Abstract
To evaluate the relative contribution of the GH/IGF axis to the development of peak bone mineral density (BMD), we measured skeletal changes in IGF-I knockout (KO), IGF-II KO, and GH-deficient lit/lit mice and their corresponding control mice at d 23 (prepubertal), 31 (pubertal), and 56 (postpubertal) in the entire femur by dual energy x-ray absorptiometry and in the mid-diaphysis by peripheral quantitative computed tomography. Lack of growth factors resulted in different degrees of failure of skeletal growth depending on the growth period and the growth factor involved. At d 23, femoral length, size, and BMD were reduced by 25–40%, 15–17%, and 8–10%, respectively, in mice deficient in IGF-I, IGF-II, and GH compared with the control mice. During puberty, BMD increased by 40% in control mice and by 15% in IGF-II KO and GH-deficient mice, whereas it did not increase in the IGF-I KO mice. Disruption of IGF-I, but not IGF-II, completely prevented the periosteal expansion that occurs during puberty, whereas it was reduced by 50% in GH-deficient mice. At d 56, femoral length, size, and BMD were reduced by 40–55%, 11–18%, and 25–32%, respectively, in mice deficient in IGF-I, IGF-II, and GH compared with the control mice. Our data demonstrate that: 1) mice deficient in IGF-I exhibit a greater impairment in bone accretion than mice deficient in IGF-II or GH; 2) GH/IGF-I, but not IGF-II, is critical for puberty-induced bone growth; and 3) IGF-I effects on bone accretion during prepuberty are mediated predominantly via mechanisms independent of GH, whereas during puberty they are mediated via both GH-dependent and GH-independent mechanisms.
Osteoporosis is a disease that affects the elderly, particularly women. It is characterized by a low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, leading to a high incidence of bone fractures (1). Because the amount of bone mass accumulated during the active growth phases early in life is an important determinant of the risk of developing senile osteoporosis (2, 3), studies on the mechanisms regulating bone accretion during postnatal growth are critical in our efforts to identify potential preventive or interventive measures that would lower the risk of developing osteoporosis.
Puberty is a crucial time in life for bone accretion, with past studies showing that BMD increases by 40–50% during this period (4–6). In terms of mechanisms that contribute to a variation in peak BMD reached immediately after the puberty, it is generally well accepted that about 70% of this variation in BMD difference can be attributed to genetic differences (7–9). Besides genetic influence, other variables that influence peak BMD include differences in physical activity, diet, vitamin D intake, calcium intake, and interaction between environmental and genetic factors (7–9). Regarding the potential regulatory molecules that contribute to the acquisition of peak BMD during postnatal growth, the GH/IGF system has received considerable attention for a number of reasons, including: 1) IGFs are the most abundant growth factors stored in bone, and they are the most abundant growth factors produced by osteoblast cells (10–12); 2) C3H/HeJ mice have 50% greater total femoral BMD and 30% higher serum and skeletal IGF-I concentration than C57BL/6J mice and a congenic mouse strain with a donated segment containing an IGF-I quantitative trait locus from C3H/HeJ on a C57BL/6J background exhibit alterations in IGF-I production as well as total femoral BMD (13, 14); and 3) the GH/IGF axis is up-regulated during puberty in both humans and mice, and this up-regulation correlates with changes in bone formation markers (15).
Consistent with the idea that the IGF system plays an important role in the regulation of peak BMD, recent in vivo studies provide direct evidence for the importance of IGF-I in the regulation of bone formation. First, Bikle et al. (16) have shown that the bone formation rate, as evaluated by histomorphometry, was reduced by 77% in the tibia of IGF-I knockout (KO) mice compared with corresponding wild-type littermates. Second, Miyakoshi et al. (17) have demonstrated that PTH increased bone formation and bone density in growing mice and that these effects of PTH were not evident in IGF-I KO mice, thus suggesting that IGF-I is required for the anabolic effects of PTH. Third, Zhao et al. (18) have shown that transgenic overexpression of IGF-I in osteoblasts increases peak BMD compared with control mice.
Although previous studies have shown that mice deficient in IGFs exhibit delayed skeletal maturations and smaller bones (19–22), the effects of a lack of IGFs on peak BMD or BMD gain during puberty have not been quantified. In terms of mechanisms that contribute to the up-regulation of the GH/IGF axis during puberty, it is known that IGF production is under the influence of both systemic (e.g. GH, PTH, progesterone, estradiol, testosterone) and local (e.g. cytokines, bone morphogenetic proteins) factors. Of the various systemic regulators, GH is probably the single most important regulator of IGF production (10, 23). Accordingly, disruption of GH production or the GH receptor leads to an impairment of skeletal maturation and smaller body and bone size (20, 24, 25).
The purpose of this study is 2-fold: 1) to evaluate the relative importance of IGF-1, IGF-II, and GH in mediating skeletal changes that occur during postnatal growth; and 2) to evaluate if IGF-I effects on peak BMD are mediated via primarily GH-dependent or both GH-dependent and GH-independent mechanisms. To address these questions, we generated mice deficient in IGF-I, IGF-II, or GH and evaluated skeletal changes at various times during postnatal growth.
Materials and Methods
Animals
Breeding pairs of IGF-I KO and IGF-II KO mice were generously provided by Dr. Argiris Efstradiatis (19, 25). Breeding pairs of C57BL/6J-Ghrhrclit/+ [GH-releasing hormone receptor (Ghrhr) gene disruption, allele name little] were obtained from The Jackson Laboratory (Bar Harbor, ME).
To generate homozygous IGF-I KO mice, breeder mice (MF1/DBA) heterozygous for the IGF-I KO allele were mated using established breeding protocol. The reason for choosing an MF1/DBA mixed genetic background to generate IGF-I KO mice was based on previous observations that the frequency of surviving nullizygotes obtained in subsequent crosses was higher in this particular genetic background compared with other genetic backgrounds (19). IGF-I null pups were identified by the characteristic small size at birth and failure to grow after birth, as well as by PCR analysis using IGF-I forward primer (5′-CCACAGGCTATGGCTCCAGCATTC-3′), IGF-I reverse primer (5′-GTCAGTGTGGCGCTCGGCAC-3′), and neo reverse primer (5′-ATCCATCTTGTTCAATGGCCGATCCC-3′), yielding a 450-bp product for the disrupted IGF-I gene and 160-bp product for the wild-type gene (19, 26). Heterozygous littermates were genotyped and used for breeding and the wild-type littermates were used as control animals in this study.
To generate mice lacking functional IGF-II genes, IGF-II KO mice was generated by disrupting one of the IGF-II alleles in cultured embryonic stem cells from a 129/SV/EV strain and injecting them into host blastocysts derived from a C57BL/6J strain (25). Male mice containing the IGF-II disrupted allele were bred with wild-type C57BL/6J female mice to generate IGF-II KO and corresponding control mice. It has been previously shown that transmission of the IGF-II mutant allele through the male germ line results in heterozygous progeny that are IGF-II deficient because of the parental imprinting of the mouse IGF-II gene (25). IGF-II KO mice were identified by their small body weight at birth and by genotyping of tail DNA as previously described (25).
