Abstract
The function of the lysosomal degradative pathway of autophagy in cellular injury is unclear as findings in nonhepatic cells have implicated autophagy as both a mediator of cell death and as a survival response. Autophagic function is impaired in steatotic and aged hepatocytes, suggesting that in these settings hepatocellular injury may be altered by the decrease in autophagy. To delineate the specific function of autophagy in the hepatocyte injury response, the effects of menadione-induced oxidative stress were examined in the RALA255-10G rat hepatocyte line when macroautophagy was inhibited by an shRNA-mediated knockdown of the autophagy gene atg5. Inhibition of macroautophagy sensitized cells to apoptotic and necrotic death from normally nontoxic concentrations of menadione. Inhibition of macroautophagy led to overactivation of the c-Jun N-terminal kinase (JNK)/c-Jun signaling pathway that induced cell death. Death occurred from activation of the mitochondrial death pathway with cellular ATP depletion, mitochondrial cytochrome c release and caspase activation. Sensitization to death from menadione occurred despite up regulation of other forms of autophagy in compensation for the loss of macroautophagy. Chaperone-mediated autophagy (CMA) also mediated resistance to menadione as CMA inhibition sensitized cells to death from menadione through a mechanism different from that of a loss of macroautophagy as death occurred in the absence of JNK/c-Jun overactivation or ATP depletion.
Conclusion
Hepatocyte resistance to injury from menadione-induced oxidative stress is mediated by distinct functions of both macroautophagy and CMA, indicating that impaired function of either form of autophagy may promote oxidant-induced liver injury.
Keywords: apoptosis, necrosis, menadione, mitochondria, c-Jun N-terminal kinase
Excessive production of reactive oxygen species (ROS) can overwhelm cellular antioxidant defenses and lead to oxidative stress that mediates cell injury and death. Oxidative stress has been implicated as a central mechanism of a variety of forms of hepatic injury including that caused by toxins,1 ischemia/reperfusion,2 viral infection3 and steatohepatitis.4,5 ROS may promote cellular injury through the direct oxidative modification of cellular macromolecules including proteins, lipids and DNA. Alternatively, recent investigations have emphasized that oxidant injury results from the direct activation of cell signaling pathways that regulate death. In the model of hepatocyte oxidative stress mediated by the superoxide generator menadione, apoptosis results from activation of the mitogen-activated protein kinase (MAPK) c-Jun N-terminal kinase (JNK) and its downstream effector c-Jun.6,7 Overactivation of the JNK/c-Jun pathway is central to many forms of hepatocyte death including chronic as well as acute oxidant stress.8–11 Although it is known that ROS can increase JNK signaling by activating upstream kinases or inactivating phosphatases, other as yet unknown mechanisms likely contribute to ROS-induced increases in JNK/c-Jun signaling.
Studies performed in nonhepatic cell types have demonstrated a relationship between the pathways of cell death and autophagy. Autophagy is a process in which cellular components are targeted to the lysosome for degradation.12 There are three types of mammalian autophagy which differ in their substrates and regulation.13 Macroautophagy sequesters whole organelles or portions of cytoplasm into autophagic vacuoles or autophagosomes that fuse with lysosomes and undergo degradation. In microautophagy the lysosomal membrane itself surrounds and sequesters portions of cytosol for degradation. In chaperone-mediated autophagy (CMA), proteins with a specific pentapeptide motif are selectively targeted for degradation by heat shock cognate chaperone of 70 kD and translocate to the lysosome for uptake by lysosome-associated membrane protein type 2A (LAMP-2A) and degradation. Autophagy has two principal functions: to remove unwanted or damaged cellular constituents and to supply the cell with substrates for energy generation in times of nutrient deprivation.14 The central role of autophagy in the elimination of damaged cellular components has suggested that it may be an important protective pathway in cellular injury, and oxidized proteins are one autophagic substrate.15 However, the relationship of autophagy to cell death is controversial. In nonhepatic cells macroautophagy has been implicated as a mechanism of cell death from a variety of stimuli including radiation and viruses.16,17 In contrast, other investigations have shown that macroautophagy mediates survival from injury induced by nutrient deprivation, ischemia/reperfusion injury and endoplasmic reticulum stress.18–20 Little is known about the function of autophagy specifically in hepatocyte injury and cell death. Autophagic function is known to be decreased in the liver under certain conditions such as steatosis and aging.21,22 Thus, if autophagy serves a protective function in hepatocytes, then these conditions that impair autophagic function may promote liver injury and resultant cell death.
To determine the function of autophagy in hepatocyte injury, the effects of a knockdown of the critical macroautophagy gene atg5 on menadione toxicity were examined in the nontransformed rat hepatocyte line RALA255-10G. Inhibition of macroautophagy sensitized these cells to apoptotic and necrotic death from normally nontoxic concentrations of menadione. Death was mediated by JNK/c-Jun overactivation that triggered the mitochondrial death pathway. Compensatory up regulation of other forms of autophagy failed to protect against cell death despite the ability of CMA to also mediate resistance to death from menadione. These findings demonstrate a critical and mutually exclusive function of both macroautophagy and CMA in hepatocyte resistance to death from oxidant stress.
Materials and Methods
This section is in the Supporting Materials.
