Summary
Sec1/Munc18 (SM) proteins bind to and function with soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs) at each vesicle fusion site in the cell. The purpose for these interactions is becoming clearer, as what had been interpreted as functional divergence between SM proteins acting at different vesicle trafficking steps, or in specialized cells, is giving way to more recent evidence for common functions among all SM proteins. What is emerging is a picture of SM proteins acting not merely as SNARE regulators, but also as central components of the membrane fusion apparatus. The available data suggest sequential models that describe how the soluble SM protein might first regulate SNARE complex assembly and then cooperate with SNAREs to stimulate membrane fusion.
Introduction
Membrane-enclosed vesicles traffic protein and lipid cargo for a wide variety of functions fundamental to eukaryotic cells, including organelle formation and maintenance, neurotransmitter secretion, protein targeting and cell growth. For accurate trafficking, these vesicles rely on conserved families of both soluble and membrane-bound intracellular proteins, which assemble together to form vesicle attachment and membrane fusion complexes in order to merge vesicles with specific target membrane compartments. The conserved nature of intracellular vesicle trafficking proteins suggests that common principles underlie the mechanism of vesicle attachment and membrane fusion [1].
Vesicle trafficking proteins fall into four major categories: i) vesicle-anchored (v) and target-membrane–anchored (t) soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs), which bring the two membranes together and catalyze fusion by assembling into tight SNARE complexes through alpha-helical sequences called SNARE motifs [2]; ii) N-ethylmaleimide–sensitive factor (NSF) and NSF attachment proteins (SNAPs), which disassemble SNARE complexes to recycle the SNAREs for another round of fusion [reviewed in [3]; [1]; [4]; [5]]; iii) Rab GTPases and multicomponent vesicle tethering complexes, which orchestrate vesicle attachment and the subsequent assembly of cognate v-t SNARE complexes [reviewed in [6]]; and iv) Sec1/Munc18 (SM) proteins, which are soluble factors that may act with the SNARE proteins before and after vesicle attachment.
The exact function(s) of SM proteins has been enigmatic, in part because multiple roles in the steps that lead to membrane fusion have been attributed to SM proteins [reviewed in [7]; [8]], and in part because there are likely some differences in their mechanisms of action. Initial studies supported functional differentiation among SM proteins, as diverse modes of interactions were observed between SM proteins and SNAREs from the syntaxin family, as well as with assembled SNARE complexes [reviewed in [7]]. Yet none of the interactions appeared to be universal: an unsatisfying result, considering the conservation of four SM protein subfamilies throughout the evolution of all eukaryotes [9] [10] [11] and their essential functions for exocytosis (Sec1/Munc18), endocytosis (Vps45), protein biosynthesis (Sly1) and degradation (Vps33).
A string of exciting new results is leading to a more cogent picture of how the functions of SM proteins and SNAREs are coupled. While SM proteins have been viewed in the past as regulators of SNARE function, the emerging picture suggests that SM proteins also cooperate with SNARE complexes to induce vesicle membrane fusion. In this review, we describe some of the recent studies, placing them in the overall context set by the earlier studies, and we discuss potential models for the mechanism of action of SM proteins.
Architecture of SM proteins
The primary amino-acid sequence of SM proteins predicts hydrophilic proteins of approximately 600 amino acids with no recognizable domains or motifs to suggest how SM proteins could promote vesicle attachment or fusion. Structural studies have revealed that the overall fold of SM proteins is highly conserved between different organisms and at different vesicle trafficking steps, supporting a commonality of function(s) [12]; [13,14]. The SM protein structure includes three domains (called domains 1–3) (Figure 1A) that together form an arch shape with a large cavity on one side, and a deep groove on the opposite side, both of which have been implicated in interactions with the SNAREs (Figure 1). Comparison of diverse SM proteins structures reveals a conformational flexibility in the orientations of domain 1 and part of domain 3 around the central cavity [13], which is likely to impact the interactions with SNAREs.