To generate mice deficient in GH, breeder mice heterozygous or homozygous for the little mutation were bred to generate homozygous and heterozygous offspring. The little mutation arose spontaneously in a production stock of the C57BL/6 strain at The Jackson Laboratory (27, 28). Homozygotes are smaller than normal from about 2 wk of age onward (27). The lit/lit mouse is a dwarf mouse strain characterized by no detectable levels of circulating GH and as a consequence low serum IGF-I levels caused by a missense mutation in the Ghrhr gene that abolishes the function of the receptor (27, 28). Homozygous lit/lit mice were identified by the body weight and reduced serum IGF-I levels.
Based on previous data that pubertal maturation in C57BL/6J females begins when serum estradiol increases on d 26 and vaginal opening occurs by d 31 (6, 29), we euthanized IGF-I-, IGF-II-, or GH-deficient mice and corresponding control mice (+/+) at d 23 (before puberty), d 35 (at the end of puberty), and d 56 (post puberty) to collect bones for phenotypic measurements. Mice were euthanized by CO2 inhalation followed by cervical dislocation. Because bone density is known to vary in different inbred strains of mice, corresponding littermate mice containing wild-type IGF-I or IGF-II alleles representing the same mixed genetic background as KO mice were used as control for comparison. Both male and female mice (equal distribution) were used in all groups. Bones and serum were stored at −70 C before analyses.
The experimental procedures performed in this study were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Studies Subcommittee at the Jerry L. Pettis Memorial Veterans Affairs Medical Center.
Bone densitometry of the femur by PIXImus
Bone mineral content (BMC) and areal BMD (aBMD) were measured by dual energy x-ray absorptiometry, using the PIXImus instrument (Lunar Corp., Madison, WI). The precision for BMC and BMD was ± 1% for repeat measurements of the same bones several times (17, 30).
Volumetric bone density (vBMD) and geometric parameters of emur
vBMD and geometric parameters at the middiaphysis or distal metaphysis were determined by peripheral quantitative computed tomography (pQCT) as described. Analysis of the scans was performed using the manufacturer-supplied software program (STRATEC MEDIZIN-TECHNIC GMBH Bone Density Software, version 5.40 C; Norland, Madison, WI). Total bone mineral density (total BMD) and geometric parameters were estimated with Loop analysis. The threshold was set at 230–630 mg/cm3. These parameters at the mid-diaphysis for each femur represent an average of three scans. The coefficient of variation for total BMD, periosteal circumference, and endosteal circumference for repeat measurements of four mouse femur (2–5 measurements) were less than 3%, less than 1%, and 2%, respectively (17, 30, 31). The longitudinal length of the femur was measured with a caliper.
Femurs dry weight
After pQCT measurement, femurs were cleaned of soft tissue and kept in a hood for 14 d (at room temperature). Femur dry weights were measured with an electronic balance (Model A-160, Denver Instrument Co., Arvada, CO).
Osteocalcin RIA
Osteocalcin levels were measured by a RIA as previously described (31). The sensitivity of the mouse osteocalcin assay was 0.5 ng/ml, and interassay variation was less than 8%.
Statistical analysis
The data are expressed as mean ± sd. Statistical analysis of the data were performed by Student’s unpaired t test.
Both male and female mice in approximately equal number were used in the study. Data from male and female mice were pooled for data analyses except for data involving gender specific differences (see Table 5).
TABLE 5.
Periosteal circumference and BMD in male and female mice lacking IGF-I, IGF-II, or GH and their corresponding control mice
| Parameter | Sex | IGF-I gene | IGF-II gene | GH RH receptor gene | |||
|---|---|---|---|---|---|---|---|
| Wild type | Knockout | Wild type | Knockout | Wild type | Knockout | ||
| Periosteal Circumference (mm) | Female | 4.67 ± 0.17 | 2.70 ± 0.09 | 4.23 ± 0.10 | 3.68 ± 0.015 | 4.48 ± 0.08 | 3.60 ± 0.16 |
| Male | 4.99 ± 0.24a | 2.83 ± 0.11a | 4.34 ± 0.10b | 3.95 ± 0.16a | 4.81 ± 0.27a | 3.79 ± 0.13a | |
| Total vBMD (mg/cm3) | Female | 788.2 ± 23.6 | 597.07 ± 9.38 | 715.0 ± 21.7 | 753.1 ± 30.9 | 467.0 ± 22.7 | 404.4 ± 65.6 |
| Male | 784.4 ± 46 | 645.2 ± 26.7a | 701.0 ± 30.7 | 703.8 ± 17.4 | 506 ± 94.2 | 428.3 ± 76.6 | |
Values (mean ± SD) are derived from 13-wk-old knockout and corresponding control mice for IGF-I line and from 8-wk-old mice for IGF-II and GHRH receptor (lit/lit) lines. Seven to 10 mice were used per group for the wild-type mice, and 5–9 mice were used per group for the various knockout lines.
Represents P < 0.05 vs. corresponding female mice.
Represents P = 0.07 vs. corresponding female mice.
Results
Body weight changes
Body weight was decreased by 67%, 40%, and 25%, respectively, in mice deficient in IGF-I, IGF-II, and GH, respectively, at the end of prepubertal growth period (Tables 1–3). A puberty-induced increase in body weight failed to occur in the IGF-I KO mice, whereas in IGF-II KO mice body weight increased by 35% during puberty (between d 23 and 31). Body weight increased by 22% in mice deficient in GH during puberty, whereas it increased by 58% in corresponding control mice. At 8 wk of age, body weight was decreased by 77%, 36%, and 47%, respectively, in mice deficient in IGF-I, IGF-II, and GH compared with corresponding control mice (Tables 1–3).
TABLE 1.
