Abstract
Inhibition of histone deacetylase (HDAC) function is a validated therapeutic strategy for cancer treatment. Of the several structurally distinct small molecule histone deacetylase inhibitors (HDACi) reported, macrocyclic depsipeptides possess the most complex cap-groups and have demonstrated excellent HDAC inhibition potency and isoform selectivity. Unfortunately, the development of macrocyclic depsipeptides has been hampered in part due to development problems characteristic of large peptides and the complex reaction schemes required for their synthesis. Herein we report that tricyclic ketolide TE-802 is an excellent mimetic for the peptide backbone of macrocyclic HDACi. Compounds derived from this template are particularly selective against HDAC 1 and 2 with nanomolar inhibitory activity. Interrogation of the association between a subset of these compounds and key HDAC isoforms, using AutoDock, enables a molecular description of the interaction between the HDAC enzyme's outer rim and the inhibitors’ macrocyclic cap group that are responsible for compound affinity and presumably isoform selectivity.
Introduction
Histone deacetylase (HDAC) and histone acetyltransferase (HAT) are two functionally opposing enzymes, many of which tightly regulate the chromatin structure and function via sustenance of equilibrium between the acetylated and deacetylated states of histones. By catalyzing the removal of acetyl groups, HDACs induce a condensed chromatin structure resulting in transcription repression, whereas acetylated histones are associated with a more accessible/open chromatin structure and activation of transcription.1-4 In addition, many non-histone proteins such as tubulin, ERα, p53, HSP 90, NF-YA, and GATA-1 have been found in an acetylated state and may be substrates of HDACs.5-10 Eighteen human HDAC isoforms are known and they are subdivided into the classical zinc dependent HDACs comprising of class I, II, and IV; and NAD+ dependent sirtuins, class III enzymes.9, 11, 12
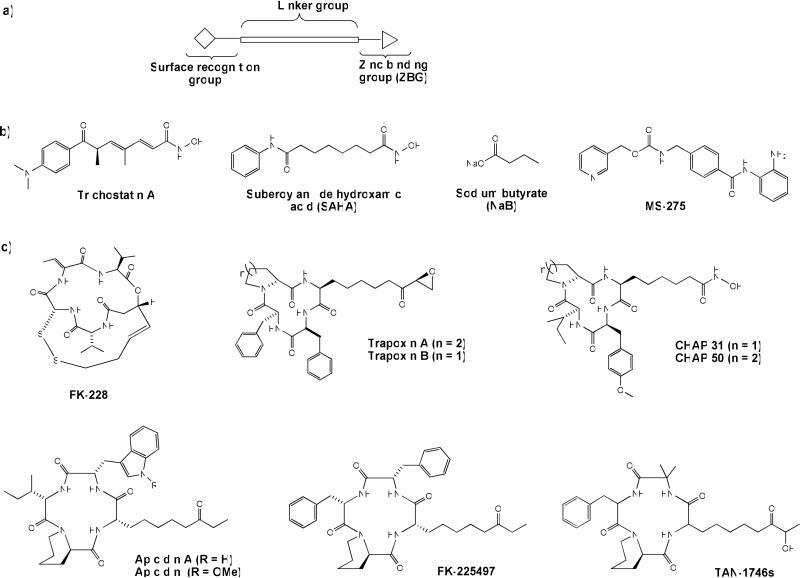
HDAC inhibitors (HDACi) are an emerging class of novel anti-cancer drugs with a demonstrated ability to arrest proliferation of nearly all transformed cell types, including epithelial (melanoma, lung, breast, pancreas, ovary, prostate, colon and bladder) and hematological (lymphoma, leukemia and multiple myeloma) tumors.13 To date, several classes of small molecule HDACi – fitting a three-motif pharmacophoric model, namely, a zinc-binding group (ZBG), a hydrophobic linker, and a recognition cap group14 (Figure 1a) - have been reported. Examples include hydroxamic acids such as trichostatin A (TSA), suberoylanilide hydroxamic acid (SAHA) (approved in 2006 by the FDA for the treatment of cutaneous T-cell lymphoma (CTCL)15, 16), benzamides, short chain fatty acids, electophilic ketones, and cyclic peptides such as FK-228 (romidepsin) which was recently approved by the FDA17, 18 for the treatment of CTCL in patients who have received at least one prior systemic therapy (Figure 1b, c).19, 20 However, most of these drugs non-specifically inhibit various HDAC isoforms. At the fore of HDAC drug development is the identification of isoform-selective HDACi with the potential for enhanced potency and reduced side effects, compared to the current pan-HDACi. However, these efforts have been so far modestly successful, resulting in only few HDACi that demonstrate partial selectivity.21, 22
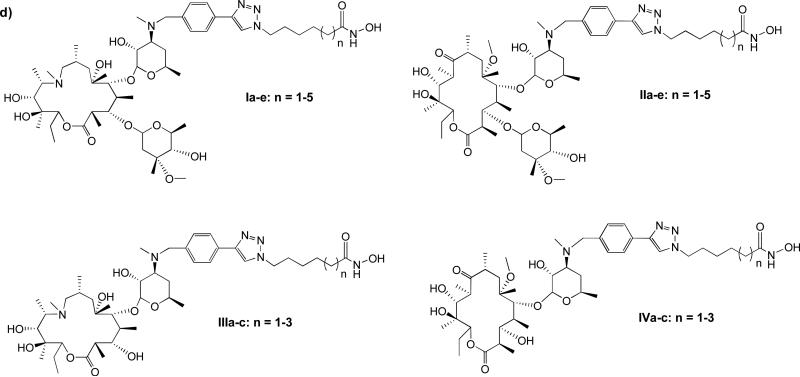
Figure 1.
a) Pharmacophoric model of HDACi; representative examples of b) acyclic, c) cyclic peptide, and d) macrolide – based HDAC inhibitors.
On the other hand, macrocyclic peptide HDACi have the most complex recognition cap group moieties and present an excellent opportunity for the selective modulation of the biological activities of HDACi. Although they possess potent HDAC inhibition activity (nanomolar range), their progress through clinical trials has been slow.17, 18, 23 The paucity of clinically effective cyclic-peptide HDACi may be in part due to development problems characteristic of large peptides, most especially poor oral bioavailability. Identification of non-peptide macrocyclic HDACi will offer a new class of macrocyclic HDACi with potentially more favorable drug-like properties. Furthermore, this will enhance our understanding of the roles of specific interactions between the enzyme outer rim and inhibitor cap groups in HDACi activity and ultimately aid in the identification of more isoform-selective HDACi.
Recently we reported that non-peptide macrocyclic skeletons derived from 14-and 15-membered macrolides are suitable as surrogates for the cap-groups of macrocyclic HDACi (Figure 1d). The resulting HDACi have improved enzyme inhibition potency and isoform-selectivity.24 Herein, we report that enhancement of the 14-membered macrolide ring hydrophobicity and rigidity facilitates specific drug interactions with the enzyme's outer rim residues, maximizes HDAC inhibition, and improves drug cytotoxicity against human cancer cell lines. Moreover, these compounds have anti-parasitic activities against causative parasites of malaria and leishmaniasis in a manner that reveals structural attributes which confer a specific anti-parasitic response.
Results and Discussion
Molecular docking analysis
Previous molecular docking studies on HDACi derived from 14- and 15-membered macrolides clarithromycin and azithromycin, respectively, using histone deacetylase-like protein (HDLP), revealed the structural basis for the enhanced activity of these macrocyclic compounds. Either ring system adopted docked orientations that displayed molecular surface complementarities between the macrolide skeleton and the enzyme outer rim.24 In these docked structures, most of the hydrophobic components of the macrolide ring optimally interact with the hydrophobic residues within HDLP hydrophobic pockets. Common to both ring systems are the vicinal-diols at C11 and C12, and C12 and C13 for clarithromycin and azithromycin, respectively, which have to be accommodated within the enzyme hydrophobic pocket. We postulated that refinement of the ring hydrophobicity through appropriate modification of the vicinal-diols could further modulate the HDAC inhibition of these non-peptide macrocyclic HDACi. In the previous report, we observed that the two macrolide skeletons contributed distinctly to the overall HDAC binding. For 14- and 15-membered macrolides with C5 and C6 linkers, the 14-membered compounds are about 2-5 fold more potent HDACi than their 15-membered counterparts.24 Based on the foregoing, we focused on the transformation of the 14-membered macrocyclic template.
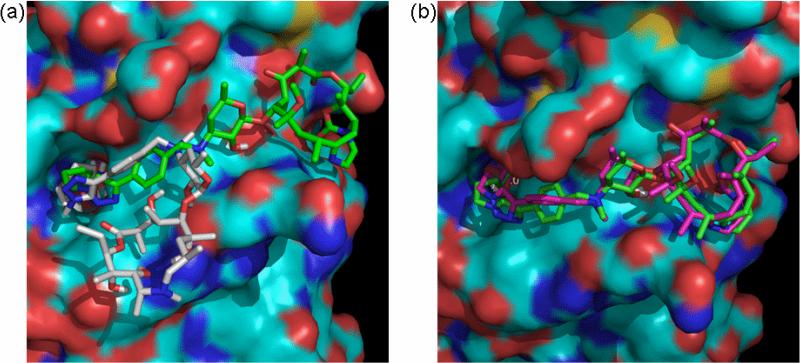
Conversion of the 14-membered ring vicinal-diols to various 5-membered carbamates is a common and synthetically tractable approach toward their transformation to relatively more hydrophobic ketolide skeletons (Figure 2).25-27 The template of ketolide 10 (TE-802),25 in which the carbamate NH group has been further substituted with a small hydrophobic group that results in a tricyclic ring system, is very attractive in this regard. The rigid template of 10 minimally perturbs the ring within the region of interest while maximizing ring hydrophobicity and should be compatible with the enzyme outer rim hydrophobic pocket. To verify this prospect, we proceeded to interrogate the binding interaction between HDAC1 homology model built from human HDAC2 X-ray structure 3MAX coordinates28 and the non-peptide macrocyclic hydroxamates 15a-b, derived from the ketolide 10, using AutoDock as described previously. We focused on the C5 and C6 linked compounds 15a-b because these spacer lengths are optimal for the anti-HDAC activity of the previous non-peptide macrocyclic HDACi.24 Molecular docking analysis revealed that the enhanced hydrophobicity and/or rigidity of the ketolide moiety of the C5 and C6 linker compounds 15a and 15b, respectively, forced their rings to adopt docked orientations that are distinct from the corresponding macrolide template whose ZBG makes similar interaction with the active site Zn2+ ion (Figure 3a). The desosamine sugar 2’-OH group of both 15a and 15b is within 1.9 Å of the backbone carbonyl group of Tyr24 with which it could potentially engage in a stabilizing H-bonding interaction (see supporting information Figure S1a). Relative to that of 15a, the ketolide ring of 15b fits optimally into a hydrophobic pocket on HDAC1 outer rim. The aryl moiety of 15b is partly stacked atop of His28 that forms the base of a hydrophobic cleft at the entrance to a pocket-like active site (Figure 3b and supporting information Figure S1b). The orientation of the aryl moiety enables the C6-linker methylene group to efficiently present the hydroxamate ZBG to the active site Zn2+ ion. To accommodate for the loss of one methylene group and consequently optimally interact with the active site zinc, the ketolide ring of 15a is slightly lifted out of the pocket while its aryl moiety is twisted, almost perpendicular to the aryl moiety of 15b, to make a minimal edge contact with His28 at the pocket entrance cleft. In this orientation, 15a is more solvent exposed relative to 15b (Figure 3b). The observed disparities in the docked orientations of 15a and 15b may have implications on their affinity for the enzyme.
