure 2. Limited expression of AR in the newborn brain.
(A–L) Coronal sections through the brain of male P1, P4, P7 and adult males bearing the AR-IPIN allele stained for βgal activity. There are few βgal+ cells in the BNST or POA at P1 (arrowhead, C). There are more βgal+ cells at P4 in these areas, and by P7 the number of cells approximates that observed in the adult brain. Scale bar equals 500 μm (top row), and 100 μm (bottom two rows).
(M–O) Nissl stained sections highlighting (red box) the BNST (M, N) and POA (M, O). n ≥ 3 at P1, P4, P7.
See also Figures S1, S2.
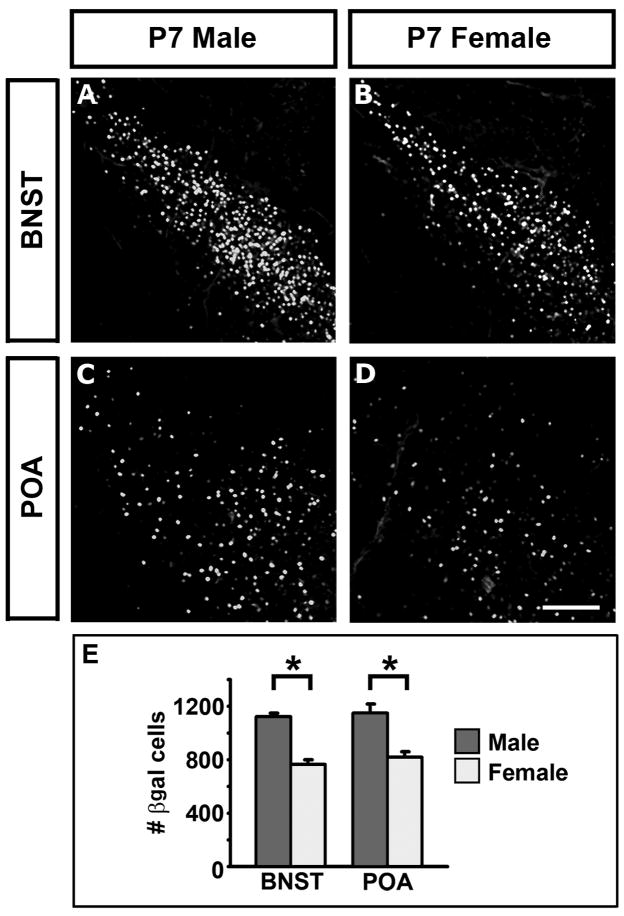
Figure 3. Sexual dimorphism in AR expression.
(A–D) Coronal sections through the BNST and POA of P7 mice bearing the AR-IPIN allele immunolabeled for βgal.
(E) There are more βgal+ cells in the BNST and POA of ARIPIN/Y males than in ARIPIN/IPIN females.
Mean ± SEM; n = 4 for each genotype, * p ≤ 0.005. Scale bar equals 100 μm.
See also Figure S2.
The fetal testis produces testosterone from E13 (Crocoll et al., 1998) and we wondered if this hormone signaled via AR in the prenatal brain to masculinize neural pathways. We did not observe AR in the brain at E13.5, using βgal expression to visualize AR positive cells in mice bearing the AR-IPIN allele (Figure S2A–D). By contrast, at E15.5 and E17.5, we could visualize AR expression in the neurons near the ArcN and VMH but not in the BNST, POA, or MeA (Figure S2E–L). The ontogeny of AR expression in the BNST, POA, and MeA makes it unlikely that this hormone receptor plays a major, cell-autonomous role in masculinizing these neural pathways for male typical behaviors prenatally or at the time of the neonatal testosterone surge.
Estrogen is necessary and sufficient for sexual differentiation of AR expression
Previous work demonstrates that adult AR expression is sexually dimorphic such that there are more AR positive neurons in the BNST, POA, and the basal forebrain in males compared to females (Shah et al., 2004). AR expression in the BNST and POA in the P7 male resembles that observed in the adult male (Figure 2G–L) whereas we did not observe AR positive cells in the basal forebrain at P7 (data not shown). We asked whether the adult pattern of sexual dimorphism in AR expression was also apparent at P7 in the BNST and POA. Immunolabeling for βgal at P7 revealed significantly more AR positive cells in the male BNST and POA than in these regions in the female (Figure 3), consistent with previous reports of sexual dimorphism in these regions (McAbee and DonCarlos, 1998; Shah et al., 2004).
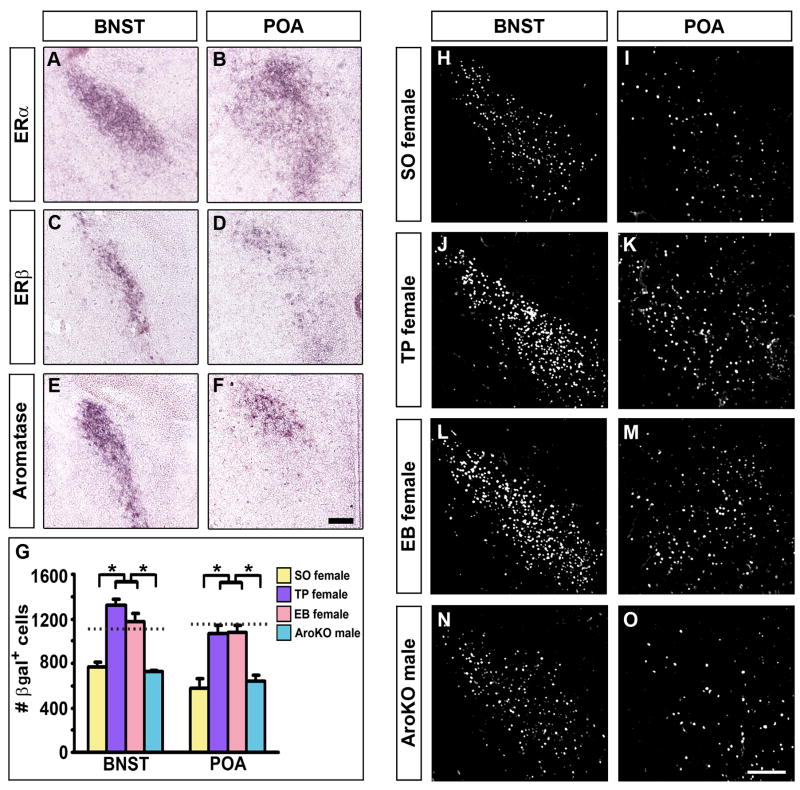
The sexual dimorphism in AR expression is unlikely to arise from testosterone signaling via AR as this receptor is expressed in few cells in these regions at the time of the testosterone surge. Rather it is likely to result from the autonomous action of forebrain patterning genes or from estrogen signaling (Arnold et al., 2003; Hoch et al., 2009; Wu et al., 2009). Previous work indicates that both nuclear estrogen receptors (ERα, β) as well as aromatase are expressed in the early neonatal brain (Harada and Yamada, 1992; Wolfe et al., 2005; Wu et al., 2009). Indeed, we observed abundant expression of ERα, β and aromatase in the BNST and POA at P1 (Figure 4A–F), consistent with the notion that testosterone may masculinize AR expression after its conversion into estrogen.
