Abstract
Inhaled glucocorticoid (GC) therapy is a vital part of the management of chronic asthma. GCs are metabolized by members of the cytochrome P450 3A family in both liver and lung, but the enzymes are differentially expressed. Selective inhibition of one or more P450 3A enzymes could substantially modify target and systemic concentrations of GCs. In this study, we have evaluated the mechanism-based inactivation of P450 3A4, 3A5 and 3A7 enzymes by GCs. Among the five major inhaled GCs approved for clinical use in the United States, fluticasone propionate (FLT) was the most potent mechanism-based inactivator of P450 3A5, the predominant P450 enzyme in the lung. FLT inactivated P450 3A5 in a time- and concentration-dependent manner with KI, kinact and partition ratio of 16 μM, 0.027 min-1 and 3, respectively. In contrast, FLT minimally inactivated P450 3A4 and did not inactivate 3A7, even with a concentration of 100 μM. The inactivation of P450 3A5 by FLT was irreversible because dialysis did not restore enzyme activity. In addition, the exogenous nucleophilic scavenger GSH did not attenuate inactivation. The prosthetic heme of P450 3A5 was not modified by FLT. The loss of P450 3A5 activity in lung cells could substantially decrease the metabolism of FLT, which would increase the effective FLT concentration at its target site, the respiratory epithelium. Also, inactivation of lung P450 3A5 could increase the absorption of inhaled FLT, which could lead to high systemic concentrations and adverse effects, such as life-threatening adrenal crises or cataracts that have been documented in children receiving high doses of inhaled GCs.
Introduction
Asthma is a chronic disease of the airways characterized by bronchoconstriction hyper-reactivity, inflammation, increased mucus production, and intermittent airway obstruction. Respiratory infections, allergens, air pollutants, temperature changes, stress, and exercise can trigger breathing difficulties with asthma, such as coughing, wheezing and shortness of breath. The mainstay of asthma management is inhaled glucocorticoid (GC)1 therapy, which directly targets inflammation in relevant airway epithelial cells. Beclomethasone dipropionate, budesonide, flunisolide, fluticasone propionate (FLT) and triamcinolone acetonide are widely used therapeutic agents for asthma in the United States (1). These drugs work via the GC receptor to regulate gene expression leading to decreased inflammation and airway mucus production (2, 3).
Although inhaled GC therapy is a vital part of the management of persistent asthma, approximately 30% of all asthmatics have some degree of steroid insensitivity or resistance (3). Insensitivity refers to a poor response to GCs when given in normal, age-appropriate, anti-inflammatory doses, while resistance implies a complete inability to respond to GCs at any dose or at any normal time interval (3). The mechanisms of these responses are not well understood.
In the human body, GCs are metabolized by members of the cytochrome P450 3A family in both liver and lung, where they are known to be differentially expressed (4). Aerosolized drug deposited in the lungs is likely to be metabolized in specific bronchial, bronchiolar, and alveolar epithelial cells, because these cells express significant amounts of P450 3A transcripts and proteins (4, 5). The main pulmonary P450 3A form in human is P450 3A5, whereas P450 3A4 is usually not found in respiratory tissues (6-10). Thus, selective inhibition of one or more P450 3A enzymes could substantially modify airway and systemic concentrations of GCs. CYP3A5 is a polymorphic gene (11, 12), and the CYP3A5*3 allele produces a non-functional truncated protein. This genotype is found in about 50% of African-Americans and greater than 80% of Caucasians. Thus, less than half of a diverse American population would be expected to have one functional CYP3A5*1 gene, and therefore exhibit any CYP3A5 enzyme activity in their liver or lung tissues.
In general, P450-mediated drug metabolism results in a formation of more polar and pharmacologically inactive metabolites. Metabolism can produce reactive electrophiles that can covalently bind to intracellular macromolecules, potentially causing drug toxicity. Reactive metabolites formed in the active site of P450s may covalently bind to the enzyme itself, causing mechanism-based inactivation (13). This type of inactivation can lead to serious pharmacokinetic variations for drugs and promote potentially dangerous drug-drug interactions (14) because of the irreversibility of the inactivation process and reduced inactivation and clearance of drugs.
While higher doses of inhaled GCs usually improve airway responses, they may produce dose-related systemic adverse effects, such as adrenal suppression, decreased bone mineral density, cataracts, and growth suppression (15-17). The safety of high doses, particularly those of FLT, has been questioned by several reports of life threatening acute adrenal crisis in children (16, 18, 19). Systemic bioavailability of inhaled GCs predominantly arises from absorption from the lung. Although the greater part (about 80%) of an inhaled dose will be deposited in the oropharynx, swallowed, and absorbed from the gastrointestinal tract, it is predominantly inactivated via first-pass hepatic metabolism including metabolism by P450 3A4 and 3A5. However, a small portion (∼20%) can be absorbed and/or metabolized in respiratory tissues with high absorption capacity (20, 21). If metabolism occurs during respiratory absorption, changes in metabolic enzymes could alter local concentrations of active drug or systemic concentrations in some cases.
Singh et al. (22) reported that the mean systemic bioavailability of a single 1000 μg inhaled dose of FLT was 21.2% (14.3-31.4%) in healthy adult volunteers and 13.3% (8.5-21.9%) in the adult patients with chronic obstructive pulmonary disease. Cmax values in diseased individuals (1,961 pg ml-1 h-1) were half of the maximum concentration in healthy patients (2,996 pg ml-1 h-1). Bioavailability after administration by the iv route was not altered by the disease. Since high first-pass, hepatic metabolism of FLT by P450 3A4 makes oral absorption of active drug into the systemic circulation negligible (<1%), systemic exposure of an inhaled dose of FLT probably occurs through lung absorption (23, 24). Thus, if respiratory absorption is the primary route controlling systemic concentrations, changes in metabolism within lung cells could substantially alter the systemic effects of FLT.
A thorough examination of the mechanism-based inactivation of CYP3A4, CYP3A5, and CYP3A7 by GCs has not been reported. Thus, the aim of this study was to evaluate mechanism-based inactivation of the three major P450 3A enzymes by inhaled GCs that are widely used in the United States. We have demonstrated that FLT is a potent mechanism-based inactivator of P450 3A5, the predominant P450 3A enzyme in the lung. The potential relevance of these findings to treatment of asthma and possible toxicities are discussed.
Experimental Procedures
Materials
FLT, beclomethasone dipropionate, budesonide, flunisolide, triamcinolone acetonide, testosterone, 6β-hydroxytestosterone and 11β-hydroxytestosterone, NADPH, glutathione (GSH) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Other reagents and solvents used were of the highest grade available. Microsomal preparations from baculovirus-infected insect cells (Supersomes) expressing human P450 enzymes (3A4, 3A5, and 3A7) were obtained from BD Gentest (Woburn, MA). The Supersomes were coexpressed with human NADPH-cytochrome P450 reductase and human cytochrome b5. at presumably optimum ratios, but recombinant CYP3A enzymes with varying ratios of P450 reductase would be required to determine if enzyme inactivation rates would be altered. The molar ratios of recombinant P450, P450 reductase, and cytochrome b5 might not be optimal for maximal turnover in these microsomal preparations. However, addition of extra recombinant reductases to determine optimal ratios would not necessarily mimic the more “normal” in vivo enzyme system.