Figure 2.
Representative examples of ketolides
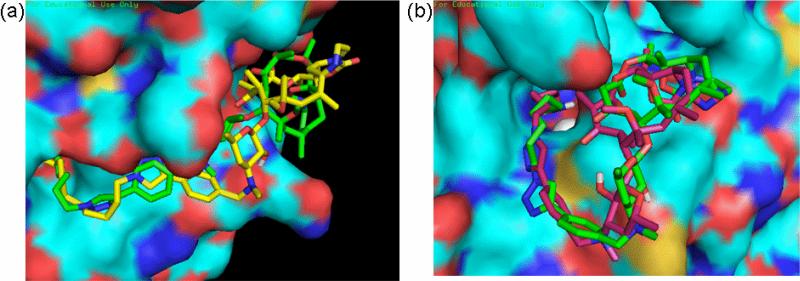
Figure 3.
Docked structures of ketolide-derived HDACi at the active site of HDAC1. (a) Superposition of the low energy conformation of IIIb (grey) and 15b (green) revealed the pocket binding preferences of inhibitors at the HDAC1 surface. (b) Relative orientation of the macrocyclic rings of 15a (pink) and 15b (green) within the hydrophobic pocket on HDAC1 outer rim.
Chemistry
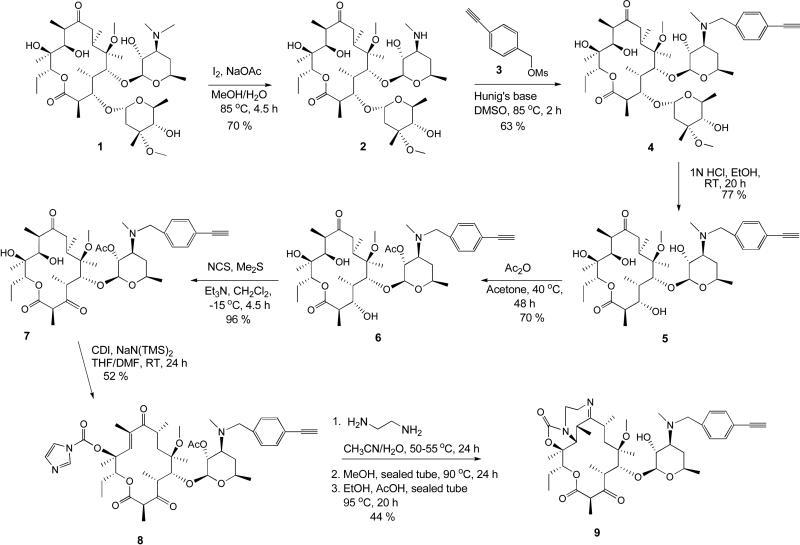
To experimentally test these in silico observations, we synthesized compounds 15a-e. Our proposed synthesis of the requisite compounds involves the intermediacy of 4-ethynylbenzylketolide 9. We envisioned that 9 could be accessed via two different routes – A and B – whereby the 4-ethynylbenzyl group is introduced early or late in the synthesis (Schemes 1 and 2 respectively). It is gratifying to note that both routes worked equally well in furnishing intermediate 9.
Scheme 1.
Synthesis of tricyclic ketolide 9 from clarithromycin 1
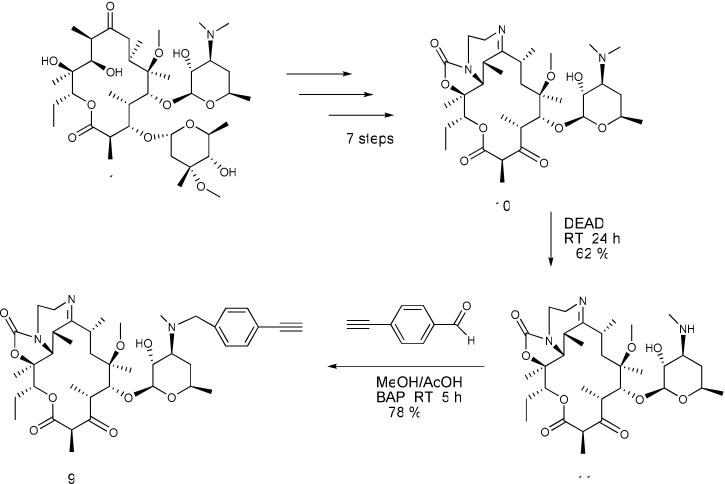
Scheme 2.
Synthesis of tricyclic ketolide 9 from ketolide 10
Demethylation of clarithromycin 1 under standard conditions gave 3’-desmethylclarithromycin 2 in 70 % yield.29 Subsequent alkylation of 2 with 4-ethynylbenzyl methanesulfonate afforded the modified 4-ethynylbenzylclarithromycin 4 which was treated with 1N HCl to selectively cleave the cladinose sugar and afford compound 5. It was observed for this reaction step that time was of essence as longer reaction times led to extensive byproducts formation. Selective acetylation of the 2’-OH group was accomplished by treating an acetone solution of 5 with acetic anhydride at 40 °C for 48 h to give compound 6 in 70 % yield. Subsequent oxidation of the 3-hydroxyl group of compound 6 to a 3-keto functional group, under anhydrous conditions with NCS, afforded the ketolide 7 in a near quantitative yield. Treatment of 7 with excess carbonyldiimidazole (CDI) and NaHMDS in a mixture of THF/DMF afforded the 12-carbamoylimidazolide ketolide 8 in 52 % yield. Transformation of 8 into the desired tricyclic ketolide 9 was achieved in two successive cyclization steps adapting literature procedures.25 Reaction of imidazolide 8 with ethylenediamine followed by intramolecular Michael addition led to the formation of 11,12-cyclic carbamate. Subsequent intramolecular dehydration completed the cyclization process, affording the desired product 9 in 44 % yield (Scheme 1).
Alternatively, intermediate 9 could be directly obtained from 10. We synthesized 10 from clarithromycin 1 according to a literature procedure.25, 26, 30, 31 Subsequent N-demethylation of 10 using diethyl azodicarboxylate (DEAD) gave the expected N-desmethyl ketolide 11 in 62 % yield.31 Reductive amination of 11 with 4-ethynylbenzaldehyde using borane-pyridine complex (BAP) afforded the requisite intermediate 9 in 78 % yield (Scheme 2).
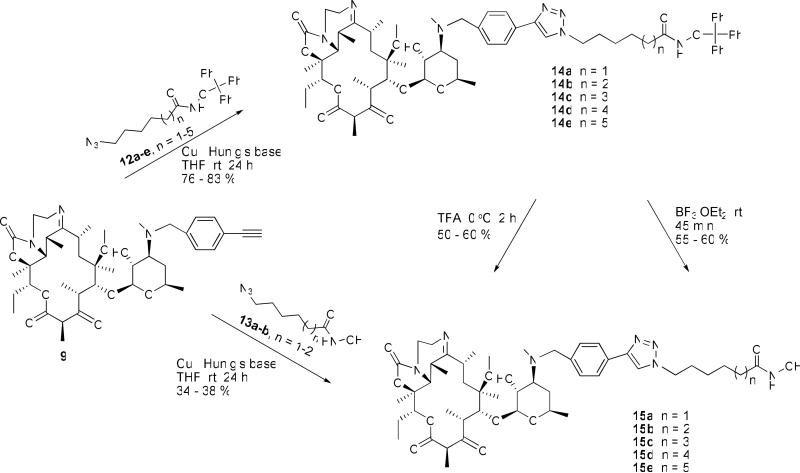
The final transformation of 9 to 15a-e was uneventful (Scheme 3). Copper (I) catalyzed cycloaddition reaction24 of 9 with O-trityl protected azidohydoxamate analogs 12a-e afforded the 1,2,3-triazole linked derivatives 14a-e. Deprotection of the trityl group of 14a-e with TFA gave the desired products 15a-e in good yields. A similar outcome was obtained when trityl group deprotection was effected with BF3.OEt2. Alternatively, the desired hydroxamates could be obtained through direct copper (I) catalyzed cycloaddition between unprotected azidohydroxamates 13 and alkyne 9, according to our published protocol.24
Scheme 3.
Synthesis of triazole-linked tricyclic ketolide hydroxamates 15
HDACi activity and selectivity of 15a-e
We first tested compounds 15a-e for their HDAC inhibitory activity (Table 1) against HeLa cell nuclear extract (which contains primarily HDACs 1 and 2), HDAC 6, and HDAC 8 using the Fluor de Lys assay kit (Enzo Life Sciences, Inc.).32-34 Results show a linker-length dependent anti-HDAC activity which peaked with compound 15b, an analog having 6 methylene linkers separating the triazole ring from the zinc binding hydroxamic acid group (Table 1). Compound 15b potently inhibits the deacetylase activity of HeLa nuclear extract with a single digit nanomolar IC50. Although compound 15a (analog having 5 methylene linkers) also has nanomolar inhibition activity against the nuclear extract, it is nevertheless over 7-fold worse than 15b. The HDAC inhibition profiles of 15a and 15b paralleled those we previously reported for the macrolide-derived HDACi of the same linker lengths.24 Additionally, this result is in close agreement with the in silico prediction using HDAC1 which revealed that 15b adopted a docked orientation with a better fit with the enzyme outer rim hydrophobic pocket (Figure 3b). The affinity of 15a for the enzyme could be compromised by the solvent exposure of its macrocyclic ring and the shortness of its linker region relative to that of 15b. These shortcomings in the docked structure of 15a may explain the 7-fold enhanced anti-HDAC activity of 15b relative to 15a.
Table 1.
HDAC inhibition activity (IC50) and isoform selectivity of tricyclic ketolide-based HDAC inhibitors
| Compd | n | Nuclear Extract (nM) | HDAC 8 (nM) | HDAC8:Nuclear Extract Isoform Selectivity | HDAC 6 (nM) | HDAC6:Nuclear Extract Isoform Selectivity |
|---|---|---|---|---|---|---|
| 15a | 1 | 7.77 | 796.2 | 102.5 | 1180.1 | 151.9 |
| 15b | 2 | 1.03 | 544.6 | 528.7 | 728.7 | 707.5 |
| 15c | 3 | 104.2 | 1909.3 | 18.3 | 1709.8 | 16.4 |
| 15d | 4 | 163.6 | 2859.9 | 17.5 | 1916.9 | 11.7 |
| 15e | 5 | 208.2 | 4557.8 | 21.9 | 3203.1 | 15.4 |
| SAHAa | 65.0 | 1860.0 | 29 | 85.5 | 1.3 |
Cited from ref. 22
Compounds 15c-e, analogs having longer methylene linkers than those of 15b, show a progressive reduction in anti-HDAC activity with the increase in linker length (Table 1). We then performed further molecular docking analyses on HDAC1 with the goal of shedding more light on the chain length dependence reduction in the anti-HDAC activities of these longer chain compounds. We observed that these longer linker compounds adopted various docked orientations which progressively extruded the ketolide ring from the outer rim pocket into solvent exposed regions on the enzyme surface. Compound 15e, analog with the longest methylene linker in the series herein disclosed, made the least interaction with the enzyme outer rim (Figure 4a). The observed extrusion of the macrocyclic ring from the enzyme surface as the methylene linker length increases could compromise the compounds’ affinity for the enzyme. These in silico observations provide a plausible structural basis for the observed chain length dependence attenuation of the anti-HDAC activities of these ketolide-derived HDACi.
