Abstract
Mammalian reproductive cycles are controlled by an intricate interplay between the hypothalamus, pituitary and gonads. Central to the function of this axis is the ability of the pituitary gonadotrope to appropriately respond to stimulation by gonadotropin-releasing hormone (GnRH). This review focuses on the role of cell signaling and in particular, mitogen-activated protein kinase (MAPK) activities regulated by GnRH that are necessary for normal fertility. Recently, new mouse models making use of conditional gene deletion have shed new light on the relationships between GnRH signaling and fertility in both male and female mice. Within the reproductive axis, GnRH signaling is initiated through discrete membrane compartments in which the receptor resides leading to the activation of the extracellular signal-regulated kinases (ERKs 1/2). As defined by gonadotrope-derived cellular models, the ERKs appear to play a central role in the regulation of a cohort of immediate early genes that regulate the expression of late genes that, in part, define the differentiated character of the gonadotrope. Recent data would suggest that in vivo, conditional, pituitary-specific disruption of ERK signaling by GnRH leads to a gender-specific perturbation of fertility. Double ERK knockout in the anterior pituitary leads to female infertility due to LH biosynthesis deficiency and a failure in ovulation. In contrast, male mice are modestly LH deficient; however, this does not have an appreciable impact on fertility.
Keywords: Hypothalamic-Pituitary-Gonadal axis, Reproduction, Gonadotropin-releasing hormone, Gonadotrope, Cell signaling, Mitogen-activated protein kinase, ERKs, Gonadotropins, Fertility, Mouse models
1. Introduction
The hypothalamic-pituitary-gonadal (HPG) axis consists of an intercommunicating set of neural and endocrine tissues that function as a highly integrated unit in the regulation of fertility. The basic organization of this physiological axis is highly conserved and underlies reproductive competence in a wide variety of diverse vertebrate species including birds, reptiles, fish, and mammals [2;76;140;168]. The gonadotrope cell of the anterior pituitary plays a particularly critical role within this system as the intermediary between the hypothalamic gonadotropin-releasing hormone (GnRH) signal and the germ cell reservoirs and steroid hormone productivity of the gonads. Expression of the GnRH receptor (GnRHR) is a defining characteristic of the gonadotrope. Binding of GnRH to its receptor triggers a complex array of intracellular signal transduction events within the gonadotrope; these signaling cascades orchestrate the overall physiological response of these cells to GnRH stimulation, culminating in the synthesis and release of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH). Thus, in both functional and anatomical senses, the GnRHR and the intracellular signal transduction systems to which it couples, form the fundamental interface between the “neuro-” and “endocrine” components of this neuroendocrine axis. The mechanisms underlying the response of the gonadotrope to hypothalamic GnRH are thus of great importance to our understanding of the molecular basis of reproductive function and fertility.
Signal transduction systems form the basic biochemical infrastructure by which cells mount specific and appropriate biological responses to a wide variety of extracellular or intracellular cues. Indeed, dysregulation of signaling processes contributes to many forms of disease including cancer, immunological disease and neuro-degenerative disorders, and targeted manipulation of signaling pathways is an area of great scientific as well as clinical therapeutic interest [20;182;298]. The signal transduction events activated by the GnRHR have themselves been the focus of extensive investigation for well over two decades (recently reviewed in detail by Naor in [199]). This work has generated a formidable body of knowledge regarding the nature and kinetics of GnRH-induced signaling networks and encompasses great advances in our understanding of GnRHR signaling in a variety of experimental systems. However, within the context of reproductive biology, significant challenges remain. The response of the differentiated gonadotrope to the GnRH signal in vivo occurs within the context of a complex and dynamic endocrine milieu. A meaningful understanding of the molecular basis of gonadotrope function therefore awaits systematic clarification of the functionality of GnRH-induced intracellular signal transduction pathways in vivo and their requirements for fertility. The development and refinement of sophisticated transgenic technologies offers a powerful tool by which to address these questions. Recent results utilizing this approach have begun to shed exciting new light on important facets of gonadotrope function in vivo. In this review, we will provide an overview of the mammalian HPG axis with a broad emphasis on the link between GnRH-induced intracellular signal transduction pathways and gonadotrope gene expression within the intact endocrine environment of the living animal.
2. The hypothalamic-pituitary-gonadal (HPG) axis
2.1 Overview of the functional organization of the mammalian HPG axis
In the classical view, the HPG axis is depicted as a tiered and linearly organized set of endocrine tissues that is dedicated to the regulation and support of reproductive activity. This axis consists of a small subset of hypothalamic neurons that express the decapeptide hormone GnRH, the gonadotrope cells of the anterior pituitary, and the gonads. Activation of this endocrine axis commences with the pulsatile secretion of GnRH from the hypothalamus. GnRH is delivered to the anterior pituitary via the hypophyseal portal circulation where it binds to the GnRHR on the surface of gonadotropes triggering the synthesis and secretion of the gonadotropins FSH and LH. The gonadotropins are dimeric glycoprotein hormones composed of distinct hormone-specific β subunits paired with a common α subunit (αGSU). The β subunits of FSH and LH, together with the αGSU and the GnRHR comprise the essential genetic signature of the differentiated gonadotrope [241]. In the male, LH stimulates production of testosterone and androgen-binding protein by testicular Leydig cells and Sertoli cells, respectively. In the female, LH stimulates the production of androgens by the thecal cells that surround the growing ovarian follicle. During the terminal stages of follicular growth, LH also drives the production of progesterone from the granulosa cells of the preovulatory follicle. FSH binds to receptors on the surface of ovarian granulosa cells stimulating the expression of aromatase enzymes that convert thecal androgens to estradiol. In the male, FSH binds to receptors on the surface of Sertoli cells and functions in concert with testosterone to promote the proliferation of spermatogonia as well as the meiosis and postmeiotic development of germ cells.
Several animal models have provided insights into the functional organization of the HPG axis. The hypogonadal (HPG) mouse fails to produce GnRH due to a deletion mutation in the gene encoding the GnRH precursor peptide [174]. HPG mice never enter puberty and display a persistent hypogonadotropic-hypogonadal phenotype which is nevertheless able to be rescued by knockin of a functional GnRH gene [175]. Early studies in sheep demonstrated that surgical ablation of the pituitary stalk (hypothalamic-pituitary disconnection, HPD) led to dramatic decreases in pituitary secretion of the gonadotropins and secondary hypogonadism [38;39]. Pulsatile administration of GnRH to these animals reestablished corresponding and appropriate pulsatile gonadotropin secretion and fertility while continuous administration of GnRH led to virtual cessation of pituitary gonadotropin production, highlighting the fundamental importance of GnRH pulsatility for suitable gonadotrope cell function [37].
In mice, elimination of the gene for either the β subunit of LH, or the LH receptor, leads to infertility in both genders associated with marked decreases in gonadal steroid hormone production, along with defective spermatogenesis and late follicular developmental arrest [148;306]. FSH levels are normal in these mice and the hypogonadal phenotype of the LHβ null mouse is able to be rescued by exogenous LH. In contrast, female mice lacking the gene for the β subunit of FSH are infertile due to a defect in ovarian follicular maturation; however, males remain fertile, despite decreased testicular size and sperm production [138]. Similarly, the phenotype of female mice lacking the FSH receptor (FSHR) is similar to that of the FSHβ-deficient animal (infertility due to follicular developmental arrest) [1;52]. However, in males, loss of the FSHR leads to a greater impairment of gonadal function than in FSHβ null mice, with significant decreases in Leydig cell numbers and levels of circulating testosterone [6]. It has been suggested that the more severe phenotype displayed by FSHR deficient animals as compared with those lacking FSH itself, is indicative of constitutive and physiologically important ligand-independent signaling activity of gonadal FSHR populations [6].
In addition to information gained from animal models, many naturally-occurring human diseases provide insight into the HPG axis function. Kallmann Syndrome (KS) is a form of hypogonadotropic-hypogonadism that results from a failure of migration of GnRH neurons from the olfactory epithelium to the hypothalamus during embryonic development and the consequent lack of hypothalamic GnRH production [77]. Individuals with KS typically are anosmic (deficient in the sense of smell) due to hypoplasia or aplasia of the olfactory tracts and in addition may display a variety of developmental abnormalities including dental or renal agenesis, palate defects, digital malformations and hearing loss [99]. Several specific mutations have been identified that collectively account for approximately 30% of reported cases of KS. The most severe form of the disease (KAL1) is an X chromosome-linked condition associated with a loss-of-function mutation in the KAL1 gene that encodes the protein anosmin-1 [77;100]. Anosmin-1 is a secreted multidomain-containing glycoprotein that is thought to guide developing axonal projections during morphogenesis of the olfactory system in part through stabilization of growth factor-receptor complexes, especially complexes between fibroblast growth factors (FGFs) and their receptors (FGFRs) [55;260]. Since olfactory axonal tracts function as a pathway along which nascent GnRH neurons migrate from their origin in the olfactory epithelium to the hypothalamus, disturbances in the development of the olfactory system may interfere with the ability of GnRH neurons to populate the hypothalamus. This leads to GnRH deficiency and hypogonadotropic-hypogonadism. Loss-of-function mutations in FGFR1 have also been associated with an autosomal dominant form of KS (KAL2); however this form of the disease is incompletely penetrant, indicating a more complex and probably oligogenic basis [56;240].
Mutations in the gene encoding the human GnRHR have been discovered that lead to hypogonadotropic-hypogonadism of variable severity [10;11;50;131;143] and reviewed in [9]. The impairment of intracellular inositol phosphate accumulation associated with some GNRHR mutations is able to be rescued by molecular chaperones, indicating that misfolding and abnormal intracellular trafficking of newly synthesized receptor proteins and failure of receptor expression may underlie the effects of some mutations [145]. This is discussed in more detail below. Naturally occurring inactivating mutations in the genes for LHβ and FSHβ as well as their receptors have also been reported. These mutations are generally associated with hypogonadal phenotypes, and given their adverse effects on reproductive function, are understandably rare [4;14;144;269].
2.2 Hypothalamic secretion of GnRH
The release of GnRH from the hypothalamus is inherently pulsatile, and this pulsatility is required for appropriate gonadotropin production by the pituitary [12;172;172;278]. The importance of GnRH pulsatility was first demonstrated in 1978 in experiments in Rhesus monkeys with surgically created lesions of the basomedial hypothalamus that eliminated the GnRH neurons [28]. Continuous administration of exogenous GnRH to these animals led to decreased production of gonadotropins and hypogonadism, while pulsatile administration of GnRH at hourly intervals restored gonadal function and reproductive cyclicity [12]. The suppression of pituitary responsiveness to GnRH following continuous exposure results at least in part from downregulation of GnRHR’s at the level of the gonadotrope [160;176;204;209;283]. However, desensitization of signaling processes downstream of the GnRHR may also play a role [156;181;217;292]. Downregulation of gonadotrope responsiveness to GnRH through continuous hormone exposure effectively uncouples the gonads from pituitary regulation, allowing for precise clinical manipulation of germ cell maturation through the administration of exogenous gonadotropins. This general strategy forms the basis for the clinical use of GnRH analogs that are prescribed for the treatment of many forms of infertility, especially in females [169;236].
Both the amplitude and frequency of GnRH pulses are tightly regulated and, in the female, vary dramatically over the course of the reproductive cycle [172]. Rapid GnRH pulsatility has been shown to promote the synthesis of αGSU and LHβ, while slower GnRH pulsatility favors production of FSHβ [92]. Different pulse frequencies of GnRH stimulation also affect the kinetics of gonadotropin release from the pituitary, with higher pulse frequencies linked to selective secretion of LH against a background of more tonic and consistent secretion of FSH [92;116;220]. The resulting pulsatile discharge of gonadotropins, especially LH, from the gonadotrope leads to corresponding oscillations in plasma gonadotropin levels, the magnitude of which may further reflect the shorter circulatory half-life of LH as compared to FSH. The responsiveness of the gonadotrope to changes in hypothalamic GnRH pulsatility is critical for the generation of the preovulatory LH surge in the female, and the mechanisms underlying the ability of the gonadotrope to interpret GnRH pulsatility has received considerable attention in a number of basic scientific arenas [47;91;113;118;126;151;171;282]. In contrast, regulated fluctuations in GnRH pulsatility play a less significant role in male reproductive function. This sexual dimorphism with regards to cyclic variation in GnRH pulsatility is thought to be based in the neuroanatomical circuitry involving the hypothalamic GnRH neurons and their various afferents. Significant gender dimorphism has been demonstrated with respect to the anatomical organization and functionality of the hypothalamic GnRH system; this dimorphism is established early in mammalian embryogenesis as a result of androgen exposure [41;43;97]. Whether any aspect(s) of gonadotrope responsiveness to differential GnRH pulse frequency reflects a similar intrinsic gender dimorphism remains an intriguing question. However, early studies in primates showed that castration can result in hypothalamic sensitivity to positive feedback effects of estrogen and female-like patterns of gonadotropin secretion in males. Thus, at least in primates, gender divergence in gonadotrope function appears to be a reversible effect of postnatal androgenic stimulation of the pituitary [120;205].
2.3 Feedback regulatory mechanisms in the HPG axis
The HPG axis is subject to both positive feed-forward and negative feedback regulation at several levels. At the level of the hypothalamus, early recognition of the pulsatile nature of GnRH secretion led to the notion of a central “pulse generator”, the inherent oscillatory activity of which controls the secretory rhythm of GnRH neurons [128]. Anatomically, this pulse generator is thought to be located predominantly within the suprachiasmatic nucleus of the hypothalamus. Interestingly, studies from dispersed hypothalamic neurons in primary culture suggest that pulsatile secretion of GnRH may be an inherent property of the neurons themselves [187]. Moreover, populations of GnRH neurons may be capable of broad scale synchronization of GnRH pulsatility, and this synchronization does not appear to require direct intercellular contact [159]. GnRH neurons express the GnRHR, which couples to different intracellular heterotrimeric G-proteins in a manner apparently dependent on ligand dose [136]. These observations have led to a model in which low doses of GnRH may exert positive autocrine and paracrine feedback effects on neuronal GnRH secretion through GnRHR-Gαs-induced increases in intracellular cyclic adenosine-monophosphate (cAMP) levels. The resulting increase in GnRH secretion subsequently propels dramatic further positive feedback effects by driving GnRHR-Gαq/11-induced increases in intracellular calcium. However, once the concentration of extracellular GnRH reaches a sufficiently high (threshold) level, GnRHR-Gαi-mediated signaling pathways are preferentially activated, leading to negative feedback suppression of secretory activity [123].
While the pulsatility of GnRH neurons is widely understood to represent an inherent cellular characteristic, the extent to which this property dictates the pattern of oscillatory release of GnRH in vivo is not clear. Furthermore, it is clear that the hypothalamic pulse generator is extensively modulated by a multitude of higher level inputs including photoperiod, environmental stress, metabolic state and nutritional status, as well as various endocrine mediators [8;24;83;132;153;185]. The central nervous system is thus constantly integrating and translating a vast diversity of environmental and physiological cues into a coordinated pattern of GnRH release from the hypothalamus. Gonadal steroid hormones play a particularly important role in feedback regulation of hypothalamic secretion of GnRH [60;84;154;216;218]. In the male, testosterone exerts negative feedback effects primarily on the release of GnRH from the hypothalamus [270]. Immunohistochemical analyses in rams suggest that, at least in this species, hypothalamic GnRH neurons do not express the androgen receptor [106]. Thus, the effects of testosterone and its metabolites are thought to be mediated by higher level afferent neuronal populations that likely include the kisspeptin neurons of the arcuate nucleus and the AVPV region [259]. In some species, a minor negative feedback effect may also be exerted at the level of the gonadotrope [270].
In the female, the feedback effects of gonadal steroids on HPG function are more complex, species-specific, and depend on the stage of the reproductive cycle. Studies in rats indicate that estrogen is capable of down-regulating the expression of GnRH within the hypothalamus, and that this effect is most pronounced during the follicular phase [213;214;312]. A direct inhibitory effect on the GnRH neuron mediated by the β isoform of estrogen receptor (ERβ) has been reported [214]. However, the negative feedback effects of estrogen remain intact in ERβ-null mice, and more recent results indicate that negative feedback by estrogen during the early follicular phase is mediated through estrogen response element (ERE)-independent signaling of ERα [84]. In contrast, during the preovulatory period, the effects of estrogen on both the hypothalamus and the pituitary become positive [197]. Study of the positive effects of estrogen in sheep initially led to a model of periodic hypothalamic GnRH release that encompasses three distinct phases of activation, transmission, and surge [64]. The activation phase is now known to involve a positive indirect effect of estrogen on hypothalamic production of GnRH, mediated through ERα expressed by afferent kisspeptin-positive neurons of the rostral peri-ventricular region of the third ventricle that synapse on the GnRH neurons [63;105;214;254]. Increasing estrogen levels during the middle to late follicular phase leads to enhanced synthesis of kisspeptin within these afferent neurons; direct kisspeptin stimulation of hypothalamic GnRH neurons mediated through the kisspeptin receptor, KISS1R, (transmission phase) then leads in turn to rapid and significant increases in hypothalamic GnRH secretion (surge phase) culminating in the massive preovulatory release of LH from the pituitary that is the definitive trigger of ovulation [42;64;105;115]. The rise in estrogen through the follicular phase has also been shown to exert positive feedback effects at the level of the pituitary where it primes the gonadotrope for the preovulatory LH surge [38;304].
