Abstract
Mg2+ is an essential ion for many cellular processes, including protein synthesis, nucleic acid stability, and numerous enzymatic reactions. Mg2+ homeostasis in mammals depends on the equilibrium between intestinal absorption, renal excretion, and exchange with bone. The transient receptor potential melastatin type 6 (TRPM6) is an epithelial Mg2+ channel, which is abundantly expressed in the luminal membrane of the renal and intestinal cells. It functions as the gatekeeper of transepithelial Mg2+ transport. Remarkably, TRPM6 combines a Mg2+-permeable channel with an α-kinase domain. Here, by the Ras recruitment system, we identified methionine sulfoxide reductase B1 (MsrB1) as an interacting protein of the TRPM6 α-kinase domain. Importantly, MsrB1 and TRPM6 are both present in the renal Mg2+-transporting distal convoluted tubules. MsrB1 has no effect on TRPM6 channel activity in the normoxic conditions. However, hydrogen peroxide (H2O2) decreased TRPM6 channel activity. Co-expression of MsrB1 with TRPM6 attenuated the inhibitory effect of H2O2 (TRPM6, 67 ± 5% of control; TRPM6 + MsrB1, 81 ± 5% of control). Cell surface biotinylation assays showed that H2O2 treatment does not affect the expression of TRPM6 at the plasma membrane. Next, mutation of Met1755 to Ala in TRPM6 reduced the inhibitory effect of H2O2 on TRPM6 channel activity (TRPM6 M1755A: 84 ± 10% of control), thereby mimicking the action of MsrB1. Thus, these data suggest that MsrB1 recovers TRPM6 channel activity by reducing the oxidation of Met1755 and could, thereby, function as a modulator of TRPM6 during oxidative stress.
Keywords: Enzymes, Kidney, Methionine, Oxidative Stress, TRP Channels, Distal Convoluted Tubule, Hydrogen Peroxide, Magnesium
Introduction
To maintain a physiological extra- and intracellular Mg2+ concentration is of great importance to keep the accurate function of more than 300 enzymatic systems and the subsequent various biological and physiological processes (1–4). The kidney is the principal organ responsible for the regulation of the body Mg2+ balance. Around 80% of the total plasma Mg2+ is ultrafiltered through the glomeruli and subsequently reabsorbed passively in the proximal tubule and the thick ascending limb of Henle's loop (5). The final urinary Mg2+ concentration is defined by active Mg2+ reabsorption in the distal convoluted tubule (DCT)3 (6).
The transient receptor potential melastatin type 6 (TRPM6) is a cation channel playing a crucial role in Mg2+ homeostasis. Mutations in TRPM6 cause hypomagnesemia with secondary hypocalcemia (7, 8). Interestingly, mice deficient of TRPM6 (TRPM6−/− mice) were essentially embryonically lethal, and the incidental TRPM6−/− mice that survived had neural tube defects (9). TRPM6 and its closest homologue TRPM7 uniquely combine an ion channel pore-forming region with a serine/threonine protein kinase domain. It is located at the carboxyl terminus and has similarities with members of the α-kinase family (10, 11). Previous studies demonstrated that receptor for activated C-kinase 1 (RACK1) and repressor of estrogen receptor activity (REA) interact with this domain and inhibit channel activity in an (auto)phosphorylation-dependent manner (12, 13). Moreover, modulation of TRPM6 channel activity by intracellular ATP requires the ATP-binding motif in the α-kinase domain (14). Although the phosphorylation activity of the TRPM6/7 α-kinase domains has been well determined, the role of these domains in regulating channel activity remains elusive (12, 15–18).
Over the last years, several studies have implicated TRPM channels in ischemia (19, 20). Sun et al. (21) showed that decreased TRPM7 channel expression significantly reduced neuronal cell death after global ischemia. Furthermore, TRPM4 channel activation in vascular smooth muscle has been shown to contribute to cell death of vascular cells during ischemic injury, and TRPM2 has been well studied in relation to oxidative stress (22–25). Accumulating evidence suggests that reactive oxygen species are not only harmful side products of cellular metabolism but also central players in cell signaling and regulation (26–29). Interestingly, renal DCT cells contain the largest number of mitochondria. However, the effect of oxidative stress on the epithelial Mg2+ channel TRPM6, expressed at the apical membrane of the DCT, has not been studied.
The aim of the present study was to investigate the role of the α-kinase domain in TRPM6 channel activity by the identification of associated proteins. To this end, the Ras recruitment system (RRS), a novel yeast two-hybrid screening system, which is designed to screen for partners of plasma membrane proteins, was applied (30). Here, we identified methionine sulfoxide reductase B1 (MsrB1) as a TRPM6-associated protein, binding to the TRPM6 α-kinase domain. Using biochemical, immunohistochemical, and electrophysiological analyses, we demonstrated a novel operation mode for MsrB1 in the regulation of TRPM6 channel activity in oxidative stress through modulating methionine oxidation in TRPM6.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Human embryonic kidney (HEK) 293 cells were grown and transfected as described previously (31), and electrophysiological recordings were performed 48 h after transfection.