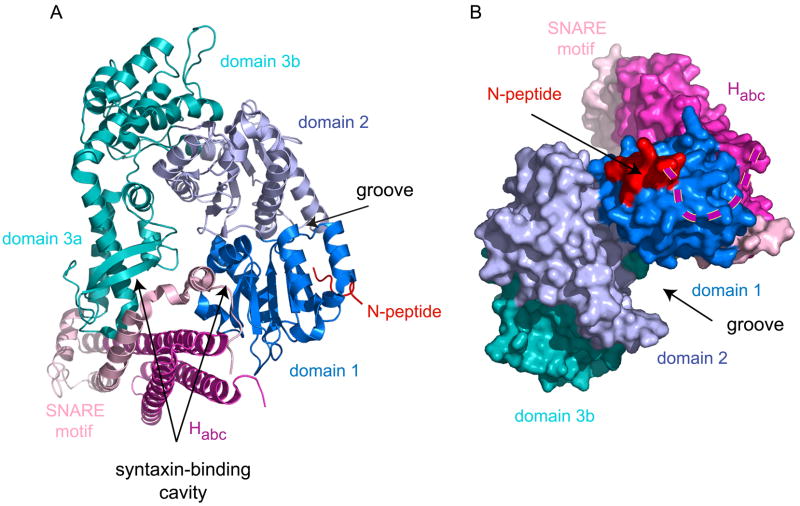
Figure 1. Structure of Munc18-1 bound to synaxin-1.
(A) A ribbon diagram illustrates the architecture of the conserved three-domain SM protein, Munc18-1, bound to the helical bundle of synaxin-1 in the closed conformation. Munc18-1 domain 1 (blue) binds the syntaxin N-peptide (red) and forms an arch with domains 2 (light blue) and 3 (teal). The cavity of the arch binds the syntaxin-1 helical bundle formed by the SNARE motif helix (light pink) bound to the Habc domain (hot pink) in the closed conformation. The location of the groove between domains 1 and 2 and the syntaxin-binding cavity are indicated with arrows.
(B) Rotated view reveals two syntaxin-binding sites and a groove implicated in SNARE complex binding. SM protein Munc18-1 [domains colored as in (A)] is viewed from the membrane end of the closed, 4-helix bundle of synaxin-1 [colored as in (A)]. The syntaxin N-peptide is bound to domain 1 of Munc18-1, and is connected to the syntaxin-1 Habc helical bundle by an unresolved linker (aa 10–26; hatched line). Mutations in the groove region of a yeast SM protein, Sec1p, abolish SNARE complex binding. The syntaxin-binding cavity and domain 3a are tilted into the page and are, therefore, not visible in this view. Images were created using Pymol™ (DeLano Scientific, Palo Alto, CA) and Protein Data Bank accession number 3C98.
An SM protein bound to the closed conformation of syntaxin
The first connection between SM proteins and SNAREs came from the identification of a tight interaction between the synaptic protein Munc18-1 and its cognate t-SNARE, syntaxin-1, which are both required for neurotransmitter release [15]. This interaction requires a closed conformation of syntaxin-1, whereby an N-terminal three-helix bundle called the Habc domain [16] binds to the SNARE motif, preventing SNARE complex assembly [17]; [18]. A co-crystal structure revealed how closed syntaxin-1 is held by the flexible cavity of Munc18-1 [12] (Figure 1). In this configuration, syntaxin-1 is prevented from premature reassembly with the other SNAREs after disassembly, and the binding interaction stabilizes both Munc18-1 and syntaxin-1 in vivo [19]; [20]. At the same time, these findings were puzzling, because an inhibitory role for Munc18-1 is at odds with its essential role in neurotransmitter release [19].
To address this apparent paradox, it was proposed that the Munc18-1/syntaxin-1 structure represented an intermediate in the opening reaction that stimulates SNARE assembly [17]; [12]; [21]. However, recent NMR data indicates that isolated syntaxin-1 forms a weak closed conformation that is only stabilized when Munc18-1 is bound [22], supporting an inhibitory role. Moreover, a mutation designed to open syntaxin-1 increases the probability of release of synaptic vesicles [20], and another synaptic protein with a key role in release, Unc13/Munc13, has been implicated in opening syntaxin-1 [23]. The MUN domain [24] of Munc13 binds to the SNARE complex and has been proposed to promote assembly [25]; [26]. Other domains of Unc13/Munc13 and diverse factors that bind to them [27]; [28] control distinct forms of presynaptic plasticity, likely by regulating the activity of the MUN domain in opening syntaxin-1 [24]. Hence, the transition from closed to open syntaxin-1 may be a key point of regulation for brain function.
