Abstract
The molecular mechanisms responsible for selenium (Se) tolerance and hyperaccumulation were studied in the Se hyperaccumulator Stanleya pinnata (Brassicaceae) by comparing it with the related secondary Se accumulator Stanleya albescens using a combination of physiological, structural, genomic, and biochemical approaches. S. pinnata accumulated 3.6-fold more Se and was tolerant to 20 μm selenate, while S. albescens suffered reduced growth, chlorosis and necrosis, impaired photosynthesis, and high levels of reactive oxygen species. Levels of ascorbic acid, glutathione, total sulfur, and nonprotein thiols were higher in S. pinnata, suggesting that Se tolerance may in part be due to increased antioxidants and up-regulated sulfur assimilation. S. pinnata had higher selenocysteine methyltransferase protein levels and, judged from liquid chromatography-mass spectrometry, mainly accumulated the free amino acid methylselenocysteine, while S. albescens accumulated mainly the free amino acid selenocystathionine. S. albescens leaf x-ray absorption near-edge structure scans mainly detected a carbon-Se-carbon compound (presumably selenocystathionine) in addition to some selenocysteine and selenate. Thus, S. albescens may accumulate more toxic forms of Se in its leaves than S. pinnata. The species also showed different leaf Se sequestration patterns: while S. albescens showed a diffuse pattern, S. pinnata sequestered Se in localized epidermal cell clusters along leaf margins and tips, concentrated inside of epidermal cells. Transcript analyses of S. pinnata showed a constitutively higher expression of genes involved in sulfur assimilation, antioxidant activities, defense, and response to (methyl)jasmonic acid, salicylic acid, or ethylene. The levels of some of these hormones were constitutively elevated in S. pinnata compared with S. albescens, and leaf Se accumulation was slightly enhanced in both species when these hormones were supplied. Thus, defense-related phytohormones may play an important signaling role in the Se hyperaccumulation of S. pinnata, perhaps by constitutively up-regulating sulfur/Se assimilation followed by methylation of selenocysteine and the targeted sequestration of methylselenocysteine.
Selenium (Se) is an essential micronutrient for many organisms, but for higher plants an essential function of Se has not yet been discovered (Zhang and Gladyshev, 2010). Most plant species accumulate less than 25 μg Se g −1 dry weight in their natural environment and cannot tolerate increased Se concentrations; these are termed nonaccumulators (White et al., 2004). In contrast, some species of the genera Stanleya (Brassicaceae) and Astragalus (Fabaceae) can hyperaccumulate Se to concentrations of 1,000 to 15,000 μg Se g−1 dry weight in their shoots (0.1%–1.5%) while growing on soils containing only 2 to 10 μg Se g−1 dry weight (Byers, 1935; Virupaksha and Shrift, 1965; Davis, 1972, 1986; Galeas et al., 2007). Hyperaccumulation is a phenomenon where plants accumulate metals or metalloids to much higher concentrations compared with nonaccumulator plants, typically more than 100-fold when growing in their natural habitat on metalliferous soils (Minguzzi and Vergnano, 1948; Jaffré et al., 1976; Brooks et al., 1977). Metals that can be hyperaccumulated by plants include nickel, zinc, cobalt, chromium, molybdenum, cadmium, arsenic, and Se (Reeves and Baker, 2000). To date, 45 plant families are documented to contain hyperaccumulators, and at least 200 metal-hyperaccumulating species have evolved worldwide (Reeves and Baker, 2000). Hyperaccumulators typically have shoot-to-root metal ratios greater than 1, and their leaves often show the highest metal concentration inside specific tissues such as epidermal cells or leaf hairs and most often inside large or small vacuoles (Krämer et al., 1997; Küpper et al., 1999, 2000, 2001; Pickering et al., 2001, 2003a; Freeman et al., 2006b; for review, see Peer et al., 2005). Both metal tolerance and hyperaccumulation are considered prerequisites for metal hyperaccumulation in plants, and these molecular mechanisms have been found to be under independent genetic control (Macnair, 1993; Macnair et al., 1999; Bert et al., 2000; Assuncao et al., 2001).
Environmental factors influencing the evolution of elemental hyperaccumulation in plants have been hypothesized to be metal tolerance, allelopathy, drought tolerance, or protection from herbivores and pathogens (Tadros, 1957; Reeves and Brooks, 1983; Baker and Brooks, 1989; Boyd and Martens, 1992; Baker and Whiting, 2002; Boyd, 2007). Depending on the plant species and the metal, different selection pressures may have resulted in the hyperaccumulator phenotype. Most studies investigating the effect of Se hyperaccumulation on herbivores and pathogens have supported a function for Se in defense (Pilon-Smits and Freeman, 2006; Boyd, 2007; Quinn et al., 2007). This phenomenon is termed the elemental defense hypothesis and has been shown in a wide range of hyperaccumulator species for many different metals (Boyd and Martens, 1992; Boyd, 2007). The hyperaccumulators Astragalus bisulcatus and Stanleya pinnata were found to predominantly accumulate Se in peripheral tissues of young leaves and reproductive organs: in structures important to protect, prone to herbivore and pathogen attacks, and/or typically associated with playing a defensive role (Pickering et al., 2003a; Freeman et al., 2006b; Galeas et al., 2007). Indeed, laboratory and field studies have shown that Se can protect plants from various herbivores and fungal pathogens (Vickerman and Trumble, 1999; Bañuelos et al., 2002; Hanson et al., 2003, 2004; Freeman et al., 2006a, 2007, 2009; Galeas et al., 2008; Quinn et al., 2008).
Se is chemically similar to sulfur (S) and is assimilated by S metabolic pathways. Plants take up selenate (SeO42−), the most abundant bioavailable form of Se in soils, via sulfate transporters in roots (Shibagaki et al., 2002; Ellis and Salt, 2003). After uptake, selenate is thought to be transported into leaves and chloroplasts (Leustek, 2002). Indistinguishable to most S enzymes, Se can be found in most S-containing metabolites (Leustek, 2002). In chloroplasts, the S assimilation pathway first reduces selenate to selenite, then selenide, which is enzymatically incorporated into selenocysteine (SeCys) and selenomethionine (SeMet; for review, see Terry et al., 2000; Ellis and Salt, 2003; Sors et al., 2005b). These two seleno-amino acids can be misincorporated into proteins, replacing Cys and Met, which causes Se toxicity (Brown and Shrift, 1981, 1982; Stadtman, 1996). It has also been shown that Se causes reactive oxygen species (ROS) formation and oxidative stress in plants (Gomes-Junior et al., 2007; Tamaoki et al., 2008b). Se toxicity in plants may directly result from the ROS generated via selenite reacting with reduced glutathione (GSH), as shown in vitro (Saitoh and Imuran, 1987). Alternatively, Se toxicity could result if Se replaces S in essential S metabolites, including proteins.
A key enzyme in Se hyperaccumulators is selenocysteine methyltransferase (SMT), which methylates SeCys and prevents it from being misincorporated into proteins, thereby preventing toxicity (Brown and Shrift, 1981, 1982; Neuhierl and Bock, 1996). Thus, the hyperaccumulator A. bisulcatus rapidly converts selenate to methylselenocysteine (MeSeCys; Virupaksha and Shrift, 1965; Dunnill and Fowden, 1967) and γ-glutamyl-methylselenocysteine (Nigam and McConnell, 1969; Freeman et al., 2006b). The gene encoding SMT was cloned from A. bisulcatus (Neuhierl and Bock, 1996) and overexpressed in Arabidopsis (Arabidopsis thaliana) and Brassica juncea; this led to enhanced Se tolerance and accumulation when plants were given selenite (SeO32−) but not when given selenate (Ellis et al., 2004; LeDuc et al., 2004). Total Se accumulation in the SMT transgenic plants was much lower than commonly found in hyperaccumulators, which suggests that SMT is an important enzyme for hyperaccumulation and tolerance, but additional processes are likely involved that have a synergistic effect on Se hyperaccumulation. Sors et al. (2005a) found no evidence that enzymatic differences in the capacity to reduce or assimilate S is important for Se hyperaccumulation in Astragalus, while SMT enzyme activity correlated with Se hyperaccumulation. Additionally, a nonaccumulator SMT homolog lacked the SMT activity in vitro, explaining why little or no detectable MeSeCys accumulation was observed in the nonaccumulator species (Sors et al., 2009). The SMT enzyme is now known to be localized predominantly within the chloroplast in Astragalus, the principal site of Se and S assimilation in plants (Sors et al., 2009). Like A. bisulcatus, the Brassicaceae hyperaccumulator S. pinnata accumulates mainly MeSeCys (Shrift and Virupaksha, 1965; Freeman et al., 2006b). In addition to the specific methylation of SeCys by SMT, it is not clear at this point which mechanisms may contribute to Se hyperaccumulation and tolerance in A. bisulcatus or other hyperaccumulators (Sors et al., 2005a, 2005b, 2009). Se hyperaccumulators do possess some unexplained physiological traits associated with growing on Se-enriched soils. S. pinnata roots have been reported to grow toward Se-rich soil in split-box experiments, and Astragalus species have been documented to grow slower and are smaller in the absence of Se in soils (Trelease and Trelease, 1938; Trelease and Beath 1949; Goodson et al., 2003).
There is substantial variation in Se accumulation (ranging from hyperaccumulation to Se secondary accumulation to Se nonaccumulation) and Se tolerance between and within Stanleya species (Beath et al., 1939; Feist and Parker, 2001; Parker et al., 2003), making it an ideal genus to compare molecular mechanisms involved in Se hyperaccumulation and tolerance. In this study, we compare Se tolerance and hyperaccumulation between the hyperaccumulator S. pinnata var pinnata (prince's plume) and the secondary accumulator Stanleya albescens (white prince's plume). We investigated the molecular mechanisms responsible for Se tolerance and hyperaccumulation using a combination of molecular, structural, genomic, biochemical, and physiological analyses. Together, our new findings give better insight into the underlying molecular mechanisms associated with Se hyperaccumulation in S. pinnata.
RESULTS
Se Tolerance Assay
Se tolerance in S. pinnata and S. albescens was first tested in seedlings by measuring root growth on vertically placed agar plates after 30 d of growth from germination (Supplemental Fig. S1). The relative tolerance index was calculated by dividing root length of seedlings grown with 40 μm selenate by the average root length of the same species grown without Se. The relative tolerance index was 2.6 for the Se hyperaccumulator S. pinnata and 0.6 for S. albescens. Thus, S. pinnata appeared to benefit from 40 μm selenate in its growth medium (P < 0.01), while S. albescens was impaired by it (P = 0.063). When grown with 40 μm selenate, S. pinnata roots were 1.6 times longer than S. albescens roots (P < 0.05). When grown without Se, the roots of S. pinnata were 2.6-fold shorter than those of S. albescens (P < 0.05).
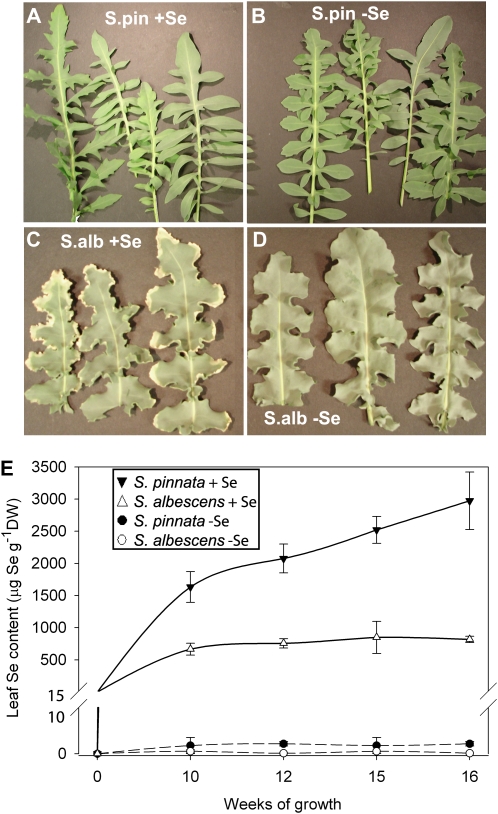
Se tolerance of the two species was then compared in more mature plants. After 16 weeks of semiarid drip system growth in the presence of 20 μm selenate, S. pinnata showed no signs of Se toxicity (Fig. 1A) but had the same phenotype as S. pinnata grown without Se (Fig. 1B). However, S. albescens grown with Se showed visible leaf chlorosis and necrosis (Fig. 1C). This phenotype was not present in S. albescens grown without selenate (Fig. 1D). Thus, both at the seedling and mature plant level, S. pinnata was completely tolerant to, or even benefited from, 20 μm selenate, while S. albescens was sensitive to this Se treatment.
Figure 1.
A to D, Photographs of leaves showing the health of each test group after 16 weeks of growth: S. pinnata + Se (A), S. pinnata – Se (B), S. albescens + Se (C), S. albescens – Se (D). E, Se accumulation in leaves after 16 weeks of growth with and without 20 μm selenate. S. pinnata + Se, black triangles; S. albescens + Se, white triangles; S. pinnata – Se, black circles; S. albescens – Se, white circles (n = 6 ± se). DW, Dry weight.
Se and S Accumulation
After 10 weeks of growth from germination in the presence of 20 μm selenate, S. pinnata had 2.6-fold higher levels of Se in its leaves than S. albescens, and after 16 weeks, S. pinnata leaf Se levels were 3.6-fold higher than those in S. albescens (2,973 ± 446 and 818 ± 49 μg Se g−1 dry weight, respectively; Fig. 1E). After 16 weeks of growth with Se, the roots of S. pinnata contained 721 μg Se g−1 dry weight, a 3.6-fold higher Se concentration compared with the roots of S. albescens, which contained 201 μg Se g−1 dry weight.
These Se levels in S. pinnata confirm that this species is a Se hyperaccumulator and are comparable to those found in its native habitat on seleniferous soil west of Fort Collins, Colorado, where S. pinnata leaves from 10 individuals averaged 3,775 ± 506 μg Se g−1 dry weight (Galeas et al., 2007). S. albescens does not appear to be a hyperaccumulator, based on these results and from field collection data (data not shown), but rather resembles a typical secondary accumulator of Se, such as B. juncea.
Since selenate and sulfate compete for uptake and assimilation in plants, the S levels in S. pinnata and S. albescens were also compared. After 16 weeks of growth with Se, S. pinnata had accumulated 1.3-fold more S than S. albescens: 7,235 ± 279 compared with 5,391 ± 32 μg S g−1 dry weight (P < 0.05). Grown without Se, the two species showed little or no significant difference in leaf S concentration: S. pinnata accumulated 13,728 ± 888 μg S g−1 dry weight while S. albescens accumulated 11,169 ± 431 μg S g−1 dry weight. Both species accumulated twice as much S when grown without Se compared with selenate-supplied plants of the same species. The direct comparison of μg Se g−1dry weight with μg S g−1 dry weight in leaves of Se-supplied plants showed a ratio of 0.43 for S. pinnata and 0.19 for S. albescens. Thus, despite its higher S levels, the hyperaccumulator still had a higher Se/S ratio. For comparison, the selenate/sulfate ratio in the supplied medium was around 0.05.
Reduced organic S metabolites have been reported to compose a large fraction of the S pool (Nikiforova et al., 2006). To determine whether levels of reduced organic S metabolites differed between the species and how they are affected by selenate treatment, the levels of total nonprotein thiols (reduced S metabolites including glutathione and Cys) were measured in young and mature leaves of S. pinnata and S. albescens grown with and without Se (Supplemental Fig. S2). Mature leaves of S. pinnata plants supplied with Se contained 60% higher nonprotein thiol levels compared with the mature leaves of S. albescens (P < 0.05); there were no significant differences between the two species when the plants were grown without Se.
Effects of Se on Leaf Physiology
As a further comparison of Se tolerance in the two species, the effect of 20 μm selenate on the photosynthetic efficiency of S. pinnata and S. albescens was analyzed after 16 weeks of growth. The light-dependent electron transport rate was highest for S. pinnata treated with Se, followed by S. pinnata and S. albescens grown without Se (Fig. 2). The lowest photosynthetic rate was observed for S. albescens grown with Se.