In addition to the feedback effects mediated by estrogen, progesterone also exerts complex modulatory feedback effects at all levels of the HPG axis. The negative feedback effects of progesterone upon hypothalamic GnRH release during the luteal phase of the cycle are well established and play an important role in limiting the potential positive effects of luteal estrogen during the postovulatory phase. As with estrogen, the feedback effects of progestins are indirect, as GnRH neurons do not express the progesterone receptor (PR) [256]. Negative feedback effects of progesterone on GnRH release can occur during either the activation or transmission phases of the cycle of GnRH secretion, and kisspeptin-positive neurons of the suprachiasmatic nucleus appear to be a major target of progesterone action in this context [40;101;225;258]. Recent results suggest that progesterone may also exert direct negative feedback effects upon the release of GnRH from GnRH-producing cells through a rapid activation of membrane-associated PR, implicating signaling events at the plasma membrane of GnRH neurons as possible regulators of the surge phase [257]. However, the effects of progesterone on hypothalamic GnRH release may also be positive. Classic studies in rats have clearly established the importance of progesterone receptor activation for the preovulatory GnRH surge and in sheep, exogenous progesterone was shown to exert a priming effect on the hypothalamus, leading to higher GnRH levels during induced preovulatory GnRH surges, and delaying the onset of the GnRH surge relative to non-progesterone treated animals [27;65;135;188;219]. Recent work in rats indicates that astrocytes may function as a hypothalamic source of progesterone that facilitates the GnRH surge [188;189]. The synthesis of progesterone by hypothalamic astrocytes is itself positively regulated by estrogen [189]. This regulation involves rapid activation of plasma membrane-associated ERα, direct interaction of ERα with metabotropic glutamate receptors, and Gαq-dependent increases in intracellular calcium. This provides an intriguing example of how estrogen may regulate biosynthetic processes through nonclassical plasma membrane-associated signaling and indirect modulation of plasma membrane ion channels [121]. As further illustration of the complex and integrated landscape of feedback regulation, expression of the PR has itself been shown to be induced by estrogen within peri-ventricular neurons of the hypothalamus, and exerts important positive regulatory effects on hypothalamic release of GnRH that may involve ligand-independent signaling. Indeed, elegant experiments involving systemic treatment with PR antagonists or intra-cerebroventricular administration of antisense oligonucleotides targeting the PR eliminated GnRH and LH surges in rats [32].
The mechanisms underlying the feedback effects of gonadal steroids on both the hypothalamic GnRH system as well as the gonadotrope have been explored in detail using a variety of transgenic models. Progesterone receptor null males are reproductively competent, while females are infertile and have complex reproductive phenotypes reflecting the diverse roles played by PR at all levels of the female HPG-genital tract axis [165]. Mice harboring a floxed PR allele allowing conditional deletion of both A and B isoforms of PR were recently described and promise to become a useful tool for conditional ablation of PR and analysis of both its transcriptional and signaling functions in either the pituitary or brain [102].
Mice lacking either kisspeptin or its receptor KISS1R display a hypogonadotropic-hypogonadal phenotype and are infertile. Predictably, the phenotype of kisspeptin null mice is able to be at least partially rescued by administration of exogenous kisspeptin, while the KISS1R null animal is nonresponsive to this treatment [141]. The requirement for ERα in mediating the positive effects of estrogen are demonstrated in the divergent phenotypes of ERα and ERβ null mice; ERα null mice are infertile and nonresponsive to estrogen feedback while ERβ null mice are subfertile with impaired ovulatory capacity but remain sensitive to the positive feedback effects of estrogen [295]. The reproductive phenotype of the ERα null was further recapitulated in a neuronal-specific ERα knockout demonstrating that the positive feedback effects of estrogen upon GnRH release from the hypothalamus are exerted specifically upon neuronal afferents that regulate the activity of hypothalamic GnRH neurons [295]. Interestingly, in mice in which the wild-type ERα sequence was substituted with a mutant form of ERα possessing a defective DNA-binding domain, the negative feedback effects of estrogen were largely maintained while positive feedback effects were absent, indicating that positive feedback requires expression of ERE-regulated genes, while negative feedback is supported by non-canonical ERE-independent ER signaling [84]. At the level of the gonadotrope, the negative effects of ERE-independent estrogen signaling were subsequently shown to involve suppression of secretion, but not synthesis of LH, highlighting the importance of non-genomic ERE-independent ER signaling [85]. Recently, the role of ERα in the gonadotrope was further investigated using a pituitary-targeted conditional knockout of ERα [82]. Mice lacking ERα specifically in the pituitary showed a sexually dimorphic phenotype with males unaffected while females displayed irregular estrous cycles and were infertile. Interestingly, serum levels of the gonadotropins were not affected by the loss of ERα in the pituitary leaving the question of whether ERα functions primarily in a signaling or transcriptional capacity within the gonadotrope unanswered.
In addition to the gonadal steroids, the peptide hormones inhibin, activin, and follistatin play important roles in both gonadal and paracrine feedback regulation of gonadotrope function (reviewed in [87]). Inhibin is a dimeric glycoprotein consisting of a common α-subunit paired with a distinct β subunit. Two β subunits have been identified within the reproductive axis (designated βA and βB); two corresponding forms of inhibin exist, consisting of αβA and αβB dimers, respectively. Activins are related hormones that consist of dimers of inhibin β subunits. The monomeric β subunits of inhibin may pair in any combination, giving rise to three activin species, βAβA, βAβB, or βBβB, referred to as activins A, B, or AB, respectively. Follistatin is an unrelated glycoprotein that modulates the bioactivity of activin dimers through direct interaction. The inhibins, activins, and follistatin were originally purified from gonadal tissue; however, they are now known to be variously expressed at all levels of the HPG axis as well as in several nonreproductive tissues including the liver and brain [129;232;239;246;280]. The α and βB subunits of inhibin/activin as well as follistatin are expressed in the pituitary, and while activin B and follistatin have been implicated in paracrine regulation of gonadotrope function, the gonads appear to be the predominant, if not the sole source of mature bioactive inhibin [68;231;232;293].
The inhibins and activins are members of the TGFβ family of mediators, and bind to the type II activin receptor, a transmembrane receptor with serine-threonine kinase activity. Binding of activin to the type II receptor leads to the recruitment of the type I receptor subunit and activation of the heterodimeric receptor-ligand complex. In contrast, binding of inhibin to the type II receptor occurs in conjunction with the inhibin coreceptor betaglycan, a cell surface proteoglycan, and prevents both activin interaction with the type II receptor and type I-II receptor dimerization [150]. Inhibin thus functions as a competitive inhibitor of activin. Follistatin binds activin within the extracellular compartment, including the plasma, and neutralizes its activity by preventing receptor interaction [198].
The inhibin-activin-follistatin system functions primarily to modulate FSH production by the gonadotrope; however, this modulation may occur at several levels. Some evidence suggests that the expression of activin A and follistatin in the hypothalamus may constitute a local autocrine or paracrine feedback loop regulating secretion of GnRH [87;137;166]. Gonadotropes express the activin receptor and activin potently upregulates FSH expression in these cells in conjunction with GnRH. Activin may also regulate the expression of both FSH and LH indirectly by augmenting GnRH-induced homologous upregulation of the GnRHR [68;206].
Binding of activin to its cognate type II receptor leads to recruitment, dimerization, and trans-phosphorylation of the type I receptor. The receptor complex then leads to the activation of the receptor-associated SMADs 2 and 3, and their association with the common mediator SMAD 4. The SMAD complex undergoes nuclear translocation and functions as a transcriptional regulator through direct binding to DNA and recruitment of various coactivators [87]. In the gonadotrope, the most thoroughly characterized gene target of activin-induced SMAD signaling is FSHβ, and indeed, in some systems, activin has been shown to be a more potent inducer of FSHβ expression than GnRH itself [286]. In LβT2 cells, pretreatment with activin suppressed GnRH-induced activation of ERK, and enhanced activation of JNK and p38 activity indicating that upstream cross-regulation may take place between activin-induced SMAD signaling and other signaling pathways induced by GnRH [44;307]. However, functional interaction between these pathways appears to occur more prominently at the point of their convergence upon specific gene promoters (reviewed recently by [186;268]). As a notable example, activin-induced SMAD signaling and GnRH-induced upregulation of AP1 have been shown to result in synergistic transcriptional activation of both the GnRHR and FSHβ through occupancy of SMAD and AP1 response elements in the promoters of these respective genes [21;44;45;62;68;88;207;284].
3. Molecular basis of the response of the gonadotrope to GnRH
3.1 Fundamental mechanisms underlying pituitary morphogenesis
The anterior lobe of the pituitary is composed of five discrete hormone producing cell types; in addition to the gonadotrope, the corticotrope, thyrotrope, somatotrope, and lactotrope cells respond to distinct hypothalamic releasing factors and produce adrenocorticotrophic hormone (ACTH), thyroid-stimulating hormone (TSH), growth hormone (GH), and prolactin (PRL), respectively. During embryogenesis, these hormone-producing cell lineages develop from a common primordium in a highly regulated spatial and temporal progression. The distinct phenotypes expressed by these cells vis-a-vis their hormone production have made the pituitary a useful model system in which to study basic mechanisms of cellular differentiation and organogenesis in vivo. A comprehensive review of pituitary development is beyond the scope of this review; however, several basic concepts related to the development of pituitary cell types are relevant to the experimental approaches and results described herein and are therefore outlined in brief. Pituitary development has been recently reviewed in detail in [311].
Proliferative expansion of the pituitary primordium and sequential appearance of lineage-committed cell types of the anterior portion of the gland occurs from e9.5 through approximately e13.5. These processes are dependent on tightly regulated spatiotemporal expression of numerous transcription factors [235;285]. The αGSU is the first hormonal subunit gene to be expressed within the developing pituitary, appearing in the ventral region of Rathke’s pouch at e10.5 [311]. The distribution and timing of αGSU promoter activation has been studied in transgenic mice using reporters linked to various portions of the αGSU promoter. A 381 base pair fragment of the murine αGSU promoter is sufficient to direct gonadotrope- and thyrotrope-specific reporter expression within the developing pituitary; however, higher levels of expression are obtained with the use of a 4.6 kb promoter region that incorporates an upstream enhancer element [122]. One study using this 4.6 kb αGSU promoter fragment demonstrated reporter expression as early as e9.5 throughout the pituitary primordium [122]. The possibility of such early, promiscuous, or misregulated activation of this transgenic promoter fragment during development is important in relation to studies described below, in which in vivo pituitary-targeted genetic recombination was accomplished using this same 4.6 kb promoter to regulate expression of Cre recombinase.
3.2 Developmental specification and differentiation of the gonadotrope lineage
The developmental events underlying fate specification of the gonadotrope lineage remain largely unclear. This is in contrast to other pituitary lineages such as the thyrotrope, somatotrope and lactotrope cells (the Pit-1 lineages) whose development and differentiation are completely dependent on expression of the POU domain-containing transcription factor Pit-1 [294]. The orphan nuclear receptor steroidogenic factor-1 (SF1) is first expressed at e13.5 and is the first gonadotrope-specific marker to appear within the developing gland [114]. Onset of expression of SF1 is occasionally cited as the defining event in gonadotrope specification. Indeed, pituitary-specific depletion of SF1 led to impaired expression of the gonadotropins as well as the GnRHR, and secondary hypogonadism [310]. However, this phenotype was able to be rescued by administration of exogenous GnRH suggesting that while SF1 is necessary for gonadotrope function, it is not required for developmental specification or differentiation of the gonadotrope lineage [310].
The gonadotrope is the last of the anterior pituitary cell types to undergo terminal differentiation as marked by the relatively late onset of expression of the genes encoding the β-subunits of FSH and LH. The mechanisms underlying the timing of initial expression of these gonadotropin subunit genes are unknown. Activation of the murine FSHβ promoter first occurs at approximately e17.5 while LHβ biosynthesis commences approximately 24 hours later. In conjunction with a cohort of relatively ubiquitous and cell type-non-specific transcription factors, SF1 plays a key role in activation of the promoters for all of the genes that comprise the genetic signature of the gonadotrope (αGSU, FSHβ, LHβ, GnRHR), and is primarily responsible for restricting expression of FSHβ, LHβ, as well as GnRHR to the gonadotrope [7].
3.3 Experimental models used in the study of gonadotrope cell function
More recent studies of GnRH-activated signal transduction pathways in the gonadotrope have relied upon and benefitted greatly from the αT3-1 and LβT2 murine gonadotrope-derived cell lines derived by the Mellon laboratory. These cell lines were generated through targeted expression of SV40 large T antigen in developing pituitary cells [110;279;294]. Both lines express the GnRHR as well as the αGSU. In addition, the LβT2 cell line expresses the β subunits of LH and FSH [86], and may thus represent a somewhat more differentiated gonadotrope phenotype.
Due to the restricted pattern of expression of the GnRHR as well as the specificity of GnRH action to gonadotropes, many aspects of gonadotrope function, particularly transcriptional responses to GnRH stimulation, can be readily examined in primary cultures of mixed pituitary cells generated by enzymatic dispersal of fresh pituitary tissue. However, measurements of GnRH-induced changes in transcript levels in primary culture systems must be interpreted with caution, since expression of a gene of interest in cell types other than the gonadotrope will contribute to baseline transcript levels within an experimental unit, and can lead to underestimation of the effects of hormone stimulation on the subpopulation of gonadotropes within that unit. Alternatively, protocols for purification of gonadotropes from dispersed pituitary tissue and their propagation in primary culture have been described; however, they are technically demanding [158]. Two intriguing new models for identification of gonadotrope cells in vivo make use of genetically modified mouse models. One approach has been to use the ovine FSHβ gene promoter regulating the expression of H-2Kk gene, a major histocompatibility protein that can be expressed on the surface of FSHβ expressing cells such as gonadotropes [297]. Since the H-2Kk protein is present on the cell surface, it can be effectively targeted using commercially available H-2Kk-specific antibodies for immunologically-based cell enrichment. Alternatively, GRIC (GnRH receptor IRES Cre) mice have Cre recombinase knocked into the GnRHR gene locus along with an internal ribosome entry site (IRES) to facilitate Cre recombinase expression in cell types endogenously expressing the GnRHR [288]. When bred to reporter mice, the GRIC Cre expression effectively allows for fluorescent marker expression in gonadotropes. Using the GRIC model system along with fluorescent reporter mouse lines, future studies may focus on fluorescently-labeled gonadotropes which could be greatly enriched by methods such as flow cytometry.
Several genetically modified mouse models have been developed that shed light on the role of specific gene products within the pituitary. Constitutive gene ablation may result in a phenotype that reflects primary pituitary dysfunction. For example, despite its broad pattern of expression, mice lacking the immediate early gene Egr1 display gender-specific infertility associated with a failure of LH production in the gonadotrope of female mice [146]. Alternatively, due to their more restricted pattern of expression, the promoters of genes encoding a number of the pituitary trophic hormones or cell type-specific receptors have been used to target transgene expression to specific pituitary cell lineages allowing conditional, Cre recombinase-mediated disruption of floxed alleles [16;33;34;82;201;288].
3.4 Structural and functional aspects of the GnRH receptor
The GnRHR is a member of the large G-protein coupled receptor (GPCR) superfamily. Three forms of the GnRHR have been identified in vertebrates, designated types I, II, and III; three corresponding forms of GnRH (type I, II, and III) have also been identified [191]. The type I receptor is the predominant form expressed in the gonadotrope, and in some mammalian species including humans is also expressed in certain extra-pituitary tissues most notably the breast, gonads, prostate, and uterus [35]. The natural ligand for the type I receptor is type I GnRH; however, type II GnRH may also engage and activate the type I receptor [177]. Indeed, in several mammalian species, the type II GnRHR has been evolutionarily silenced at the genomic level. Thus, in humans, the biological effects of GnRH II or its analogues are thought to be mediated by the type I receptor [190].
