Abstract
Brush borders and plasma membranes have been purified from mucosal epithelial cells of rabbit ileum under control conditions and after treatment for 3 hr with cholera toxin in vivo. The activity of several enzymes in these preparations was measured. It was concluded that adenyl cyclase, like NaK-ATPase, seems not to be a normal constituent of brush borders. Both these enzymes are present in plasma membrane preparations derived largely from the basal and lateral margins of the epithelial cells, both may be phospholipid dependent enzymes and both are affected by cholera toxin. Adenyl cyclase activity is increased while NaK-ATPase is decreased. The activities of alkaline phosphatase, leucineaminopeptidase, 5′-nucleotidase, glucose-6-phosphatase, and Mg-ATPase were not found to be affected by the toxin. Cholera toxin, which makes contact with the luminal side of the epithelial cells, in the natural disease and in the experimental model, would appear to exert its pathologic effect on adenyl cyclase at the opposite (basal and lateral) side of the cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANDES D., ZETTERQVIST H., SHELDON H. Histochemical techniques for electron microscopy: alkaline phosphatase. Nature. 1956 Feb 25;177(4504):382–383. doi: 10.1038/177382a0. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK S. L., Jr The localization of alkaline phosphatase in tissues of mice, using the electron microscope. Am J Anat. 1961 Jul;109:57–83. doi: 10.1002/aja.1001090106. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. C., Rohde J. E., Sharp G. W. Intestinal adenyl-cyclase activity in human cholera. Lancet. 1971 May 8;1(7706):939–941. doi: 10.1016/s0140-6736(71)91443-7. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Rohde J. E., Sharp G. W. Properties of adenyl cyclase from human jejunal mucosa during naturally acquired cholera and convalescence. J Clin Invest. 1972 Apr;51(4):731–740. doi: 10.1172/JCI106867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., HABBU M. K. Experimental cholera in infant rabbits: a method for chemotherapeutic investigation. Br J Pharmacol Chemother. 1955 Jun;10(2):153–159. doi: 10.1111/j.1476-5381.1955.tb00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. 3. Mg2+-ATPase,(Na+-K+-Mg2+)-ATPase and 5'-nucleotidase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jul 13;120(3):369–382. doi: 10.1016/0926-6585(66)90304-9. [DOI] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Field M., Plotkin G. R., Silen W. Effects of vasopressin, theophylline and cyclic adenosine monophosphate on short-circuit current across isolated rabbit ileal mucosa. Nature. 1968 Feb 3;217(5127):469–471. doi: 10.1038/217469a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., MII S., KOHOUT P. M. Studies on the terminal electron transport system. I. Succinic dehydrogenase. J Biol Chem. 1955 Dec;217(2):551–567. [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
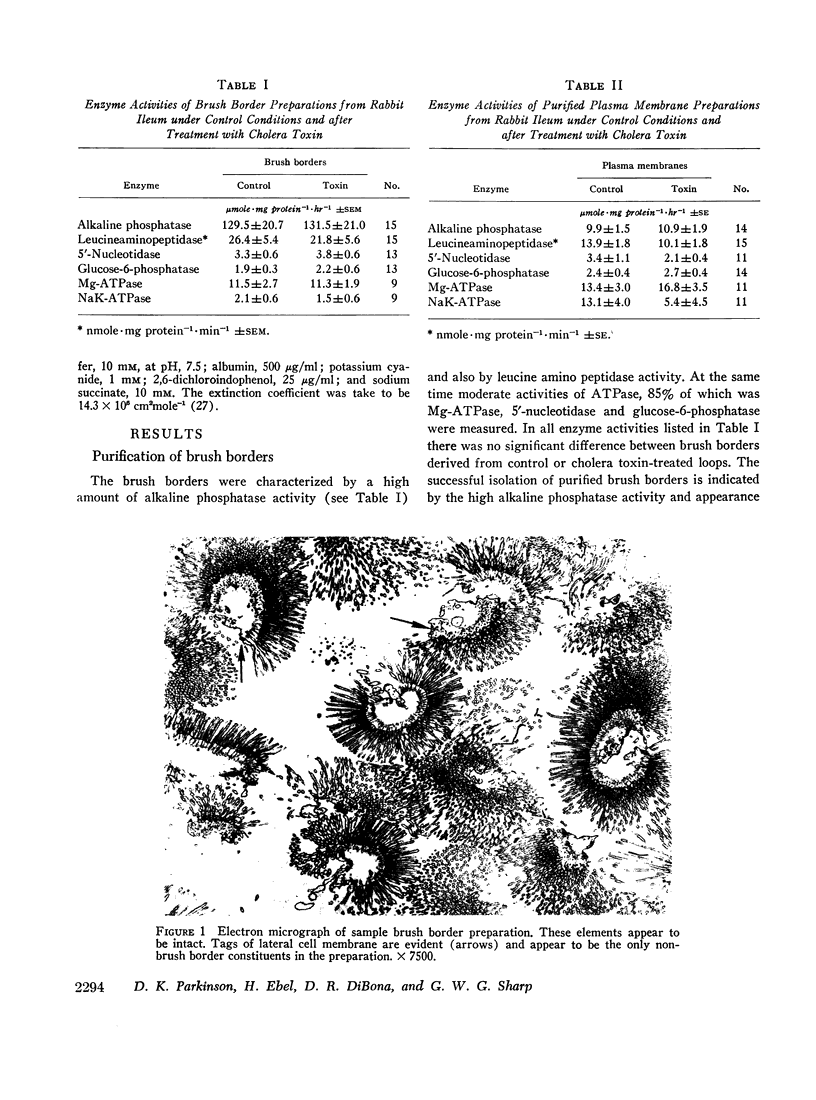
- Hirschhorn N., Rosenberg I. H. Sodium-potassium stimulated adenosine triphosphatase of the small intestine of man: studies in cholera and other diarrheal diseases. J Lab Clin Med. 1968 Jul;72(1):28–39. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Richardson S. H. An ion translocase system from rabbit intestinal mucosa. Preparation and properties of the (Na+K+)-activated ATPase. Biochim Biophys Acta. 1968 Jun 11;150(4):572–577. doi: 10.1016/0005-2736(68)90046-1. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C., Steenburg R. W., Pierce N. F. Experimental cholera. A canine model. Lancet. 1966 Jul 23;2(7456):206–207. doi: 10.1016/s0140-6736(66)92484-6. [DOI] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S., Lipson L. C., Parkinson D. K. Action of cholera toxin to stimulate adenyl cyclase. Trans Assoc Am Physicians. 1971;84:200–211. [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- TUPPY H., WIESBAUER U., WINTERSBERGER E. [Amino acid-p-nitroanilide as a substrate for aminopeptidases and other proteolytic enzymes]. Hoppe Seylers Z Physiol Chem. 1962 Nov 15;329:278–288. doi: 10.1515/bchm2.1962.329.1.278. [DOI] [PubMed] [Google Scholar]