Results
Inhibition of Macroautophagy Sensitizes RALA Hepatocytes to Death from Menadione
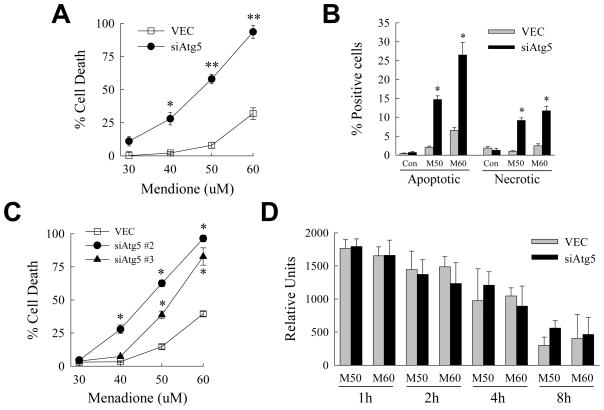
To determine whether autophagy regulates hepatocyte injury from oxidant stress, the effect of a genetic knockdown of the critical macroautophagy gene atg5 on cell death from menadione-induced oxidant stress was examined. Control VEC cells infected with lentiviral vector alone, and siAtg5 cells stably infected with a lentivirus expressing an shRNA to Atg5, were established as previously described.22 Cells were treated for 24 h with increasing concentrations of menadione. The inhibition of macroautophagy significantly increased cell death at all concentrations of menadione by MTT assay (Fig. 1A). Increased siAtg5 cell death was confirmed by quantification of the numbers of steady-state apoptotic and necrotic cells 12 h after menadione treatment by fluorescence microscopy of acridine orange/ethidium bromide costained cells. Inhibition of macroautophagy led to significantly greater numbers of apoptotic and necrotic cells with menadione treatment (Fig. 1B). Levels of apoptosis were greater than those of necrosis, and the numbers of necrotic cells may have been inflated by secondary necrosis of apoptotic cells, suggesting that the primary mechanism of cell death was apoptotic.
Fig. 1.
Inhibition of macroautophagy sensitizes to death from menadione. (A) VEC and siAtg5 cells were treated with the indicated menadione concentrations for 24 h and the percentage of cell death determined by MTT assay (*P<0.001 and **P<0.000001 as compared to VEC cells treated with the same menadione concentration; n=7). (B) VEC and siAtg5 cells were treated with 50 (M50) or 60 (M60) μM menadione for 12 h, costained with acridine orange and ethidium bromide, and the percentages of apoptotic and necrotic cells determined by fluorescence microscopy as described in Materials and Methods (*P<0.0001 as compared to the same treatment in VEC cells; n=6–7). (C) Percentage of cell death by MTT assay in VEC, siAtg5 #2 and siAtg5 #3 cells after 24 h of treatment with the indicated concentrations of menadione (*P<0.0001 as compared to the same treatment in VEC cells; n=6–8). (D) Relative levels of ROS in the two cell types at different times after treatment with 50 or 60 μM menadione (n=3–6).
To insure that findings in siAtg5 cells resulted from an inhibition of macroautophagy and not an shRNA off-target effect, menadione toxicity was examined in RALA hepatocytes expressing two other distinct Atg5 shRNAs. Cells expressing either of these two alternative Atg5 shRNAs were sensitized to death from menadione (Fig. 1C). Thus, macroautophagy was critical for RALA hepatocyte resistance to death from menadione-induced oxidant stress.
Knockdown of Atg5 does not Alter Levels of Oxidant Stress
Although the known function of Atg5 is restricted to the autophagic pathway, an explanation for the increased sensitivity of siAtg5 cells to menadione toxicity could be that the loss of this protein somehow amplified the level of oxidant stress induced by menadione. To exclude this possibility, ROS levels in response to menadione were measured in the two cell types by the lucigenin assay. No ROS were detected in untreated VEC or siAtg5 cells (data not shown). Levels of ROS were increased at 1–8 h after menadione treatment and were equivalent in the two cells types (Fig. 1D). No significant ROS production was found in either cell type at subsequent times. Therefore the effects of the Atg5 knockdown were not secondary to alterations in menadione metabolism or ROS production.
Inhibition of Macroautophagy Promotes Caspase-dependent Cell Death by Activation of the Mitochondrial Death Pathway
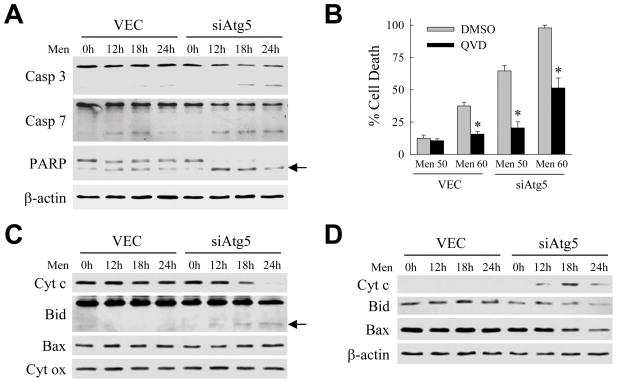
The finding that siAtg5 cell death from menadione occurred in part by apoptosis suggested that a loss of macroautophagy may sensitize these cells to caspase-dependent death. Western blot analysis of menadione-treated VEC and siAtg5 cells demonstrated decreased levels of procaspase 3 and 7 along with greater increases in the cleaved, active forms of these enzymes in siAtg5 cells (Fig. 2A). Menadione-treated siAtg5 cells had elevated caspase activity as demonstrated by loss of full-length poly-ADP ribose polymerase (PARP) and increased levels of the cleaved form of PARP with menadione treatment (Fig. 2A). Caspase activation mediated death in both VEC cells treated with toxic concentrations of menadione, and in siAtg5 cells treated with low or high menadione concentrations, as the caspase inhibitor Q-VD-OPh significantly inhibited cell death (Fig. 2B).
