Abstract
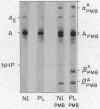
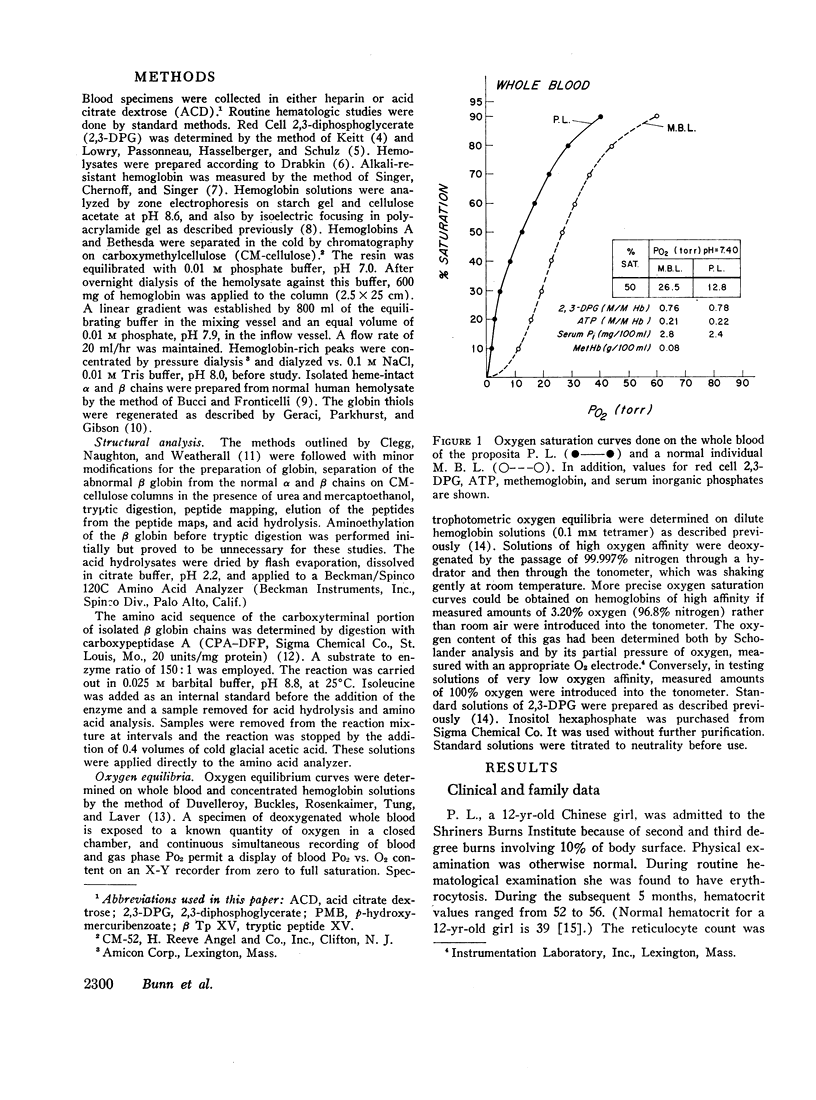
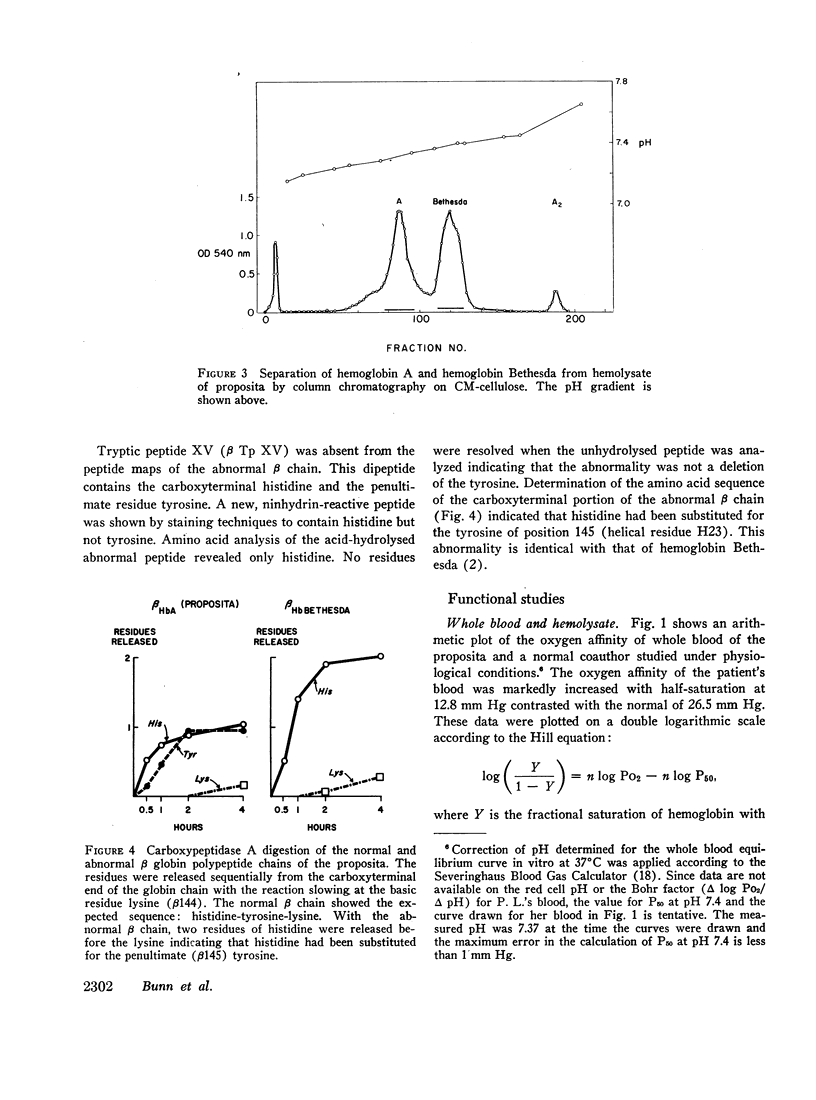
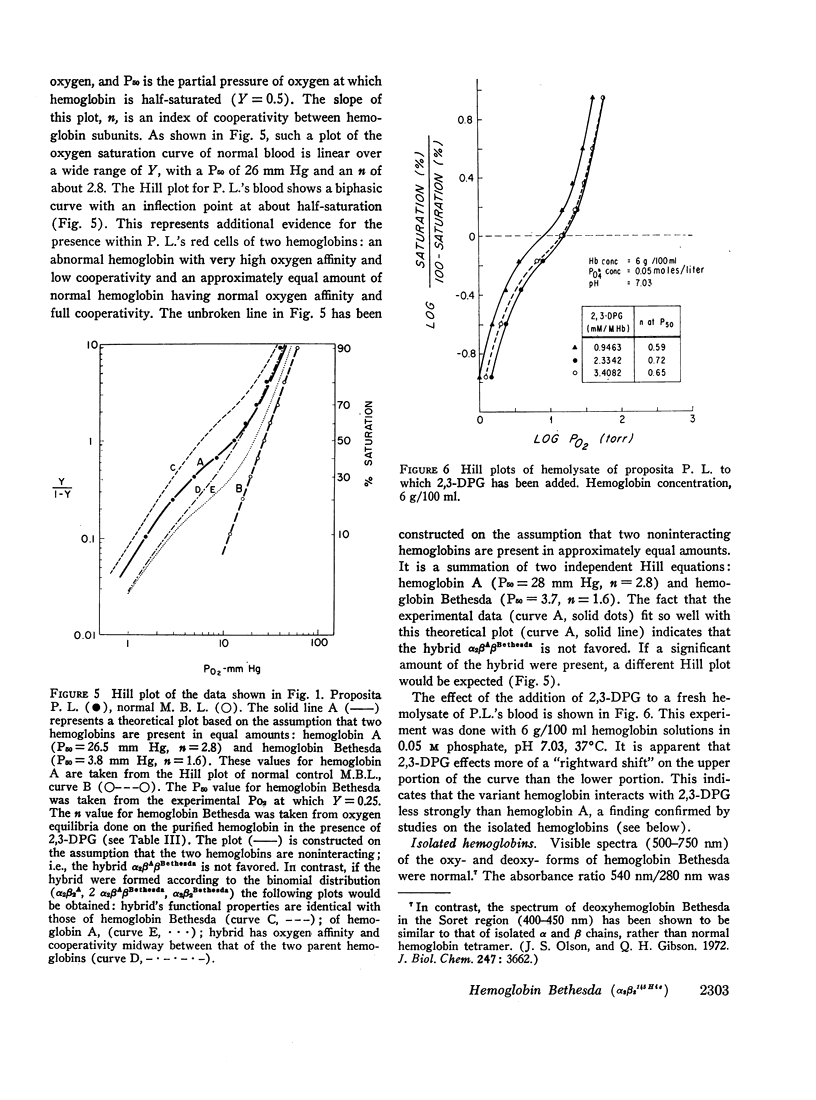
Studies have been performed on a 12-yr-old Chinese girl with compensatory erythrocytosis due to the presence of hemoglobin Bethesda comprising about 45% of the red cell hemoglobin. Her parents and three siblings were normal. The oxygen affinity of her blood was markedly increased: under physiological conditions (pH 7.40, 37°C). P50 was 12.8 mm Hg (normal = 26.5 mm Hg). The red cell 2,3-diphosphoglycerate (2.3-DPG) level was normal. The abnormal hemoglobin could not be separated from hemoglobin A by zone electrophoresis at pH 8.6 or isoelectric focusing on polyacrylamide gel. However, after the hemoglobin was split into free α and β chains by treatment with p-hydroxymercuribenzoate (PMB) or 6 M urea, an abnormal β chain was readily demonstrated having a higher isoelectric point (more positive net charge) than normal βA. Structural analysis of the variant β chain demonstrated the substitution of histidine for tyrosine at position 145: hemoglobin Bethesda (α2β2145His). From earlier chemical and crystallographic studies, it has been postulated that this residue is a critical determinant of hemoglobin