Figure 4.
Comparison of the orientations of (a) the C6-linker compound 15b (green) and C9-linker compound 15e (yellow) at the outer rim of HDAC1; (b) the C5-linker compound 15a (pink) and C6-linker compound 15b (green) at the outer rim of HDAC8.
To obtain evidence for the HDAC isoform selectivity, we tested all triketolide HDACi herein described against HDAC 6 and HDAC 8 using the Fluor de Lys assay. Compared to SAHA, compounds 15a and 15b are more selective for the nuclear extract (HDACs 1/2) with selectivity indices comparable to those of their 14-membered macrolide congeners.24 Compounds 15c-e are less selective for either HDAC isoform compared to 15a-b. However, 15c-e have improved HDAC 6 and comparable HDAC 8 isoform selectivity relative to SAHA (Table 1). The linker length dependent dissipation of HDAC isoform selectivity could also be due to the relief of the attractive interactions between the ketolide macrocycle and the enzyme hydrophobic cleft, a concomitant effect of the increase in compounds’ spacer length. HDAC8 could be particularly sensitive to changes in linker length because it has a much shallower active site pocket relative to HDAC1.35 To gain insight into the structural basis of the observed isoform selectivity, we performed molecular docking analysis on the HDAC8 structure reported by Somoza et al.35 In contrast to their docked structures on HDAC1, the orientations of 15a and 15b that enable interaction of the ZBG with the active site Zn2+ ion are those which adopted a closed conformation (Figure 4b). In this conformation, the macrolactonic ring of either compound is nestled atop of the entrance to the enzyme active site while the desosamine sugar, the triazolylaryl moiety and a portion of the metylene linker are lifted off the enzyme surface and wrapped back under the macrolactonic ring to enable the ZBG interact with the Zn2+ ion at the base of a shallow active site pocket. This closed conformation is inaccessible to 15e, analog with the longest methylene linkers in the series herein disclosed. Instead, 15e adopted a conformation in which its macrocyclic moiety is flanged into a distinct solvent exposed patch on the enzyme surface to give its long linker moiety enough room to present the ZBG to the enzyme active site (see supporting information Figure S1c). Analysis of X-ray data have revealed significant inhibitor specific changes in the enzyme active site topology of HDAC8.35 It is therefore possible that the observed HDAC1 isoform selectivity may be partly due to the inability of these ketolide based HDACi to efficiently induce active site conformational changes that facilitate HDAC8 specific inhibitor association with the enzyme active site.36
Recent studies have shown that the use of fluorescently-labeled substrates can perturb enzyme activity and the effect of inhibitors and activators on the deacetylases.37, 38 In one example, the discovery that resveratrol and various analogues activate the deacetylase sirtuin 1 (SIRT1) was found to be an artifact of the fluorescent assay.39 Indeed, many recommended Fluor de Lys substrates have been shown not to be active towards their corresponding enzymes, including HDAC8, absent the fluorophore.40, 41 In light of these data, we sought to validate that compounds 15a-e do inhibit HDAC8 using the label-free SAMDI mass spectrometry assay.
The SAMDI technique, wherein self-assembled monolayers (SAM) of alkanethiolates on gold are analyzed by matrix assisted laser desorption/ionization (MALDI) mass spectrometry, has been used to characterize many biochemical activities, including the deacetylase enzyme family.41-43 The SAMDI assay is also amenable to analyze homogenous reactions, as the substrates are immobilized to the SAMs after the reaction has been terminated.44 We employed the SAMDI technique to measure the inhibition constant (Ki) of compounds 15a-e against HDAC8 using a previously identified preferred HDAC8 substrate, Ac-GRKAcFGC-NH2.41
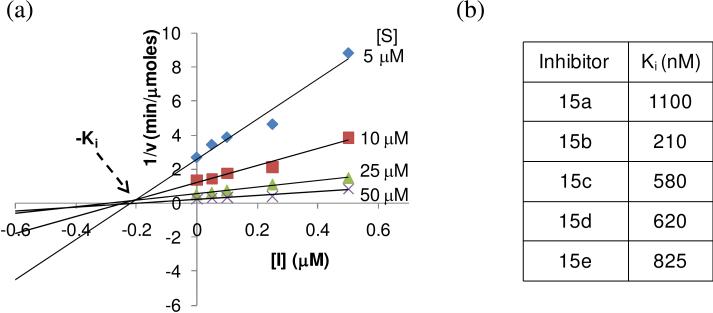
In a first example, the reactions were setup in a 96-well plate with 1 M HDAC8, varying concentrations of substrate (5 μM, 10 μM, 25 μM, and 50 μM), and inhibitor 15b (0 nM, 50 nM, 100 nM, 250 nM, and 500 nM). The reactions were stopped at different time points and applied to arrays of gold circles presenting maleimide-terminated SAMs to immobilize the substrate via Michael addition of the cysteine thiol with maleimide. SAMDI spectra were then acquired for each reaction and a shift of m/z 42 is indicative of a deacetylation reaction (Supplementary Figure S1). The amount of product formed was plotted over time for each reaction condition to get the initial rates of reaction. A Dixon plot (1/v vs. [I]) shows lines corresponding to different substrate concentrations which intersect around 200 nM, which is the Ki for compound 15b (Figure 5a). We then measured the Ki for the remaining compounds and found that 15b has the highest affinity for HDAC8. Consistent with the in silico data, we observed a weak affinity for compound 15a for HDAC8. The Ki's for 15c-e are rather comparable (Figure 5b), yet do mimic the trend observed with the fluorescent assay that as the length of the hydroxamic acid linker increases, the inhibitor affinity decreases. Finally, we note that while we observe a similar inhibition pattern with 15a-e using the two assays, there is a disparity in the absolute magnitude of the inhibition constants. This is anticipated due to the nature of each assay—including the use of either a labeled and unlabeled substrate—and the parameters that were measured to compare inhibitor potency.
Figure 5.
Determination of Ki for compounds 15a-e using SAMDI mass spectrometry. (a) Dixon plot of compound 15b. (b) Ki values for compounds 15a-e as determined by SAMDI.
Encouraged by the in vitro HDAC inhibition results, we studied the effect of 15a-e on the viability of three representative cancer cell lines – prostate (DU-145), lung (A549), and breast cancer (MCF-7) and a non-transformed cell - human lung fibroblast cell (Hs1.Lu). We included non-transformed cell line in our investigation in order to obtain evidence for compound selective toxicity. Drug concentrations necessary for 50% inhibition of cell viability (EC50) were quantitatively measured using MTS colorimetric assay as we previously described.24, 45 Table 2 shows the EC50 values of each compound. Compounds 15a-e inhibit the proliferation of all transformed cells studied with anti-proliferative activity that closely matched their in vitro anti-HDAC activity. Specifically, compound 15b has the most potent anti-proliferative activity with high nanomolar efficacy against all three cancer cell lines. Moreover, none of the compounds show any discernable toxicity against normal Hs1.Lu cells at drug concentrations in excess of 10 μM (Table 2). These data showed that the triketolide hydroxamates reported here are selectively toxic to the transformed cells, a trait that tracks with those of many HDACi.
Table 2.
Anti-proliferative activity (μM) of tricyclic ketolide-based HDAC inhibitors
| Compd | n | DU-145 (μM) | A549 (μM) | MCF-7 (μM) | Hs1.Lu Normal Lung Fibroblast (μM) |
|---|---|---|---|---|---|
| 15a | 1 | 1.64 | 1.17 | 1.26 | > 10 |
| 15b | 2 | 0.82 | 0.66 | 0.75 | > 10 |
| 15c | 3 | 3.70 | 1.64 | 1.35 | > 10 |
| 15d | 4 | 3.89 | 2.48 | 2.56 | > 10 |
| 15e | 5 | 4.83 | 2.81 | 3.68 | > 10 |
Rapidly growing eukaryotes including the human pathogenic apicomplexan protozoans and trypanosomatids, such as the causative agents of malaria and leishmaniasis respectively, have been shown to be responsive to HDACi.46-50 These earlier observations demonstrate HDACs as promising targets in the fight against the often fatal diseases caused by these parasites. However, the structure activity relationship (SAR) of the antimalarial and antileishmanial activities of HDACi is not entirely clear. To further profile the biological activities of these triketolide HDACi, we investigated their anti-parasitic activities against Plasmodium falciparum and Leishmania donovani, the causative parasites of human malaria and leishmaniasis respectively.
The antimalarial activities of compounds 15a-e closely paralleled their anti-HDAC activity against HeLa nuclear extract HDAC. They potently inhibit the proliferation of both the sensitive and resistant strains of P. falciparum with IC50 ranging from 0.12 g/mL to 1.5 g/mL (Table 3). Again, 15b has the most potent antimalarial activity which is between 2- to 4-fold more potent than the control compound SAHA. Moreover, 15b is several folds more selectively toxic to either strains of P. falciparum compared to SAHA. This result suggests that the parasite HDACs could be one of the compound intracellular targets. To obtain a direct evidence for the involvement of P. falciparum HDACs as one of the potential intracellular targets for these compounds, we investigated their effects on the activity of P.falciparum HDAC1 (pf-HDAC1).50 All the compounds demonstrate inhibitory activity against pf-HDAC1 with IC50 values that followed a similar pattern as their activity against the HeLa nuclear extract HDAC (Table 4). The anti pf-HDAC1 activity of compound 15b, the most potent in this series, is about 3-fold enhanced relative to the previously reported macrolide HDACi chemotypes with the same linker length.50 The enhanced anti pf-HDAC1 activity of 15b could be due to a better fit to the enzyme afforded by its rigid tricyclic ring. Overall, this result confirms that these ketolide based HDACi partly derived their antimalarial activity through intracellular inhibition of pf-HDAC1 activity.
Table 3.