Body weight and skeletal changes in the femurs of mice lacking a functional IGF-I gene and corresponding age-matched control mice
| Parameter | Day 23 | Day 31 | Day 56 | Day 90 | ||||
|---|---|---|---|---|---|---|---|---|
| Wild type n = 20 |
Knockout n = 8 |
Wild type n = 20 |
Knockout n = 8 |
Wild type n = 14 |
Knockout n = 10 |
Wild type n = 19 |
Knockout n = 15 |
|
| Body weight (g) |
12.33 ± 1.92 | 4.01 ± 1.13a | 20.33 ± 2.97 | 4.28 ± 0.76a | 26.91 ± 3.33 | 6.10 ± 0.93a | 31.02 ± 5.72 | 8.47 ± 1.31a |
| Dry weight (mg) |
14.74 ± 2.33 | 4.26 ± 0.77a | 24.52 ± 3.06 | 4.81 ± 1.05a | 38.51 ± 2.95 | 7.58 ± 1.03a | 47.82 ± 3.80 | 10.56 ± 2.14a |
| Length (mm) |
10.96 ± 0.45 | 7.03 ± 0.39a | 12.81 ± 0.49 | 7.61 ± 0.42a | 15.12 ± 0.53 | 9.07 ± 0.38a | 15.90 ± 0.39 | 10.20 ± 0.76a |
| BMC (mg) |
5.72 ± 1.72 | 0.72 ± 0.32a | 12.42 ± 1.89 | 1.46 ± 0.55a | 21.57 ± 2.23 | 2.74 ± 0.74a | 27.30 ± 2.10 | 3.90 ± 1.80a |
| aBMD (mg/cm2) |
26.30 ± 3.27 | 18.02 ± 0.96a | 37.09 ± 2.84 | 20.14 ± 1.97a | 50.06 ± 3.78 | 22.13 ± 2.09a | 56.20 ± 2.80 | 25.50 ± 4.10a |
| vBMD (mg/cm3) |
356.9 ± 49.7 | 253.7 ± 61.17a | 470.5 ± 53.4 | 324.1 ± 27.0a | 680.9 ± 46.4 | 461.6 ± 42.4a | 786.6 ± 33.2 | 619.5 ± 73.0a |
| Cortical BMD (mg/cm3) |
671.5 ± 58.7 | 482.3 ± 57.4a | 852.1 ± 52.5 | 533.7 ± 30.3a | 1023.9 ± 31.0 | 695.4 ± 35.4a | 1137.3 ± 80.3 | 815.8 ± 30.1a |
| Peri. Circ. (mm) |
3.80 ± 0.15 | 2.36 ± 0.25a | 4.23 ± 0.19 | 2.45 ± 0.16a | 4.56 ± 0.19 | 2.61 ± 0.15a | 4.81 ± 0.26 | 2.76 ± 0.12a |
| Endo. Circ. (mm) |
2.70 ± 0.14 | 1.77 ± 0.10a | 2.85 ± 0.16 | 1.70 ± 0.10a | 2.75 ± 0.18 | 1.58 ± 0.14a | 2.78 ± 0.18 | 1.48 ± 0.14a |
Values are mean ± SD and include data from both male and female mice of approximately equal number. pQCT measurements were made at mid-diaphysis of the femur.
P < 0.01 vs. corresponding age-matched wild-type mice.
TABLE 3.
Body weight and skeletal changes in the femur of mice lacking GH (lit/lit) and corresponding age-matched control (lit/+) mice
| Parameter | Day 23 | Day 31 | Day 56 | |||
|---|---|---|---|---|---|---|
| lit/+ n = 24 |
lit/lit n = 17 |
lit/+ n = 13 |
lit/it n = 10 |
lit/+ n = 14 |
lit/lit n = 11 |
|
| Body weight (g) | 8.10 ± 1.23 | 6.05 ± 0.84a | 12.85 ± 1.25 | 7.38 ± 0.93a | 19.79 ± 2.70 | 10.53 ± 0.67a |
| Dry weight (mg) | 9.84 ± 1.59 | 6.96 ± 1.11a | 18.30 ± 3.55 | 9.57 ± 0.99a | 31.19 ± 6.68 | 12.39 ± 2.79a |
| Length (mm) | 9.76 ± 0.46 | 8.74 ± 0.43a | 11.84 ± 0.44 | 9.47 ± 0.2a | 13.95 ± 0.08 | 10.39 ± 0.91a |
| BMC (mg) | 2.45 ± 1.12 | 1.51 ± 0.35a | 8.64 ± 1.60 | 3.67 ± 0.43a | 15.26 ± 4.02 | 5.68 ± 1.83a |
| aBMD (mg/cm2) | 21.14 ± 1.83 | 19.50 ± 0.91a | 29.43 ± 2.58 | 22.54 ± 0.85a | 38.52 ± 5.05 | 26.06 ± 3.36a |
| vBMD (mg/cm3) | 237.3 ± 33.5 | 221.8 ± 52.0 | 410.4 ± 29.1 | 364.0 ± 23.7a | 485.2 ± 65.1 | 414.7 ± 68.84a |
| Peri. Circ. (mm) | 3.57 ± 0.26 | 3.29 ± 0.16a | 4.22 ± 0.08 | 3.61 ± 0.11a | 4.63 ± 0.25 | 3.68 ± 0.18a |
| Endo. Circ. (mm) | 2.78 ± 0.22 | 2.62 ± 0.28a | 2.92 ± 0.06 | 2.54 ± 0.09a | 3.17 ± 0.11 | 2.51 ± 0.15a |
Values are mean ± SD and include data from both male and female mice of approximately equal number. pQCT measurements were made at mid-diaphysis of the femur.
P < 0.01 vs. corresponding age-matched wild-type mice.
Femur weight changes
Femur dry weight was reduced by 71%, 36%, and 29%, respectively, in mice deficient in IGF-I, IGF-II, and GH at d 23 (Tables 1–3). Femur weight did not increase significantly during puberty in IGF-I KO mice, whereas it increased by about 40% in mice deficient in IGF-II or GH. Femur weight was increased by about 60% in control mice during puberty. Femur dry weight was reduced by 80%, 34%, and 60%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with corresponding control mice at 8 wk of age (Tables 1–3).
Femur length
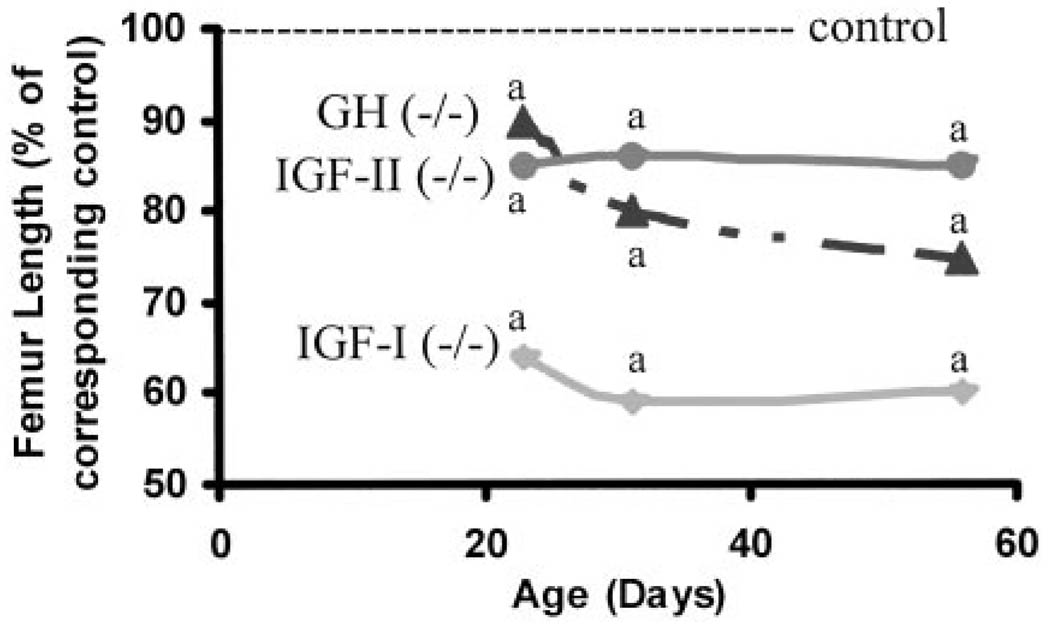
Femur length was decreased by 36%, 15%, and 10%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with corresponding control mice at d 23 (Fig. 1). Femur length increased by 8%, 13%, and 8%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with 17%, 13%, and 21% in corresponding control mice during puberty (Tables 1–3). At d 56, femur length was reduced by 40%, 14% and 25%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with corresponding control mice. The rates of gain in femur length during pubertal and postpubertal growth periods were significantly reduced in IGF-I KO and GH-deficient mice (Fig. 2). In contrast, the rate of gain in femur length was unaffected in IGF-II KO mice during pubertal and postpubertal growth periods (data not shown).