Single Time- -Dependent Inactivation of P450 3A4, 3A5 and 3A7 by Five GCs
Primary incubations included five GCs (0 or 100 μM), 2 mM NADPH, P450 3A enzymes (500 pmol/mL) and 100 mM potassium phosphate buffer (pH 7.4). GCs were added to the incubation mixture in DMSO at a final concentration of ≤ 2%. The primary mixture was incubated in a 37 °C water bath for 0 and 30 min. At each preincubation time point, aliquots (10 μL) of the incubation mixtures containing 5 pmol of P450 3A4 were transferred to a secondary incubation to a final volume of 100 μL, which included 200 μM testosterone, 2 mM NADPH, and 100 μM potassium phosphate (pH 7.4). The secondary mixture was incubated in a 37 °C shaking water bath for 10 min (P450 3A4), 15 min (3A5) and 40 min (3A7) and stopped by the addition of 100 μL of ice-cold acetonitrile containing 20 μM of 11β-hydroxytestosterone as an internal standard. The supernatants obtained after centrifugation (20000 ×g for 3 min) were concentrated using a centrifugal evaporator and then reconstituted with 20% acetonitrile. High performance liquid chromatography (HPLC) analysis was performed on an Agilent 1100 system (Agilent Technologies, Inc., Palo Alto, CA). 6β-Hydroxytestosterone formation was quantified by separation with a Phenomenex Luna C18-2 (4.6 × 150 mm, 5 μm particle; Torrance, CA) reverse-phase column at a flow rate of 1 mL/min using a mobile phase of acetonitrile (solvent A) and 1 mM ammonium acetate (solvent B). Gradient elution was performed using the following program: 0 min, 20% A; 15 min, 55% A; 16 min, 100% A; 19 min, 100% A; 20 min, 20% A; 32 min, 20% A. The eluate from the HPLC column was monitored by UV absorption at 254 nm to determine the ratio of 6β-hydroxytestosterone to 11β-hydroxytestosterone in each sample. The incubations and analyses were performed three times for each sample. The effect of each GC on the inactivation of each P450 3A enzyme was evaluated by comparing the loss of testosterone 6β-hydroxylation activity during the 30-min preincubation time period.
Time- and Concentration-Dependent Inactivation of P450 3A4 and 3A5 by FLT
Primary incubations included FLT (0, 10, 20, 50, 100 μM for P450 3A4, and 0, 1, 2, 5, 10 μM for P450 3A5), 2 mM NADPH, P450 3A4 or 3A5 enzymes (500 pmol/mL) and 100 mM potassium phosphate buffer (pH 7.4). The primary mixture was incubated in a 37 °C water bath for 0, 10, 20 and 30 min. The other procedures were the same as that of the previous section.
Partition Ratio
The partition ratio was estimated using the titration method (25). Primary reaction mixtures for P450 3A5 were prepared as described above, with the exception that increasing concentrations of the inactivator, FLT (0, 0.5, 1, 2, 3, 5, 10, 15, 20, 25 μM), were added to the primary mixture, until complete inactivation was obtained. The primary incubations were conducted for 30 min. The rest of the assay procedure was identical to that described for the inactivation studies. The partition ratio was obtained by plotting the percent activity remaining versus [FLT]/[P450 3A5]. The partition coefficient was then extrapolated from the intercept between the linear regression line obtained at low concentrations of FLT and the straight line obtained at saturating FLT concentrations.
Protective Effect of GSH on Inactivation and Irreversibility of Inactivation
Separate incubations in the presence or absence of FLT (20 μM) with or without GSH (4 mM) were performed for 30 min and assayed for residual activity as described above to evaluate if GSH attenuated inactivation of P450 3A5 enzyme. The remaining portion without GSH addition was dialyzed against 100 mM potassium phosphate buffer (pH 7.4) containing 10% glycerol at 4°C for 4 h using Slide-A-Lyzer Mini dialysis units with a molecular weight cut-off of 3500 (Pierce Chemical, Rockford, IL). The dialysis buffer was changed every hour. The dialyzed samples were then assayed for testosterone 6β-hydroxylation activity.
Heme Adduct Analysis
Analysis for FLT adducts to the prosthetic heme was accomplished after incubating FLT (100 μM) with 50 pmol of recombinant P450 3A5 in 100 mM potassium phosphate (pH 7.4), at 37 °C for 30 min. Incubations (in triplicate) were initiated by addition of 2 mM NADPH in a final volume of 50 μL. Incubations without NADPH served as controls. 1-Aminobenzotriazole was used as a positive control for heme destruction during oxidation (26). The reactions were stopped by placing the incubations on ice and adding 10 μL of 0.3% aqueous trifluoroacetic acid (TFA). The incubation mixtures were directly injected onto a Jupiter C4 column (5 mL particles, 2.0 × 150 mm; Phenomenex Inc.) coupled with an Agilent 1100 HPLC system. The mobile phase consisted of 0.05% TFA in acetonitrile (solvent A) and 0.05% TFA in water (solvent B) with the gradient program that was set as 0 min, 30% A; 30 min, 80% A. The flow rate was 0.2 mL/min. Heme was monitored by absorption at 405 nm.
Analysis of Alkylated Peptides from Inactivated P450 3A5 Digests
A potential FLT adduct to P450 3A5 apoprotein was analyzed with the following procedure. One hundred pmol of recombinant P450 3A5 was incubated with 100 μM FLT and 2 mM NADPH 100 mM potassium phosphate (pH 7.4) at 37 °C for 60 min. The reactions were initiated by addition of 2 mM NADPH in a final volume of 500 μL. Incubations without FLT were the controls. After incubation, protein was precipitated by centrifugation. Protein precipitate was collected by the addition of 0.5 mL acetonitrile to the residual supernatant and by centrifugation. The pooled protein precipitate was solubilized with NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA) and heated in boiling water for ∼10 min. The proteins were separated by SDS-PAGE using precast NuPAGE 4-12% Bis-Tris Gels (1.0 mm thickness, Invitrogen). The proteins were separated by electrophoresis overnight at 200 V and 120 mA for 50 min, after which the proteins were stained with 0.25 % (w/v) Coomassie brilliant blue R-250 in a mixture of 45 % methanol and 9 % acetic acid in water for about 45 min and destained with 7% methanol/7% acetic acid in water. The Coomassie-stained bands corresponding to P450 3A5 protein (∼50 kDa) were excised, minced, and digested in-gel with trypsin or trypsin/chymotrypsin (Pierce Chemical, Rockford, IL). Briefly, the excised gel pieces were destained with 25 mM ammonium bicarbonate containing 50% acetonitrile. The proteins were reduced with 50 mM tris[2-carboxyethyl]phosphine (10 min, 60 °C) followed by alkylation of free sulfhydryl groups with 100 mM iodoacetoamide (60 min, room temperature, in the dark). The proteins were also analyzed without the reduction and alkylation steps. After washing, the gel pieces were dehydrated with acetonitrile, air-dried and rehydrated in 10-20 μL of 25 mM ammonium acetate containing 10 ng of trypsin/μL or 10-20 μL of 5 ng/μL of trypsin + 5 ng/μL of chymotrypsin and 2 mM calcium chloride. The digestion was performed at 37 °C overnight. The digested peptides were extracted from the gel with acetonitrile in water containing 0.1% formic acid, and concentrated by centrifugal evaporation. The peptide mixtures were then analyzed using a liquid chromatography-mass spectrometry (LC-MS) system. The peptides were separated on a Jupiter Proteo 90 A column (4 mm particles, 0.5 × 150 mm; Phenomenex Inc.). The mobile phase consisted of 0.1% formic acid in acetonitrile (solvent A) and 0.1% formic acid in water (solvent B) with the gradient program that was set as 0 min, 5% C; 52.5 min, 50% A; 71.0 min, 95% A; 75.9 min, 95% A; 76.0 min, 5% A; 90.0min, 5% A. Using a flow splitter, the flow from the pump (0.25 mL/min) was adjusted to a flow rate of approximately 15-20 μL/min. Electrospray ionization (ESI) mass spectra were recorded with a LCQ Advantage Mass Spectrometer (ThermoFischer Scientific) in a positive ion mode. The heated capillary temperature was set at 250 °C, the spray voltage was set at 2.5 kV, and the capillary voltage was 22 V. Full scan ESI mass spectra (m/z 400-1600) and data dependent MS/MS spectra for the top three most intense ions were recorded. Dynamic exclusion was used to maximize the detection of peptides. The LC-MS data were analyzed using SEQUEST and P-Mod (version 2) software (27) to identify peptides and potentially modified peptides by comparing the observed spectra to theoretical spectra for P450 3A5. Monoisotopic precursor and fragment ions with a mass tolerance of 2 amu, less than two missed cleavages, as well as static modifications of Cys-carbamidomethylation (+57.02), Met-oxidation (+15.99), and adduction of possible reactive metabolites of FLT (+498.17, +514.16 and +516.18) to nucleophilic amino acids were the criteria used for SEQUEST. LC-MS data were separately processed through P-Mod software to search for possible chemical modifications.