Figure 2.
Light intensity-dependent electron transport rate after 16 weeks of growth. S. pinnata + Se, black circles; S. albescens + Se, black squares; S. pinnata – Se, white triangles; S. albescens – Se, white diamonds. The relative electron transport rate was calculated from the product of ΦPSII and light intensity (μmol m−2 s−1). Data represent averages of six different plants ± sd. Significant differences between each treatment and their appropriate controls (grown without Se), using Student's t test (P < 0.05), are denoted with asterisks.
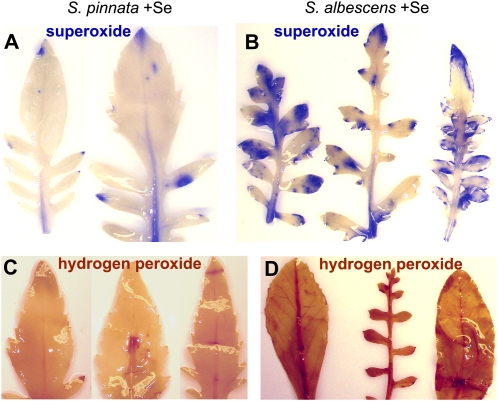
ROS are formed when stress or malfunctions impede electron flow in the photosynthetic electron transport chain. A Se-associated reduction in electron flux like that observed for S. albescens may therefore give rise to ROS production. Increased ROS may also be formed directly from selenite and GSH in vitro (Saitoh and Imuran, 1987). Se treatment has been shown previously to induce ROS accumulation in Arabidopsis leaves and coffee (Coffea arabica) cells (Gomes-Junior et al., 2007; Tamaoki et al., 2008b). The production of ROS in the two Stanleya species was analyzed in situ using stains sensitive to superoxide and hydrogen peroxide. In plants treated with 20 μm selenate for 10 weeks, the superoxide accumulation in S. pinnata leaves was lower compared with S. albescens leaves (Fig. 3, A and B). Similarly, the hydrogen peroxide accumulation in leaves of S. pinnata treated with 20 μm selenate for 10 weeks was lower compared with S. albescens leaves grown with Se (Fig. 3, C and D). Thus, when grown with Se, S. pinnata photosynthesis was not negatively affected and ROS were not accumulated. However, S. albescens photosynthesis was negatively affected when grown with Se, and superoxide and hydrogen peroxide radicals accumulated. These results confirm that the secondary accumulator S. albescens is sensitive to 20 μm selenate while the hyperaccumulator S. pinnata is not.
Figure 3.
Se-induced superoxide and hydrogen peroxide visualized by in situ ROS staining in 10-week-old leaves. A and B, Superoxide accumulation in S. pinnata + 20 μm selenate (A) and S. albescens + 20 μm selenate (B), monitored in situ through the precipitation of purple formazan from the reaction of nitroblue tetrazolium with superoxide. C and D, Hydrogen peroxide accumulation in S. pinnata + 20 μm selenate (C) and S. albescens + 20 μm selenate (D). Hydrogen peroxide was visualized in situ as reddish-brown precipitate.
Quantification of Antioxidant and ROS-Scavenging Capacity
The observed differences between S. pinnata and S. albescens in Se-induced ROS led us to investigate the levels of the important antioxidant glutathione in young leaves from both species (Fig. 4A). When grown without Se, S. pinnata contained 1.2-fold more GSH, 4.3-fold more oxidized glutathione (GSSG), and 1.4-fold more total glutathione than S. albescens (P < 0.05). When grown with Se, S. pinnata contained a 1.4-fold higher level of GSH, a 1.2-fold higher GSSG level, and a 1.3-fold higher total glutathione level than S. albescens (P < 0.05). The glutathione redox state (ratio of GSH to GSSG) in plants grown without Se was 3.6 for S. pinnata and 13.2 (a relatively normal ratio) for S. albescens, while plants grown with Se showed a ratio of 4.0 for S. pinnata and 3.4 for S. albescens (Fig. 4A). Thus, in the Se-sensitive species S. albescens, there was a significant drop in the reduction state of its glutathione pool when treated with 20 μm selenate (P < 0.05), while in the Se-tolerant S. pinnata, the ratio of GSH to GSSG was unaffected. In both species, treatment with Se caused a significant concentration decrease in total glutathione relative to plants grown without Se (P < 0.05).
Figure 4.
Quantification of the antioxidants glutathione and ascorbate and the free radical-scavenging capacities in S. pinnata and S. albescens young leaves grown with and without 20 μm selenate. A, Glutathione concentrations (nmol g−1 fresh weight [FW]), distinguishing GSH (white bars) and GSSG (hatched bars), in plants grown without (–) Se and with (+) Se. B, AsA concentrations (nmol g−1 fresh weight) in plants treated without (–) Se (white bars) and with (+) 20 μm selenate (gray bars). C, ABTS radical-scavenging capacity (μmol TEAC g−1 dry weight [DW]). D, DPPH radical-scavenging capacity (μmol TEAC g−1 dry weight). Data represent averages of three different plants ± sd. Significant differences using Student's t test at P < 0.05 are denoted with unique letters.
In order to further examine antioxidant levels, we measured ascorbic acid (AsA) concentrations in young leaves of both species, another important antioxidant molecule in plants (Smirnoff et al., 2001). When grown without Se, S. pinnata had a 1.3-fold higher AsA concentration than S. albescens (P < 0.05; Fig. 4B), but when grown with Se, no significant difference in AsA concentration was observed between the species. Taken together, these findings indicate a constitutive increase in levels of the key antioxidant molecules glutathione and ascorbate in young leaves of the hyperaccumulator S. pinnata when compared with the secondary accumulator S. albescens.
Because S. pinnata had higher constitutive levels of both glutathione and AsA, we examined the total antioxidant activity in young leaves of these two species by two different methods, 2,2′-azino-bis(3-ethylbenzo-thiazoline)-6-sulfonic acid (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH), which both measure free radical-scavenging capacities. These tests showed similar results: when grown without Se, S. pinnata possessed a 1.5-fold higher radical-scavenging capacity than S. albescens (Fig. 4, C and D). However, when grown with Se, no significant differences were observed between the species.
SMT Protein Levels and Incorporation of Se into Protein
Since the enzyme SMT is thought to be important for Se tolerance and hyperaccumulation in Astragalus hyperaccumulators by preventing nonspecific incorporation of SeCys into proteins (Brown and Shrift, 1981, 1982; Neuhierl and Bock, 1996), SMT levels were compared between S. pinnata and S. albescens. Using polyclonal antibodies raised against AbSMT from A. bisulcatus (Fabaceae), immunoblots of leaf extracts from both Stanleya species clearly detected a single protein band that had the same size as the AbSMT (36.7 kD; Fig. 5). Semiquantification of the SMT protein band (Table I) from plants of both species grown with Se indicated that S. pinnata had on average seven times more SMT signal than S. albescens (Fig. 5).
Figure 5.
Immunoblots on total protein extracts from young leaves taken from S. pinnata (lanes 1–4) and S. albescens (lanes 5–8) treated with 20 μm selenate. Blots were decorated with polyclonal antibody raised against the A. bisulcatus SMT 36.7-kD protein (Neuhierl et al., 1999).
Table I. Semiquantification of the SMT immunoreactive band.
A 1:4,000 A. bisulcatus SMT antibody and 30 μg of total protein were loaded for each plant.
| Plant Species | Mean SMT Pixel No. ± se |
| S. pinnata | 91 ± 36 |
| S. albescens | 13 ± 12 |
The relative Se protein incorporation coefficient was then calculated to investigate whether the SMT protein levels correlate with the incorporation of Se into total proteins. As shown in Table II, leaves from the hyperaccumulator S. pinnata had a slightly higher relative Se protein incorporation coefficient compared with the secondary accumulator S. albescens.
Table II. Relative Se protein incorporation coefficient.
Data represent averages from three samples of two different plants pooled.
| Plant Species | Se (μg g−1 Total Protein)/Leaf Se (μg g−1 Dry Weight) |
| S. pinnata | 0.18 ± 0.02 |
| S. albescens | 0.13 ± 0.01 |
Se Speciation and Spatial Distribution
To further investigate the underlying mechanisms associated with the enhanced Se tolerance and accumulation in S. pinnata, we probed the distribution and chemical speciation of Se in young leaves of S. pinnata and S. albescens using micro x-ray fluorescence (μ-XRF) and x-ray absorption near-edge structure (XANES). In S. pinnata leaves, 99% of total Se was detected in organic forms corresponding with standards containing carbon-Se-carbon (C-Se-C) bonds (Table III). In S. pinnata, these forms were previously found using radioautography to be 83% MeSeCys and 17% selenocystathionine (SeCyst; Shrift and Virupaksha, 1965). We later reconfirmed this result using liquid chromatography-mass spectrometry (LC-MS) and found a similar 80%:20% ratio of MeSeCys and SeCyst in S. pinnata, respectively (Freeman et al., 2006b). The XANES spectra of Se in S. albescens were different and fitted best with a composition of 75% of a C-Se-C compound, 20% SeCys, and 5% selenate (Table III). Using LC-MS, we further investigated the chemical composition of the free organic Se pool in S. albescens and found that in both young and old leaves, the total free detectable organic Se pool in S. albescens was entirely SeCyst with no other forms detected (Supplemental Fig. S5). Together, these findings indicate that the total Se composition in young leaves of the secondary accumulator S. albescens was 75% C-Se-C, 20% SeCys, and 5% selenate, with the free organic Se being found as SeCyst, the only C-Se-C compound detected. Since research has shown that as A. bisulcatus leaves age, Se speciation changes from mostly the C-Se-C form MeSeCys to selenate (Pickering et al., 2000, 2003b), we also analyzed the Se speciation of S. pinnata leaves of different ages. The Se speciation in old S. pinnata leaves did not change and was largely the same as it was in young leaves (Table III).
Table III. Composition of Se in leaves by microfocused XANES.
Se composition calculated from XANES. Data are average percentages from three spectra. ND, Not detected.
| Plant Species | SeO4 | SeCys | SeCystine | C-Se-C Compounds |
| S. pinnata young leaf | ND | ND | ND | 99.5 |
| S. pinnata medium leaf | ND | ND | ND | 99.4 |
| S. pinnata old leaf | 1.4 | ND | ND | 98.4 |
| S. albescens young leaf | 4.8 | 19.4 | ND | 75.5 |
In order to compare the localization of Se in young leaves of S. pinnata and S. albescens, we used μ-XRF. Figure 6, A and B, shows a map of total Se (in red) and calcium (in blue). Similar to what we found under different laboratory growth conditions and in the field (Freeman et al., 2006b), the S. pinnata young leaf accumulated Se in distinct globular areas particularly near the leaf margins and tip (Fig. 6A). In contrast, the distribution of Se in S. albescens was diffuse throughout the entire leaf edge and not localized in discrete areas (Fig. 6B). Therefore, the hyperaccumulator S. pinnata and the secondary accumulator S. albescens showed markedly different Se speciation in their leaves as well as different tissue Se sequestration patterns.
Figure 6.
Localization and speciation of Se in S. pinnata and S. albescens. A and B, μ-XAS maps showing spatial distribution of total Se imaged in red and calcium imaged in blue, both at normal gain, in young leaves of S. pinnata (A) and S. albescens (B). Bars = 1 mm. The inset shows a higher magnification image of S. pinnata cells containing high levels of Se, from the area in the white box. White circles show locations of XANES speciation scans reported in Table III. C, Scanning electron microscopy and EDS analysis of the distribution of Se in different tissues exposed by freeze fracturing fully hydrated frozen leaves of S. pinnata with positive (+) Se detection and negative (−) Se detection in the various cells. D, S. albescens, showing a single enlarged peripheral epidermal cell, positive (+) for Se. Bars in C and D = 50 μm. E, Se distribution in peripheral epidermal cells of S. pinnata with positive (+) Se detection. Bar = 30 μm. F, Epidermal cell of S. pinnata at higher magnification; light etching of this sample reveals membranes allowing identification of organelles and areas of high Se concentration with positive (+) Se detection and negative (−) Se detection. Bar = 10 μm. G, Epidermal cell wall of S. pinnata without detectable signal negative (−) for Se. Bar = 10 μm. H, Upper epidermal surface of S. pinnata; a line scan taken across a rupture reveals a positive peak only over a Se-rich epidermal cell immediately beneath the cuticle. Bar = 35 μm.
To investigate the localization of Se in more detail at the cellular and subcellular levels, we used energy-dispersive spectroscopy (EDS), which determines the location of Se at a higher magnification than μ-XRF, but with lower sensitivity. This technique uses a scanning electron microscope and EDS to probe freeze-fractured, flash-frozen, fully hydrated leaves for their Se distribution. In S. pinnata leaves, Se was detected in all epidermal cells tested, and these levels decreased in epidermal cells closer to the mid vein (Fig. 6, C and E). In S. albescens, there was only one enlarged peripheral epidermal cell that showed a slightly detectable signal for Se (Fig. 6D). No Se was detected in vascular or mesophyll tissues of S. pinnata. In a particular epidermal cell of S. pinnata, the highest concentrations of Se were located in an organelle resembling a small vacuole (Fig. 6F, top left). The epidermal cell wall of S. pinnata was also tested, and no Se was detected (Fig. 6G). A line scan with energy insufficient to penetrate through the leaf cuticle was taken across the leaf surface, and a rupture revealed the edge of a Se-rich epidermal cell immediately beneath the cuticle, indicating that Se was not accumulated in the cuticular layer (Fig. 6H). Se was also not detected in any of the cuticular wax crystals tested. A few leaf hairs were found on field samples and in another S. pinnata accession; however, no Se was detected in these leaf hairs (data not shown). Thus, S. pinnata appears to sequester most Se in the symplast (perhaps in small vacuoles) of epidermal cells, particularly in cells near the margins and tips of leaves.
Gene Expression Analyses
Genetic Similarity Test
Based on sequence identity of the internal transcribed spacer regions 1 and 2 and the 5.8S ribosomal RNA gene, S. pinnata (GenBank accession no. AF531620) is 83% identical to the Brassicaceae model species Arabidopsis (W.A. Peer and D.E. Salt, personal communication). In order to test whether S. pinnata and S. albescens show an equal degree of similarity to Arabidopsis, which would make it possible to use Arabidopsis macroarrays to compare gene expression patterns in the two Stanleya species, we sequenced PCR products obtained from S. pinnata and S. albescens and compared the sequences with those of Arabidopsis. Seven independent genes, all involved in S uptake or assimilation, were selected for the analysis (Supplemental Table S6) because expression of these genes was expected to differ between the two Stanleya species. After BLAST alignment, the average genetic similarity was calculated from the seven different gene sequences (Supplemental Table S6). S. pinnata and S. albescens were on average 88% ± 2% and 89% ± 2% identical to Arabidopsis, respectively, and 94% ± 2% identical to each other based on DNA sequence alignments. In view of the equal genetic similarity of the two Stanleya species to Arabidopsis and the high cDNA sequence identity to each other, we decided to use Arabidopsis macroarrays to compare gene expression profiles in these two Stanleya species.
Experimental Design
Sets of custom Arabidopsis cDNAs containing 324 different Arabidopsis genes potentially involved with Se tolerance or hyperaccumulation were manually spotted onto nylon membranes (Tamaoki et al., 2008b) and hybridized with root or shoot cDNA obtained from S. pinnata or S. albescens plants treated with or without Se. Genes that showed a 2-fold or greater higher expression level in young leaves and roots of S. pinnata compared with S. albescens grown with and without Se are listed in Tables IV to VII. Since these Stanleya genes have not yet been named, we will refer to the genes that show differential expression in the two Stanleya species by the names of the Arabidopsis cDNAs with which they hybridized on the macroarrays. Genes whose mRNA levels were higher in leaves of S. pinnata than S. albescens are organized into functional groups and summarized in Supplemental Figure S3. Genes that were induced by Se in both species are also provided in Supplemental Tables S1 to S3. Below, we present the differences in gene expression between the Stanleya species organized by functional group.
