Abstract
We observed naphthyl-keratin adducts and dose-related metabolic enzyme induction at the mRNA level in reconstructed human epidermis in vitro after exposure to naphthalene. Immunofluorescence detection of 2-naphthyl-keratin-1 adducts confirmed the metabolism of naphthalene and adduction of keratin. We also observed naphthyl-keratin adducts in dermal tape-strip samples collected from naphthalene-exposed workers at levels ranging from 0.004 to 6.104 pmole adduct/μg keratin. We have demonstrated the ability of the human skin to metabolize naphthalene and to form naphthyl-keratin adducts both in vitro and in vivo. The results indicate the potential use of keratin adducts as biomarkers of dermal exposure.
Keywords: biomarkers, dermal exposure, jet fuel, keratin adducts, naphthalene (CAS 91-20-3)
Introduction
Human skin is a large and structurally complex organ of many layers, which possesses different biological properties and characteristics. Keratinocytes in the basal layer proliferate and start differentiating and migrating to the surface to form the stratum corneum, which takes approximately four weeks (Furukawa et al., 2008). The differentiating basal keratinocytes express various differentiation markers, including large amounts of keratin 1 (K1) and keratin 10 (K10) proteins (Schweizer et al., 1983; Eichner et al., 1986). The cells increase in size once they enter the spinous layer and migrate further into the granular layer, at which time all the cytoplasmic organelles are eliminated, and the keratins become the principal proteins in the cytoplasm (Fuchs et al., 1980; Albers et al., 1992). Keratinocytes in the epidermis contains a myriad of transport and metabolic enzymes providing active uptake and biotransformation of many xenobiotics, such as polycyclic aromatic hydrocarbons (PAHs) (Baron et al., 2001; Gibbs et al., 2007). PAHs absorbed into the skin layer are metabolized to electrophiles, which may adduct nucleophilic sites on keratin proteins while keratinocytes differentiate, mature, and migrate upward to the stratum corneum (Watt, 1988). We hypothesized that these metabolites are likely to adduct the nucleophilic cysteine residues on the head region of the N-terminal of K1 and K10 proteins, which are the preferred adduction sites on proteins, albumin, and hemoglobin (Yeowell-O’Connell et al., 1996; Waidyanatha et al., 2002) when co-expressed in the suprabasal layer of the epidermis.
Naphthalene, the simplest form of a PAH, is a widely used industrial chemical and is a major constituent in jet fuels (JP-8) (McDougal et al., 2000) to which humans are environmentally and occupationally exposed. Naphthalene exposure has become a significant health concern due to evidence for its carcinogenic properties in mice and rats (NTP, 2000; North et al., 2008). This has propelled several agencies to classify naphthalene as a possible human carcinogen (US EPA, 1998; NTP, 2000; IARC, 2002; DFG, 2008). Naphthalene toxicity is also associated with other disorders, such as hemolytic anemia in children (Zinkham et al., 1958; Santhanakrishnan et al., 1973), cataracts (Ghetti et al., 1956), and laryngeal tumors (Wolf, 1978). Although there are many hydrocarbon species in the JP-8 mixture, naphthalene has been successfully established as the surrogate marker for jet-fuel (JP-8) exposure from both inhalation (Egeghy et al., 2003) and dermal (Chao et al., 2006; Kim et al., 2007) exposure routes. Furthermore, few chemicals had been shown to have the same potency as naphthalene to form reactive metabolites that covalently bind to various macromolecules, such as albumin and hemoglobin (Waidyanatha et al., 2002). Naphthalene metabolites in urine and their adduction to albumin have also been established as biomarkers of JP-8 and other PAH exposure (Serdar et al., 2004; Waidyanatha et al., 2004). Multiple studies have shown the existence of several cytochrome P450s isoforms in the skin, which is a major family of enzymes that metabolize endogenous substrates, drugs, and xenobiotics (Janmohamed et al., 2001; Saeki et al., 2002; Yengi et al., 2003; Du et al., 2004). Naphthalene is quickly absorbed through the epidermis (Kim et al., 2006), due to its hydrophobicity and small molecular weight, and may be metabolized by P450 enzymes associated with PAH metabolism, such as 1A1, 1A2, 1B1, 2E1, and 3A5 (Gabbani et al., 1999; Godschalk et al., 2001; Lee et al., 2001; Shimada, 2006). We hypothesized that naphthalene exposure induces the expression of P450 enzymes in the skin, which transforms naphthalene to reactive electrophilic molecules (e.g., naphthalene epoxide). These molecules may react with the sulfhydryl group of the cysteine residue in the head region of K1 and K10 expressed de novo in the viable epidermis, forming 1-naphthyl–K1 (1NK1), 2-naphthyl–K1 (2NK1), 1-naphthyl–K10 (1NK10), or 2-naphthyl–K10 (2NK10) adducts.
To investigate the potential for the formation of these naphthyl-keratin adducts in the skin of exposed individuals, we developed specific polyclonal antibodies and enzyme-linked immunosorbent assays (ELISA) to detect 1- and 2-naphthyl-keratin adducts (Kang-Sickel et al. 2008). Here, we investigated the effects of dermal exposure to naphthalene at the molecular level using three-dimensional (3D) reconstructed human skin in vitro and in situ immunofluorescence, and then in vivo using specific ELISA developed previously to analyze tape-strip samples collected from U.S. Air Force (USAF) personnel, who were routinely exposed to naphthalene-containing jet-fuel (JP-8). We also validated the approach of utilizing ELISA to quantitatively measure these adducts, which will facilitate investigation of naphthalene/JP-8 exposure assessment, particularly regarding dermal contact.