In Vitro Antileishmanial (μg/mL) and Antimalarial (μg/mL) activities of tricyclic ketolide-based HDAC inhibitor derivatives
| Compound | n | Antileishmanial Activitya | Antimalarial Activityb | Cytotoxicity (VERO)b IC50 (μg/ml) | S.I. D6 (W2) | ||
|---|---|---|---|---|---|---|---|
| IC50 (μg/ml) | IC90 (μg/ml) | Plasmodium falciparum (D6 clone) IC50 (μg/ml) | Plasmodium falciparum (W2 clone) IC50 (μg/ml) | ||||
| 15a | 1 | NA | NA | 0.897 ± 0.198 | 1.789 ± 0.254 | NC | >5.3 (>2.68) |
| 15b | 2 | NA | NA | 0.137 ± 0.027 | 0.133 ± 0.027 | NC | >35.0 (>36.0) |
| 15c | 3 | 19.7 ± 2.4 | 35.8 ± 4.4 | 1.317 ± 0.231 | 1.475 ± 0.244 | NC | >3.6 (>3.3) |
| 15d | 4 | 14.9 ± 1.7 | 32.3 ± 3.1 | 1.296 ± 0.129 | 1.376 ± 0.079 | NC | >3.7 (>3.5)) |
| 15e | 5 | 4.8 ± 0.5 | 23.7 ± 2.4 | 1.195 ± 0.058 | 0.897 ± 0.025 | NC | >4.0 (>5.3) |
| Chloroquine | - | NT | NT | 0.012 ± 0.002 | 0.147 ± 0.018 | NT | NT |
| Artemisinin | - | NT | NT | 0.0066 ± 0.0007 | 0.0091 ± 0.0011 | NT | NT |
| Pentamidine | - | 1.17 ± 0.25 | 2.45 ± 0.34 | NT | NT | NT | NT |
| Amphotericine B | - | 0.079 ± 0.012 | 0.395 ± 0.054 | NT | NT | NT | NT |
| SAHA | - | 21.5 ± 3.51 | 51.7 ± 8.1 | 0.279 ± 0.077 | 0.392 ± 0.045 | 1.375 ± 0.255 | 4.9 (3.5) |
Maximum tested concentration = 40 μg/ml
Maximun tested concentration= 4.8 μg/ml
NA = Not active up to highest concentration tested
NC = Not cytotoxic up to the highest concentration tested
NT = Not tested
S.I.= Selectivity Index (IC50 vero/IC50)
Table 4.
In Vitro Inhibition of pf-HDAC1 by tricyclic ketolide-based HDACi
| Compound | n | IC50 (nM) |
|---|---|---|
| 15a | 1 | 36±2.9 |
| 15b | 2 | 10±0.5 |
| 15c | 3 | 40±1.8 |
| 15d | 4 | 290±16 |
| 15e | 5 | 304±17 |
| SAHA | - | 130±9.2 |
Interestingly however, compounds 15a-b are devoid of antileishmanial activity against the promastigote stage of L. donovani, while compounds 15c-e have a moderate to good activity which peaks with 15e, the analog with the longest methylene spacer in the series disclosed in this manuscript (Table 3). This result is in contrast to the antimalarial activity that mirrors compound anti-HDAC activity and it may have implication in the organization of the active sites of the relevant P. falciparum and L. donovani HDAC isozymes. We have observed a similar methylene spacer-length dependence in the antileishmanial activity of macrocyclic HDACi derived from 14- and 15-membered macrolides.46, 50 The foregoing observations further support the suitability of HDAC inhibition as a viable therapeutic strategy to curb infections caused by apicomplexan protozoans and trypanosomatids.
Conclusion
We report that tricyclic ketolide is an excellent mimetic for the peptide backbone of macrocyclic HDACi. The tricyclic ketolide herein described has a rigid hydrophobic skeleton which, as we confirmed by molecular docking analysis, favorably interacts with the HDAC enzyme's outer rim groups thereby resulting in enhanced HDAC inhibition profile. Compounds derived from this template are particularly selective against HeLa nuclear extract HDACs (1/2) with nanomolar inhibitory activity and have improved drug cytotoxicity against human cancer cell lines. Many of these compounds have superior anti-parasitic activities against causative parasites of malaria and leishmaniasis compared to clinically useful SAHA. The pattern of the antimalarial and antileishmanial activities of these compounds reveals structural attributes which confer a specific anti-parasitic response. Finally, ketolides like their 14- and 15-membered macrolide counterparts have selective tissue/cell distribution profile. For example, ketolides have been reported to exceptionally accumulate in the phagocytes, in particular, human polymorphonuclear neutrophils (PMNs), and other phagocytic and epithelial cell lines.51 Therefore, these ketolide derived HDACi could find application in targeting tumor-associated macrophages and further clarify the roles of HDACi in the inhibition of macrophage migration inhibitory factor (MIF) gene, a phenomenon that has been suggested to be essential part of their antitumorigenic effects.52
Experimental Section
Materials and methods
All commercially available starting materials were used without further purification. Clarithromycin 1 was purchased from Greenfield Chemical. 4-Ethynylbenzyl alcohol, ethyl 6-bromohexanoate, ethyl 7-bromoheptanoate, 8-bromooctanoic acid, and methyl 10-bromodecanoate were purchased from Sigma Aldrich. 9-Bromononanoic acid was purchased from Karl Industries Inc. Reaction solvents were either high performance liquid chromatography (HPLC) grade or American Chemical Society (ACS) grade and used without further purification. HDAC fluorimetric assay kit and recombinant HDACs were procured from Enzo Life Sciences, BIOMOL International, PA. Analtech silica gel plates (60 F254) were used for analytical TLC, and Analtech preparative TLC plates (UV 254, 2000 μm) were used for purification. UV light and anisaldehyde/iodine stain were used to visualize the spots. 200-400 Mesh silica gel was used in column chromatography. Nuclear magnetic resonance (NMR) spectra were recorded on a Varian-Gemini 400 magnetic resonance spectrometer. 1H NMR spectra were recorded in parts per million (ppm) relative to the peak of CDCl3, (7.24 ppm). 13C spectra were recorded relative to the central peak of the CDCl3 triplet (77.0 ppm) and were recorded with complete hetero-decoupling. High-resolution mass spectra were recorded at the Georgia Institute of Technology mass spectrometry facility in Atlanta. Melting points were recorded uncorrected on a MEL-TEMP II apparatus. HPLC assays were performed on a Beckman Coulter instrument using a Phenomenex RP C-18 column (250 × 4.6 mm), eluting with A: 0.1% formic acid/acetonitrile and B: 0.1% formic acid/water at a gradient of 5 – 50% over 30 min, detecting at 251 nm and a flow rate of 1 ml/min. Sample concentrations were 250 μM, injecting 5 μL. 3’-Desmethylclarithromycin 2, 4-ethynylbenzylclarithromycin 4, descladinose-4-ethynylbenzylclarithromycin 5, and azidoalkylhydroxamic acid (13a-b) were synthesized as we previously reported.24 Azido-O-trityl alkylhydroxamate derivatives (12a-e) were synthesized by adapting literature protocol.46
2’-O-Acetyl-descladinose-4-ethynylbenzylclarithromycin (6)
To a solution of descladinose-4-ethynylbenzylclarithromycin 5 (3.80 g, 5.50 mmol) in acetone (20 ml) was added Ac2O (0.62 g, 6.00 mmol) and the resulting mixture was stirred at 40 °C for 36 h. The reaction mixture was diluted with EtOAc (100ml) and washed with aqueous NaHCO3 (70 ml) and saturated brine (70 ml). Purification on silica column (6:1 CH2Cl2/acetone) afforded 2.8 g (70 %) of the title compound as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 0.80 (3H, t, J = 7.2 Hz), 0.90 (3H, d, J = 7.2 Hz), 1.08-1.47 (12H, m), 1.58 (6H, s), 1.63-2.05 (7H, m), 2.08 (3H, s), 2.16 (3H, s), 2.42-2.80 (3H, m), 2.92 (3H, s), 2.94-3.00 (1H, m), 3.03-3.68 (3H, m), 3.79 (2H, s), 3.94 (1H, s), 4.08 (1H, m), 4.54 (1H, d, J = 8.0 Hz), 4.80 (1H, m), 5.15 (1H, dd, J = 11.6, 2.4 Hz), 7.17 (2H, d, J = 8.4 Hz), 7.38 (2H, d, J = 8.0 Hz); 13C NMR (CDCl3, 100 MHz) δ 8.0, 10.7, 12.8, 15.5, 16.4, 18.1, 19.5, 21.3, 21.5, 21.6, 31.1, 31.9, 36.0, 37.0, 37.5, 38.7, 44.3, 45.7, 50.0, 58.4, 62.5, 68.9, 69.8, 71.6, 74.4, 76.9, 77.0, 77.8, 78.1, 81.3, 83.9, 99.9, 120.7, 128.4, 132.2, 141.1, 170.1, 174.9, 221.2; HRMS (FAB, mnba) calc for [C40H61NO11 + H]+ 732.4323 , found 732.4311.
2-Oxo-2’-O-acetyl-descladinose-4-ethynylbenzylclarithromycin (7)
Methyl sulfide (1.42 g, 22. 80 mmol), was added to a mixture of N-chlorosuccinimide (2.61 g, 19.50 mmol) and CH2Cl2 (10 ml) while maintaining the temperature at -15 °C. Compound 6 (10.0 g, 13. 7 mmol) dissolved in CH2Cl2 (50 ml) was added to the reaction flask, followed by Et3N (1.56 g, 15.4 mmol) and stirred at -15 °C for 4.5 h. The reaction mixture was poured into EtOAc (350 ml) and 0.5 N aqueous NaOH (250 ml). The organic layer was separated, washed with saturated brine (250 ml) and dried over Na2SO4. Solvent was evaporated off and the crude was purified on silica column (2:3:0.1 EtOAc/Hexane/Et3N) to afford 9.54 g (96 %) of the title compound as off-white solid. 1H NMR (CDCl3, 400 MHz) δ 0.80-0.86 (6H, m), 1.09-1.57 (12H, m), 1.58 (6H, s), 1.62-2.02 (7H, m), 2.05 (3H, s), 2.15 (3H, s), 2.44-2.80 (2H, m), 2.92 (3H, s), 2.95-3.00 (1H, m), 3.05-3.66 (4H, m), 3.80 (1H, q, J = 14.0, 6.8 Hz), 3.89 (1H, s), 4.12 (1H, m), 4.38 (1H, d, J = 8.0 Hz), 4.79-4.83 (1H, m), 5.14 (1H, dd, J = 11.2, 2.0 Hz), 7.16 (2H, d, J = 7.6 Hz), 7.38 (2H, d, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.8, 12.4, 14.4, 14.5, 16.5, 17.9, 19.6, 21.3, 21.5, 31.3, 36.9, 37.6, 39.2, 45.1, 46.3, 49.7, 51.1, 58.5, 62.5, 69.2, 69.6, 71.5, 74.1, 77.0, 77.1, 77.2, 78.1, 83.9, 101.1, 120.7, 128.5, 132.2, 140.9, 169.6, 170.0, 205.7, 221.1; HRMS (FAB, mnba) calc for [C40H59NO11 + H]+ 730.4166, found 730.4132.
