Abstract
The kinetics of the induction of rat kidney phosphoenolpyruvate carboxykinase activity after triamcinolone and ammonium chloride administration have been investigated with a view to the further differentiation of the two processes.
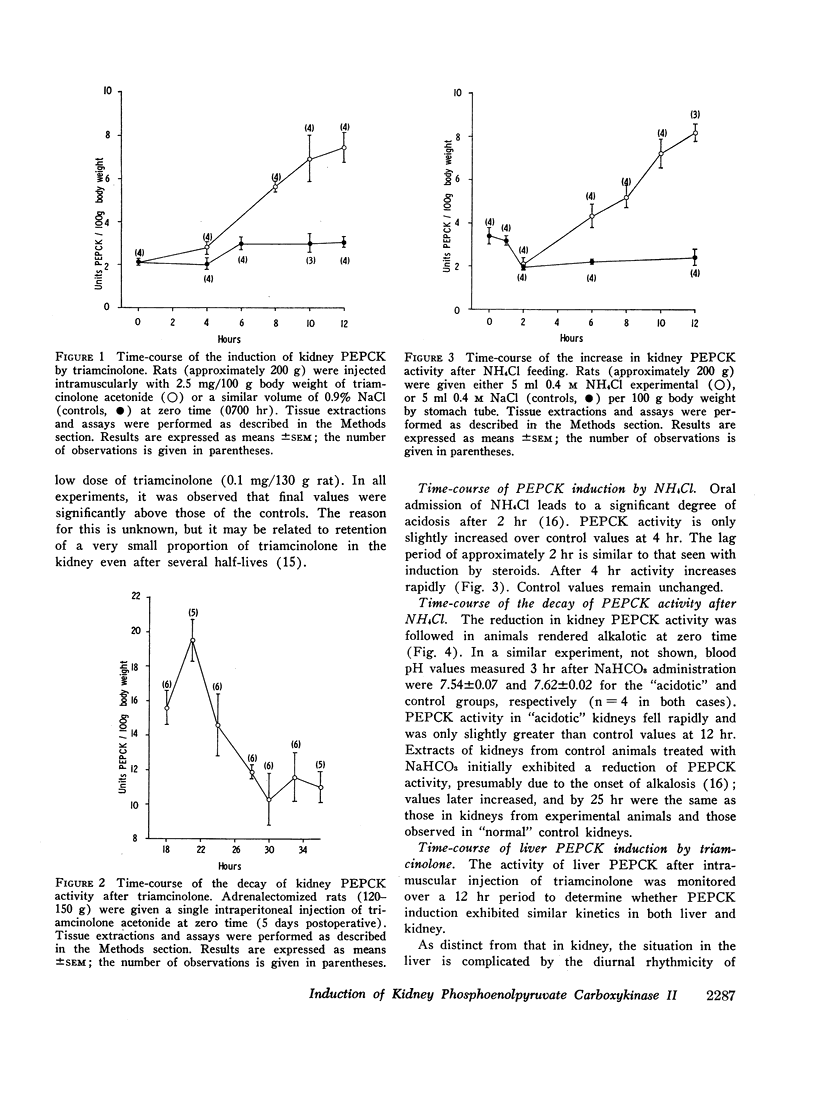
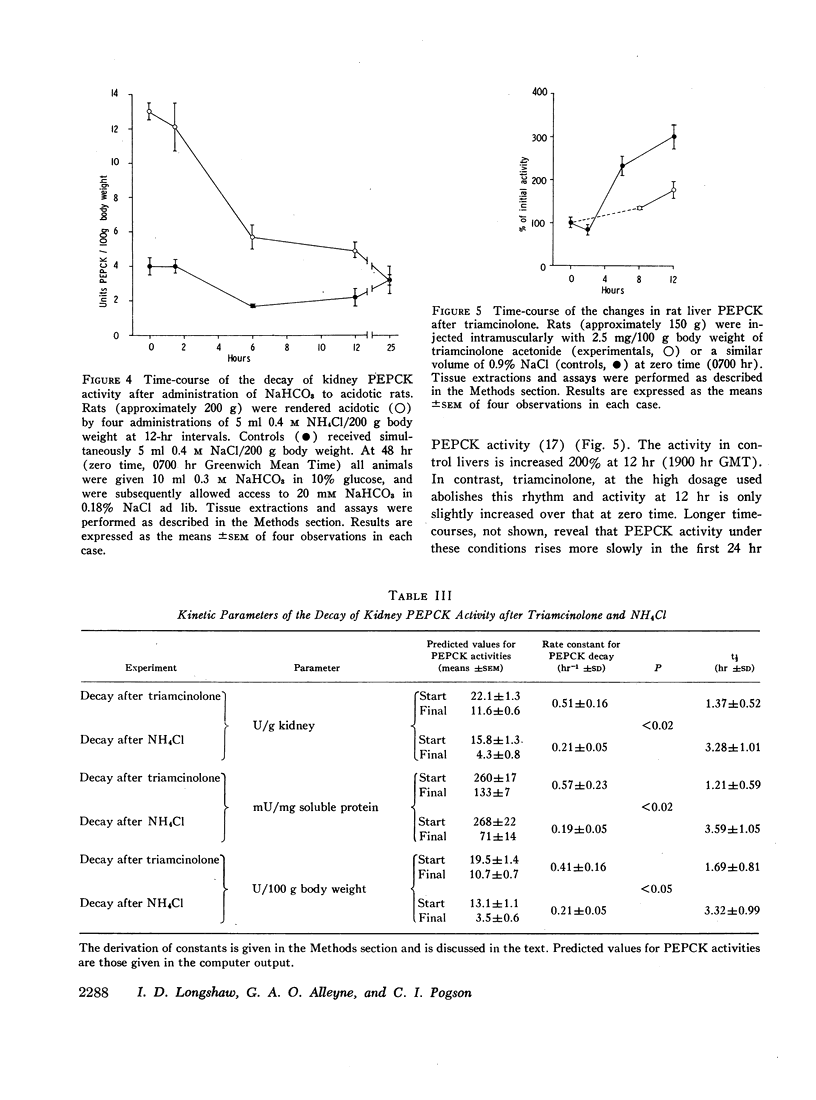
The half-life of kidney phosphoenolpyruvate carboxykinase activity, as measured from the decay curve after a single doses of triamcinolone, is approximately 1.4 hr. This compares with a half-life for the enzyme from acidotic kidney of approximately 3.4 hr.
Analysis of the data indicates that the induction of phosphoenolpyruvate carboxykinase activity by triamcinolone may be attributed to an increase in de novo protein