Figure 4. Estrogen masculinizes AR expression.
(A–F) Coronal sections through the BNST and POA of P1 males labeled for ERα, ERβ, or aromatase mRNA.
(G–O) There are more βgal+ cells in the P7 BNST and POA of ARIPIN/IPIN females treated at P1 with testosterone (TP female) or estrogen (EB female) compared to vehicle (SO female) or to aromatase−/−; ARIPIN/Y (AroKO) males. Horizontal dashed lines represent the mean value in WT males shown in Figure 3. Mean ± SEM; n = 4 for each group of mice, * p ≤ 0.01 by Tukey’s posthoc test following one-way ANOVA. Scale bar equals 200 μm (A–F) and 100 μm (H–O).
If testosterone does masculinize AR expression in the BNST and POA subsequent to aromatization to estrogen, then either of these two hormones should be sufficient to drive male pattern differentiation of AR in these regions. Indeed, we find that administering testosterone or estrogen to P1 females masculinizes the number of AR positive cells in the P7 BNST and POA (Figure 4G–M), consistent with previous pharmacological studies in other vertebrates (Kim et al., 2004;McAbee and DonCarlos, 1999a, b). To determine whether the conversion of testosterone to estrogen is essential for the development of these sex differences in AR expression, we examined the BNST and POA of P7 aromatase−/− males (Honda et al., 1998). In these animals, the number of AR positive cells is indistinguishable from that observed in control females, and significantly lower than in control males (Figure 4G, N, O). Taken together, these results show that estrogen controls the sexual differentiation of AR expression in the BNST and POA in males. We note that the few AR positive cells in the P1 BNST and POA may also respond to the testosterone surge at birth by inducing sexual differentiation of AR in neighboring cells that do not express this receptor; in such a scenario, both estrogen and testosterone signal via their cognate receptors to regulate the sex difference in AR expression in a redundant manner. Nevertheless, our findings demonstrate that estrogen is necessary and sufficient to drive masculinization of AR expression in these brain regions.
Genetic deletion of AR in the nervous system
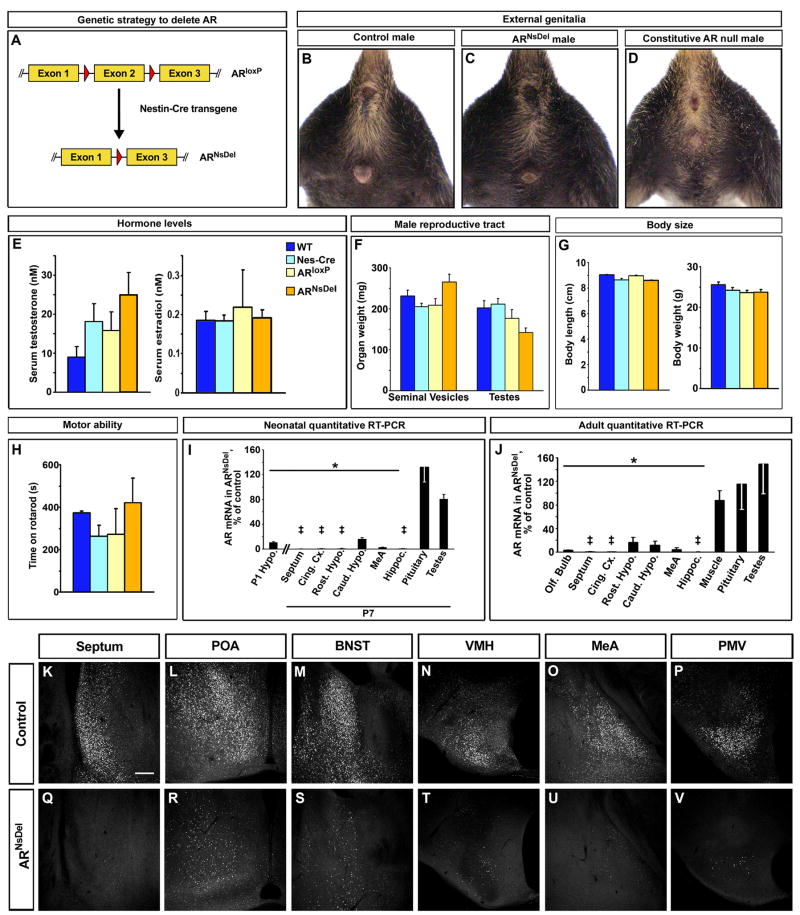
Our results suggest that testosterone serves primarily as a precursor for estrogen during the neonatal period of sexual differentiation of the brain. Consequently, testosterone signaling via AR is unlikely to be essential for the differentiation of the male typical repertoire of dimorphic behaviors. Constitutive deletion of AR in all tissues results in feminization of the external genitalia, and eventual testicular atrophy, leading to a loss of circulating testosterone in adults (Lyon and Hawkes, 1970; Sato et al., 2004). To bypass the requirement for AR in the testes, we used a Cre-loxP strategy to engineer a deletion of AR specifically in the nervous system. We crossed mice bearing a previously described loxP-flanked allele of AR (ARloxP) (De Gendt et al., 2004) to animals harboring the Nestin-Cre transgene (Nes-Cre) (Tronche et al., 1999) that drives Cre recombinase specifically in neural stem cells and glia (Figure 5A). This strategy should yield an early, nervous system restricted deletion of AR in males carrying the X-linked ARloxP and the Nes-Cre transgenic alleles. Indeed, in contrast to the external phenotypes observed in constitutive AR mutant males, an examination of adult ARloxP/Y; Nes-Cre (ARNsDel) mice suggests normal AR function in non-neural tissues (Figure 5B–D, F): the genitalia of these mice are masculinized, and the testes and seminal vesicles, sensitive peripheral tissues responsive to AR signaling, are similar in weight to those of their control littermates (wildtype (WT), ARloxP/Y, and Nes-Cre males). While there appears to be an elevation in circulating testosterone in ARNsDel mice, this is not statistically different when compared to the titers of this hormone in males of the control genotypes (p = 0.157, Kruskal-Wallis test for multiple group comparisons) (Figure 5E). The titers of circulating estrogen in these mutants are also comparable to those of the control males (Figure 5E). These findings suggest that testicular function is likely unimpaired in the absence of AR in the nervous system. Indeed, we find that ARNsDel mutants can sire litters when co-housed with wildtype females (data not shown), and that AR transcript levels in the testis as well as the pituitary are comparable between ARNsDel mice and their controls (Figure 5I, J).