Methods
In vitro human epidermis
The 3-D human skin tissue reconstruct (EFT-300™, MatTek, Ashford, MA) is a commercially available in vitro skin model. EFT-300 is a full-thickness skin model that consists of normal, human-derived epidermal keratinocytes and fibroblasts to form a multi-layered, well-differentiated skin model. It contains stratum corneum, epidermis, and dermis, and is metabolically active, thus, making it a suitable in vitro tool for skin toxicology studies. The tissue constructs on cellulose membrane support and insert with the appropriate air-liquid interface were cultured in 6-well plates containing 1 mL of fresh media in each well. Upon arrival to the laboratory, the tissue constructs were placed in a 37°C, 5% CO2 incubator overnight for equilibration. Before each new media exchange and naphthalene exposure, the used media was aspirated and 1 mL of fresh, pre-warmed media was added into each well below the tissue insert. Based upon preliminary dose range-finding studies, four skin inserts were treated every other day for each of the six treatment groups: untreated, vehicle only (acetone), 0.02 nmol, 0.2 nmol, 2 nmol, or 20 nmol of naphthalene per well for each exposure. After three repeated exposures every other day, the reconstructed skin was removed from the insert, and tissues from each of the six treatment groups were collected for viability study and determination for gene expression (cDNA microarray). In addition, to demonstrate the capacity of human skin to metabolize naphthalene and to form naphthyl-keratin adducts, one tissue from each of the six treatment groups was collected for hematoxylin and eosin (H&E) staining and immunofluorescence microscopy.
Viability
One skin insert from each treatment group was washed with phosphate buffered saline (PBS) three times and placed into a clean plate containing 300 μL of MTT agent (MatTek) and incubated at 37°C for 3 hr. At the end of incubation, each skin insert was transferred into a fresh plate containing 2 mL of MTT extraction solution and placed on a shaker for 2 hr. An aliquot of each extracted sample solution (200 μL) was placed into a new plate and the optical densities were measured at 570 nm and the background readings subtracted at 650 nm (Mosmann, 1983).
RNA expression
The epidermal tissue was homogenized, and the total RNAs were isolated using Qiagen RNeasy protocol. Quantified total RNA (250 ng) was amplified and SuperArray HS-019 Human GEArray series Q arrays (Cat. No. HS-019, Bioscience Corp., Frederick, MD) were used to determine the relative expressions of genes that are considered relevant to skin metabolism of endogenous and exogenous substrates. RNA (5 μg) was used to generate biotin-16-dUTP-labeled cDNA probes according to manufacturer’s instructions. The cDNA probes were denatured and hybridized at 60°C with the SuperArray membrane, which was washed and exposed with chemiluminescent substrate. Acetone is an endogenous substrate for CYP2E1, and was used as a delivery vehicle in vitro for naphthalene. Therefore, the mRNA transcription profile data were normalized against acetone control to deduct the proportion of expression induced by the vehicle, as well as the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), to control for differences between samples (Fluorchem 8900, Alpha Innotech, San Leandro, CA).
Immunohistochemistry/immunofluorescence
One tissue from each of the six treatment groups was placed in a histology cassette and fixed in 60% of ethanol, 30% water, and 10% formalin (37% formaldehyde solution), for 2–4 hr, and then fixed with 70% ethanol overnight at 4°C. The tissues were then dehydrated, embedded in paraffin, and sectioned. The tissue sections were deparaffinized and dehydrated through a series of ethanol. Antigen retrieval was performed using citrate buffer and the sections were blocked with 1X Autobuffer (Biomeda, Foster City, CA). The affinity-purified rabbit anti-2NK1 antibody (Kang-Sickel et al., 2008) was applied onto the tissue section and incubated in the dark for one hour. After rinsing with PBS, the secondary antibody, Alexa Fluor 546 conjugated chicken anti-rabbit IgG (Molecular Probe) was applied and incubated in the dark for one hour. The sections were washed 3 times in 1X PBS, coverslipped with Vectashield Hard Set mounting medium with DAP-1 stain, and images captured under the inverse microscope (Olympus IX70) with camera (Sony DXC-s 500) (Ponec et al., 2002).
Detection of naphthyl-keratin adducts in the skin of occupationally exposed workers
Dermal tape-strip samples were obtained from 105 fuel-cell maintenance workers exposed to jet fuel (JP-8) at five USAF bases (described in Chao et al. 2005). Dermal samples were collected post-exposure using adhesive tape-strips (2.5 cm × 4.0 cm, surface area 10 cm2 Cover-Roll™, Beiersdorf AG, Germany) from 3–5 exposed body regions and three sequential tapes were collected from each site. Each tape was applied onto the skin surface with a constant pressure and removed at approximately 45-degree angle after one minute. The tape was rolled with the adhesive side facing out and placed into a 2-mL cryovial and stored in −80°C until further analysis. Dermal samples were also collected from individuals who declared that they had not been exposed to naphthalene or jet-fuel (exposure controls). The study was approved by the Institutional Review Board in the Office of Human Research Ethics at the University of North Carolina at Chapel Hill. Participation of the human subjects did not occur until after informed consent was obtained.
Keratin protein quantitation
Three sequential tapes collected from one exposed site were pooled and extracted with 3 mL of the extraction solution (8 M urea, 50 mM Tris, 0.1 M b-mercaptoethanol, 0.1% sodium azide), vortexed for 15 sec, and placed on a shaker overnight at 150 rpm. The Bradford assay was performed using human epidermis keratin solution (Sigma-Aldrich) as standard with serial dilution from 500 μg/mL. Each sample (100 μL) was placed into a cuvette and 1 mL of Bradford reagent (Amresco Inc., Solon, OH) was added, vortexed, and read at 595 nm (Cary 100, Varian Instrument Co, Palo Alto, CA). The keratin concentration of each sample was determined by interpolation and reference to a standard curve (Kang-Sickel et al., 2008). The remaining sample from each extraction is used for adduct quantitation using ELISA described below.