Statistical analysis
All data were reported as mean ± standard deviation. The differences between enzyme turnover rates were calculated using student's t-test and differences were considered significant with a probability of p ≤ 0.01.
Results
Inhibitory Potential of Five GCs for P450 3A4, 3A5 and 3A7 enzymes
We used a standard 6β-hydroxylation of testoterone HPLC method to determine enzyme activities (28). The recombinant 3A4, 3A5 and 3A7 enzymes exhibited reasonable activities of 620, 490 and 150 pmol product/(min × pmol P450), respectively. Incubations were run for 10, 15 and 40 min for 3A4, 3A5 and 3A7, respectively, to provide sufficient product. These times were within the linear portion of enzyme turnover. Testosterone 6β-hydroxylation activity was measured with a standard curve, using 11β-hydroxytestosterone as the internal standard.
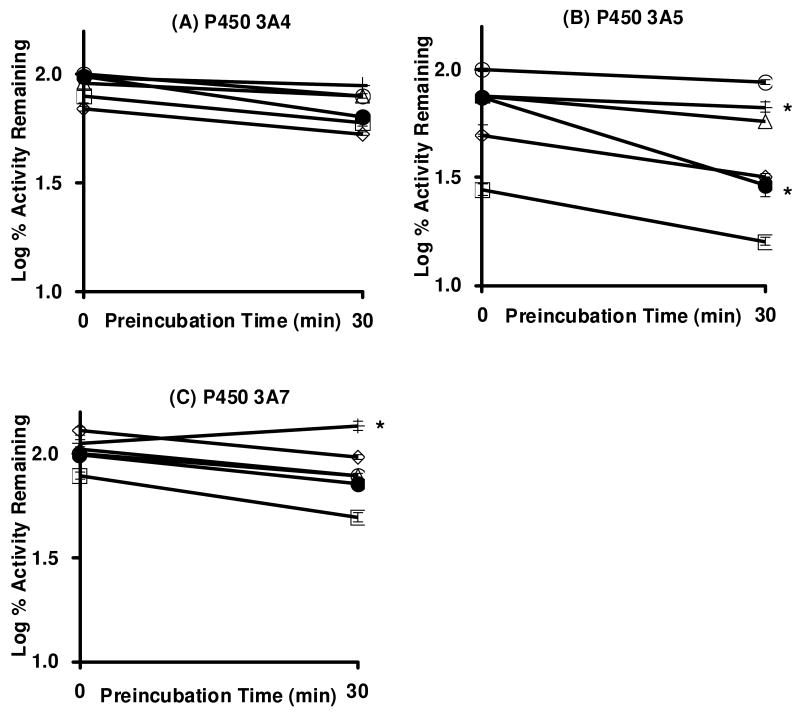
Single time- and concentration-dependent inactivation assays of P450 3A4, 3A5 and 3A7 enzymes were performed in triplicate to evaluate the inhibitory potential of five GCs. High concentration of GC (100 μM) and relatively long preincubation time (30 min) were employed to assess the potential of GCs to reduce the testosterone 6β-hydroxylation activity of each enzyme. Among the combination of five GCs and three enzymes, FLT (●) significantly reduced the testosterone 6β-hydroxylation activity of P450 3A5 (Figure 1B), and the slope of the line was statistically different from the slope of the control line. FLT was a potent competitive inhibitor of CYP3A5 (Fig 1, zero time point of preincubation with the 100 μM concentration), but lower concentrations did not show such potent competitive inhibition (Fig 2). FLT also reduced P450 3A4 activity, albeit to a lesser extent, and the slope was not statistically different from the slope of the control line. FLT did not inactivate P450 3A7. None of the other 4 GCs were mechanism-based inactivators of P450 3A4, 3A5, or 3A7, because the slopes were not statistically reduced from the slopes of the control. Interestingly, TRI appeared to protect P450 3A7 and 3A4 from inactivation (Figure 1A, 1C), but we do not have an explanation for this minor effect.
Figure 1.
Single time-dependent loss of testosterone 6β-hydroxylation activity of CYP3A4, 3A5 and 3A7 enzymes following incubation with DMSO (○) or 100 μM of beclomethasone dipropionate (□), budesonide (◇), flunisolide (△), FLT (●), and triamcinolone acetonide (+) in the presence of NADPH. The data represent the mean and standard deviations from three separate experiments, albeit most of the standard deviations are smaller than the data label. The lines with an asterisk indicate statistically significant (p < 0.01) slope differences in testosterone 6β-hydroxylation activity from the control.
Figure 2.
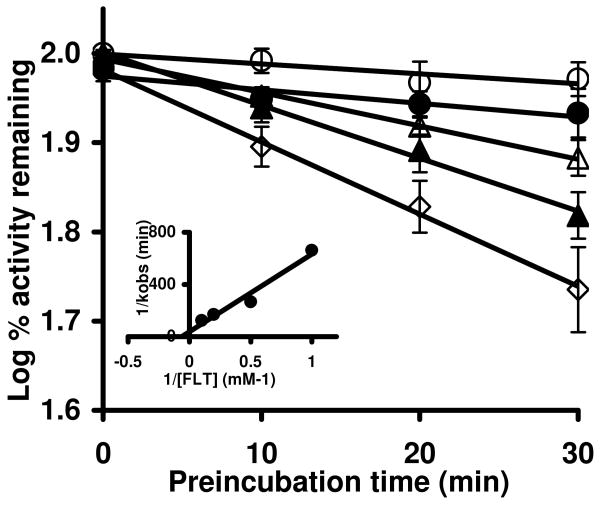
Time- and concentration-dependent inactivation of P450 3A5 with increasing concentrations of FLT. Incubation and assay conditions were described under Materials and Methods. The concentrations of FLT were 0 (○), 1 (●), 2 (△), 5 (▲), and 10 (◇) μM. Control incubations were done in the presence of NADPH, but without FLT. The data shown represent the mean and standard deviations from three separate experiments. The slopes of the lines represent the observed first-order rate constants (kobs) of the inactivation reaction at a given FLT concentration. The inset shows the double-reciprocal Kitz-Wilson plot for P450 3A5 inactivation. The KI (obtained from the x-intercept) was 16.1 μM, and the maximal rate of inactivation kinact (obtained from the y-intercept) was 0.027/min-1.