Figure 5. Targeted deletion of AR in the nervous system.
(A) Genetic strategy to delete AR in the nervous system.
(B–D) Adult external genitalia and milk line are masculinized in control and ARNsDel males, but not in constitutively null AR males.
(E) Similar levels of serum testosterone and estrogen in all males (n ≥ 12/genotype).
(F) Similar weight of testes and seminal vesicles in all males (n ≥ 7/genotype).
(G) Similar body length (snout to base of tail, n ≥ 4/genotype) and weight (n ≥ 12/genotype) in all males.
(H) No difference in time to fall from rotarod between control and ARNsDel males (n ≥ 4/genotype).
(I, J) Reduction in normalized AR mRNA in the brain of P1 (I), P7 (I), and adult (J) ARNsDel males shown as percent of AR mRNA levels in controls (Hypo., hypothalamus; Cing. Cx., cingulate cortex; Rost. Hypo., rostral hypothalamus; Caud. Hypo., caudal hypothalamus; Hippoc., hippocampus). Similar AR mRNA levels between ARNsDel and control males in other tissues. Mean ± SEM; ‡ mRNA < 0.5% of control, * p < 5×10−4, n = 4 for each genotype. (K–V) Fewer AR immunolabeled cells are visualized in coronal sections through septum, POA, BNST, VMH, MeA and ventral premamillary nucleus (PMV) in ARNsDel males compared to control males. Scale bar equals 100 μm.
See also Figure S3.
Inadvertent deletion of AR in non-neural tissues such as muscle could result in a failure to thrive, a generalized motor deficit or muscular weakness. However, ARNsDel mice appear indistinguishable from their controls in body length and weight (Figure 5G). We also did not observe abnormal gait or gross motor deficits in our behavioral assays with these mutants (data not shown). In addition, ARNsDel males were similar to their control littermates in general motor activity and social interactions such as grooming (Figure S3A–C). When assayed for motor performance on the rotarod, ARNsDel mice performed equivalently to control males (Figure 5H). In accord with these findings, quantitative RT-PCR (qPCR) also reveals comparable levels of AR mRNA in skeletal muscle obtained from ARNsDel and control males (Figure 5J).
In contrast to these findings in non-neural tissues, we observe a profound reduction in AR expression in the brain of adult ARNsDel males. Using qPCR, we find a large diminution in the levels of AR mRNA in various brain regions known to express this receptor (Shah et al., 2004; Simerly et al., 1990), including in the MeA, BNST, POA and other parts of the hypothalamus, olfactory bulbs, cingulate cortex, lateral septum, and the hippocampus (Figure 5J). Nes-Cre drives recombination in neural stem cells, and we therefore asked if the postnatal expression of AR was abolished in ARNsDel pups. We find a dramatic decrease in AR expression at P1 as well as P7 in these mutants compared to their control male littermates (Figure 5I). In agreement with these results, immunolabeling reveals very few AR positive cells in the forebrain of adult ARNsDel males compared to controls (Figure 5K–V). Taken together, our genetic strategy yields male mice that have intact peripheral masculinization and circulating testosterone and a deletion of AR that appears restricted to the nervous system. These mutants therefore afford the opportunity to assess the contribution of testosterone signaling via AR in the neural circuits that control male typical behaviors.