Fig 1.
Femur length in mice lacking functional IGF-I (−/−), IGF-II (P-), and GH (lit/lit) during postnatal growth. Values represent the percentage of corresponding control mice and are mean of 8–24 per group. Data represent pooled data from male and female mice. a, P < 0.05 vs. corresponding age-matched control mice.
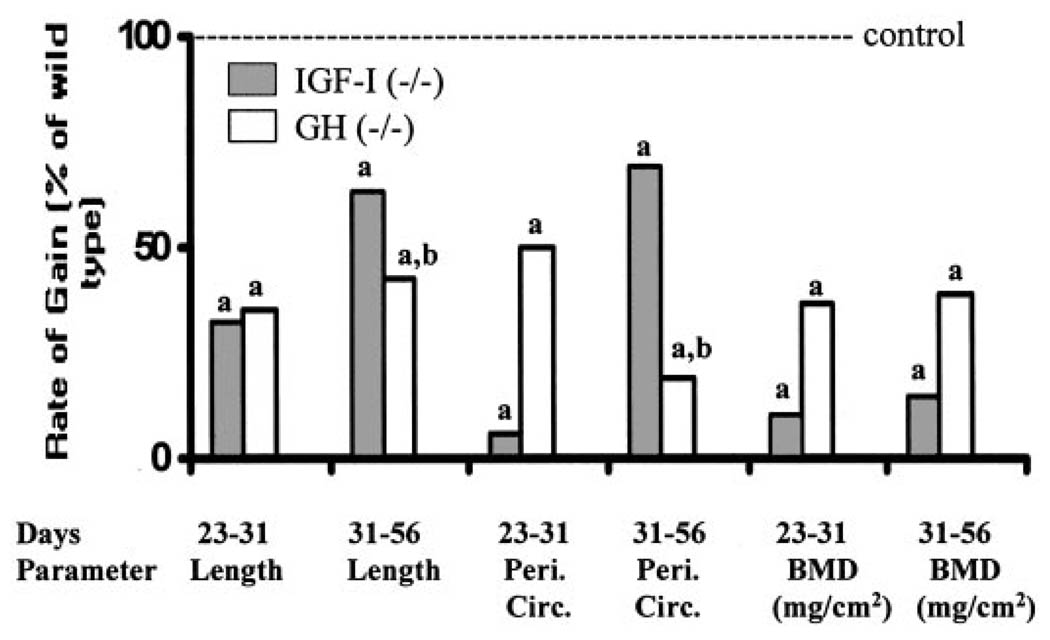
Fig 2.
Rate of gain in length, periosteal circumference, and BMD during pubertal (d 23–31) and postpubertal (d 31–56) growth phases in mice lacking functional IGF-I (−/−), and GH (lit/lit). Values represent percentage of corresponding control mice and are mean of 8–24 per group. Data represent pooled data from male and female mice. a, P < 0.05 vs. corresponding age-matched control mice; b, P < 0.05 vs. IGF-I KO mice.
PIXImus data
Femoral bone mineral content (BMC) was decreased by 88%, 45%, and 39%, respectively, at d 23 in mice deficient in IGF-I, IGF-II or GH compared with control mice (Tables 1–3). The rate of gain in BMC during puberty was decreased by 90%, 50%, and 65%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with corresponding control mice (Tables 1–3). At d 56, femur BMC was reduced by 88%, 38%, and 63%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with control mice.
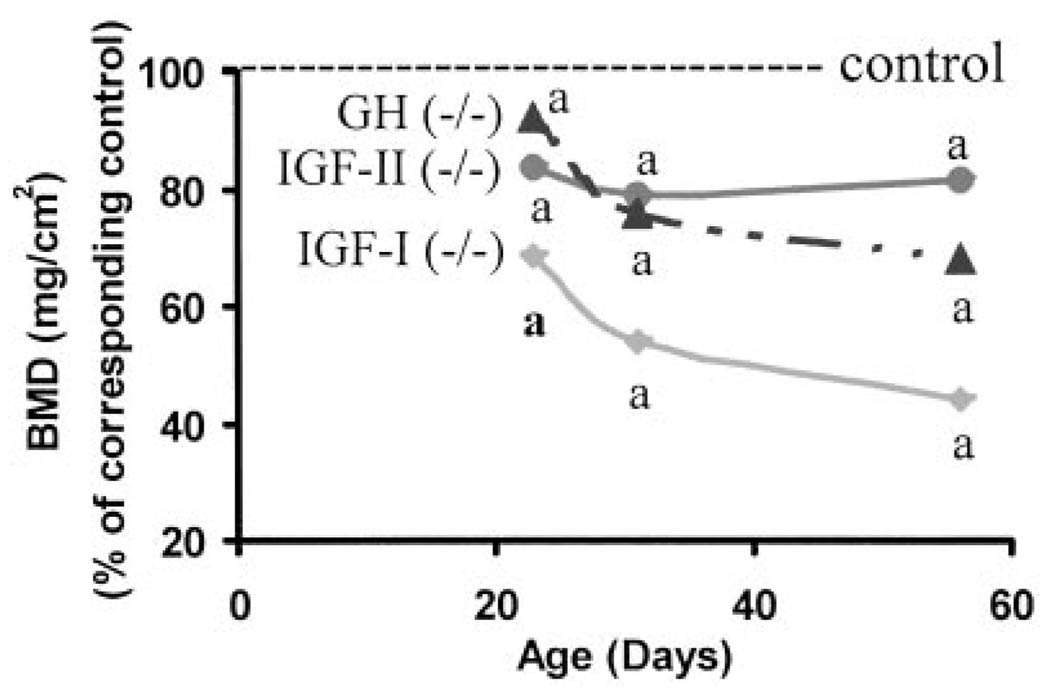
Areal BMD (mg/cm2) of femur was decreased by 31%, 17% and 8%, respectively, in mice deficient in IGF-I, IGF-II, or GH, respectively, at d 23 compared with corresponding control littermates (Tables 1–3, Fig. 3). BMD increased by nearly 40% in control mice between d 23 and 31, which represents the period of puberty in mice. In contrast, the increase in BMD failed to occur in IGF-I KO mice (Fig. 2). BMD increased by 12% and 15%, respectively, during puberty in IGF-II KO and GH-deficient lit/lit mice. At d 56,BMD was decreased by 56%, 18%, and 32%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with corresponding control mice (Fig. 3). The rate of gain in BMD was significantly reduced in IGF-I KO and GH-deficient mice during both pubertal and postpubertal growth periods (Fig. 2). The lack of IGF-II had a significant effect on the rate of gain in areal BMD during pubertal but not during postpubertal growth period (data not shown).