A function for SM proteins in gating SNARE assembly does not, however, appear to be universal. By contrast to syntaxin-1, the closed conformation of the yeast homolog, Sso1p, is highly stable [29], and Sso1p does not bind to its cognate SM protein, Sec1p [30]; [31]. Furthermore, at the yeast vacuole, the syntaxin Vam3p does not adopt a closed conformation [32]. Moreover, several studies showed that most syntaxins bind to their cognate SM proteins using a different mode, involving the syntaxin amino terminus [14]; [33]; [34] (see below).
Taken together, these findings suggested that the Munc18-1/syntaxin-1 interaction arose from specific requirements of regulated exocytosis in higher eukaryotes. However, enticing new data indicate that the regulatory function is not limited to the synapses of higher eukaryotes: the yeast endosomal syntaxin Tlg2p also adopts a closed conformation that binds to its cognate SM protein Vps45p [35], and the same conclusion may apply to their mammalian homologues [36]. Moreover, the Unc13/Munc13 MUN domain was recently found to have homology with components of tethering complexes involved in diverse types of membrane traffic [37], suggesting that the mechanism of syntaxin-1 opening may be shared in other systems. Hence, even if some mechanisms of regulation of the conformational transition of neuronal syntaxin-1 are specific to the synapse, these recent data suggest the Munc18-1/closed syntaxin-1 structure may represent a more general complex than previously suggested.
An interaction with the syntaxin N-terminus
Although it seems clear that binding of SM proteins to closed syntaxin conformations plays an important regulatory role, a more general function for SM proteins may involve an interaction at the syntaxin amino terminus, with a conserved N-peptide motif [reviewed in [38]]. Multiple findings have supported the functional importance of syntaxin N-peptide binding to SM proteins in vivo, including the disruption of the Golgi structure and of neurotransmitter release caused by interference with this interaction [34]; [39]; [40]; [41]. However, other studies have revealed milder or no functional effects upon disruption of syntaxin N-peptide binding to SM proteins [42]; [43]; [44]. Although widespread, binding of SM proteins to the syntaxin N-peptide motif is not universal. For example, it is clear that syntaxin N-peptide motifs are not involved in the function of Sec1p [31] and Vps33p [45], two SM proteins that also do not interact with closed syntaxin.
In a recent study, the N-peptide interaction between synaxin 1 and Munc18-1 was shown to inhibit assembly of syntaxin with other SNAREs, preventing formation of the SNARE complex [36]. Re-examination of the original Munc18-1/synaxin 1 X-ray diffraction data [12] has revealed how this SM protein simultaneously binds to the syntaxin N-peptide and the helical bundle of the closed conformation [36] (Figure 1A). A view from the membrane end suggests how this configuration might influence SNARE assembly (Figure 1B). While the connection between the N-peptide and the first helix of the Habc domain is not resolved, the SM protein-bound syntaxin may overlap a binding site for an assembled SNARE complex, which has recently been mapped for yeast Sec1p to a nearby “groove” region [11]. Note that, although the interaction of the syntaxin-1 N-peptide with Munc18-1 hinders the transition from the Munc18-1/closed syntaxin-1 complex to the SNARE complex [36], other factors in the cell, such as Munc13, likely facilitate this transition [25]; [26], and interactions with the N-peptide and Habc domain could keep Munc18-1 bound to syntaxin-1 after opening the closed conformation [39]; [46].
How might N-peptide binding be required for synaptic vesicle fusion? One possibility places the assembly regulation function of syntaxin-binding SM proteins as an obligatory intermediate on the pathway to SM protein stimulated vesicle fusion (Figure 2A, B). Capture of the SM protein by either a tight N-peptide or closed-conformation interaction would not only ensure efficient and highly regulated SNARE complex assembly (and prevention of rapid, inappropriate re-assembly after disassembly), but would also concentrate the SM protein at vesicle docking sites, where it could act on SNARE complexes to stimulate membrane fusion.