Fig. 2.
Cell death in menadione-treated siAtg5 cells is caspase dependent and associated with activation of the mitochondrial death pathway. (A) Protein was isolated from VEC and siAtg5 cells untreated or treated with 50 μM menadione for the indicated number of hours. Aliquots of protein were immunoblotted with antibodies for caspase 3 (Casp 3), caspase 7 (Casp 7), poly-ADP ribose polymerase (PARP) and β-actin as a loading control. Arrow indicates the cleaved form of PARP. (B) Percentage cell death in VEC and siAtg5 cells treated with 50 or 60 μM menadione after pretreatment with the vehicle dimethyl sulfoxide (DMSO) or Q-VD-OPh (QVD) (*P<0.0001 as compared to DMSO-treated cells; n=4). (C) Immunoblots of mitochondrial protein isolates from VEC and siAtg5 cells untreated or treated with 50 μM menadione for the indicated number of hours. Proteins were immunoblotted with antibodies for cytochrome c (Cyt c), Bid, Bax, and cytochrome oxidase (Cyt ox). tBid is indicated by the arrow. (D) Immunoblots of cytosolic protein isolates from the same cells probed with the indicated antibodies.
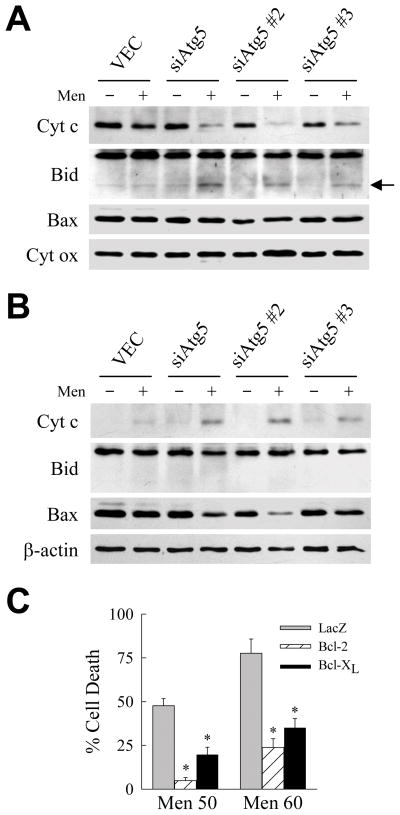
Caspase activation in hepatocytes occurs frequently through activation of the mitochondrial death pathway and release of the pro-apoptotic mitochondrial protein cytochrome c in a process regulated by Bcl-2 family members.23 Immunoblots of mitochondrial protein isolates demonstrated the loss of cytochrome c and the appearance of tBid, the truncated pro-apoptotic form of Bid, in menadione-treated siAtg5 but not VEC cells (Fig. 2C). Levels of the mitochondrial protein cytochrome oxidase, which is not released by mitochondrial death pathway activation, were unaffected by menadione in both cell types indicating equal sample loading (Fig. 2C). Cytochrome c was detected in the cytosol in parallel with the loss of this protein from mitochondria together with decreases in cytosolic levels of Bid and to a lesser extent Bax (Fig. 2D). Mitochondrial cytochrome c release and accumulation of tBid also occurred with menadione treatment of the cells expressing the two alternative Atg5 shRNAs (Fig. 3A and 3B). Finally, activation of the mitochondrial death pathway mediated cell death in menadione-treated siAtg5 cells as adenoviral overexpression of the anti-apoptotic factor Bcl-2 or Bcl-XL blocked cell death in comparison to cells infected with a control adenovirus Ad5LacZ expressing β-galactosidase (Fig. 3C).
Fig. 3.
Menadione toxicity in cells with an inhibition of macroautophagy is mediated by the mitochondrial death pathway. (A) Immunoblots of mitochondrial proteins isolated from VEC, siAtg5, siAtg5 #2 and siAtg5 #3 cells untreated or treated with 50 μM menadione for 12 h and probed for cytochrome c (Cyt c), Bid, Bax and cytochrome oxidase (Cyt ox). Arrow indicates tBid. (B) Cytosolic protein isolates from the same cells immunoblotted with the indicated antibodies. (C) Percentage cell death by MTT assay in siAtg5 cells infected with adenoviruses expressing β-galactosidase (LacZ), Bcl-2 or Bcl-XL and treated with 50 or 60 μM menadione for 24 h (*P<0.001 as compared to LacZ-infected cells; n=6–7).
Loss of Macroautophagy Promotes JNK/c-Jun Overactivation
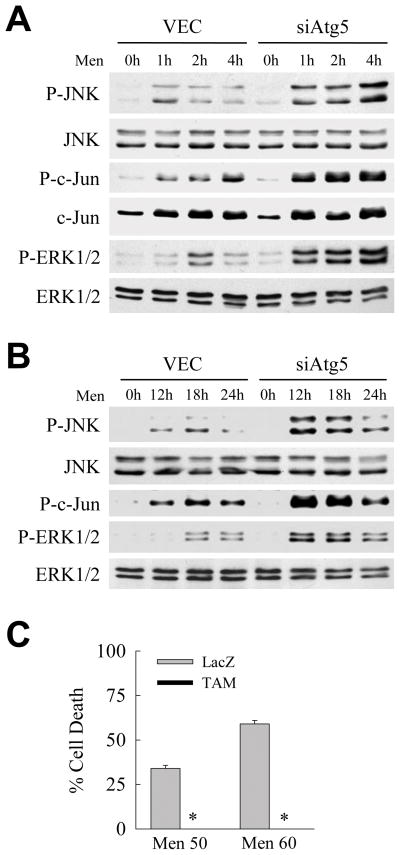
Previous studies have identified overactivation of the MAPK JNK as a mechanism of hepatocyte death from oxidant stress including that from menadione.6,24 To examine whether a loss of macroautophagy affected JNK signaling, levels of JNK activation were compared in the presence and absence of macroautophagy. The levels of JNK/c-Jun activation immediately after menadione treatment were increased in siAtg5 cells as indicated by increased levels of phospho-JNK and phospho-c-Jun at 1–4 h (Fig. 4A). These increased levels of JNK activation were maintained for greater than 18 h in siAtg5 cells (Fig. 4B). Similarly overactivation of the MAPK ERK1/2 also occurred in menadione-treated siAtg5 cells (Fig. 4A and 4B).