Fig 3.
Femur BMD in mice lacking functional IGF-I (−/−), IGF-II (P-), and GH (lit/lit) during postnatal growth. Values represent percentage of corresponding control mice and are mean of 8–24 per group. Data represent pooled data from male and female mice. a, P < 0.05 vs. corresponding age-matched control mice.
pQCT data
Because mice deficient in IGF-I, IGF-II, or GH are smaller, and because body size can influence BMD measurements by PIXImus, we also measured BMD by pQCT, which measures BMD per unit volume of bone and is not influenced by body size. Total volumetric BMD (mg/cm3) at the mid-diaphysis of the femur was reduced by 29% in IGF-I KO mice at d 23, whereas it was increased by 15% in IGF-II KO mice compared with control mice (Tables 1–3). In contrast, total volumetric BMD was not different at d 23 in GH-deficient mice compared with control mice. Total volumetric BMD was decreased by 32% and 15%, respectively, in mice deficient in IGF-I or GH at d 56, whereas it was not significantly different in IGF-II KO mice compared with corresponding control mice (Tables 1–3). Consistent with the decreased volumetric BMD at the mid diaphysis, at the distal metaphysis both total BMD and cortical BMD were decreased by 36% and 30%, respectively, in IGF-I KO mice at 8 wk of age compared with corresponding control mice (Table 4).
TABLE 4.
Bone size and BMD changes in the distal metaphysis of femurs of mice lacking a functional IGF-I gene and corresponding age-matched control mice
| Parameter | Wild type | Knockout |
|---|---|---|
| Total vBMD (mg/cm3) | 538.0 ± 75.5 | 343.5 ± 35.9a |
| Cortical BMD (mg/cm3) | 845.4 ± 60.2 | 596.6 ± 40.6a |
| Periosteal circumference (mm) | 5.69 ± 0.37 | 3.49 ± 0.36a |
| Endosteal circumference (mm) | 3.83 ± 0.46 | 2.46 ± 0.29a |
Values are mean ± SD and include data from both male and female mice of approximately equal number. pQCT measurements were made at the distal metaphysis of the femur.
P < 0.01 vs. corresponding age-matched control mice.
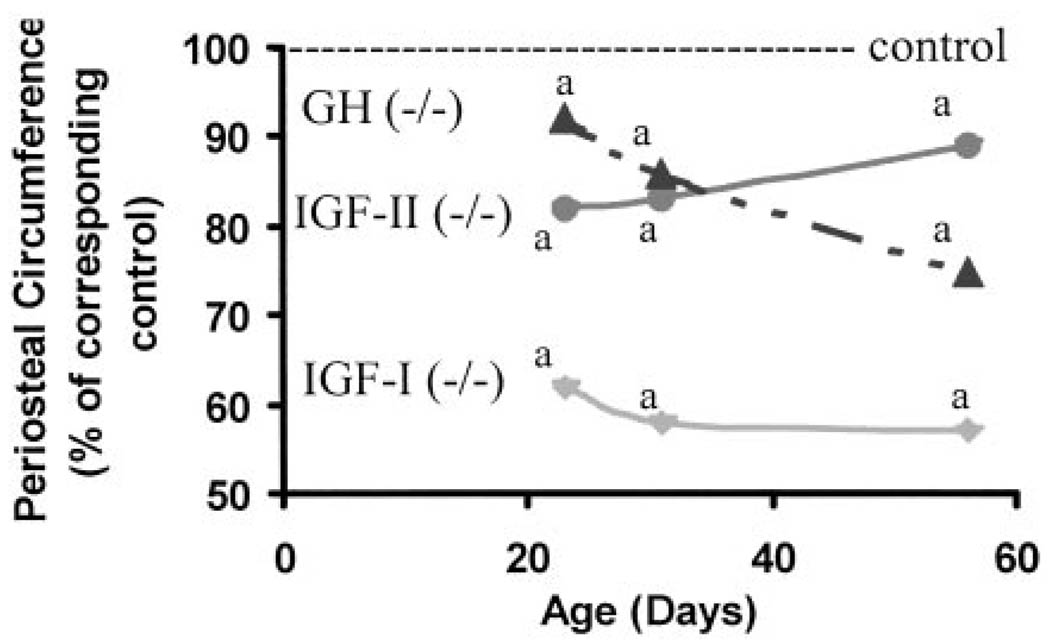
Periosteal circumference at the mid-diaphysis of the femur was decreased by 38%, 16%, and 8%, respectively, in mice deficient in IGF-I, IGF-II, or GH at d 23 compared with corresponding control mice (Fig. 4). Although periosteal circumference increased by 12–15% during puberty in control mice, no significant increase in periosteal circumference occurred in IGF-I KO mice (Tables 1–3). Disruption of IGF-II had no effect on periosteal expansion during puberty, whereas periosteal circumference increased by 50% of corresponding control mice in GH-deficient lit/lit mice. At d 56, periosteal circumference was reduced by 43%, 11%, and 21%, respectively, in mice deficient in IGF-I, IGF-II, or GH compared with control mice. The rate of gain in periosteal circumference was significantly reduced in IGF-I KO and GH-deficient mice compared with corresponding control mice both during pubertal and postpubertal growth periods (Fig. 3). In contrast, the rate of gain in periosteal circumference was not impaired in IGF-II KO mice compared with corresponding control mice either during pubertal or postpubertal growth period (data not shown).
Fig 4.
Periosteal circumference in mice lacking functional IGF-I (−/−), IGF-II (P-), and GH (lit/lit) during postnatal growth. Values represent percentage of corresponding control mice and are mean of 8–24 per group. Data represent pooled data from male and female mice. a, P < 0.05 vs. corresponding age-matched control mice.
Consistent with the data on periosteal circumference, endosteal circumference was correspondingly reduced in mice deficient in IGF-I, IGF-II, or GH. At d 56, endosteal circumference was reduced by 43%, 15%, and 21%, respectively, in mice lacking IGF-I, IGF-II or GH compared with control mice (Tables 1–3).
Both periosteal and endosteal circumference were also significantly decreased at the distal metaphysis in IGF-I KO mice at 8 wk of age compared with corresponding control mice (Table 4).
Gender-specific differences
There were no significant differences for any of the skeletal parameters between males and females for age groups 23 d and 31 d. Therefore, the data from both males and females were pooled for analyses. At 56 d of age, significant difference was found between male and female mice for certain skeletal parameters (e.g. periosteal circumference). To evaluate if GH/IGF axis mediate gender specific effects on the skeleton, we compared bone size and BMD in male vs. female mice lacking IGF-I, IGF-II, or GH and their corresponding control mice (Table 5). As expected, male mice exhibited larger periosteal circumference compared with corresponding female mice. However, the difference in periosteal circumference between male and female mice was maintained in all three transgenic lines, thus suggesting that GH/IGF axis is not a major player in contributing to gender specific effect on bone size. The total volumetric BMD was not different between male vs. female mice, consistent with our previous data (6).