Time- and Concentration-Dependent Inactivation of P450 3A4 and 3A5 by FLT
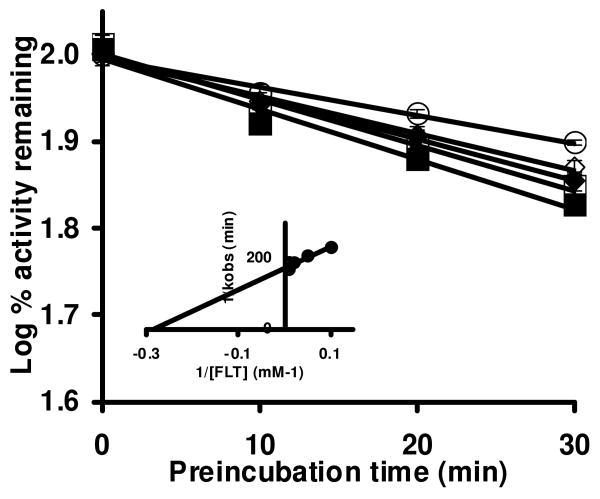
Based on the single time- and concentration-dependent assay (Figure 1), a detailed evaluation of time- and concentration-dependent loss of P450 3A4 and 3A5 enzyme activities was conducted. The loss of testosterone 6β-hydroxylation activity was measured with the indicated FLT concentrations and a plot of log percent remaining activity versus time was performed. Incubations of P450 3A5 and 3A4 with FLT produced a time- and concentration-dependent loss of 6β-hydroxylation activity (Figures 2 and 3). The loss in 6β-hydroxylation activity of P450 3A5 (Figure 2) increased with increasing concentrations of FLT, and a maximal loss of 45 ± 6 % (mean ± S.D. of three separate experiments) was observed after a 30-min preincubation with 10 μM FLT (Figure 2A). The loss in P450 3A5 activity in control incubations (DMSO) after a 30-min preincubation was 6 ± 4 %. In contrast, the loss in 6β-hydroxylation activity of P450 3A4 was modest even when higher concentrations of FLT were used (Figure 3). Although a maximal 33 ± 1 % loss of P450 3A4 enzyme activity was observed after a 30-min preincubation with 100 μM of FLT, 21 ± 1 % of P450 3A4 activity was lost after a 30-min preincubation without FLT. The slopes of the lines, based upon triplicate determinations, are the observed first-order rate constants (kobs) of the inactivation reaction at specific FLT concentrations. The kinetic constants for the inactivation for both enzymes were determined from a double-reciprocal Kitz-Wilson plot of kobs against FLT concentration (25, 29) (Figure 2 and 3, inset). The inactivator concentration at half-maximal inactivation rate, KI, was determined to be 16 μM for P450 3A5 and 3.5 μM for 3A4. The inactivation rate constant, kinact, was 0.027 min-1 for P450 3A5 and 0.006 min-1 for 3A4. When the data were fit to a direct plot of appropriate inactivation equations (30), the results were: KI, of 5.6 μM for P450 3A5 and 3.9 μM for 3A4 and kinact, was 0.013 min-1 for P450 3A5 and 0.006 min-1 for 3A4.
Figure 3.
Time- and concentration-dependent inactivation of P450 3A4 by increasing concentrations of FLT. Incubation and assay conditions were as described under Materials and Methods. The concentrations of FLT were 0 (○), 10 (◇), 20 (◆), 50 (□), and 100 (■) μM. Control incubations were done in the presence of NADPH, but without FLT. The data shown represent the means and standard deviations from three separate experiments. The slopes of the lines represent the observed first-order rate constants (kobs) of the inactivation reaction at a specific FLT concentration. The inset shows the double-reciprocal Kitz-Wilson plot for P450 3A4 inactivation. The KI (obtained from the x-intercept) was 3.5 μM, and the maximal rate of inactivation kinact (obtained from the y-intercept) was 0.006/min-1.
Partition Ratio
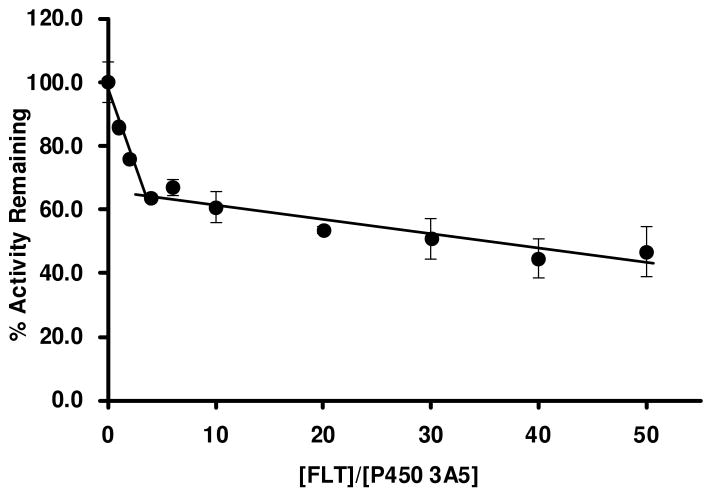
The partition ratio, defined as the number of molecules of the inactivator metabolized per molecule of the enzyme inactivated (13), was estimated using the titration method (25). The percent enzymatic activity remaining, after a 30-min preincubation (longest time point tested), was plotted against the ratio of the concentrations of FLT to P450 3A5. Figure 4 shows the plots of the percentage activity remaining versus the ratio of [FLT]/[P450 3A5]. The x-intercept between the linear regression line obtained at low concentrations of FLT and the straight line obtained at saturating FLT concentrations was extrapolated graphically to obtain the turnover number (P + 1), which is equal to the partition ratio + 1. The partition ratio was then calculated from the turnover number to be 3. The inactivation of CYP3A4 by FLT was not efficient, and FLT concentrations were solubility-limited. We estimated from the data in Fig 3 that it was approximately 30.
Figure 4.
Partition ratio for FLT-mediated inactivation of P450 3A5 showing loss of enzyme activity as a function of the ratio of [FLT]/[P450 3A5]. The enzyme was incubated with increasing concentrations of FLT (0, 0.5, 1, 2, 3, 5, 10, 15, 20, 25 μM) for 30 min as described under Materials and Methods. Percent activity remaining after complete inactivation (30 min) was plotted against the molar ratio of FLT to P450 3A5. The partition ratio was obtained from the intercept of the linear regression line containing the lower inactivator concentrations and the straight line drawn from the higher inactivator concentrations. The partition ratio for FLT-mediated inactivation of P450 3A5 was found to be 3. The data points were the means of three separate experiments.
Effect of GSH on Inactivation
In a mechanism-based inactivation process, addition of exogenous nucleophiles such as GSH should not protect the enzyme against inactivation, because the reactive intermediate inactivates the enzyme before diffusing out of the active site (13). To determine if GSH attenuates FLT-mediated inactivation of P450 3A5, 4 mM of GSH was included in the primary incubation. As shown in Table 1, a 30-min preincubation with 20 μM FLT produced 70 ± 1 % (mean ± S.D. of three separate experiments) loss of P450 3A5 activity, while inclusion of 4 mM GSH produced 68 ± 5 % loss. Thus, inclusion of GSH did not ameliorate inactivation of P450 3A5 by FLT.
Table 1. Irreversibility of P450 3A5 inactivation and lack of protection by GSH.
| Sample | Before Dialysis | After dialysis | |
|---|---|---|---|
| 0 min | 30 min | ||
| % | |||
| P450 3A5 Control | 100 ± 5 | 79 ± 3 | 59 ± 9 |
| P450 3A5 + 20 μM FLT | 87 ± 4 | 30 ± 1 | 17 ± 1 |
| P450 3A5 + 20 μM FLT + 4 mM GSH | 89 ± 4 | 32 ± 5 | NDa |
CYP3A5 was inactivated for 30 min with 20 μM FLT with or without 4 mM GSH as described under Materials and Methods, and the remaining activity was measured before and after dialysis. Control incubations lacked FLT but contained 2 % DMSO. The data points represent the means ± S.D. of three separate experiments.
Not Determined
Irreversibility of P450 3A5 Inactivation by FLT
To determine if the loss of enzyme activity was reversible, FLT-inactivated P450 3A5 incubation mixtures were dialyzed against phosphate buffer containing glycerol. Table 1 shows the percentage of testosterone 6β-hydroxylation activity that was measured before and after dialysis. Four-hour dialysis of the incubation mixture after the primary incubation did not restore the activity of P450 3A5. In fact, the residual P450 3A5 enzyme had lower catalytic activity than the enzyme that had not been treated with FLT.