AR increases the frequency of male sexual behavior
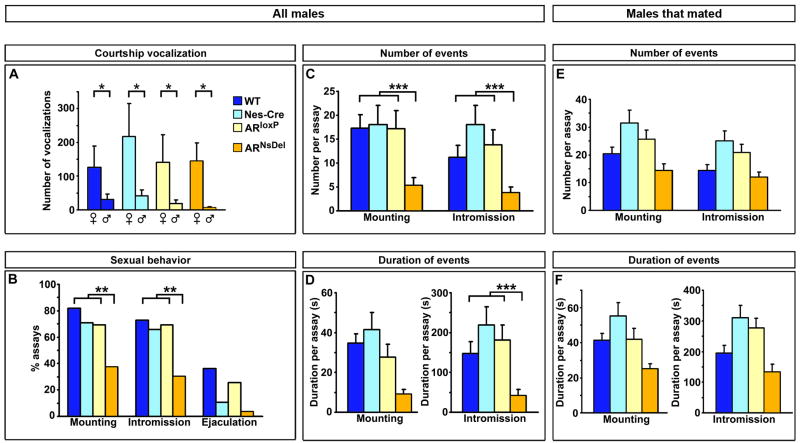
In order to determine whether AR mediated signaling in the nervous system is essential for male sexual behavior, we examined the behavior of ARNsDel mice in mating assays. In mice, mating consists of a series of stereotyped routines that include mounting, intromission or penetration (as visualized by pelvic thrusting), and ejaculation. These behaviors can be reliably elicited in a 30 minute assay in which a WT estrous female is introduced into the cage of a singly housed WT male (Mandiyan et al., 2005; McGill, 1962). A statistically similar percent of ARNsDel residents (42%; n = 24 mice) mounted at least once when tested in 2–3 assays for sexual behavior with different estrous females when compared to control residents (Figure S4A). When we analyzed the percent of all assays with mounts, we observed mating in fewer assays with ARNsDel residents compared to males in the control cohort (Figure 6B). Notably, we find that some of the ARNsDel mice never mate, whereas when they do exhibit sexual behavior, these mutants mate in most assays (85% ± 6), similar to control residents (Figure S4G).
Figure 6. AR increases the frequency of male mating.
(A) ARNsDel and control males emit more ultrasonic vocalizations to female than male intruders.
(B) ARNsDel males mount and intromit females in fewer assays than controls.
(C) As a group, ARNsDel males exhibit fewer mounts and intromissions than control males.
(D) No statistical difference between ARNsDel and control males in time spent mounting, but as a group, ARNsDel mice intromit for a shorter duration than controls.
(E, F) Once male mating is initiated, there is no difference between ARNsDel males and their control cohorts in the total number (E) or duration (F) of mounts and intromissions.
Mean ± SEM; * p < 0.033, n ≥ 3/genotype; ** p < 0.05, post hoc Bonferroni’s correction for Fisher’s exact test, n ≥ 12/genotype; *** p < 0.05, Tukey’s test following Kruskal-Wallis comparison, n ≥ 12/genotype.
The lowered probability of initiating mating across all assays was also reflected in the diminution of the number of mounts and intromissions as well as in the duration of intromissions exhibited by ARNsDel animals (Figure 6C, D). Male mice do not always achieve ejaculation within a 30 minute assay (McGill, 1962), and we observed ejaculation at a similar, low frequency in males of all genotypes (Figures 6B and S4A). The deficits in some parameters of sexual behavior in ARNsDel mice reflected an analysis of all assays, including those in which the resident did not initiate mating. We also examined these behavioral parameters by restricting our analysis to include only the assays in which males mated. Strikingly, this analysis revealed that once ARNsDel mice initiate sexual behavior, they mate in a manner similar to their controls (Figures 6E, F and S4C–F; Supplementary Movies S1, S2).
Thus, while ARNsDel mutants are less likely to mate, once sexual behavior is initiated, its display appears similar to that of wildtype males. This suggests that AR controls the probability of triggering male mating but not the pattern of this complex behavioral routine. Alternatively, the lowered likelihood of initiating mating behavior could simply reflect a large variability in the extent of AR deletion in the brain. In this scenario, ARNsDel mice who do not mate may have little residual AR in the brain compared to the mutants who do initiate sexual behavior. Several lines of evidence favor the notion that AR regulates the probability of triggering male mating. First, qPCR analysis of AR deletion in individual ARNsDel males reveals a consistent, strong diminution of AR message in all mutants, regardless of their performance in mating assays (Figure S3D). Second, when ARNsDel mutants who did not mate in any of the three 30 minute mating assays were co-housed with females, they successfully sired litters (SAJ, preliminary observations). Finally, only a subset of constitutive AR mutant males attempted to mate when supplemented with testosterone (or estrogen) at doses that recapitulate wildtype circulating levels of this steroid hormone (Olsen, 1992; Sato et al., 2004) (MVW, unpublished observations). Taken together, these findings are consistent with the notion that AR functions in the brain to regulate the likelihood of initiating male sexual behavior.
Chemosensory cues emanating from females are critical for triggering male sexual behavior, and WT males engage in extensive anogenital chemoinvestigation of females prior to initiating sexual behavior (Keverne, 2004; Mandiyan et al., 2005; Yoon et al., 2005). The reduced frequency of male sexual behavior we observe with ARNsDel mice may be a consequence of deficits in such chemoinvestigation. However, we find that ARNsDel animals chemoinvestigate conspecifics in a manner comparable to their control male littermates (Figures S4B, S5C). Previous work shows that, unlike WT males, constitutive AR mutants have a preference for male rather than female odors (Bodo and Rissman, 2007). Chemosensory cues from the two sexes leads to gender discrimination, one consequence of which is female-directed ultrasonic vocalizations by the resident male (Nyby et al., 1977; Pankevich et al., 2004; Stowers et al., 2002). We observe that ARNsDel mice vocalize to female, but not to male, intruders in their cage in a manner similar to their control counterparts, suggesting that sex discrimination is intact in these animals (Figure 6A). Taken together, these results suggest that the reduced frequency of male sexual behavior of ARNsDel mice is not a consequence of reduced chemoinvestigation of females or an inability to distinguish the sexes.