The type I GnRHR was first cloned in 1992 from cDNA derived from the murine αT3-1 gonadotrope-derived cell line [222;277]. Subsequent cloning of GnRHR’s from human, rat, sheep, pig, and horse demonstrated that the receptor is highly conserved, with greater than 80% amino acid homology among these mammalian species [226]. The deduced sequence predicts a 327 (or, in some species, including humans, 328) amino acid (aa) receptor with seven transmembrane domains, a 35 aa amino-terminal extracellular domain with 2 putative glycosylation sites, and a conspicuously short 1-2 aa carboxyl-terminal cytoplasmic domain. An additional glycosylation site is predicted within the first extracellular loop [226;277]. While the crystal structure of the GnRHR has not been determined, its homology with other members of the GPCR family, including rhodopsin, has allowed model-based predictions of the overall topology of the receptor, as well as the structural basis of its interaction with ligands [248]. Many of these predictions have been verified by site-directed mutagenesis studies in recombinant receptors expressed in cell culture systems. For example, alanine substitution of asparagine residues 18 or 102 (predicted glycosylation sites) led to decreased receptor glycosylation, and while this had no effect on ligand affinity, overall receptor expression was decreased, indicating a role for glycosylation of these residues in receptor stability or appropriate membrane targeting [48]. Similar methods have identified critical residues involved in ligand binding within the second and seventh transmembrane domains, as well as the third extracellular loop [72;73;127] . Insight into the structural basis of the interaction of the receptor with GnRH has facilitated the development of receptor antagonists, as well as high-affinity ligands that function as super-agonists [48]. Antide [N-Ac-D-Nal(2)1,d-pCl-Phe2,D-PaI(3)3,Lys(Nic)5,D-Lys(Nic)6,Lys(iPr)8, D-Ala10]GnRH and buserelin (des-GLY10 [D-Ser(t-But)6 ]-LH-RH Ethylamide) are examples of GnRHR antagonist, and super-agonist, respectively, that are commonly used in molecular and cellular studies of gonadotrope function. Both compounds were used in the investigations described here.
The lack of a carboxyl-terminal intracellular domain renders the mammalian type I GnRHR a structurally and functionally unique member of the GPCR family [226]. The C-terminal tail domain of the prototypical GPCR is an important target for phosphorylation, typically mediated by a G-protein coupled receptor kinase (GRKs) [224]. C-terminal tail phosphorylation generates a docking site for members of the β-arrestin family of scaffolding proteins, which upon binding, mediate rapid desensitization and dynamin-dependent internalization of a receptor [163]. Lack of a C-terminal tail implies that the GnRHR may be resistant to rapid desensitization and internalization, and this has been confirmed experimentally for this receptor in a variety of settings [283;291;291;292]. In addition, numerous studies have demonstrated rapid desensitization and internalization of chimeric receptors in which the C-terminal tail of various GPCRs was fused to the C-terminus of the mammalian type I GnRHR [29;70;98;103;104;107;209;283]. Collectively, these results establish a causal link between the absence of the C-terminal tail and the delayed internalization kinetics of the type I GnRHR. Exposure of gonadotropes to GnRH does lead to receptor internalization; however, this process occurs more slowly than with other GPCR’s, and appears to be independent of dynamin [108]. Indeed, the GnRHR has been described as a “naturally-occurring internalization deficient mutant” GPCR [180].
Appropriate plasma membrane expression of the GnRHR is of obvious importance for responsiveness of the gonadotrope to GnRH. In turn, surface expression of functional receptors requires proper intracellular folding, glycosylation, and trafficking of newly synthesized receptor proteins. Naturally-occurring mutations have been described in the human GnRHR gene that lead to hypogonadotropic-hypogonadism of variable clinical severity [269]. These mutations have been mapped to various locations throughout the length of the GnRHR coding sequence. Functional analysis of recombinant receptors harboring many of these mutations indicates that ligand binding and coupling to intracellular effectors are unimpaired, but that the mutant receptors undergo improper folding and either aberrant intracellular trafficking or premature degradation [145]. Loss of newly synthesized receptors due to stochastic processes of misfolding and premature degradation is common for some membrane receptors, and underscores the dynamic nature of receptor biosynthesis and recycling [212;247]. In many instances, cell permeable ligand-mimetics may shift the equilibrium of this process in favor of effective receptor expression by improving the efficiency with which nascent receptors adopt a proper conformation during folding [211;287]. This has been shown for the GnRHR where treatment of cells with the peptidomimetic GnRH antagonist indole IN3 was able to establish GnRH responsiveness in cells expressing a variety of mutant receptors that otherwise were hypothesized to have undergone misrouting and premature degradation [145]. This has stimulated interest in the mechanisms underlying appropriate plasma membrane targeting of the human GnRHR as well as the development of pharmacological agents that would allow manipulation of cell surface receptor density by altering the efficiency of folding and trafficking.
3.5 GnRH-induced signal transduction in the gonadotrope
The signaling events initiated by engagement of the GnRHR have been the subject of considerable investigation and have recently been reviewed in detail [199]. As with other GPCR’s, ligand binding induces a conformational change in the receptor that leads to dissociation and activation of a heterotrimeric G-protein which, in the pituitary, generally involves the Gαq/11 subunit [89]. While fluctuation of the receptor between active and inactive conformations is a fundamental process in GnRH signaling, recent work suggests that the receptor may be able to be stabilized in a number of different active conformations following the binding of different ligands [161;191;215]. The ability of these different conformations to lead to variable downstream signaling activities has led to the concept of ligand-induced selective signaling [191]. The molecular basis of selective signaling in response to specific ligands has been analyzed in the human GnRHR using point mutagenesis and domain swap techniques disclosing specific residues and extracellular domains that play key roles in determining ligand specificity [161;265]. Furthermore, elegant studies of the Xenopus GnRHR revealed a novel inside-out regulatory feedback mechanism in which agonist-induced activation of PKC led to an increase in the inherent affinity of the receptor for its ligand [31]. Interestingly, the human GnRHR was not subject to this feedback regulation; thus the significance of this inside-out signaling mechanism for gonadotrope responsiveness to hypothalamic GnRH remains unclear.
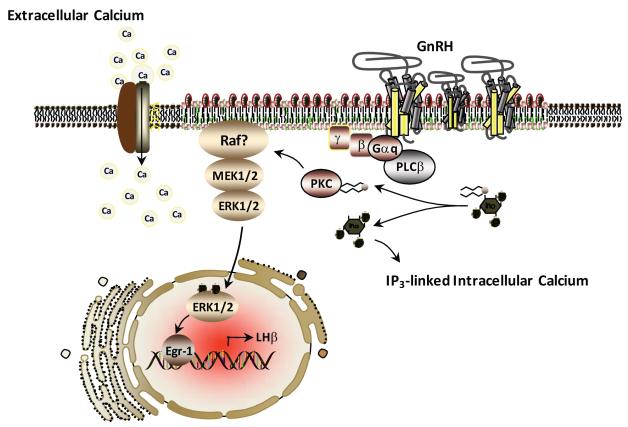
In the gonadotrope, GTP loading of Gαq/11 leads to activation of phospholipase (PL)Cβ, and subsequent elaboration of the second messengers, phophatidylinositol-triphosphate (IP3) and diacylglycerol (DAG). Generation of IP3 leads to an initial rapid rise in intracellular calcium concentration resulting from release of intracellular calcium stores. DAG leads to activation of PKC isozymes and contributes to a more sustained rise in intracellular calcium concentration, which derives from influx of extracellular calcium through L-type voltage gated channels [13]. These events are prerequisite for activation of mitogen-activated protein kinase (MAPK) activity, in particular the extracellular signal-regulated kinase (ERK) pathway [194] (see Figure 1). The mitogen-activated protein kinase (MAPK) pathways are a highly conserved set of signal transduction cascades that mediate cellular responses to an enormous variety of environmental stimuli. In mammals, there are 4 predominant MAPK pathways: the ERK, jun-N-terminal kinase (JNK), p38, and ERK5/Big MAP kinase (ERK/BMK) pathways. The basic organization of these pathways is similar, consisting of a multi-level phosphotransfer system; activation of the pathway begins with phosphorylation of an upstream MAP kinase-kinase kinase (MAPKKK), which phosphorylates and activates an intermediate level MAP kinase kinase (MAPKK), which in turn activates the terminal MAP kinase (MAPK). In addition to these core kinases, numerous scaffolding and adaptor proteins have been shown to play important roles in the functional organization of this pathway [193]. Activated MAP kinases phosphorylate numerous substrates throughout the cell including elements of the transcriptional machinery, chromatin components, cytoskeletal structures, and other downstream enzymes [210;305].
Figure 1.
Model for GnRH receptor (GnRHR) signaling within the pituitary gonadotrope that leads to LH biosynthesis. The GnRHR resides within discrete plasma membrane compartments (membrane rafts) characterized by increased presence of cholesterol (green within membrane) and sphingolipids (orange within membranes). Activation of the ERK signaling pathway occurs within the membrane rafts facilitated by local influx of extracellular calcium through voltage dependent calcium channels (VGCC).
3.5.1 Membrane-associated signaling events
The delayed kinetics of GnRHR internalization underscores the importance of the plasma membrane as a platform for organization of the early signaling events that occur following ligand binding. This is in contrast to other GPCRs that undergo rapid internalization and that may nucleate active signaling complexes on the surface of endosomes or in association with cytoskeletal structures [80;183;184;252;274]. The GnRHR has been shown to couple to multiple G-proteins depending on cell type and experimental model, and controversy prevails regarding the relative importance of G-protein families for gonadotrope function. Studies based on overexpression of the GnRHR in heterologous cell systems indicate that the receptor is capable of interacting with both Gαs and Gαi subtypes; however, the in vivo relevance of these findings remains unclear [67;139;152]. Studies using the both αT3-1 and LβT2 cell lines showed that GnRH stimulation leads to increases in intracellular cAMP; however, adenylate cyclase activation was apparently independent of Gαs activation and was mediated by events downstream of Gαq/11 [89;157]. The ability of the GnRHR to couple to Gαi in extra-pituitary tissues has been convincingly demonstrated, and signaling through Gαi has been shown to play a role in regulating the pulse frequency of GnRH release from the hypothalamus [35;136;177]. However, the role of Gαi signaling in the gonadotrope remains unclear. Overall, the signaling events initiated by GnRHR occupancy are thought predominantly to reflect the activation of Gαq/11 family members [89;112;250;251;261]. Notably, Gαq and Gα11 appear to be redundant in their ability to mediate the effects of GnRHR activation since mice lacking either species of G-protein remain fertile [262]. Gαq/11 comprises a group of pertussis toxin-insensitive palmitoylated GTP-binding proteins that lead to selective activation of PLCβ isoforms.
Detailed structural and functional analysis of protein domains within PLCβ indicate that these enzymes undergo constitutive and reversible membrane association mediated by the C-terminal region as well as the N-terminal plekstrin homology domain [58]. Activation of PLCβ does not involve membrane recruitment. Catalytic activity of PLCβ is stimulated by interaction with GTP-loaded Gαq/11 subunits, and leads to the elaboration of IP3 and DAG within the plasma membrane. PLCβ also acts as a Gαq/11 GTPase activating protein (GAP) leading to negative feedback effects on the signaling input by stimulating hydrolysis of Gαq/11 bound GTP [36]. PLCβ isoforms are also present within the nucleus, where they catalyze a nuclear cycle of inositol-phosphate (IP) turnover that has been shown to be important in cellular responses to various mitogenic stimuli [173]. Interestingly, nuclear PLCβ1 is a direct ERK substrate, and ERK-mediated phosphorylation of S982 within the C-terminal domain is required for PLCβ1 activation by IGF-1 [302]. Of note, S982 resides within a regulatory region of the C-terminal domain that is also required for interaction with Gαq/11 [125]. Whether nuclear IP turnover plays any role in GnRH action, or whether GnRH-activated PLC isoforms are regulated by phosphorylation at the level of the plasma membrane is unknown.
In contrast to PLCβ, activation of PKC isoforms involves plasma membrane recruitment. Three major classes of PKC have been characterized, namely the conventional, novel, and atypical PKCs [273]. All are activated through the synergistic effects of DAG and phosphatidylserine. In addition, activation of the conventional PKCs requires calcium. αT3-1 cells express a variety of PKCs representing all classes including the α, β, ε, θ, and ζ isoforms [134;167;229]. Fractionation experiments indicated that the α, ε, and ζ isoforms may be the major PKCs activated by GnRH in these cells [134].
In αT3-1 cells, PKC activity is required for ERK pathway activation by GnRH; however the mechanism underlying this requirement is unknown [228;266]. Raf-1 kinase is widely believed to be the key upstream activator of the ERK signaling module in the gonadotrope, and direct activation of Raf-1 by PKC has been suggested as a mechanism by which GnRH-induced signaling events at the plasma membrane may link to an ERK cascade that ultimately converges on the nucleus [54;130]. Membrane recruitment is a critical step in the complex series of events that leads to Raf-1 activation; thus, recruitment and activation of Raf-1 by membrane-bound PKC is a conceptually attractive scenario. However, little evidence exists to support direct activation of Raf-1 by PKC. Furthermore, the importance of Raf-1 itself in GnRH-induced ERK activation has not been conclusively demonstrated, either in the αT3-1 cells or LβT2 cell lines, or in vivo. The architecture of this portion of the GnRH signal transduction network, particularly the link between signaling activities at the plasma membrane and the ERK module, thus remain unclear.
3.5.3 Membrane compartments and the GnRHR
In contrast to the homogenous fluid mosaic model of cellular membrane organization originally proposed by S.J. Singer and G. Nicholson in 1972, contemporary views propose the plasma membrane to be a heterogeneous assembly of spatially and functionally distinct domains that may differ with respect to both lipid and protein composition [22;61;255]. This membrane raft hypothesis is predicated upon the notion that different lipid species within a membrane bilayer may differ in their affinities for each other and for membrane proteins, and that this may facilitate the formation of biochemically and biophysically distinct lipid domains for which integral and peripheral membrane proteins may have different relative affinities. The GnRHR was shown to partition completely into low-bouyant density membrane fractions following sucrose density gradient centrifugation of both αT3-1 cell homogenates and whole mouse pituitaries, suggesting that the receptor may have an inherent preference for a membrane raft environment [19;202] (see Figure 1). Disruption of the biophysical properties of the plasma membrane through depletion of sterols uncoupled the GnRHR-G-protein complex from PLCβ and blocked the ability of GnRH to activate the ERK pathway. These observations led to the hypothesis that the ability of the GnRHR to signal appropriately is affected by the biophysical properties of the plasma membrane of the gonadotrope, and may require association with specialized plasma membrane microdomains such as cholesterol/sphingolipids enriched rafts.
Fluoresence recovery after photobleaching (FRAP) studies with fluorochrome labeled GnRHR showed that treatment with GnRH agonist, but not the GnRH antagonist, caused a dose-dependent decrease in the lateral mobility of receptors within the membrane [111]. Fluorescence resonance energy transfer (FRET) between receptors was not detected; however treatment with either GnRH agonist or antagonist caused a dose-dependent increase in FRET consistent with receptor dimerization [111]. This indicates that agonist-induced receptor clustering may facilitate coupling of the receptor with the downstream intracellular signaling apparatus. Consistent with this hypothesis, several key signaling intermediates known or presumed to play a role in the GnRHR-ERK pathway were also found to associate at least partially with low density membranes including Gαq/11, calmodulin, isoforms of the 14-3-3 family of adaptor proteins, Raf-1, MEKs, and the ERKs themselves [19;202;227]. Thus, laterally segregated lipid microdomains within the plasma membrane of the gonadotrope may play an important role in the regulation of signaling dynamics following ligand occupancy of the GnRHR.
3.5.4 Calcium signaling in the gonadotrope
In the gonadotrope, GnRH induces a rapid biphasic elevation of intracellular calcium [263]. The initial sharp rise in cytosolic calcium reflects predominantly the release of calcium from intracellular storage depots, particularly the endoplasmic reticulum (ER). Following this initial spike, a more sustained phase of calcium elevation occurs, due primarily to influx of extracellular calcium via L-type voltage gated channels (VGCC; Figure 1). Release of intracellular calcium from the ER is triggered by IP3 through direct interaction with IP3 receptors on the surface of the ER. Calcium influx through VGCC is dependent on the activity of PKC isozymes [195].
Calcium influx via VGCC is uniquely required for ERK activation in the gonadotrope, although the mechanisms underlying this requirement remain unclear [194;195]. In previous work from this laboratory, GnRH stimulation led to rapid calcium loading of the calcium sensing protein calmodulin, which was itself shown to be required for ERK activation independently of the calmodulin-dependent protein kinase CamKII [227]. In vitro reconstitution experiments indicated direct calcium-dependent interaction between calmodulin and Raf-1. In addition, calmodulin was also recovered from membrane rafts following sucrose density gradient centrifugation of αT3-1 cell homogenates, suggesting that calmodulin may serve as a link between VGCC calcium and the ERK pathway, and that this linkage may be regulated through association with membrane rafts [227]. The cellular effects of calcium signals have long been known to be highly compartmentalized (calcium signaling via discrete microdomain) in a wide variety of excitable and nonexcitable cells [15]. Thus, our observations raise the intriguing possibility that the contribution of VGCC calcium to GnRH-induced ERK activation in the gonadotrope represents a similarly compartmentalized event, possibly organized through association with plasma membrane rafts.