ELISA for adduct quantitation
The sensitivities and specificities of the antibodies used in ELISA were tested and validated previously, and a standard curve was developed for each of the four naphthyl-keratin adducts (1NK1, 2NK1, 1NK10, 2NK10) (Kang-Sickel et al., 2008). The ELISA for adduct quantitation was performed by coating polystyrene plates with 100 μL of a serial dilution of an antigen standard (1NK1, 2NK1, 1NK10, 2NK10), starting at 500 ng antigen/mL in Voller’s buffer (100 mM sodium bicarbonate) to a well (in replicate). The extracted tape-stripped samples were coated in triplicates, along with samples collected from individuals not exposed to JP8 (exposure controls), on the same plate, sealed, and incubated at 4°C overnight. The plates were washed 4 times with 250 μL of 0.1% Tween 20 in PBS (PBST) and incubated with 150 μL of blocking buffer (5% skim milk in PBST) at 37°C to prevent non-specific binding of subsequent reagents. The plates were washed as described above and 100 μL of each affinity-purified antibody ranging from 0.8 to 7 μg/mL in PBST was added to the appropriate plate and incubated for 1 hr at 37°C. The plates were washed again and 100 μL of a 1:4000 dilution of goat anti-rabbit HRP-conjugate was added. The plates were incubated for 1 h, washed, and 100 μL of TMB-ELISA substrate (Pierce) was added and allowed to react at room temperature for 30 min. The reaction was stopped by adding 100 μL of 2 M sulfuric acid and absorbance determined at 450 nm (Emax, Molecular Devices, Sunnyvale, CA). The amount of keratin adduct in each sample was determined based on the standard curve, adjusted for the exposure control samples. The adduct level was then adjusted for the amount of keratin recovered from each tape-strip sample (Kang-Sickel et al., 2008).
Data analysis
All statistical analyses were conducted using the SAS software (V.9.1.3, SAS Institute, Cary, NC) at a significance level of 0.1. The amount of each of the four adducts measured from each sample was adjusted for the amount of keratin removed from the tape-strip samples to obtain naphthyl-keratin adduct/keratin ratio (pmole/μg), to account for the variability in the keratin amount that may occur in each tape-strip sample. Natural log-transformations were made to the four adduct/keratin ratios, K1 (1NK1 + 2NK1) ratio, K10 (1NK10 + 2NK10) ratio, and total adduct/keratin ratio, to satisfy normality assumption prior to statistical analysis. The total adduct/keratin ratio was selected for further analysis since it represents the sum of all possible naphthyl-keratin adducts formed in the epidermis.
The USAF personnel were assigned to three a priori determined exposure categories (high, medium, or low) (Chao et al., 2005). The least-square t-tests with Tukey’s adjustment was performed to investigate differences in adduct levels between bases, exposure groups, task performed, demographic factors such as race, and gender, smoking status, and the use of personal protective equipment (e.g., booties, gloves, respirators, cotton overalls). The Pearson’s correlation was used to investigate the effect of the average temperature and humidity of the sampling month between each base.
Results
In vitro human epidermis
The absence of naphthalene toxicity to the reconstructed epidermis under these exposure levels and conditions was demonstrated by the similar response of all treatment groups in the MTT assay (Figure 1). The MTT assay is based on the ability of a mitochondrial dehydrogenase in viable cells to cleave the tetrazolium rings of the pale yellow MTT and form a dark blue formazan precipitate, which is largely impermeable to cell membranes and thus accumulates within healthy cells. Lysis and solubilization of the cells by the addition of a detergent results in the liberation and solubilization of the crystals. The number of viable cells is directly proportional to the level of the formazan created, which can be quantified using a colorimetric assay. This assured the validity of the observed dose related mRNA responses to naphthalene-induced metabolism in the absence of toxicity.
Figure 1.
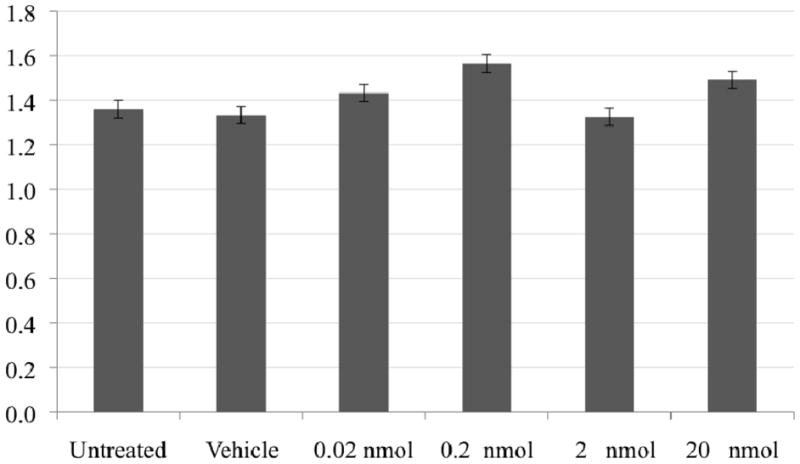
Viability (mitochondrial dehydrogenase activity) of the human reconstructed epidermis (untreated, acetone vehicle, or naphthalene exposure) was not compromised under these conditions and, thus, may allow observation of normal homeostatic responses in response to xenobiotic exposure.
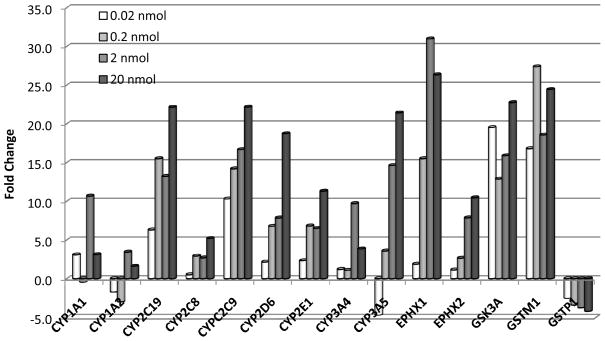
An increase in relative transcript expression levels of a number of cytochrome P450 genes (CYP1A1, CYP2C19, CYP2C8, CYP2C9, CYP2D6, CYP2E1, CYP3A4, and CYP3A5), epoxide hydrolase 1 and 2 (EPHX1, EPHX2), and glutathione S-transferase mu 1 (GSTM1) were observed in HS-019 SuperArrays (Figure 2). Acetone is an endogenous substrate for CYP2E1, especially under caloric restriction and ketosis, and may work with increased naphthalene substrate to saturate phase 1 and phase 2 metabolism enzymes. These data were normalized against the consistently expressed GAPDH transcript, and the vehicle (acetone) contribution to induction was deducted, thus demonstrating the dose-response relationship induced by exposure to naphthalene alone. This robust activity suggests that many cytochrome P450 genes exist in human epidermis, and can be induced by naphthalene. These results demonstrate the capability of human epidermis to express and induce enzymes that metabolize xenobiotics, such as naphthalene. These genes are, thus, candidates for genotyping and phenotyping individuals for susceptibility to naphthalene exposure and associated disease.