function. Hemoglobin Bethesda was separated from hemoglobin A by column chromatography. Oxygen equilibria of purified hemoglobin Bethesda revealed an extremely high oxygen affinity (exceeding that of isolated α and β chains), and markedly reduced cooperativity. The Bohr effect of hemoglobin Bethesda was 1/3 that of hemoglobin A. However, hemoglobin Bethesda showed a significant interaction with 2.3-DPG and inositol hexaphosphate.
Full text
PDF

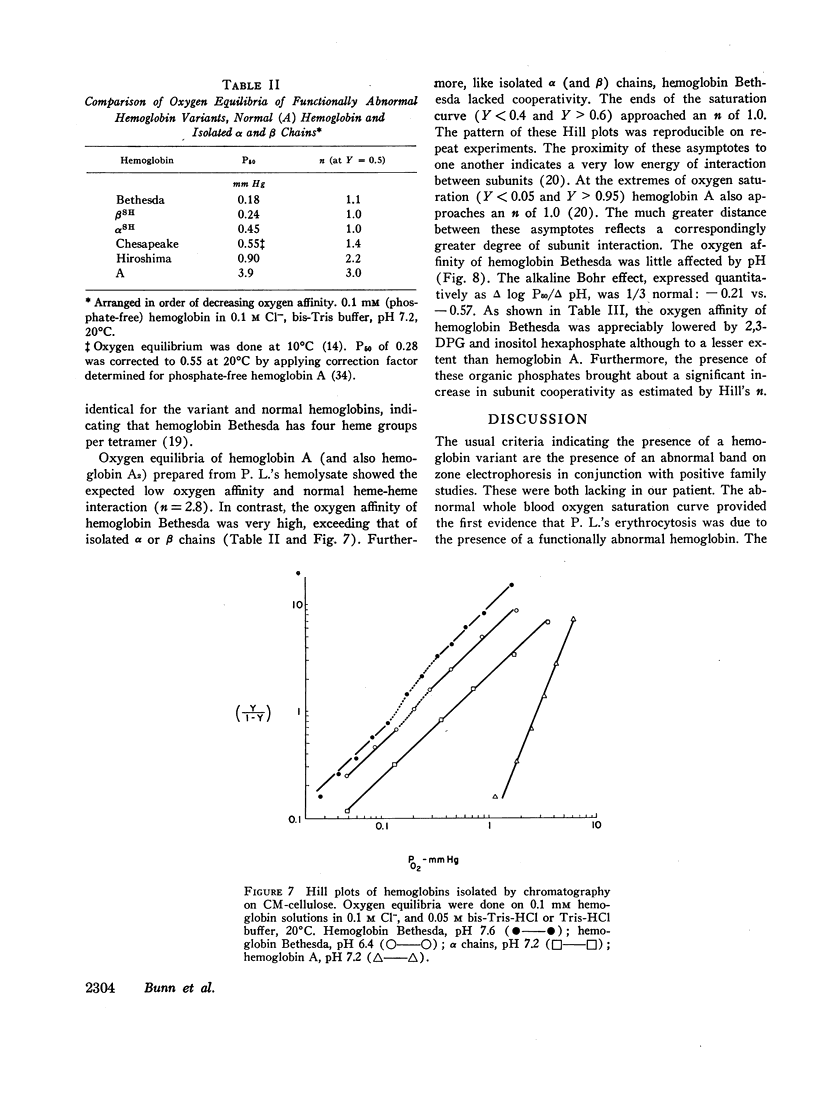
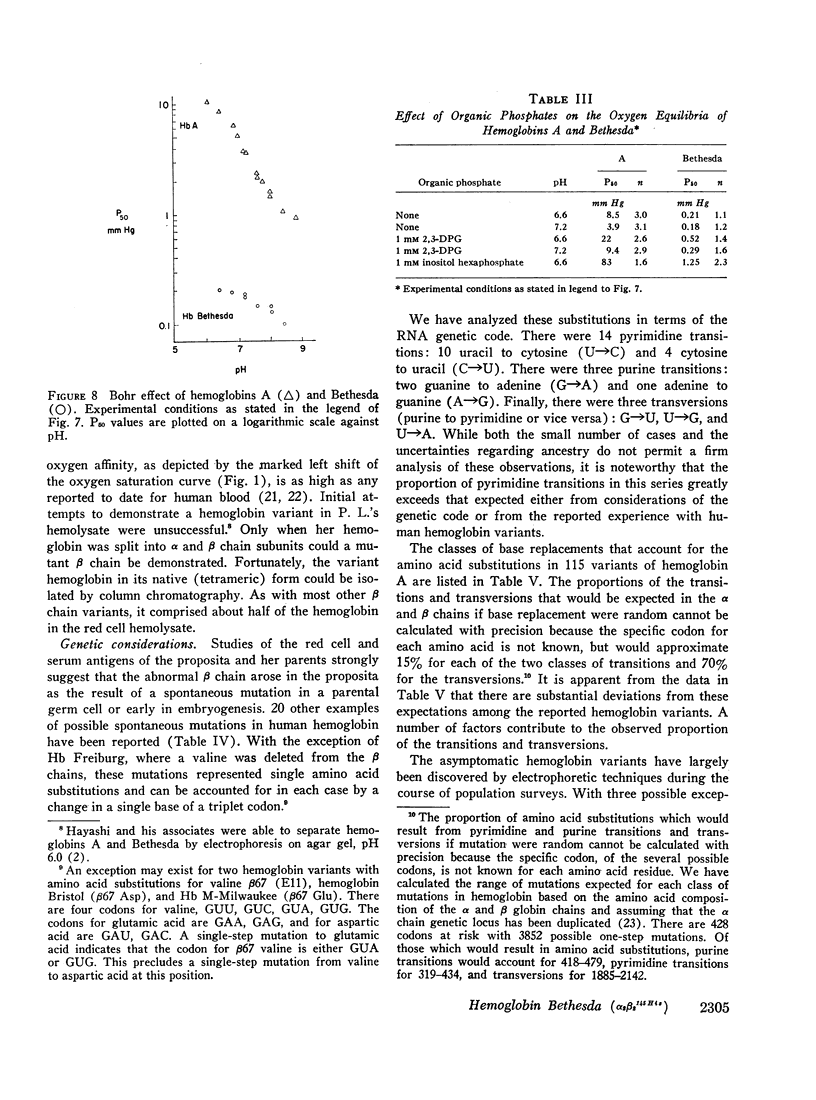
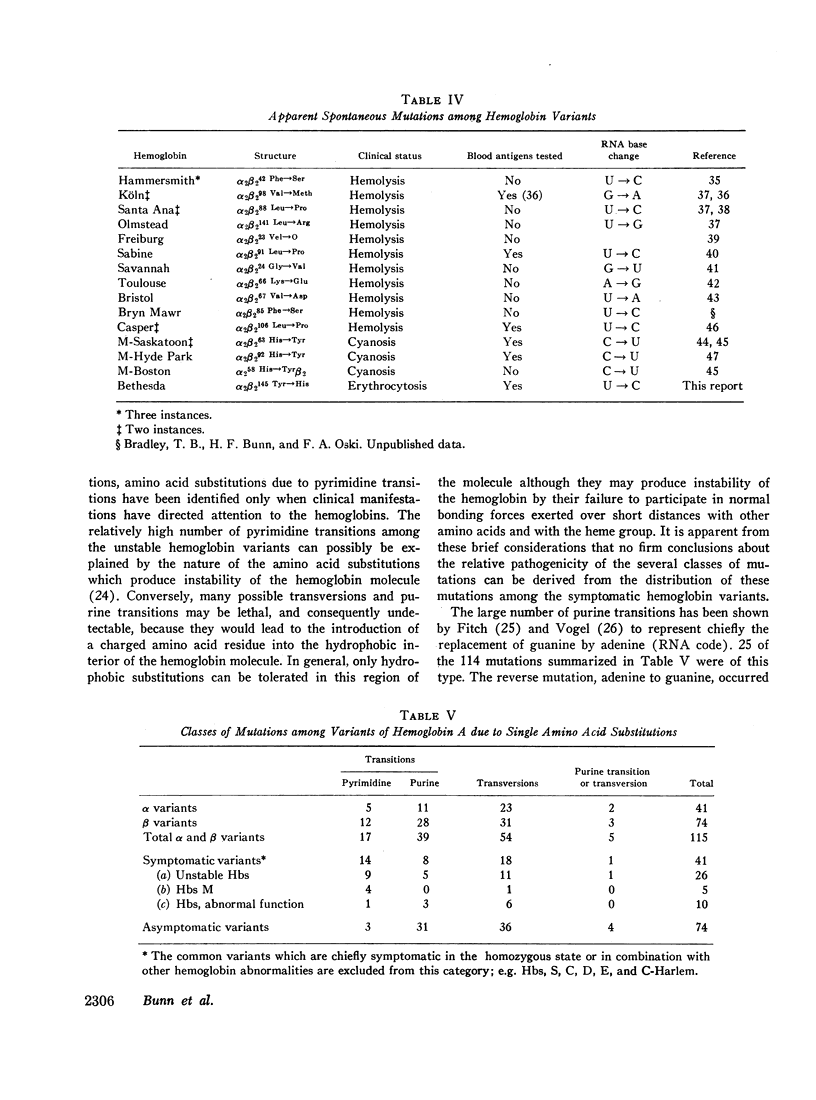



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Parer J. T., Stamatoyannopoulos G. Erythrocytosis associated with hemoglobin Rainier: oxygen equilibria and marrow regulation. J Clin Invest. 1969 Aug;48(8):1376–1386. doi: 10.1172/JCI106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becroft D. M., Douglas R., Carrell R. W., Lehmann H. Haemoglobin M Hyde Park: a hereditary methaemoglobinaemia in a Caucasian child. N Z Med J. 1968 Aug;68(435):72–76. [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F. Dissociation of haemoglobin Chesapeake into subunits. Nature. 1970 Aug 22;227(5260):839–840. doi: 10.1038/227839a0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Ransil B. J., Chao A. The interaction between erythrocyte organic phosphates, magnesium ion, and hemoglobin. J Biol Chem. 1971 Sep 10;246(17):5273–5279. [PubMed] [Google Scholar]