Serum osteocalcin levels
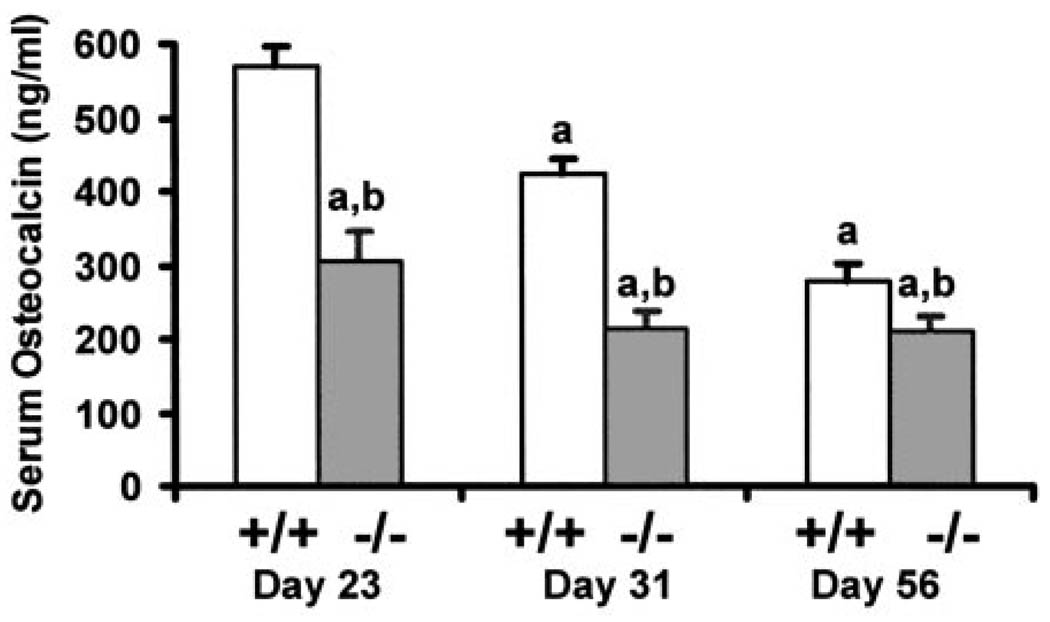
Serum osteocalcin was decreased 54% in IGF-I KO mice at d 23 compared with control mice (Fig. 5), whereas it was not significantly different in IGF-II KO mice (data not shown). Serum osteocalcin level was decreased by 24% in IGF-I KO at d 56, whereas it was not different in IGF-II KO mice compared with control mice.
Fig 5.
Serum osteocalcin level in IGF-I KO mice and corresponding control mice during postnatal growth. Values are mean ± SD of 8–20 mice per group. Data represent pooled data from male and female mice. a, P < 0.05 vs. d 23; b, P < 0.05 vs. corresponding age-matched control mice.
Discussion
The salient features of our study are as follows: 1) It provides direct evidence that IGF-I but not IGF-II is critical for the increased BMD that occurs during puberty in mice; 2) It provides direct evidence that the increases in both longitudinal bone growth and bone size during puberty require IGF-I; 3) It provides evidence that mice deficient in IGF-I exhibit greater impairment in bone accretion than mice deficient in GH during postnatal growth (i.e. non-GH IGF-I effect); and 4) It provides evidence that skeletal changes that occur during the prepubertal growth phase are mediated predominantly via a GH-independent mechanism, whereas the changes that occur during the pubertal and postpubertal growth phases are predominantly mediated via GH-dependent mechanisms.
Our finding that areal BMD increases by 40% during puberty in control mice, whereas no significant increase occurs in IGF-IKO mice, provides the first direct evidence that IGF-I is critical for the increased BMD that occurs during puberty. In contrast to IGF-I, IGF-II plays a minimal role in regulating BMD accretion during puberty. This is not entirely surprising based on what is known about the developmental switch from IGF-II to IGF-I during postnatal growth in mice. In this regard, it is known that IGF-II is produced in greater abundance during embryonic growth, whereas, during postnatal growth, IGF-I production increases even as IGF-II decreases (33–35). The finding that peak BMD was reduced to a greater extent in mice deficient in GH compared with IGF-II KO mice also provides further evidence that IGF-I, and not IGF-II, is critical in regulating BMD accretion during puberty.
The rapid accretion of bone during the period of sexual maturation is associated with increases in both longitudinal bone growth as well as bone size. In terms of potential signaling molecules that contribute to the rapid increase in longitudinal bone growth during puberty, the GH/IGF axis is a potential candidate because IGF-I gene deletion results in dwarfism in mice (19–22) and extreme short stature in humans (36). Accordingly, we found that the rate of gain in femur length during the pubertal growth period is reduced by 70% in mice lacking IGF-I or GH compared with corresponding control mice. In terms of the target cell type that contributes to reduced longitudinal bone growth in mice deficient in IGF-I, Wang et al. (37) have demonstrated that the terminal hypertrophic chondrocytes, which form the scaffold on which long bone growth extends, are reduced in linear dimension by 30% in IGF-I null mice, accounting for most of their decreased longitudinal growth. Thus, IGF-I is critical for the augmented longitudinal growth that occurs during the period of sexual maturation.
Our findings that periosteal circumference failed to increase in IGF-I KO mice provide direct evidence that the puberty-induced increase in periosteal circumference requires IGF-I. If the effect of IGF-I on periosteal circumference is direct, we should be able to correct the deficiency in periosteal expansion by exogenous administration of IGF-I during puberty. Accordingly, we recently found that treatment of IGF-I-deficient midi mice (IGF-Im/m mice), which exhibit more than 60% reduction in circulating IGF-I levels, with IGF-I during puberty significantly increased periosteal circumference (30). Thus, IGF-I is an essential regulator of periosteal circumference during puberty.
IGF-I effects on target tissues are known to be mediated via IGF-I’s actions as an endocrine hormone and local autocrine/paracrine growth factor. Of the various regulators of IGF-I action, GH is probably the single most important regulator of IGF-I function, capable of regulating both endocrine (liver derived) and local (bone derived) IGF-I actions (23, 38, 39). In addition to GH, IGF-I production in bone is also under the influence of a variety of other systemic (PTH, estradiol, testosterone, thyroid hormone) and local (bone morphogenetic proteins, TGFβ1, ILs) regulators (10, 11, 40). If GH is the major regulator of IGF-I, then we would anticipate mice deficient in GH to exhibit similar phenotypic changes as that of IGF-I KO mice. In this regard, we found that bone length, periosteal circumference, and BMD were decreased much more in IGF-IKO mice compared with lit/lit mice at d 23, thus suggesting that IGF-I effects on bone accretion during the prepubertal growth phase are mediated primarily independent of GH. These data suggest that other mechanisms besides GH regulate the effects of IGF-I during the prepubertal growth phase in mice.