Figure 2.
Naphthalene significantly (p ≤ 0.05) induced many xenobiotic-metabolizing genes in the reconstructed human skin. Relative gene expression normalized to GAPDH and vehicle control (acetone).
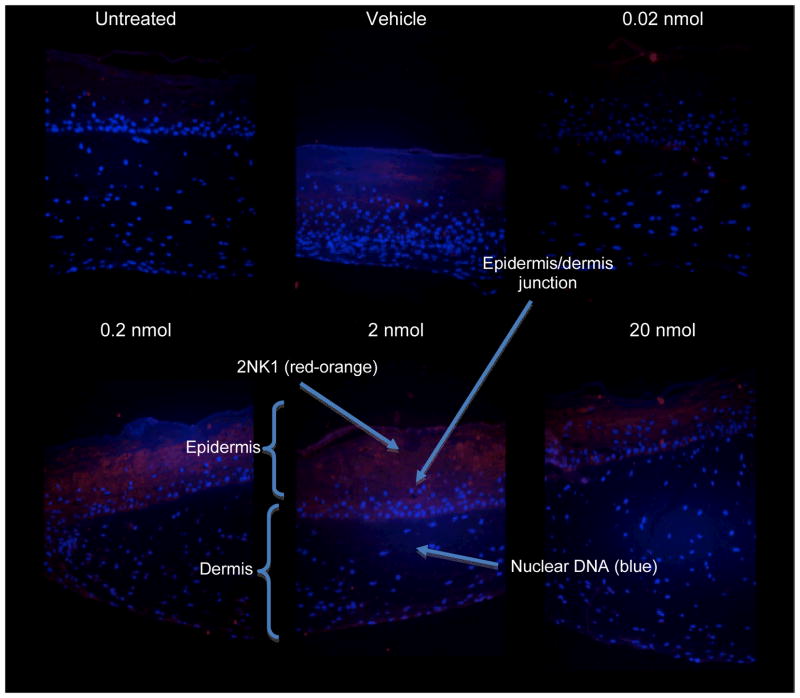
Immunofluorescence staining for 2NK1 adducts was performed on the cross-section of the paraffin-embedded tissue samples from the six treatment groups. We observed that signal intensity of 2NK1 adducts in the keratinocytes increased with naphthalene dose (Figure 3). The affinity-purified rabbit anti–2NK1 polyclonal antibody retained a low level of cross-reactivity to keratin as observed in the untreated and vehicle (acetone) treated skin, but still detected a naphthalene dose-related increase in adduct levels that were in agreement with gene induction in SuperArray. Further, the 2NK1 adduct detection signals concentrated in the epidermis, primarily the suprabasal layer. This observation confirms the metabolizing capacity of keratinocytes, the core components of the epidermis, which differentiate and form the non-viable stratum corneum, from which the tape-strip samples were obtained.
Figure 3.
A dose-related increase in detectable 2NK1 adduct levels was observed in the epidermis, but not in the dermis of reconstructed skin, after three topical exposures to naphthalene (every other day). Anti-2NK1-red-orange (Alexa Fluor 546); nuclear DNA-blue (DAP-1).
Detection of naphthyl-keratin adducts in the skin of occupationally exposed workers
Keratin protein quantities in the tape-strip skin samples collected from 105 USAF personnel occupationally exposed to JP-8 ranged from 29.6 to 1877.3 μg/ml, with mean and standard deviation of 652 ± 382 μg/ml. The variation in the amount of keratin recovered from individual tape-strips may reflect the difference in perspiration and other personal factors affecting adhesion and removal of the tape. Hence, the amount of adducts was adjusted for the amount of keratin removed in each tape-strip sample to obtain a normalized adduct-keratin ratio.
The four naphthyl-keratin adducts were detected in the tape-stripped skin samples at levels from 0.004 to 6.104 pmole adduct/μg keratin with the highest observed for 2NK10 and the lowest for 1NK10; the mean naphthyl-keratin adduct levels were 0.19 ± 0.16 (1NK1), 0.56 ± 0.32 (1NK10), 0.14 ± 0.12 (2NK1), and 2.11 ± 0.88 (2NK10) pmole adduct/μg keratin (Table 1). Significant differences were observed between the mean levels of 1NK1 and 2NK1 (p = 0.0149), 1NK10 and 2NK10 (p < 0.0001), and the total naphthyl-bound K1 (1NK1 + 2NK1) and total naphthyl-bound K10 (1NK10 + 2NK10) (p < 0.0001). The mean levels of total 1-naphthyl (1NK1 + 1NK10) were significantly lower than the total 2-naphthyl (2NK1 + 2NK10) adduct levels (p < 0.0001).
Table 1.
The means and standard deviations for levels of four individual naphthyl-keratin adducts (1NK1, 2NK1, 1NK10, 2NK10), total keratin-1 adducts, total keratin-10 adducts, total 1-naphthyl keratin adducts, and total 2-naphthyl keratin adducts (pmole adduct/μg keratin) measured in the epidermis of the US Air Force personnel exposed to jet fuel.