- Cabannes R., Labie D., Rosa J., Ruffié J., Bierme R. Une nouvelle hémoglobine par mutation portée sur la chaine beta, l'hémoglobine I-Toulouse(alpha2-beta2 66 lysglu) C R Acad Sci Hebd Seances Acad Sci D. 1969 Jul 7;269(1):111–112. [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H. The unstable haemoglobin haemolytic anaemias. Semin Hematol. 1969 Apr;6(2):116–132. [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- DACIE J. V., GRIMES A. J., MEISLER A., STEINGOLD L., HEMSTED E. H., BEAVEN G. H., WHITE J. C. HEREDITARY HEINZ-BODY ANAEMIA. A REPORT OF STUDIES ON FIVE PATIENTS WITH MILD ANAEMIA. Br J Haematol. 1964 Jul;10:388–402. doi: 10.1111/j.1365-2141.1964.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Dacie J. V., Shinton N. K., Gaffney P. J., Jr, Lehmann H. Haemoglobin Hammersmith (beta-42 (CDI) Phe replaced by ser). Nature. 1967 Nov 18;216(5116):663–665. doi: 10.1038/216663a0. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- Duvelleroy M. A., Buckles R. G., Rosenkaimer S., Tung C., Laver M. B. An oxyhemoglobin dissociation analyzer. J Appl Physiol. 1970 Feb;28(2):227–233. doi: 10.1152/jappl.1970.28.2.227. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Extensions of the allosteric model for haemoglobin. Nature. 1971 Mar 26;230(5291):224–227. doi: 10.1038/230224a0. [DOI] [PubMed] [Google Scholar]
- FARMER M. B., LEHMANN H., RAINE D. N. TWO UNRELATED PATIENTS WITH CONGENITAL CYANOSIS DUE TO HAEMOGLOBINOPATHY M. Lancet. 1964 Oct 10;2(7363):786–789. doi: 10.1016/s0140-6736(64)90563-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks V. F., Opfell R. W., Burgert E. O., Jr Three families with unstable hemoglobinopathies (Köln, Olmsted and Santa Ana) causing hemolytic anemia with inclusion bodies and pigmenturia. Am J Med. 1969 Mar;46(3):344–359. doi: 10.1016/0002-9343(69)90037-0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Evidence suggesting a non-random character to nucleotide replacements in naturally occurring mutations. J Mol Biol. 1967 Jun 28;26(3):499–507. doi: 10.1016/0022-2836(67)90317-8. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Hayashi A., Stamatoyannopoulos G., Yoshida A., Adamson J. Haemoglobin Rainier: beta-145 (HC2) tyrosine leads to cysteine and haemoglobin Bethesda: beta-145 (HC2) tyrosine leads to histidine. Nat New Biol. 1971 Apr 28;230(17):264–267. doi: 10.1038/newbio230264a0. [DOI] [PubMed] [Google Scholar]