Fig. 4.
JNK/c-Jun overactivation occurs with menadione treatment in cells with an inhibition of macroautophagy. (A) Immunoblots of total protein from VEC and siAtg5 cells treated with 50 μM menadione for the times shown and probed for phospho-JNK (P-JNK), total JNK, phospho-c-Jun (P-c-Jun), total c-Jun, phospho-ERK1/2 (P-ERK1/2) and total ERK1/2. (B) Immunoblots of proteins from cells treated with menadione for 12–24 h. (C) Percentages of cell death in siAtg5 cells infected with Ad5LacZ (LacZ) or Ad5TAM (TAM) and treated with 50 or 60 μM menadione (*P<0.00001 when compared to Ad5LacZ-infected cells; n=7).
To determine whether the overactivation of JNK/c-Jun signaling that occurred with a knockdown of macroautophagy was mechanistically involved in cell death from menadione, the effects of blocking JNK/c-Jun signaling on menadione toxicity were examined. The JNK/c-Jun signaling pathway was blocked by adenoviral expression of the c-Jun dominant negative TAM,25 which has been shown previously to block death from menadione in wild-type cells.6 siAtg5 cells infected with Ad5LacZ were sensitive to death from menadione similar to uninfected siAtg5 cells (Fig. 4C). Cell death from menadione was completely suppressed in TAM-expressing cells (Fig. 4C). Loss of macroautophagy therefore sensitized cells to death from menadione through overactivation of JNK/c-Jun signaling.
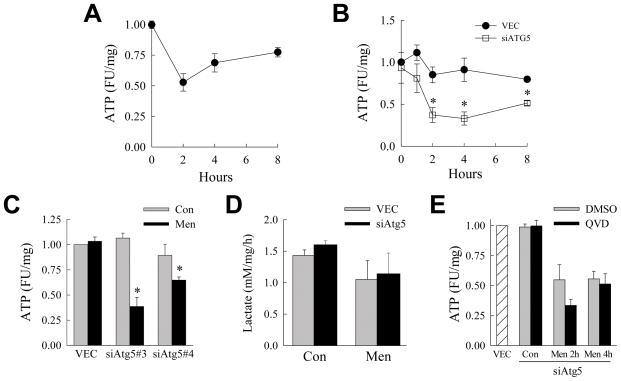
Menadione Induces ATP Depletion in siAtg5 Cells
Activation of the mitochondrial death pathway promotes organelle dysfunction that can lead to ATP depletion.26 Macroautophagy preserves cellular energy levels during times of stress.14 We therefore investigated the possibility that energy homeostasis was compromised in hepatocytes lacking macroautophagy and challenged with menadione. VEC cells treated with a toxic concentration of menadione underwent a marked reduction in ATP within 2 h of treatment (Fig. 5A), indicating that menadione toxicity was associated with an impairment in ATP generation. When VEC and siAtg5 cells were treated with the low concentration of menadione not toxic to VEC cells, ATP depletion occurred selectively in siAtg5 cells but not VEC cells (Fig. 5B). Significant ATP depletion also occurred with menadione treatment in cells expressing the two alternative Atg5 shRNAs (Fig. 5C). Thus, an inhibition of autophagy led to RALA hepatocyte ATP depletion from a level of oxidant stress that failed to affect the metabolic homeostasis of normal cells.
Fig. 5.
Inhibition of macroautophagy leads to ATP depletion in response to menadione. (A) Relative ATP levels in fluorescence units (FU) in VEC cells treated with 60 μM menadione for the indicated times (n=5–8). (B) ATP levels in VEC and siAtg5 cells treated with 50 μM menadione for the times shown (*P<0.01 as compared to VEC cells; n=3–4). (C) ATP levels in VEC, siAtg5 #2 and siAtg5 #3 cells untreated (Con) and after 2 h of 50 μM menadione (Men) (*P<0.03 as compared to untreated cells; n=4–6). (D) Rates of lactate production in VEC and siAtg5 cells untreated and treated with 50 μM menadione for 2 h (n=3). (E) Levels of ATP in untreated VEC cells and siAtg5 cells untreated (Con) or treated with 50 μM menadione for 2 or 4 h after pretreatment with dimethylsulfoxide (DMSO) or Q-VD-OPh (QVD) ( n=4).
An alternative possibility to a menadione-induced impairment in mitochondrial energy generation was that the inhibition of macroautophagy interfered with ATP production by the cytosolic glycolytic pathway. To examine for changes in glycolysis, levels of the glycolytic by product lactate were determined. Lactate levels were equivalent in untreated VEC and siAtg5 cells (Fig. 5D). There was a slight although not statistically significant decrease in lactate production over the 2 h after menadione treatment in both cell types (Fig. 5D). The level of glycolysis was therefore unaffected by the inhibition of macroautophagy. Consistent with the lack of any contribution of glycolysis to the ATP depletion that occurred in siAtg5 cells, supplementation of the cells with methyl pyruvate failed to reverse the ATP depletion that occurred in these cells with menadione treatment (data not shown).