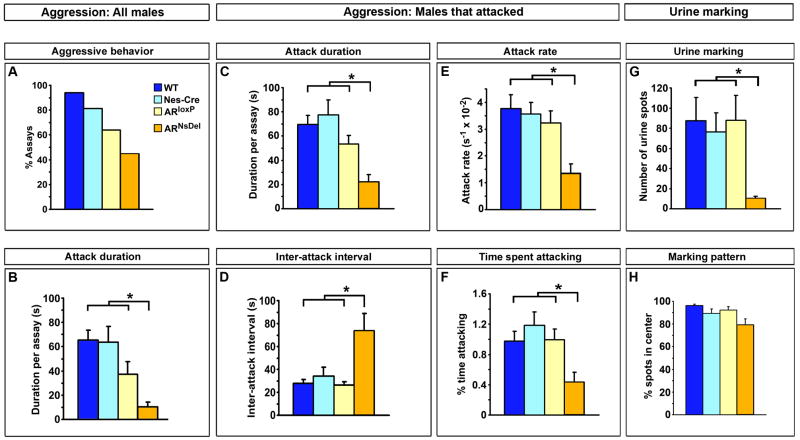
AR controls the degree of male territorial behaviors
We next tested ARNsDel animals in assays of male territorial behaviors. Singly housed WT male but not female resident mice attack male intruders in their homecage (resident-intruder aggression test) (Miczek et al., 2001). ARNsDel males were less aggressive than control residents by several measures. Although there was no statistical difference in the number of attacks or in the percent of tests with aggression (Figures 7A, S5A, B), the mutants spent significantly less time fighting compared to control residents, and this deficit persisted even when we restricted our analysis to the assays in which we observed aggressive interactions (Figure 7B, C). The reduction in total duration of attacks cannot be explained by alterations in chemoinvestigation, the latency to first attack, or by the total attack number, as these parameters were statistically similar in all resident males (Figures S5B–E). Rather, our analysis revealed a deficit in the pattern of aggression following the first fight initiated by ARNsDel males (Figures 7D–F and S5F). Compared to the control residents, ARNsDel mice spent less time fighting with the intruder and exhibited a longer interval between successive attacks (Figure 7D, F). While ARNsDel residents and their controls attacked a similar number of times in an assay (Figure S5E), the mutants exhibited a lower attack rate (Figure 7E), initiating fewer attacks per unit time following the first fight. Unlike the attack number metric, a measurement of attack rate eliminates from analysis the variable latency to the first attack in any particular assay, and as such represents a corrected, perhaps more sensitive, measure of the frequency of fighting. As part of territorial behavior, resident WT male mice mark their territory by depositing many urine spots across the cage floor, whereas females pool their urine in one or a few large spots in a corner of the cage (Desjardins et al., 1973; Kimura and Hagiwara, 1985). Thus, there is a dimorphism in the number as well as the pattern of urine marks deposited by male and female mice. The male pattern of urine marking appears independent of AR function in the nervous system as ARNsDel residents also distribute their urine marks across the cage floor, similar to WT males (Figure 7H). By contrast, we find that ARNsDel residents deposit fewer urine marks compared to control resident males (Figure 7G). Importantly, ARNsDel males deposit more urine marks (10.2 ± 2.1 spots) than WT females who pool urine (1.7 ± 0.5 spots) (Wu et al., 2009), suggesting that AR is not required to masculinize this parameter of urine marking, but rather AR enhances the display of this behavior. Taken together, these findings show that AR functions in the nervous system to control specific parameters of male typical urine marking and fighting.
Figure 7. AR increases the levels of male territorial displays.
(A) No statistical difference between ARNsDel and control males in percent assays containing aggression.
(B) As a group, ARNsDel residents attack WT male intruders for a shorter duration compared to controls.
(C) When ARNsDel residents fight, they do so for a shorter duration compared to controls.
(D–F) In assays with fighting, ARNsDel residents exhibit an increase in time between fights (D), attack at a slower rate (E) and for a smaller percent of the duration of the assay (F) compared to controls.
(G) ARNsDel males deposit fewer urine marks compared to controls.
(H) No difference between ARNsDel and control males in the percent of urine marks away from cage perimeter.
Mean ± SEM; * p < 0.05, Tukey’s test following Kruskal-Wallis comparison, n ≥ 12/genotype.
See also Figure S5.
DISCUSSION
We find that male mice lacking AR in the nervous system can initiate masculine sexual and territorial displays. However, these mutants exhibit striking deficits in the pattern or the extent of these behaviors. Taken together, our findings demonstrate that AR is not essential for the masculinization of mating, aggression, and urine marking. Rather, AR signaling serves to amplify the display of this behavioral repertoire in males.
Our genetic strategy permits us to define a functional contribution of AR signaling in the neural circuits that mediate male behaviors. Using an approach identical to ours, a recent study also showed deficits in mating and fighting in males lacking AR in the nervous system (Raskin et al., 2009). Differences in the phenotypes reported in that study compared to our findings likely arise from variations in the experimental design or strain differences. Here, we have significantly refined the analysis of ARNsDel mutants to provide new mechanistic insight into the role of AR in masculinizing the brain and behavior. We have compared ARNsDel males to each of the control genotypes (WT, Nes-Cre, ARloxP/Y) to assess the contributions of these distinct genetic backgrounds to all mutant phenotypes. We have also developed analytical tools to examine in an extensive manner the behavioral deficits in sexual and territorial behaviors of ARNsDel males. In the absence of AR function in the nervous system, males discriminate between the sexes and initiate appropriate behavioral responses, mating with females and fighting with males. Our analysis of male mating suggests that AR in the brain regulates the probability of triggering sexual behavior but not the pattern of various components of male mating. Our analysis of territorial behaviors reveals a previously unreported role of AR in the brain in controlling the duration and pattern of inter-male aggression. We find that ARNsDel mutants mark their territory in a male pattern, but they deposit far fewer urine marks, indicating a deficit in this component of male territorial display. Our studies also indicate that it is unlikely that the masculine behaviors observed in AR mutants result from a failure to delete AR prior to the early neonatal sexual differentiation of the brain. Indeed, we find that there is minimal AR expression in regions known to be critical for dimorphic behaviors during this period, when gonadal hormones orchestrate sexual differentiation of the brain (Meisel and Sachs, 1994; Morris et al., 2004; Motelica-Heino et al., 1993), and this sparse neonatal AR expression is largely eliminated in ARNsDel males. Importantly, we demonstrate that sexual differentiation of AR expression itself is controlled by estrogen signaling. The sparse perinatal expression of AR in the brain suggests that the behavioral phenotype of ARNsDel mice results from activational rather than organizational effects of testosterone acting on AR. We cannot exclude the possibility that AR also functions during the later postnatal period, including puberty, to influence the maturation of the neural circuits that drive male behaviors (Schulz et al., 2009). Regardless of the exact timepoint at which AR functions to control behaviors, our findings indicate that AR is not a master regulator for male behaviors, but rather, it serves as a gain control mechanism to regulate the extent of male sexual and territorial displays.
