Abstract
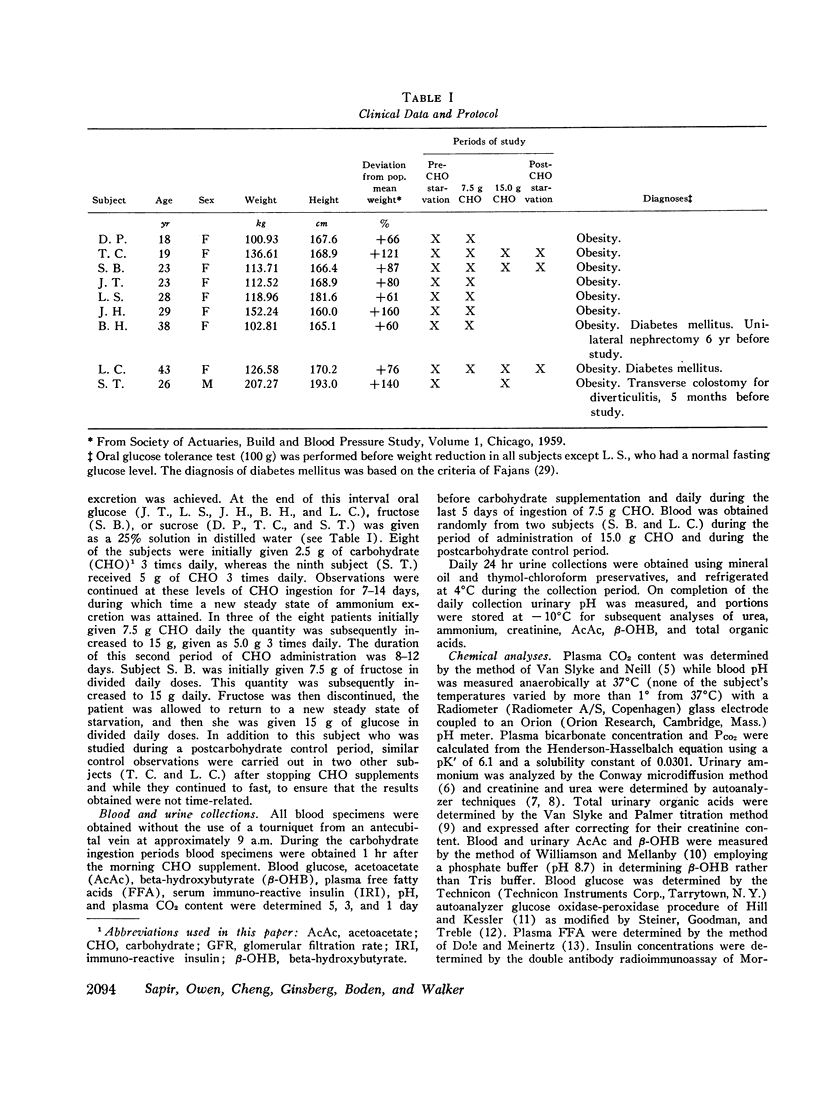
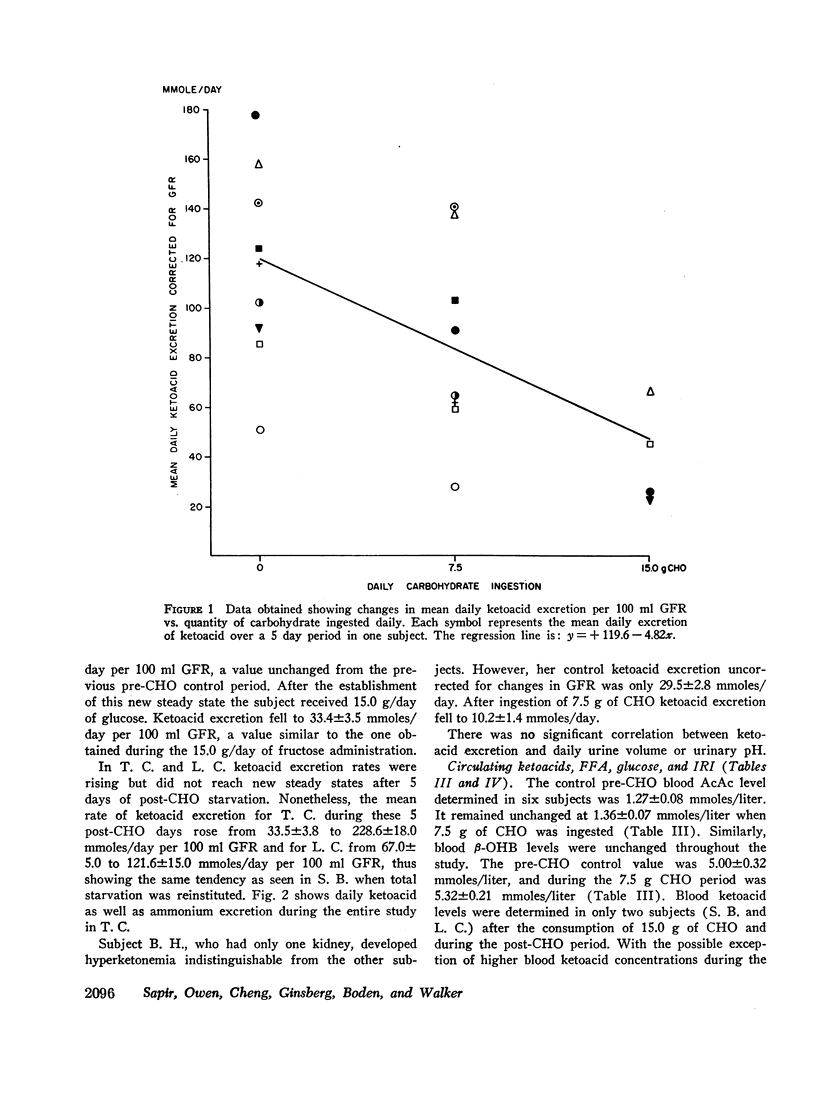
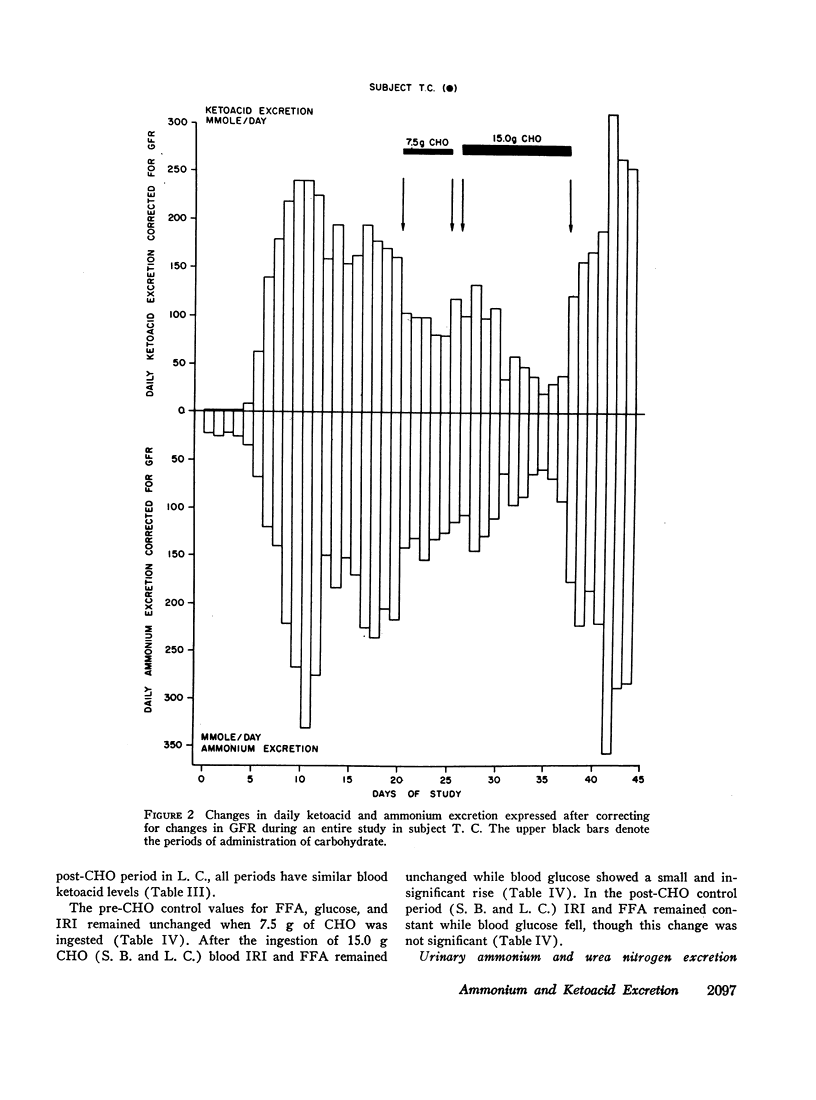
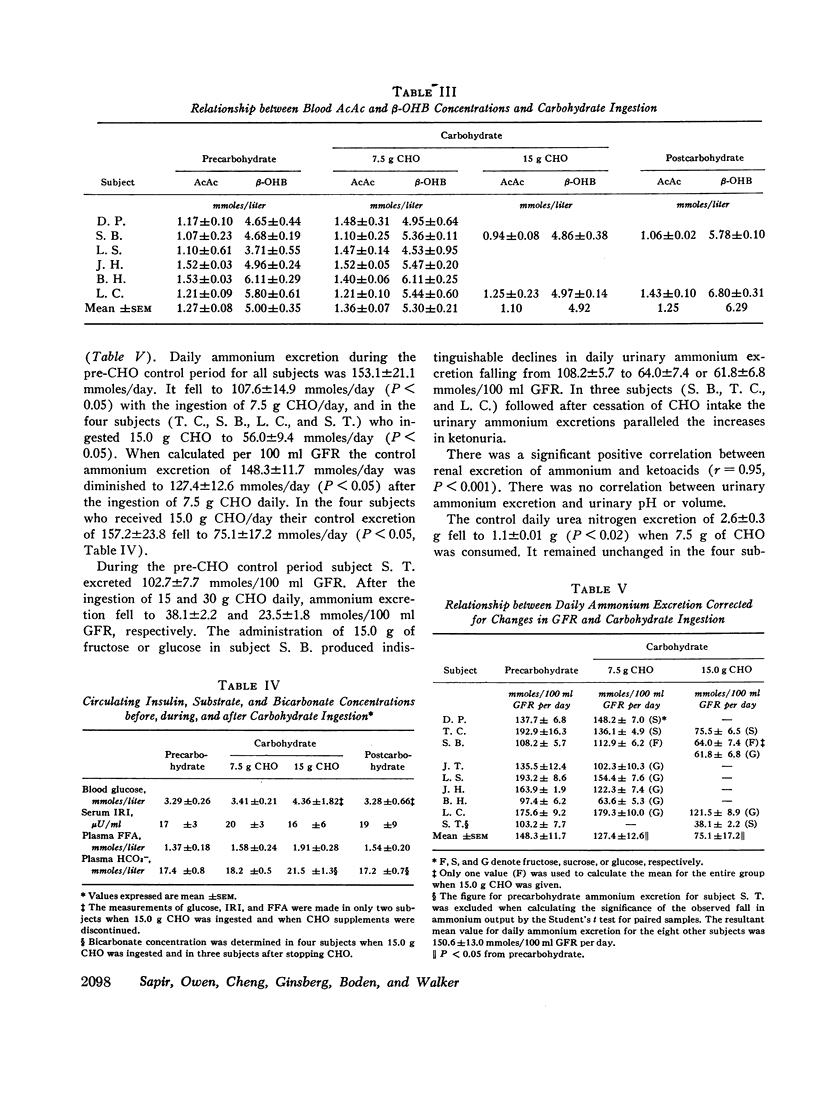
The metabolic effects of oral ingestion of minute quantities of carbohydrate during prolonged starvation were studied in nine obese subjects. Measurements were made during a control period of total starvation, during the ingestion of 7.5 g carbohydrate daily, and finally during the ingestion of 15.0 g carbohydrate daily. Daily ketoacid excretion fell after carbohydrate ingestion and was significantly correlated (r = 0.62, P < 0.01) with the amount of carbohydrate administered. Despite this fall in ketoacids, the concentration of blood ketoacids, plasma free fatty acids, and serum insulin remained constant throughout the study. Urinary ammonium excretion, closely correlated with ketoacid output (r = 0.95, P < 0.001), also fell