| Naphthyl-keratin adducts | Mean | STD | Min | Max |
|---|---|---|---|---|
| 1NK1 | 0.1857a | 0.1620 | 0.0407 | 1.1732 |
| 2NK1 | 0.1421 | 0.1206 | 0.0063 | 0.6192 |
| 1NK10 | 0.5593b | 0.3171 | 0.0044 | 1.5079 |
| 2NK10 | 2.1083 | 0.8782 | 0.9349 | 6.1039 |
| Total keratin-1 adducts (1NK1 + 2NK1) | 0.3278c | 0.2211 | 0.0901 | 1.4921 |
| Total keratin-10 adducts (1NK10 + 2NK10) | 2.6676 | 1.0581 | 1.1726 | 6.2250 |
| Total 1-naphthyl keratin adducts (1NK1 + 1NK10) | 0.7450d | 0.3217 | 0.2746 | 1.6628 |
| Total 2-naphthyl keratin adducts (2NK1 + 2NK10) | 2.2504 | 0.9558 | 0.9894 | 6.4228 |
Significantly different from 2NK1 (p = 0.0149)
Significantly different from 2NK10 (p < 0.0001)
Significantly different from total naphthyl-bound K10 (p < 0.0001)
Significantly different from total 2-naphthyl keratin adduct levels (p < 0.0001)
The means and standard deviations of the log-transformed total naphthyl-keratin adduct levels were 1.060 ± 0.400, 0.835 ± 0.312, and 1.070 ± 0.299 pmole/μg keratin for high-, medium-, and low-exposure groups (as classified by a priori assigned exposure category), respectively (Table 2). The adduct levels were significantly different between the high- and medium-exposure groups (p = 0.069), but not between the medium- and low-exposure groups, and between the high- and low-exposure groups. The low-exposure group had the highest adduct levels while the high-exposure group had the second highest levels among the exposure groups. Upon closer examination, it was discovered that majority of workers in the low-exposure group (17 of 19) had participated in tasks such as repairing fuel lines, fuel tanks, draining and refueling, and reported contact with jet fuel, even though they did not partake in fuel-cell maintenance on the sampling day. Hence, these workers were assigned to low-exposure group in the overall study (Chao et al., 2005). It is very possible that these workers had no or minimal contact with JP-8 on the sampling day, but that they had had considerable exposure to JP-8 previously during their daily duties that in turn resulted in significant naphthyl-keratin adduct formation in the skin.
Table 2.
The means and standard deviations of the log-transformed total naphthyl-keratin adduct levels [ln(pmole adduct/μg keratin)] measured in the epidermis of the US Air Force personnel exposed to jet fuel.
| Exposure Group | Base A | Base B | Base C | Base D | Base E | All Bases |
|---|---|---|---|---|---|---|
| All | 0.642±0.210a (n = 30) | 1.021±0.259 (n = 22) | 1.444±0.367b (n = 15) | 1.270±0.201b (n = 22) | 1.019±0.258 (n = 16) | 1.025±0.377 (n = 105) |
| High | 0.630±0.224a (n = 22) | 1.082±0.248 (n = 15) | 1.546±0.364b (n = 9) | 1.315±0.175c (n = 16) | 1.157±0.150 (n = 7) | 1.060±0.400* (n = 69) |
| Medium | 0.541±0.107 (n = 4) | 0.884±0.273 (n = 6) | 1.113±0.319 (n = 2) | NA | 0.899±0.359 (n = 5) | 0.835±0.312 (n = 17) |
| Low | 0.811±0.100d (n = 4) | 0.930 (n = 1) | 1.380±0.366 (n = 4) | 1.153±0.232 (n = 6) | 0.930±0.196 (n = 4) | 1.070±0.299 (n = 19) |
| Aircraft type | Single-seat training/combat | Fighter | Transporte | Transporte | Fighter, refueling | |
| Fuel capacity (L) | 6,200 | 20,000 | 26,000–36,500 | 33,700 | 20,000 | |
n = Number of subjects.
NA = Not applicable; no workers in this category.
Significantly lower than all the other bases.
Significantly higher than bases A, B, and E.
Significantly higher than bases A and B.
Significantly lower than base C.
Significant difference between transport planes (base C and base D) and fighter jets (base B and base E) (p < 0.0001)
Significant difference between high- and medium-exposure group (p = 0.069).
Significant differences in the total adduct levels were observed between the five USAF bases, with the highest levels observed at base C and the lowest at base A (Table 2). These differences are likely the result of the types of aircrafts serviced in each base. The bases with the highest adduct levels (C and D) serviced transport planes. These aircrafts have 26,000–36,500 L fuel tanks and are capable of carrying large cargos. The base with the lowest adduct levels (A) provided support for small fighter jets (fuel tank capacity 6,200 L). Bases B and E serviced primarily the F-series fighter jets (fuel tank capacity 20,000 L) and the measured adduct levels were significantly lower compared to the bases C and D (p < 0.0001) and significantly higher when compared to base A (p < 0.0001).
The type and use of personal protective equipment (PPE) such as gloves, booties, and aprons were significant in affecting the adduct levels in all subjects (p = 0.005, 0.016, 0.042, respectively; data not shown). Wearing gloves was found to be associated with lower adduct levels, indicating their protection against dermal exposure to JP-8. However, wearing booties or aprons were associated with increased adduct formation in the epidermis. Because both booties and aprons were made of cotton material, the increased level of keratin adducts may have been due to cotton’s inadequacy in protecting skin from JP-8 exposure or, worse, cotton fabric may have served as a wicking conductor for dermal absorption of JP-8 (Carlton et al., 2000).
No significant difference was found between races, genders, smoking status, and, interestingly, between workers with or without skin irritation. However, slightly higher adduct levels were observed in workers who reported skin irritation (3.182 vs. 2.871 pmole adduct/μg keratin). The tasks of replacing foam and pumping contaminated air out of the tank instead of pumping fresh air into fuel tank were also associated with increased adduct levels (p = 0.006 and p = 0.001, respectively).
In addition to the types of aircrafts serviced in these bases, the differences in adduct levels may also be influenced by the temperatures and humidity of the geographical locations of the bases, as well as differences in the maintenance practices, personal hygiene, and the type and use of PPE. The results showed that the adduct levels strongly correlated with the average low temperature of the sampling month (r = 0.908, p = 0.033) and moderately with average high temperature of the sampling month (r = 0.826, p = 0.085) (data not shown).
Discussion
The data obtained from the in vitro reconstructed human epidermis confirm the existence and dose related induction of both phase I and phase II xenobiotic-metabolizing enzymes (Figure 2), including CYP2E1, in the human epidermis as reported previously (Pavek et al., 2008). CYP families 1, 2, and 3 are major xenobiotic-metabolizing enzymes in humans (Nelson, 2002), and CYP1A1, 1B1, 2B6, 2E1, and 3A are expressed significantly greater in normal human skin keratinocytes relative to fibroblasts (Baron et al., 2001). This corroborates our new findings of the dose-responsive induction of CYP2E1, involved in naphthalene metabolism, along with several other xenobiotic-metabolizing enzymes (Figure 2), and 2NK1-adduct formation that occurred in the viable reconstructed epidermis (Figure 3), but not the dermis. Naphthalene undergoes oxidative metabolism by cytochrome P450 enzymes resulting in the production of reactive epoxides (Jerina et al., 1970; Wilson et al., 1996). These epoxides react with sulfhydryl group of the cysteine residue in the exposed head region of K1 or K10, forming naphthyl-keratin adducts, as we observed in the USAF personnel; or they may react with other cellular components, such as membrane lipids and DNA inducing adducts and mutations, increasing the risk for cancer.