The findings of this study demonstrate that the rate of gain in femur length during puberty was approximately 35% of normal in both IGF-I KO and GH-deficient lit/lit mice, thus suggesting that IGF-I effects on longitudinal growth are mainly mediated via GH (Table 6). In contrast, periosteal circumference increased by 50% in mice lacking GH compared with control mice during puberty, whereas no increase in periosteal circumference occurred in mice lacking IGF-I. These data suggest that the increase in periosteal circumference during puberty is IGF-I-dependent and mediated equally via both GH-independent and GH-dependent mechanisms. Similarly, the rate of gain in areal BMD during puberty appears to be mediated via both GH-dependent and GH-independent mechanisms.
TABLE 6.
Relative contribution of IGF-I, IGF-II, and GH to skeletal changes that occur during postnatal growth in mice
| Prepubertal | Pubertal | Postpubertal | |
|---|---|---|---|
| Length | IGF-I>IGF-II>GH | IGF-I=GH>IGF-II | GH>IGF-I>IGF-II |
| Size | IGF-I>IGF-II>GH | IGF-I>GH>IGF-II | GH>IGF-I>IGF-II |
| BMD | IGF-I>IGF-II>GH | IGF-I>GH>IGF-II | IGF-I>GH>IGF-II |
Data represent pooled data from male and female mice.
Interestingly, gain in length and periosteal circumference during the post pubertal period (d 31–56) are impaired to a greater extent in GH-deficient lit/lit mice compared with IGF-I KO mice, thus suggesting that the GH effects on longitudinal bone growth and periosteal expansion during the post pubertal period may be mediated, in part, by an IGFI-independent mechanism. Regarding potential messenger molecules that could contribute to IGF-independent effects of GH, IGFBP-5 is a candidate because GH is a major regulator of IGFBP-5 levels in serum and GH increases IGFBP-5 production in osteoblasts (41) and because IGFBP-5 treatment increases bone formation parameters in vitro and in vivo in part by an IGF-independent mechanism (42). The issue of whether some of the IGF-independent effects of GH on longitudinal growth and periosteal expansion are mediated via IGFBP-5 acting as a growth factor independent of IGF-I is an interesting possibility that requires further studies.
Our study addresses some very important fundamental issues. Therefore, the conclusions made in this study should be viewed in the context of the following pitfalls: 1) IGF-I and IGF-II KO mice and their corresponding control mice used in these studies were developed in mixed genetic backgrounds, whereas little mutation (lit/lit) arose spontaneously in a production of the C57BL/6 strain in The Jackson Laboratory. To minimize the genetic background effect, we took several steps, including use of corresponding littermates that contained a wild-type gene but of the same mixed genetic background as controls, use of mice from several litters, and use of large number of mice (n = 8–24) for each group; 2) IGF-I, IGF-II, and GH-deficient mice are smaller in size compared with corresponding control mice. One could therefore argue that some or all of the observed BMD phenotypic differences in IGF-I- or GH-deficient mice could be attributed to differences in body size. This is not necessarily true based on the significant difference between IGF-I KO and control mice in cortical bone density (mg/cm3), which is not influenced by size. Furthermore, the differences in body size (40%) in IGF-II KO and corresponding control mice were not associated with corresponding changes in either total or cortical BMD; and 3) The gain in femur length and periosteal circumference, but not BMD, were influenced to a much greater extent in GH-deficient lit/lit mice compared with IGF-I KO mice during postpubertal growth period.
In conclusion, the raise in peak BMD and bone size, two important determinants of bone strength, starts long before puberty (presumably in utero) and continues post puberty. Some aspects of the GH/IGF system is involved throughout from prepubertal, pubertal, and postpubertal growth phases. Our data also provide evidence that both local IGF production and systemic IGF production (mediated by GH) influence the raise in BMD and bone size during postnatal growth.
TABLE 2.
Body weight and skeletal changes in the femur of mice lacking the functional paternal IGF-II gene and corresponding age-matched control mice
| Parameter | Day 23 | Day 31 | Day 56 | |||
|---|---|---|---|---|---|---|
| Wild type n = 18 |
Knockout n = 16 |
Wild type n = 21 |
Knockout n = 20 |
Wild type n = 18 |
Knockout n = 18 |
|
| Body weight (g) | 11.52 ± 0.93 | 6.94 ± 0.89a | 16.15 ± 1.75 | 9.41 ± 1.93a | 20.86 ± 1.91 | 13.41 ± 1.83a |
| Dry weight (mg) | 14.91 ± 1.09 | 9.66 ± 1.62a | 23.53 ± 2.17 | 13.59 ± 2.33a | 33.43 ± 2.88 | 22.11 ± 3.19a |
| Length (mm) | 11.27 ± 0.29 | 9.56 ± 0.38a | 12.72 ± 0.36 | 10.82 ± 0.48a | 14.68 ± 0.38 | 12.69 ± 0.57a |
| BMC (mg) | 6.93 ± 0.78 | 3.77 ± 0.88a | 11.58 ± 1.34 | 6.06 ± 1.45a | 17.66 ± 1.78 | 10.99 ± 2.36a |
| aBMD (mg/cm2) | 28.56 ± 1.76 | 23.76 ± 2.39a | 35.51 ± 2.00 | 27.94 ± 2.48a | 45.87 ± 2.53 | 37.44 ± 3.91a |
| vBMD (mg/cm3) | 425.9 ± 51.9 | 494.3 ± 46.1a | 549.1 ± 31.4 | 542.1 ± 64.9 | 708.0 ± 26.8 | 727.0 ± 34.8 |
| Cortical BMD (mg/cm3) | 754.7 ± 38.4 | 738.3 ± 45.45 | 882.0 ± 33.6 | 821.2 ± 56.3 | 1038.7 ± 22.2 | 1002.0 ± 35.1 |
| Peri. Circ. (mm) | 3.66 ± 0.16 | 3.05 ± 0.16a | 4.00 ± 0.12 | 3.31 ± 0.19a | 4.29 ± 0.13 | 3.80 ± 0.20a |
| Endo. Circ. (mm) | 2.48 ± 0.14 | 1.87 ± 0.09a | 2.54 ± 0.11 | 1.99 ± 0.15a | 2.52 ± 0.09 | 2.10 ± 0.11a |
Values are mean ± SD and include data from both male and female mice of approximately equal number. pQCT measurements were made at mid-diaphysis of the femur.
P < 0.01 vs. corresponding age-matched wild-type mice.
Acknowledgments
We are grateful to Dr. Argiris Efstratiadis (Columbia University, New York, NY) for providing us breeding pairs of IGF-I and IGF-II KO mice for our studies. We would also like to acknowledge the secretarial assistance provided by Sean Belcher and technical assistance by Alice Kramer.