12-Carbamoylimidazole Ketolide (8)
To a suspension of 7 (1.50 g, 2.06 mmol) in a mixture of anhydrous THF (20 ml) and anhydrous DMF (7 ml), was added CDI (1.30 g, 8.20 mmol) followed by a solution of NaN(TMS)2 1.0 M in THF (2.60 ml, 2.60 mmol) over 75 min and the resulting mixture was stirred at RT for 24 h. The reaction mixture was diluted with EtOAc (50 ml), washed with aqueous NaHCO3 (20 ml) and saturated brine (20 ml) and dried over Na2SO4. Solvent was evaporated off and the crude was purified on silica column (3:2:0.1 EtOAc/Hexane/Et3N) to yield 52 % (0.86 g) of the title compound. 1H NMR (CDCl3, 400 MHz) δ 0.91 (3H, t, 7.6 Hz), 1.09 (3H, d, J = 7.2 Hz ), 1.14-1.50 (9H, m), 1.80 (3H, s), 1.83 (3H, s), 1.56-1.94 (7H, m), 2.00 (3H, s), 2.05 (3H, s), 2.12 (3H, s), 2.64-2.80 (1H, m), 3.02 (3H, s), 2.95-3.70 (4H, m), 3.73 (1H, q, J = 14.0, 6.8 Hz), 4.10-4.06 (2H, m), 4.30 (1H, d, J = 7.6 Hz), 4.77-4.80 (1H, m), 5.68 (1H, dd, J = 11.2, 2.0 Hz), 6.77 (1H, s), 7.03 (1H, s), 7.16 (2H, d, J = 7.6 Hz), 7.36 (3H, m), 8.06 (1H, s); 13C NMR (CDCl3, 100 MHz) δ 10.7, 13.5, 14.4, 15.2, 19.1, 20.4, 21.1, 21.2, 21.5, 22.8, 31.2, 36.8, 47.6, 50.5, 51.2, 58.5, 60.6, 62.8, 69.3, 71.6, 78.7, 83.9, 84.7, 117.3, 120.7, 128.4, 131.1, 132.2, 137.3, 138.8, 140.9, 146.2, 169.1, 169.8; HRMS (FAB, mnba) calc for [C44H59N3O11 + H]+ 806.4228, found 806.4210
4-Ethynylbenzyltricyclic Ketolide (9)
Protocol A
To a solution of 8 (0.60 g, 0. 74 mmol) in CH3CN (10 ml) and H2O (1 ml), was added ethylenediamine (0.44 g, 7.40 mmol) and the mixture was heated in a sealed tube at 50-55 °C for 24 h. Solvent was evaporated off and the reaction mixture partitioned between H2O (15 ml) and CH2Cl2 (20 ml) and separated. The organic layer was washed with saturated brine (20 ml), dried over Na2SO4, and solvent was evaporated off in vacuo. The crude product was dissolved in anhydrous MeOH (15 ml) and heated at 90 °C in a sealed tube for 24 h. MeOH was evaporated and the product purified on silica column (EtOAc/Et3N 12:0.1) to give 0.27 g (48 %) of the intermediate product. EtOH (6 ml) and AcOH (0.043 g, 0.71 mmol) were added to the pure intermediate product (0.27 g, 0.34 mmol) and the mixture was heated at 95 °C in a sealed tube for 20 h. The reaction mixture was suspended in dilute NH4OH (10 ml) and CH2Cl2 (15 ml) and the organic layer separated, washed with saturated brine (10 ml), and dried over Na2SO4. Purification on silica column (EtOAc/MeOH/Et3N 10:0.5:0.05) afforded 0.24 g (92 %); 44 % overall yield of compound 9.
Protocol B – reductive amination
A solution of 11 (0.20 g, 0.32 mmol) and 4-ethynylbenzaldehyde (0.21 g, 1.60 mmol) in MeOH (4 ml) and acetic acid (37 μL, 0.64 mmol) was stirred for 30 min at room temperature. Borane-pyridine complex (80 μL, 0.64 mmol) was added and the reaction stirred for 5 h. The reaction was diluted with EtOAc (20 ml) and washed with saturated NaHCO3 (20 ml) and saturated brine (20 ml). The organic layer was dried over Na2SO4, concentrated in vacuo and purified by preparative TLC (12:1 CH2Cl2/MeOH) to afford 0.18 g (78 %) of compound 9 as a creamish solid. 1H NMR (CDCl3, 400 MHz) δ 0.80 (3H, t, J = 7.2 Hz), 1.00 (3H, d, J = 6. 8 Hz ), 1.14-1.55 (12H, m), 1.30 (3H, s), 1.43 (3H, s), 1.60-1.96 (6H, m), 2.12 (3H, s), 2.45-2. 95 (6H, m), 3.03 (3H, s) 3.23-3.43 (4H, m), 3.64-3.78 (4H, m), 3. 94 (1H, m), 4.16 (1H, d, J = 8.4 Hz), 4.25 (1H, d, J = 7.6 Hz), 4.90 (1H, d, J = 10.0 Hz), 7.18 (2H, d, J = 8.0 Hz), 7.39 (2H, d, J = 7.2 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.6, 11.1, 13.0, 14.6, 16.6, 19.3, 19.8, 21.4, 22.3, 29.7, 36.5, 37.1, 38.7, 42.5, 43.0, 48.3, 49.3, 49.7, 51.4, 57.6, 60.1, 65.6, 69.7, 70.5, 76.7, 78.7, 79.4, 81.7, 83.6, 104.0, 121.0, 128.8, 132.4, 140.0, 156.3, 169.8, 181.5, 204.4; HRMS (FAB, mnba) calc for [C41H59N3O9 + H]+ 738.4330, found 738.4332
Representative Procedure for the Synthesis of O-trityl Protected Tricyclic Ketolide Hydroxamate. Tricyclic Ketolide-N-benzyltriazolyl-O-tritylhexahydroxamate (14a)
4-Ethynylbenzyltricyclic ketolide 9 (0.10 g, 0.14 mmol) and 6-azido-O-trityl hexahydroxamate 12a (0.06 g, 0.14 mmol) were dissolved in anhydrous THF (7 mL) and stirred under argon at room temperature. Copper (I) iodide (0.012 g, 0.063 mmol) and Hunig's base (0.6 mL) were added to the reaction mixture, and stirring continued for 24 h. The reaction mixture was diluted with 1:4 NH4OH/saturated NH4Cl (50 mL) and extracted with 10% MeOH/CH2Cl2 (3 × 30 mL). The organic layers were combined, dried over Na2SO4 and concentrated in vacuo. The crude product was purified by prep TLC (silica, 12:1:0.1 CH2Cl2/MeOH/conc. NH4OH) to give 127 mg (78 %) of 14a as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 0.85 (3H, t, J = 7.6 Hz), 1.1 (3H, d, J = 6.8 Hz), 1.20-1.39 (17H, m), 1.46-2.00 (15H, m), 2.19 (3H, s), 2.58-2.80 (6H, m), 2.84-3.00 (1H, m), 3.02-3.17 (1H, m), 3.31-3.60 (3H, m), 3.64-3.87 (5H, m), 3.96-4.0 (1H, m), 4.22 (1H, d, J = 8.4 Hz), 4.29-4.32 (3H, m), 4.93 (1H, dd, J = 10.0, 1.6 Hz), 7.23-7.45 (17H, m), 7.71 (1H, s), 7.79 (2H, d, J = 8.0 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.6, 16.7, 19.3, 19.9, 21.4, 22.3, 22.8, 26.0, 29.6, 30.2, 31.0, 36.5, 37.0, 38.8, 42.5, 43.0, 48.3, 49.3, 49.7, 50.2, 51.4, 53.7, 57.7, 60.1, 65.6, 69.8, 70.5, 76.7, 78.7, 79.4, 81.8, 93.1, 104.1, 119.7, 126.0, 128.2, 128.4, 129.2, 129.4, 130.0, 138.9, 141.2, 147.6, 156.3, 169.8, 177.0, 181.5, 204.5; HRMS (ESI) calc for [C66 H85 N7 O11 + H]+ 1152.6379, found 1152.6367.
Tricyclic Ketolide-N-benzyltriazolyl-O-tritylheptahydroxamate (14b)
Reaction of 4-ethynylbenzyltricyclic ketolide 9 (0.10 g, 0.14 mmol) and 7-azido-O-trityl heptahydroxamate 12b (0.06 g, 0.14 mmol) within 24 h as described for the synthesis of 14a gave 133 mg (83 %) of 14b as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 0.85 (3H, t, J = 7.6 Hz), 1.06 (3H, d, J = 6.8 Hz), 1.20-1.42 (19H, m), 1.45-1.98 (15H, m), 2.20 (3H, s), 2.57-2.79 (6H, m), 2.85-3.00 (1H, m), 3.03-3.17 (1H, m), 3.30-3.57 (3H, m), 3.72-3.83 (5H, m), 3.96-4.00 (1H, m), 4.22 (1H, d, J = 8.8 Hz), 4.29-4.35 (3H, m), 4.95 (1H, dd, J = 10.4, 2.4 Hz), 7.22-7.45 (17H, m), 7.71 (1H, s), 7.78 (2H, d, J = 8.4 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.6, 16.6, 19.3, 19.9, 21.4, 22.3, 26.3, 29.6, 30.2, 36.5, 37.0, 38.8, 42.5, 43.0, 48.3, 49.3, 49.7, 50.4, 51.4, 53.7, 57.7, 60.1, 65.5, 69.7, 70.5, 76.7, 78.7, 79.4, 81.8, 92.8, 104.1, 119.7, 126.0, 128.4, 129.2, 129.4, 130.0, 132.3, 138.9, 141.3, 147.6, 156.3, 169.8, 177.6, 182.5, 204.5; HRMS (ESI) calc for [C67 H87 N7 O11 + H]+ 1166.6536, found 1166.6478
Tricyclic Ketolide-N-benzyltriazolyl-O-trityloctahydroxamate (14c)
Reaction of 4-ethynylbenzyltricyclic ketolide 9 (0.08 g, 0.10 mmol) and 8-azido-O-trityl octahydroxamate 12c (0.05 g, 0.10 mmol) in anhydrous THF (5 ml) within 22 h as described for the synthesis of 14a gave 94 mg (79 %) of 14c as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 0.83 (3H, t, J = 7.2 Hz), 1.04 (3H, d, J = 7.2 Hz), 1.15-1.41 (21H, m), 1.45-1.95 (15H, m), 2.18 (3H, s), 2.55-2.73 (6H, m), 2.87-2.97 (1H, m), 3.03-3.10 (1H, m), 3.28-3.57 (3H, m), 3.70-3.82 (5H, m), 3.93-3.97 (1H, m), 4.21 (1H, d, J = 8.4 Hz), 4.27-4.34 (3H, m), 4.94 (1H, dd, J = 12.8, 2.4 Hz), 7.25-7.42 (17H, m), 7.71 (1H, s), 7.76 (2H, d, J = 8.0 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.6, 16.6, 19.3, 19.9, 21.4, 22.3, 26.4, 28.8, 29.6, 30.4, 36.6, 37.0, 38.8, 42.5, 42.9, 48.3, 49.3, 49.7, 50.6, 51.4, 53.7, 57.7, 60.1, 65.5, 69.8, 70.5, 76.7, 78.7, 79.4, 81.8, 93.8, 104.1, 119.6, 126.0, 127.7, 128.3, 128.8, 129.2, 129.4, 130.0, 132.7, 138.9, 141.3, 147.7, 156.3, 169.8, 177.0, 182.4, 204.4; HRMS (ESI) calc for [C68 H89 N7 O11 + H]+ 1180.6692, found 1180.6637