The total naphthyl-K1 levels observed were significantly lower than the total naphthyl-K10 (p < 0.0001). These results confirmed our previously reported observation using samples collected from 13 individuals occupationally exposed to jet fuel (Kang-Sickel et al., 2008). K1 and K10 are normally present in 1:1 ratio in healthy human skin and form the intermediate filaments, which provide structure and strength to the squame. However, K10 up-regulation and K1 down-regulation in keratinocyte cell cultures exposed to JP-8 has been reported (Witzmann et al., 2005). In addition, inter-individual differences in xenobiotic-metabolizing enzyme function due to non-synonymous SNPs and structural variants may affect metabolic activity and an individual’s ability and efficiency to form reactive metabolites. Individual differences in detoxification capacity may alter susceptibility to exposure related disease (Alexandrie et al., 2000). Associations between diseases, such as cancer, and genetic polymorphisms (SNPs and copy number variants) of xenobiotic-metabolizing enzymes, such as cytochrome P450s and GSTs, have been reported (Figueroa et al., 2008; Majumdar et al., 2008; Torresan et al., 2008). More studies are needed to elucidate the relationship between individual genetic polymorphisms and their effects on exposure biomarkers and disease risk. Further, more research is required to understand naphthyl-keratin adducts and urinary naphthol levels, and the implications for an individual’s long-term disease susceptibility to naphthalene exposure.
The significant differences in adduct levels between the five USAF bases are particularly interesting. Some workers routinely enter the wing tanks of transport planes (bases C and D), providing a significant potential for dermal contact with jet fuel, while the wing tanks of some fighter jets are too small for entry, which may reduce workers’ potential for exposure. In addition, significantly higher adduct levels were observed in workers participating in tasks involving foam replacement and ventilation. Similarly, Carlton and Smith (2000) observed significantly higher air concentrations of benzene in workers involved in foam procedures compared to other tasks. These workers were likely exposed to the residual fuel in the wet foams during routine maintenance. The hydrophilic property of polyurethane foam, may allow aromatic hydrocarbons, such as naphthalene, to be absorbed into the foam compared to that of the alkane hydrocarbons, which are less hydrophilic. Therefore, the foam may absorb more aromatic hydrocarbons from the fuel and, thus, increasing the exposure potential during foam handling.
This research supports the use of naphthyl-keratin adducts as biomarkers of exposure when assessing dermal exposure to naphthalene, as a marker for jet fuel exposure, based on the dose-related enzyme expression and adduct formation observed both in vitro and in vivo. These data also confirm our approach to use non-invasive sampling of skin by tape stripping and ELISA analysis of epitope-specific antigens to determine prior and cumulative chronic exposure to naphthalene, as a marker for JP-8 exposure. By studying the adduction of keratin occurring in the viable epidermis and subsequently in the stratum corneum available for noninvasive sampling, we were able to investigate both external and internal factors contributing to keratin protein adduction during dermal exposure, including exposure levels, tasks performed, and use of PPE. Further, our study exemplifies the inherent problem of exposure misclassification in epidemiology studies that rely on a priori determined job groups without the knowledge of quantitative exposure or biomarker of exposure data.
Quantification of dermal exposure is especially relevant for the USAF personnel who enter fuel tanks to perform maintenance tasks and whose dermal protection is insufficient with cotton overalls. Urinary naphthols have been suggested as sensitive biomarkers for exposure to ambient PAH in the general population (Yang et al., 1999; Serdar et al., 2004). However, no consideration was made to quantify dermal exposures to PAHs in these studies. We observed in our previous studies that dermal exposure, along with inhalation exposure, is an important route for jet fuel exposure and that dermal exposure can contribute significantly to the total body burden (Chao et al., 2005; Chao et al., 2006; Kim et al., 2007). Future studies are warranted to examine the relationship between keratin adducts and urinary naphthols as informative biomarkers of systemic exposure, and their relationship in regard to acute and chronic exposure to jet fuel and to validate the use of keratin adducts as biomarkers of jet fuel exposure. The relationship between individual differences in metabolic capacity and levels of these biomarkers should also be taken into account. Accurate estimation of the contribution of dermal exposure to the systemic dose is required in order to complement and improve current exposure and risk assessment models for long-term health effects of jet fuel (naphthalene) exposure, a facet that is missing from current epidemiological research.
Conclusions
In summary, we have demonstrated ability of the human epidermis to metabolize naphthalene and to form naphthyl-keratin adducts both in vitro and in vivo. We also demonstrated the feasibility of utilizing ELISA to quantitate specific naphthyl-keratin adducts from the tape-striped samples collected from the skin of workers routinely exposed jet fuel. The ability to quantitate keratin adducts as biomarkers of dermal exposure will enable us to distinguish systemic dose resulting from dermal exposure from other routes of exposure. Since determination of exposure biomarkers from all sources of exposure contact is critical to understanding systemic exposure and toxicity, the techniques described in this study will allow us to better estimate total systemic dose for inclusion in exposure- and risk-assessment models, and possibly to illustrate the mechanisms of the xenobiotics-metabolizing pathways.
Acknowledgments
The authors thank the workers for their participation in the study. The authors declare they have no competing financial interests. This work was supported by U.S. Air Force (Texas Tech University subcontract 1331/0489-01), National Institute of Environmental Health Sciences (grant number P42ES05948 and the Division of Intramural Research ES021134), and National Institute for Occupational Safety and Health (grant numbers T42/CCT422952, T42/008673).
Footnotes
Declaration of interest: The authors report no conflicts of interest.