Recent studies have also identified proline-rich tyrosine kinase 2 (Pyk2) as a calcium-dependent enzyme required for GnRH-induced ERK activation in both αT3-1 and LβT2 cells. In LβT2 cells, Pyk2 was shown to act as a scaffold for the assembly of a signaling complex containing c-Src, Grb2, and Sos, and this complex was suggested to form a direct link between GnRH-induced calcium currents and canonical Ras-dependent activation of the ERK pathway [178]. Work from this laboratory also identified Pyk2 as a catalytic activity required for GnRH-induced ERK activation in αT3-1 cells and showed that GnRH induces rapid phosphorylation of Pyk2 in mouse gonadotropes in vivo [300]. In agreement with previous results demonstrating a requirement for calmodulin for GnRH-induced ERK activation, this study documented a direct interaction between calcium-activated calmodulin and the catalytic domain of Pyk2 [227;300]. Pyk2 thus appears to play a key role in linking VGCC-mediated calcium signals with the ERK pathway in gonadotropes.
3.5.5 GnRH-induced ERK signaling
In the gonadotrope, GnRH stimulation leads to activation of the ERK, JNK, and p38 pathways [149;192;229;253]. Several studies link activation of these pathways to transcriptional regulation of the gonadotropin genes. A recent report indicates that ERK5/BMK is also activated by GnRH in the LβT2 cell line and may play a role in upregulation of FSHβ in the gonadotrope [151]. In the context of this review, we will focus on regulation of ERK activity by GnRH (Figure 1).
The ERK pathway is involved in the transduction of signals emanating from a multitude of different cell surface receptors. The best characterized of these are the receptor tyrosine kinases (RTKs). Indeed, the ERK proteins were initially identified as kinase activities induced in cells by RTK ligands including a variety of mitogenic peptides and insulin [17;264]. These ligands stimulate the ERK pathway through a classical mechanism involving activation of the low molecular weight GTPase Ras. The ability of ERKs to mediate the proliferative effects of RTK ligands has been demonstrated in a variety of settings, and has been the focus of intense investigation in the areas of basic cell biology as well as drug discovery and anti-cancer therapeutics [230;249]. The ERK pathway is also activated following engagement of many GPCR [289]. Agonists for these receptors include a wide variety of small molecules as well as numerous neurotransmitters, peptides hormones, cytokines, nucleotides and amino acids, ions, and lipids [238]. GPCRs are linked to a bewildering array of cellular functions. While the ERK pathway has been shown to be important in mediating the mitogenic effects of many GPCR ligands, ERKs also play a role in the specific responses of some highly differentiated cells to certain agonists [238;281].
The ability of the ERK pathway to elicit dramatically diverse biological responses in different contexts points to complex and varied mechanisms of signal interpretation that have evolved in cells. Early insights into ERK signal specificity came from studies of the PC12 rat pheochromocytoma cell line in which it was shown that sustained ERK activation induced by nerve growth factor (NGF) led to neuronal differentiation while transient ERK activation following EGF treatment elicited a non-differentiating proliferative response [170]. One established mechanism by which cells may interpret differences in ERK signal duration derives from study of the ERK-dependent immediate early response protein c-Fos. As a component of the dimeric transcription factor AP-1, c-Fos is capable of coupling ERK pathway activity with the expression of a wide variety of genes. Up-regulation of c-Fos occurs rapidly following ERK activation; however, in the absence of post-translational modification, the newly synthesized protein undergoes rapid degradation. ERK-dependent phosphorylation of the C-terminus of c-Fos stabilizes the protein, prolonging its half-life and allowing its nuclear accumulation [196]. C-terminal phosphorylation also targets the protein for secondary ERK-dependent phosphorylation of internal residues 325 and 331, which enhances its transcriptional activation function [196]. However, this only occurs under conditions of relatively sustained ERK activation. These dual ERK-dependent phosphorylation events that sequentially stabilize and activate c-Fos provide an example of a simple but elegant mechanism by which cells may link ERK signals of variable duration with different transcriptional outputs.
Protein-protein interactions mediated through defined docking domains are also important in the establishment of ERK signal specificity. ERKs contain 2 key interaction domains: the common docking (CD) domain on the face of the protein opposite the catalytic site, and a separate interaction domain at the edge of the activation site [147;267]. The former binds to cognate ‘D’ domains of interactors such as ribosomal S6 kinases (RSK), MEK1/2, and certain MAP kinase phosphatases (MKPs) / dual-specificity phosphatases (DUSPs). The latter domain binds to the ‘docking for ERK [Phe-X-Phe]’ (DEF) domain of many ERK substrates including c-Fos and Egr1 [147;267]. Docking interactions are prerequisite for appropriate substrate recognition by ERKs and also guide the interaction of ERKs with various scaffolding and adaptor proteins. These interactions may contribute to signal specificity by confining ERK activity to particular subcellular compartments. For example, docking interactions underlie the nuclear translocation of activated ERKs that occurs in most cell types in response to various stimuli [23;124]. The MAPKKs MEK 1 and 2 can play an important role in this process. The MEK proteins contain a nuclear export sequence that restricts them to the cytosol [78;79]. Under resting conditions, stable associations between MEKs and inactive ERKs mediated through D domain-CD domain interactions may tether ERKs within the cytosolic compartment [78]. Upon pathway activation, however, the MEK-ERK association is destabilized allowing dissociation and nuclear translocation of activated ERKs.
In addition to their established tendency to undergo nuclear translocation, ERKs may also be recruited to extranuclear membranes, and may be activated within the context of membrane bound signaling complexes [271;275]. The DEF domain containing protein kinase suppressor of ras (KSR) is a scaffold protein that has been shown to facilitate Ras-dependent activation of membrane targeted ERKs through nucleation of signaling complexes containing all the core kinases of the ERK pathway [237]. KSR has also been shown to bind Gβγ dimers and to facilitate Ras-independent activation of membrane-associated ERKs following occupancy of certain GPCRs [46]. Docking interactions also underlie the organization of ERK signaling complexes by β–arrestins following desensitization and internalization of GPCRs [164]. Some data suggests that arrestin-associated complexes may serve to restrict activated ERKs to the cytosolic compartment thereby selectively targeting extranuclear substrates [164;272]. Similarly, formation of focal adhesions may involve the recruitment, activation, and retention of ERKs at sites of adhesion through interactions with p125-focal-adhesion kinase (FAK) or Pyk2 [71]. The activity of this pool of ERKs may be restricted to highly compartmentalized phosphorylation of cytoskeletal components of the adhesion complex.
3.5.6 Mechanisms of ERK activation by GPCR’s
In the classical paradigm of GPCR signaling, ligand binding stabilizes the receptor in an active conformation leading to nucleotide exchange of its G-protein α subunit and the dissociation of the α subunit as well as the dimeric βγ subunit from the receptor. The free α subunit then regulates the activity of various enzymatic effectors involved in the production of second messengers [81]. Historically, studies of GPCR signaling have emphasized the role of these receptors in highly differentiated cellular functions such as neurotransmission and excitation-contraction coupling. However, in recent years, appreciation of the role of GPCRs in cellular growth control has led to an interest in the mechanisms by which these receptors may activate established mitogenic pathways, in particular the ERK pathway [238].
The mechanisms of ERK activation by GPCRs vary considerably with both receptor and cell type. In some settings, GPCRs have been shown to cause phosphorylation and activation of RTKs such as the epidermal growth factor receptor (EGFR) or the platelet-derived growth factor receptor (PDGFR) in a process referred to as transactivation [243]. The most widely accepted view of RTK transactivation is based on the phenomenon of ectodomain shedding. According to this model, GPCR ligands lead to activation of ADAMTS family transmembrane matrix metalloproteinases (MMPs) through the activity of various intracellular effectors such as calcium, reactive oxygen species, Gβγ dimers, PKC isoforms, and the non-receptor tyrosine kinases c-src and Pyk2. MMPs in turn catalyze the proteolytic release of membrane bound RTK ligands leading to conventional ligand-dependent “outside-in” activation of RTKs and canonical Ras-dependent activation of the Raf-MEK-ERK cascade [208;244]. One report demonstrated the importance of EGFR transactivation through ectodomain shedding for GnRH-induced ERK activation in αT3-1 cells [233]. However, this may reflect a unique property of the specific cell clones used in those experiments as others, including ourselves, have been unable to demonstrate EGF responsiveness in αT3-1 cells cultured in our laboratory. Similarly, c-Src has been implicated as a link between the GnRHR and the ERK pathway through the activation of Ras [13;178]. However, other reports indicate that GnRH-induced ERK activation is Ras-independent [223]. Thus, the mechanisms by which the unique GnRHR couples to the ERK module via ectodomain shedding and or activation of c-Src in vivo remain unclear. The molecular architecture of this region of GnRH signaling cascade would therefore seem to be a promising area of investigation.
GPCRs may also regulate ERK activation through second messenger-dependent enzyme systems. For example, in the HEK293 cell model, activation of ERK through the endogenously expressed β(2)-adrenergic receptor was shown to involve cAMP-dependent activation of PKA, and subsequent activation of B-Raf via the small Ras-family GTPase Rap-1 [245]. β-arrestins have been shown to be capable of linking numerous GPCRs to the ERK module through nucleation of signaling complexes containing internalized receptors along with all the core components of the ERK pathway [164]. Finally Gβγ dimers may couple to the ERK pathway independent of their cognate α-subunit through the direct or indirect activation of the activity of phosphatidylinositol-3-kinase (PI3-kinase) or PLCβ [162].
In gonadotropes, GnRH stimulation leads to relatively transient activation of ERKs [155;200;228;253;266]. Studies on the mechanism of GnRH-induced ERK activation in gonadotropes have yielded conflicting results depending on the model systems employed. Nevertheless, some generalizations seem valid. Unlike other GPCRs, the GnRHR is not phosphorylated following ligand binding and the lack of a C-terminal tail precludes direct interaction with β-arrestins [109;291]. Therefore arrestin-mediated mechanisms of ERK activation are unlikely to apply [30]. In contrast, the requirement for extracellular calcium influx and activation of PKC isoforms for ERK activation has been widely demonstrated [195;200]. Previous work from this laboratory showed further that GnRH-induced ERK activation was blocked by inhibition of calmodulin [227]. Direct activation of Raf-1 by PKC is often cited as mechanism by GnRH may drive ERK pathway activation in a Ras-independent manner. This hypothesis derives largely from a study in which PKC was shown to phosphorylate Raf-1 in vitro. However, in light of the complex nature of Raf-1 activation, this hypothesis may be largely discounted [130]. Moreover, while Raf-1 is widely assumed to be the predominant upstream activator of the ERK pathway in gonadotrope cells, experimental evidence in support of this is lacking. Current views highlight the role of Raf-1 as an important regulator of apoptosis that is not required for ERK activation in all settings, and suggest that the alternative isoform B-Raf may be of greater overall importance as a MAPKKK than Raf-1 [5]. Indeed, within the pituitary, activation of the ERK pathway in both corticotrope and somatotrope cells following stimulation with the GPCR ligands corticotropin-releasing hormone (CRH) or growth hormone-releasing hormone (GHRH) does not involve Raf-1, but proceeds through a pathway involving Gαs, PKA, Rap-1 and B-raf [133;234]. Similar studies of the role of Raf kinase isoforms regulating ERK activation in gonadotropes following GnRH stimulation have not been reported.
4. Signal transduction and the response of the gonadotrope to variation in GnRH pulse frequency
4.1. Cell signaling and integration of GnRH pulse frequency
Over two decades, clinical observation and experimental study have confirmed the requirement for pulsatile GnRH stimulation for appropriate gonadotrope function and fertility [12;172;172;278]. Excessively low GnRH pulsatility leads to hypothalamic amenorrhea and infertility in women, while chronically high GnRH pulsatility contributes to infertility associated with the polycystic ovarian syndrome. Further, specific variations in GnRH pulse frequency are essential to the normal estrous cycle in females [96;221]. Lower frequency GnRH stimulation predominates over the follicular phase of the cycle favoring production and secretion of FSH and follicular recruitment and growth, while a precisely coordinated transition to higher frequency stimulation is required to drive the preovulatory LH surge [92]. While pulsatile hormone release is a common feature of many endocrine tissues, the striking responsiveness of the gonadotrope to GnRH pulse frequency has made this cell an attractive model for investigation of the mechanisms underlying stimulus-frequency interpretation [282].
While the specific biochemical processes by which the gonadotrope mounts specific responses to changing GnRH pulse frequencies have not been clarified, selective activation and termination of signaling activity is thought to play a major role in translating a given GnRH pulse frequency into selective expression of a given gene [69]. Calcium signaling has been implicated in pulse frequency interpretation in the gonadotrope [25;94]. Exposure of perifused rat pituitary cells to pulses of the calcium channel agonist BayK8644 revealed highly specific transcriptional response patterns of the gonadotropin subunits, with more rapid calcium pulse frequencies leading to preferential expression of αGSU and LHβ, while slower pulse frequencies caused preferential expression of FSHβ [95]. Indeed the patterns of gonadotropin transcriptional response in this system were very similar to the patterns of response seen with GnRH stimulation. Activation of calmodulin by calcium loading constitutes an important signaling hub in the gonadotrope, and results from our laboratory show that calmodulin loading is required for ERK activation by GnRH [227]. Calmodulin loading also leads to rapid activation of calcium/calmodulin-dependent kinase II (CamK II) in the gonadotrope [90;93]. GnRH has been shown to induce oscillatory calcium signals in the gonadotrope, and the activation of CamK II is sensitive to both the frequency and amplitude of intracellular calcium oscillations [49;276]. The activation of CamK II is not itself dependent on GnRH pulse frequency; however, the rapid deactivation kinetics of CamK II suggest that sustained CamK II activity may occur preferentially under conditions of rapid GnRH pulsatility, which is consistent with a role for this enzyme in mediating differential responses to variable and physiologically relevant GnRH pulse frequencies [25;90;93]. Therefore, CamK II is an attractive candidate molecule with respect to the ability of the gonadotrope to decode GnRH pulse frequency.
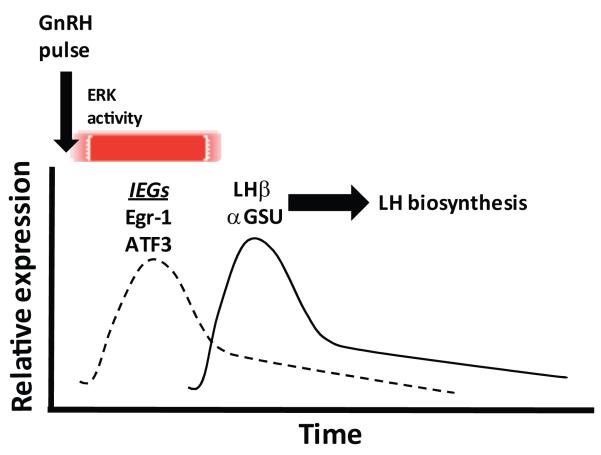
The MAP kinase pathways, most notably the ERK signaling system, have also been implicated in GnRH pulse frequency interpretation in the gonadotrope [25;26;117]. In perifused LβT2 cells as well as primary gonadotropes, slower pulse frequency of GnRH stimulation led to a more rapid onset and sustained pattern of ERK activation as compared with higher frequency stimulation. In addition, slower pulse frequencies resulted in higher levels of nuclear phospho-ERK, suggesting that ERK-dependent transcriptional processes may be enhanced under conditions of lower pulse frequency [117]. Interestingly, pharmacological inhibition of ERK signaling in perifused LβT2 cells as well as in primary pituitary cell cultures blocked the stimulatory effect of GnRH on both FSHβ and LHβ transcription [117]. The ERK dependence of LHβ transcription is consistent with many previous studies showing the importance of the ERK-dependent transcription factor Egr1 for LHβ expression [57;74;142;146;296]. In contrast, the role of ERK signaling in mediating upregulation of FSHβ by GnRH is less established and the observed requirement for ERK activity for FSHβ expression may appear difficult to reconcile with our data showing that FSHβ expression remains largely intact in mice with ERK-deficient gonadotropes [18]. However, the perifusion system in which ERK-dependence of FSHβ expression was observed was designed to test the effects of GnRH in isolation from other endocrine signals, particularly activin. Thus, whether our results represent an inherent ERK-independence of FSHβ expression in differentiated gonadotropes in vivo, or whether a collateral activin-mediated mechanism supporting FSHβ expression is at play in this setting is unclear.