Tricyclic Ketolide-N-benzyltriazolyl-O-tritylnonahydroxamate (14d)
Reaction of 4-ethynylbenzyltricyclic ketolide 9 (0.08 g, 0.10 mmol) and 9-azido-O-trityl nonahydroxamate 12d (0.05 g, 0.10 mmol) in anhydrous THF (5 ml) within 20 h as described for the synthesis of 14a gave 93 mg (76 %) of 14d as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 0.85 (3H, t, J = 8.0 Hz), 1.05 (3H, d, J = 6.8 Hz), 1.16-1.44 (23H, m), 1.45-1.96 (15H, m), 2.20 (3H, m), 2.57-2.78 (6H, m), 2.90-2.99 (1H, m), 3.02-3.12 (1H, m), 3.30-3.57 (3H, m), 3.72-3.84 (5H, m), 3.96-4.00 (1H, m), 4.29 (1H, d, J = 8.4 Hz), 4.31 (1H, d, J = 7.2 Hz), 4.37 (2H, t, J = 7.2 Hz), 4.95 (1H, dd, J = 12.8, 2.4 Hz), 7.19-7.45 (17H, m), 7.72 (1H, s), 7.78 (2H, d, J = 8.0 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.6, 16.6, 19.3, 19.9, 21.4, 22.3, 26.6, 28.9, 29.2, 29.6, 29.9, 30.5, 36.5, 37.0, 37.1, 38.8, 42.5, 43.0, 48.3, 49.3, 49.7, 50.6, 51.4, 57.7, 60.1, 65.5, 65.6, 69.7, 70.5, 76.7, 78.7, 79.4, 81.8, 94.3, 104.1, 119.6, 126.0, 128.3, 128.8, 129.0, 129.2, 129.4, 130.0, 132.3, 138.9, 141.3, 147.6, 156.3, 169.8, 178.0, 181.5, 204.5; HRMS (ESI) calc for [C69 H91 N7 O11 + H]+ 1194.6849, found 1194.6838
Tricyclic Ketolide-N-benzyltriazolyl-O-trityldecahydroxamate (14e)
Reaction of 4-ethynylbenzyltricyclic ketolide 9 (0.08 g, 0.10 mmol) and 10-azido-O-trityl decahydroxamate 12e (0.05 g, 0.10 mmol) in anhydrous THF (5 ml) within 20 h as described for the synthesis of 14a gave 98 mg (80 %) of 14e as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 0.83 (3H, t, J = 6.8 Hz), 1.03 (3H, d, J = 7.2 Hz), 1.15-1.40 (25H, m), 1.44-1.96 (15H, m), 2.17 (3H, s), 2.57-2.78 (6H, m), 2.87-2.95 (1H, m), 3.03-3.08 (1H, m), 3.28-3.53 (3H, m), 3.70-3.82 (5H, m), 3.92-3.96 (1H, m), 4.20 (1H, d, J = 8.4 Hz), 4.29 (1H, d, J = 6.8 Hz), 4.33 (2H, t, J = 7.2 Hz), 4.93 (1H, d, J = 10.0 Hz), 7.26-7.43 (17H, m), 7.73 (1H, s), 7.76 (2H, d, J = 8.0 Hz); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.6, 16.6, 19.3, 19.8, 21.4, 22.3, 26.6, 29.1, 29.3, 29.6, 29.9, 30.5, 36.6, 37.0, 38.8, 42.5, 43.0, 48.3, 49.3, 49.7, 50.6, 51.4, 57.7, 60.1, 65.5, 69.7, 70.5, 76.7, 78.7, 79.4, 81.8, 93.4, 104.1, 199.6, 126.0, 128.3, 129.2, 129.4, 130.0, 138,9, 147.6, 156.3, 169.8, 177.6, 182.0, 204.5; HRMS (ESI) calc for [C70 H93 N7 O11 + H]+ 1208.7005, found 1208.6888
Tricyclic Ketolide-N-benzyltriazolylhexahydroxamic acid (15a)
Method A
To a solution of 14a (0.12 g, 0.11 mmol) in methylene chloride (1 ml) at 0 °C, was added thioanisole (0.2 ml) and TFA (0.2 ml) dropwise. Stirring was continued at 0 °C for 2 h after which TLC analysis indicated complete consumption of the starting material. Excess TFA and solvent were evaporated off and the residue was immediately placed in the ice-bath followed by addition of PBS buffer (10 ml). Saturated NaHCO3 was added dropwise until the pH was neutral. Extraction with 20 % MeOH/ CH2Cl2 (10 ml × 3), drying over Na2SO4, and evaporation of solvent afforded crude product which was then purified by prep TLC (silica, 12:1:0.1 CH2Cl2/MeOH/conc. NH4OH) to afford 47 mg (50 %) of 15a as a yellow-white solid.
Method B
To a mixture of 14a (0.14 g, 0.12 mmol) in CH2Cl2/MeOH (2 ml: 2 ml) was added BF3.OEt2 (0.03 g, 0.24 mmol) and the mixture was stirred at room temperature for 45 min. Dilute NaHCO3 was added until pH = 8 (approx. 20 ml), and the suspension was extracted with 10 % MeOH/ CH2Cl2 (10 ml × 4), dried over Na2SO4 and concentrated in vacuo. The crude product was purified by prep TLC (silica, 12:1:0.1 CH2Cl2/MeOH/conc. NH4OH) to give 64 mg (60 %) of 15a as a yellow-white solid.
Method C
4-Ethynylbenzyltricyclic ketolide 9 (0.05 g, 0.07 mmol) and 6-azidohexahydroxamic acid24 13a (0.03 g, 0.15 mmol) were dissolved in anhydrous THF (7 mL) with stirring under argon at room temperature. Copper (I) iodide (0.01 g, 0.04 mmol) and Hunig's base (0.5 mL) were added to the reaction mixture, and stirring continued for 12 h. The reaction mixture was diluted with 10% MeOH/CH2Cl2 (20 mL) and washed with 1:4 NH4OH/saturated NH4Cl (3 × 10 mL) and saturated NH4Cl (10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by prep TLC (silica, 12:1:0.1 CH2Cl2/MeOH/conc. NH4OH) to give 20 mg (34 %) of 15a as a brown-white solid; mp 127-130 °C; retention time 20.167 min. 1H NMR (CDCl3, 400 MHz) δ 0.85 (3H, t, J = 8.0 Hz), 1.05 (3H, d, J = 7.2 Hz), 1.20-2.00 (30H, m), 2.13-2.18 (2H, m), 2.20 (3H, s), 2.58-2.72 (6H, m), 2.92-2.98 (1H, m), 3.05-3.09 (1H, m), 3.30-3.55 (3H, m), 3.70-3.84 (5H, m), 3.95-3.98 (1H, m), 4.22 (1H, d, J = 8.0 Hz ), 4.31-4.38 (3H, m), 4.95 (1H, d, J = 8.8 Hz ), 7.33 (2H, d, J = 8.0 Hz), 7.77 (3H, m); 13C NMR (CDCl3, 100 MHz) δ 10.6, 11.1, 13.1, 14.6, 16.5, 19.3, 19.8, 21.4, 22.3, 24.7, 25.9, 29.7, 29.9, 30.0, 36.6, 37.1, 38.8, 42.5, 42.8, 49.4, 49.6, 50.3, 51.4, 53.7, 57.7, 60.1, 65.5, 69.7, 70.5, 76.7, 78.7, 79.3, 81.8, 104.1, 120.1, 126.0, 129.5, 129.7, 139.1, 147.7, 156.3, 169.8, 171.1, 182.3, 204.5; HRMS (ESI) calc for [C47 H71 N7 O11 + H]+ 910.5284, found 910.5279.
Tricyclic Ketolide-N-benzyltriazolylheptahydroxamic acid (15b)
The reaction of 14b (0.13 g, 0.11 mmol) in CH2Cl2 (1 ml), thioanisole (0.3 ml) and TFA (0.3 ml), according to method A, for 3 h afforded 56 mg (55 %) of 15b as a yellow-white solid. Similarly, the reaction of 14b (0.15 g, 0.18 mmol) and BF3.OEt2 (0.04 g, 0.26 mmol), according to method B, afforded 65 mg (55 %) of 15b as a yellow-white solid. Also, the reaction of 4-ethynylbenzyltricyclic ketolide 9 (0.05 g, 0.06 mmol) and 7-azidoheptahydroxamic acid 13b (0.02 g, 0.11 mmol) within 12 h, according, to method C, gave 22 mg (38 %) of 15b as a brown-white solid; mp 128-131 °C; retention time 21.233 min. 1H NMR (CDCl3, 400 MHz) δ 0.83 (3H, t, J = 7.6 Hz), 1.03 (3H, d, J = 6.4 Hz), 1.18-1.90 (32H, m), 2.10-2.13 (2H, m), 2.17 (3H, s), 2.58-2.73 (6H, m), 2.93-2.98 (1H, m), 3.03-3.07 (1H, m), 3.28-3.60 (3H, m), 3.70-3.81 (5H, m), 3.98 (1H, m), 4.20 (1H, d, J = 8.4 Hz ), 4.29-4.38 (3H, m), 4.93 (1H, d, J = 10.4 Hz ), 7.30 (2H, d, J = 7.6 Hz), 7.75 (2H, d, J = 7.6 Hz), 7.78 (1H, s); 13C NMR (CDCl3, 100 MHz) δ 10.6, 11.1, 13.1, 14.6, 16.5, 19.3, 19.8, 21.4, 22.3, 25.2, 26.0, 28.2, 29.7, 29.9, 30.1, 36.6, 37.0, 38.8, 42.5, 42.7, 48.2, 49.3, 49.5, 50.4, 51.4, 53.7, 57.7, 60.1, 65.5, 69.7, 70.5, 76.7, 78.7, 79.3, 81.8, 104.0, 120.0, 126.0, 129.4, 129.8, 139.1, 147.7, 156.3, 169.8, 171.2, 182.4, 204.5; HRMS (ESI) calc for [C48 H73 N7 O11 + H]+ 924.5440, found 924.5422.
Tricyclic Ketolide-N-benzyltriazolyloctahydroxamic acid (15c)
The reaction of 14c (0.09 g, 0.08 mmol) in CH2Cl2 (1 ml), thioanisole (0.2 ml) and TFA (0.2 ml), according to method A, afforded 37 mg (51 %) of 15c as a yellow-white solid; mp 113-115 °C; retention time 22.533 min. 1H NMR (CDCl3, 400 MHz) δ 0.83 (3H, t, J = 6.4 Hz), 1.03 (3H, d, J = 5.6 Hz), 1.18-1.60 (21H, m), 1.66-2.1 (15H, m), 2.18 (3H, s), 2.56-2.70 (6H, m), 2.90-2.98 (1H, m), 3.02-3.07 (1H, m), 3.28-3.58 (3H, m), 3.70-3.81 (5H, m), 3.94-3.97 (1H, m), 4.20 (1H, d, J = 7.2 Hz), 4.28 (1H, d, J = 6.0 Hz), 4.35 (2H, m), 4.93 (1H, d, J = 10.4 Hz), 7.30 (2H, d, J = 7.2 Hz), 7.73-7.76 (3H, m); 13C NMR (CDCl3, 100 MHz) δ 10.6, 11.1, 13.1, 14.4, 14.6, 16.5, 19.3, 19.8, 21.3, 21.4, 22.3, 25.2, 26.1, 28.3, 29.7, 29.9, 30.2, 32.9, 36.6, 37.1, 38.8, 42.5, 42.7, 48.2, 49.4, 49.5, 50.5, 51.4, 57.7, 60.1, 60.6, 65.5, 69.7, 70.5, 76.7, 78.7, 79.3, 81.8, 104.0, 119.9, 126.0, 129.5, 129.8, 139.0, 147.7, 156.3, 169.8, 171.7, 182.4, 204.5; HRMS (ESI) calc for [C49 H75 N7 O11 + + H]+ 938.5597, found 938.5556.