Figure 2. Simplified models for SM protein function in vesicle fusion.
Before fusion of the secretory vesicle (1), the t-SNARE syntaxin (magenta) adopts a closed conformation and is sequestered from the other SNAREs by an SM protein (blue ellipse). When the secretory vesicle comes into close apposition with the plasma membrane, the SM protein-bound t-SNARE assembles with the v-SNARE (green), pinning together the two membranes. The SM protein then binds to the SNARE complex and activates membrane fusion using an unknown mechanism.
(A) In one model, the SM protein translocates from the syntaxin N-terminal domain (2) to bind the assembled SNARE complexes at the membrane-proximal end before membrane mixing (3). Here, the SM protein is proposed to provide leverage for the SNAREs in order to achieve membrane fusion (4) [see ref. 65].
(B) In another model, SNARE complex assembly induces hemifusion (2), before the SM protein translocates from the syntaxin N-terminal domain to the membrane-proximal end of the assembled SNARE complexes, where it stimulates full fusion (3) by altering the curvature preference of the membrane fusion intermediate (C).
(C) How protein shape can change membrane curvature at key steps in fusion. A planar segment of a vesicle membrane (vm) containing unpaired v-SNAREs is shown below the unpaired t-SNAREs in a segment of the plasma membrane [pm; “apposition,” in (B)]. The luminal leaflet of the vesicle and the extracellular leaflet of the plasma membrane are distal (blue), whereas the leaflets facing the cytoplasm are proximal (red) to each other. Each SNARE is anchored across both leaflets of a membrane bilayer by a helical transmembrane domain (black rectangle). In step (1), SNAREs assemble between the two membranes to form trans SNARE complexes, and the proximal leaflets fuse to form a transient stalk intermediate. In step (2), the distal leaflets meet in the center of the stalk to form a new bilayer, called the hemifusion diaphragm (hd). The cone shape (triangle with cytoplasmic apex) of the trans SNARE complexes stabilizes the hemifusion intermediate by promoting negative curvature in the proximal leaflets [“hemifusion,” in (B)]. In step (3), hemifusion is resolved to full fusion, and the vesicle lumen becomes continuous with the extracellular space [“full fusion,” in (B)]. The SM protein changes the geometry of the SNARE complex from a cone to an inverted cone shape, simultaneously inducing positive membrane curvature (exaggerated to emphasize an outward pulling force) and formation of the cis SNARE complex, in order to destabilize the hemifusion diaphragm and complete membrane fusion.
SM proteins and membrane fusion
In addition to a regulatory role with syntaxin at the SNARE complex assembly step, there is functional evidence that SM proteins are required at a later step, for fusion of the vesicle and target membranes. In S. cerevisiae, sec1 mutants have been isolated with a temperature sensitive block after SNARE complex assembly, but before secretory vesicle fusion [11]; [47]. These mutations cluster in the groove region of Sec1p (Figure 1B), a region distinct from the sites of sec1 mutations that block secretion at a step before SNARE complex assembly [11]. Evidence from studies in chromaffin cells also indicates that Munc18-1 acts at multiple steps in exocytosis [48]. Overexpression of the t-SNARE, SNAP-25, rescues the vesicle attachment defect, but not the vesicle fusion defect observed in chromaffin cells from Munc18-1 KO mice [49]. These and other observations [reviewed in [7]] underscore a fundamental requirement of SM proteins for membrane fusion.