Heme Adduct Analysis
The FLT-inactivated P450 3A5 incubation mixture was analyzed by HPLC to determine if a heme adduct of FLT could be detected. Heme and potential heme adducts were detected by monitoring at 405 nm as described in Materials and Methods. The positive control (1-aminobenzotriazole plus NADPH) showed a modified heme peak in the HPLC chromatogram, as well as a decrease in the native heme peak area (data not shown). In contrast, the heme elution profile was the same between incubations with or without FLT, in the presence or absence of NADPH (data not shown). Also, NADPH-dependent loss of the native heme was not observed, nor was there a change in P450 content, measured by reduced CO-binding spectra (data not shown). These observations suggest that that the prosthetic heme of P450 3A5 was neither modified nor destroyed by FLT to any substantial extent.
Analysis of Alkylated Peptides
Apoprotein of FLT-inactivated P450 3A5 was separated by SDS-PAGE, in-gel digested with protease and analyzed by LC-MS to detect FLT-adducted peptides. Two digestion conditions (trypsin or trypsin/chymotrypsin), either with or without reduction and alkylation conditions were employed to thoroughly analyze the digested peptides. LC-MS analysis of the digested peptides was obtained under various conditions The LC-MS data were analyzed using SEQUEST and P-Mod software to identify tryptic and tryptic+chymotryptic peptides and potentially modified forms of these peptides. Using SEQUEST, we considered adduction by the following possible reactive metabolites of FLT (+498.17-a dehydrogenated metabolite, +514.16-an oxygenated and dehydrogenated metabolite and +516.18-an oxygenated metabolite), based on results from metabolism studies of FLT and similar compounds, to all nucleophilic amino acids (Lys, Arg, Cys, His, Ser, Glu, and Asp) and included these as variable modifications in the search criteria settings. P-Mod software was also used to search for all modified peptides. This is not a biased method, and can potentially identify unknown modifications. The input data for P-Mod were 1) theoretical peptides produced by trypsin or trypsin+chymotrypsin digestion of CYP3A5 (less than two missed cleavages were also included), and 2) LC-MS/MS raw data. The software automatically identifies peptides based on MS/MS fragment ions. It also reports potential modifications (e.g. +57 for cysteine alkylation by iodoacetamide, +16 for methionine oxidation, etc..) of peptides based on the spectral data and assigns a probability score. P-Mod results indicated a number of potential modifications, however, none of them were considered to be peptides modified by fluticasone since they were either observed also in controls or were masses that could not be rationalized.
A list of the peptides that we were able to routinely detect with high confidence, and comments related to the relative detection in incubations with maximum FLT-mediated inactivation, is shown in Table 2. From this table it is evident that ∼20% of 3A45 was not detected. Several theoretical peptides were not observed from inactivated 3A5, which could have been potential sites of modification. However, as indicated above, no rational and/or unique modifications were observed.
Table 2.
CYP3A5 tryptic and tryptic/chymotryptic peptides identified from enzyme incubations with FLT.
| AA Position | Peptide AA Sequence* | Comment**# |
|---|---|---|
| 1-28 | MDLIPNLAVETWLLLAVSLVLLYLYGTR | |
| 29-34 | THGLFK | |
| 36-46 | LGIPGPTPLPL | |
| 36-51 | LGIPGPTPLPLLGNVL | |
| 36-54 | LGIPGPTPLPLLGNVLSYR | |
| 60-66 | FDTECYK | Absent in FLT+; No adduct found |
| 60-67 | FDTECYKK | |
| 71-75 | MWGTY | |
| 71-91 | MWGTYEGQLPVLAITDPDVIR | Absent in FLT-; No adduct found |
| 76-91 | EGQLPVLAITDPDVIR | |
| 97-105 | ECYSVFTNR | Absent in FLT+; No adduct found |
| 97-106 | ECYSVFTNRR | |
| 107-115 | SLGPVGFMK | |
| 116-127 | SAISLAEDEEWK | Absent in FLT+; No adduct found |
| 116-128 | SAISLAEDEEWKR | |
| 128-143 | RIRSLLSPTFTSGKLK | |
| 131-141 | SLLSPTFTSGK | |
| 142-156 | LKEMFPIIAQYGDVL | |
| 142-158 | LKEMFPIIAQYGDVLVR | |
| 144-152 | EMFPIIAQY | Absent in FLT-; No adduct found |
| 144-158 | EMFPIIAQYGDVLVR | |
| 167-173 | GKPVTLK | Absent in FLT+; No adduct found |
| 173-179 | KDIFGAY | Absent in FLT-; No adduct found |
| 174-179 | DIFGAY | Absent in FLT-; No adduct found |
| 174-196 | DIFGAYSMDVITGTSFGVNIDSL | Absent in FLT+; No adduct found |
| 180-189 | SMDVITGTSF | |
| 180-208 | SMDVITGTSFGVNIDSLNNPQDPFVESTK | |
| 190-209 | GVNIDSLNNPQDPFVESTKK | |
| 237-243 | NVSLFPK | Absent in FLT-; No adduct found |
| 244-251 | DTINFLSK | |
| 267-282 | HRLDFLQLMIDSQNSK | |
| 267-283 | HRLDFLQLMIDSQNSKK | Absent in FLT-; No adduct found |
| 269-282 | LDFLQLMIDSQNSK | |
| 269-288 | LDFLQLMIDSQNSKETESHK | |
| 289-302 | ALSDLELAAQSIIF | Absent in FLT+; No adduct found |
| 294-302 | ELAAQSIIF | Absent in FLT-; No adduct found |
| 317-330 | TLYELATHPDVQQK | |
| 320-330 | ELATHPDVQQK | |
| 334-342 | EIDAVLPNK | |
| 343-365 | APPTYDAVVQMEYLDMVVNETLR | |
| 366-372 | LFPVAIR | |
| 366-379 | LFPVAIRLERTCKK | |
| 380-390 | DVEINGVFIPK | |
| 391-399 | GSMVVIPTY | |
| 391-406 | GSMVVIPTYALHHDPK | |
| 407-418 | YWTEPEEFRPER | |
| 408-418 | WTEPEEFRPER | Absent in FLT-; No adduct found |
| 415-422 | RPERFSKK | Absent in FLT+; No adduct found |
| 419-439 | FSKKKDSIDPYIYTPFGTGPR | Absent in FLT+; No adduct found |
| 422-439 | KKDSIDPYIYTPFGTGPR | |
| 423-439 | KDSIDPYIYTPFGTGPR | |
| 424-439 | DSIDPYIYTPFGTGPR | |
| 430-434 | IYTPF | Absent in FLT-; No adduct found |
| 440-445 | NCIGMR | Absent in FLT+; No adduct found |
| 446-452 | FALMNMK | |
| 458-462 | VLQNF | Absent in FLT+; No adduct found |
| 469-475 | ETQIPLK | |
| 469-490 | ETQIPLKLDTQGLLQPEKPIVLK | |
| 476-490 | LDTQGLLQPEKPIVLK |
Bold: Segments of 3A5 peptides that were not observed in peptides derived from 3A5 that was incubated with FLT.
Peptides were identified using Sequest with the following criteria: enzyme=trypsin (KR) or trypsin/chymotrypsin (KRFWYL) fully enzymatic, up to 3 missed cleavage sites,; peptide tolerance=2.0 amu, fragment ion tolerance=2.0 amu, up to 3 post-translational modifications/peptide, static modifications of 15.99 for methionine and 57.02 for cysteine alkylation by iodoacetamide. Peptides were also filtered with the following criteria: peptide probability <1×10-2, peptide score (Xcorr) >1.0.