DNA Constructs
The α-kinase domain of mouse (1759–2028) TRPM6 was cloned into the pMet425-Myc-Ras (kind gift from Dr. A. Aronheim, Haifa, Israel) by PCR using mouse kidney cDNA as template. The α-kinase domain of human (1759–2022) TRPM6 was cloned into the pEBG vector using human TRPM6 in pCINeo/IRES-GFP (32) as template. Full-length mouse MsrB1 cDNA was cloned into pCB7 by PCR using mouse kidney cDNA and FLAG-tagged at the N-terminal tail. Wild-type human TRPM6 in the pCINeo/IRES-GFP vector was HA-tagged at the N-terminal tail as described previously (32). TRPM6 M1755A, TRPM6 M1775A, TRPM6 M1904A, and TRPM6 M1947A mutants were created using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. All constructs were verified by sequence analysis.
RRS Screening
RRS screening was performed as described previously (30). Briefly, cdc25-2 strains (kind gift from Dr. A. Aronheim) were co-transformed with the pMet425-Myc-Ras-TRPM6 α-kinase domain and mouse kidney cDNA library. Transformants were grown on selectable minimal glucose plates for 5 days at 25 °C followed by subsequent replica plating onto minimal galactose plates, incubating for 5–7 days at 37 °C. Library plasmids of positive colonies growing on galactose plates at 37 °C were isolated and further analyzed by DNA sequencing. The positive colonies were co-transformed with the pMet425-Myc-Ras-TRPM6 α-kinase domain into cdc25-2 cells to confirm specificity of interaction.
RT-PCR
Total RNA isolation from mouse tissue and reverse transcription were performed as described previously (33). MsrB1 and β-actin were amplified by PCR and subsequently analyzed by agarose gel electrophoresis.
Electrophysiology
Patch clamp experiments were performed in the tight seal whole-cell configuration at room temperature using an EPC-10 patch clamp amplifier computer controlled by the Pulse software (HEKA Elektronik, Lambrecht, Germany). Electrode resistances were 2–5 megaohms, and capacitance and access resistance were monitored continuously. A ramp protocol, consisting of linear voltage ramp from −100 to +100 mV (within 450 ms), was applied every 2 s from a holding potential of 0 mV. Current densities were obtained by normalizing the current amplitude to the cell membrane capacitance. The time course of current development was determined by measuring the current at +80 and −80 mV. I/V relations were established from the ramp protocols. The analysis and display of patch clamp data were performed using Igor Pro software (WaveMetrics, Lake Oswego, OR). The standard pipette solution contained 150 mm NaCl, 10 mm EDTA, and 10 mm HEPES-NaOH, pH 7.2. The extracellular solution contained 150 mm NaCl, 10 mm HEPES-NaOH, pH 7.4, supplemented with 1 mm CaCl2. To avoid breakdown, hydrogen peroxide (H2O2) was stored at 4 °C prior to use and added to the perfusate immediately (<1 min) prior to making recordings.
Co-precipitation Assay
HEK293 cells were transiently co-transfected with MsrB1 and pEBG-TRPM6-α-kinase or pEBG empty vector. 24 h after transfection, cells were lysed for 1 h on ice in lysis buffer (150 mm NaCl, 5 mm EDTA, 50 mm Tris-NaOH, pH 7.5, 0.33% (v/v) Triton X-100 including the protease inhibitors leupeptin (0.01 mg/ml), pepstatin (0.05 mg/ml), and phenylmethylsulfonyl fluoride (1 mm)). After centrifugation, supernatants of the lysates were incubated overnight with glutathione-Sepharose 4B beads at 4 °C. In the co-precipitation experiment with full-length TRPM6, HEK293 cells were co-transfected with pEBG-MsrB1 and TRPM6 pCIneo/IRES-GFP or pCIneo/IRES-GFP empty vector and subsequently lysed and incubated on glutathione-Sepharose 4B beads as described above. After extensive washing, the bound proteins were eluted with SDS-PAGE loading buffer. The co-precipitation was analyzed using the anti-MsrB1 antibody (kind gift from Dr. J. Moskovitz, Lawrence, KS), mouse anti-HA (Sigma) antibody, or anti-GST antibody (Sigma).
Cell Surface Labeling with Biotin
HEK293 cells, in poly-l-lysine (Sigma)-coated 10-cm dishes, were transiently transfected with 15 μg of HA-TRPM6. 72 h after transfection, cells were treated with 1 mm H2O2 for 10 min at 37 °C. Cell surface labeling with NHS-LC-LC-biotin (Pierce, Etten-Leur, The Netherlands) was performed as described previously (34). 1 h after homogenizing, biotinylated proteins were precipitated using NeutrAvidin-agarose beads (Pierce). TRPM6 expression was analyzed by immunoblot for the precipitates (plasma membrane fraction) and for the total cell lysates using the mouse anti-HA antibody.