Table IV. Constitutive difference (−Se; S. pinnata/S. albescens) 10 week, shoot, gene sets 1 and 2.
| Gene Name | Annotation | Gene Identifier | Average | SD | P |
| S assimilation genes | |||||
| CS26 | Cysteine synthase 26 | At3g03630 | 3.45 | 0.24 | 0.03 |
| IMPase | Myoinositol monophosphatase | At4g39120 | 3.00 | 0.33 | 0.03 |
| CYSC1 | Cysteine synthase isomer | At3g61440 | 2.41 | 0.21 | 0.02 |
| CS | Cysteine synthase pyridoxal-5′-phosphate-dependent | At1g55880 | 2.18 | 0.05 | 0.01 |
| Antioxidant and redox control genes | |||||
| GSTF6 | Glutathione S-transferase F6 | At1g02930 | 3.06 | 0.14 | 0.01 |
| GRXC10 | Glutaredoxin | At5g11930 | 2.60 | 0.57 | 0.08 |
| CAT3 | Catalase, putative | At1g20620 | 2.60 | 0.47 | 0.02 |
| mtDHAR | Dehydroascorbate reductase, mitochondrion | At1g19570 | 2.00 | 0.45 | 0.03 |
| GRX | Glutaredoxin | At5g58530 | 1.95 | 0.06 | 0.03 |
| Defense-related genes | |||||
| PDF1.2 | Plant defensin 1.2 | At5g44420 | 31.16 | 5.11 | 0.03 |
| PR4 | Pathogenesis-related protein 4 | At3g04720 | 4.60 | 0.15 | 0.00 |
| Pin2 | Proteinase inhibitor 2 | At2g02100 | 4.12 | 0.31 | 0.03 |
| PR2 | Pathogenesis-related protein 2 | At3g57260 | 3.97 | 0.49 | 0.01 |
| PR1 | Pathogenesis-related protein 1 | At2g14610 | 3.87 | 0.19 | 0.01 |
| PR5 | Pathogenesis-related protein 5 | At1g75040 | 3.76 | 0.06 | 0.01 |
| ACS6 | 1-Aminocyclopropane-1-carboxylate synthase 6 | At4g11280 | 3.63 | 0.52 | 0.04 |
| VSP1 | Vegetative storage protein 1 | At5g24780 | 3.02 | 0.45 | 0.04 |
| Molecular chaperone genes | |||||
| BiP2 | Luminal-binding protein 2 | At5g42020 | 4.38 | 0.78 | 0.02 |
| BiP1 | Luminal-binding protein 1 | At5g28540 | 3.29 | 0.64 | 0.03 |
Table VI. Induced difference (+Se; S. pinnata/S. albescens) 10 week, shoot, gene sets 1 and 2.
| Gene Name | Annotation | Gene Identifier | Average | SD | P |
| Sulfate transporter genes | |||||
| Sultr4;1 | Sulfate transporter | At5g13550 | 2.59 | 0.08 | 0.02 |
| Sultr1;2 | Sulfate transporter | At1g78000 | 2.30 | 0.25 | 0.02 |
| Sultr3;3 | Sulfate transporter | At1g23090 | 2.10 | 0.01 | 0.02 |
| Sultr4;2 | Sulfate transporter | At3g12520 | 2.06 | 0.34 | 0.06 |
| Sultr2;1 | Sulfate transporter | At5g10180 | 2.08 | 0.04 | 0.01 |
| S assimilation genes | |||||
| CYSC1 | Cysteine synthase isomer | At3g61440 | 3.71 | 0.01 | 0.02 |
| SAT52 | Serine acetyltransferase 52 | At5g56760 | 3.60 | 0.15 | 0.04 |
| OASA1 | O-Acetylserine (thiol)-lyase | At3g22460 | 2.97 | 0.54 | 0.02 |
| IMPase | Myoinositol monophosphatase | At4g39120 | 2.50 | 0.16 | 0.02 |
| APR3 | 5′-Adenylylsulfate reductase 3 | At4g21990 | 2.42 | 0.47 | 0.06 |
| ATCYSD2 | Cysteine synthase | At5g28020 | 2.40 | 0.52 | 0.05 |
| Allinase | Cysteine sulfoxide lyase, Allinase family protein | At4g24670 | 2.20 | 0.08 | 0.02 |
| AHL | 3′-Phosphoadenosine-5′-phosphate phosphatase | At5g54390 | 2.19 | 0.42 | 0.06 |
| APR1 | 5′-Adenylylsulfate reductase 1 | At4g04610 | 2.12 | 0.01 | 0.01 |
| AKN4 | Adenylylsulfate (APS) kinase 4 | At5g67520 | 2.08 | 0.36 | 0.06 |
| APR2 | 5′-Adenylylsulfate reductase 2 | At1g62180 | 2.07 | 0.20 | 0.02 |
| SIR | Sulfite reductase | At5g04590 | 2.04 | 0.42 | 0.06 |
| Antioxidant and redox control genes | |||||
| GSTU1 | Glutathione S-transferase U1 | At2g29490 | 2.56 | 0.04 | 0.01 |
| CAT3 | Catalase, putative | At1g20620 | 2.49 | 0.37 | 0.01 |
| VTC1 | GDP-d-mannose pyrophosphorylase, AsA biosynthesis | At2g39770 | 2.38 | 0.18 | 0.01 |
| ATFD | Ferredoxin | At1g10960 | 2.34 | 0.27 | 0.03 |
| GPX1 | Glutathione peroxidase, chloroplast | At2g25080 | 2.27 | 0.06 | 0.01 |
| GRXS6 | Glutaredoxin | At3g62930 | 2.14 | 0.24 | 0.04 |
| ATP2a | Peroxidase 21 | At2g37130 | 2.09 | 0.37 | 0.03 |
| CAT1 | Cytosolic catalase | At1g20630 | 2.02 | 0.04 | 0.06 |
| Defense-related genes | |||||
| PDF1.2 | Plant defensin 1.2 | At5g44420 | 19.70 | 4.84 | 0.05 |
| Pin2 | Proteinase inhibitor 2 | At2g02100 | 4.02 | 0.86 | 0.04 |
| PR1 | Pathogenesis-related protein 1 | At2g14610 | 3.00 | 0.78 | 0.04 |
| ACS6 | 1-Aminocyclopropane-1-carboxylate synthase 6 | At4g11280 | 2.75 | 0.53 | 0.04 |
| VSP1 | Vegetative storage protein 1 | At5g24780 | 2.49 | 0.17 | 0.01 |
| CRA1 | Encodes a 12S seed storage protein | At5g44120 | 2.37 | 0.16 | 0.03 |
| SAL1 | 3′(2′),5′-Bisphosphate nucleotidase, putative | At5g63980 | 2.22 | 0.16 | 0.01 |
| ERD5 | Proline oxidase | At5g38710 | 2.10 | 0.18 | 0.04 |
| CNX1 | Calnexin 1 | At5g61790 | 2.02 | 0.37 | 0.03 |
| CRT1 | Calreticulin 1 | At1g56340 | 1.97 | 0.25 | 0.02 |
| NPR1 | Nonexpresser of PR genes 1 | At1g64280 | 1.93 | 0.03 | 0.04 |
| Molecular chaperone genes | |||||
| HSP17.6-CII | 17.6-kD class II heat shock protein | At5g12020 | 2.22 | 0.45 | 0.05 |
| HSP17.4C-CI | Heat shock protein 17.4 C-CI | At1g53540 | 2.20 | 0.01 | 0.02 |
| HSP17.4A-CI | Heat shock protein 17.4 A-CI | At1g59860 | 2.16 | 0.01 | 0.01 |
| Selenoprotein genes | |||||
| SFP | Selenoprotein family protein | At1g05720 | 2.07 | 0.04 | 0.01 |
| SBP | Putative Se-binding protein | At2g03880 | 2.23 | 0.15 | 0.02 |
| FeS cluster-related genes | |||||
| AtSufE | S acceptor interacts/activates Cys desulfurases | At4g26500 | 2.63 | 0.71 | 0.05 |
| NFU4 | Nitrogen fixation NifU-like family protein | At3g20970 | 2.57 | 0.36 | 0.06 |
| TIC55 | Translocation inner envelope membrane of plastids | At2g24820 | 2.37 | 0.11 | 0.01 |
| ISU1 | FeS cluster assembly complex protein | At4g22220 | 2.27 | 0.42 | 0.06 |
| ATXDH1 | Xanthine dehydrogenase | At4g34900 | 2.26 | 0.40 | 0.05 |
| CpSufD | FeS metabolism-associated domain-containing protein | At1g32500 | 2.24 | 0.08 | 0.01 |
| AtMtNifS | Cysteine desulfurase | At5g65720 | 2.08 | 0.11 | 0.03 |
| CNX5 | Molybdopterin synthase sulfurylase | At5g55130 | 2.08 | 0.38 | 0.06 |
Genes Involved in S/Se Metabolism
Since S and Se are thought to be metabolized by the same pathways, we compared the expression levels of S-associated genes between the two Stanleya species. In leaves of S. pinnata grown without Se, four S assimilation-related genes were more highly expressed compared with S. albescens; three are Cys synthase-encoding genes and one is a myoinositol monophosphatase-like gene that is thought to be involved with S metabolic processes and/or His biosynthesis (Table IV). In roots of S. pinnata grown without Se, 22 different S assimilation genes were more highly expressed compared with S. albescens (Table V), including sulfate transporter genes, sulfate activation and reduction genes, genes mediating Cys and Met synthesis, and glutathione synthesis genes.
Table V. Constitutive difference (−Se; S. pinnata/S. albescens) 10 week, root, gene set 1.
| Gene Name | Annotation | Gene Identifier | Average | SD | P |
| Sulfate transporter genes | |||||
| Sultr4;1 | Sulfate transporter | At5g13550 | 2.94 | 0.44 | 0.03 |
| Sultr1;2 | Sulfate transporter | At1g78000 | 2.84 | 0.08 | 0.02 |
| Sultr3;2 | Sulfate transporter | At4g02700 | 2.75 | 0.04 | 0.01 |
| S assimilation-related genes | |||||
| APR1 | 5′-Adenylylsulfate reductase 1 | At4g04610 | 7.66 | 0.31 | 0.02 |
| APS2 | ATP-sulfurylase 2 | At1g19920 | 5.14 | 0.53 | 0.04 |
| APR3 | 5′-Adenylylsulfate reductase 3 | At4g21990 | 4.79 | 0.43 | 0.04 |
| APR2 | 5′-Adenylylsulfate reductase 2 | At1g62180 | 3.23 | 0.02 | 0.00 |
| ATMS2 | Methionine synthase cytosolic | At3g03780 | 3.19 | 0.42 | 0.02 |
| APS1 | ATP sulfurylase 1 | At3g22890 | 3.12 | 0.03 | 0.01 |
| OASA1 | O-Acetylserine (thiol)-lyase | At3g22460 | 3.09 | 0.07 | 0.00 |
| CYSC1 | Cysteine synthase isomer | At3g61440 | 2.92 | 0.58 | 0.04 |
| ATMS1 | Methionine synthase cytosolic | At5g17920 | 2.87 | 0.46 | 0.04 |
| ATCYSD2 | Cysteine synthase | At5g28020 | 2.66 | 0.06 | 0.01 |
| AKN1 | Adenylylsulfate (APS) kinase 1 | At2g14750 | 2.66 | 0.47 | 0.04 |
| GSH2 | Glutathione synthetase | At5g27380 | 2.58 | 0.06 | 0.02 |
| SAT52 | Serine acetyltransferase 52, cytosolic | At5g56760 | 2.57 | 0.11 | 0.01 |
| CYS-3A | Cysteine synthase | At4g14880 | 2.38 | 0.50 | 0.05 |
| GSH1 | γ-Glutamylcysteine synthetase | At4g23100 | 2.55 | 0.01 | 0.01 |
| SAT1 | Serine acetyltransferase 1, mitochondrial | At3g13110 | 2.41 | 0.59 | 0.05 |
| CS26 | Cysteine synthase 26 | At3g03630 | 2.17 | 0.11 | 0.01 |
| CYSD1 | Cysteine synthase | At3g04940 | 2.15 | 0.05 | 0.00 |
| SAT106 | Serine acetyltransferase 106 | At2g17640 | 2.07 | 0.17 | 0.02 |
| Antioxidant and redox control genes | |||||
| ATFD3 | Ferredoxin | At2g27510 | 4.53 | 0.49 | 0.03 |
| GRXS15 | Glutaredoxin | At3g15660 | 3.07 | 0.28 | 0.01 |
| GPX2 | GSH peroxidase | At2g31570 | 3.04 | 0.14 | 0.02 |
| GRXC10 | Glutaredoxin | At5g11930 | 2.29 | 0.44 | 0.04 |
| VTC4 | l-Galactose-1-phosphate phosphatase, AsA biosynthesis | At3g02870 | 2.17 | 0.04 | 0.03 |
| Defense-related genes | |||||
| Pin2 | Proteinase inhibitor 2 | At2g02100 | 5.10 | 0.37 | 0.01 |
| PR5 | Pathogenesis-related protein 5 | At1g75040 | 3.65 | 0.43 | 0.01 |
| VSP1 | Vegetative storage protein 1 | At5g24780 | 3.09 | 0.02 | 0.01 |
| ACS6 | 1-Aminocyclopropane-1-carboxylate synthase 6 | At4g11280 | 2.57 | 0.21 | 0.02 |
| PR1 | Pathogenesis-related protein 1 | At2g14610 | 2.21 | 0.02 | 0.01 |
| Molecular chaperone genes | |||||
| HSP17.6-CII | 17.6-kD class II heat shock protein | At5g12020 | 3.18 | 0.24 | 0.04 |
| HSP17.4C-CI | Heat shock protein 17.4 C-CI | At1g53540 | 2.09 | 0.09 | 0.01 |
| Selenoprotein genes | |||||
| MZN1.9 | Selenoprotein-related | At5g58640 | 2.08 | 0.23 | 0.05 |
| SFP | Selenoprotein family protein | At1g05720 | 2.93 | 0.42 | 0.01 |
| FeS cluster-related genes | |||||
| CpSufE2 | FeS metabolism-associated domain-containing protein | At1g67810 | 2.83 | 0.21 | 0.01 |
| NFU4 | Nitrogen fixation NifU-like family protein | At3g20970 | 2.22 | 0.04 | 0.01 |
| IscA-like 1 | HesB-like domain-containing protein | At2g16710 | 2.29 | 0.24 | 0.03 |
| ABA3 | MoCo sulfurase family protein | At5g44720 | 2.77 | 0.01 | 0.01 |
| AtMtNifS | Cysteine desulfurase | At5g65720 | 2.11 | 0.15 | 0.03 |
| ISU1 | FeS cluster assembly complex protein | At4g22220 | 2.92 | 0.12 | 0.01 |
In leaves of plants grown with Se, there was higher expression of 17 S assimilation genes in S. pinnata compared with S. albescens (Table VI), responsible for sulfate transport, sulfate reduction, sulfite reduction, and Cys synthesis. In roots of Se-treated plants, 10 S assimilation-related genes showed higher expression in S. pinnata compared with S. albescens (Table VII), involved in sulfate transport, sulfate reduction, and Cys and Met synthesis.