Peptide/AA adducts and potential “other” modifications were analyzed with Pmod VII and Sequest by adding static modifications of potential FLT metabolites to select amino acids in the search criteria settings.
Sequence coverage for 3A5 from control samples was 81.4% and 76.4% in incubations with FLT.
Discussion
Effective GC therapy for asthma that minimizes the risks of systemic adverse effects requires the comprehensive characterization of respiratory metabolism of GCs. Among the enzymes that catalyze the biotransformation of inhaled GCs, P450 3A enzymes are considered to be most important, because they are primarily responsible for the detoxication of GCs in the airway cells as well as in the liver. In the present study, we have evaluated the mechanism-based loss of P450 3A enzyme activity by five GCs widely used in the United States. FLT was an inactivator of P450 3A5, but the other four GCs did not inactivate the P450 3A enzymes, because they did not lose activities after relatively long incubations (30 min) at a high concentration (100 μM), and the slopes of the rates of enzyme activity loss were not statistically different from the slope of the control incubations. The apparent protective effect of triamcinolone observed for P450 3A7 and 3A4 (Figure 1) might be caused by an allosteric activation of the enzymes by a metabolite of triamcinolone that was formed during the preincubation. Additional experiments confirmed that FLT was a potent inactivator of P450 3A5 and a weak inactivator of P450 3A4. The inactivation of P450 3A5 by FLT was irreversible, and the exogenous protective nucleophile GSH did not attenuate inactivation (Table 1). These multiple outcomes firmly establish FLT as a typical and potent mechanism-based inactivator (13, 25), with a reasonably low KI of 16 μM and a relatively slow kinact of 0.027 min-1. These inactivation kinetics values were comparable to those of the prototype inactivator 17α-ethynylestradiol which is bioactivated in vitro to covalently form adducts with the heme and the apoprotein of P450 3A5, with KI, kinact and partition ratio of 16 μM, 0.06 min-1 and 25, respectively (31).
Theoretically, the clinical pharmacokinetic effect of a P450 inactivator is a function of its KI, kinact and partition ratio and the synthesis rate of new enzyme. A number of drugs have been reported to be mechanism-based inactivators of P450 3A4. Clinically important inhibitors include some macrolide antibiotics (e.g. clarithromycin and erythromycin), antituberculosis agent (e.g. isoniazid), antiepileptics (e.g. carbamazepine), anticancer agents (e.g. tamoxifen and CPT-11), anti-HIV agents (e.g. ritonavir and delavirdine), antihypertensives (e.g. verapamil), steroids and their modulators (e.g. gestodene). and certain compounds from herbal medicines (32). KI (μM) and kinact (min-1) values of most of these inactivators ranges from 0.1 to 50 μM, and from 0.02 to 0.4 min-1, respectively. These in vitro values are comparable to the in vitro values of FLT inactivation of P450 3A5. Kinetic parameters and molecular mechanism for the inactivation for P450 3A4 and 3A5 have been extensively characterized (31), and the values are also similar to those of FLT.
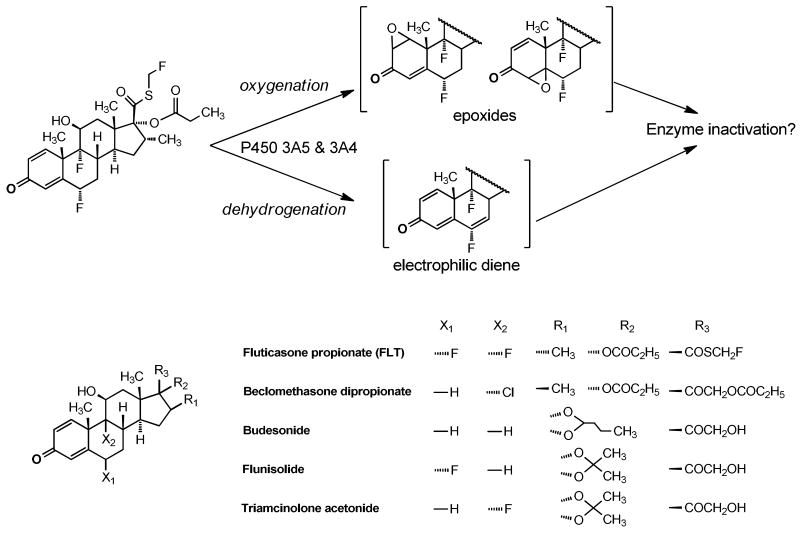
Inactivation of P450 3A5 with FLT exhibited a very potent partition ratio of 3 (Figure 4). Partition ratios, ranging from almost zero to several thousand, can be viewed as the average number of catalytic events of the enzyme before it is inactivated (13), and the number of 3 is among the lowest values ever reported for a P450 enzyme (33-35). This low partition ratio suggests that FLT is a highly efficient inactivator of P450 3A5 with minimal branching to non-inactivating metabolic pathways, and that the reactive intermediate of FLT is too reactive to diffuse from the active site of P450 3A5, which is supported by the failure of dialysis to restore activity. FLT has a quinoid structure, and potentially bioactivated by P450s to produce epoxides or an electrophilic Michael acceptor diene that could alkylate nucleophilic residues of the enzyme (Figure 5). However, Figure 5 shows that all of the GCs examined have the same quinoid structural moiety, and an explanation for the highly selective inactivation by FLT is not obvious.
Figure 5.
Potential bioactivation pathways to reactive electrophilic intermediates of FLT by P450 3A enzymes are illustrated. Nucleophilic residues of the proteins could add to the epoxides or diene to inactivate the enzymes. Structures of beclomethasone dipropionate, budesonide, flunisolide, fluticasone propionate, and triamcinolone acetonide are shown.
We tried to determine if a heme and/or protein adduct of FLT could be detected after P450 3A5 inactivation. However, neither the heme elution profiles (HPLC) nor the peak areas of native heme were changed to any substantial degree. These results suggest that the prosthetic heme of P450 3A5 is not the main target of reactive metabolite of FLT.
To determine if FLT intermediates targeted P450 3A5 apoprotein, P450 3A5 protein was separated by SDS-PAGE, digested in-gel with protease, and analyzed by LC-MS. Several conditions were employed to obtain an array of digested peptides, but identification of an alkylated peptide was not successful, even though peptide coverage was complete enough to confirm the identity of the native P450 3A5 sequence.
The principal metabolite of FLT in humans is a 17β-carboxylic acid derivative with negligible GC activity (36). This metabolite is produced by P450 3A4, 3A5 and 3A7 enzymes with almost identical in vitro intrinsic clearance values (Vmax/Km) for the three enzymes. (37). Pearce et al. (37) showed that FLT was metabolized by the three CYP3A enzymes to its major metabolite at a rate about 25-fold higher than CYP2C19, and the 3A and 2C19 enzymes were the only recombinant human enzymes that metabolized FLT. Other enzymes tested were 1A1, 1B1, 2A6, 2B6, 2C9, 2C18, 2D6, and 2E1. Additionally, specific inhibitors of CYP3A enzymes ameliorated FLT metabolism in human liver microsomes, but inhibitors/inactivators of six other P450s did not inhibit turnover. Finally, regression analysis of FLT metabolism with ten P450 enzyme activities in fourteen human liver microsomal samples correlated only to CYP3A enzyme activity. Thus, the CYP3A enzymes are the predominant catalysts of FLT hepatic metabolism. Based on this observation, contribution of non-CYP3A enzymes to the pulmonary metabolism of FLT is probably minimal.