4.2 Intracellular negative feedback and phosphatase activity
The mechanism by which slower GnRH pulse frequency leads to more sustained ERK activation may ultimately be linked not only to feed-forward activation kinetics, but also to intracellular feedback processes of ERK signal termination. The dual specificity phosphatases (DUSPs) are a heterogeneous family of regulatory phosphatases that bind and dephosphorylate MAP kinases through D-domain-dependent interactions. As such, the DUSPs have received much attention as negative regulators of MAP kinase signaling [3;51;51;53;119;308]. Microarray analysis showed that DUSPs 1 and 4 (MKP1 and 2) are upregulated following GnRH stimulation in LβT2 cells [299]. Results from this laboratory have shown further that DUSPs 1 and 4 are upregulated by GnRH both in αT3-1 cells and in vivo and that this upregulation is correlated with decreases in both ERK and JNK activity [308]. DUSP 4 upregulation in αT3-1 cells was itself dependent upon ERK activation and Egr1 expression, pointing to a potential feedback loop underlying termination of the ERK signal [309]. We have observed that DUSP4 transcript levels are not increased in response to GnRH stimulation in primary pituitary cell cultures from mice with pituitary-targeted deletion of ERK 1 and 2, as they are in cells from control animals (S Bliss & M Roberson, unpublished results). However, more recent results using heterologous expression of the GnRHR in HeLa cells have challenged this notion, showing that dephosphorylation of GnRH-activated ERK, while dependent on de novo protein synthesis, is not affected by combined suppression of DUSPs 1, 2, and 4 accomplished through the use of siRNA [3]. Interestingly, in this same system, the more sustained ERK activation that occurred following specific activation of PKC was sensitive to the effects of DUSP knockdown. Interpretation of our own studies in αT3-1 cells as well as results in HeLa cells is complicated by potential cell-type specificity and the in vitro nature of the studies. Indeed it is notable that the murine DUSP knockout models that have been reported to date (involving DUSPs 1, 2, 10, and 14) are all fertile [242]. Thus, the potential for functional redundancy between DUSP family members is likely a complicating issue in the determination of the role of phosphatase shaping of the magnitude and duration of the ERK response to GnRH stimulation.
5. Importance of ERK signaling for gonadotrope function and fertility
In gonadotropes, a substantial fraction of active ERK undergoes rapid nuclear translocation, and numerous studies using a variety of cellular models point to the importance of ERK signaling in GnRH-induced transcriptional responses [59;75;155;178;194;300;301]. The immediate early gene response to GnRH stimulation was examined by microarray analysis in the LβT2 cell line where 28 immediate early genes were shown to be significantly upregulated within 60 minutes of GnRH exposure [299]. The role of ERK signaling in the induction of several of these genes, including c-Fos, Egr1, and LRG21/ATF3, is established [59;155;227] (Figure 2). As a component of the dimeric transcription factor AP-1, c-Fos has been implicated in GnRH-induced expression of FSHβ, as well as in homologous upregulation of the GnRHR [44;45;284;290]. Egr1 plays an established role in the transcriptional upregulation of LHβ, and also contributes to the expression of the dual specificity phosphatase MKP2/DUSP4 [296;309]. ERKs have also been shown to contribute to GnRH-induced transcriptional upregulation of the αGSU through putative phosphorylation of Ets family transcription factors associated with the αGSU promoter [228]. ATF3 may also enhance expression of at least the human αGSU through dimerization with c-Jun [301]. ERK signaling is thus linked to the expression of the full complement of genes (GnRHR, FSHβ, LHβ, αGSU) that comprise the genetic signature of the gonadotrope. Whether ERK signaling contributes to the expression of other members of this immediate early gene repertoire in the gonadotrope remains to be determined.
Figure 2.
Temporal relationship between transient ERK activation, immediate early gene (IEGs) upregulation and activation of late genes that lead to LH biosynthesis following a pulse of GnRH.
Despite the vast amount of data that has been published regarding the organization and function of the ERK signaling pathway in a variety of cellular models, little is known about the importance of ERK signaling for the function of differentiated cells in vivo. This is particularly true with respect to GnRH-induced ERK activation in the gonadotrope. Much of the data on ERK signaling in the gonadotrope is based on the assumption that ERK1 and ERK2 are functionally redundant; thus the question of whether ERK1 and ERK2 serve differential roles in these cells remains largely unexplored. However, it has been shown that ERK1 and 2 can serve quite distinct roles in some differentiated cell types [179]. In addition, while the ERK1 null mouse is viable and fertile and displays only subtle phenotypic defects, attempts to address the in vivo requirements for ERK signaling generally have been complicated by the embryonic lethality of the ERK2 null genotype [203;303].
To address the requirement of ERK signaling for the function of differentiated gonadotropes in vivo, and to evaluate the functional redundancy between ERK1 and ERK2 in these cells, our laboratory recently developed a murine model of gonadotrope-targeted ERK pathway ablation [18]. This model was generated by crossing αGSU-Cre expressing animals with mice harboring a floxed allele at the ERK2 locus. In a background of normal ERK1 alleles, both male and female mice with ERK2-deficient gonadotropes (conditional ablation) were viable and fertile. In light of the lack of reproductive phenotype in the ERK1 null mouse, these results indicated that either ERK1 or ERK2 are capable of supporting gonadotrope function in a redundant manner in vivo. Crossing the gonadotrope-targeted ERK2 conditional knockout onto the ERK1 null background generated ERK1/2 compound knockouts at significantly less than the expected Mendelian frequency, suggesting that early and promiscuous expression of the Cre recombinase (as outlined earlier) may have impacted viability in a subset of these animals. However, in compound knockouts that survived to adulthood, males were reproductively normal, while females demonstrated anovulatory infertility. Exogenous replacement of gonadotropins effectively rescued the anovulatory phenotype. These studies also made use of the increased frequency of GnRH pulsatility following gonadectomy (classical ablation studies effectively removing gonadal steroid negative feedback) to examine if GnRH hyperstimulation of the gonadotrope resulted in normal upregulation of gonadotropin subunit genes and gonadotropin secretion in the absence of ERK signaling. The female infertility reflected primarily a lack of both basal and inducible synthesis of LHβ and LH secretion, and this defect was in turn linked to a failure in the ability of gonadotropes to upregulate Egr1 in response to GnRH. ERK signaling was also shown to be required for normal expression of the common αGSU in this paradigm. In contrast, expression of FSHβ and the GnRHR transcripts were relatively maintained in ERK-deficient animals of both genders following castration. Interestingly, despite interruption of the ERK pathway in thyrotropes, ERK1/2 compound null animals of both genders remained euthyroid, indicating that ERK signaling is not required for thyrotrope function. These results indicated that GnRH-induced ERK signaling is essential for fertility in the female mouse, and the sexually dimorphic requirement for this signaling pathway appeared to reflect the importance of ERK activity and Egr1 inducibility in mediating the transcriptional response of the gonadotrope to high frequency GnRH stimulation, the subsequent accumulation of LH within the gonadotrope necessary for the generation of the LH surge at the time of ovulation. Accumulation of high levels of LH within the male gonadotrope is simply not necessary to maintain normal male reproductive function.
6. Conclusions and future directions
Over the past two decades, a great deal of mechanistic detail has emerged from research examining GnRHR behavior and GnRH actions in pituitary gonadotropes leading to the induction of a complex signaling network largely attributable to the creation and utility of gonadotrope cell lines such as αT3-1 and LβT2 cells. The GnRHR is a truly unique member of the GPCR superfamily, playing a central role in the regulation of gonadotrope cell function. The GnRHR is an exclusive and constitutive occupant in discrete membrane rafts and productive signaling at least to the ERK pathway requires this compartmentalized membrane association and organization. This need is clearly compatible with the need for putatively localized calcium signals supporting ERK activation. However, integration of ERK signaling in gonadotropes in response to GnRH at the level of PKC and specific MAPKKK involved is not currently clear and remains the focus of our continued efforts. Future studies will be greatly augmented with our ability to isolate responses to GnRH and ERK signaling using the GRIC-Cre mouse line both to facilitate gene excision exclusively in gonadotropes as well as visualize gonadotrope populations with the use of fluorescent reporter mouse lines. Collectively these studies are leading toward elucidation of a more complete understanding of the immediate early gene repertoire contributing to late expression of gene products that define the behavior and identity of the gonadotrope.
One of the goals of this review was to outline the importance of GnRH-induced ERK signaling within the gonadotrope of the anterior pituitary. However, the role and requirement of ERK signaling within each tiered level of the HPG axis is of considerable importance. This is exemplified by the recent elegant studies of a double ERK conditional knockout within ovarian granulosa cells recently reported by the Richards group [66]. These studies provide intriguing evidence that gonadotropin-induced ERK signaling within ovarian granulosa cells is required for resumption of meiosis by the oocyte, ovulation and subsequent luteinization of follicle cells following ovulation. Like our studies of GnRH-induced ERK activation and upregulation of Egr-1 activity regulating LHβ subunit expression in the anterior pituitary, LH-induced ERK activity and subsequent induction of C/EBPβ activity underlies a critical pathway directing ovulation and luteinization within the ovary. Thus, ERK activity is required for normal fertility at both the pituitary and ovarian levels within the HPG axis. Future studies will focus on a possible role of ERKs within hypothalamic neurons and germ cells themselves. Clearly the enticing prediction is that ERK signaling integrates control of fertility at several levels with the HPG axis, at least in female mice.
While GnRH signaling through the ERK pathway is clearly central to endocrine regulation of fertility, this signaling cascade reflects only a small portion of the global signaling network induced by a pulse of GnRH. Studies focusing on the role and requirement of JNK and p38 MAPK signaling may well be highly informative and set the framework for pharmacological and therapeutic manipulations to both inhibit and enhance fertility.
Acknowledgements
The Roberson lab has ongoing support from an NIH/NICHD grant R01HD34722 to MSR. The authors thank all of the present and former members of the Roberson lab for all of their efforts. Our apologies to the many labs and investigators that have contributed to this field whose research we did not cite due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- [2].Al-Kindi AY, Mahmoud Y, Woller MJ. Ultrastructural changes in granulosa cells and plasma steroid levels after administration of luteinizing hormone-releasing hormone in the Western painted turtle, Chrysemys picta. Tissue Cell. 2001;33:361–367. doi: 10.1054/tice.2001.0188. [DOI] [PubMed] [Google Scholar]
- [3].Armstrong SP, Caunt CJ, McArdle CA. Gonadotropin-releasing hormone and protein kinase C signaling to ERK: spatiotemporal regulation of ERK by docking domains and dual-specificity phosphatases. Mol Endocrinol. 2009;23:510–519. doi: 10.1210/me.2008-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arnhold IJ, Lofrano-Porto A, Latronico AC. Inactivating mutations of luteinizing hormone beta-subunit or luteinizing hormone receptor cause oligo-amenorrhea and infertility in women. Horm Res. 2009;71:75–82. doi: 10.1159/000183895. [DOI] [PubMed] [Google Scholar]
- [5].Baccarini M. Second nature: biological functions of the Raf-1 “kinase”. FEBS Lett. 2005;579:3271–3277. doi: 10.1016/j.febslet.2005.03.024. [DOI] [PubMed] [Google Scholar]
- [6].Baker PJ, Pakarinen P, Huhtaniemi IT, Abel MH, Charlton HM, Kumar TR, O’Shaughnessy PJ. Failure of normal Leydig cell development in follicle-stimulating hormone (FSH) receptor-deficient mice, but not FSHbeta-deficient mice: role for constitutive FSH receptor activity. Endocrinology. 2003;144:138–145. doi: 10.1210/en.2002-220637. [DOI] [PubMed] [Google Scholar]
- [7].Bakke M, Zhao L, Parker KL. Approaches to define the role of SF-1 at different levels of the hypothalamic-pituitary-steroidogenic organ axis. Mol Cell Endocrinol. 2001;179:33–37. doi: 10.1016/s0303-7207(01)00468-3. [DOI] [PubMed] [Google Scholar]
- [8].Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- [9].Bedecarrats GY, Kaiser UB. Mutations in the human gonadotropin-releasing hormone receptor: insights into receptor biology and function. Semin Reprod Med. 2007;25:368–378. doi: 10.1055/s-2007-984743. [DOI] [PubMed] [Google Scholar]
- [10].Bedecarrats GY, Linher KD, Janovick JA, Beranova M, Kada F, Seminara SB, Conn P. Michael, Kaiser UB. Four naturally occurring mutations in the human GnRH receptor affect ligand binding and receptor function. Mol Cell Endocrinol. 2003;205:51–64. doi: 10.1016/s0303-7207(03)00201-6. [DOI] [PubMed] [Google Scholar]
- [11].Bedecarrats GY, Linher KD, Kaiser UB. Two common naturally occurring mutations in the human gonadotropin-releasing hormone (GnRH) receptor have differential effects on gonadotropin gene expression and on GnRH-mediated signal transduction. J Clin Endocrinol Metab. 2003;88:834–843. doi: 10.1210/jc.2002-020806. [DOI] [PubMed] [Google Scholar]
- [12].Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- [13].Benard O, Naor Z, Seger R. Role of dynamin, Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J Biol Chem. 2001;276:4554–4563. doi: 10.1074/jbc.M006995200. [DOI] [PubMed] [Google Scholar]
- [14].Berger K, Souza H, Brito VN, d’Alva CB, Mendonca BB, Latronico AC. Clinical and hormonal features of selective follicle-stimulating hormone (FSH) deficiency due to FSH beta-subunit gene mutations in both sexes. Fertil Steril. 2005;83:466–470. doi: 10.1016/j.fertnstert.2004.06.069. [DOI] [PubMed] [Google Scholar]
- [15].Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- [16].Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- [17].Blackshear PJ, Nemenoff RA, Avruch J. Insulin and growth factors stimulate the phosphorylation of a Mr-22000 protein in 3T3-L1 adipocytes. Biochem J. 1983;214:11–19. doi: 10.1042/bj2140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–1101. doi: 10.1210/me.2009-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, Roberson MS. Signaling complexes associated with the type I gonadotropin-releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Mol Endocrinol. 2007;21:538–549. doi: 10.1210/me.2006-0289. [DOI] [PubMed] [Google Scholar]
- [20].Borders AS, de Almeida L, Van Eldik LJ, Watterson DM. The p38alpha mitogen-activated protein kinase as a central nervous system drug discovery target. BMC Neurosci. 2008;9(Suppl 2):S12. doi: 10.1186/1471-2202-9-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Braden TD, Conn PM. Activin-A stimulates the synthesis of gonadotropin-releasing hormone receptors. Endocrinology. 1992;130:2101–2105. doi: 10.1210/endo.130.4.1312442. [DOI] [PubMed] [Google Scholar]
- [22].Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- [23].Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Burcelin R, Thorens B, Glauser M, Gaillard RC, Pralong FP. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology. 2003;144:4484–4491. doi: 10.1210/en.2003-0457. [DOI] [PubMed] [Google Scholar]
- [25].Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79:947–953. doi: 10.1095/biolreprod.108.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of Lhb and Egr1 gene expression by GNRH pulses in rat pituitaries is both c-Jun N-terminal kinase (JNK)- and extracellular signal-regulated kinase (ERK)-dependent. Biol Reprod. 2009;81:1206–1215. doi: 10.1095/biolreprod.109.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Caraty A, Skinner DC. Progesterone priming is essential for the full expression of the positive feedback effect of estradiol in inducing the preovulatory gonadotropin-releasing hormone surge in the ewe. Endocrinology. 1999;140:165–170. doi: 10.1210/endo.140.1.6444. [DOI] [PubMed] [Google Scholar]
- [28].Carmel PW, Araki S, Ferin M. Pituitary stalk portal blood collection in rhesus monkeys: evidence for pulsatile release of gonadotropin-releasing hormone (GnRH) Endocrinology. 1976;99:243–248. doi: 10.1210/endo-99-1-243. [DOI] [PubMed] [Google Scholar]