Immunoblotting
Protein samples were denatured by incubation for 30 min at 37 °C in Laemmli buffer and then subjected to SDS-PAGE. Immunoblots were incubated with either mouse anti-HA or rabbit anti-MsrB1 antibody. Subsequently, blots were incubated with sheep horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Sigma) and then visualized using the enhanced chemiluminescence system.
Immunohistochemistry
Immunohistochemistry was performed as described previously (35). Briefly, mouse kidney sections were incubated for 16 h at 4 °C with rabbit anti-MsrB1 and guinea pig anti-TRPM6. To visualize TRPM6, tyramide signal amplification kit (PerkinElmer Life Sciences, Zaventem, Belgium) was used after incubation with biotin-coated goat anti-mouse secondary antibody. Images were taken with a Bio-Rad MRC 100 confocal laser scanning microscope.
Statistical Analysis
Values are expressed as mean ± S.E. Statistical significance between groups was determined by analysis of variance followed by Bonferroni's multiple comparison test. Differences between the means of two groups were analyzed by an unpaired Student's t test. p < 0.05 was considered statistically significant.
RESULTS
MsrB1 Interacts with TRPM6 α-Kinase Domain
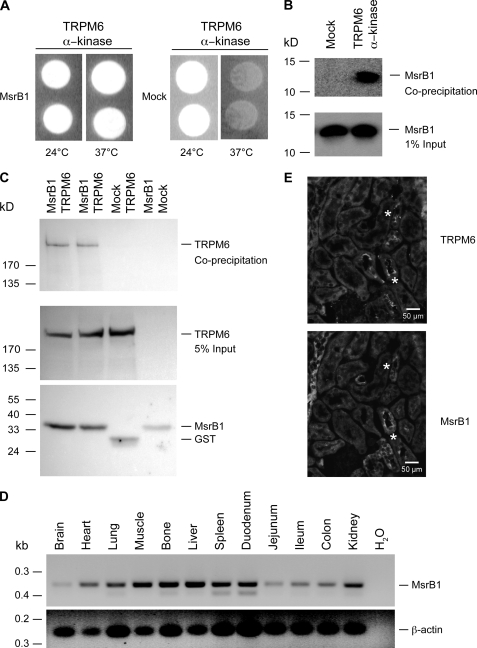
To identify proteins interacting with the α-kinase domain of TRPM6, we applied the RRS. When compared with the conventional yeast two-hybrid screening system, RRS is more appropriate to detect interaction partners of plasma membrane proteins (30). In this approach, MsrB1, a methionine sulfoxide reductase (36), was identified as an interacting protein of the TRPM6 α-kinase domain. Subsequently, MsrB1 cDNA was co-transformed with the α-kinase domain of TRPM6 into cdc25-2 yeast strain to confirm the interaction. As shown in Fig. 1A, whereas the cdc25-2 strains co-transformed with MsrB1 and the TRPM6 α-kinase domain grow at 37 °C, yeast co-transformed with the control vector and TRPM6 α-kinase domain only survived at 24 °C. The association between TRPM6 and MsrB1 was further substantiated by co-precipitation studies of glutathione S-transferase (GST) and GST-TRPM6-kinase in MsrB1-expressing HEK293 cells. MsrB1 co-precipitated with the GST-α-kinase but not with GST alone (Fig. 1B, upper panel). MsrB1 was equally expressed in the tested conditions (Fig. 1B, lower panel). Furthermore, co-precipitation studies of full-length TRPM6 with MsrB1 in HEK293 cells showed that full-length TRPM6 associates with GST-MsrB1 but not with GST alone (Fig. 1C, upper panel). TRPM6 and MsrB1 were expressed in all conditions tested (Fig. 1C, middle and bottom panel).
FIGURE 1.
Interaction and co-expression between TRPM6 and MsrB1. A, complementation of the cdc25-2 mutation through the interaction of TRPM6 α-kinase domain with MsrB1. The temperature-sensitive cdc25-2 yeast strain was co-transformed with TRPM6 α-kinase domain and MsrB1 or control plasmid (Mock) and incubated on galactose-containing plates either at 25 °C or at 37 °C. B, co-precipitation studies of GST and GST-α-kinase in MsrB1-expressing HEK293 cells (top panel). MsrB1 input (1%) expression was analyzed by immunoblotting (bottom panel). C, co-precipitation of GST-MsrB1 in TRPM6-expressing HEK293 cells (top panel). TRPM6 input (5%) and MsrB1 precipitation expression were analyzed by immunoblotting (middle panel and bottom panel). D, distribution of MsrB1 (top panel) mRNA expression analyzed by RT-PCR on various tissues. β-Actin was used as a positive control (bottom panel). E, immunohistochemical analysis of TRPM6 (upper panel) and MsrB1 (lower panel) in serial mouse kidney sections. * indicates overlapping immunopositive tubules for TRPM6 and MsrB1.