Biochemical studies have uncovered a direct interaction with assembled SNARE complexes, placing SM proteins at the site of the membrane fusion reaction. Binding to SNARE complexes was initially observed for two yeast SM proteins, Sly1p [50]; [43] and Sec1p [30]. More recently, a direct interaction with SNARE complexes has been observed for Munc18-1 [51]; [52], Munc18c [53] and Vps45 [42]; [36]; [35]. For many SM proteins, the discovery of an interaction with the N-peptide motif offered a trivial explanation: SM proteins could remain bound to the syntaxin amino terminus, while the carboxy-terminal SNARE motif helix participated in the four-helix bundle that forms the SNARE complex. In this way, the function of the conserved N-peptide interaction would be to concentrate SM protein at membrane fusion sites (Figure 2A). However, as mentioned above, N-peptide binding is not universally required and, therefore, may not represent the configuration required for the membrane fusion function of SM proteins. Moreover, mutations that disrupt binding of Munc18-1 to the syntaxin-1 N-terminal region disrupt vesicle priming at a release-ready state (thought to be the SNARE complex assembly step), but do not affect fusion of primed vesicles, suggesting that the N-peptide interaction represents a function required upstream of membrane fusion [46]. A strong clue for the functional significance of an SM protein-SNARE complex interaction was provided by the finding that yeast Sec1p does not bind the N-peptide or closed conformation of its cognate syntaxin, Sso1p, but instead binds the helical bundle of the assembled SNARE complex [31]. Although Munc18-1 binding to the SNARE four-helix bundle has also been reported [51]; [54]; [55], more studies are required to determine whether the Sec1p result represents a general interaction required for SM protein function in membrane fusion.
Recently, several groups have reported SM protein-stimulation of SNARE-mediated lipid mixing, a limited assay for membrane fusion [56]; [51]; [57]. At high concentrations, SNAREs promote lipid mixing in the absence of other factors [58], although lipid mixing depends on the physical state of the vesicles and may be accompanied by disruption of membrane integrity [59]; [60]. Uncovering the stimulatory effect of Munc18-1 on SNARE-dependent lipid mixing required lowering SNARE concentrations [51]; [61]. In similar experiments, but with giant unilamellar vesicles, Munc18-1 was found to be required for lipid mixing [62]. In these experiments, the effect of Munc18-1 depended on its interaction with the syntaxin-1 N-peptide [51]; [62]. Subsequent studies of single vesicle fusion events revealed a stimulatory role for Munc18-1 that does not require the syntaxin N-peptide or Habc domain [63], suggesting strongly that Munc18-1 binds and acts with the SNARE complex helical bundle to stimulate fusion. Recent data suggest that Munc18-1 can bind to the membrane-proximal region of the v-SNARE, synaptobrevin [55], which would place Munc18-1 at the end of the SNARE complex adjacent to the membrane, where it could perform its required function in membrane fusion.
In another system, the yeast vacuolar membrane fusion machinery was reconstituted in liposomes to recapitulate the protein and lipid requirements for homotypic vacuole fusion in the cell [57]; [64]. Experiments using this comprehensive reconstitution assay revealed a strict dependence of lipid mixing on the Vps33p-HOPS tethering complex, which is required to promote formation of trans SNARE complexes between fusing membranes. In a similar analysis, the reconstitution of the endosomal fusion machinery in a contents mixing assay revealed a strong dependence on a protein complex including the SM protein, Vps45 [54]. However, the specific roles of the SM protein within the vacuolar and endosomal protein complexes remain to be discovered.
Models for SM protein and SNARE induced membrane fusion
Based in part on the results presented above, a consensus is building that SM proteins function both as regulators of SNARE complex assembly at the vesicle attachment step, and as a part of the core machinery at the membrane fusion step. Syntaxin interactions and the involvement of other factors, such as Rab-GTPases and tethering complexes, are likely to underlie the regulatory function, but how might a soluble SM protein cooperate with SNARE complexes to induce membrane fusion?
We envision two hypothetical models. In both models, we assume that the SM protein is attracted to the site of fusion by virtue of binding to the cognate syntaxin or through some other nearby interaction, and that subsequent translocation to a binding site on the SNARE complex helical bundle is key for membrane fusion (Figure 2A,B).
One model (Figure 2A) proposes that the bulk of the SM protein bound to the assembling SNARE complex prevents diffusion of the SNAREs to the center of the intermembrane space, where they could hinder fusion. In this configuration, the SM protein provides a key asymmetry that helps to apply torque to the two membranes [65]. Note that, if Munc18-1 were bound to the membrane adjacent region of syntaptobrevin, it would be difficult to complete C-terminal assembly of the SNARE complex without strongly bending the membranes; in addition, two basic regions of Munc18-1 could participate in membrane bending to induce fusion [55].