Table VII. Induced difference (+Se; S. pinnata/S. albescens) 10 week, root, gene set 1.
| Gene Name | Annotation | Gene Identifier | Average | SD | P |
| Sulfate transporter genes | |||||
| Sultr3;2 | Sulfate transporter | At4g02700 | 2.28 | 0.78 | 0.09 |
| S assimilation-related genes | |||||
| IMPase | Myoinositol monophosphatase | At4g39120 | 3.29 | 1.29 | 0.06 |
| CYSD1 | Cysteine synthase | At3g04940 | 3.19 | 0.86 | 0.03 |
| ATMS3 | Methionine synthase, chloroplastic | At5g20980 | 2.56 | 0.23 | 0.01 |
| APR2 | 5′-Adenylylsulfate reductase 2 | At1g62180 | 2.52 | 0.74 | 0.06 |
| CS26 | Cysteine synthase 26 | At3g03630 | 2.42 | 0.06 | 0.01 |
| SAT52 | Serine acetyltransferase 52, cytosolic | At5g56760 | 2.25 | 0.18 | 0.01 |
| APR3 | 5′-Adenylylsulfate reductase 3 | At4g21990 | 2.23 | 0.46 | 0.05 |
| ATMS2 | Methionine synthase cytosolic | At3g03780 | 2.15 | 0.15 | 0.01 |
| CBL | Cystathionine β-lyase | At3g57050 | 2.06 | 0.24 | 0.03 |
| Antioxidant and redox control genes | |||||
| GRXC9 | Glutaredoxin affected by Se | At1g28480 | 4.31 | 0.51 | 0.00 |
| GRXS15 | Glutaredoxin | At3g15660 | 4.20 | 0.47 | 0.01 |
| ATFD3 | Ferredoxin | At2g27510 | 2.66 | 0.28 | 0.01 |
| GR2 | Glutathione reductase | At3g54660 | 2.26 | 0.28 | 0.02 |
| CXIP2 | Glutaredoxin | At2g38270 | 2.20 | 0.40 | 0.03 |
| GRXC5 | Glutaredoxin | At4g28730 | 2.17 | 0.27 | 0.03 |
| GRX | Glutaredoxin | At3g57070 | 2.03 | 0.27 | 0.03 |
| Defense-related genes | |||||
| ACS6 | 1-Aminocyclopropane-1-carboxylate synthase 6 | At4g11280 | 3.18 | 0.88 | 0.04 |
| Pin2 | Proteinase inhibitor 2 | At2g02100 | 2.42 | 0.40 | 0.03 |
| GLUT | UDP-glucoronosyl/UDP-glucosyl transferase protein | At1g05680 | 2.42 | 0.44 | 0.02 |
| RD29B | Response to water deprivation, salt, and abscisic acid | At5g52300 | 2.32 | 0.52 | 0.04 |
| STZ | Salt tolerance zinc finger | At1g27730 | 2.28 | 0.28 | 0.02 |
| MTN3 | Nodulin MtN3 family protein | At5g13170 | 2.16 | 0.57 | 0.05 |
| DDF1 | Encodes a member of the DREB subfamily A-1 | At1g12610 | 2.09 | 0.51 | 0.06 |
| Molecular chaperone genes | |||||
| HSP17.6A | Heat shock protein 17.6A | At5g12030 | 2.35 | 0.66 | 0.06 |
| ATHSP17.4 | Heat shock protein 17.4 | At3g46230 | 2.06 | 0.41 | 0.05 |
| FeS cluster-related genes | |||||
| PSAA | Encodes psaA protein reaction center for PSI | AtCg00350 | 3.13 | 0.13 | 0.03 |
| NFU2 | Nitrogen fixation NifU-like family protein | At5g49940 | 2.46 | 0.23 | 0.02 |
| TIC55 | Translocation inner envelope membrane of plastids | At4g25650 | 2.44 | 0.87 | 0.07 |
| TIC55 | Translocation inner envelope membrane of plastids | At2g24820 | 2.29 | 0.19 | 0.01 |
| IscA-like 1 | HesB-like domain-containing protein | At2g16710 | 2.27 | 0.69 | 0.07 |
| SIRB | Sirohydrochlorin ferrochelatase | At1g50170 | 2.18 | 0.56 | 0.06 |
| PSAC | Encodes the PsaC subunit of PSI | AtCg01060 | 2.07 | 0.69 | 0.09 |
| CpSufB | FeS metabolism-associated domain-containing protein | At4g04770 | 2.05 | 0.06 | 0.03 |
| RDH2 | Thiosulfate:cyanide sulfurtransferase | At1g16460 | 1.99 | 0.30 | 0.02 |
Plants have not been shown to have Se-specific enzymes, but they have Se-binding protein (SBP)-like genes. In roots of plants grown without Se, a higher level of expression was observed in S. pinnata than S. albescens for two SBP-like genes: MZN1.9 and SFP (Table V). In leaves of plants grown with Se, S. pinnata again showed a higher level of gene expression than S. albescens for two SBP-like genes, SFP and SBP (Table VI).
Molecular Chaperones and Cofactor Assembly Genes
We examined the expression of molecular chaperone genes because they are associated with the responses to various abiotic stresses and are crucial for helping proteins fold under adverse conditions. In plants grown in the absence of Se, two molecular chaperone genes (BiP1 and BiP2) were more highly expressed in S. pinnata compared with S. albescens (Table IV). In roots of plants grown without Se, a higher level of expression was observed in S. pinnata for two heat shock protein (HSP) genes (Table V). In leaves of plants grown with selenate, S. pinnata showed a higher expression than S. albescens for three genes encoding HSP (Table VI). In the roots of Se-supplied plants, the transcript levels of two HSPs were higher in S. pinnata compared with S. albescens (Table VII).
We examined the expression of genes related to biosynthesis of iron-sulfur (FeS) clusters and molybdenum cofactor (MoCo) because these cofactors are associated with many important processes that may be needed for coping with Se stress. In the absence of Se, no gene expression differences were observed for these genes in the leaves of S. pinnata compared with S. albescens (Table IV). However, in roots of plants grown without Se, a higher level of gene expression was observed in S. pinnata compared with S. albescens for six genes (Table V), including Cys desulfurases, activators of Cys desulfurases, and various scaffold proteins for FeS assembly. When plants were grown with selenate, the expression of six cofactor assembly genes was higher in leaves of S. pinnata versus S. albescens (Table VI), encoding a Cys desulfurase, a Cys desulfurase activator, several FeS scaffold proteins, and molybdopterin synthase sulfurylase. Two FeS cluster-containing proteins were also more highly expressed (TIC55 and ATXDH1). In roots of plants grown with selenate, the expression levels of three FeS scaffold-encoding genes were higher in S. pinnata compared with S. albescens as well as five genes encoding FeS cluster-containing proteins (TIC55, SIRB, PsaC, PSAA, and RDH2).
Antioxidant and Redox Control Genes
Because S. pinnata had higher antioxidant levels and radical-scavenging capacities than S. albescens and because Se generated more oxidative stress in leaves of S. albescens than in S. pinnata leaves, we also examined the expression of antioxidant and redox control genes. In leaves of plants grown without Se, five antioxidant or redox control genes were more highly expressed in S. pinnata than in S. albescens (Table IV), encoding a glutathione S-transferase, two glutaredoxins, a catalase, and a dehydroascorbate reductase. In roots of plants grown without Se, five different antioxidant or redox control genes had higher expression in S. pinnata than in S. albescens (Table V), encoding a ferredoxin, two glutaredoxins, a glutathione peroxidase, and an l-Gal-1-P phosphatase. In Se-supplied plants, eight genes encoding antioxidant or redox control enzymes showed higher expression in leaves of S. pinnata versus S. albescens (Table VI), encoding a glutathione S-transferase, two catalases, a GDP-d-Man pyrophosphorylase, a ferredoxin, a glutathione peroxidase, a glutaredoxin, and an ATP-dependent peroxidase. In roots of plants grown with Se, S. pinnata showed higher expression levels than S. albescens for seven antioxidant and redox control genes (Table VII), encoding several glutaredoxins, a ferredoxin, and a glutathione reductase.
Defense-Associated Genes
Expression of eight defense-related genes was higher in young leaves of S. pinnata than in S. albescens when grown without selenate (Table IV), encoding a plant defensin, PDF1.2 (this gene showed the largest difference in expression level between S. pinnata and S. albescens of all genes tested), four pathogenesis-related (PR) proteins, Proteinase Inhibitor2 (Pin2), 1-Aminocyclopropane-1-Carboxylate Synthase6 (ACS6; involved in ethylene synthesis), and Vegetative Storage Protein1 (VSP1). Five of these genes were also expressed at a higher basal levels in the roots of S. pinnata than in S. albescens (Pin2, PR1 and PR5, VSP1, and ACS6; Table V). In leaves of plants grown with Se, the expression of 11 defense-related genes was higher in S. pinnata than S. albescens (Table VI), including five of the genes also up-regulated in leaves of plants grown without Se (PDF1.2, Pin2, PR1, ACS6, and VSP1). In roots of plants grown with Se, the expression of seven defense-related genes was higher in S. pinnata than S. albescens (Table VII), including ACS6 and Pin2.
Verification of Gene Expression Patterns Using Northern Blotting and Reverse Transcription-PCR
To verify the gene expression patterns found in the macroarray studies with an independent experimental approach and biological replicate, northern-blot analysis and semiquantitative reverse transcription (RT)-PCR were performed for selected genes. Consistent with the macroarray data, the basal expression levels of defense-associated genes Pin2, PDF1.2, and ACS6 were higher in S. pinnata than in S. albescens when grown without selenate (Supplemental Fig. S4, A and B). Moreover, the expression levels of S-associated genes, Cys synthases, SAT52, SAT1, APS, GSH1, and GSH2 were constitutive in S. pinnata. Together, the results from the macroarray, northern-blot analysis, and semiquantitative RT-PCR approaches indicate that the expression of several key genes involved in S uptake, S assimilation, and defense are more enhanced in S. pinnata than in S. albescens.
Tissue Levels of Signaling Molecules and Total Phenolics
Because the gene expression analyses showed differences in constitutive (−Se) and inducible (+Se) expression levels of genes associated with the biosynthesis of, or the response to, phytohormones such as methyl jasmonic acid (MeJA), jasmonic acid (JA), ethylene, and salicylic acid (SA; Table IV), we measured the plant concentrations of these hormones in young leaves of both species grown with or without Se. In addition, we measured the levels of the JA precursor methyl-linolenic acid (MeLin) in the same young leaves. When grown without Se, MeLin concentration was 4.5-fold higher in S. pinnata than in S. albescens (P < 0.05; Fig. 7A), but in the presence of Se, no significant difference was observed in MeLin concentrations between both species. As for JA, in the absence of Se, S. pinnata leaves had 420 ± 137 nmol g−1 fresh weight JA, while S. albescens did not contain any detectable JA (Fig. 7B). In contrast, when grown with Se, S. pinnata JA levels were not detectable in young leaves, while in S. albescens, JA was present at a very low level (12 ± 8 nmol g−1 fresh weight). MeJA, which is thought to be a highly bioactive hormone, was found in S. pinnata young leaves grown without Se at 59 ± 13 nmol g−1 fresh weight, while it was barely detectable (less than 1 nmol g−1 fresh weight) in S. albescens grown without Se (Fig. 7C). In the presence of Se, S. pinnata young leaves had 3.7-fold more MeJA than S. albescens leaves (P < 0.05), with levels of 41 ± 8 and 11 ± 3 nmol g−1 fresh weight, respectively.
Figure 7.
Quantification of the hormones MeJA and JA, their initial precursor MeLin, the potent defense response elicitor free SA, the hormone ethylene, and total phenolics in S. pinnata and S. albescens young leaves grown without (–) Se (white bars) and with (+) 20 μm selenate (gray bars). Data represent averages of three different plants ± sd. Significant differences (P < 0.05) using Student's t test are denoted with unique letters. DW, Dry weight; FW, fresh weight; GAE, gallic acid equivalents.
As for the phytohormone ethylene, we found that in whole young plants of S. pinnata grown without Se, ethylene production was 1.6-fold lower than in S. albescens (P < 0.05; Fig. 7D). In contrast, in whole young plants grown with Se, S. pinnata produced 1.6 times more ethylene than S. albescens (P < 0.05). We also measured levels of the signaling molecule SA and found that in young leaves grown without Se, the concentration of free SA was 10.8 times higher in S. pinnata than in S. albescens (Fig. 7E). When grown with Se, the levels of SA in S. pinnata young leaves were 4.6-fold higher than S. albescens (P < 0.05).
Finally, total phenolics were measured in the young leaves. Phenolic compounds are often associated with abiotic stress defense and signaling disease resistance and are considered a good indicator of the antioxidant capacity of leaves. We found that in plants grown without Se, the total phenolics levels of S. pinnata were 1.6-fold higher compared with S. albescens (Fig. 7F), and in plants grown with Se, the S. pinnata total phenolics levels were 1.3-fold higher than S. albescens (P < 0.05).
Thus, in comparison with the secondary accumulator S. albescens, the hyperaccumulator S. pinnata showed a trend for higher levels of JA, its precursor MeLin and active derivative MeJA, as well as higher SA and total phenolics. Ethylene in whole young plants showed mixed results. In the absence of Se, it was present at lower levels in S. pinnata, but it appeared to be induced more strongly by Se in the hyperaccumulator, so that its level was higher in S. pinnata than in S. albescens whole young plants when grown in the presence of selenate.
Physiological Effects of MeJA, 1-Aminocyclopropane-1-Carboxylate, and O-Acetylserine on Se Accumulation in Shoots
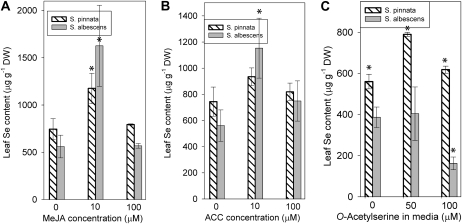
To obtain further insight into the effects of the phytohormones and signaling molecules measured previously on shoot Se accumulation, we applied these compounds as foliar spray treatments to the shoots of young plants. MeJA at 10 μm was effective at increasing Se accumulation in both S. pinnata and S. albescens shoots (Fig. 8A). Six-week-old plants of S. pinnata grown in the presence of 20 μm selenate that had been sprayed daily with 10 μm MeJA during the last 3 weeks had accumulated 1.6-fold more Se than water controls (P < 0.05). Similarly, S. albescens accumulated 2.8-fold more Se after 10 μm MeJA treatment compared with water controls (P < 0.05). At 100 μm MeJA, both species did not accumulate more Se than water controls.
Figure 8.
Phytohormone and elicitor precursor spray treatments of shoots and OAS-supplemented medium caused differential Se accumulation in shoots of S. pinnata and S. albescens. A and B, Daily foliar spray treatments for 3 weeks of MeJA (A) and ACC (B) with water-treated controls in comparison with Se accumulation (μg Se g−1 leaf dry weight [DW]) in 6-week old S. pinnata and S. albescens shoots. C, OAS-supplemented growth medium versus Se accumulation in S. pinnata and S. albescens (μg Se g−1 leaf dry weight) in 5-week-old shoots. All plants were germinated and grown throughout in the presence of 20 μm SeO4 in prewashed Turface with fertilizer or half-strength MS salts + vitamins. Data represent averages of three to five different plants ± sd. Significant differences between each treatment and their appropriate water controls using Student's t test (P < 0.05) are denoted with asterisks.
1-Aminocyclopropane-1-carboxylate (ACC) is an ethylene precursor often used in ethylene elicitor experiments. Spraying young S. pinnata shoots with 10 μm ACC every day for 3 weeks did not significantly affect shoot Se accumulation compared with water controls (Fig. 8B). However, S. albescens accumulated 2-fold more Se compared with its water control when 10 μm ACC was applied (P < 0.05). At the 100 μm ACC treatment level, both species did not accumulate more Se than their water controls and the plants were smaller in size, indicating inhibition of growth at this ACC concentration.
Because of the expression differences observed between S. pinnata and S. albescens with respect to genes involved in S transport and assimilation, we tested the effect of treatment with O-acetylserine (OAS) on the ability of both species to accumulate Se. It is known that OAS, the carbon substrate for Cys biosynthesis, up-regulates the key genes involved in S transport, reduction, and assimilation. After 30 d of growth in medium containing 20 μm selenate but no OAS (Fig. 8C), the Se accumulation in S. pinnata young shoots was 1.5-fold higher than in S. albescens (P < 0.05). After growth in medium containing both 20 μm selenate and 50 μm OAS, S. pinnata had accumulated 1.4-fold more Se than its control grown without OAS (P < 0.05), while S. albescens accumulation was not affected by the same treatment. At 100 μm OAS, S. pinnata had accumulated marginally more Se than its control grown without OAS. However, S. albescens young shoots showed a decrease in Se content by 2.4-fold compared with its control grown without OAS (P < 0.05). Thus, OAS stimulated Se accumulation in S. pinnata young shoots but not in S. albescens.