Among the three major P450 3As, P450 3A5 is the major form in the human lung and may be the major constitutive respiratory P450 enzyme. Significant amounts of P450 3A5 protein are expressed in bronchial epithelium, bronchial glands, alveolar epithelium, vascular and capillary epithelium and alveolar macrophages (6-8). In contrast, the same cell types have little or no P450 3A4 expression (9). Recent comprehensive transcriptional analysis of bronchial and peripheral human lung tissues strongly confirmed the importance of 3A5 not 3A4 in the human respiratory tract (10). These observations suggest that the loss of pulmonary P450 3A5 removes the principal metabolic pathway of FLT within the airway cells.
Although P450 3A5 is the predominant P450 3A form in the lung, the CYP3A5 gene is polymorphic. Several polymorphisms lead to complete absence of functional P450 3A5 enzyme The CYP3A5*3 allele is very common (4, 11, 38), which leads to expression of active enzyme in less than 50% of African-Americans, and less than 20% of Caucasians. In addition, GCs are potent inducers of P450 3A5 mRNA in human A549 lung adenocarcinoma cells (39), and may elicit similar responses in vivo. We believe that the activity/expression patterns of the P450 3A5 enzyme, determined by complex combination of genetic polymorphisms, developmental expression patterns, and environmental factors, play significant roles in the responses of asthmatics to GCs. The results obtained in this study demonstrate that FLT is a potential environmental (therapeutic) factor that decreases the amount of P450 3A5 enzyme, particularly in populations expressing high levels of pulmonary P450 3A5.
Human lung has efficient metabolic functions to protect against foreign chemicals entering the body. There is still great controversy regarding the importance of pulmonary metabolism of xenobiotics, due to the low levels of some enzymes when the enzyme activities are expressed as their activity in the whole human lung. However, the airway epithelium is exposed to much higher concentrations than the concentrations that would be expected in hepatocytes after inhalation. Therefore, decreased metabolism in airway cells could significantly alter the respiratory effects of GCs. (40). In particular, inhaled lipophilic compounds such as benzo[a]pyrene deposited in the airway epithelium are extensively retained and expansively metabolized (41, 42).
The balance between inactivation and synthesis of new enzyme determines the kinetics of degradation. We expect that 3A5 synthesis would not compensate for the efficient inactivation of this enzyme by FLT, which has a remarkable partition ratio of 3. The clinical dose of inhaled FLT is as low as several hundred micrograms/day and only ∼20% of the inhaled dose is deposited in the lung (24). However, if the rate of inactivation exceeds the rate of the synthesis of new enzyme, the long-term administration and irreversibility of P450 3A5 inactivation would probably eventually decrease the enzymatic activity of P450 3A5. The loss of P450 3A5 activity in the airway cells will increase the concentration of FLT in the same cells.
The main determinant of systemic bioavailability of inhaled FLT is the direct absorption from the lung, because the swallowed fraction absorbed from the gastrointestinal tract is extensively metabolized in the liver, so the systemic bioavailability of FLT is less than 1% (23, 24). However, the low systemic concentrations of FLT should be increased by reduced airway metabolism if P450 3A5 were inactivated by FLT. The occurrence of life-threatening adrenal failure in asthmatic children receiving FLT (16, 18, 19) was attributed solely to the high GC activity and lipophilicity of FLT, which could lead to more extensive binding within systemic tissues and prolonged retention (15, 18). In addition to this postulated mechanism, we propose that the gradual inactivation of pulmonary P450 3A5 by repeated FLT inhalation, followed by increased absorption from the lung, could explain the unfortunate systemic adverse effects of FLT in children.
In conclusion, we have demonstrated that FLT is a potent mechanism-based inactivator of P450 3A5, the predominant P450 3A isoform in lung. Long-term inhalation of FLT could decrease the amount of active P450 3A5 enzyme in the lung, leading to the decreased clearance of FLT, followed by the possibility of increased target and systemic concentrations of FLT.
Acknowledgments
This work was supported by NIH Grants GM074249 from the National Institute of General Medical Sciences, HD060559 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; Primary Children's Medical Center Research Foundation, Salt Lake City, Utah; and Daiichi Sankyo Co., Ltd., Tokyo, Japan. The authors thank Ms. Erin Romero for construction of the Table of Contents graphic design.
Footnotes
The abbreviations used are: GC, glucocorticoid; FLT, fluticasone propionate; TFA, trifluoroacetic acid
References
- 1.Undem BJ. Pharmacotherapy of Asthma. In: Brunton LL, Lazo JS, Parker KL, editors. The Pharmacological Basis of Therapeutics. The McGraw-Hill Companies; New York: 2005. pp. 717–736. [Google Scholar]
- 2.Leung DYM, Bloom JW. Update on Glucocorticoid Action and Resistance. Journal of allergy and Clinical Immunology. 2003;111:3–22. doi: 10.1067/mai.2003.97. [DOI] [PubMed] [Google Scholar]
- 3.Mjaanes CM, Whelan GJ, Szefler SJ. Corticosteroid therapy in Asthma: Predictors of Responsiveness. Clinics in Chest Medicine. 2006;27:119–132. doi: 10.1016/j.ccm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JY, Wang Y, Prakash C. Xenobiotic-metabolizing enzymes in human lung. Current drug metabolism. 2006;7:939–948. doi: 10.2174/138920006779010575. [DOI] [PubMed] [Google Scholar]
- 5.Bernauer U, Heinrich-Hirsch B, Tonnies M, Peter-Matthias W, Gundert-Remy U. Characterisation of the xenobiotic-metabolizing Cytochrome P450 expression pattern in human lung tissue by immunochemical and activity determination. Toxicol Lett. 2006;164:278–288. doi: 10.1016/j.toxlet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Anttila S, Hukkanen J, Hakkola J, Stjernvall T, Beaune P, Edwards RJ, Boobis AR, Pelkonen O, Raunio H. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am J Respir Cell Mol Biol. 1997;16:242–249. doi: 10.1165/ajrcmb.16.3.9070608. [DOI] [PubMed] [Google Scholar]
- 7.Kivisto KT, Fritz P, Linder A, Friedel G, Beaune P, Kroemer HK. Immunohistochemical localization of cytochrome P450 3A in human pulmonary carcinomas and normal bronchial tissue. Histochem Cell Biol. 1995;103:25–29. doi: 10.1007/BF01464472. [DOI] [PubMed] [Google Scholar]
- 8.Kivisto KT, Griese EU, Fritz P, Linder A, Hakkola J, Raunio H, Beaune P, Kroemer HK. Expression of cytochrome P 450 3A enzymes in human lung: a combined RT-PCR and immunohistochemical analysis of normal tissue and lung tumours. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:207–212. doi: 10.1007/BF00168759. [DOI] [PubMed] [Google Scholar]
- 9.Mace K, Bowman ED, Vautravers P, Shields PG, Harris CC, Pfeifer AM. Characterisation of xenobiotic-metabolising enzyme expression in human bronchial mucosa and peripheral lung tissues. Eur J Cancer. 1998;34:914–920. doi: 10.1016/s0959-8049(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 10.Leclerc J, Tournel G, Courcot-Ngoubo Ngangue E, Pottier N, Lafitte JJ, Jaillard S, Mensier E, Lhermitte M, Broly F, Lo-Guidice JM. Profiling gene expression of whole cytochrome P450 superfamily in human bronchial and peripheral lung tissues: Differential expression in non-small cell lung cancers. Biochimie. 2010;92:292–306. doi: 10.1016/j.biochi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Goldstein JA. Functionally defective or altered CYP3A4 and CYP3A5 single nucleotide polymorphism and their detection with genotyping tests. Pharmacogenomics. 2005;6:357–371. doi: 10.1517/14622416.6.4.357. [DOI] [PubMed] [Google Scholar]