MsrB1 Co-expresses with TRPM6 in Kidney
To address the tissue distribution of MsrB1, reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed on a panel of mouse tissues. The expected DNA fragment of MsrB1 was detected in all tissues as indicated in Fig. 1D. The integrity of the cDNA was confirmed by the detection of β-actin. To further study the co-expression of MsrB1 and TRPM6 in kidney, immunohistochemistry was performed on serial mouse kidney sections. This analysis indicated 70% immunopositive staining for MsrB1 in the TRPM6-expressing DCT segment, which has been implicated in active Mg2+ reabsorption (32) (Fig. 1E).
H2O2 Inhibits TRPM6 Channel Activity
Considering that MsrB1 is a stress protein that mainly exerts its function during oxidative stress (36), we hypothesized that MsrB1 regulates TRPM6 channel activity during oxidative stress. Therefore, we examined the effect of H2O2 on TRPM6 channel activity. To this end, HEK293 cells expressing TRPM6 were treated with 1 mm H2O2 during whole-cell patch clamp recordings. As shown in Fig. 2, A and B, H2O2 caused a significant inhibition of the TRPM6-mediated current (67 ± 5% of control, n = 13, p < 0.05) when compared with the non-treated cells (n = 11). Furthermore, we demonstrate that H2O2 inhibits TRPM6 channel activity in a dose-dependent effect with an IC50 of 148 μm (Fig. 2C).
FIGURE 2.
H2O2 effect on TRPM6 channel activity and plasma membrane expression. A, time course of the current density (pA/pF) at +80 mV of TRPM6-transfected HEK293 cells, where 1 mm H2O2 was added to the bath solution after 200 s (arrow). B, current-voltage (I/V) relation of TRPM6-transfected cells perfused with standard extracellular solution (solid line) and 1 mm H2O2 (dashed line). C, the percentage of inhibition of the TRPM6 current at +80 mV after 1000 s in response to different H2O2 concentrations, normalized to 1 mm H2O2. D, TRPM6-expressing HEK293 cells were treated with 1 mm H2O2 and subsequently subjected to cell surface biotinylation assays. TRPM6 expression was analyzed by immunoblotting for the plasma membrane (PM) fraction (top panel) and for the total cell lysates (bottom panel). A representative immunoblot of three independent experiments is shown. As negative controls, mock-transfected cells (Mock) were used. E, densitometry quantification of TRPM6 surface expression with/without H2O2 treatment. OD, optical density.
H2O2 Treatment Does Not Affect TRPM6 Cell Surface Expression
Next, the influence of H2O2 on the amount of TRPM6 channels at the plasma membrane was investigated by cell surface biotinylation experiments. As shown in Fig. 2D (upper panel), treatment with H2O2 did not affect the plasma membrane abundance of TRPM6. Of note, the protein levels of TRPM6 were equal in all tested conditions as verified by the total cell lysates (Fig. 2D, bottom panel).
MsrB1 Prevents the Inhibitory Effect of H2O2
Next, the effect of MsrB1 on TRPM6 channel activity was investigated. Patch clamp analysis demonstrated that MsrB1 has no effect on TRPM6 channel activity (TRPM6 + empty vector, 250 ± 42 pA/pF; TRPM6 + MsrB1, 224 ± 37 pA/pF, p > 0.2). To investigate the role of MsrB1 on TRPM6 channel activity during oxidative stress, TRPM6 and MsrB1 were co-expressed in HEK293 cells and treated with H2O2 during whole-cell patch clamp recordings. Treatment with H2O2 significantly decreased TRPM6 current (67 ± 5% of control, n = 13, p < 0.05), whereas co-expression of MsrB1 significantly attenuated this inhibition (81 ± 5% of control, n = 13, p < 0.05) (Fig. 3, A and B).
FIGURE 3.
MsrB1 reduces the inhibitory effect of H2O2 on TRPM6. A, time course of the current density (pA/pF) at +80 mV of TRPM6 and TRPM6 + MsrB1-transfected HEK293 cells, where 1 mm H2O2 was added to the bath solution after 200 s (arrow). B, normalized values of the current at +80 mV after 800 s of TRPM6 and TRPM6 + MsrB1, treated with (open bars) or without (closed bars) H2O2. * indicates p < 0.05.