We find minimal AR expression in various brain regions of ARNsDel males, demonstrating that most neurons do not need to signal via this receptor to drive male sexual and territorial behaviors. As we have deleted AR in the developing and adult animal, we cannot exclude the possibility that masculine differentiation of behaviors in these mutants reflects compensatory mechanisms that are activated in the absence of AR signaling. Mice exhibit a large array of behavioral dimorphisms beyond sexual and territorial displays, and it will be important in future studies to determine whether AR function in the nervous system is essential for the appropriate display of such behaviors (Zuloaga et al., 2008a; Zuloaga et al., 2008b). Nevertheless, our study indicates that AR functions in the nervous system to control various parameters of male sexual and territorial behaviors, but it is not essential to masculinize this behavioral repertoire in mice.
A model for hormonal control of male sexual and territorial behaviors
Testosterone is essential for male behaviors. We set out to distinguish two competing models of testosterone’s function in male behaviors: in one scenario, testosterone simply serves as a prohormone for estrogen in the brain, and it is estrogen signaling via its cognate receptors that masculinizes the brain and behavior (Figure 1A). Alternatively, testosterone may serve not only as a precursor for estrogen, but it may also signal via AR to control male behaviors (Figure 1B). We find that male mice mutant for AR in the nervous system do not exhibit male typical levels of mating and territorial behaviors. AR can regulate the activity and expression of aromatase (Roselli et al., 2009), and therefore might serve to amplify male behaviors by regulating the levels of local estrogen synthesis. However, supplementation of constitutive AR mutants with estrogen does not restore mating and territorial displays to wildtype levels (Olsen, 1992; Sato et al., 2004; Scordalakes and Rissman, 2004). Such studies therefore suggest that AR in the brain may also control the expression of other genes that modulate the levels of male behavioral displays. Irrespective of the exact molecular mechanisms, our data demonstrate that testosterone signaling via AR is essential for wildtype male behavior.
The dual requirement for estrogen and testosterone in masculinizing the brain for sexual and territorial behaviors immediately poses the question of whether these two signaling pathways operate independently or via epistatic interactions. Mice of both sexes exhibit male mating behavior, whose display can be modulated by sensory as well as hormonal cues (Edwards and Burge, 1971; Jyotika et al., 2007; Kimchi et al., 2007; Martel and Baum, 2009). These findings suggest that the neural circuit for male mating is present in both sexes. Nevertheless, the neural control of some components of sexually dimorphic behaviors is thought to differentiate under the control of the perinatal testosterone surge (Arnold et al., 2003; Morris et al., 2004). The sparse expression of AR in the perinatal period, however, suggests that the masculinization of neural pathways in response to the testosterone surge at birth proceeds primarily under the control of estrogen.
We have recently demonstrated that the sexual differentiation of aromatase expressing neurons in the BNST and the MeA is independent of AR and is controlled by estrogen (Wu et al., 2009). Similarly, estrogen has been shown to regulate the dimorphic expression of other genes in these regions as well as in the POA (Amateau and McCarthy, 2004; Scordalakes and Rissman, 2004; Simerly et al., 1997). It is difficult to completely exclude a function of AR in sexual differentiation of these limbic regions (Bodo and Rissman, 2008; Han and De Vries, 2003). However, our data constrains such a requirement to operate via a cell non-autonomous mechanism as AR is not expressed in the vast majority of cells in these areas at the time of the testosterone surge. By contrast, the perinatal expression of AR we observe in the vicinity of the VMH could potentially direct the previously described sexual differentiation of this nucleus (Dugger et al., 2007) in a cell autonomous manner. We show here that the masculinization of AR expression in the BNST and POA, two limbic regions previously implicated in sexual and territorial behaviors, is controlled by estrogen signaling. This postnatal sexual differentiation of AR is unlikely to result from estrogen regulated neurogenesis as neurons that populate these regions are born prenatally (al-Shamma and De Vries, 1996; Bayer, 1980; Bayer and Altman, 1987). Previous work has implicated dimorphic apoptosis as playing a critical role in the sexual differentiation of the BNST, POA, and other brain regions (Arai et al., 1996; Davis et al., 1996; Forger, 2009; Holmes et al., 2009; Waters and Simerly, 2009; Wu et al., 2009). It is therefore possible that the dimorphism in AR expression is a consequence of estrogen regulated cell survival. Estrogen may also control the dimorphism in AR expression by directly regulating the transcription of this gene via its nuclear hormone receptors. Regardless of the exact mechanism, our findings indicate that estrogen signaling drives the sexual differentiation of AR expression, and that it is also likely to control much of the perinatal masculinization of the brain.
The behavioral deficits of ARNsDel males are strikingly reminiscent of the behavioral phenotype of females treated with neonatal estrogen. As adults, such neonatally estrogen treated females respond to endogenous estrogen by exhibiting male patterns of mating and territorial displays at reduced levels compared to WT males (Wu et al., 2009) but similar to those observed in the ARNsDel males. Unlike ARNsDel males, however, these females do not have masculine levels of circulating testosterone, and depend on ovarian hormones to demonstrate male typical behaviors. Upon provision of exogenous testosterone in adult life, such neonatally estrogen treated females appear to mate, fight, and mark territory in a manner comparable to WT males. Taken together, these complementary findings suggest that testosterone signals via AR in the adult male to augment the male pattern behaviors that have differentiated under the control of estrogen signaling. Such a model is also consistent with the observation that testosterone signaling via AR is insufficient to elicit masculinized sexual or territorial behaviors in male mice doubly mutant for ERα, β. These diverse findings suggest a model for the control of male pattern behaviors in which estrogen masculinizes the neural circuits for mating, fighting and territory marking, and testosterone and estrogen signaling generates the male typical levels of these behaviors. It will be interesting in future studies to identify the molecular and circuit level mechanisms that are controlled by these hormones.