- 13.Kent UM, Juschyshyn MI, Hollenberg PF. Mechanism-based inactivators as probes of cytochrome P450 structure and function. Current drug metabolism. 2001;2:215–243. doi: 10.2174/1389200013338478. [DOI] [PubMed] [Google Scholar]
- 14.Kalgutkar AS, Obach RS, Maurer TS. Mechanism-based inactivation of cytochrome P450 enzymes: chemical mechanisms, structure-activity relationships and relationship to clinical drug-drug interactions and idiosyncratic adverse drug reactions. Curr Drug Metab. 2007;8:407–447. doi: 10.2174/138920007780866807. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999;159:941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 16.Randell TL, Donaghue KC, Ambler GR, Cowell CT, Fitzgerald DA, van Asperen PP. Safety of the newer inhaled corticosteroids in childhood asthma. Paediatr Drugs. 2003;5:481–504. doi: 10.2165/00128072-200305070-00005. [DOI] [PubMed] [Google Scholar]
- 17.Peters SP. Safety of inhaled corticosteroids in the treatment of persistent asthma. J Natl Med Assoc. 2006;98:851–861. [PMC free article] [PubMed] [Google Scholar]
- 18.Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87:457–461. doi: 10.1136/adc.87.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodlie M, McKean MC. Strategies to screen for adrenal suppression in children with asthma: there is no consensus among UK centres. Thorax. 2008;63:841–842. doi: 10.1136/thx.2008.100222. [DOI] [PubMed] [Google Scholar]
- 20.Lipworth BJ. New perspectives on inhaled drug delivery and systemic bioactivity. Thorax. 1995;50:105–110. doi: 10.1136/thx.50.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohatagi S, Appajosyula S, Derendorf H, Szefler S, Nave R, Zech K, Banerji D. Risk-benefit value of inhaled glucocorticoids: a pharmacokinetic/pharmacodynamic perspective. J Clin Pharmacol. 2004;44:37–47. doi: 10.1177/0091270003260334. [DOI] [PubMed] [Google Scholar]
- 22.Singh SD, Whale C, Houghton N, Daley-Yates P, Kirby SM, Woodcock AA. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2003;55:375–381. doi: 10.1046/j.1365-2125.2003.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falcoz C, Oliver R, McDowall JE, Ventresca P, Bye A, Daley-Yates PT. Bioavailability of orally administered micronised fluticasone propionate. Clin Pharmacokinet. 2000;39 1:9–15. doi: 10.2165/00003088-200039001-00002. [DOI] [PubMed] [Google Scholar]
- 24.Crim C, Pierre LN, Daley-Yates PT. A review of the pharmacology and pharmacokinetics of inhaled fluticasone propionate and mometasone furoate. Clin Ther. 2001;23:1339–1354. doi: 10.1016/s0149-2918(01)80113-2. [DOI] [PubMed] [Google Scholar]
- 25.Silverman RB. Mechanism-based enzyme inactivators. Methods Enzymol. 1995;249:240–283. doi: 10.1016/0076-6879(95)49038-8. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz de Montellano PR, Mathews JM. Autocatalytic alkylation of the cytochrome P-450 prosthetic haem group by 1-aminobenzotriazole. Isolation of an NN-bridged benzyne-protoporphyrin IX adduct. Biochem J. 1981;195:761–764. doi: 10.1042/bj1950761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen BT, Davey SW, Ham AJ, Liebler DC. P-Mod: an algorithm and software to map modifications to peptide sequences using tandem MS data. J Proteome Res. 2005;4:358–368. doi: 10.1021/pr0498234. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Yost GS. Metabolic activation of a novel 3-substituted indole-containing TNF-alpha inhibitor: dehydrogenation and inactivation of CYP3A4. Chem Res Toxicol. 2008;21:374–385. doi: 10.1021/tx700294g. [DOI] [PubMed] [Google Scholar]
- 29.Kitz R, Wilson IB. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962;237:3245–3249. [PubMed] [Google Scholar]
- 30.Grimm SW, Einolf HJ, Hall SD, He K, Lim HK, Ling KH, Lu C, Nomeir AA, Seibert E, Skordos KW, Tonn GR, Van Horn R, Wang RW, Wong YN, Yang TJ, Obach RS. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of America. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:1355–1370. doi: 10.1124/dmd.109.026716. [DOI] [PubMed] [Google Scholar]
- 31.Lin HL, Hollenberg PF. The inactivation of cytochrome P450 3A5 by 17alpha-ethynylestradiol is cytochrome b5-dependent: metabolic activation of the ethynyl moiety leads to the formation of glutathione conjugates, a heme adduct, and covalent binding to the apoprotein. J Pharmacol Exp Ther. 2007;321:276–287. doi: 10.1124/jpet.106.117861. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Chan E, Lim LY, Boelsterli UA, Li SC, Wang J, Zhang Q, Huang M, Xu A. Therapeutic drugs that behave as mechanism-based inhibitors of cytochrome P450 3A4. Current drug metabolism. 2004;5:415–442. doi: 10.2174/1389200043335450. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Lin HL, Walker VJ, Hamdane D, Hollenberg PF. tert-Butylphenylacetylene is a potent mechanism-based inactivator of cytochrome P450 2B4: inhibition of cytochrome P450 catalysis by steric hindrance. Mol Pharmacol. 2009;76:1011–1018. doi: 10.1124/mol.109.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiba M, Nishime JA, Lin JH. Potent and selective inactivation of human liver microsomal cytochrome P-450 isoforms by L-754,394, an investigational human immune deficiency virus protease inhibitor. J Pharmacol Exp Ther. 1995;275:1527–1534. [PubMed] [Google Scholar]
- 35.Kunze KL, Trager WF. Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline. Chem Res Toxicol. 1993;6:649–656. doi: 10.1021/tx00035a009. [DOI] [PubMed] [Google Scholar]
- 36.Harding SM. The human pharmacology of fluticasone propionate. Respir Med. 1990;84 A:25–29. doi: 10.1016/s0954-6111(08)80004-2. [DOI] [PubMed] [Google Scholar]
- 37.Pearce RE, Leeder JS, Kearns GL. Biotransformation of fluticasone: in vitro characterization. Drug Metab Dispos. 2006;34:1035–1040. doi: 10.1124/dmd.105.009043. [DOI] [PubMed] [Google Scholar]
- 38.Daly AK. Significance of the minor cytochrome P450 3A isoforms. Clin Pharmacokinet. 2006;45:13–31. doi: 10.2165/00003088-200645010-00002. [DOI] [PubMed] [Google Scholar]
- 39.Hukkanen J, Vaisanen T, Lassila A, Piipari R, Anttila S, Pelkonen O, Raunio H, Hakkola J. Regulation of CYP3A5 by glucocorticoids and cigarette smoke in human lung-derived cells. J Pharmacol Exp Ther. 2003;304:745–752. doi: 10.1124/jpet.102.038208. [DOI] [PubMed] [Google Scholar]
- 40.Hukkanen J, Pelkonen O, Hakkola J, Raunio H. Expression and regulation of xenobiotic-metabolizing cytochrome P450 (CYP) enzymes in human lung. Crit Rev Toxicol. 2002;32:391–411. doi: 10.1080/20024091064273. [DOI] [PubMed] [Google Scholar]
- 41.Gerde P, Muggenburg BA, Thornton-Manning JR, Lewis JL, Pyon KH, Dahl AR. Benzo[a]pyrene at an environmentally relevant dose is slowly absorbed by, and extensively metabolized in, tracheal epithelium. Carcinogenesis. 1997;18:1825–1832. doi: 10.1093/carcin/18.9.1825. [DOI] [PubMed] [Google Scholar]
- 42.Gerde P, Muggenburg BA, Lundborg M, Dahl AR. The rapid alveolar absorption of diesel soot-adsorbed benzo[a]pyrene: bioavailability, metabolism and dosimetry of an inhaled particle-borne carcinogen. Carcinogenesis. 2001;22:741–749. doi: 10.1093/carcin/22.5.741. [DOI] [PubMed] [Google Scholar]