The temporal relationship of ATP depletion to the other cell death events in menadione-treated siAtg5 cells was determined. The decrease in ATP was completely blocked by TAM expression in siAtg5 cells (data not shown), indicating that ATP depletion was downstream of JNK/c-Jun overactivation. Cytochrome c release did not occur until long after the time of ATP depletion (data not shown), and caspase inhibition with Q-VD-OPh that blocked cell death had no effect on menadione-induced ATP depletion (Fig. 5E). In the absence of macroautophagy, menadione therefore induced JNK/c-Jun overactivation that led to ATP depletion, cytochrome c release and effector caspase activation.
Oleate Blocks Death from Menadione in the Absence of Macroautophagy
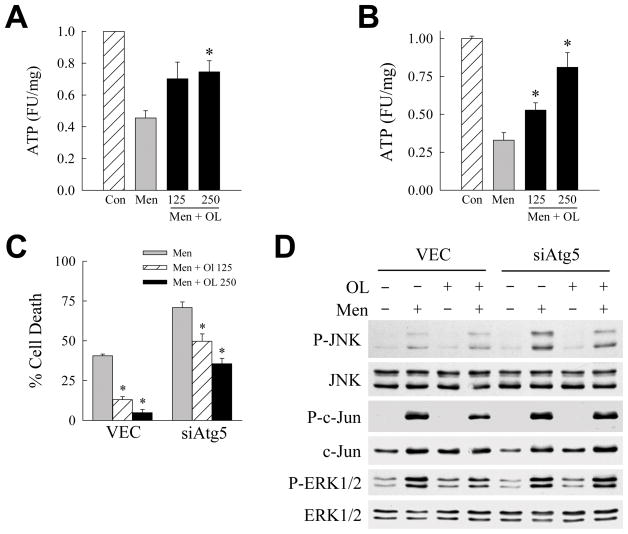
The finding that ATP depletion occurred upstream of caspase activation implied that the decrease in ATP was the mechanism of mitochondrial cytochrome c release and cell death. To determine whether the susceptibility of siAtg5 cells to ATP depletion was causal in their sensitization to death from menadione, the effects of energy repletion on cell death were determined. To replete cellular ATP, menadione-treated cells were cotreated with the fatty acid oleate which undergoes mitochondrial β-oxidation to generate ATP. Oleic acid cotreatment was effective in preventing ATP depletion that occurred in either VEC cells treated with high dose menadione (Fig. 6A), or siAtg5 cells treated with low dose menadione (Fig. 6B). Oleic acid had no effect on menadione-induced ROS production as determined by lucigenin assay (data not shown).
Fig. 6.
Oleate blocks death from menadione. (A) ATP levels in VEC cells untreated (Con) or treated with 60 μM menadione alone or in combination with 125 or 250 μM oleic acid (Men + OL) (*P<0.02 as compared to cells treated with menadione alone; n=4–6). (B) Levels of ATP in siAtg5 cells untreated (Con) or treated with 50 μM menadione alone or with oleic acid (*P<0.02 as compared to cells treated with menadione alone; n=3–5). (C) Percentage cell death in VEC and siAtg5 cells treated with menadione alone (Men; 60 μM for VEC cells and 50 μM for siAtg5 cells) or menadione plus 125 or 250 μM oleic acid (*P<0.003 as compared to cells treated with menadione alone; n=7). (D) Immunoblots of total protein from VEC and siAtg5 cells untreated or treated with 50 μM menadione (Men) and/or 0.25 μM oleic acid (OL) for 1 h and probed with the antibodies shown.
Oleate significantly inhibited cell death from menadione in control cells or cells sensitized to menadione toxicity from an inhibition of macroautophagy (Fig. 6C). Consistent with the protective effect of oleate occurring through the normalization of ATP levels, delay of oleate treatment until 2 h after menadione administration, the point at which maximal ATP depletion had already occurred, failed to block cell death (data not shown). In addition, the fatty acid palmitate which failed to reverse menadione-induced ATP depletion also did not prevent siAtg5 cell death from menadione (data not shown). Alternatively, these findings are consistent with the mechanism of oleate’s inhibition of cell death being a direct protective effect on the mitochondria which secondarily preserved ATP production. Oleate treatment failed to decrease JNK/c-Jun overactivation (Fig. 6D), consistent with previous data that activation of this MAPK pathway occurred upstream of ATP depletion. These findings together with those from the Bcl-2/Bcl-XL overexpression studies demonstrate that the inability of siAtg5 cells to maintain mitochondrial function with menadione challenge was the mechanism of their sensitization to death from oxidant stress.