- [29].Castro-Fernandez C, Conn PM. Regulation of the gonadotropin-releasing hormone receptor (GnRHR) by RGS proteins: role of the GnRHR carboxyl-terminus. Mol.Cell Endocrinol. 2002;191:149–156. doi: 10.1016/s0303-7207(02)00082-5. [DOI] [PubMed] [Google Scholar]
- [30].Caunt CJ, Finch AR, Sedgley KR, Oakley L, Luttrell LM, McArdle CA. Arrestin-mediated ERK activation by gonadotropin-releasing hormone receptors: receptor-specific activation mechanisms and compartmentalization. J.Biol.Chem. 2006;281:2701–2710. doi: 10.1074/jbc.M507242200. [DOI] [PubMed] [Google Scholar]
- [31].Caunt CJ, Hislop JN, Kelly E, Matharu AL, Green LD, Sedgley KR, Finch AR, McArdle CA. Regulation of gonadotropin-releasing hormone receptors by protein kinase C: inside out signalling and evidence for multiple active conformations. Endocrinology. 2004;145:3594–3602. doi: 10.1210/en.2004-0092. [DOI] [PubMed] [Google Scholar]
- [32].Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- [33].Charles MA, Mortensen AH, Potok MA, Camper SA. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis. 2008;46:507–514. doi: 10.1002/dvg.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- [35].Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275:5479–5495. doi: 10.1111/j.1742-4658.2008.06677.x. [DOI] [PubMed] [Google Scholar]
- [36].Chidiac P, Ross EM. Phospholipase C-beta1 directly accelerates GTP hydrolysis by Galphaq and acceleration is inhibited by Gbeta gamma subunits. J Biol Chem. 1999;274:19639–19643. doi: 10.1074/jbc.274.28.19639. [DOI] [PubMed] [Google Scholar]
- [37].Clarke IJ, Burman KJ, Doughton BW, Cummins JT. Effects of constant infusion of gonadotrophin-releasing hormone in ovariectomized ewes with hypothalamo-pituitary disconnection: further evidence for differential control of LH and FSH secretion and the lack of a priming effect. J Endocrinol. 1986;111:43–49. doi: 10.1677/joe.0.1110043. [DOI] [PubMed] [Google Scholar]
- [38].Clarke IJ, Cummins JT. Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology. 1984;39:267–274. doi: 10.1159/000123990. [DOI] [PubMed] [Google Scholar]
- [39].Clarke IJ, Cummins JT, de Kretser DM. Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology. 1983;36:376–384. doi: 10.1159/000123484. [DOI] [PubMed] [Google Scholar]
- [40].Clarkson J, de Tassigny X. d’Anglemont, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- [43].Corbier P. Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology. 1985;116:142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- [44].Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. p38 mitogen-activated protein kinase is critical for synergistic induction of the FSH(beta) gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol.Endocrinol. 2007;21:3071–3086. doi: 10.1210/me.2007-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J.Biol.Chem. 2004;279:152–162. doi: 10.1074/jbc.M304697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Crespo P, Cachero TG, Xu N, Gutkind JS. Dual effect of beta-adrenergic receptors on mitogen-activated protein kinase. Evidence for a beta gamma-dependent activation and a G alpha s-cAMP-mediated inhibition. J Biol Chem. 1995;270:25259–25265. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- [47].Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125:917–924. doi: 10.1210/endo-125-2-917. [DOI] [PubMed] [Google Scholar]
- [48].Davidson JS, Flanagan CA, Zhou W, Becker II, Elario R, Emeran W, Sealfon SC, Millar RP. Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: role in receptor expression but not ligand binding. Mol Cell Endocrinol. 1995;107:241–245. doi: 10.1016/0303-7207(94)03449-4. [DOI] [PubMed] [Google Scholar]
- [49].De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- [50].de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- [51].Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- [52].Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dimitri CA, Dowdle W, MacKeigan JP, Blenis J, Murphy LO. Spatially separate docking sites on ERK2 regulate distinct signaling events in vivo. Curr Biol. 2005;15:1319–1324. doi: 10.1016/j.cub.2005.06.037. [DOI] [PubMed] [Google Scholar]
- [54].Dobkin-Bekman M, Naidich M, Pawson AJ, Millar RP, Seger R, Naor Z. Activation of mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol Cell Endocrinol. 2006;252:184–190. doi: 10.1016/j.mce.2006.03.035. [DOI] [PubMed] [Google Scholar]
- [55].Dode C, Hardelin JP. Kallmann syndrome: fibroblast growth factor signaling insufficiency? J Mol Med. 2004;82:725–734. doi: 10.1007/s00109-004-0571-y. [DOI] [PubMed] [Google Scholar]
- [56].Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pecheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- [57].Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–13876. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- [58].Drin G, Scarlata S. Stimulation of phospholipase Cbeta by membrane interactions, interdomain movement, and G protein binding--how many ways can you activate an enzyme? Cell Signal. 2007;19:1383–1392. doi: 10.1016/j.cellsig.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL. GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol. 2002;16:221–233. doi: 10.1210/mend.16.2.0779. [DOI] [PubMed] [Google Scholar]
- [60].Dyer RG, Robinson JE. The LHRH pulse generator. J Endocrinol. 1989;123:1–2. doi: 10.1677/joe.0.1230001. [DOI] [PubMed] [Google Scholar]
- [61].Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- [62].Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol. 2003;206:93–111. doi: 10.1016/s0303-7207(03)00235-1. [DOI] [PubMed] [Google Scholar]
- [63].Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18:806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- [64].Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology. 1997;138:5408–5414. doi: 10.1210/endo.138.12.5558. [DOI] [PubMed] [Google Scholar]
- [65].Everett JW. Progesterone and estrogen in the experimental control of ovulation time and other features of the estrous cycle in the rat. Endocrinology. 1948;43:389–405. doi: 10.1210/endo-43-6-389. [DOI] [PubMed] [Google Scholar]
- [66].Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Faure M, Voyno-Yasenetskaya TA, Bourne HR. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- [68].Fernandez-Vazquez G, Kaiser UB, Albarracin CT, Chin WW. Transcriptional activation of the gonadotropin-releasing hormone receptor gene by activin A. Mol Endocrinol. 1996;10:356–366. doi: 10.1210/mend.10.4.8721981. [DOI] [PubMed] [Google Scholar]
- [69].Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol Reprod. 2006;74:993–998. doi: 10.1095/biolreprod.105.049049. [DOI] [PubMed] [Google Scholar]
- [70].Finch AR, Caunt CJ, Armstrong SP, McArdle CA. Agonist-induced internalization and downregulation of gonadotropin-releasing hormone receptors. Am.J.Physiol Cell Physiol. 2009;297:C591–C600. doi: 10.1152/ajpcell.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Flanagan CA, Rodic V, Konvicka K, Yuen T, Chi L, Rivier JE, Millar RP, Weinstein H, Sealfon SC. Multiple interactions of the Asp(2.61(98)) side chain of the gonadotropin-releasing hormone receptor contribute differentially to ligand interaction. Biochemistry. 2000;39:8133–8141. doi: 10.1021/bi000085g. [DOI] [PubMed] [Google Scholar]
- [73].Flanagan CA, Zhou W, Chi L, Yuen T, Rodic V, Robertson D, Johnson M, Holland P, Millar RP, Weinstein H, Mitchell R, Sealfon SC. The functional microdomain in transmembrane helices 2 and 7 regulates expression, activation, and coupling pathways of the gonadotropin-releasing hormone receptor. J Biol Chem. 1999;274:28880–28886. doi: 10.1074/jbc.274.41.28880. [DOI] [PubMed] [Google Scholar]
- [74].Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15:77–87. doi: 10.1093/molehr/gan079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fowkes RC, King P, Burrin JM. Regulation of human glycoprotein hormone alpha-subunit gene transcription in LbetaT2 gonadotropes by protein kinase C and extracellular signal-regulated kinase 1/2. Biol Reprod. 2002;67:725–734. doi: 10.1095/biolreprod67.3.725. [DOI] [PubMed] [Google Scholar]
- [76].Francis RC, Soma K, Fernald RD. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci U S A. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- [78].Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997;272:32642–32648. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- [79].Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- [80].Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- [81].Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- [82].Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology. 2008;149:20–27. doi: 10.1210/en.2007-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Glass JD, Ferreira S, Deaver DR. Photoperiodic adjustments in hypothalamic amines, gonadotropin-releasing hormone, and beta-endorphin in the white-footed mouse. Endocrinology. 1988;123:1119–1127. doi: 10.1210/endo-123-2-1119. [DOI] [PubMed] [Google Scholar]
- [84].Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Graham KE, Nusser KD, Low MJ. LbetaT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J.Endocrinol. 1999;162:R1–R5. doi: 10.1677/joe.0.162r001. [DOI] [PubMed] [Google Scholar]
- [87].Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22:253–267. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- [88].Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB. Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-beta gene. Mol Endocrinol. 2005;19:237–254. doi: 10.1210/me.2003-0473. [DOI] [PubMed] [Google Scholar]
- [89].Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275:9193–9200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- [90].Haisenleder DJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription: evidence for the involvement of calcium/calmodulin-dependent kinase II (Ca/CAMK II) activation in rat pituitaries. Endocrinology. 2003;144:2768–2774. doi: 10.1210/en.2002-0168. [DOI] [PubMed] [Google Scholar]
- [91].Haisenleder DJ, Cox ME, Parsons SJ, Marshall JC. Gonadotropin-releasing hormone pulses are required to maintain activation of mitogen-activated protein kinase: role in stimulation of gonadotrope gene expression. Endocrinology. 1998;139:3104–3111. doi: 10.1210/endo.139.7.6091. [DOI] [PubMed] [Google Scholar]
- [92].Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- [93].Haisenleder DJ, Ferris HA, Shupnik MA. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology. 2003;144:2409–2416. doi: 10.1210/en.2002-0013. [DOI] [PubMed] [Google Scholar]
- [94].Haisenleder DJ, Workman LJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin subunit transcriptional responses to calcium signals in the rat: evidence for regulation by pulse frequency. Biol Reprod. 2001;65:1789–1793. doi: 10.1095/biolreprod65.6.1789. [DOI] [PubMed] [Google Scholar]
- [95].Haisenleder DJ, Yasin M, Marshall JC. Gonadotropin subunit and gonadotropin-releasing hormone receptor gene expression are regulated by alterations in the frequency of calcium pulsatile signals. Endocrinology. 1997;138:5227–5230. doi: 10.1210/endo.138.12.5611. [DOI] [PubMed] [Google Scholar]
- [96].Hall JE, Taylor AE, Hayes FJ, Crowley WF., Jr. Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest. 1998;21:602–611. doi: 10.1007/BF03350785. [DOI] [PubMed] [Google Scholar]
- [97].Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod. 1985;32:855–864. doi: 10.1095/biolreprod32.4.855. [DOI] [PubMed] [Google Scholar]
- [98].Hanyaloglu AC, Vrecl M, Kroeger KM, Miles LE, Qian H, Thomas WG, Eidne KA. Casein kinase II sites in the intracellular C-terminal domain of the thyrotropin-releasing hormone receptor and chimeric gonadotropin-releasing hormone receptors contribute to beta-arrestin-dependent internalization. J.Biol.Chem. 2001;276:18066–18074. doi: 10.1074/jbc.M009275200. [DOI] [PubMed] [Google Scholar]
- [99].Hardelin JP, Dode C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2:181–193. doi: 10.1159/000152034. [DOI] [PubMed] [Google Scholar]
- [100].Hardelin JP, Julliard AK, Moniot B, Soussi-Yanicostas N, Verney C, Schwanzel-Fukuda M, Ayer-Le Lievre C, Petit C. Anosmin-1 is a regionally restricted component of basement membranes and interstitial matrices during organogenesis: implications for the developmental anomalies of X chromosome-linked Kallmann syndrome. Dev Dyn. 1999;215:26–44. doi: 10.1002/(SICI)1097-0177(199905)215:1<26::AID-DVDY4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [101].Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP. Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specific period of time immediately after activation of the gonadotropin-releasing hormone surge-generating system. Endocrinology. 1999;140:827–834. doi: 10.1210/endo.140.2.6490. [DOI] [PubMed] [Google Scholar]
- [102].Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. Genesis. 2006;44:391–395. doi: 10.1002/dvg.20227. [DOI] [PubMed] [Google Scholar]
- [103].Heding A, Vrecl M, Bogerd J, McGregor A, Sellar R, Taylor PL, Eidne KA. Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics. J Biol Chem. 1998;273:11472–11477. doi: 10.1074/jbc.273.19.11472. [DOI] [PubMed] [Google Scholar]
- [104].Heding A, Vrecl M, Hanyaloglu AC, Sellar R, Taylor PL, Eidne KA. The rat gonadotropin-releasing hormone receptor internalizes via a beta-arrestin-independent, but dynamin-dependent, pathway: addition of a carboxyl-terminal tail confers beta-arrestin dependency. Endocrinology. 2000;141:299–306. doi: 10.1210/endo.141.1.7269. [DOI] [PubMed] [Google Scholar]
- [105].Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology. 1996;63:120–131. doi: 10.1159/000126948. [DOI] [PubMed] [Google Scholar]
- [107].Hislop JN, Caunt CJ, Sedgley KR, Kelly E, Mundell S, Green LD, McArdle CA. Internalization of gonadotropin-releasing hormone receptors (GnRHRs): does arrestin binding to the C-terminal tail target GnRHRs for dynamin-dependent internalization? J.Mol.Endocrinol. 2005;35:177–189. doi: 10.1677/jme.1.01809. [DOI] [PubMed] [Google Scholar]
- [108].Hislop JN, Everest HM, Flynn A, Harding T, Uney JB, Troskie BE, Millar RP, McArdle CA. Differential internalization of mammalian and non-mammalian gonadotropin-releasing hormone receptors. Uncoupling of dynamin-dependent internalization from mitogen-activated protein kinase signaling. J Biol Chem. 2001;276:39685–39694. doi: 10.1074/jbc.M104542200. [DOI] [PubMed] [Google Scholar]
- [109].Hislop JN, Madziva MT, Everest HM, Harding T, Uney JB, Willars GB, Millar RP, Troskie BE, Davidson JS, McArdle CA. Desensitization and internalization of human and xenopus gonadotropin-releasing hormone receptors expressed in alphaT4 pituitary cells using recombinant adenovirus. Endocrinology. 2000;141:4564–4575. doi: 10.1210/endo.141.12.7813. [DOI] [PubMed] [Google Scholar]
- [110].Horn F, Bilezikjian LM, Perrin MH, Bosma MM, Windle JJ, Huber KS, Blount AL, Hille B, Vale W, Mellon PL. Intracellular responses to gonadotropin-releasing hormone in a clonal cell line of the gonadotrope lineage. Mol Endocrinol. 1991;5:347–355. doi: 10.1210/mend-5-3-347. [DOI] [PubMed] [Google Scholar]
- [111].Horvat RD, Roess DA, Nelson SE, Barisas BG, Clay CM. Binding of agonist but not antagonist leads to fluorescence resonance energy transfer between intrinsically fluorescent gonadotropin-releasing hormone receptors. Mol Endocrinol. 2001;15:695–703. doi: 10.1210/mend.15.5.0633. [DOI] [PubMed] [Google Scholar]
- [112].Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992;6:1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- [113].Hughes VA, Boepple PA, Crowley WF, Jr., Seminara SB. Interplay between dose and frequency of GnRH administration in determining pituitary gonadotropin responsiveness. Neuroendocrinology. 2008;87:142–150. doi: 10.1159/000112421. [DOI] [PubMed] [Google Scholar]
- [114].Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- [115].Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- [116].Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1995;92:12280–12284. doi: 10.1073/pnas.92.26.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology. 2005;146:5503–5513. doi: 10.1210/en.2004-1317. [DOI] [PubMed] [Google Scholar]
- [118].Kanasaki H, Mutiara S, Oride A, Purwana IN, Miyazaki K. Pulse frequency-dependent gonadotropin gene expression by adenylate cyclase-activating polypeptide 1 in perifused mouse pituitary gonadotroph LbetaT2 cells. Biol Reprod. 2009;81:465–472. doi: 10.1095/biolreprod.108.074765. [DOI] [PubMed] [Google Scholar]
- [119].Karlsson M, Mandl M, Keyse SM. Spatio-temporal regulation of mitogen-activated protein kinase (MAPK) signalling by protein phosphatases. Biochem Soc Trans. 2006;34:842–845. doi: 10.1042/BST0340842. [DOI] [PubMed] [Google Scholar]
- [120].Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference bewteen primates and rodents. Science. 1973;179:484–486. doi: 10.1126/science.179.4072.484. [DOI] [PubMed] [Google Scholar]
- [121].Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol.Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kendall SK, Gordon DF, Birkmeier TS, Petrey D, Sarapura VD, O’Shea KS, Wood WM, Lloyd RV, Ridgway EC, Camper SA. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone alpha-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Mol Endocrinol. 1994;8:1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- [123].Khadra A, Li YX. A model for the pulsatile secretion of gonadotropin-releasing hormone from synchronized hypothalamic neurons. Biophys J. 2006;91:74–83. doi: 10.1529/biophysj.105.080630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- [125].Kim CG, Park D, Rhee SG. The role of carboxyl-terminal basic amino acids in Gqalpha-dependent activation, particulate association, and nuclear localization of phospholipase C-beta1. J Biol Chem. 1996;271:21187–21192. doi: 10.1074/jbc.271.35.21187. [DOI] [PubMed] [Google Scholar]