Involvement of Met1755 in H2O2-inhibited TRPM6 Activity
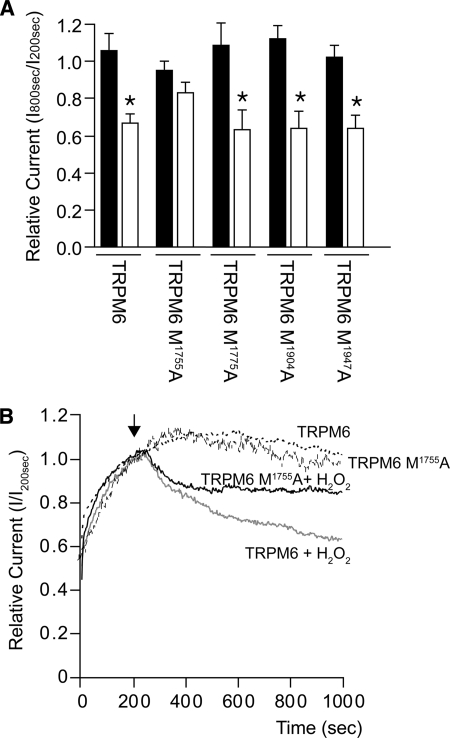
Because MsrB1 binds to the TRPM6 α-kinase domain and recovers the inhibitory effect of H2O2 on TRPM6 channel activity, we hypothesized that MsrB1 could reduce methionine oxidation of the α-kinase domain. Therefore, the methionine residues located on the periphery of the TRPM6 α-kinase domain three-dimensional structure,4 which might be more vulnerable to H2O2, were mutated into alanines (namely M1755A, M1775A, M1947A, and M1904A as analyzed by Yasara software). Subsequent patch clamp analysis showed that only the TRPM6 M1755A mutant was not significantly inhibited by H2O2 (83 ± 6% of control, n = 12, p > 0.05) (Fig. 4, A and B). Of note, the other mutants were significantly inhibited by H2O2 (M1775A, 63 ± 10%, n = 8; M1904A, 64 ± 9%, n = 8; M1947A, 64 ± 7%, n = 10) (Fig. 4A).
FIGURE 4.
TRPM6 M1755A is not significantly inhibited by H2O2. A, normalized values of the current at +80 mV after 800 s of TRPM6 and all TRPM6 Met/Ala mutants, treated with (open bars) or without (closed bars) 1 mm H2O2. * indicates p < 0.05. B, time course of the current density (pA/pF) at +80 mV of TRPM6 and TRPM6 M1755A-transfected HEK293 cells, where 1 mm H2O2 was added to the bath solution after 200 s (arrow).
DISCUSSION
In the present study, we identified MsrB1 as a new TRPM6-associated protein and showed that MsrB1 recovers TRPM6 channel activity via reducing the oxidation state of Met1755 during oxidative stress. First, MsrB1 directly binds to the TRPM6 α-kinase domain and co-precipitates full-length TRPM6. Second, MsrB1 is co-expressed with TRPM6 in the renal DCT. Third, H2O2 inhibits TRPM6 channel activity without affecting the plasma membrane expression. Fourth, MsrB1 prevents the inhibitory effect of H2O2 on TRPM6 channel activity. Finally, H2O2 has no significant effect on the TRPM6 M1755A mutant.
TRPM6 belongs to the TRPM subfamily of the TRP channels and is so far the only known channel directly mediating active transepithelial Mg2+ transport (4, 7, 8, 32). However, the molecular regulation of this channel remains elusive. Here, we used a novel yeast two-hybrid procedure, RRS, to screen proteins interacting with the TRPM6 α-kinase domain. Our data showed that MsrB1 binds to the α-kinase domain of TRPM6, resulting in the recruitment of Ras to the membrane and subsequent complementation of the temperature-sensitive cdc25-2 mutation, so we identified MsrB1 as a new interacting protein of the TRPM6 α-kinase domain. This interaction has been confirmed by a subsequent GST co-precipitation assay in HEK293 cells with full-length TRPM6. Importantly, MsrB1 and TRPM6 are co-expressed in the renal DCT, which further substantiates the physiological relevance of the interaction between both proteins.
MsrB1 is an oxidoreductase that catalyzes the thiol-dependent reduction of methionine sulfoxide (36). MsrB1 belongs to the Msr family composed of MsrA and MsrB. Mammals contain one MsrA and three MsrBs that are highly abundant in kidney, liver, heart, and nervous tissue (37–41). These enzymes protect cells from oxidative stress via the repair of oxidative damage to proteins and thereby restore biological activity. They can be involved in reactive oxygen species-mediated signal transduction through modulation of the function of target proteins (36, 42–44). Accumulating data showed that reversible methionine oxidation and reduction play a dynamic role in a variety of cellular signaling pathways (45, 46). For example, the methionine residues of Helix-3, Ca2+/calmodulin-dependent protein kinase II (CamKII), shaker voltage-dependent K+ channel, and Slo1 K+ channels can be oxidized and hereby regulate their function (46–49). It has been shown that oxidation of a methionine residue in the shaker voltage-dependent K+ channel disrupts its inactivation. This effect can be reversed by co-expression with MsrA1 (47).