DISCUSSION
The data presented here provide new insight into Se tolerance and sequestration mechanisms in the Se hyperaccumulator S. pinnata. To our knowledge, these data are novel, since earlier work on Se tolerance and accumulation mechanisms have mainly been performed on the hyperaccumulator A. bisulcatus, the secondary accumulator B. juncea, and the nonaccumulator Arabidopsis. Some of the mechanisms proposed here for S. pinnata appear to be different compared with A. bisulcatus (e.g. with respect to up-regulation of S assimilation genes), but there are also interesting parallels (e.g. the main form of free Se accumulated and the preferential accumulation in the epidermis). This article also describes, to our knowledge, the first transcriptome analysis of any Se hyperaccumulator and identifies new genes that may contribute to the Se hyperaccumulation phenotype of the Brassicaceae family member S. pinnata.
The hyperaccumulator S. pinnata was completely tolerant to 20 μm selenate, while S. albescens suffered toxicity at this concentration, judged from visible leaf chlorosis and necrosis, accumulation of ROS, and decreased photosynthetic performance. Shoot Se accumulation was approximately 3.6-fold higher in S. pinnata, demonstrating that the enhanced Se tolerance of S. pinnata is due to detoxification and not exclusion. Our biochemical studies offer some insight into the molecular mechanisms potentially mediating these differences in Se tolerance and accumulation.
One important molecular mechanism for Se tolerance in S. pinnata is likely the chemical form into which inorganic Se is converted and then hyperaccumulated. The free Se in young leaves of S. pinnata consists of approximately 80% MeSeCys and approximately 20% selenocystathionine, with no detectable inorganic forms, as judged from LC-MS; similarly, XANES Se analysis of S. pinnata found greater than 98% of Se as C-Se-C forms (Shrift and Virupaksha, 1965; Freeman et al., 2006b; this study). In contrast, the secondary accumulator S. albescens contained only selenocystathionine as the detectable free organic Se form in both young and old leaves, as judged from LC-MS. XANES Se speciation analysis demonstrated for S. albescens that C-Se-C forms represented 75% of total Se, along with 20% SeCys and 5% selenate. The high MeSeCys levels in S. pinnata may be explained by its increased level of SMT protein, which was detected on average 7-fold more than in S. albescens by immunoblotting. Accumulation of Se as MeSeCys is thought to offer a safe way to sequester Se, since this amino acid does not get misincorporated into proteins and thus likely contributes to the enhanced Se tolerance and hyperaccumulation of S. pinnata (Brown and Shrift, 1981, 1982; Neuhierl and Bock, 1996). While the only free organic form found in S. albescens, selenocystathionine, may offer some protection from Se incorporation into protein, it may be more toxic than MeSeCys. Selenocystathionine is a minor constituent of total Se in hyperaccumulators, while MeSeCys is usually the predominant form in hyperaccumulators. Moreover, species such as A. bisulcatus or Astragalus racemosus with the highest Se hyperaccumulation (5,000–10,000 μg total Se g−1 dry weight) typically contain exclusively MeSeCys, while species such as S. pinnata, Neptunia amplexicaulis, and Astragalus pectinatus, which show less extreme Se hyperaccumulation (2,000–5,000 μg total Se g−1 dry weight), typically contain a mixture of 70% to 80% MeSeCys and 20% to 30% SeCyst (Horn and Jones, 1941; Shrift and Virupaksha, 1965; Peterson and Butler, 1967; Freeman et al., 2006b). Species such as the Se accumulator Morinda reticulata that accumulate approximately 90% of total Se as SeCyst closely match the secondary accumulator S. albescens in their total Se accumulation when fed Se (Peterson and Butler, 1971). Moreover, SeCyst is a metabolic intermediate between SeCys and SeMet, and both amino acids can be toxic to plants when incorporated into protein (for review, see Pickering et al., 2003b). MeSeCys, on the other hand, is the result of a branching pathway that moves Se away from incorporation into protein. It is possible that the SeCyst accumulation found in both S. pinnata and S. albescens offers some protection from Se incorporation into proteins in the form of SeMet, but it may not protect against SeCys being misincorporated into protein, which likely is more toxic than SeMet misincorporation, in view of the important functions of Cys residues in the disulfide bonds often required for proper protein structure and function. The SeCys and selenate found to make up the additional 25% of total Se in S. albescens are more toxic than MeSeCys or SeCyst and most likely further contribute to toxicity. Incidentally, it is interesting that this secondary accumulator, S. albescens, accumulated such a large fraction of Se in organic form. Other secondary accumulators and nonaccumulators were reported to accumulate mainly selenate when supplied with selenate, with a minor fraction of organic Se with a XANES spectrum similar to SeMet (de Souza et al., 1998; Van Hoewyk et al., 2005). These results, however, may be artifactual and completely due to the short time after selenate treatment before these plants were harvested. Based on XANES alone, SeMet, MeSeCys, and SeCyst cannot be distinguished (all contain C-Se-C), and thus it is possible that this minor organic fraction was SeCyst. Mass spectrometry studies did indeed reveal the presence of SeCyst in B. juncea, Lecythis minor, and Arabidopsis, all secondary accumulators or nonaccumulator plants (Montes-Bayon et al., 2002; Dernovics et al., 2007).
It is surprising that the hyperaccumulator S. pinnata did not show a lower level of Se incorporation into protein than the nonaccumulator S. albescens. For comparison, Se incorporation into protein in the hyperaccumulator A. bisulcatus (0.09 ± 0.015) was lower than in the nonaccumulator Astragalus drummondii (0.343 ± 0.097; μg Se g−1 total protein/μg leaf Se g−1dry weight). Despite their similar levels of Se incorporation into total protein, S. pinnata accumulated 3.6-fold more Se and did not suffer any Se toxicity, while S. albescens clearly did. This could suggest that the observed Se toxicity is also due to some other process than Se incorporation into protein (e.g. oxidative stress caused by inorganic Se) or that Se incorporation in S. pinnata proteins happens in less harmful amino acids (SeMet rather than SeCys) or proteins or in a more regulated manner than in S. albescens. Past bioinformatic analysis has not revealed any essential selenoproteins in higher plants (no SeCys insertion sequence has been found; Stillwell and Berry, 2005; Zhang and Gladyshev, 2010), but it cannot be excluded that Se is incorporated posttranslationally into some proteins (e.g. by modifying a Ser residue to a SeCys enzymatically). In this context, the early report by Shrift and Virupaksha (1965) that S. pinnata showed a minor Se-enriched radioactive band with an RF greater than glutathione is interesting. When this band was hydrolyzed with 4 n HCl, chromatography showed several ninhydrin-positive spots with small amounts of radioactivity being present at the position of SeCys. These results first indicated that S. pinnata has a peptide that contains SeCys and other amino acids (Shrift and Virupaksha, 1965). Also interesting is that the gene that showed the biggest up-regulation in S. pinnata, PDF1.2, encodes a small and extremely Cys-rich pathogen defensin protein (Broekaert et al., 1995; Penninckx et al., 1996). Overproduction of a similar plant defensin from the zinc hyperaccumulator Arabidopsis halleri (AhPDF1.1) in Arabidopsis led to a significant increase in resistance to zinc and selenite (Mirouze et al., 2006; Tamaoki et al., 2008a). Considering the known role for Se in S. pinnata elemental defense (for review, see Quinn et al., 2007), one may envision the presence of a SeCys-rich toxic defense protein. Misincorporation rates alone for SeCys into highly expressed Cys-rich PDF protein in the hyperaccumulator plant may contribute to enhanced elemental defense and Se tolerance. It can also be hypothesized that Se is bound by particular S. pinnata proteins rather than being present in the primary protein sequence as SeCys. Our macroarray results did show evidence of increased expression of two SBP-encoding genes, but whether the Se bound by such a protein would still be present after TCA precipitation and acetone wash is questionable.
The tolerance of S. pinnata to Se may also involve the sequestration of Se in specific epidermal locations. XRF mapping showed that in S. pinnata, Se was accumulated in discrete “hot spots” along the leaf margins, while in S. albescens, the distribution of Se was diffuse throughout the leaf. EDS showed Se levels to be highest in S. pinnata epidermal cells around the leaf edges, with decreasing epidermal Se concentrations closer to the mid vein. No Se was detected in vascular, spongy parenchyma, or palisade parenchyma cells of S. pinnata, suggesting the S. pinnata peripheral epidermal cell clusters are the predominant sites of Se sequestration. In S. albescens, EDS only detected Se in a single, epidermal cell. Inside S. pinnata epidermal cells, the highest concentrations of Se were present in an organelle that resembles a small vacuole. The cell walls of S. pinnata had no detectable Se. Se was not detected in the cuticle or in any cuticular wax crystals tested. This is noteworthy because an earlier finding reported a potential seleniferous leaf wax in Stanleya bipinnata (McColloch et al., 1963). No Se was detected in leaf hairs, the predominant site of sequestration in another hyperaccumulator, A. bisulcatus (Freeman et al., 2006b). Thus, S. pinnata appears to sequester most of its Se in the symplast, in small vacuoles of epidermal cells, particularly near the leaf edges. The unique Se transport and sequestration mechanisms into and out of these localized cells is unknown and deserves further exploration as one of the possible key mechanisms for Se hypertolerance and hyperaccumulation. It is possible that Se accumulation in localized areas both prevents plants from Se toxicity and provides a storage mechanism for organic nontoxic Se to remobilize for future biotic defense in growing young leaves and reproductive parts.
Another mechanism contributing to S. pinnata's Se tolerance may be its capacity to prevent or reduce Se-associated oxidative stress. The decreased photosynthetic performance in Se-treated S. albescens may have been caused by Se-induced problems with the photosynthetic machinery, either via selenate-mediated oxidative stress or via replacement of S amino acids by their Se analogs in photosynthetic proteins. Such negative effects of Se on photosynthesis may be further magnified by a subsequent increase in ROS generation. Electrons lost from inefficient photosynthetic electron transport can react with molecular oxygen, forming superoxide, which then is converted to hydrogen peroxide and other free radical intermediates. Visualization of ROS in situ using hydrogen peroxide- or superoxide-sensitive dyes showed that S. pinnata accumulated lower levels of ROS than S. albescens. Prolonged exposure to ROS may have caused a programmed cell death cascade in S. albescens leaves, leading to the observed chlorosis and necrotic lesions. Leaves of S. pinnata contained higher levels of the ROS-scavenging metabolites GSH and AsA compared with S. albescens leaves, suggesting a higher free radical-scavenging capacity that would explain the observed lower levels of ROS accumulation in S. pinnata in the presence of Se. The high level of GSSG in S. pinnata suggests that ROS were generated in this hyperaccumulator but that the increased glutathione pool was potentially able to better scavenge the Se-associated free radicals before they could cause oxidative stress. Our previous study with the nonhyperaccumulator Arabidopsis indicated that an optimal level of ROS generation is necessary for selenite resistance in this species (Tamaoki et al., 2008b). We hypothesized that ROS production in Arabidopsis may be required as signal molecules for the activation of pathways that lead to Se resistance. In analogy, it is possible that 20 μm Se treatment leads to optimal ROS levels for Se tolerance in S. pinnata, while in S. albescens, this same Se treatment leads to excess levels of ROS, damaging photosystems and triggering programmed cell death pathways.
Perhaps further lowering oxidative stress, S. pinnata showed higher expression levels of several molecular chaperone-encoding genes (luminal-binding proteins, HSPs). The enhanced expression level of molecular chaperones may contribute to S. pinnata's capacity to prevent oxidative stress by helping repair any Se-induced problems with protein folding or stability. Moreover, there was a trend for genes involved in the biosynthesis of FeS clusters or MoCo to be more highly expressed in S. pinnata compared with S. albescens; a similar pattern was observed for the expression levels of several genes encoding proteins that require these cofactors to function. Since FeS clusters and MoCo are sensitive to oxidative stress, and the absence of the cofactor usually leads to degradation of the cofactor-requiring protein, the up-regulation of these genes may reflect a better capacity in the hyperaccumulator to reconstitute these cofactors, which may in turn explain their better photosynthetic capacity.
S. pinnata showed constitutive up-regulation of genes involved in biosynthesis of the phytohormones MeJA, JA, SA, and ethylene. These hormones are typically associated with stress and defense responses in plants. JA and ethylene were shown earlier to also be involved in selenite resistance in Arabidopsis (Tamaoki et al., 2008b). A more Se-resistant accession of this species produced higher levels of JA and ethylene in response to Se treatment, and genes involved in the synthesis of these hormones were more highly induced. Moreover, knockout mutants unable to synthesize or respond to these hormones showed reduced resistance, while supply of the hormones to a more Se-sensitive accession enhanced resistance. We hypothesized that the positive effects of these hormones on Se tolerance may be due to up-regulation of S uptake and assimilation. MeJA has been shown to up-regulate genes involved in primary and secondary S-related pathways (Jost et al., 2005). After selenite treatment, genes encoding key reactions of sulfate assimilation and glutathione synthesis were indeed up-regulated in Arabidopsis (Tamaoki et al., 2008b). Constitutive up-regulation of S assimilation may enable the plant to more efficiently prevent Se analogs from replacing S in proteins and other S compounds.
In S. pinnata, many important S assimilation pathway genes were found to be constitutively up-regulated compared with S. albescens. The constitutively up-regulated sulfate/selenate assimilation pathway in roots and shoots may explain the higher total S and Se levels in the hyperaccumulator as well as the higher levels of reduced antioxidant thiols. When grown with Se, S. pinnata had accumulated 1.3-fold more S than S. albescens, and mature leaves of S. pinnata plants with Se contained 60% higher nonprotein thiol levels compared with the mature leaves of S. albescens. When grown with Se, S. pinnata contained a 1.4-fold higher level of GSH, a 1.2-fold higher GSSG level, and a 1.3-fold higher total glutathione level than S. albescens. In the Se-sensitive species S. albescens, there was a significant drop in the reduction state of its glutathione pool when treated with Se, while in the Se-tolerant S. pinnata, the ratio of GSH to GSSG was unchanged. It is highly plausible that part of the Se tolerance mechanism in S. pinnata is its ability to prevent S starvation in the presence of Se and that S. albescens is suffering from S deficiency when grown with Se.
The genetic mechanisms likely responsible for the increased Se and S accumulation ability of the Se hyperaccumulator S. pinnata are also discovered herein, because in S. pinnata leaves grown without Se, three Cys synthase genes were constitutively up-regulated (Fig. 4A). In roots of S. pinnata grown without Se, 22 different S assimilation genes were more highly expressed compared with S. albescens (Table V). These included a sulfate transporter gene, Sultr1;2, previously shown to result in selenate resistance in Arabidopsis when knocked out (Shibagaki et al., 2002). This implicates Sultr1;2 in the nonspecific transport of selenate into Arabidopsis roots; perhaps a homolog in S. pinnata has evolved to function mainly in selenate uptake. In this context, it is interesting that the Se/S ratio, a direct μg comparison, was 0.43 for S. pinnata and 0.19 for S. albescens, while the selenate/sulfate ratio in the supplied medium was 0.05. The hyperaccumulator had both higher S and Se levels than the nonhyperaccumulator, but it appeared to have a higher preference for Se relative to S. This is in support of the presence of a specialized selenate transporter in the hyperaccumulator. A high Se/S ratio is thought to be one of the typical characteristics of hyperaccumulators (White et al., 2004, 2007). In agreement with our observed constitutive up-regulation of genes responding to MeJA, JA, and SA signaling in S. pinnata, the levels of these hormones were constitutively higher in the hyperaccumulator leaves and they were induced in response to Se in S. albescens roots. External supply of MeJA or ACC resulted in enhanced levels of Se accumulation in S. albescens over this short-term experiment. These results indicate that internal MeJA and/or ethylene levels cause a higher Se accumulation capacity in Stanleya species, with the hyperaccumulator having higher constitutive levels of these hormones than the secondary accumulator. Taken together, constitutive up-regulation of S assimilation mechanisms, mediated by MeJA, JA, and/or SA, may be an important underlying molecular mechanism for S. pinnata's Se tolerance and hyperaccumulation.