- [126].Kirk SE, Dalkin AC, Yasin M, Haisenleder DJ, Marshall JC. Gonadotropin-releasing hormone pulse frequency regulates expression of pituitary follistatin messenger ribonucleic acid: a mechanism for differential gonadotrope function. Endocrinology. 1994;135:876–880. doi: 10.1210/endo.135.3.8070381. [DOI] [PubMed] [Google Scholar]
- [127].Kitanovic S, Yuen T, Flanagan CA, Ebersole BJ, Sealfon SC. Insertional mutagenesis of the arginine cage domain of the gonadotropin-releasing hormone receptor. Mol Endocrinol. 2001;15:390–397. doi: 10.1210/mend.15.3.0611. [DOI] [PubMed] [Google Scholar]
- [128].Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- [129].Kogawa K, Ogawa K, Hayashi Y, Nakamura T, Titani K, Sugino H. Immunohistochemical localization of follistatin in rat tissues. Endocrinol Jpn. 1991;38:383–391. doi: 10.1507/endocrj1954.38.383. [DOI] [PubMed] [Google Scholar]
- [130].Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- [131].Kottler ML, Chauvin S, Lahlou N, Harris CE, Johnston CJ, Lagarde JP, Bouchard P, Farid NR, Counis R. A new compound heterozygous mutation of the gonadotropin-releasing hormone receptor (L314X, Q106R) in a woman with complete hypogonadotropic hypogonadism: chronic estrogen administration amplifies the gonadotropin defect. J Clin Endocrinol Metab. 2000;85:3002–3008. doi: 10.1210/jcem.85.9.6783. [DOI] [PubMed] [Google Scholar]
- [132].Kovacs P, Parlow AF, Karkanias GB. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology. 2002;76:357–365. doi: 10.1159/000067585. [DOI] [PubMed] [Google Scholar]
- [133].Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638–1651. doi: 10.1210/mend.16.7.0863. [DOI] [PubMed] [Google Scholar]
- [134].Kratzmeier M, Poch A, Mukhopadhyay AK, McArdle CA. Selective translocation of non-conventional protein kinase C isoenzymes by gonadotropin-releasing hormone (GnRH) in the gonadotrope-derived alpha T3-1 cell line. Mol Cell Endocrinol. 1996;118:103–111. doi: 10.1016/0303-7207(96)03788-4. [DOI] [PubMed] [Google Scholar]
- [135].Krey LC, Tyrey L, Everett JW. The estrogen-induced advance in the cyclic LH surge in the rat: dependency on ovarian progesterone secretion. Endocrinology. 1973;93:385–390. doi: 10.1210/endo-93-2-385. [DOI] [PubMed] [Google Scholar]
- [136].Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci U S A. 2003;100:2969–2974. doi: 10.1073/pnas.0535708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kumar TR, Agno J, Janovick JA, Conn PM, Matzuk MM. Regulation of FSHbeta and GnRH receptor gene expression in activin receptor II knockout male mice. Mol Cell Endocrinol. 2003;212:19–27. doi: 10.1016/j.mce.2003.09.019. [DOI] [PubMed] [Google Scholar]
- [138].Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- [139].Kuphal D, Janovick JA, Kaiser UB, Chin WW, Conn PM. Stable transfection of GH3 cells with rat gonadotropin-releasing hormone receptor complementary deoxyribonucleic acid results in expression of a receptor coupled to cyclic adenosine 3′,5′-monophosphate-dependent prolactin release via a G-protein. Endocrinology. 1994;135:315–320. doi: 10.1210/endo.135.1.8013367. [DOI] [PubMed] [Google Scholar]
- [140].Lake JI, Lange HS, O’Brien S, Sanford SE, Maney DL. Activity of the hypothalamic-pituitary-gonadal axis differs between behavioral phenotypes in female white-throated sparrows (Zonotrichia albicollis) Gen Comp Endocrinol. 2008;156:426–433. doi: 10.1016/j.ygcen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- [141].Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- [142].Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191. doi: 10.1210/me.2006-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Layman LC, Cohen DP, Jin M, Xie J, Li Z, Reindollar RH, Bolbolan S, Bick DP, Sherins RR, Duck LW, Musgrove LC, Sellers JC, Neill JD. Mutations in gonadotropin-releasing hormone receptor gene cause hypogonadotropic hypogonadism. Nat Genet. 1998;18:14–15. doi: 10.1038/ng0198-14. [DOI] [PubMed] [Google Scholar]
- [144].Layman LC, Lee EJ, Peak DB, Namnoum AB, Vu KV, van Lingen BL, Gray MR, McDonough PG, Reindollar RH, Jameson JL. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N Engl J Med. 1997;337:607–611. doi: 10.1056/NEJM199708283370905. [DOI] [PubMed] [Google Scholar]
- [145].Leanos-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–4828. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- [146].Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- [147].Lee T, Hoofnagle AN, Kabuyama Y, Stroud J, Min X, Goldsmith EJ, Chen L, Resing KA, Ahn NG. Docking motif interactions in MAP kinases revealed by hydrogen exchange mass spectrometry. Mol Cell. 2004;14:43–55. doi: 10.1016/s1097-2765(04)00161-3. [DOI] [PubMed] [Google Scholar]
- [148].Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- [149].Levi NL, Hanoch T, Benard O, Rozenblat M, Harris D, Reiss N, Naor Z, Seger R. Stimulation of Jun N-terminal kinase (JNK) by gonadotropin-releasing hormone in pituitary alpha T3-1 cell line is mediated by protein kinase C, c-Src, and CDC42. Mol Endocrinol. 1998;12:815–824. doi: 10.1210/mend.12.6.0120. [DOI] [PubMed] [Google Scholar]
- [150].Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- [151].Lim S, Pnueli L, Tan JH, Naor Z, Rajagopal G, Melamed P. Negative feedback governs gonadotrope frequency-decoding of gonadotropin releasing hormone pulse-frequency. PLoS One. 2009;4:e7244. doi: 10.1371/journal.pone.0007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lin X, Janovick JA, Brothers S, Blomenrohr M, Bogerd J, Conn PM. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998;12:161–171. doi: 10.1210/mend.12.2.0056. [DOI] [PubMed] [Google Scholar]
- [153].Lincoln GA, Clarke IJ. Absence of photoperiodic modulation of gonadotrophin secretion in HPD rams following chronic pulsatile infusion of GnRH. J Neuroendocrinol. 1998;10:461–471. doi: 10.1046/j.1365-2826.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- [154].Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology. 1998;139:4092–4101. doi: 10.1210/endo.139.10.6253. [DOI] [PubMed] [Google Scholar]
- [155].Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells. Mol Endocrinol. 2002;16:419–434. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- [156].Liu F, Austin DA, Webster NJ. Gonadotropin-releasing hormone-desensitized LbetaT2 gonadotrope cells are refractory to acute protein kinase C, cyclic AMP, and calcium-dependent signaling. Endocrinology. 2003;144:4354–4365. doi: 10.1210/en.2003-0204. [DOI] [PubMed] [Google Scholar]
- [157].Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lloyd JM, Childs GV. Differential storage and release of luteinizing hormone and follicle-releasing hormone from individual gonadotropes separated by centrifugal elutriation. Endocrinology. 1988;122:1282–1290. doi: 10.1210/endo-122-4-1282. [DOI] [PubMed] [Google Scholar]
- [159].Lopez FJ, Merchenthaler IJ, Moretto M, Negro-Vilar A. Modulating mechanisms of neuroendocrine cell activity: the LHRH pulse generator. Cell Mol Neurobiol. 1998;18:125–146. doi: 10.1023/A:1022531411717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Loumaye E, Catt KJ. Homologous regulation of gonadotropin-releasing hormone receptors in cultured pituitary cells. Science. 1982;215:983–985. doi: 10.1126/science.6296998. [DOI] [PubMed] [Google Scholar]
- [161].Lu ZL, Gallagher R, Sellar R, Coetsee M, Millar RP. Mutations remote from the human gonadotropin-releasing hormone (GnRH) receptor-binding sites specifically increase binding affinity for GnRH II but not GnRH I: evidence for ligand-selective, receptor-active conformations. J.Biol.Chem. 2005;280:29796–29803. doi: 10.1074/jbc.M413520200. [DOI] [PubMed] [Google Scholar]
- [162].Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- [163].Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- [164].Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- [166].MacConell LA, Lawson MA, Mellon PL, Roberts VJ. Activin A regulation of gonadotropin-releasing hormone synthesis and release in vitro. Neuroendocrinology. 1999;70:246–254. doi: 10.1159/000054483. [DOI] [PubMed] [Google Scholar]
- [167].MacEwan DJ, Johnson MS, Mitchell R. Protein kinase C isoforms in pituitary cells displaying differential sensitivity to phorbol ester. Mol Cell Biochem. 1999;202:85–90. doi: 10.1023/a:1007090718274. [DOI] [PubMed] [Google Scholar]
- [168].Mahesh VB, Muldoon TG, Smanik EJ, Young HK. Interaction between the ovaries and the hypothalamic pituitary axis in the regulation of gonadotropin secretion. Prog Clin Biol Res. 1982;112(Pt A):165–182. [PubMed] [Google Scholar]
- [169].Manna C, Rahman A, Sbracia M, Pappalardo S, Mohamed EI, Linder R, Nardo LG. Serum luteinizing hormone, follicle-stimulating hormone and oestradiol pattern in women undergoing pituitary suppression with different gonadotrophin-releasing hormone analogue protocols for assisted reproduction. Gynecol.Endocrinol. 2005;20:188–194. doi: 10.1080/09513590400027141. [DOI] [PubMed] [Google Scholar]
- [170].Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- [171].Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP. Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res. 1991;47:155–187. doi: 10.1016/b978-0-12-571147-0.50009-3. [DOI] [PubMed] [Google Scholar]
- [172].Marshall JC, Kelch RP. Gonadotropin-releasing hormone: role of pulsatile secretion in the regulation of reproduction. N Engl J Med. 1986;315:1459–1468. doi: 10.1056/NEJM198612043152306. [DOI] [PubMed] [Google Scholar]
- [173].Martelli AM, Gilmour RS, Bertagnolo V, Neri LM, Manzoli L, Cocco L. Nuclear localization and signalling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature. 1992;358:242–245. doi: 10.1038/358242a0. [DOI] [PubMed] [Google Scholar]
- [174].Mason AJ, Hayflick JS, Zoeller RT, Young WS, III, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- [175].Mason AJ, Pitts SL, Nikolics K, Szonyi E, Wilcox JN, Seeburg PH, Stewart TA. The hypogonadal mouse: reproductive functions restored by gene therapy. Science. 1986;234:1372–1378. doi: 10.1126/science.3097822. [DOI] [PubMed] [Google Scholar]
- [176].Mason DR, Arora KK, Mertz LM, Catt KJ. Homologous down-regulation of gonadotropin-releasing hormone receptor sites and messenger ribonucleic acid transcripts in alpha T3-1 cells. Endocrinology. 1994;135:1165–1170. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- [177].Maudsley S, Davidson L, Pawson AJ, Chan R, de Maturana R. Lopez, Millar RP. Gonadotropin-releasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Galphai-coupling state of the type I GnRH receptor. Cancer Res. 2004;64:7533–7544. doi: 10.1158/0008-5472.CAN-04-1360. [DOI] [PubMed] [Google Scholar]
- [178].Maudsley S, Naor Z, Bonfil D, Davidson L, Karali D, Pawson AJ, Larder R, Pope C, Nelson N, Millar RP, Brown P. Proline-rich tyrosine kinase 2 mediates gonadotropin-releasing hormone signaling to a specific extracellularly regulated kinase-sensitive transcriptional locus in the luteinizing hormone beta-subunit gene. Mol Endocrinol. 2007;21:1216–1233. doi: 10.1210/me.2006-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouyssegur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- [180].McArdle CA, Davidson JS, Willars GB. The tail of the gonadotrophin-releasing hormone receptor: desensitization at, and distal to, G protein-coupled receptors. Mol Cell Endocrinol. 1999;151:129–136. doi: 10.1016/s0303-7207(99)00024-6. [DOI] [PubMed] [Google Scholar]
- [181].McArdle CA, Willars GB, Fowkes RC, Nahorski SR, Davidson JS, Forrest-Owen W. Desensitization of gonadotropin-releasing hormone action in alphaT3-1 cells due to uncoupling of inositol 1,4,5-trisphosphate generation and Ca2+ mobilization. J Biol Chem. 1996;271:23711–23717. doi: 10.1074/jbc.271.39.23711. [DOI] [PubMed] [Google Scholar]
- [182].McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Franklin RF, Steelman LS. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul. 2009 doi: 10.1016/j.advenzreg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- [184].McLaughlin NJ, Banerjee A, Khan SY, Lieber JL, Kelher MR, Gamboni-Robertson F, Sheppard FR, Moore EE, Mierau GW, Elzi DJ, Silliman CC. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J Immunol. 2008;180:8192–8203. doi: 10.4049/jimmunol.180.12.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Meczekalski B, Podfigurna-Stopa A, Warenik-Szymankiewicz A, Genazzani AR. Functional hypothalamic amenorrhea: current view on neuroendocrine aberrations. Gynecol Endocrinol. 2008;24:4–11. doi: 10.1080/09513590701807381. [DOI] [PubMed] [Google Scholar]
- [186].Melamed P. Hormonal signaling to follicle stimulating hormone beta-subunit gene expression. Mol.Cell Endocrinol. 2010;314:204–212. doi: 10.1016/j.mce.2009.05.012. [DOI] [PubMed] [Google Scholar]
- [187].Melrose P, Gross L, Cruse I, Rush M. Isolated gonadotropin-releasing hormone neurons harvested from adult male rats secrete biologically active neuropeptide in a regular repetitive manner. Endocrinology. 1987;121:182–189. doi: 10.1210/endo-121-1-182. [DOI] [PubMed] [Google Scholar]
- [188].Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- [189].Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- [190].Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- [191].Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- [192].Mitchell R, Sim PJ, Leslie T, Johnson MS, Thomson FJ. Activation of MAP kinase associated with the priming effect of LHRH. J.Endocrinol. 1994;140:R15–R18. doi: 10.1677/joe.0.140r015. [DOI] [PubMed] [Google Scholar]
- [193].Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- [194].Mulvaney JM, Roberson MS. Divergent signaling pathways requiring discrete calcium signals mediate concurrent activation of two mitogen-activated protein kinases by gonadotropin-releasing hormone. J Biol Chem. 2000;275:14182–14189. doi: 10.1074/jbc.275.19.14182. [DOI] [PubMed] [Google Scholar]
- [195].Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem. 1999;274:29796–29804. doi: 10.1074/jbc.274.42.29796. [DOI] [PubMed] [Google Scholar]
- [196].Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- [197].Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, Parducz A. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- [198].Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- [199].Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [200].Naor Z, Benard O, Seger R. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab. 2000;11:91–99. doi: 10.1016/s1043-2760(99)00232-5. [DOI] [PubMed] [Google Scholar]
- [201].Nasonkin IO, Potok MA, Camper SA. Cre-mediated recombination in pituitary somatotropes. Genesis. 2009;47:55–60. doi: 10.1002/dvg.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278:31593–31602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- [203].Nekrasova T, Shive C, Gao Y, Kawamura K, Guardia R, Landreth G, Forsthuber TG. ERK1-deficient mice show normal T cell effector function and are highly susceptible to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:2374–2380. doi: 10.4049/jimmunol.175.4.2374. [DOI] [PubMed] [Google Scholar]
- [204].Nett TM, Crowder ME, Moss GE, Duello TM. GnRH-receptor interaction. V. Down-regulation of pituitary receptors for GnRH in ovariectomized ewes by infusion of homologous hormone. Biol Reprod. 1981;24:1145–1155. [PubMed] [Google Scholar]
- [205].Norman RL, Spies HG. Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology. 1986;118:2608–2610. doi: 10.1210/endo-118-6-2608. [DOI] [PubMed] [Google Scholar]
- [206].Norwitz ER, Xu S, Jeong KH, Bedecarrats GY, Winebrenner LD, Chin WW, Kaiser UB. Activin A augments GnRH-mediated transcriptional activation of the mouse GnRH receptor gene. Endocrinology. 2002;143:985–997. doi: 10.1210/endo.143.3.8663. [DOI] [PubMed] [Google Scholar]
- [207].Norwitz ER, Xu S, Xu J, Spiryda LB, Park JS, Jeong KH, McGee EA, Kaiser UB. Direct binding of AP-1 (Fos/Jun) proteins to a SMAD binding element facilitates both gonadotropin-releasing hormone (GnRH)- and activin-mediated transcriptional activation of the mouse GnRH receptor gene. J Biol Chem. 2002;277:37469–37478. doi: 10.1074/jbc.M206571200. [DOI] [PubMed] [Google Scholar]
- [208].Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- [209].Pawson AJ, Faccenda E, Maudsley S, Lu ZL, Naor Z, Millar RP. Mammalian type I gonadotropin-releasing hormone receptors undergo slow, constitutive, agonist-independent internalization. Endocrinology. 2008;149:1415–1422. doi: 10.1210/en.2007-1159. [DOI] [PubMed] [Google Scholar]
- [210].Pearson G, Robinson F, Gibson T. Beers, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- [211].Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- [213].Petersen SL, McCrone S, Keller M, Shores S. Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology. 1995;136:3604–3610. doi: 10.1210/endo.136.8.7628399. [DOI] [PubMed] [Google Scholar]
- [214].Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- [215].Pfleger KD, Pawson AJ, Millar RP. Changes to gonadotropin-releasing hormone (GnRH) receptor extracellular loops differentially affect GnRH analog binding and activation: evidence for distinct ligand-stabilized receptor conformations. Endocrinology. 2008;149:3118–3129. doi: 10.1210/en.2008-0002. [DOI] [PubMed] [Google Scholar]
- [216].Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF, Jr., Hayes FJ. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab. 2008;93:2686–2692. doi: 10.1210/jc.2007-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Poulin B, Rich N, Mas JL, Kordon C, Enjalbert A, Drouva SV. GnRH signalling pathways and GnRH-induced homologous desensitization in a gonadotrope cell line (alphaT3-1) Mol Cell Endocrinol. 1998;142:99–117. doi: 10.1016/s0303-7207(98)00114-2. [DOI] [PubMed] [Google Scholar]