synthesis. Induction by acidosis is qualitatively distinct and is partly attributed to a reduction in the rate of decay of phosphoenolpyruvate carboxykinase activity.
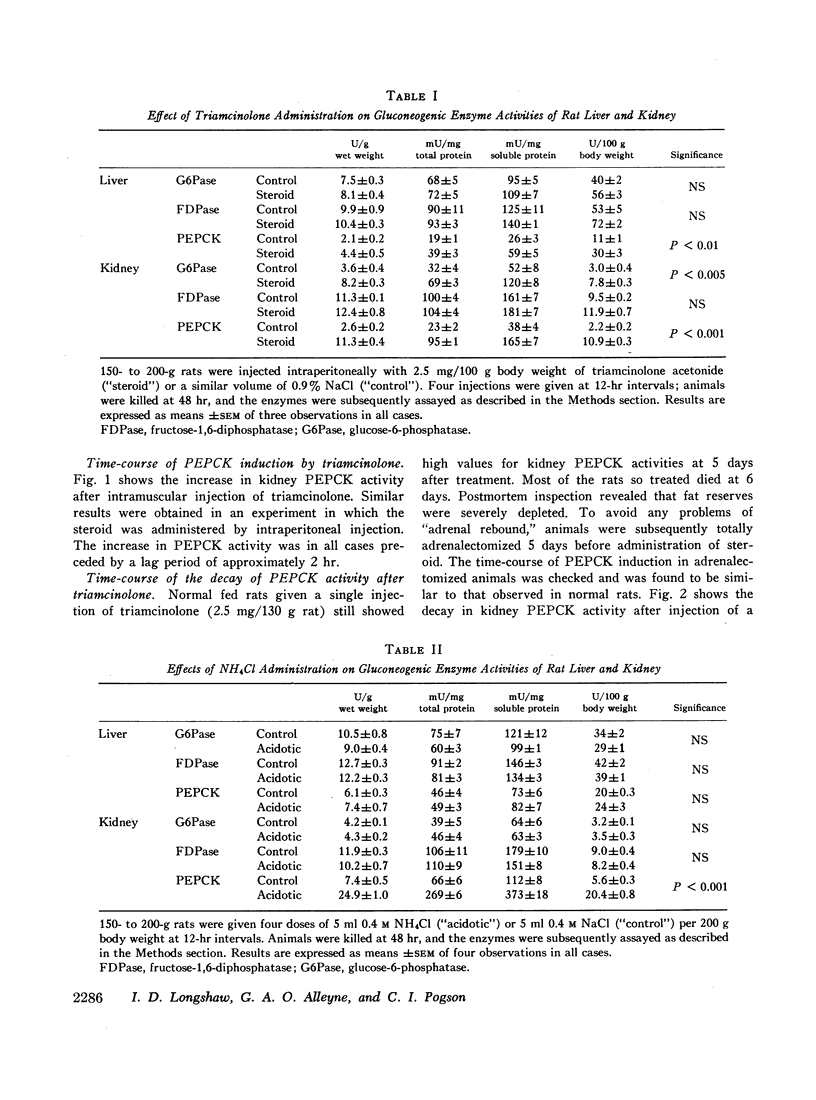
The activities of the gluconeogenic enzymes glucose-6-phosphatase, fructose-1,6-diphosphatase, and phosphoenolpyruvate carboxykinase in both liver and kidney have been measured in animals separately treated with triamcinolone and ammonium chloride. Triamcinolone significantly increases the activities of liver phosphoenolpyruvate carboxykinase, kidney glucose-6-phosphatase, and kidney phosphoenolpyruvate carboxykinase only; ammonium chloride stimulates a 200% increase in kidney phosphoenolpyruvate carboxykinase, but has no effect on the other enzymes.