Tricyclic Ketolide-N-benzyltriazolylnonahydroxamic acid (15d)
The reaction of 14d (0.09 g, 0.08 mmol) in CH2Cl2 (1 ml), thioanisole (0.2 ml) and TFA (0.2 ml), according to method A, afforded 43 mg (60 %) of 15d as a yellow-white solid; mp 112-115 °C; retention time 23.983 min.1H NMR (CDCl3, 400 MHz) δ 0.83 (3H, t, J = 7.2 Hz), 1.03 (3H, d, J = 7.2 Hz), 1.17-1.60 (23H, m), 1.66-2.14 (15H, m), 2.17 (3H, s), 2.58-2.73 (6H, m), 2.87-2.95 (1H, m), 3.00-3.07 (1H, m), 3.27-3.55 (3H, m), 3.70-3.82 (5H, m), 3.93-3.97 (1H, m), 4.20 (1H, d, J = 8.8 Hz), 4.28 (1H, d, J = 7.2 Hz), 4.35 (2H, t, J = 6.8 Hz), 4.92 (1H, dd, J = 10.0, 1.2 Hz), 7.30 (2H, d, J = 8.0 Hz), 7.75 (3H, m); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.6, 16.5, 19.3, 19.8, 21.4, 22.3, 25.4, 26.3, 28.6, 28.8, 28.9, 29.6, 29.9, 30.3, 32.9, 36.6, 37.1, 38.8, 42.5, 42.8, 48.2, 49.3, 49.6, 50.6, 51.4, 57.7, 60.1, 65.5,, 69.7, 70.5, 76.7, 78.7, 79.3, 81.8, 104.1, 119.8, 126.0, 129.5, 129.8, 139.0, 147.7, 156.3, 169.8, 171.3, 182.9, 204.5; HRMS (ESI) calc for [C50 H77 N7 O11 + H]+ 952.5753, found 952.5728.
Tricyclic Ketolide-N-benzyltriazolyldecahydroxamic acid (15e)
The reaction of 14e (0.10 g, 0.08 mmol) in CH2Cl2 (1 ml), thioanisole (0.2 ml) and TFA (0.2 ml), according to method A, afforded 42 mg (55 %) of 15e as a yellow-white solid; mp 123-126 °C; retention time 25.583 min.1H NMR (CDCl3, 400 MHz) δ 0.83 (3H, t, J = 7.2 Hz), 1.03 (3H, d, J = 6.4 Hz), 1.18-1.61 (25H, m), 1.66-2.14 (15H, m), 2.18 (3H, s), 2.59-2.73 (6H, m), 2.89-2.94 (1H, m), 3.01-3.07 (1H, m), 3.28-3.54 (3H, m), 3.70-8.83 (5H, m), 3.93-3.97 (1H, m), 4.20 (1H, d, J = 8.4 Hz), 4.28 (1H, d, J = 6.8 Hz), 4.36 (2H, t, J = 7.2 Hz), 4.92 (1H, dd, J = 10.4, 1.6 Hz), 7.30 (2H, d, J = 8.0 Hz), 7.76 (3H, m); 13C NMR (CDCl3, 100 MHz) δ 10.7, 11.1, 13.1, 14.4, 14.6, 16.5, 19.3, 19.9, 21.4, 22.3, 25.5, 26.3, 28.7, 28.9, 29.0, 29.6, 29.9, 30.3, 33.1, 36.6, 37.1, 38.8, 42.5, 42.7, 48.2, 49.4, 49.5, 50.6, 51.4, 57.7, 60.1, 60.6, 65.5, 69.7, 70.5, 76.7, 78.7, 79.3, 81.8, 104.0, 119.8, 126.0, 129.5, 129.8, 139.0, 147.7, 156.3, 169.8, 171.7, 182.9, 204.5; HRMS (ESI) calc for [C51 H79 N7 O11 + H]+ 966.5910, found 966.5919.
Biological Assays
In vitro HDAC inhibition
(a) Fluorescence Assay: In vitro activity against HeLa nuclear extract was determined as previously described.24, 32, 45 Briefly, 15 μL of HeLa nuclear extract was mixed with 5 μL of 10× compound and 5 μL of assay buffer. Fluorogenic substrate (25 μL) was added, and reaction was allowed to proceed for 15 min at room temperature and then stopped by addition of a developer containing TSA. Fluorescence was monitored after 15 min at excitation and emission wavelengths of 360 and 460 nm, respectively. Assays against HDAC 8 and HDAC 6 were performed according to manufacturer's instructions. (b) SAMDI Assay: The maleimide-presenting SAMs and expression of HDAC8 enzyme were prepared as previously reported.41 The peptide substrate (Ac-GRKAcFGC-NH2) was synthesized using standard FMOC solid phase peptide synthesis protocols using reagents from Anaspec (San Jose, CA). The enzyme was diluted to 1 μM in buffer (25 mM Tris-HCl (pH 8.0) containing 137 mM NaCl, 2.7 mM KCl, and 1 mM MgCl2) with varying concentrations of inhibitor (0 nM, 50 nM, 100 nM, 250 nM, and 500 nM) for 5 minutes at room temperature. The reaction was initiated upon substrate addition (5 μM, 10 μM, 25 μM, and 50 μM) to a final volume of 20 μL per reaction and the plate was incubated at 37°C. At each time point (0, 3, 6, 9, and 12 minutes), 3 μL aliquots were transferred to a separate plate and the reaction was terminated by heating to 80°C for one minute. After collecting all time points, the aliquots were transferred to the maleimide-presenting arrays and incubated at 37°C for one hour to allow the immobilization of the peptide substrate. The arrays were then washed with water, ethanol, and dried under nitrogen. Matrix (2, 4, 6-trihydroxyacetophenone, .01 μL, 20 mg/ mL in acetone) was applied, air dried, and the arrays were analyzed by SAMDI MS. (c) Pf-HDAC1 inhibition studies were performed as described in ref 47.
Mass Spectrometry and Data Analysis
Mass analysis was performed using an Applied Biosystems 4800 MALDI TOF/TOF mass spectrometer (Framingham, MA). A 355 nm Nd:YAG laser was used as a desorption ionization source and all spectra were acquired with 20 kV accelerating voltage in positive reflector mode. The extent of reaction was calculated as previously reported.41 Briefly, the relative amount of each compound was calculated by measuring the relative peak intensities of each molecular ion peak (M+) [amount of X = Ix/(Ix + Iy] where x refers to the deacetylated peak and y is the parent ion. For Ki analysis, the moles of deacetylated peptide was plotted over time and the slope of the linear portion was measured to give the initial velocity of the reaction. A Dixon plot was then created ([I] vs. 1/v) and the intersecting point corresponds to the Ki of the inhibitor.
Cell Growth Inhibition
All cell lines were maintained in a 37 °C environment containing 5% CO2 with recommended media (ATCC). For cell growth inhibition, cells were passaged the day before dosing into a 96-well cell culture plate and allowed to grow for a minimum of 24 h prior to drug addition. All compounds were diluted first to 1000X tested concentration in DMSO and then to test concentrations in cell media so that the DMSO concentration would be constant across all wells at 0.1% in a 100 μl volume. Cells were dosed for 72 h and then 20 μl of MTS reagent was added and allowed to incubate for 2 h. Absorbance was then determined using an absorbance plate reader set to 490 nm.
Antimalarial and Antileishmanial Assays
In vitro antimalarial and antileishmanial activities were determined as previously described.46 Briefly, antimalarial activity of the compounds was determined in vitro on chloroquine sensitive (D6, Sierra Leone) and resistant (W2, IndoChina) strains of Plasmodium falciparum. The 96 well microplate assay is based on evaluation of the effect of the compounds on growth of asynchronous cultures of P. falciparum, determined by the assay of parasite lactate dehydrogenase (pLDH) activity.53 The appropriate dilutions of the compounds were prepared in DMSO or RPMI-1640 medium and added to the cultures of P. falciparum (2% hematocrit, 2% parasitemia) set up in clear flat bottomed 96 well plates. The plates were placed into the humidified chamber and flushed with a gas mixture of 90% N2, 5% CO2 & 5% O2. The cultures were incubated at 37°C for 72 h. Growth of the parasite in each well was determined by pLDH assay using Malstat® reagent.53 The medium and RBC controls were also set-up in each plates. The standard antimalarial agents, chloroquine and artemisinin, were used as the positive controls while DMSO was tested as the negative control. Antileishmanial activity of the compounds was tested in vitro on a culture of Leishmania donovani promastigotes. In a 96 well microplate assay compounds with appropriate dilution were added to the leishmania promastigotes culture (2 × 106 cell/mL). The plates were incubated at 26°C for 72 h and growth of leishmania promastigotes was determined by Alamar blue assay.54 Pentamidine and Amphotericin B were tested as standard antileishmanial agents. All the compounds were simultaneously tested for cytotoxicty on VERO (monkey kidney epithilial) cells by Neutral Red assay.55 IC50 value for each compound was computed from the growth inhibition curve.
Supplementary Material
Acknowledgements
This work was financially supported by Georgia Institute of Technology, by the Blanchard fellowship and by NIH Grant R01CA131217 (A.K.O.). NCNPR is partially supported by USDA-ARS scientific cooperative agreement no 58-6408-2-0009. W.G. is a recipient of a GAANN predoctoral fellowship from the Georgia Tech Center for Drug Design, Development and Delivery. Z.G.L. is supported by the ARCS Foundation Chicago Chapter. M.M. is supported by NIH (RO1 GM084188).
Abbreviations
- HDAC
Histone deacetylase
- HAT
Histone acetyltransferase
- HDACi
Histone deacetylase inhibitors
- HDLP
Histone deacetylase-like protein
- SAHA
Suberoylanilide hydroxamic acid
- TSA
Trichostatin A
- ZBG
Zinc-binding group
- MALDI
Matrix assisted laser desorption/ ionization
- SAM
Self-assembled monolayers
Footnotes
Supporting Information Available: 1H NMR and 13C NMR spectral information, HPLC traces and pf-HDAC1 activity dose-response curves of 15a-e, and molecular modeling outputs. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jenuwein T, Allis CD. Translating the Histone Code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RW. Histone-Deacetylase Inhibitors: Novel Drugs for the Treatment of Cancer. Nat. Rev. Drug Discov. 2002;1(4):287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 3.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006;5(9):755–768. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 4.Somech R, Izraeli S, Simon AJ. Histone deacetylase inhibitors – a new tool to treat cancer. Cancer Treat. Rev. 2004;30(5):461–472. doi: 10.1016/j.ctrv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396(6711):594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 6.Gu W, Roeder RG. Activation of p53 Sequence-Specific DNA Binding by Acetylation of the p53 C-Terminal Domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 7.Jin S, Scotto KW. Transcriptional Regulation of the MDR1 Gene by Histone Acetyltransferase and Deacetylase Is Mediated by NF-Y. Mol. Cell Biol. 1998;18(7):4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigushin DM, Coombes RC. Targeted Histone Deacetylase Inhibition for Cancer Therapy. Curr. Cancer Drug Targets. 2004;4(2):205–218. doi: 10.2174/1568009043481560. [DOI] [PubMed] [Google Scholar]
- 9.Yang X-J, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 2008;9(3):206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Fang H, Jiao J, Xu W. The Structure and Function of Histone Deacetylases: The Target for Anti-cancer Therapy. Curr. Med. Chem. 2008;15(27):2840–2849. doi: 10.2174/092986708786242796. [DOI] [PubMed] [Google Scholar]
- 12.Neugebauer RC, Sippl W, Jung M. Inhibitors of NAD+ dependent histone deacetylases (sirtuins). Curr. Pharm. Des. 2008;14(6):562–73. doi: 10.2174/138161208783885380. [DOI] [PubMed] [Google Scholar]
- 13.Kelly WK, O'Connor OA, Marks PA. Histone deacetylase inhibitors: from target to clinical trials. Expert Opin. Investig. Drugs. 2002;11:1695–1713. doi: 10.1517/13543784.11.12.1695. [DOI] [PubMed] [Google Scholar]
- 14.Miller TA, Witter DJ, Belvedere S. Histone Deacetylase Inhibitors. J. Med. Chem. 2003;46(24):5097–5116. doi: 10.1021/jm0303094. [DOI] [PubMed] [Google Scholar]
- 15.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nat. Rev. Drug Discov. 2007;6(1):21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 16. http://www.fda.gov/cder/Offices/OODP/whatsnew/vorinostat.htm.