The same hormones, MeJA, JA, and SA, function to up-regulate defense-associated genes when plants are attacked by pathogens or herbivores (Turner et al., 2002; Wang et al., 2002; Durrant and Dong, 2004). Thus, it is not surprising that S. pinnata showed constitutive up-regulation of many defense-associated genes, including the Cys-rich PDF gene mentioned above. Whether the up-regulation of this class of genes plays any role in Se tolerance or accumulation at this point is unclear. The upstream signal transduction changes that constitutively up-regulate S assimilation (via MeJA, JA, SA, or ethylene) may simply also turn on these defense-related genes, without having an additional effect on Se metabolism. Alternatively, it is possible that these genes, traditionally associated with biotic stress resistance, also serve functions in abiotic stress resistance and are regulated by the relative oxidative status of the plant. ROS-antioxidant interaction is inherently involved in many different stresses and responses of plants to their environment (Foyer and Noctor, 2005). An elemental stimulus such as Se that disrupts cellular redox balance could serve as an inducer for sets of defense-related genes, including PR proteins. Low levels of ascorbate and changes in the cellular glutathione pool can also elicit pathogen resistance responses (Mou et al., 2003; Pastori et al., 2003; Barth et al., 2004; Gomez et al., 2004).
Earlier studies have particularly focused on the Se hyperaccumulator Astragalus and on SMT as a key enzyme for Se hyperaccumulation and tolerance (Neuhierl and Bock, 1996; Ellis et al., 2004; Sors et al., 2005b, 2009). Comparative Astragalus studies found a correlation between hyperaccumulation and SMT but no difference in the enzymatic rates of S assimilation between various hyperaccumulator and nonaccumulator species (Sors et al., 2006). In S. pinnata, SMT protein levels were also elevated compared with the secondary accumulator. This likely constitutes one of the underlying mechanisms for Se tolerance and hyperaccumulation in this species, since the main form of Se accumulated in S. pinnata is MeSeCys, while in the secondary accumulator it is Se-cystathionine. However, the elevated SMT and MeSeCys level appears to be but one piece of the puzzle controlling Se hyperaccumulation. Sequestration of MeSeCys in the vacuoles of specialized epidermal cells may be another. Moreover, it appears that the S assimilation pathway is constitutively up-regulated in the S. pinnata hyperaccumulator, judged from the higher levels of transcripts observed and the higher total S and GSH levels. The enhanced transcript levels of genes involved in S assimilation in S. pinnata compared with S. albescens, particularly in the absence of Se and in the roots, indicate that the expression of these genes is constitutively up-regulated in the Se hyperaccumulator S. pinnata. The expression of similar genes was also frequently induced by Se in the secondary accumulator S. albescens. It may be proposed that S. pinnata has lost the ability to sense its overall S status and behaves as if it is continuously S starved. This in turn may affect cellular redox state, S and Se levels, and thus Se hyperaccumulation. The up-regulated S assimilation pathway may alleviate a bottleneck for both sulfate and selenate transport followed by assimilation into Cys/SeCys, and the elevated SMT levels further convert SeCys into nontoxic MeSeCys. The key to the up-regulation of these S-related pathways in S. pinnata appears to be controlled in part through constitutively elevated levels of the signaling molecules MeJA, JA, and SA. The upstream mechanisms that result in the up-regulation of these hormone levels will be an interesting topic for further study. Future, more comprehensive transcriptomic studies may reveal one or more key genes upstream of the ones observed in this study that respond to Se and up-regulate the genes causing the elevated hormone and S levels.
Identification and comparison of these key genes would open the possibility of transferring the entire Se hyperaccumulator syndrome to fast-growing nonaccumulator species with favorable properties for phytoremediation or for producing Se-enriched biofortified food. Further research may also reveal hyperaccumulator-specific signal transduction mechanisms and Se transporters for the uptake of selenate and for the transport and sequestration of other selenocompounds, particularly MeSeCys.
MATERIALS AND METHODS
Plant Growth
Stanleya pinnata seeds were obtained from native plants growing in the field in Pine Ridge Natural Area west of Fort Collins, Colorado. Stanleya albescens seeds were collected from the Uncompahgre plateau in western Colorado.
During plant plate growth experiments, plants were grown for 30 d on square agar plates containing half-strength Murashige and Skoog salt plus B5 vitamins, with and without 40 μm selenate. Suc was excluded from all experiments. Seeds from S. pinnata and S. albescens were grown on separate plates to exclude any phytotoxic effects of seeds from one species to those of the other.
When growing plants to measure Se accumulation, physiology of Se toxicity, SMT immunoblot, and Se protein incorporation, macroarray and metabolic analyses experiments followed an arid western plant growth protocol previously used for Se hyperaccumulators and described by Sors et al. (2005a). Twenty plants per treatment from each species were grown via a controlled drip system in a sterilized, pathogen-free laboratory growth room on presoaked and water-rinsed gravel (Turface) in round 25-cm-diameter pots, for 16 weeks, with and without 20 μm selenate. No sign of any pathogen infection was ever observed in these plants. They were fertilized with (in mg L−1) 200 nitrogen, 29 phosphorus, 167 potassium, 67 calcium, 30 magnesium, and micronutrients. Nutrients were supplied from 1 g L−1 15-5-15 commercial fertilizer formulation (Miracle Gro Excel Cal-Mag; Scotts). Adjustment of pH to 5.7 to 6.0 was achieved via 93% sulfuric acid at 0.08 mL L−1. Next to each seedling, this solution was dripped into each pot four times weekly, 20 mL of solution during weeks 1 to 5, 50 mL during weeks 5 to 10, and 100 mL during weeks 10 to 16, for each mature plant used. Plants were grown at 24°C/20°C (day/night) with a 16-h photoperiod and a full-spectrum photosynthetic photon flux density of 300 μmol m−2 s−1.
Leaf Harvest
Young leaves from plants were harvested and flash frozen after 10 weeks. Leaf number for all treatments and species at 10 weeks was on average 11.2 ± 1.7. No visible toxicity sign of any kind was observed in leaves when they were harvested at 10 weeks, and a portion was stored at −80°C for genetic and biochemical analysis. Fresh leaves at 10 weeks were used for in situ ROS tests. After 16 weeks, plants were used for chlorophyll fluorescence measurements before leaves were harvested for toxicity images and total Se analysis.
Quantification of Se and S Accumulation
Plant materials were rinsed with distilled water and dried at 45°C for 48 h. Replicates were acid digested and analyzed for Se and S by inductively coupled plasma atomic emission spectrometry as described by Pilon-Smits et al. (1999).
Quantification of Se Tolerance Index
For quantification of selenate resistance, plants were grown on vertically placed agar plates for 30 d with or without 40 μm selenate and root length was measured. The Se tolerance index was calculated for both species as root length grown in the presence of selenate divided by mean root length on control medium as described by Zhang et al. (2006) and Tamaoki et al. (2008b). Ten replicate plants were measured for each plant species and treatment.
Chlorophyll Fluorescence Measurements
Light intensity-dependent electron transport rate was measured after 16 weeks of growth from six plants per treatment using the method described by Abdel-Ghany et al. (2005). Relative electron transport rate was calculated from the product of ΦPSII (for electron flux through PSII) and light intensity (μmol m−2 s−1). The resulting chlorophyll fluorescence parameter represents the efficiency of PSII photochemistry (Genty et al., 1989).
In Situ ROS Detection
Se-induced in situ accumulation of superoxide was detected with nitroblue tetrazolium (Boehringer Mannheim) as described by Jabs et al. (1996). To visualize in situ accumulation of hydrogen peroxide, 3,3′-diaminobenzidine staining was performed as described by Torres et al. (2002).
Antioxidant Analyses
To measure GSH and GSSG levels, 100 mg of fresh leaves was homogenized in 2 mL of cold 5% (w/v) metaphosphoric acid. GSH and GSSG contents were measured as described by Yoshida et al. (2006).
For measurement of AsA content, leaves were freeze dried using a Genesis Freeze Drier (Virtis). Lyophilized samples were then weighed to determine percentage dry matter content and ground in preparation for extraction. The dried samples were ground into a fine powder using a mortar and pestle and sieved with a No. 20 Tyler sieve (WS Tyler). For sample extraction, 5 mL of 80% acetone (Fisher Scientific) and 200 mg of powder from each replicate were placed in 15-mL centrifuge tubes. The tubes were thoroughly mixed, rotated in the dark (4°C) for 15 min, and then centrifuged (4°C, 4,000 rpm) for 15 min. One milliliter of supernatant fluid was transferred to a microcentrifge tube and vacufuged at 45°C to dryness (approximately 2–3 h). Samples were stored at −20°C until analytical tests were completed. AsA (vitamin C) content was determined using a HPLC method as described by Esparaza Rivera et al. (2006) and modified from Galvis Sanchez et al. (2003). Freeze-dried samples were extracted with a 5% (w/v) aqueous solution of metaphosphoric acid containing 1% (w/v) dithiothreitol (Promega) and then allowed to rotate for 15 min at 4°C. The samples were centrifuged for 5 min at 4,000 rpm and 4°C before the supernatant was filtered through a 0.45-μm nylon syringe filter. The extraction process was repeated, and the supernatant from both extractions was placed in an amber HPLC vial. AsA standards were made by mixing 100 mg of dithiothreitol (Promega), 10 mg of AsA (Sigma-Aldrich), and 10 mL of 100% methanol before diluting to five concentrations for the standard curve. All samples were analyzed by HPLC (Hewlett-Packard model 1050 series) using an Inertsil C4 column run with a phosphoric acid/methanol gradient, and absorbance was read at 254 nm and analyzed using Chem Station for LC Rev A 09.01 software (Agilent Technologies).
Antioxidant Capacity
Leaves were freeze dried using a Genesis Freeze Drier (Virtis). Lyophilized samples were then weighed to determine percentage dry matter content and ground in preparation for extraction. The dried samples were ground into a fine powder using a mortar and pestle and sieved with a No. 20 Tyler sieve (WS Tyler). For sample extraction, 5 mL of 80% acetone (Fisher Scientific) and 200 mg of powder from each replicate were placed in 15-mL centrifuge tubes. The tubes were vortexed until thoroughly mixed, rotated in the dark (4°C) for 15 min, and then centrifuged (4°C, 4,000 rpm) for 15 min. One milliliter of supernatant was transferred to a microcentrifuge tube and vacufuged at 45°C to dryness (approximately 2–3 h). Samples were stored at −20°C until analytical tests were completed.
The ABTS diammonium salt assay was used to estimate antioxidant capacity. This assay is based upon measuring the capacity of an extract to scavenge and detoxify the ABTS˙+ radical and is considered an estimate of hydroxyl-scavenging activity (Miller and Rice-Evans, 1996). The protocol used was based on the microplate method described by Esparaza Rivera et al. (2006) as modified from Miller and Rice-Evans (1996). The ABTS˙+ solution was prepared by mixing 40 mg of ABTS˙+ (Calbiochem, EMD Biosciences), 15 mL of distilled water, and 2.0 ± 0.5 g of MnO2 (Sigma-Aldrich). After 20 min, the MnO2 was removed using double filtration, first with vacuum filtration and second with a 0.2-μm syringe filter. The absorbance value of the ABTS˙+ solution was read at 734 nm in the Spectra Max Plus (Molecular Devices) spectrophotometer using Softmax Pro software (Molecular Devices) and adjusted to 0.70 absorbance units by adding 5.0 mm phosphate buffer solution. Once the ABTS˙+ solution was adjusted, it was held at 30°C and used within 4 h. Vacufuged samples were reconstituted with 1 mL of 80% acetone (Fisher Scientific). Twenty-five microliters of each reconstituted sample was mixed with 250 μL of the ABTS˙+ solution, and the absorbance value was read after exactly 60 s at 30°C in a temperature-controlled microplate reader. ABTS˙+ antioxidant capacity was reported as Trolox equivalent antioxidant capacity (TEAC) per gram of sample on a fresh weight basis and was calculated by comparing with a Trolox (Calbiochem) standard curve. Analyses were run in triplicate at three dilutions for a total of nine assays per sample.
The DPPH+ assay was also used to estimate antioxidant capacity and was measured using the method of Lu and Foo (2000) with some modifications. Vacufuged samples were reconstituted with 1.0 mL of 5.0 mm phosphate buffer solution. A 0.1 mm DPPH+ solution was made by mixing 7.89 mg of DPPH+ with 100% methanol. Absorbance was read in the Spectra Max Plus (Molecular Devices) spectrophotometer using Softmax Pro software (Molecular Devices) at 515 nm and adjusted to 0.95 absorbance units. Fifteen microliters of the reconstituted samples were mixed with 285 μL of the DPPH+ solution and read at 515 nm exactly after being held for 3 min at 25°C. The results were compared with a Trolox (Calbiochem) standard curve and expressed as TEAC 100 g−1 fresh weight.
Western Blot
SDS-PAGE was performed according to Laemmli (1970) using 12.5% gels. Immunoblotting experiments were carried out according to Neuhierl et al. (1999) using 30 μg of total leaf protein loaded from each plant and an anti-Astragalus bisulcatus SMT antibody at a 1:4,000 dilution. Quantification of immunoreactive bands was achieved using the ImageJ program (National Institutes of Health; http://rsb.info.nih.gov/ij). A. bisulcatus SMT protein was predicted from its sequence to be 36.7 kD (Neuhierl et al., 1999).
Se Protein Incorporation
Protein was extracted as described by Garifullina et al. (2003), and a portion was quantified by bicinchoninic acid protein assay from Pierce, according to the manufacturer's directions. Total protein concentrations were then adjusted to be equal, precipitated with TCA, and washed two times with acetone. The acetone-washed total protein pellet was then acid digested and assayed by inductively coupled plasma atomic emission spectrometry, and the amount of Se in total proteins was normalized to total Se in leaves to calculate the total Se protein incorporation coefficient by the method of Garifullina et al. (2003).
Se Standards
Na2SeO4 (S8295), Na2SeO3 (S1382), SeCystine (S1650), and SeMet (S3132) were obtained from Sigma-Aldrich. MeSeCys, γ-glutamylmethylselenocysteine, and SeGSH2 standards were obtained from PharmaSe. SeCys was obtained by reducing SeCystine at 25°C overnight in 100 mm sodium borohydride at a 1:1 molar ratio. Gray and red elemental Se were generously provided by Amy Ryser and Dan Strawn.
μ-XRF/μ-XAS
Samples were washed to remove any external Se, flash frozen in liquid nitrogen, and shipped on dry ice to the Advanced Light Source at the Lawrence Berkeley Laboratory for microspectroscopic analysis on Beamline 10.3.2 (Marcus et al., 2004). The total distribution of Se was mapped by scanning the samples in the microfocused x-ray monochromatic beam at 13,085 eV. Samples were mounted onto a Peltier stage kept at −33°C to reduce potential radiation damage. μ-SXRF mapping of Se was performed first on all samples. Large maps were collected using a 7-μm (horizontal) × 7-μm (vertical) beam at 13,085 eV sampled in 20-μm × 20-μm pixels. The Kα fluorescence line intensities of Se (and other elements of interest) were measured with a seven-element GE solid-state detector and normalized to the incident beam intensity and dwell time. The chemical forms of Se on spots of interest in sample X were further investigated using microfocused Se K-edge XANES. XANES provides information about the oxidation state and, when compared with well-characterized Se standard compounds, information about its chemical speciation (Pickering et al., 1999). Aqueous solutions of the various selenocompounds were used as standard materials. Red Se (white line maximum set at 13,074.73 eV) was used for energy calibration. μ-XRF and XANES data analyses were performed with a suite of LabVIEW programs (National Instruments) available at Beamline 10.3.2 (http://xraysweb.lbl.gov/uxas/Beamline/Software/Software.htm).