A second model (Figure 2B,C) proposes that SM proteins stimulate vesicle membrane fusion by destabilizing a hemi-fusion intermediate state. The conserved helical-bundle shape of the SNARE complex could be responsible for inducing the negative curvature required for the stalk and hemi-fusion membrane intermediates, and there is evidence to support stabilization of hemifusion by trans SNARE complex assembly [66–68].
Resolving the hemi-fusion intermediate to complete membrane fusion, however, has been difficult to envision. At low concentrations of fusion proteins, the hemi-fusion intermediate has been shown to become quite stable, if not resolved in a timely fashion [69]; [68]. By binding and changing the shape of the imbedded SNAREs (see legend, Figure 2C), SM proteins could transiently disfavor negative curvature for more positive curvature, much like the effect induced on local positive membrane curvature when attachment proteins bind to receptors [for a review, see [70]]. If the shape change occurs simultaneously for SNARE complexes that rim the intersection of two fusing membranes, a reversal in curvature preference could generate enough outward force to destabilize the hemi-fusion diaphragm and complete the membrane-fusion reaction. Protein-induced curvature at the membrane fusion site has been proposed previously as a mechanism for stimulation of membrane fusion [71]; [72].
Conclusions
Recent work has simplified the picture of SM proteins acting with SNAREs to control vesicle attachment and membrane fusion, yet several questions remain to be answered. Is binding to the syntaxin N-peptide inhibitory or activating? A sequential model for regulation of SNARE assembly, followed by cooperation with SNARE complexes in vesicle fusion, might explain how SM proteins could be both inhibitory and stimulatory, especially in tightly regulated, rapid-response systems, such as synaptic transmission. Is a steric hindrance model or one that involves changes in membrane curvature informative for SM protein activation of membrane fusion, or is the correct mechanism altogether something else? Here, there may be lessons to be learned from membrane fission, where analogous membrane intermediates may be involved. Before vesicle docking, are SM proteins required at an early step, acting with vesicle tethering complexes and Rab-GTPases for selective vesicle attachment to target membranes? The isolation and structural mapping of additional SM protein mutants in model systems will help to separate early and late functions for SM proteins and provide a closer look at the surfaces used for selective vesicle trafficking to and fusion with target membranes. In the near future, we can expect high-resolution structures of SM protein-SNARE complex assemblies to help inform the mechanisms of regulation and fusion activation. The ultimate challenge will be to determine the sequence of steps taken as dynamic interactions between soluble and membrane-anchored proteins force two membranes through the dramatic changes that result in the irreversible and vital consequences of membrane fusion.
Acknowledgments
This work was supported by National Institutes of Health General Medical Sciences grants NS40944 and NS37200 (J.R.), and funding from the Department of Biochemistry & Biophysics, Texas A&M University (C.M.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chavela M. Carr, Email: chave@tamu.edu.
Josep Rizo, Email: jose@arnie.swmed.edu.
References
- 1.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Söllner T, Whiteheart SW, Brunner M, Erdjument–Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 4.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Rizo J, Sudhof TC. SNAREs and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 8.Toonen RF, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Arac D, Dulubova I, Pei J, Huryeva I, Grishin NV, Rizo J. Three-dimensional structure of the rSly1 N-terminal domain reveals a conformational change induced by binding to syntaxin 5. J Mol Biol. 2005;346:589–601. doi: 10.1016/j.jmb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Koumandou VL, Dacks JB, Coulson RM, Field MC. Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol Biol. 2007;7:29. doi: 10.1186/1471-2148-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Hashizume K, Cheng YS, Hutton JL, Chiu CH, Carr CM. Yeast Sec1p functions before and after vesicle docking. Mol Biol Cell. 2009;20:4673–4685. doi: 10.1091/mbc.E09-02-0172. This paper shows that yeast Sec1p is required before and after SNARE complex assembly, in support of general requirements for SM proteins in both vesicle attachment and fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 13.Bracher A, Weissenhorn W. Crystal structures of neuronal squid Sec1 implicate inter-domain hinge movement in the release of t-SNAREs. J Mol Biol. 2001;306:7–13. doi: 10.1006/jmbi.2000.4347. [DOI] [PubMed] [Google Scholar]