Constitutive Autophagic Function Mediates Resistance to Menadione
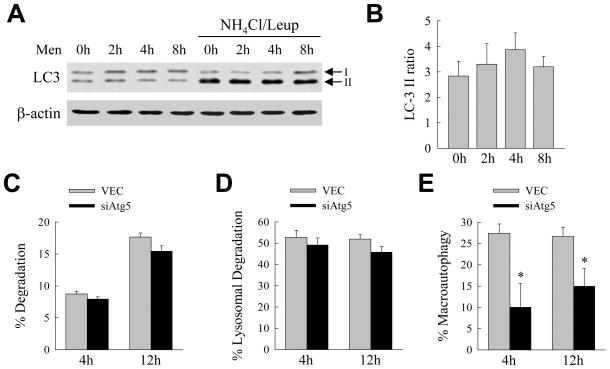
Cells have constitutive levels of autophagy that can be increased in response to stress, including that from oxidants.27 To determine whether hepatocellular resistance to oxidant stress depended on basal autophagic function, or the ability of RALA hepatocytes to increase autophagy in response to this challenge, levels of autophagy were determined by measure of autophagic flow.28 Autophagic flow was determined by the rate of accumulation of the autophagosome-associated form of LC3, LC3-II, with inhibition of lysosomal function by ammonium chloride and leupeptin. LC3-II levels were equivalent in untreated and menadione-treated cells in the absence and presence of the lysosomal inhibitors ammonium chloride and leupeptin by immunoblotting (Fig. 7A). The ratio of LC3-II in the presence of ammonium chloride/leupeptin to that in the absence of lysosomal inhibitors was unchanged with menadione treatment indicating that autophagic function was unaltered by menadione-induced oxidant stress (Fig. 7B). Basal levels of macroautophagy therefore mediated RALA hepatocyte resistance to menadione cytotoxicity.
Fig. 7.
Other forms of autophagy are up regulated in compensation for the loss of macroautophagy. (A) Immunoblots of proteins isolated from VEC cells untreated and treated with menadione for the times shown in the absence or presence of ammonium chloride/leupeptin (NH4Cl/Leup) and probed for LC3 and β-actin. Ammonium chloride/leupeptin was added to the cells 2 h prior to isolation. The LC3-I and LC3-II bands are indicated by arrows. (B) Ratio of the signal intensity of the LC3-II bands in the presence of inhibitors to that in the absence of inhibitors as determined by densitometric scanning of 3 immunoblots. (C) Percentage of protein degradation in VEC and siAtg5 cells at the indicated times as measured by metabolic pulse-chase labeling (n=4). (D) Percentage of lysosomal degradation in the same cells as determined by the amount of protein degradation that was inhibited by ammonium chloride/leupeptin (n=4) (E) Percentage of lysosomal degradation that was mediated by macroautophagy which was the amount inhibited by 3-methyladenine (*P<0.05 when compared to VEC cells; n=4).
Compensatory Up Regulation of other Forms of Autophagy Occurs in siAtg5 Cells
The findings of increased death from menadione in RALA hepatocytes with a knockdown of Atg5 are in contrast to those we have previously reported in murine embryonic fibroblasts (MEFs).29 MEFs from atg5 null mice were sensitized to death receptor-mediated apoptosis but had increased resistance to menadione toxicity.29 This protection against death from menadione was mediated by the up regulation of a second form autophagy, chaperone-mediated autophagy (CMA), that occurred in atg5−/− MEFs in compensation for the loss of macroautophagy.29 These prior studies suggested that findings of increased death from menadione in RALA hepatocytes may have reflected an inability of these cells to compensate for the loss of macroautophagy with an increase in CMA. To examine this possibility, levels of lysosomal protein degradation were determined in cells with an Atg5 knockdown.
By pulse-chase metabolic labeling the rate of total protein degradation was equivalent in VEC and siAtg5 cells at 4 and 12 h (Fig. 7C). The proportion of protein degradation that was inhibited by ammonium chloride/leupeptin and therefore lysosome dependent was also equivalent in the two cell types (Fig. 7D). The amount of lysosomal degradation secondary to macroautophagy was estimated as the fraction that was blocked by the pharmacological inhibitor 3-methyladenine. As expected, levels of macroautophagy were significantly decreased in siAtg5 cells (Fig. 7E). Maintenance of levels of total lysosomal protein degradation in siAtg5 cells despite the decrease in macroautophagy indicated that other forms of autophagy were up regulated in these cells. Thus, similar to findings in atg5−/− MEFs, siAtg5 cells increased other forms of autophagy in response to the loss of macroautophagy.
CMA Regulates RALA Hepatocyte Resistance to Death from Menadione
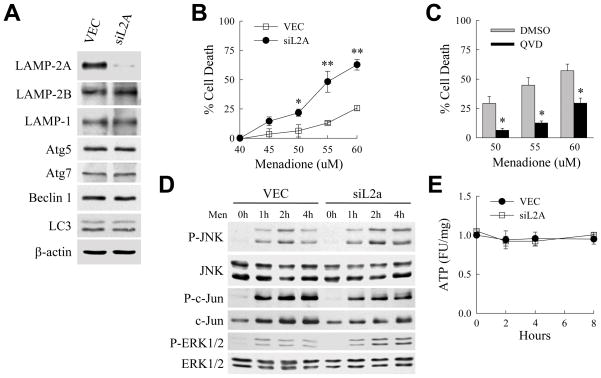
The sensitization of siAtg5 cells to death from menadione despite a compensatory increase in other forms of lysosomal degradation, suggested that these alternative forms of autophagy may not be protective against menadione-induced oxidant stress in RALA hepatocytes. To exclude this possibility, the effect of a loss of CMA on cell death from menadione was examined. siL2A cells with a stable knockdown of the critical CMA receptor LAMP-2A were generated. The knockdown was specific for LAMP-2A as levels of other LAMPs, mediators of macroautophagy and LC3 were unchanged (Fig. 8A). Menadione treatment of siL2A cells revealed that the loss of CMA sensitized cells to death from menadione similar to the effect of an inhibition of macroautophagy (Fig. 8B).
Fig. 8.