In the present study, we showed that MsrB1 interacts with the TRPM6 α-kinase domain but does not affect the channel activity in normoxic conditions. However, H2O2 is a product during oxidative stress and has been studied in relation to potassium channel function and the TRPM2 channel (reviewed in Refs. 50 and 51). Here, we demonstrated that H2O2 significantly decreases the TRPM6-mediated current in HEK293 cells in a dose-dependent manner. As H2O2 did not change the surface expression of TRPM6, it possibly regulates TRPM6 channel activity directly through modulation of the channel conductance. Importantly, the decreased TRPM6 channel activity can be partly recovered by co-expression with MsrB1. This partial recovery could be explained by the fact that Msrs function optimally at 37 °C (52), whereas our experiments were performed at room temperature. Another possible explanation is the time lapse of MsrB1 functioning, which is likely slower than the oxidation by H2O2.
Free and protein-bound methionine residues are among the most susceptible to oxidation by reactive oxygen species (53). It is proposed that surface-exposed methionine residues in a protein constitute an antioxidant defense mechanism because various oxidants can easily react with these residues to form methionine sulfoxide. Reduction back to methionine by methionine sulfoxide reductases could catalytically drive this antioxidant system as has been suggested by several studies (54–56). Therefore, the methionine residues located on the periphery of the TRPM6 α-kinase domain three-dimensional structure4 were examined. Four methionine residues (Met1755, Met1775, Met1904, and Met1947) were mutated into alanines, and their channel activity was studied upon H2O2 treatment. Mutation of Met1755 to Ala significantly attenuates the effect of H2O2 on TRPM6 channel activity, mimicking the MsrB1 effect (co-expression of MsrB1, 81 ± 5% of control; M1755A, 83 ± 6% of control). These data suggest that Met1755 is a crucial target of H2O2. Notably, renal DCT cells contain the largest number of mitochondria, the major source of endogenous H2O2, per unit length of any cell along the nephron, underlying a vast source of intracellular H2O2 (57). TRPM6 is predominantly expressed in DCT and is inhibited by H2O2. Therefore, it is conceivable that the metabolic state of DCT cells, which influences the abundance of reactive oxygen species generated from mitochondria, modulates the Mg2+ reabsorption via TRPM6.
Taken together, our study demonstrated that H2O2 inhibits TRPM6 channel activity and pointed out Met1775 as an important target for channel oxidation. Moreover, its interacting protein MsrB1 dynamically regulates this oxidative effect on TRPM6. These data provide insight into the molecular basis of TRPM6 channel regulation and transepithelial Mg2+ (re)absorption. Our findings present the first example of modulating TRP channel activity by methionine oxidation and contribute to a further understanding of the regulation of TRP channels by novel post-translational modifications.
Acknowledgments
We greatly thank Dr. A. Aronheim for the RRS system and Dr. J. Moskovitz for the rabbit anti-MsrB1 antibody. We thank Dr. S. Verkaart for valuable discussion.
This work was supported by the Netherlands Organization for Scientific Research (ZonMw Grants 9120.6110, 9120.8026), a European Young Investigator award from the European Science Foundation, and the Dutch Kidney Foundation (Grant C03.6017).
H. Venselaar, personal communication.
- DCT
- distal convoluted tubule
- TRP
- transient receptor potential
- TRPM
- transient receptor potential melastatin
- RRS
- Ras recruitment system
- Msr
- methionine sulfoxide reductase
- pF
- picofarads.