The induction processes whereby triamcinolone increases phosphoenolpyruvate carboxykinase activities in liver and kidney differ quantitatively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleyne G. A. Renal metabolic response to acid-base changes. II. The early effects of metabolic acidosis on renal metabolism in the rat. J Clin Invest. 1970 May;49(5):943–951. doi: 10.1172/JCI106314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUCE H. M., PARKES A. S. Feeding and breeding of laboratory animals; a complete cubed diet for mice and rats. J Hyg (Lond) 1949 Jun;47(2):202–208. doi: 10.1017/s0022172400014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- FLORINI J. R., PEETS E. A., BUYSKE D. A. Plasma half-life, tissue distribution, and excretion of triamcinolone-H3. J Pharmacol Exp Ther. 1961 Mar;131:287–293. [PubMed] [Google Scholar]
- FREEDLAND R. A., HARPER A. E. Metabolic adaptations in higher animals. I. Dietary effects on liver glucose-6-phosphatase. J Biol Chem. 1957 Oct;228(2):743–751. [PubMed] [Google Scholar]
- Flores H., Alleyne G. A. Phosphoenolpyruvate carboxykinase of kidney. Subcellular distribution and response to acid-base changes. Biochem J. 1971 Jun;123(1):35–39. doi: 10.1042/bj1230035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. O., Lardy H. A., Ray P. D., Johnston J. B. Alteration of rat liver phosphoenolpyruvate carboxykinase activity by L-tryptophan in vivo and metals in vitro. Biochemistry. 1967 Jul;6(7):2120–2128. doi: 10.1021/bi00859a033. [DOI] [PubMed] [Google Scholar]
- Freedland R. A., Avery E. H., Taylor A. R. Effect of thyroid hormones on metabolism. II. The effect of adrenalectomy or hypophysectomy on responses of rat liver enzyme activity to l-thyroxine injection. Can J Biochem. 1968 Feb;46(2):141–150. doi: 10.1139/o68-021. [DOI] [PubMed] [Google Scholar]
- Haining J. L. Kinetics of induction of rat liver enzymes by glucocorticoids. Mol Pharmacol. 1970 Jul;6(4):444–447. [PubMed] [Google Scholar]
- Haining J. L. On the kinetics of liver enzyme regression following induction. Arch Biochem Biophys. 1971 May;144(1):204–208. doi: 10.1016/0003-9861(71)90469-3. [DOI] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R. Rapid protein catabolism in mammalian cells is obscured by reutilization of amino acids. Biochem Biophys Res Commun. 1971 Apr 2;43(1):76–81. doi: 10.1016/s0006-291x(71)80088-8. [DOI] [PubMed] [Google Scholar]
- Longshaw I. D., Pogson C. I. The effect of steroids and ammonium chloride acidosis on phosphoenolpyruvate carboxykinase in rat kidney cortex. I. Differentiation of the inductive process and characterization of enzyme activities. J Clin Invest. 1972 Sep;51(9):2277–2283. doi: 10.1172/JCI107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Phillips L. J., Berry L. J. Circadian rhythm of mouse liver phosphoenolpyruvate carboxykinase. Am J Physiol. 1970 May;218(5):1440–1444. doi: 10.1152/ajplegacy.1970.218.5.1440. [DOI] [PubMed] [Google Scholar]
- Poole B. The kinetics of disappearance of labeled leucine from the free leucine pool of rat liver and its effect on the apparent turnover of catalase and other hepatic proteins. J Biol Chem. 1971 Nov;246(21):6587–6591. [PubMed] [Google Scholar]
- Reshef L., Ballard F. J., Hanson R. W. The role of the adrenals in the regulation of phosphoenolpyruvate carboxykinase of rat adipose tissue. J Biol Chem. 1969 Oct 25;244(20):5577–5581. [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G., SINGHAL R. L., STAMM N. B., SRIVASTAVA S. K. HORMONAL INDUCTION AND SUPPRESSION OF LIVER ENZYME BIOSYNTHESIS. Fed Proc. 1965 May-Jun;24:745–754. [PubMed] [Google Scholar]
- Wicks W. D. Differential effects of glucocorticoids and adenosine 3',5'-monophosphate on hepatic enzyme synthesis. J Biol Chem. 1971 Jan 10;246(1):217–223. [PubMed] [Google Scholar]