- Hollán S. R., Szelényi J. G., Miltényi M., Charlesworth D., Lorkin P. A., Lehmann H. Unstable haemoglobin disease caused by Hb Santa Ana- 88 (F4) Leu leads to Pro. Haematologia (Budap) 1970;4(2):141–155. [PubMed] [Google Scholar]
- Huisman T. H., Brown A. K., Efremov G. D., Wilson J. B., Reynolds C. A., Uy R., Smith L. L. Hemoglobin Savannah (B6(24) beta-glycine is greater than valine): an unstable variant causing anemia with inclusion bodies. J Clin Invest. 1971 Mar;50(3):650–659. doi: 10.1172/JCI106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPHSON A. M., WEINSTEIN H. G., YAKULIS V. J., SINGER L., HELLER P. A new variant of hemoglobin M disease: hemoglobin M-Chicago. J Lab Clin Med. 1962 Jun;59:918–925. [PubMed] [Google Scholar]
- Jones R. T., Brimhall B., Huisman T. H., Kleihauer E., Betke K. Hemoglobin Freiburg: abnormal hemoglobin due to deletion of a single amino acid residue. Science. 1966 Nov 25;154(3752):1024–1027. doi: 10.1126/science.154.3752.1024. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Reduced nicotinamide adenine dinucleotide-linked analysis of 2,3-diphosphoglyceric acid: spectrophotometric and fluorometric procedures. J Lab Clin Med. 1971 Mar;77(3):470–475. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Differences between alpha- and beta-chain mutants of human haemoglobin and between alpha- and beta-thalassaemia. Possible duplication of the alpha-chain gene. Br Med J. 1968 Dec 21;4(5633):748–750. doi: 10.1136/bmj.4.5633.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. R., Weed R. I., Stamatoyannopoulos G., Yoshida A. Hemoglobin Köln disease occurring as a fresh mutation: erythrocyte metabolism and survival. Blood. 1971 Dec;38(6):715–729. [PubMed] [Google Scholar]
- Morimoto H., Lehmann H., Perutz M. F. Moleuclar pathology of human haemoglobin: stereochemical interpretation of abnormal oxygen affinities. Nature. 1971 Aug 6;232(5310):408–413. doi: 10.1038/232408a0. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Gibson Q. H., Charache S. Relation between structure and function in Hemoglobin Chesapeake. Biochemistry. 1967 Aug;6(8):2395–2402. doi: 10.1021/bi00860a015. [DOI] [PubMed] [Google Scholar]
- Novy M. J., Edwards M. J., Metcalfe J. Hemoglobin Yakina. II. High blood oxygen affinity associated with compensatory erythrocytosis and normal hemodynamics. J Clin Invest. 1967 Nov;46(11):1848–1854. doi: 10.1172/JCI105675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Kimura M. Amino acid composition of proteins as a product of molecular evolution. Science. 1971 Oct 8;174(4005):150–153. doi: 10.1126/science.174.4005.150. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- SINGER K., CHERNOFF A. I., SINGER L. Studies on abnormal hemoglobins. I. Their demonstration in sickle cell anemia and other hematologic disorders by means of alkali denaturation. Blood. 1951 May;6(5):413–428. [PubMed] [Google Scholar]
- Schneider R. G., Ueda S., Alperin J. B., Brimhall B., Jones R. T. Hemoglobin sabine beta 91 (f 7) leu to pro. An unstable variant causing severe anemia with inclusion bodies. N Engl J Med. 1969 Apr 3;280(14):739–745. doi: 10.1056/NEJM196904032801402. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Steadman J. H., Yates A., Huehns E. R. Idiopathic Heinz body anaemia: Hb-Bristol (beta67 (E11) Val to Asp). Br J Haematol. 1970 Apr;18(4):435–446. doi: 10.1111/j.1365-2141.1970.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Imai K., Shimizu K. Effect of inositol hexaphosphate and other organic phosphates on the cooperativity in oxygen binding of human hemoglobins. Biochem Biophys Res Commun. 1971 Aug 6;44(3):682–686. doi: 10.1016/s0006-291x(71)80137-7. [DOI] [PubMed] [Google Scholar]
- Vogel F. Point mutations and human hemoglobin variants. Humangenetik. 1969;8(1):1–26. doi: 10.1007/BF00286751. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]