- 14.Bracher A, Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. Embo J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez I, Ubach J, Dulubova I, Zhang X, Sudhof TC, Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 17.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. Embo J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 20•.Gerber SH, Rah JC, Min SW, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. This paper shows that knockin mice bearing a mutation that helps to open syntaxin-1 exhibit an increase in the probability of vesicle release, suggesting that the closed conformation gates entry of syntaxin-1 into SNARE complexes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munson M, Chen X, Cocina AE, Schultz SM, Hughson FM. Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Lu J, Dulubova I, Rizo J. NMR analysis of the closed conformation of syntaxin-1. J Biomol NMR. 2008;41:43–54. doi: 10.1007/s10858-008-9239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, Grishin NV, Rosenmund C, Rizo J. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 25.Guan R, Dai H, Rizo J. Binding of the Munc13-1 MUN domain to membrane-anchored SNARE complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- 26.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure. 2008;16:308–320. doi: 10.1016/j.str.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Sudhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? Embo J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Hu B, Zieba A, Neumann NG, Kasper-Sonnenberg M, Honsbein A, Hultqvist G, Conze T, Witt W, Limbach C, et al. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. J Neurosci. 2009;29:12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson KL, Munson M, Miller RB, Filip TJ, Fairman R, Hughson FM. Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- 30.Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Togneri J, Cheng YS, Munson M, Hughson FM, Carr CM. Specific SNARE complex binding mode of the Sec1/Munc-18 protein, Sec1p. Proc Natl Acad Sci U S A. 2006;103:17730–17735. doi: 10.1073/pnas.0605448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 33.Dulubova I, Yamaguchi T, Gao Y, Min SW, Huryeva I, Sudhof TC, Rizo J. How Tlg2p/syntaxin 16 'snares' Vps45. Embo J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 35•.Furgason ML, MacDonald C, Shanks SG, Ryder SP, Bryant NJ, Munson M. The N-terminal peptide of the syntaxin Tlg2p modulates binding of its closed conformation to Vps45p. Proc Natl Acad Sci U S A. 2009;106:14303–14308. doi: 10.1073/pnas.0902976106. This paper provides strong evidence that Vps45p binds to a closed conformation of Tlg2p, suggesting that the Munc18-1/closed syntaxin-1 interaction may be more general than previously thought. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. Embo J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. This paper provides evidence that Munc18-1 binds to the N-petide motif as well as the closed conformation of syntaxin-1, and includes evidence that both binding sites are also used by mammalian Vps45, suggesting a dual interaction with syntaxin may be shared among SM proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pei J, Ma C, Rizo J, Grishin NV. Remote homology between Munc13 MUN domain and vesicle tethering complexes. J Mol Biol. 2009;391:509–517. doi: 10.1016/j.jmb.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munson M, Bryant NJ. A role for the syntaxin N-terminus. Biochem J. 2009;418:e1–3. doi: 10.1042/BJ20082389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Sudhof TC. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J Neurosci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEwen JM, Kaplan JM. UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol Biol Cell. 2008;19:3836–3846. doi: 10.1091/mbc.E08-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson JR, Ferdek P, Lian LY, Barclay JW, Burgoyne RD, Morgan A. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem J. 2009;418:73–80. doi: 10.1042/BJ20081956. [DOI] [PubMed] [Google Scholar]
- 42.Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ. The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J Cell Biol. 2006;173:927–936. doi: 10.1083/jcb.200512024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng R, Gallwitz D. Multiple SNARE interactions of an SM protein: Sed5p/Sly1p binding is dispensable for transport. Embo J. 2004;23:3939–3949. doi: 10.1038/sj.emboj.7600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han L, Jiang T, Han GA, Malintan NT, Xie L, Wang L, Tse FW, Gaisano HY, Collins BM, Meunier FA, et al. Rescue of Munc18-1 and -2 double knockdown reveals the essential functions of interaction between Munc18 and closed syntaxin in PC12 cells. Mol Biol Cell. 2009;20:4962–4975. doi: 10.1091/mbc.E09-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Deak F, Xu Y, Chang WP, Dulubova I, Khvotchev M, Liu X, Sudhof TC, Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. The results in this paper provide evidence that Munc18-1 binding to the SNARE complex through the syntaxin-1 N-terminal region is important for priming synaptic vesicles to a release-ready state, but not for fusion of the primed vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulyas-Kovacs A, de Wit H, Milosevic I, Kochubey O, Toonen R, Klingauf J, Verhage M, Sorensen JB. Munc18-1: sequential interactions with the fusion machinery stimulate vesicle docking and priming. J Neurosci. 