Inhibition of CMA sensitizes cells to death from menadione. (A) Total protein from VEC and siL2A cells immunoblotted with the indicated antibodies. (B) Percentage cell death by MTT assay in VEC and siL2A cells treated with the indicated concentrations of menadione for 24 h (*P<0.04 and **P<0.01 as compared to VEC cells treated with the same menadione concentration; n=4). (C) Percentage cell death in siL2A cells treated with dimethyl sulfoxide (DMSO) vehicle or Q-VD-OPh (QVD) and menadione at the concentrations shown (*P<0.003 as compared to DMSO-treated cells; n=6). (D) Immunoblots of total protein isolated from VEC and siAtg5 cells untreated or treated with 50 μM menadione for the indicated times. (E) ATP levels in VEC and siL2A cells after treatment with 50 μM menadione (n=3).
To determine whether the loss of CMA sensitized RALA hepatocytes to death by a mechanism different from that which mediated death from an inhibition of macroautophagy, siL2A cell death from menadione was examined for caspase dependence. Q-VD-OPh significantly decreased cell death in siL2A cells treated with menadione (Fig. 8C), indicating that cell death from either an inhibition of macroautophagy or CMA occurred predominantly through caspase-dependent apoptosis. However, in contrast to the overactivation of JNK/c-Jun signaling that occurred with menadione treatment in cells with an inhibition of macroautophagy, siL2a cells were sensitized to death in the absence of any greater increase in JNK signaling (Fig. 8D). Levels of phospho-c-Jun were in fact somewhat decreased in siL2A cells after menadione treatment and levels of phospho-ERK1/2 were equivalent to those in VEC cells (Fig. 8D). In the absence of JNK/c-Jun overactivation, siL2A cells maintained normal levels of ATP in response to menadione treatment (Fig. 8E), in contrast to the ATP depletion that occurred in siAtg5 cells. Thus, similar to macroautophagy CMA mediated resistance to caspase-dependent apoptosis from menadione-induced oxidant stress, but through a distinct mechanism that did not involve the maintenance of energy homeostasis.
Discussion
The relationship between the pathways of cell death and autophagy remain controversial. Experimental evidence has suggested that autophagy may mediate cell death, and autophagy has been classified as one of three morphological forms of cell death together with apoptosis and necrosis.30 However, a number of issues have been raised about the validity of the studies that led to this conclusion,30 and other investigations have suggested an alternative function for macroautophagy as a pathway critical for cell survival.18,19,29 The present study is one of the first to specifically examine this question in hepatocytes, and demonstrates that macroautophagy is essential for RALA hepatocyte resistance to cell death from menadione-induced oxidative stress. Oxidant-induced death in cells lacking macroautophagy was predominantly caspase-dependent apoptosis. Caspase activation was triggered by the mitochondrial death pathway as apoptosis was associated with ATP depletion and mitochondrial cytochrome c release, and blocked by oleate which normalized ATP levels or Bcl-2/Bcl-XL overexpression.
In contrast to the concept that oxidant stress induces autophagy, the level of autophagic flow was not increased in RALA hepatocytes after menadione treatment, identical to our previous results in MEFs.29 It was possible that autophagy was already maximized in these cells secondary to culture in serum-free medium. However, the cells received fresh medium containing amino acids and glucose to maintain normal levels of macroautophagy and autophagic flow remained similar to serum-supplemented cells (data not shown). The ability to augment autophagic function may have been restricted in part by the decrease in ATP that occurred from menadione treatment as macroautophagy requires ATP.31 These findings give further support to the concept that constitutive as well as induced levels of macroautophagy regulate cellular physiology. Basal levels of macroautophagy are critical for RALA hepatocyte resistance to oxidative stress, consistent with autophagy being a survival pathway in these cells.
The increased sensitivity of RALA hepatocytes with an inhibition of macroautophagy to death from menadione-mediated oxidant stress contrasts with our previous findings in MEFs with a genetic knockout of Atg5.29 MEFs from atg5 null mice were sensitized to death from tumor necrosis factor and Fas but protected from menadione toxicity.29 The resistance of atg5 null MEFs to menadione-induced death was secondary to increased CMA that compensated for the loss of macroautophagy.29 Crosstalk among the different autophagic pathways is known to result in the up regulation of one type of autophagy when another form is inhibited, and atg5 null MEFs have increased levels of CMA.32 A compensatory response occurred in RALA hepatocytes with an inhibition of macroautophagy as demonstrated by the maintenance of normal levels of lysosomal protein degradation in siAtg5 cells despite the decrease in degradation mediated through macroautophagy. Thus, crosstalk among autophagic pathways also occurred in RALA hepatocytes, and the sensitization of these cells to death from menadione did not result from a failure to up regulate other forms of autophagy.
CMA also functioned to protect RALA hepatocytes from menadione cytotoxicity as a knockdown of the CMA receptor LAMP-2A sensitized cells to death from this oxidant stress. This finding together with that of the compensatory up regulation of forms of autophagy other than macroautophagy in siAtg5 cells suggested that these cells should be resistant to menadione toxicity similar to atg5 null MEFs. The fact that siAtg5 cells were sensitized to death from menadione indicates that macroautophagy and CMA have nonredundant functions in hepatocyte resistance to oxidant stress such that the increase in CMA could not substitute for the loss of macroautophagy in these cells. Thus, the function of autophagy in resistance to oxidant stress differed in an important way in RALA hepatocytes from that in MEFs. Macroautophagy functioned to limit JNK/c-Jun signaling and maintain energy homeostasis as RALA hepatocytes lacking macroautophagy developed ATP depletion from menadione treatment that led to cell death. In contrast, sensitization to menadione-induced death by the loss of CMA occurred despite normal levels of JNK activation and cellular ATP. These mechanistic differences in the protective effects of macroautophagy and CMA during hepatocyte oxidative stress are consistent with the specific functions of these two pathways. Macroautophagy mediates the bulk degradation of whole organelles including damaged mitochondria,33 and may therefore have the protective function of limiting activation of the apoptotic pathway by removing such mitochondria. In contrast, CMA removes selective proteins including oxidized proteins which may promote cellular injury downstream of JNK activation and ATP depletion.15 Whatever the mechanism, the present findings demonstrate that the two forms of autophagy have disparate functions in RALA hepatocytes, and therefore the up regulation of one form cannot compensate for the loss of the other. The difference in the responses of hepatocytes and MEFs with an inhibition of macroautophagy to menadione challenge emphasizes the importance of delineating cell type specific functions of the various forms of autophagy.