EDS
Young leaves from mature plants of S. pinnata and S. albescens were washed to remove any external Se. They were then placed in plastic test tubes with the base of the petiole immersed in water to prevent dehydration and sealed before overnight shipping to Rothamsted Research. EDS was used to analyze for Se on the fully hydrated freeze-fractured leaves. Leaf tissue (7 mm × 5 mm) was cut from each specimen using a sterile blade, mounted on a cryo stub using Tissue-Tek OCT compound (Sakura), and plunge frozen in preslushed liquid nitrogen. The specimen was transferred under vacuum to the GATAN Alto 2100 cryo chamber (Gatan) with temperature maintained at −180°C. Here, it was fractured, etched to remove any contaminating ice, and coated with silver for examination. The specimen was then passed into the JEOL LV6360 scanning electron microscope and mounted on the stage with the temperature maintained at −160°C and with parameters set for EDS analysis using the OXFORD INCA 2000 microanalysis system (Oxford Instruments). The L line for Se was chosen to avoid peak interference on the Kα line and an acceleration voltage of 5,000 eV.
Macroarray Gene Expression Analysis
Expression of 324 different genes (listed in Supplemental Tables S1 and S3) was analyzed by custom-made cDNA macroarrays using cDNA clones from the Arabidopsis Biological Resource Center and RIKEN BioResource Center. Twenty-three genes were redundant between gene sets 1 and 2. These cDNA clones were resequenced for confirmation before use. Seventy nanograms of each PCR-amplified sample was blotted onto Hybond N+ nylon membranes with Multi Pin Blotter 96 (Atto). Each gene was spotted on a membrane in duplicate. λDNA was used as a negative control. The constitutively expressed gene EF1α (At5G12110) was included as an internal standard. For the macroarray studies, young leaves from both species were harvested after 10 weeks of growth in the arid western plant growth protocol with or without 20 μm sodium selenate. Then shoots were separated from roots and frozen with liquid nitrogen for total RNA extraction. Total RNA was extracted from shoots and roots using the RNeasy Plant Mini kit (Qiagen). Hybridization, probe labeling, and signal detection were carried out according to Tamaoki et al. (2003). The signal intensity of each spot was obtained as described previously (Tamaoki et al., 2003). In brief, we subtracted the value of the signal intensity of the negative control (λDNA) from the signal intensity of each spot and then normalized the signal intensities against the intensity of EF1α, the expression of which had been confirmed to be unchanged with or without 20 μm selenate (Supplemental Fig. S4). Macroarray analysis was carried out two times using biological replicate samples, and each macroarray membrane contained duplicate spots for each gene. Thus, the average, sd, and P value of each gene were calculated from fold induction values calculated from four independent spots. From the four independent signal intensities of each spot, we calculated the difference in gene expression between the two Stanleya species without selenate (designated as constitutive difference) or with selenate (designated as induced difference). The average, sd, and P value of each gene were calculated from fold induction values using GeneSpring GX10 software (Agilent Technologies).
Expression Analysis via Semiquantitative RT-PCR
Total RNA was isolated from shoots as described above. Five micrograms of DNase-treated total RNA was reverse transcribed using the First-Strand cDNA Synthesis kit (Fermentas International) following the manufacturer's instructions. PCR was carried out as described previously (Schiavon et al., 2007). After the separation of each band with agarose gel electrophoresis, the signal intensity of each band was measured with the ImageJ program. Obtained signal intensities were normalized against the intensity of EF1α, a constitutively expressed control gene for RT-PCR. A list of primers used in these experiments is presented as Supplemental Table S5.
Expression Analysis via Northern-Blot Analysis
For northern-blot analysis, total RNA was isolated from shoots as described above. An aliquot (10 μg) of each RNA preparation was separated by denaturing agarose gel electrophoresis and transferred onto Hybond N+ membranes (GE Healthcare). The cDNA fragments used for hybridization as probes were obtained from the RT-PCR analysis as described above. These cDNA fragments were sequenced for confirmation before use. Probes were made by cutting plasmids with suitable restriction enzymes and then labeled with [α-32P]dCTP. After the hybridization, the signal intensity of each band was measured with the ImageJ program.
LC-MS Metabolite Quantification
LC-MS analysis of organic selenocompounds in plant tissues was performed as described by Freeman et al. (2006b). This method does not detect Se that is incorporated in proteins.
LC-MS of MeJA, SA, and JA levels in shoot tissue was determined in 10-week old plants grown as described above with or without 20 μm sodium selenate. The extracts were prepared as described by Wilbert et al. (1998). The extracts were analyzed by LC-MS using a Hewlett-Packard Agilent 1100 series HPLC apparatus and a Finnigan LcQDuo thermoquest MS system equipped with Xcalibur software. Through 30-μL injections, these extracts were separated at 40°C using a Phenomenex Hypersil 5-mm C18 (ODS) column (250 × 2 mm, 5 mm), at a flow rate of 0.32 mL min−1, using two eluents: (A) water + 0.1% formic acid; and (B) 100% methanol + 0.1% formic acid. The following gradient program was used during the 23-min run: 0 to 7 min, 50% A and 50% B; 7 to 9 min, 30% A and 70% B; 9 to 12 min, 100% B; 12 to 13 min, 50% A and 50% B; with a 10-min postrun column wash of 50% A and 50% B. Standard curves were established using chemicals purchased from Sigma Chemical. MeJA (catalog no. 392707) had a retention time of 2.5 min, SA (catalog no. A-6262) had a retention time of 4.45 min, and JA (catalog no. J2500) had a retention time of 6.85 min. Through MS, the different metabolites were measured at their appropriate masses, and retention times were observed for each of the standards. The MS detector settings were 1 to 3.5 min in positive ion mode using parameters generated with the MeJA standard and the automated tune program, 3.5 to 5.5 min in negative ion mode using parameters generated with the SA standard and the automated tune program, 5.5 to 13 min in negative ion mode using parameters generated with the JA standard and the automated tune program. Samples were kept at room temperature (25°C) in the autosampler. The previously published (Wilbert et al., 1998) and observed precursor and product ions for these standards were exactly the same.
GC of Ethylene and GC-MS of MeLin
GC was used to measure ethylene evolution of 5-week-old plants, as described by Tamaoki et al. (2008b). Two plants were enclosed into a 60-mL Labco Exetainer vial (Labco Limited) for GC analysis and incubated for 24 h with illumination in a growth chamber. Ethylene generated during the incubation was measured by injecting 25 mL of head space gas into a Fisions 8000 gas chromatograph with a flame ionization detector. A 2-m Altec Hayesep N 80/100 column was used with isothermic oven temperature at 70°C and flame ionization detection temperature at 200°C. The program was 4 min in length, with the ethylene peak eluting at 1.40 min. Ethylene peak area was determined by the PeakSimple program (version 3.39, six channel; SRI Instruments).
Frozen young leaves were extracted for MeLin analysis by placing 100 mg of frozen tissue into 1 mL of ice-cold ultrapure methanol at 4°C with occasional vortexing until leaves were fully extracted and 100% clear opaque and the methanol was green. Samples were then centrifuged for 10 min at 15,800g. MeLin was measured using an Agilent 6890 Plus/5973 N GC-MS system equipped with a 7683 autosampler tray and autoinjector module with Chemstation software and data system. The column used was a 30-m Supelco SPB-1 column with a 250-μm diameter, 0.25-μm film thickness, and a maximum temperature of 320°C. Initial flow was 1.0 mL min−1, with an average velocity of 37 cm s−1 and a nominal pressure of 8.64 p.s.i. The elution profile of the oven was a controlled temperature ramp of 70°C at 2 min and 200°C at 20 min with a 320°C 5-min postrun column cleanup. Sample volume injected was 2.0 μL with no postinjection dwell time. Retention time for MeLin in leaves was 14.13 min and was quantified using an appropriate standard curve that was established using a standard purchased from Sigma Chemical (catalogue no. L2626). MeLin in leaves from both species matched the Wiley 6N mass database used on this GC-MS system with an exact 99% quality. The authentic MeLin standard had the exact same retention time as the plant MeLin at 14.13 min and also matched the Wiley 6N mass database with 99% quality.
Total Phenolics Content
Leaves were freeze dried using a Genesis Freeze Drier (Virtis). Lyophilized samples were then weighed to determine percentage dry matter content and ground in preparation for extraction. The dried samples were ground into a fine powder using a mortar and pestle and sieved with a No. 20 Tyler sieve (WS Tyler). For sample extraction, 5 mL of 80% acetone (Fisher Scientific) and 200 mg of powder from each replicate were placed in 15-mL centrifuge tubes. The tubes were vortexed until thoroughly mixed, rotated in the dark (4°C) for 15 min, and then centrifuged (4°C, 4,000 rpm) for 15 min. One milliliter of supernatant was transferred to an Eppendorf tube and vacufuged at 45°C to dryness (approximately 2–3 h). Samples were stored at −20°C until analytical tests were completed. Total phenolics content was measured using a microplate-based Folin-Ciocalteu assay adapted from Spanos and Wrolstad (1990), Singleton et al. (1999), and Esparaza Rivera et al. (2006). Vacufuged extractions were reconstituted with 1.0 mL of 80% acetone, and 100 μL of this solution was diluted with 900 μL of nanopure water. In triplicate, 35 μL of the diluted sample was pipetted into microplate wells. Using a multichannel pipette, 150 μL of 0.2 m Folin-Ciocalteu reagent (Sigma-Aldrich) was added to all wells. The plate was shaken for 30 s and held for 5 min at room temperature, followed by the addition of 115 μL of 7.5% (w/v) Na2CO3 (Fisher Scientific) to all wells and rotational shaking for 30 s. The plate was held for an additional 5 min at room temperature and incubated at 45°C for 30 min followed by cooling to room temperature for 1 h. Plates were then read at 765 nm in a Spectra Max Plus (Molecular Devices) spectrophotometer using Softmax Pro software (Molecular Devices). Total phenolic content was calculated by comparing with a gallic acid (Sigma Chemical) standard curve and expressed as mg 100 −1 g fresh weight.
In Vitro Treatments
Both Stanleya plant species were grown in Turface along with the same fertilizer and 20 μm selenate used in the long-term arid drip system. Hormone and elicitors were sprayed onto the shoots, 20 mL daily at 10 or 100 μm MeJA or ACC, along with a water control for the last 3 weeks before the 6-week-old S. pinnata and S. albescens shoots were harvested and dried for Se analyses. S. pinnata was then grown in Turface and fertilized as described above, and 20 μm selenate, hormone, and elicitors were sprayed onto the shoots: 20 mL daily five times per week at 10, 100, or 500 μm MeJA, JA, SA, or ACC, along with a water control for the last 2 weeks before the 3-week-old S. pinnata shoots were harvested and dried for Se analyses. Finally, seeds from both plant species were sterilized using 20% bleach and 70% methanol and then rinsed 10 times with sterile distilled, deionized water and germinated on half-strength Murashige and Skoog + B5 vitamins with no Suc. All plants were germinated and grown in the presence of 20 μm selenate with no added OAS, or with medium containing 20 μm selenate and 50 or 100 μm OAS. After 30 d, shoots from both species were harvested and dried for Se analyses.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Root lengths of S. pinnata and S. albescens grown on vertically placed agar plates.
Supplemental Figure S2. Total nonprotein thiols.
Supplemental Figure S3. Graphic depiction of the macroarray expression differences.
Supplemental Figure S4. Northern blot and semiquantitative RT-PCR.
Supplemental Figure S5. Liquid chromatographic separation and positive mass spectra for SeCyst.
Supplemental Table S1. Shoots, gene set 1, Se induction.
Supplemental Table S2. Roots, gene set 1, Se induction.
Supplemental Table S3. Shoots, gene set 2, Se induction.
Supplemental Table S4. Northern and semiquantitative RT-PCR quantification.
Supplemental Table S5. Arabidopsis primers.
Supplemental Table S6. Percentage genetic relatedness.
Supplementary Material
Acknowledgments
We are grateful to David E. Salt and Wendy A. Peer for their Se hyperaccumulator growth protocol, internal transcribed spacer sequence comparison, and chromosome size measurement. We also thank August Bock for providing the SMT antibodies, Donald Dick for helping us with bioanalytical chemistry, and Chris Cohu for helping us with our photosynthetic measurements.