This material is based upon work supported in part by NIH (AR-31062), the National Medical Testbed, and the U.S. Department of the Army. The view, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as a position, policy, decision, or endorsement of the Federal Government or the National Medical Technology Testbed, Inc. All work was performed in facilities provided by the Department of Veterans Affairs.
Abbreviations
- aBMD
Areal BMD
- BMC
bone mineral content
- BMD
bone mineral density
- KO
knockout
- pQCT
peripheral quantitative computed tomography
- vBMD
volumetric BMD
References
- 1.Riggs BL, Melton LJ., 3rd The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S–511S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 2.Johnston CC, Jr, Slemenda CW. Peak bone mass, bone loss and risk of fracture. Osteoporos Int. 1994;4:43–45. doi: 10.1007/BF01623435. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E, Tsalamandris C, Formica C, Hopper JL, McKay J. Reduced femoral neck bone density in the daughters of women with hip fractures: the role of low peak bone density in the pathogenesis of osteoporosis. J Bone Miner Res. 1994;9:739–743. doi: 10.1002/jbmr.5650090520. [DOI] [PubMed] [Google Scholar]
- 4.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med. 1991;325:1597–1600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 5.Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, Mohan S. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. 1999;84:2807–2814. doi: 10.1210/jcem.84.8.5905. [DOI] [PubMed] [Google Scholar]
- 6.Richman C, Kutilek S, Miyakoshi N, Srivastava AK, Beamer WG, Donahue LR, Rosen CJ, Wergedal JE, Baylink DJ, Mohan S. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res. 2001;16:386–397. doi: 10.1359/jbmr.2001.16.2.386. [DOI] [PubMed] [Google Scholar]
- 7.Harris M, Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Genetic and environmental correlations between bone formation and bone mineral density: a twin study. Bone. 1998;22:141–145. doi: 10.1016/s8756-3282(97)00252-4. [DOI] [PubMed] [Google Scholar]
- 8.Recker RR, Deng HW. Role of genetics in osteoporosis. Endocrine. 2002;17:55–66. doi: 10.1385/ENDO:17:1:55. [DOI] [PubMed] [Google Scholar]
- 9.Eisman JA. Genetics of osteoporosis. Endocr Rev. 1999;20:788–804. doi: 10.1210/edrv.20.6.0384. [DOI] [PubMed] [Google Scholar]
- 10.Mohan S, Baylink DJ. IGF system components and their role in bone metabolism. In: Roberts CT, editor. IGFs in health and disease. Totawa, NJ: Humana Press; 1999. pp. 457–496. [Google Scholar]
- 11.Delany AM, Pash JM, Canalis E. Cellular and clinical perspectives on skeletal insulin-like growth factor I. J Cell Biochem. 1994;55:328–333. doi: 10.1002/jcb.240550309. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CJ, Donahue LR. Insulin-like growth factors and bone: the osteoporosis connection revisited. Proc Soc Exp Biol Med. 1998;219:1–7. doi: 10.3181/00379727-219-44310. [DOI] [PubMed] [Google Scholar]
- 13.Rosen CJ, Dimai HP, Vereault D, Donahue LR, Beamer WG, Farley J, Linkhart S, Linkhart T, Mohan S, Baylink DJ. Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone. 1997;21:217–223. doi: 10.1016/s8756-3282(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 14.Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, Churchill G, Adamo ML, Powell DR, Turner RT, Muller R, Beamer WG. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17:570–579. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- 15.Holmes SJ, Shalet SM. Role of growth hormone and sex steroids in achieving and maintaining normal bone mass. Horm Res. 1996;45:86–93. doi: 10.1159/000184765. [DOI] [PubMed] [Google Scholar]
- 16.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 17.Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001;142:4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]
- 18.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 19.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 20.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 21.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 22.Liu JL, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178–5184. doi: 10.1210/endo.140.11.7151. [DOI] [PubMed] [Google Scholar]
- 23.Daughaday WH. Growth hormone axis overview—somatomedin hypothesis. Pediatr Nephrol. 2000;14:537–540. doi: 10.1007/s004670000334. [DOI] [PubMed] [Google Scholar]
- 24.Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjogren K, Bohlooly YM, Olsson B, Coschigano K, Tornell J, Mohan S, Isaksson OG, Baumann G, Kopchick J, Ohlsson C. Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor −/− mice. Biochem Biophys Res Commun. 2000;267:603–608. doi: 10.1006/bbrc.1999.1986. [DOI] [PubMed] [Google Scholar]
- 26.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 27.Donahue LR, Beamer WG. Growth hormone deficiency in ’little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 28.Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- 29.Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stabnov L, Kasukawa Y, Guo R, Amaar Y, Wergedal JE, Baylink DJ, Mohan S. Effect of insulin-like growth factor-1 (IGF-1) plus alendronate on bone density during puberty in IGF-1-deficient MIDI mice. Bone. 2002;30:909–916. doi: 10.1016/s8756-3282(02)00738-x. [DOI] [PubMed] [Google Scholar]
- 31.Mohan S, Kutilek S, Zhang C, Shen HG, Kodama Y, Srivastava AK, Wergedal JE, Beamer WG, Baylink DJ. Comparison of bone formation responses to parathyroid hormone(1–34), (1–31), and (2–34) in mice. Bone. 2000;27:471–478. doi: 10.1016/s8756-3282(00)00355-0. [DOI] [PubMed] [Google Scholar]
- 32.Mohan S, Baylink DJ. Development of a simple valid method for the complete removal of insulin-like growth factor (IGF)-binding proteins from IGFs in human serum and other biological fluids: comparison with acid-ethanol treatment and C18 Sep-Pak separation. J Clin Endocrinol Metab. 1995;80:637–647. doi: 10.1210/jcem.80.2.7531716. [DOI] [PubMed] [Google Scholar]
- 33.Moses AC, Nissley SP, Short PA, Rechler MM, White RM, Knight AB, Higa OZ. Increased levels of multiplication-stimulating activity, an insulin-like growth factor, in fetal rat serum. Proc Natl Acad Sci USA. 1980;77:3649–3653. doi: 10.1073/pnas.77.6.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Ercole AJ, Applewhite GT, Underwood LE. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980;75:315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- 35.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 36.Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Zhou J, Bondy CA. Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 1999;13:1985–1990. doi: 10.1096/fasebj.13.14.1985. [DOI] [PubMed] [Google Scholar]
- 38.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conover CA. In vitro studies of insulin-like growth factor I and bone. Growth Horm IGF Res. 2000;10 Suppl B:S107–S110. doi: 10.1016/s1096-6374(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 41.Thoren M, Hilding A, Brismar T, Magnusson P, Degerblad M, Larsson L, Saaf M, Baylink DJ, Mohan S. Serum levels of insulin-like growth factor binding proteins (IGFBP)-4 and -5 correlate with bone mineral density in growth hormone (GH)-deficient adults and increase with GH replacement therapy. J Bone Miner Res. 1998;13:891–899. doi: 10.1359/jbmr.1998.13.5.891. [DOI] [PubMed] [Google Scholar]
- 42.Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]