References
- Albers K, Fuchs E. The molecular biology of intermediate filament proteins. Int Rev Cytol. 1992;134:243–79. doi: 10.1016/s0074-7696(08)62030-6. [DOI] [PubMed] [Google Scholar]
- Alexandrie AK, Warholm M, Carstensen U, Axmon A, Hagmar L, Levin JO, Ostman C, Rannug A. CYP1A1 and GSTM1 polymorphisms affect urinary 1-hydroxypyrene levels after PAH exposure. Carcinogenesis. 2000;21(4):669–76. doi: 10.1093/carcin/21.4.669. [DOI] [PubMed] [Google Scholar]
- Baron JM, Holler D, Schiffer R, Frankenberg S, Neis M, Merk HF, Jugert FK. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J Invest Dermatol. 2001;116(4):541–8. doi: 10.1046/j.1523-1747.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- Baron JM, Merk HF. Drug metabolism in the skin. Curr Opin Allergy Clin Immunol. 2001;1(4):287–91. doi: 10.1097/01.all.0000011028.08297.b3. [DOI] [PubMed] [Google Scholar]
- Carlton GN, Smith LB. Exposures to jet fuel and benzene during aircraft fuel tank repair in the U.S. Air Force. Appl Occup Environ Hyg. 2000;15(6):485–91. doi: 10.1080/104732200301278. [DOI] [PubMed] [Google Scholar]
- Chao YC, Gibson RL, Nylander-French LA. Dermal exposure to jet fuel (JP-8) in US Air Force personnel. Ann Occup Hyg. 2005;49(7):639–45. doi: 10.1093/annhyg/mei021. [DOI] [PubMed] [Google Scholar]
- Chao YC, Kupper LL, Serdar B, Egeghy PP, Rappaport SM, Nylander-French LA. Dermal exposure to jet fuel JP-8 significantly contributes to the production of urinary naphthols in fuel-cell maintenance workers. Environ Health Perspect. 2006;114(2):182–5. doi: 10.1289/ehp.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DFG. List of MAK and BAT Values 2008: Maximum Concentrations and Biological Tolerance Values at the Workplace. Report 44. Wiley, John & Sons; 2008. [Google Scholar]
- Du L, Hoffman SM, Keeney DS. Epidermal CYP2 family cytochromes P450. Toxicol Appl Pharmacol. 2004;195(3):278–87. doi: 10.1016/j.taap.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Hauf-Cabalo L, Gibson R, Rappaport SM. Benzene and naphthalene in air and breath as indicators of exposure to jet fuel. Occup Environ Med. 2003;60(12):969–76. doi: 10.1136/oem.60.12.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R, Sun TT, Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986;102(5):1767–77. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JD, Sakoda LC, Graubard BI, Chanock S, Rubertone MV, Erickson RL, McGlynn KA. Genetic variation in hormone metabolizing genes and risk of testicular germ cell tumors. Cancer Causes Control. 2008;19(9):917–29. doi: 10.1007/s10552-008-9153-6. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19(4):1033–42. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Furukawa F, Kanehara S, Harano F, Shinohara S, Kamimura J, Kawabata S, Igarashi S, Kawamura M, Yamamoto Y, Miyachi Y. Effects of adenosine 5′-monophosphate on epidermal turnover. Arch Dermatol Res. 2008;300(9):485–93. doi: 10.1007/s00403-008-0882-x. [DOI] [PubMed] [Google Scholar]
- Gabbani G, Pavanello S, Nardini B, Tognato O, Bordin A, Fornasa CV, Bezze G, Clonfero E. Influence of metabolic genotype GSTM1 on levels of urinary mutagens in patients treated topically with coal tar. Mutat Res. 1999;440(1):27–33. doi: 10.1016/s1383-5718(99)00013-3. [DOI] [PubMed] [Google Scholar]
- Ghetti G, Mariani L. Ocular changes caused by naphthalene; clinical and experimental studies. Med Lav. 1956;47(10):533–8. [PubMed] [Google Scholar]
- Gibbs S, van de Sandt JJ, Merk HF, Lockley DJ, Pendlington RU, Pease CK. Xenobiotic metabolism in human skin and 3D human skin reconstructs: a review. Curr Drug Metab. 2007;8(8):758–72. doi: 10.2174/138920007782798225. [DOI] [PubMed] [Google Scholar]
- Godschalk RW, Ostertag JU, Zandsteeg AM, Van Agen B, Neuman HA, Van Straaten H, Van Schooten FJ. Impact of GSTM1 on aromatic-DNA adducts and p53 accumulation in human skin and lymphocytes. Pharmacogenetics. 2001;11(6):537–43. doi: 10.1097/00008571-200108000-00008. [DOI] [PubMed] [Google Scholar]
- IARC. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum. 2002;82:1–556. [PMC free article] [PubMed] [Google Scholar]
- Janmohamed A, Dolphin CT, Phillips IR, Shephard EA. Quantification and cellular localization of expression in human skin of genes encoding flavin-containing monooxygenases and cytochromes P450. Biochem Pharmacol. 2001;62(6):777–86. doi: 10.1016/s0006-2952(01)00718-3. [DOI] [PubMed] [Google Scholar]
- Jerina DM, Daly JW, Witkop B, Zaltzman-Nirenberg P, Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970;9(1):147–56. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Kang-Sickel JC, Fox DD, Nam TG, Jayaraj K, Ball LM, French JE, Klapper DG, Gold A, Nylander-French LA. S-arylcysteine-keratin adducts as biomarkers of human dermal exposure to aromatic hydrocarbons. Chem Res Toxicol. 2008;21(4):852–8. doi: 10.1021/tx7003773. [DOI] [PubMed] [Google Scholar]