- [218].Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Rao IM, Mahesh VB. Role of progesterone in the modulation of the preovulatory surge of gonadotropins and ovulation in the pregnant mare’s serum gonadotropin-primed immature rat and the adult rat. Biol.Reprod. 1986;35:1154–1161. doi: 10.1095/biolreprod35.5.1154. [DOI] [PubMed] [Google Scholar]
- [220].Reame N, Sauder SE, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J Clin Endocrinol Metab. 1984;59:328–337. doi: 10.1210/jcem-59-2-328. [DOI] [PubMed] [Google Scholar]
- [221].Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851–858. doi: 10.1210/jcem-61-5-851. [DOI] [PubMed] [Google Scholar]
- [222].Reinhart J, Mertz LM, Catt KJ. Molecular cloning and expression of cDNA encoding the murine gonadotropin-releasing hormone receptor. J Biol Chem. 1992;267:21281–21284. [PubMed] [Google Scholar]
- [223].Reiss N, Llevi LN, Shacham S, Harris D, Seger R, Naor Z. Mechanism of mitogen-activated protein kinase activation by gonadotropin-releasing hormone in the pituitary of alphaT3-1 cell line: differential roles of calcium and protein kinase C. Endocrinology. 1997;138:1673–1682. doi: 10.1210/endo.138.4.5057. [DOI] [PubMed] [Google Scholar]
- [224].Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [225].Richter TA, Robinson JE, Evans NP. Progesterone blocks the estradiol-stimulated luteinizing hormone surge by disrupting activation in response to a stimulatory estradiol signal in the ewe. Biol Reprod. 2002;67:119–125. doi: 10.1095/biolreprod67.1.119. [DOI] [PubMed] [Google Scholar]
- [226].Rispoli LA, Nett TM. Pituitary gonadotropin-releasing hormone (GnRH) receptor: structure, distribution and regulation of expression. Anim Reprod Sci. 2005;88:57–74. doi: 10.1016/j.anireprosci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [227].Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol. 2005;19:2412–2423. doi: 10.1210/me.2005-0094. [DOI] [PubMed] [Google Scholar]
- [228].Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA. A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol. 1995;15:3531–3539. doi: 10.1128/mcb.15.7.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Roberson MS, Zhang T, Li HL, Mulvaney JM. Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology. 1999;140:1310–1318. doi: 10.1210/endo.140.3.6579. [DOI] [PubMed] [Google Scholar]
- [230].Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- [231].Roberts VJ, Peto CA, Vale W, Sawchenko PE. Inhibin/activin subunits are costored with FSH and LH in secretory granules of the rat anterior pituitary gland. Neuroendocrinology. 1992;56:214–224. doi: 10.1159/000126231. [DOI] [PubMed] [Google Scholar]
- [232].Robertson DM, Hayward S, Irby D, Jacobsen J, Clarke L, McLachlan RI, de Kretser DM. Radioimmunoassay of rat serum inhibin: changes after PMSG stimulation and gonadectomy. Mol Cell Endocrinol. 1988;58:1–8. doi: 10.1016/0303-7207(88)90047-0. [DOI] [PubMed] [Google Scholar]
- [233].Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem. 2003;278:47307–47318. doi: 10.1074/jbc.M304377200. [DOI] [PubMed] [Google Scholar]
- [234].Romano D, Pertuit M, Rasolonjanahary R, Barnier JV, Magalon K, Enjalbert A, Gerard C. Regulation of the RAP1/RAF-1/extracellularly regulated kinase-1/2 cascade and prolactin release by the phosphoinositide 3-kinase/AKT pathway in pituitary cells. Endocrinology. 2006;147:6036–6045. doi: 10.1210/en.2006-0325. [DOI] [PubMed] [Google Scholar]
- [235].Rosenfeld MG, Briata P, Dasen J, Gleiberman AS, Kioussi C, Lin C, O’Connell SM, Ryan A, Szeto DP, Treier M. Multistep signaling and transcriptional requirements for pituitary organogenesis in vivo. Recent Prog Horm Res. 2000;55:1–13. [PubMed] [Google Scholar]
- [236].Rothman MS, Wierman ME. The role of gonadotropin releasing hormone in normal and pathologic endocrine processes. Curr Opin Endocrinol Diabetes Obes. 2007;14:306–310. doi: 10.1097/MED.0b013e3281e2c9fc. [DOI] [PubMed] [Google Scholar]
- [237].Roy F, Laberge G, Douziech M, Ferland-McCollough D, Therrien M. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 2002;16:427–438. doi: 10.1101/gad.962902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- [239].Sakai R, Shiozaki M, Tabuchi M, Eto Y. The measurement of activin/EDF in mouse serum: evidence for extragonadal production. Biochem Biophys Res Commun. 1992;188:921–926. doi: 10.1016/0006-291x(92)91143-e. [DOI] [PubMed] [Google Scholar]
- [240].Salenave S, Chanson P, Bry H, Pugeat M, Cabrol S, Carel JC, Murat A, Lecomte P, Brailly S, Hardelin JP, Dode C, Young J. Kallmann’s syndrome: a comparison of the reproductive phenotypes in men carrying KAL1 and FGFR1/KAL2 mutations. J Clin Endocrinol Metab. 2008;93:758–763. doi: 10.1210/jc.2007-1168. [DOI] [PubMed] [Google Scholar]
- [241].Salisbury TB, Binder AK, Nilson JH. Welcoming beta-catenin to the gonadotropin-releasing hormone transcriptional network in gonadotropes. Mol Endocrinol. 2008;22:1295–1303. doi: 10.1210/me.2007-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Salojin K, Oravecz T. Regulation of innate immunity by MAPK dual-specificity phosphatases: knockout models reveal new tricks of old genes. J Leukoc Biol. 2007;81:860–869. doi: 10.1189/jlb.1006639. [DOI] [PubMed] [Google Scholar]
- [243].Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23:991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- [244].Schafer B, Marg B, Gschwind A, Ullrich A. Distinct ADAM metalloproteinases regulate G protein-coupled receptor-induced cell proliferation and survival. J Biol Chem. 2004;279:47929–47938. doi: 10.1074/jbc.M400129200. [DOI] [PubMed] [Google Scholar]
- [245].Schmitt JM, Stork PJ. beta 2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf. J Biol Chem. 2000;275:25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- [246].Schneider O, Nau R, Michel U. Comparative analysis of follistatin-, activin beta A- and activin beta B-mRNA steady-state levels in diverse porcine tissues by multiplex S1 nuclease analysis. Eur J Endocrinol. 2000;142:537–544. doi: 10.1530/eje.0.1420537. [DOI] [PubMed] [Google Scholar]
- [247].Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- [248].Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- [249].Sebolt-Leopold JS. Development of anticancer drugs targeting the MAP kinase pathway. Oncogene. 2000;19:6594–6599. doi: 10.1038/sj.onc.1204083. [DOI] [PubMed] [Google Scholar]
- [250].Shah BH, MacEwan DJ, Milligan G. Gonadotrophin-releasing hormone receptor agonist-mediated down-regulation of Gq alpha/G11 alpha (pertussis toxin-insensitive) G proteins in alpha T3-1 gonadotroph cells reflects increased G protein turnover but not alterations in mRNA levels. Proc Natl Acad Sci U S A. 1995;92:1886–1890. doi: 10.1073/pnas.92.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Shah BH, Milligan G. The gonadotrophin-releasing hormone receptor of alpha T3-1 pituitary cells regulates cellular levels of both of the phosphoinositidase C-linked G proteins, Gq alpha and G11 alpha, equally. Mol Pharmacol. 1994;46:1–7. [PubMed] [Google Scholar]
- [252].Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- [253].Sim P, Mitchell R, Thorfinn L. Activation of MAP kinase in alpha T3-1 cells by luteinising hormone-releasing hormone. Biochem Soc Trans. 1993;21:357S. doi: 10.1042/bst021357s. [DOI] [PubMed] [Google Scholar]
- [254].Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [255].Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- [256].Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142:573–579. doi: 10.1210/endo.142.2.7956. [DOI] [PubMed] [Google Scholar]
- [257].Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- [259].Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- [260].Soussi-Yanicostas N, de Castro F, Julliard AK, Perfettini I, Chedotal A, Petit C. Anosmin-1, defective in the X-linked form of Kallmann syndrome, promotes axonal branch formation from olfactory bulb output neurons. Cell. 2002;109:217–228. doi: 10.1016/s0092-8674(02)00713-4. [DOI] [PubMed] [Google Scholar]
- [261].Stanislaus D, Janovick JA, Brothers S, Conn PM. Regulation of G(q/11)alpha by the gonadotropin-releasing hormone receptor. Mol Endocrinol. 1997;11:738–746. doi: 10.1210/mend.11.6.0005. [DOI] [PubMed] [Google Scholar]
- [262].Stanislaus D, Janovick JA, Ji T, Wilkie TM, Offermanns S, Conn PM. Gonadotropin and gonadal steroid release in response to a gonadotropin-releasing hormone agonist in Gqalpha and G11alpha knockout mice. Endocrinology. 1998;139:2710–2717. doi: 10.1210/endo.139.6.5942. [DOI] [PubMed] [Google Scholar]
- [263].Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–499. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- [264].Sturgill TW, Ray LB. Muscle proteins related to microtubule associated protein-2 are substrates for an insulin-stimulatable kinase. Biochem Biophys Res Commun. 1986;134:565–571. doi: 10.1016/s0006-291x(86)80457-0. [DOI] [PubMed] [Google Scholar]
- [265].Sun YM, Flanagan CA, Illing N, Ott TR, Sellar R, Fromme BJ, Hapgood J, Sharp P, Sealfon SC, Millar RP. A chicken gonadotropin-releasing hormone receptor that confers agonist activity to mammalian antagonists. Identification of D-Lys(6) in the ligand and extracellular loop two of the receptor as determinants. J.Biol.Chem. 2001;276:7754–7761. doi: 10.1074/jbc.M009020200. [DOI] [PubMed] [Google Scholar]
- [266].Sundaresan S, Colin IM, Pestell RG, Jameson JL. Stimulation of mitogen-activated protein kinase by gonadotropin-releasing hormone: evidence for the involvement of protein kinase C. Endocrinology. 1996;137:304–311. doi: 10.1210/endo.137.1.8536629. [DOI] [PubMed] [Google Scholar]
- [267].Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- [268].Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol.Cell Endocrinol. 2010;314:192–203. doi: 10.1016/j.mce.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- [270].Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod. 2001;64:735–742. doi: 10.1095/biolreprod64.3.735. [DOI] [PubMed] [Google Scholar]
- [271].Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- [272].Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- [273].Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:D1134–D1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- [274].Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res. 2004;94:1133–1141. doi: 10.1161/01.RES.0000126048.32383.6B. [DOI] [PubMed] [Google Scholar]
- [275].Torii S, Kusakabe M, Yamamoto T, Maekawa M, Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7:33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- [276].Tse A, Tse FW, Almers W, Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993;260:82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- [277].Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- [278].Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56:729–737. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- [280].Tuuri T, Eramaa M, Hilden K, Ritvos O. The tissue distribution of activin beta A- and beta B-subunit and follistatin messenger ribonucleic acids suggests multiple sites of action for the activin-follistatin system during human development. J Clin Endocrinol Metab. 1994;78:1521–1524. doi: 10.1210/jcem.78.6.8200957. [DOI] [PubMed] [Google Scholar]
- [281].Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol. 2006;5:14. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus SM. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- [283].Vrecl M, Heding A, Hanyaloglu A, Taylor PL, Eidne KA. Internalization kinetics of the gonadotropin-releasing hormone (GnRH) receptor. Pflugers Arch. 2000;439:R19–R20. [PubMed] [Google Scholar]
- [284].Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149:5577–5591. doi: 10.1210/en.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet. 1998;14:284–290. doi: 10.1016/s0168-9525(98)01476-0. [DOI] [PubMed] [Google Scholar]
- [286].Weiss J, Crowley WF, Jr., Halvorson LM, Jameson JL. Perifusion of rat pituitary cells with gonadotropin-releasing hormone, activin, and inhibin reveals distinct effects on gonadotropin gene expression and secretion. Endocrinology. 1993;132:2307–2311. doi: 10.1210/endo.132.6.8504735. [DOI] [PubMed] [Google Scholar]
- [287].Welch WJ, Howard M. Antagonists to the rescue. J Clin Invest. 2000;105:853–854. doi: 10.1172/JCI9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–2711. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- [289].Werry TD, Christopoulos A, Sexton PM. Mechanisms of ERK1/2 regulation by seven-transmembrane-domain receptors. Curr Pharm Des. 2006;12:1683–1702. doi: 10.2174/138161206776873725. [DOI] [PubMed] [Google Scholar]
- [290].White BR, Duval DL, Mulvaney JM, Roberson MS, Clay CM. Homologous regulation of the gonadotropin-releasing hormone receptor gene is partially mediated by protein kinase C activation of an activator protein-1 element. Mol Endocrinol. 1999;13:566–577. doi: 10.1210/mend.13.4.0262. [DOI] [PubMed] [Google Scholar]
- [291].Willars GB, Heding A, Vrecl M, Sellar R, Blomenrohr M, Nahorski SR, Eidne KA. Lack of a C-terminal tail in the mammalian gonadotropin-releasing hormone receptor confers resistance to agonist-dependent phosphorylation and rapid desensitization. J Biol Chem. 1999;274:30146–30153. doi: 10.1074/jbc.274.42.30146. [DOI] [PubMed] [Google Scholar]
- [292].Willars GB, Royall JE, Nahorski SR, El-Gehani F, Everest H, McArdle CA. Rapid down-regulation of the type I inositol 1,4,5-trisphosphate receptor and desensitization of gonadotropin-releasing hormone-mediated Ca2+ responses in alpha T3-1 gonadotropes. J Biol Chem. 2001;276:3123–3129. doi: 10.1074/jbc.M008916200. [DOI] [PubMed] [Google Scholar]
- [293].Wilson ME, Handa RJ. Activin subunit, follistatin, and activin receptor gene expression in the prepubertal female rat pituitary. Biol Reprod. 1998;59:278–283. doi: 10.1095/biolreprod59.2.278. [DOI] [PubMed] [Google Scholar]
- [294].Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- [295].Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol. 1999;13:752–763. doi: 10.1210/mend.13.5.0276. [DOI] [PubMed] [Google Scholar]
- [297].Wu JC, Su P, Safwat NW, Sebastian J, Miller WL. Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology. 2004;145:5832–5839. doi: 10.1210/en.2004-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Wu T, Mohan C. The AKT axis as a therapeutic target in autoimmune diseases. Endocr Metab Immune Disord Drug Targets. 2009;9:145–150. doi: 10.2174/187153009788452417. [DOI] [PubMed] [Google Scholar]
- [299].Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276:47195–47201. doi: 10.1074/jbc.M108716200. [DOI] [PubMed] [Google Scholar]
- [300].Xie J, Allen KH, Marguet A, Berghorn KA, Bliss SP, Navratil AM, Guan JL, Roberson MS. Analysis of the calcium-dependent regulation of proline-rich tyrosine kinase 2 by gonadotropin-releasing hormone. Mol Endocrinol. 2008;22:2322–2335. doi: 10.1210/me.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol. 2005;19:2624–2638. doi: 10.1210/me.2005-0056. [DOI] [PubMed] [Google Scholar]
- [302].Xu A, Suh PG, Marmy-Conus N, Pearson RB, Seok OY, Cocco L, Gilmour RS. Phosphorylation of nuclear phospholipase C beta1 by extracellular signal-regulated kinase mediates the mitogenic action of insulin-like growth factor I. Mol Cell Biol. 2001;21:2981–2990. doi: 10.1128/MCB.21.9.2981-2990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Yao Y, Li W, Wu J, Germann UA, Su MS, Kuida K, Boucher DM. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci U S A. 2003;100:12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Yin P, Kawashima K, Arita J. Direct actions of estradiol on the anterior pituitary gland are required for hypothalamus-dependent lactotrope proliferation and secretory surges of luteinizing hormone but not of prolactin in female rats. Neuroendocrinology. 2002;75:392–401. doi: 10.1159/000059436. [DOI] [PubMed] [Google Scholar]
- [305].Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- [306].Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- [307].Zhang H, Bailey JS, Coss D, Lin B, Tsutsumi R, Lawson MA, Mellon PL, Webster NJ. Activin modulates the transcriptional response of LbetaT2 cells to gonadotropin-releasing hormone and alters cellular proliferation. Mol.Endocrinol. 2006;20:2909–2930. doi: 10.1210/me.2006-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Zhang T, Roberson MS. Role of MAP kinase phosphatases in GnRH-dependent activation of MAP kinases. J Mol Endocrinol. 2006;36:41–50. doi: 10.1677/jme.1.01881. [DOI] [PubMed] [Google Scholar]
- [309].Zhang T, Wolfe MW, Roberson MS. An early growth response protein (Egr) 1 cis-element is required for gonadotropin-releasing hormone-induced mitogen-activated protein kinase phosphatase 2 gene expression. J Biol Chem. 2001;276:45604–45613. doi: 10.1074/jbc.M107075200. [DOI] [PubMed] [Google Scholar]
- [310].Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–154. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]
- [311].Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87:933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
- [312].Zoeller RT, Seeburg PH, Young WS., III In situ hybridization histochemistry for messenger ribonucleic acid (mRNA) encoding gonadotropin-releasing hormone (GnRH): effect of estrogen on cellular levels of GnRH mRNA in female rat brain. Endocrinology. 1988;122:2570–2577. doi: 10.1210/endo-122-6-2570. [DOI] [PubMed] [Google Scholar]