significantly after carbohydrate ingestion. No significant changes were present in extracellular or urinary pH. Urea nitrogen excretion did not change when urinary ammonium output fell. These results indicate that: the excretion of ketoacids and ammonium in starving man is exquisitely sensitive to minute amounts of ingested carbohydrate; the change in ketonuria appears to be due to increased renal ketoacid reabsorption after carbohydrate ingestion; and the nitrogen-sparing effect of reducing renal ammonium output in starvation can be dissociated from nitrogen sparing occurring because of changes in urine urea excretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill G. J., Jr, Owen O. E., Morgan A. P. The consumption of fuels during prolonged starvation. Adv Enzyme Regul. 1968;6:143–150. doi: 10.1016/0065-2571(68)90011-3. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Felig P., Marliss E. B., Cahill G. F., Jr Metabolic response to human growth hormone during prolonged starvation. J Clin Invest. 1971 Feb;50(2):411–421. doi: 10.1172/JCI106508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. L., Blackfan K. D., Hamilton B. A STUDY OF THE DIURETIC ACTION OF ACID PRODUCING SALTS. J Clin Invest. 1925 Apr;1(4):359–388. doi: 10.1172/JCI100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt M. W. Observations on the Effect of Various Carbohydrates on the Ketosis of Starvation in Human Subjects. Biochem J. 1925;19(6):948–957. doi: 10.1042/bj0190948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL J. B., KESSLER G. An automated determination of glucose utilizing a glucose oxidase-peroxidase system. J Lab Clin Med. 1961 Jun;57:970–980. [PubMed] [Google Scholar]
- MADISON L. L., MEBANE D., UNGER R. H., LOCHNER A. THE HYPOGLYCEMIC ACTION OF KETONES. II. EVIDENCE FOR A STIMULATORY FEEDBACK OF KETONES ON THE PANCREATIC BETA CELLS. J Clin Invest. 1964 Mar;43:408–415. doi: 10.1172/JCI104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSH W. H., FINGERHUT B., MILLER H. AUTOMATED AND MANUAL DIRECT METHODS FOR THE DETERMINATION OF BLOOD UREA. Clin Chem. 1965 Jun;11:624–627. [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Unger R. H., Soeldner J. S., Cahill G. F., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970 Dec;49(12):2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh G., Alley R. A., Robbins T. J., Narduzzi J. V., Kenny F. M., Danowski T. S. Adrenocortical indices during fasting in obesity. J Clin Endocrinol Metab. 1969 Mar;29(3):373–376. doi: 10.1210/jcem-29-3-373. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]
- WRONG O., DAVIES H. E. The excretion of acid in renal disease. Q J Med. 1959 Apr;28(110):259–313. [PubMed] [Google Scholar]