References
- Abdel-Ghany SE, Müller-Moulé P, Niyogi KK, Pilon M, Shikanai T. (2005) Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell 17: 1233–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assuncao AGL, Martins PDC, Folter SD, Vooijs R, Schat H, Aarts MGM. (2001) Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 24: 217–226 [Google Scholar]
- Baker AJM, Brooks RR. (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1: 81–126 [Google Scholar]
- Baker AJM, Whiting SM. (2002) In search of the Holy Grail—a further step in understanding metal hyperaccumulation? New Phytol 155: 1–7 [DOI] [PubMed] [Google Scholar]
- Bañuelos G, Vickerman DB, Trumble JT, Shannon MC, Davis CD, Finley JW, Mayland HF. (2002) Biotransfer possibilities of selenium from plants used in phytoremediation. Int J Phytorem 4: 315–331 [Google Scholar]
- Barth C, Moeder W, Klessing DF, Conklin PL. (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134: 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beath OA, Gilbert CS, Eppson HF. (1939) The use of indicator plants in locating seleniferous soils in the Western United States. I. General. Am J Bot 26: 257–269 [Google Scholar]
- Bert V, Macnair MR, De Laguerie P, Samumitou-Laprade P, Petit D. (2000) Zinc tolerance and accumulation in metallicolous and nonmetallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol 146: 225–233 [DOI] [PubMed] [Google Scholar]
- Boyd RS. (2007) The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant Soil 293: 153–176 [Google Scholar]
- Boyd RS, Martens SN. (1992) The raison d'etre for metal hyperaccumulation by plants. Baker AJM, Proctor J, Reeves RD, , The Ecology of Ultamafic (Serpentine) Soils. Intercept, Andover, MD, pp 279–289 [Google Scholar]
- Broekaert WF, Terras FRG, Cammue BPA, Osborn RW. (1995) Plant defensins: novel antimicrobial peptides as components of the host defence system. Plant Physiol 108: 1353–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks RR, Lee J, Reeves RD, Jaffré T. (1977) Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J Geochem Explor 7: 49–77 [Google Scholar]
- Brown TA, Shrift A. (1981) Exclusion of selenium from proteins in selenium tolerant Astragalus species. Plant Physiol 67: 1951–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Shrift A. (1982) Selenium-toxicity and tolerance in higher plants. Biol Rev Camb Philos Soc 57: 59–84 [Google Scholar]
- Byers HG. (1935) Selenium occurrence in certain soils in the United States, with a discussion of related topics. US Dept Agric Tech Bull 482: 1–47 [Google Scholar]
- Davis AM. (1972) Selenium accumulation in Astragalus species. Agron J 6: 751–754 [Google Scholar]
- Davis AM. (1986) Selenium accumulation in Astragalus and Lupinus species. Agron J 78: 727–729 [Google Scholar]
- de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai JC, Honma TSU, Yeh L, Terry N. (1998) Rate-limiting steps in selenium volatilization by Brassica juncea. Plant Physiol 117: 1487–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernovics M, García-Barrera T, Bierla K, Preudíhomme H, Lobinski R. (2007) Standardless identification of selenocystathionine and its γ-glutamyl derivatives in monkeypot nuts by 3D liquid chromatography with ICP-MS detection followed by nanoHPLC-Q-TOF-MS/MS. Analyst 132: 439–449 [DOI] [PubMed] [Google Scholar]
- Dunnill PM, Fowden L. (1967) The amino acids of the genus Astragalus. Phytochemistry 6: 1659–1663 [Google Scholar]
- Durrant WE, Dong X. (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Ellis DR, Salt DE. (2003) Plants, selenium and human health. Curr Opin Plant Biol 6: 273–279 [DOI] [PubMed] [Google Scholar]
- Ellis DR, Sors TG, Brunk DG, Albrecht C, Orser C, Lahner B, Wood KV, Harris HH, Pickering IJ, Salt DE. (2004) Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biol 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparaza Rivera JR, Stone MB, Stushnoff C, Pilon-Smits E, Kendall PA. (2006) Effects of ascorbic acid applied by two hydrocooling methods on physical and chemical properties of green leaf lettuce stored at 5°C. J Food Sci 71: S270–S276 [Google Scholar]
- Feist LJ, Parker DR. (2001) Ecotypic variation in selenium accumulation among populations of Stanleya pinnata. New Phytol 149: 61–69 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Lindblom SD, Quinn CF, Marcus MA, Fakra S, Pilon-Smits EAH. (2007) Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytol 175: 490–500 [DOI] [PubMed] [Google Scholar]
- Freeman JL, Quinn CF, Lindblom SD, Klamper EM, Pilon-Smits EAH. (2009) Selenium protects the hyperaccumulator Stanleya pinnata against black tailed prairie dog herbivory in native habitats. Am J Bot 96: 1075–1085 [DOI] [PubMed] [Google Scholar]
- Freeman JL, Quinn CF, Marcus MA, Fakra S, Pilon-Smits EAH. (2006a) Selenium tolerant diamondback moth disarms hyperaccumulator plant defense. Curr Biol 16: 2181–2192 [DOI] [PubMed] [Google Scholar]
- Freeman JL, Zhang LH, Marcus MA, Fakra S, Pilon-Smits EAH. (2006b) Spatial imaging, speciation and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol 142: 124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeas ML, Klamper EM, Bennett LE, Freeman JL, Kondratieff BC, Quinn CF, Pilon-Smits EAH. (2008) Selenium hyperaccumulation reduced plant arthropod loads in the field. New Phytol 177: 715–724 [DOI] [PubMed] [Google Scholar]
- Galeas ML, Zhang LH, Freeman JL, Wegner M, Pilon-Smits EAH. (2007) Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related non-accumulators. New Phytol 173: 517–525 [DOI] [PubMed] [Google Scholar]
- Galvis Sanchez AC, Gil-Izquierdo A, Gil MI. (2003) Comparative study of six pear cultivars in terms of their phenolic and ascorbic acid contents and antioxidant capacity. J Sci Food Agric 83: 995–1003 [Google Scholar]
- Garifullina GF, Owen JD, Lindblom SD, Tufan H, Pilon M, Pilon-Smits EAH. (2003) Expression of mouse selenocysteine lyase in Brassica juncea chloroplasts affects selenium tolerance and accumulation. Physiol Plant 118: 538–544 [Google Scholar]
- Genty B, Briantais JM, Baker NR. (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Gomes-Junior RA, Gratao PL, Gaziola SA, Mazzafera P, Lea PJ, Azevedo RA. (2007) Selenium-induced oxidative stress in coffee cell suspension cultures. Funct Plant Biol 34: 449–456 [DOI] [PubMed] [Google Scholar]
- Gomez LD, Noctor G, Knight M, Foyer CH. (2004) Regulation of calcium signaling and gene expression by glutathione. J Exp Bot 55: 1851–1859 [DOI] [PubMed] [Google Scholar]
- Goodson CC, Parker RD, Amrhein C, Zhang Y. (2003) Soil selenium uptake and root system development in plant taxa differing in Se accumulating capability. New Phytol 159: 391–401 [DOI] [PubMed] [Google Scholar]
- Hanson B, Garifullina GF, Lindbloom SD, Wangeline A, Ackley A, Kramer K, Norton AP, Lawrence CB, Pilon-Smits EAH. (2003) Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol 159: 461–469 [DOI] [PubMed] [Google Scholar]
- Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH. (2004) Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol 162: 655–662 [DOI] [PubMed] [Google Scholar]
- Horn MJ, Jones DB. (1941) Isolation from Astragalus pectinatus of a crystalline amino acid complex containing selenium and sulfur. J Biol Chem 139: 649–660 [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273: 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jaffré T, Brooks RR, Lee J, Reeves RD. (1976) Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193: 579–580 [DOI] [PubMed] [Google Scholar]
- Jost R, Altschmied L, Bloem E, Bogs J, Gershenzon J, Hähnel U, Hänsch R, Hartmann T, Kopriva S, Kruse C, et al. (2005) Expression profiling of metabolic genes in response to methyl jasmonate reveals regulation of genes of primary and secondary sulfur-related pathways in Arabidopsis thaliana. Photosynth Res 86: 491–508 [DOI] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao FJ, McGrath SP. (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212: 75–84 [DOI] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao FJ, Wieshammer G, McGrath SP. (2001) Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J Exp Bot 52: 2291–2300 [DOI] [PubMed] [Google Scholar]
- Küpper H, Zhao FJ, McGrath SP. (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119: 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U, Grime GW, Smith JAC, Hawes CR, Baker AJM. (1997) Micro-PIXE as a technique for studying nickel localization in leaves of the hyperaccumulator plant Alyssum lesbiacum. Nucl Instrum Methods Phys Res 130: 346–350 [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- LeDuc DL, Tarun AS, Montes-Bayon M, Meija J, Malit MF, Wu CP, AbdelSamie M, Chiang CY, Tagmount A, de Souza M, et al. (2004) Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol 135: 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T. (2002) Sulfate metabolism. Somerville CR Meyerowitz EM, , The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0017, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YR, Foo LY. (2000) Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem 68: 81–85 [Google Scholar]
- Macnair M. (1993) The genetics of metal tolerance in vascular plants. New Phytol 8: 143–148 [DOI] [PubMed] [Google Scholar]
- Macnair MR, Bert V, Huitson SB, Saumitou-Laprade P, Petit D. (1999) Zinc tolerance and hyperaccumulation are genetically independent characters. Proc R Soc Lond B Biol Sci 266: 2175–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus MA, MacDowell AA, Celestre R, Manceau A, Miller T, Padmore HA, Sublett RE. (2004) Beamline 10.3.2 at ALS: a hard x-ray microprobe for environmental and materials sciences. J Synchrotron Radiat 11: 239–247 [DOI] [PubMed] [Google Scholar]
- McColloch RJ, Hamilton JW, Brown SK. (1963) An apparent seleniferous wax from Stanleya bipinnata. Biochem Biophys Res Commun 11: 7–13 [Google Scholar]
- Miller NJ, Rice-Evans CA. (1996) Spectrophotometric determination of antioxidant activity. Redox Rep 2: 161–171 [DOI] [PubMed] [Google Scholar]
- Minguzzi C, Vergnano O. (1948) Il contenuto di nichel nelle ceneri di Alyssum bertolonii Desv. Mem Soc Tosc Sci Nat Ser A 55: 49–77 [Google Scholar]
- Mirouze M, Sels J, Richard O, Czernic P, Loubet S, Jacquier A, François IEJA, Cammue BPA, Lebrun M, Berthomieu P, et al. (2006) A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J 47: 329–342 [DOI] [PubMed] [Google Scholar]
- Montes-Bayon M, LeDuc DL, Terry N, Caruso J. (2002) Selenium speciation in wild-type and genetically modified Se accumulating plants with HPLC separation and ICP-MS/ES-MS detection. J Anal At Spectrom 17: 872–879 [Google Scholar]
- Mou Z, Fan W, Dong X. (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Neuhierl B, Bock A. (1996) On the mechanism of selenium tolerance in selenium-accumulating plants: purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisulcatus. Eur J Biochem 239: 235–238 [DOI] [PubMed] [Google Scholar]
- Neuhierl B, Thanbichler M, Lottspeich F, Bock A. (1999) A family of S-methylmethionine-dependent thiol/selenol methyltransferases: role in selenium tolerance and evolutionary relation. J Biol Chem 274: 5407–5414 [DOI] [PubMed] [Google Scholar]
- Nigam SN, McConnell WB. (1969) Seleno amino compounds from Astragalus bisulcatus isolation and identification of g-glutamyl-Se-methylseleno-cysteine and Se-methylselenocysteine. Biochim Biophys Acta 192: 185–190 [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Bielecka M, Gakière B, Krueger S, Rinder J, Kempa S, Morcuende R, Scheible WR, Hesse H, Hoefgen R. (2006) Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids 30: 173–183 [DOI] [PubMed] [Google Scholar]
- Parker DR, Laura JF, Varvel TW, Thomason DN, Zhang Y. (2003) Selenium phytoremediation potential of Stanleya pinnata. Plant Soil 249: 157–165 [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Baxter IR, Richards EL, Freeman JL, Murphy AS. (2005) Phytoremediation and hyperaccumulator plants. Klomp LWJ, Martinoia E, Tamás MJ, , Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man. Topics in Current Genetics 14. Springer-Verlag, New York, pp 299–340 [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samblanx GW, Buchala A, Métraux J-P, Manners JM, Broekaert WF. (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8: 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PJ, Butler GW. (1967) Significance of selenocystathionine in an Australian selenium-accumulating plant, Neptunia amplexicaulis. Nature 213: 599–600 [Google Scholar]
- Peterson PJ, Butler GW. (1971) The occurrence of selenocystathionine in Morinda reticulata Benth., a toxic seleniferous plant. Aust J Biol Sci 24: 175–177 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, George GN, Van Fleet-Stalder V, Chasteen TG, Prince RC. (1999) X-ray absorption spectroscopy of selenium-containing amino acids. J Biol Inorg Chem 4: 791–794 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Hirsch G, Prince RC, Yu EY, Salt DE, George GN. (2003a) Imaging of selenium in plants using tapered metal monocapillary optics. J Synchrotron Radiat 10: 289–290 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, Salt DE, George GN. (2000) Quantitative, chemically specific imaging of selenium transformation in plants. Proc Natl Acad Sci USA 97: 10717–10722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering IJ, Wright C, Bubner B, Ellis D, Persans M, Yu E, George GN, Prince RC, Salt DE. (2003b) Chemical form and distribution of selenium and sulfur in the selenium hyperaccumulator Astragalus bisulcatus. Plant Physiol 131: 1460–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Freeman JL. (2006) Environmental cleanup using plants: biotechnological advances and ecological considerations. Front Ecol Environ 4: 203–210 [Google Scholar]
- Pilon-Smits EAH, Hwang S, Lytle CM, Zhu YL, Tai JC, Bravo RC, Chen Y, Leustek T, Norman T. (1999) Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction and tolerance. Plant Physiol 119: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CF, Freeman JL, Galeas ML, Klamper EM, Pilon-Smits EAH. (2008) The role of selenium in protecting plants against prairie dog herbivory: implications for the evolution of selenium hyperaccumulation. Oecologia 155: 267–275 [DOI] [PubMed] [Google Scholar]
- Quinn CF, Galeas ML, Freeman JL, Pilon-Smits EAH. (2007) Selenium: deterrence, toxicity and adaptation. Integr Environ Assess Manag 3: 460–462 [DOI] [PubMed] [Google Scholar]
- Reeves R, Brooks R. (1983) European species of Thlaspi L (Cruciferae) as indicators of nickel and zinc. J Geochem Explor 18: 275–283 [Google Scholar]
- Reeves RD, Baker AJM. (2000) Phytoremediation of toxic metals. Raskin I, Ensley BD, , Using Plants to Clean Up the Environment. John Wiley & Sons, New York, pp 193–229 [Google Scholar]
- Saitoh Y, Imuran N. (1987) Active oxygen generation by the reaction of selenite with reduced glutathione in vitro: Joint Japan-USA Congress of Pharmaceutical Sciences Honolulu Hawaii USA December 2-7. J Pharm Sci 76: S135. [PubMed] [Google Scholar]
- Schiavon M, Zhang LH, Abdel-Ghany SE, Pilon M, Malagoli M, Pilon-Smits EAH. (2007) Variation in copper tolerance in Arabidopsis thaliana accessions Columbia, Landsberg erecta and Wassilewskija. Physiol Plant 129: 342–350 [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP. (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29: 475–486 [DOI] [PubMed] [Google Scholar]
- Shrift A, Virupaksha TK. (1965) Seleno-amino acids in selenium-accumulating plants. Biochim Biophys Acta 100: 65–75 [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299: 152–178 [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA. (2001) Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Physiol Plant Mol Biol 52: 437–467 [DOI] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE. (2005a) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42: 785–797 [DOI] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Salt DE. (2005b) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86: 373–389 [DOI] [PubMed] [Google Scholar]
- Sors TG, Martin CP, Salt DE. (2009) Characterization of selenocysteine methyltransferases from Astragalus species with contrasting selenium accumulation capacity. Plant J 59: 110–122 [DOI] [PubMed] [Google Scholar]
- Spanos GA, Wrolstad RE. (1990) Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J Agric Food Chem 38: 1565–1571 [Google Scholar]
- Stadtman TC. (1996) Selenocysteine. Annu Rev Biochem 65: 83–100 [DOI] [PubMed] [Google Scholar]
- Stillwell RJ, Berry MJ. (2005) Expanding the repertoire of the eukaryotic selenoproteome. Proc Natl Acad Sci USA 102: 16123–16124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros TM. (1957) Evidence of the presence of an edapho-biotic factor in the problem of serpentine tolerance. Ecology 38: 14–23 [Google Scholar]
- Tamaoki M, Freeman JL, Marques L, Pilon-Smits EAH. (2008a) New insights into the role of ethylene and jasmonic acid in the acquisition of selenium resistance in plants. Plant Signal Behav 3: 865–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Freeman JL, Pilon-Smits EAH. (2008b) Cooperative ethylene and jasmonic acid signaling regulates selenate resistance in Arabidopsis. Plant Physiol 146: 1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Matsuyama T, Kanna M, Nakajima N, Kubo A, Aono M, Saji H. (2003) Differential ozone sensitivity among Arabidopsis accessions and its relevance to ethylene synthesis. Planta 216: 552–560 [DOI] [PubMed] [Google Scholar]
- Terry N, Zayed AM, de Souza MP, Tarun AS. (2000) Selenium in higher plants. Annu Rev Plant Biol 51: 401–432 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease SF, Beath OA. (1949) Selenium. Published by the authors, New York [Google Scholar]
- Trelease SF, Trelease HM. (1938) Selenium as a stimulating and possibly essential element for indicator plants. Am J Bot 25: 372–380 [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoewyk D, Garifullina GF, Ackley AR, Abdel-Ghany SE, Marcus MA, Fakra S, Ishiyama K, Inoue E, Pilon M, Takahashi H, et al. (2005) Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiol 139: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman DB, Trumble JT. (1999) Feeding preferences of Spodoptera exigua in response to form and concentration of selenium. Arch Insect Biochem 42: 64–73 [DOI] [PubMed] [Google Scholar]
- Virupaksha TK, Shrift A. (1965) Biochemical differences between selenium accumulator and non-accumulator Astragalus species. Biochim Biophys Acta 107: 69–80 [DOI] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR. (2002) Ethylene biosynthesis and signaling networks. Plant Cell (Suppl) 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Marshall B, Broadley MR. (2007) Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann Bot (Lond) 100: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meachan MC, Mead A, Harriman M, Trueman LJ. (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55: 1927–1937 [DOI] [PubMed] [Google Scholar]
- Wilbert SM, Ericsson LH, Gordon MP. (1998) Quantification of jasmonic acid, methyl jasmonate, and salicylic acid in plants by capillary liquid chromatography electrospray tandem mass spectrometry. Anal Biochem 257: 186–194 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Tamaoki M, Shikano T, Nakajima N, Ogawa D, Ioki M, Aono M, Kubo A, Kamada H, Inoue Y, et al. (2006) Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol 47: 304–308 [DOI] [PubMed] [Google Scholar]
- Zhang LH, Byrne PF, Pilon-Smits EAH. (2006) Mapping quantitative trait loci associated with selenate tolerance in Arabidopsis thaliana. New Phytol 170: 33–42 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gladyshev VN. (2010) General trends in trace element utilization revealed by comparative genomic analyses of Co, Cu, Mo, Ni and Se. J Biol Chem 285: 3393–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.