- Kim D, Andersen ME, Chao YC, Egeghy PP, Rappaport SM, Nylander-French LA. PBTK modeling demonstrates contribution of dermal and inhalation exposure components to end-exhaled breath concentrations of naphthalene. Environ Health Perspect. 2007;115(6):894–901. doi: 10.1289/ehp.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Andersen ME, Nylander-French LA. Dermal absorption and penetration of jet fuel components in humans. Toxicol Lett. 2006;165(1):11–21. doi: 10.1016/j.toxlet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Lee CY, Lee JY, Kang JW, Kim H. Effects of genetic polymorphisms of CYP1A1, CYP2E1, GSTM1, and GSTT1 on the urinary levels of 1-hydroxypyrene and 2-naphthol in aircraft maintenance workers. Toxicol Lett. 2001;123(2–3):115–24. doi: 10.1016/s0378-4274(01)00374-5. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Mondal BC, Ghosh M, Dey S, Mukhopadhyay A, Chandra S, Dasgupta UB. Association of cytochrome P450, glutathione S-transferase and N-acetyl transferase 2 gene polymorphisms with incidence of acute myeloid leukemia. Eur J Cancer Prev. 2008;17(2):125–32. doi: 10.1097/CEJ.0b013e3282b6fd68. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Pollard DL, Weisman W, Garrett CM, Miller TE. Assessment of skin absorption and penetration of JP-8 jet fuel and its components. Toxicol Sci. 2000;55(2):247–55. doi: 10.1093/toxsci/55.2.247. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nelson DR. Introductory remarks on human CYPs. Drug Metab Rev. 2002;34(1–2):1–5. doi: 10.1081/dmr-120001385. [DOI] [PubMed] [Google Scholar]
- North DW, Abdo KM, Benson JM, Dahl AR, Morris JB, Renne R, Witschi H. A review of whole animal bioassays of the carcinogenic potential of naphthalene. Regul Toxicol Pharmacol. 2008;51(2 Suppl):S6–14. doi: 10.1016/j.yrtph.2007.09.022. [DOI] [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of naphthalene (cas no. 91-20-3) in F344/N rats (inhalation studies) Natl Toxicol Program Tech Rep Ser. 2000;(500):1–173. [PubMed] [Google Scholar]
- Pavek P, Dvorak Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr Drug Metab. 2008;9(2):129–43. doi: 10.2174/138920008783571774. [DOI] [PubMed] [Google Scholar]
- Ponec M, Boelsma E, Gibbs S, Mommaas M. Characterization of reconstructed skin models. Skin Pharmacol Appl Skin Physiol. 2002;15(Suppl 1):4–17. doi: 10.1159/000066682. [DOI] [PubMed] [Google Scholar]
- Saeki M, Saito Y, Nagano M, Teshima R, Ozawa S, Sawada J. mRNA expression of multiple cytochrome p450 isozymes in four types of cultured skin cells. Int Arch Allergy Immunol. 2002;127(4):333–6. doi: 10.1159/000057751. [DOI] [PubMed] [Google Scholar]
- Santhanakrishnan BR, Ranganathan G, Raju VB. Naphthalene induced haemolytic anaemia with haemoglobinuria. Indian J Pediatr. 1973;40(304):195–7. doi: 10.1007/BF02755155. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Winter H. Keratin biosynthesis in normal mouse epithelia and in squamous cell carcinomas. mRNA-dependent alterations of the primary structure of distinct keratin subunits in tumors. J Biol Chem. 1983;258(21):13268–72. [PubMed] [Google Scholar]
- Serdar B, Egeghy PP, Gibson R, Rappaport SM. Dose-dependent production of urinary naphthols among workers exposed to jet fuel (JP-8) Am J Ind Med. 2004;46(3):234–44. doi: 10.1002/ajim.20049. [DOI] [PubMed] [Google Scholar]
- Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21(4):257–76. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Torresan C, Oliveira MM, Torrezan GT, de Oliveira SF, Abuazar CS, Losi-Guembarovski R, Lima RS, Urban CA, Cavalli IJ, Ribeiro EM. Genetic polymorphisms in oestrogen metabolic pathway and breast cancer: a positive association with combined CYP/GST genotypes. Clin Exp Med. 2008;8(2):65–71. doi: 10.1007/s10238-008-0159-x. [DOI] [PubMed] [Google Scholar]
- US EPA. Toxicological Review of Naphthalene (CAS No. 91-20-3) in Support of Summary Information on the Integrated Risk Information System (IRIS) Cincinnati: US Environmental Protection Agency, National Center for Environmental Assessment; 1998. [Google Scholar]
- Waidyanatha S, Troester MA, Lindstrom AB, Rappaport SM. Measurement of hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone after administration of naphthalene to F344 rats. Chem Biol Interact. 2002;141(3):189–210. doi: 10.1016/s0009-2797(02)00048-0. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, Zheng Y, Serdar B, Rappaport SM. Albumin adducts of naphthalene metabolites as biomarkers of exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev. 2004;13(1):117–24. doi: 10.1158/1055-9965.epi-03-0150. [DOI] [PubMed] [Google Scholar]
- Watt FM. Proliferation and terminal differentiation of human epidermal keratinocytes in culture. Biochem Soc Trans. 1988;16(5):666–8. doi: 10.1042/bst0160666. [DOI] [PubMed] [Google Scholar]
- Wilson AS, Davis CD, Williams DP, Buckpitt AR, Pirmohamed M, Park BK. Characterisation of the toxic metabolite(s) of naphthalene. Toxicology. 1996;114(3):233–42. doi: 10.1016/s0300-483x(96)03515-9. [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Monteiro-Riviere NA, Inman AO, Kimpel MA, Pedrick NM, Ringham HN, Riviere JE. Effect of JP-8 jet fuel exposure on protein expression in human keratinocyte cells in culture. Toxicol Lett. 2005;160(1):8–21. doi: 10.1016/j.toxlet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Wolf O. Cancer of the larynx in naphthalene cleaners. Z Gesamte Hyg. 1978;24(10):737–9. [PubMed] [Google Scholar]
- Yang M, Koga M, Katoh T, Kawamoto T. A study for the proper application of urinary naphthols, new biomarkers for airborne polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol. 1999;36(1):99–108. doi: 10.1007/s002449900447. [DOI] [PubMed] [Google Scholar]
- Yengi LG, Xiang Q, Pan J, Scatina J, Kao J, Ball SE, Fruncillo R, Ferron G, Roland Wolf C. Quantitation of cytochrome P450 mRNA levels in human skin. Anal Biochem. 2003;316(1):103–10. doi: 10.1016/s0003-2697(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Yeowell-O’Connell K, McDonald TA, Rappaport SM. Analysis of hemoglobin adducts of benzene oxide by gas chromatography-mass spectrometry. Anal Biochem. 1996;237(1):49–55. doi: 10.1006/abio.1996.0199. [DOI] [PubMed] [Google Scholar]
- Zinkham WH, Childs B. A defect of glutathione metabolism in erythrocytes from patients with a naphthalene-induced hemolytic anemia. Pediatrics. 1958;22(3):461–71. [PubMed] [Google Scholar]