REFERENCES
- 1.Vetter T., Lohse M. J. (2002) Curr. Opin. Nephrol. Hypertens. 11, 403–410 [DOI] [PubMed] [Google Scholar]
- 2.Grubbs R. D., Maguire M. E. (1987) Magnesium 6, 113–127 [PubMed] [Google Scholar]
- 3.Romani A., Scarpa A. (1992) Arch. Biochem. Biophys. 298, 1–12 [DOI] [PubMed] [Google Scholar]
- 4.Konrad M., Schlingmann K. P., Gudermann T. (2004) Am. J. Physiol. Renal Physiol. 286, F599–F605 [DOI] [PubMed] [Google Scholar]
- 5.Grimellec C. L., Poujeol P., Rouffignia C. (1975) Pflugers. Arch. 354, 117–131 [DOI] [PubMed] [Google Scholar]
- 6.Dai L. J., Ritchie G., Kerstan D., Kang H. S., Cole D. E., Quamme G. A. (2001) Physiol. Rev. 81, 51–84 [DOI] [PubMed] [Google Scholar]
- 7.Walder R. Y., Landau D., Meyer P., Shalev H., Tsolia M., Borochowitz Z., Boettger M. B., Beck G. E., Englehardt R. K., Carmi R., Sheffield V. C. (2002) Nat. Genet. 31, 171–174 [DOI] [PubMed] [Google Scholar]
- 8.Schlingmann K. P., Weber S., Peters M., Niemann Nejsum L., Vitzthum H., Klingel K., Kratz M., Haddad E., Ristoff E., Dinour D., Syrrou M., Nielsen S., Sassen M., Waldegger S., Seyberth H. W., Konrad M. (2002) Nat. Genet. 31, 166–170 [DOI] [PubMed] [Google Scholar]
- 9.Walder R. Y., Yang B., Stokes J. B., Kirby P. A., Cao X., Shi P., Searby C. C., Husted R. F., Sheffield V. C. (2009) Hum. Mol. Genet. 18, 4367–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Runnels L. W., Yue L., Clapham D. E. (2001) Science 291, 1043–1047 [DOI] [PubMed] [Google Scholar]
- 11.Ryazanova L. V., Dorovkov M. V., Ansari A., Ryazanov A. G. (2004) J. Biol. Chem. 279, 3708–3716 [DOI] [PubMed] [Google Scholar]
- 12.Cao G., Thébault S., van der Wijst J., van der Kemp A., Lasonder E., Bindels R. J., Hoenderop J. G. (2008) Curr. Biol. 18, 168–176 [DOI] [PubMed] [Google Scholar]
- 13.Cao G., van der Wijst J., van der Kemp A., van Zeeland F., Bindels R. J., Hoenderop J. G. (2009) J. Biol. Chem. 284, 14788–14795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thébault S., Cao G., Venselaar H., Xi Q., Bindels R. J., Hoenderop J. G. (2008) J. Biol. Chem. 283, 19999–20007 [DOI] [PubMed] [Google Scholar]
- 15.Schmitz C., Dorovkov M. V., Zhao X., Davenport B. J., Ryazanov A. G., Perraud A. L. (2005) J. Biol. Chem. 280, 37763–37771 [DOI] [PubMed] [Google Scholar]
- 16.Clark K., Middelbeek J., Dorovkov M. V., Figdor C. G., Ryazanov A. G., Lasonder E., van Leeuwen F. N. (2008) FEBS Lett. 582, 2993–2997 [DOI] [PubMed] [Google Scholar]
- 17.Clark K., Middelbeek J., Morrice N. A., Figdor C. G., Lasonder E., van Leeuwen F. N. (2008) PLoS ONE 3, e1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita M., Kozak J. A., Shimizu Y., McLachlin D. T., Yamaguchi H., Wei F. Y., Tomizawa K., Matsui H., Chait B. T., Cahalan M. D., Nairn A. C. (2005) J. Biol. Chem. 280, 20793–20803 [DOI] [PubMed] [Google Scholar]
- 19.Rempe D. A., Takano T., Nedergaard M. (2009) Nat. Neurosci. 12, 1215–1216 [DOI] [PubMed] [Google Scholar]
- 20.McNulty S., Fonfria E. (2005) Pflugers. Arch. 451, 235–242 [DOI] [PubMed] [Google Scholar]
- 21.Sun H. S., Jackson M. F., Martin L. J., Jansen K., Teves L., Cui H., Kiyonaka S., Mori Y., Jones M., Forder J. P., Golde T. E., Orser B. A., Macdonald J. F., Tymianski M. (2009) Nat. Neurosci. 12, 1300–1307 [DOI] [PubMed] [Google Scholar]
- 22.Fonfria E., Marshall I. C., Benham C. D., Boyfield I., Brown J. D., Hill K., Hughes J. P., Skaper S. D., McNulty S. (2004) Br. J. Pharmacol. 143, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., Shimizu N., Kurose H., Okada Y., Imoto K., Mori Y. (2002) Mol. Cell 9, 163–173 [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Chu X., Tong Q., Cheung J. Y., Conrad K., Masker K., Miller B. A. (2003) J. Biol. Chem. 278, 16222–16229 [DOI] [PubMed] [Google Scholar]
- 25.Naziroğlu M. (2007) Neurochem. Res. 32, 1990–2001 [DOI] [PubMed] [Google Scholar]
- 26.Thannickal V. J., Fanburg B. L. (2000) Am. J. Physiol. Lung. Cell Mol. Physiol. 279, L1005–L1028 [DOI] [PubMed] [Google Scholar]
- 27.Ushio-Fukai M. (2009) Curr. Opin. Nephrol. Hypertens. 18, 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohchi C., Inagawa H., Nishizawa T., Soma G. (2009) Anticancer Res. 29, 817–821 [PubMed] [Google Scholar]
- 29.Eckers A., Klotz L. O. (2009) Redox. Rep. 14, 141–146 [DOI] [PubMed] [Google Scholar]