EXPERIMENTAL PROCEDURES
Animals
Mice were housed in a rodent barrier facility at UCSF with a 12:12 hour light:dark cycle. All studies with animals were done in accordance with UCSF IACUC protocols. The AR-IPIN knock-in and aromatase knockout mice have been described previously (Honda et al., 1998; Shah et al., 2004). Animals bearing the ARloxP allele (De Gendt et al., 2004) or Nes-Cre transgene (Tronche et al., 1999) were maintained on a mixed background (C57Bl/6J and 129/Sv). We mated females heterozygous for ARloxP to hemizygous Nes-Cre males to generate males bearing both alleles (ARloxP/Y; Nes-Cre) as well as control males (WT, ARloxP/Y, and Nes-Cre). Animals were weaned and group-housed by sex at 3 weeks of age.
Behavioral assays
We used adult, singly housed male mice in behavioral assays, which were performed in the dark cycle. The behavioral testing and analysis was done as described previously (Wu et al., 2009). In brief, the male was first tested for urine marking for 1 hour in a fresh cage, and then returned to the homecage. Males were subsequently tested for male mating for 30 min with an estrous intruder female. Following mating tests, mice were tested for aggression for 15 min in the resident-intruder paradigm, using an adult male intruder who was group-housed with other intruders between testing sessions. The males were subsequently tested for ultrasonic vocalizations for 3 min in response to an intruder in their cage. Each resident was tested for vocalization separately with a male and a female intruder. All animals were tested 2–3 times each in assays of mating and aggression, and once each for urine marking and ultrasonic vocalization. Experimental animals were always exposed to intruder mice they had not encountered previously, and each assay was separated by ≥ 2 days.
Histology
We used age matched mice for histological experiments. We visualized βgal activity in 20 μm thick sections using brightfield optics. Fluorescent immunolabeled sections (20 μm thick, P7; 65 μm thick, adult) were imaged using confocal microscopy. The primary antisera used in this study are monoclonal rabbit anti-AR (1:750, Epitomics) and mouse anti-βgal (1:2500, Promega). The anti-AR antibody appears specific to AR as we did not observe AR+ cells in various brain regions in constitutive AR mutant males (Tfm) (Figure S3). Staining for βgal activity and fluorescent immunolabeling were performed as described previously (Shah et al., 2004; Wu et al., 2009). Quantitation of cell numbers was performed using stereology and other experimental approaches.
Brain regions were identified based on landmarks as defined in standard atlases of the mouse brain (Paxinos and Franklin, 2001; Paxinos et al., 2007). At P1 and P7, βgal expressing cells in the anterior hypothalamus and BNST were found within the POA and posterior medial component of the medial subdivision of the BNST (also referred to as the principal nucleus of the BNST). Thus, the differences in cell number we observe between males and females and other experimental animals in these regions cannot be accounted for simply by changes in local distribution or cell density. Similar results were obtained when the quantitation was done by a second investigator.
Data analysis
Quantitation of behavioral and histological data was performed blind to relevant variables, including sex, genotype, and hormone treatment. To analyze categorical data, we used Fisher’s exact test and a post-hoc Bonferroni’s correction for multiple group comparisons. For other comparisons, we first analyzed the distribution of data with Lillefors’ goodness-of-fit test of normality. Datasets not violating this test were analyzed with parametric tests (Student’s t-test for 2 groups or one-way ANOVA), otherwise we used non-parametric analyses (Kolmogorov-Smirnov (KS) test for 2 groups or Kruskal-Wallis test). We used Tukey’s post hoc test following one-way ANOVA and Kruskal-Wallis tests to determine which groups differed significantly. For all experiments, we deemed an effect of the ARloxP/Y; Nes-Cre (ARNsDel) genotype to be statistically significant only if this genotype differed from each of the control cohorts (WT, ARloxP/Y, and Nes-Cre).
qPCR
At each age, we collected tissue from ARNsDel mice and each control group to quantitate AR mRNA levels. Each tissue sample for individual animals was processed separately for RNA extraction, cDNA synthesis, and qPCR. We used separate qPCR reactions to detect AR and the ubiquitous ribosomal protein Rpl32, which was used for normalization of AR expression. As AR expression was similar across all control genotypes but was significantly different from ARNsDel mice in all brain regions, the normalized AR mRNA levels from the control cohorts were combined and compared to those of ARNsDel males. For visualization purposes, this data is presented in Figures 5(I, J) and S3D as the percent of AR mRNA in ARNsDel males in various tissues compared to the control cohort.
Hormones
Serum testosterone and estradiol titers were determined with kits from DRG International and Cayman Chemicals, respectively. We induced estrus in adult ovariectomized mice with injections of estrogen and progesterone as described previously (Wu et al., 2009). For hormonal manipulation of neonates, females were treated on the day of birth (P1) with a single 50 μL subcutaneous injection of hormone or vehicle. We injected either 100 μg testosterone propionate (Sigma) or 5 μg of estradiol benzoate dissolved in sesame oil.