- 17. http://www.medicalnewstoday.com/articles/170146.php.
- 18. http://www.celgene.com/utility/celgene-inter.aspx?source=product&display=www.istodax.com&exit_url=www.istodax.c om.
- 19.Al-Janadi A, Chandana SR, Conley BA. Histone Deacetylation: An Attractive Target for Cancer Therapy? Drugs R D. 2008;9(6):369–383. doi: 10.2165/0126839-200809060-00003. [DOI] [PubMed] [Google Scholar]
- 20.Jones P, Steinkühler C. From Natural Products to Small Molecule Ketone Histone Deacetylase Inhibitors: Development of New Class Specific Agents. Curr. Pharm. Des. 2008;14(6):545–561. doi: 10.2174/138161208783885317. [DOI] [PubMed] [Google Scholar]
- 21.Itoh Y, Suzuki T, Miyata N. Isoform-Selective Histone Deacetylase Inhibitors. Curr. Pharm. Des. 2008;14(6):529–544. doi: 10.2174/138161208783885335. [DOI] [PubMed] [Google Scholar]
- 22.Bieliauskas AV, Pflum MKH. Isoform-selective histone deacetylase inhibitors. Chem. Soc. Rev. 2008;37:1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. http://www.clinicaltrials.gov/ct/show/NCT00106431.
- 24.Oyelere AK, Chen PC, Guerrant W, Mwakwari SC, Hood R, Zhang Y, Fan Y. Non-Peptide Macrocyclic Histone Deacetylase Inhibitors. J. Med. Chem. 2009;52:456–468. doi: 10.1021/jm801128g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashimura M, Asaka T, Misawa Y, Matsumoto K, Morimoto S. Synthesis and Antibacterial Activity of the Tricyclic Ketolides TE-802 and Its Analogs. J. Antibiot. 2001;54(8):664–678. doi: 10.7164/antibiotics.54.664. [DOI] [PubMed] [Google Scholar]
- 26.Plata DJ, Leanna MR, Rasmussen M, McLaughlin MA, Condon SL, Kerdesky FAJ, King SA, Peterson MJ, Stoner EJ, Wittenberger SJ. The synthesis of ketolide antibiotic ABT-773 (cethromycin). Tetrahedron. 2004;60(45):10171–10180. [Google Scholar]
- 27.Beebe X, Yang F, Bui MH, Mitten MJ, Ma Z, Nilius AM, Djuric SW. Synthesis and antibacterial activity of 6-O-arylpropargyl-9-oxime-11,12-carbamate ketolides. Bioorg. & Med. Chem. Lett. 2004;14(10):2417–2421. doi: 10.1016/j.bmcl.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Estiu G, Wiest O. HDAC1 Homology Model. Personal Communication. [Google Scholar]
- 29.Randolph o. T., Waid P, Nichols C, Sauer D, Haviv F, Diaz G, Bammert G, Besecke LM, Segreti JA, Mohning KM, Bush EN, Wegner CD, Greer J. Nonpeptide Luteinizing Hormone-Releasing Hormone Antagonists Derived from Erythromycin A: Design, Synthesis, and Biological Activity of Cladinose Replacement Analogues. J. Med. Chem. 2004;47(5):1085–1097. doi: 10.1021/jm030418i. [DOI] [PubMed] [Google Scholar]
- 30.Agouridas C, Denis A, Auger J-M, Benedetti Y, Bonnefoy A, Bretin F, Chantot J-F, Dussarat A, Fromentin C, D'Ambrières SG, Lachaud S, Laurin P, Martret OL, Loyau V, Tessot N. Synthesis and Antibacterial Activity of Ketolides (6-O-Methyl-3-oxoerythromycin Derivatives): A New Class of Antibacterials Highly Potent Against Macrolide-Resistant and - Susceptible Respiratory Pathogens. J. Med. Chem. 1998;41(21):4080–4100. doi: 10.1021/jm980240d. [DOI] [PubMed] [Google Scholar]
- 31.Denis A, Renou C. Novel N-demethylation of ketolide: application to the solution phase parallel synthesis of N-desosaminyl-substituted ketolides using ion exchange resins. Tetrahedron Lett. 2002;43(23):4171–4174. [Google Scholar]
- 32.HDAC Fluorimetric Assay/Drug Discovery Kit – AK-500 Manual. Fluorescent Assay System; ENZO Life Sciences International, I., Plymouth Meeting, PA.2009. [Google Scholar]
- 33.HDAC 8 Fluorimetric Drug Discovery Kit – AK-518 Manual; ENZO Life Sciences International, I., Plymouth Meeting, PA.2009. [Google Scholar]
- 34.HDAC 6 – SE-508 Manual; ENZO Life Sciences International, I., Plymouth Meeting, PA.2009. [Google Scholar]
- 35.Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, Jennings AJ, Luong C, Arvai A, Buggy JJ, Chi E, Tang J, Sang B-C, Verner E, Wynands R, Leahy EM, Dougan DR, Snell G, Navre M, Knuth MW, Swanson RV, McRee DE, Tari LW. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure. 2004;12:1325–1334. doi: 10.1016/j.str.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 36.KrennHrubec K, Marshall BL, Hedglin M, Verdin E, Ulrich SM. Design and evaluation of “linkerless” hydroxamic acids as selective HDAC8 inhibitors. Bioorg. & Med. Chem. Lett. 2007;17:2874–2878. doi: 10.1016/j.bmcl.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 37.Borra MT, Smith BC, Denu JM. Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 38.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific Activation of Sirtuins by Resveratrol. J. Biol. Chem. 2005;280(17):17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 39.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J. Biol. Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beher D, Wu J, Cumine S, Kim KW, Lu S-C, Atangan L, Wang M. Resveratrol is Not a Direct Activator of SIRT1 Enzyme Activity. Chem. Biol. & Drug Des. 2009;74(6):619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 41.Gurard-Levin ZA, Kim J, Mrksich M. Combining Mass Spectrometry and Peptide Arrays to Profile the Specificities of Histone Deacetylases. ChemBioChem. 2009;10(13):2159–2161. doi: 10.1002/cbic.200900417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mrksich M. Mass Spectrometry of Self-Assembled Monolayers: A New Tool for Molecular Surface Science. ACS Nano. 2008;2(1):7–18. doi: 10.1021/nn7004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurard-Levin ZA, Mrksich M. The Activity of HDAC8 Depends on Local and Distal Sequences of Its Peptide Substrates. Biochem. 2008;47(23):6242–6250. doi: 10.1021/bi800053v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Min D-H, Yeo W-S, Mrksich M. A Method for Connecting Solution-Phase Enzyme Activity Assays with Immobilized Format Analysis by Mass Spectrometry. Anal. Chem. 2004;76(14):3923–3929. doi: 10.1021/ac049816z. [DOI] [PubMed] [Google Scholar]
- 45.Chen PC, Patil V, Guerrant W, Green P, Oyelere AK. Synthesis and structure–activity relationship of histone deacetylase (HDAC) inhibitors with triazole-linked cap group. Bioorg. & Med. Chem. 2008;16(9):4839–4853. doi: 10.1016/j.bmc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 46.Patil V, Guerrant W, Chen PC, Gryder B, Benicewicz DB, I.Khan S, Tekwani BL, Oyelere AK. Antimalarial and antileishmanial activities of histone deacetylase inhibitors with triazole-linked cap group. Bioorg. & Med. Chem. 2010;18(1):415–425. doi: 10.1016/j.bmc.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews KT, Tran TN, Lucke AJ, Kahnberg P, Le GT, Boyle GM, Gardiner DL, Skinner-Adams TS, Fairlie DP. Potent Antimalarial Activity of Histone Deacetylase Inhibitor Analogues. Antimicrob. Agents Chemother. 2008;52:1454–1461. doi: 10.1128/AAC.00757-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agbor-Enoh S, Seudieu C, Davidson E, Dritschilo A, Jung M. Novel Inhibitor of Plasmodium Histone Deacetylase that Cures P. berghei-Infected Mice. Antimicrob. Agents Chemother. 2009;53:1727–1734. doi: 10.1128/AAC.00729-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dow GS, Chen Y, Andrews KT, Caridha D, Gerena L, Gettayacamin M, Johnson J, Melendez V, Obaldia N, III, Tran TN, Kozikowski AP. Antimalarial Activity of Phenylthiazolyly-Bearing Hydroxamate-Based Histone Deacetylase Inhibitors. Antimicrob. Agents Chemother. 2008;52:3467–3477. doi: 10.1128/AAC.00439-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerrant W, Mwakwari SC, Chen PC, Khan SI, Tekwani BL, Oyelere AK. A Structure Activity Relationship Study of the Antimalarial and Antileishmanial Activities of Non-peptide Macrocyclic Histone Deacetylase Inhibitors. ChemMedChem. 2010 doi: 10.1002/cmdc.201000087. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosnar M, Kelneric Z, Munic V, Erakovic V, Parnham MJ. Cellular Uptake and Efflux of Azithromycin, Erythromycin, Clarithromycin, Telithromycin, and Cethromycin. Antimicrob. Agents Chemother. 2005;49(6):2372–2377. doi: 10.1128/AAC.49.6.2372-2377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lugrin J, Ding XC, Le Roy D, Chanson A-L, Sweep FC, Calandra T, Roger T. Histone deacetylase inhibitors repress macrophage migration inhibitory factor (MIF) expression by targeting MIF gene transcription through a local chromatin deacetylation. Biochim. Biophys. Acta - Mol. Cell Res. 2009;1793(11):1749–1758. doi: 10.1016/j.bbamcr.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinriches DJ. Parasite Lactate Dehydrogenase as an Assay for Plasmodium falciparum Drug Sensitivity. Am. J. Trop. Med. Hyg. 1993;48(6):742–747. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 54.Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue®. Parasitol. Int. 2009;48(3):265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 55.Babich H, Borenfreund E. Cytotoxicity of T-2 toxin and its metabolites determined with the neutral red cell viability assay. Appl. Environ. Microbiol. 1991;57(7):2101–2103. doi: 10.1128/aem.57.7.2101-2103.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.