JNK activation has a central role in many forms of hepatocellular injury including that from menadione-induced oxidant stress.6,24 Death in cells with a loss of macroautophagy resulted in JNK/c-Jun overactivation that initiated the sequence of ATP depletion, cytochrome c release, caspase cleavage and cell death. Although the inverse relationship of JNK regulating autophagic function has been previously described in nonhepatic cells,34 the present studies are the first to indicate that macroautophagy functions to down regulate JNK activation. The mechanism by which macroautophagy regulates JNK signaling remains to be determined but was not specific for this kinase as increased activation of the MAPK ERK1/2 also occurred in the absence of macroautophagy. One possibility is that the sequestration of mitochondria after early injury from menadione may act to limit MAPK activation. The findings do demonstrate a novel relationship between JNK/c-Jun signaling and cellular energy homeostasis that is mediated by macroautophagy. This effect was specific for macroautophagy as a knockdown of CMA failed to affect JNK signaling. CMA was nonetheless still protective against menadione toxicity through another mechanism.
The findings suggest that pathophysiological conditions associated with an impairment of hepatic macroautophagic function may promote liver injury from oxidative stress. Recently we have demonstrated that increased lipid accumulation in hepatocytes leads to an impairment in macroautophagy.22 Oxidative stress has been implicated as a causal factor in the development of nonalcoholic fatty liver disease, as both human and experimental NAFLD are associated with oxidative stress and the accumulation of lipid peroxidation products.5,35,36 Steatosis may therefore not only provide a direct substrate that promotes oxidative stress, but also indirectly promote oxidant injury by decreasing autophagic function. Another setting of compromised autophagic function is in aging. Decreased macroautophagy and CMA are physiological consequences of aging.21 This impairment in autophagy may act to sensitize aged individuals to hepatocyte injury from sources of hepatic oxidant stress such as toxins or drugs. Investigations of individual variations in autophagic function may therefore offer new insights into the susceptibility to liver diseases mediated by oxidant stress.
Supplementary Material
Acknowledgments
Supported in part by NIH grants DK044234, DK061498 and AG031782.
We thank Dr. David Brenner for providing the adenoviruses, Dr. Xiao-Ming Yin for the Bid antibody and Dr. Ana Maria Cuervo for the LAMP-2A and LAMP-2B antibodies and her helpful discussions.
Abbreviations
- CMA
chaperone-mediated autophagy
- ERK1/2
extracellular signal-regulated kinase 1/2
- JNK
c-Jun N-terminal kinase
- LAMP
lysosome-associated membrane protein
- LC3
microtubule-associated protein light chain 3
- MAPK
mitogen-activated protein kinase
- MEF
murine embryonic fibroblast
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PARP
poly (ADP-ribose) polymerase
- Q-VD-OPh
Q-Val-Asp-OPh
- ROS
reactive oxygen species
Contributor Information
Yongjun Wang, Email: yongjun.wang@einstein.yu.edu.
Rajat Singh, Email: rajat.singh@einstein.yu.edu.
Youqing Xiang, Email: xiangyq@hotmail.com.
References
- 1.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 2.Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology. 2001;33:902–914. doi: 10.1053/jhep.2001.23073. [DOI] [PubMed] [Google Scholar]
- 3.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 4.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 6.Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology. 2003;37:1405–1413. doi: 10.1053/jhep.2003.50233. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem. 2004;279:31089–31097. doi: 10.1074/jbc.M404170200. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Lo CR, Czaja MJ. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 9.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 10.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 11.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFα- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 12.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 13.Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- 14.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22:830–844. doi: 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 18.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 20.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 21.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conde dlR, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, et al. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918–929. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Bradham CA, Hatano E, Brenner DA. Dominant-negative TAK1 induces c-Myc and G0 exit in liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1279–G1289. doi: 10.1152/ajpgi.2001.281.5.G1279. [DOI] [PubMed] [Google Scholar]
- 26.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol. 2007;22:S31–S37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaushik S, Cuervo AM. Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med. 2006;27:444–454. doi: 10.1016/j.mam.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Singh R, Massey AC, Kane SS, Kaushik S, Grant T, et al. Loss of macroautophagy promotes or prevents fibroblast apoptosis depending on the death stimulus. J Biol Chem. 2008;283:4766–4777. doi: 10.1074/jbc.M706666200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plomp PJ, Gordon PB, Meijer AJ, Hoyvik H, Seglen PO. Energy dependence of different steps in the autophagic-lysosomal pathway. J Biol Chem. 1989;264:6699–6704. [PubMed] [Google Scholar]
- 32.Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19:2179–2192. doi: 10.1091/mbc.E07-11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: Impact on disease progression. Hepatology. 2006;43:506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 36.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.