Supplementary Material
Acknowledgments
We thank K. de Gendt, G. Verhoeven, M. Breedlove for providing us with the ARloxP/+ mouse strain, and L. Frank, E. Tumer for advice on statistics. We thank T. Clandinin, D. Julius, H. Ingraham and S. Lomvardas for comments on the manuscript, and D. Anderson, R. Axel and members of the Shah laboratory for discussions. Histological images were acquired at the UCSF Nikon Imaging Center. This work was supported by NSF graduate fellowships (SAJ, EJF); NIH NRSA F32 (JT; #HD0612472); Edward Mallinckrodt, Jr. Foundation, McKnight Foundation for Neuroscience and NIH (NMS; #R01NS049488, DP1OD006425).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Shamma HA, De Vries GJ. Neurogenesis of the sexually dimorphic vasopressin cells of the bed nucleus of the stria terminalis and amygdala of rats. J Neurobiol. 1996;29:91–98. doi: 10.1002/(SICI)1097-4695(199601)29:1<91::AID-NEU7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann NY Acad Sci. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of the preoptic area: time and site of origin, migratory routes, and settling patterns of its neurons. J Comp Neurol. 1987;265:65–95. doi: 10.1002/cne.902650106. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149:4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins D, Yahr P. Lesions of the sexually dimorphic area disrupt mating and marking in male gerbils. Brain Res Bull. 1984;13:185–193. doi: 10.1016/0361-9230(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Crocoll A, Zhu CC, Cato AC, Blum M. Expression of androgen receptor mRNA during mouse embryogenesis. Mech Dev. 1998;72:175–178. doi: 10.1016/s0925-4773(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm Behav. 1971;2:49–58. [Google Scholar]
- Finney HC, Erpino MJ. Synergistic effect of estradiol benzoate and dihydrotestosterone on aggression in mice. Horm Behav. 1976;7:391–400. doi: 10.1016/0018-506x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21:393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual Differentiation of the Brain. Cambridge, MA: MIT Press; 1980. [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Harada N, Yamada K. Ontogeny of aromatase messenger ribonucleic acid in mouse brain: fluorometrical quantitation by polymerase chain reaction. Endocrinology. 1992;131:2306–2312. doi: 10.1210/endo.131.5.1425429. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Rubenstein JL, Pleasure S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol. 2009;20:378–386. doi: 10.1016/j.semcdb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Holmes MM, McCutcheon J, Forger NG. Sex differences in NeuN- and androgen receptor-positive cells in the bed nucleus of the stria terminalis are due to Bax-dependent cell death. Neuroscience. 2009;158:1251–1256. doi: 10.1016/j.neuroscience.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Glidewell-Kenney C, Jameson JL. Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5 alpha-reductase inhibitor. J Steroid Biochem Mol Biol. 2006;98:133–138. doi: 10.1016/j.jsbmb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Coats JK, Shah NM. A genetic approach to dissect sexually dimorphic behaviors. Horm Behav. 2008;53:627–637. doi: 10.1016/j.yhbeh.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kim YH, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Horm Behav. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1998;91:215–222. [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009;29:7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139:1738–1745. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999a;140:3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Regulation of androgen receptor messenger ribonucleic acid expression in the developing rat forebrain. Endocrinology. 1999b;140:1807–1814. doi: 10.1210/endo.140.4.6632. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE. Sexual behavior in three inbred strains of mice. Behaviour. 1962;19:341–350. [Google Scholar]
- Meisel RL, Sachs BD. The Physiology of Male Sexual Behavior. In: Knobil E, Neill JD, editors. Physiology of Reproduction. New York: Raven Press; 1994. pp. 3–106. [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Motelica-Heino I, Castanier M, Corbier P, Edwards DA, Roffi J. Testosterone levels in plasma and testes of neonatal mice. J Steroid Biochem. 1988;31:283–286. doi: 10.1016/0022-4731(88)90351-2. [DOI] [PubMed] [Google Scholar]
- Motelica-Heino I, Edwards DA, Roffi J. Intermale aggression in mice: does hour of castration after birth influence adult behavior? Physiol Behav. 1993;53:1017–1019. doi: 10.1016/0031-9384(93)90284-m. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ. The metabolism of androgens in central neuroendocrine tissues. J Steroid Biochem. 1975;6:993–997. doi: 10.1016/0022-4731(75)90340-4. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus) Anim Behav. 1977;25:333–341. doi: 10.1016/0003-3472(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Geller LN, Lai EV. TfM mutation and masculinization versus feminization of the mouse central nervous system. Cell. 1974;3:235–242. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Genetic influences on sexual behavior differentiation. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York: 1992. pp. 1–40. [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang H. Atlas of the Developing Mouse Brain at E17.5, P0, and P6. London: Academic Press; 2007. [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Bronson FH, Whitsett JM. Neonatal castration and intermale aggression in mice. Physiol Behav. 1972;8:265–268. doi: 10.1016/0031-9384(72)90371-x. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5alpha-androstane-3beta,17beta-diol (3betaAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci USA. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Toda K, Okada T, Takeda K, Akira S, Saibara T, Shiraishi M, Onishi S, Shizuta Y. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17beta-oestradiol to aromatase gene (Cyp19) knockout mice. J Endocrinol. 2001a;168:455–463. doi: 10.1677/joe.0.1680455. [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001b;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Wahlgren A, Svechnikov K, Strand ML, Jahnukainen K, Parvinen M, Gustafsson JA, Soder O. Estrogen receptor beta selective ligand 5alpha-Androstane-3beta, 17beta-diol stimulates spermatogonial deoxyribonucleic acid synthesis in rat seminiferous epithelium in vitro. Endocrinology. 2008;149:2917–2922. doi: 10.1210/en.2007-1126. [DOI] [PubMed] [Google Scholar]
- Wallis CJ, Luttge WG. Maintenance of male sexual behavior by combined treatment with oestrogen and dihydrotestosterone in CD-1 mice. J Endocrinol. 1975;66:257–262. doi: 10.1677/joe.0.0660257. [DOI] [PubMed] [Google Scholar]
- Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Nadler RD. Suppression of the development of female mating behavior by estrogen administered in infancy. Science. 1963;141:273–274. doi: 10.1126/science.141.3577.273. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008a;54:758–766. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008b;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.