- 30.Aronheim A. (2001) Methods 24, 29–34 [DOI] [PubMed] [Google Scholar]
- 31.Topala C. N., Groenestege W. T., Thébault S., van den Berg D., Nilius B., Hoenderop J. G., Bindels R. J. (2007) Cell Calcium 41, 513–523 [DOI] [PubMed] [Google Scholar]
- 32.Voets T., Nilius B., Hoefs S., van der Kemp A. W., Droogmans G., Bindels R. J., Hoenderop J. G. (2004) J. Biol. Chem. 279, 19–25 [DOI] [PubMed] [Google Scholar]
- 33.van de Graaf S. F., Hoenderop J. G., Gkika D., Lamers D., Prenen J., Rescher U., Gerke V., Staub O., Nilius B., Bindels R. J. (2003) EMBO J. 22, 1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gkika D., Topala C. N., Chang Q., Picard N., Thébault S., Houillier P., Hoenderop J. G., Bindels R. J. (2006) EMBO J. 25, 4707–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoenderop J. G., Dardenne O., Van Abel M., Van Der Kemp A. W., Van Os C. H., St.-Arnaud R., Bindels R. J. (2002) FASEB J. 16, 1398–1406 [DOI] [PubMed] [Google Scholar]
- 36.Grimaud R., Ezraty B., Mitchell J. K., Lafitte D., Briand C., Derrick P. J., Barras F. (2001) J. Biol. Chem. 276, 48915–48920 [DOI] [PubMed] [Google Scholar]
- 37.Moskovitz J., Weissbach H., Brot N. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 2095–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuschel L., Hansel A., Schönherr R., Weissbach H., Brot N., Hoshi T., Heinemann S. H. (1999) FEBS Lett. 456, 17–21 [DOI] [PubMed] [Google Scholar]
- 39.Fomenko D. E., Novoselov S. V., Natarajan S. K., Lee B. C., Koc A., Carlson B. A., Lee T. H., Kim H. Y., Hatfield D. L., Gladyshev V. N. (2009) J. Biol. Chem. 284, 5986–5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung S., Hansel A., Kasperczyk H., Hoshi T., Heinemann S. H. (2002) FEBS Lett. 527, 91–94 [DOI] [PubMed] [Google Scholar]
- 41.Hansel A., Jung S., Hoshi T., Heinemann S. H. (2003) Redox. Rep. 8, 384–388 [DOI] [PubMed] [Google Scholar]
- 42.Stadtman E. R., Moskovitz J., Berlett B. S., Levine R. L. (2002) Mol. Cell. Biochem. 234–235, 3–9 [PubMed] [Google Scholar]
- 43.Moskovitz J. (2005) Curr. Pharm. Des. 11, 1451–1457 [DOI] [PubMed] [Google Scholar]
- 44.Cabreiro F., Picot C. R., Friguet B., Petropoulos I. (2006) Ann. N.Y. Acad. Sci. 1067, 37–44 [DOI] [PubMed] [Google Scholar]
- 45.Bigelow D. J., Squier T. C. (2005) Biochim. Biophys. Acta. 1703, 121–134 [DOI] [PubMed] [Google Scholar]
- 46.Colombo G., Meli M., Morra G., Gabizon R., Gasset M. (2009) PLoS. ONE 4, e4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciorba M. A., Heinemann S. H., Weissbach H., Brot N., Hoshi T. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9932–9937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santarelli L. C., Wassef R., Heinemann S. H., Hoshi T. (2006) J Physiol. 571, 329–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson J. R., Joiner M. L., Guan X., Kutschke W., Yang J., Oddis C. V., Bartlett R. K., Lowe J. S., O'Donnell S. E., Aykin-Burns N., Zimmerman M. C., Zimmerman K., Ham A. J., Weiss R. M., Spitz D. R., Shea M. A., Colbran R. J., Mohler P. J., Anderson M. E. (2008) Cell 133, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisfeld J., Lückhoff A. (2007) Handb. Exp. Pharmacol. 179, 237–252 [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Gutterman D. D. (2002) Clin. Exp. Pharmacol. Physiol. 29, 305–311 [DOI] [PubMed] [Google Scholar]
- 52.Moskovitz J., Berlett B. S., Poston J. M., Stadtman E. R. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9585–9589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stadtman E. R., Levine R. L. (2003) Amino Acids 25, 207–218 [DOI] [PubMed] [Google Scholar]
- 54.Levine R. L., Mosoni L., Berlett B. S., Stadtman E. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 15036–15040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao Y., Yin D., Jas G. S., Kuczer K., Williams T. D., Schöneich C., Squier T. C. (1996) Biochemistry 35, 2767–2787 [DOI] [PubMed] [Google Scholar]
- 56.Nabuchi Y., Fujiwara E., Ueno K., Kuboniwa H., Asoh Y., Ushio H. (1995) Pharm. Res. 12, 2049–2052 [DOI] [PubMed] [Google Scholar]
- 57.Madsen K. M., Verlander J. W., Tisher C. C. (1988) J. Electron. Microsc. Tech. 9, 187–208 [DOI] [PubMed] [Google Scholar]