2007;27:8676–8686. doi: 10.1523/JNEUROSCI.0658-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.de Wit H, Walter AM, Milosevic I, Gulyas-Kovacs A, Riedel D, Sorensen JB, Verhage M. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138:935–946. doi: 10.1016/j.cell.2009.07.027. This paper shows that docking defects in chromaffin cells from Munc18-1 knockout mice can be rescued by promoting formation of t-SNARE complexes, but the fusion defect is not rescued. These data provide compelling evidence for a role of Munc18-1 downstream of docking. [DOI] [PubMed] [Google Scholar]
- 50.Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 51.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latham CF, Lopez JA, Hu SH, Gee CL, Westbury E, Blair DH, Armishaw CJ, Alewood PF, Bryant NJ, James DE, et al. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 54••.Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. This paper presents a reconstitution of the endosomal membrane fusion machinery using a contents mixing assay. The reconstituion reveals strong requirements for many of the components known to be important for fusion in vivo, including a complex that contains Vps45p. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Su L, Rizo J. Binding of Munc18–1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry. 2010;49:1568–1576. doi: 10.1021/bi9021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott BL, Van Komen JS, Irshad H, Liu S, Wilson KA, McNew JA. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J Cell Biol. 2004;167:75–85. doi: 10.1083/jcb.200405018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. Embo J. 2008;27:2031–2042. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Arac D, Wang TM, Gilpin CJ, Zimmerberg J, Rizo J. SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys J. 2006;90:2062–2074. doi: 10.1529/biophysj.105.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J. 2006;90:1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodkey TL, Liu S, Barry M, McNew JA. Munc18a scaffolds SNARE assembly to promote membrane fusion. Mol Biol Cell. 2008;19:5422–5434. doi: 10.1091/mbc.E08-05-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Tareste D, Shen J, Melia TJ, Rothman JE. SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc Natl Acad Sci U S A. 2008;105:2380–2385. doi: 10.1073/pnas.0712125105. This paper shows that Munc18-1 stimulates adhesion and lipid mixing of t-SNARE containing vesicles with giant vesicles containing v-SNAREs. Under the conditions of these experiments, Removal of the syntaxin Habc domain prevented vesicle adhesion (and lipid mixing), and a mutation in the syntaxin-1 N-peptide disrupted lipid mixing but not adhesion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Diao J, Su Z, Lu X, Yoon T-Y, Shin Y-K, Ha T. Single-Vesicle Fusion Assay Reveals Munc18-1 Binding to the SNARE Core Is Sufficient for Stimulating Membrane Fusion. ACS Chem Neurosci. 2010;1:168–174. doi: 10.1021/cn900034p. This paper provides evidence that Munc-18 stimulates membrane fusion by binding to the SNARE complex helical bundle in the absence of the N-peptide or Habc domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci U S A. 2009;106:17626–17633. doi: 10.1073/pnas.0903801106. This paper describes a comprehensive reconstitution of the yeast vacuolar fusion machinery, whereby lipid mixing between liposomes depends strictly on numerous lipids and proteins found to be key for fusion in vivo, including the Vps33p-HOPS complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Yoon TY, Okumus B, Zhang F, Shin YK, Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc Natl Acad Sci U S A. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Zhang F, Su Z, McNew JA, Shin YK. Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- 68.Jun Y, Wickner W. Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0700970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chernomordik LV, Leikina E, Kozlov MM, Frolov VA, Zimmerberg J. Structural intermediates in influenza haemagglutinin-mediated fusion. Mol Membr Biol. 1999;16:33–42. doi: 10.1080/096876899294733. [DOI] [PubMed] [Google Scholar]
- 70.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 71.Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 72.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]