Abstract
Background
Dopamine (DA) is a major neurotransmitter playing an important role in the regulation of vertebrate reproduction. We developed a novel method for the comparison of transcriptomic and proteomic data obtained from in vivo experiments designed to study the neuroendocrine actions of DA.
Methods and Findings
Female goldfish were injected (i.p.) with DA agonists (D1-specific; SKF 38393, or D2-specific; LY 171555) and sacrificed after 5 h. Serum LH levels were reduced by 57% and 75% by SKF 38393 and LY 171555, respectively, indicating that the treatments produced physiologically relevant responses in vivo. Bioinformatic strategies and a ray-finned fish database were established for microarray and iTRAQ proteomic analysis of the hypothalamus, revealing a total of 3088 mRNAs and 42 proteins as being differentially regulated by the treatments. Twenty one proteins and mRNAs corresponding to these proteins appeared on both lists. Many of the mRNAs and proteins affected by the treatments were grouped into the Gene Ontology categorizations of protein complex, signal transduction, response to stimulus, and regulation of cellular processes. There was a 57% and 14% directional agreement between the differentially-regulated mRNAs and proteins for SKF 38393 and LY 171555, respectively.
Conclusions
The results demonstrate the applicability of advanced high-throughput genomic and proteomic analyses in an amendable well-studied teleost model species whose genome has yet to be sequenced. We demonstrate that DA rapidly regulates multiple hypothalamic pathways and processes that are also known to be involved in pathologies of the central nervous system.
Introduction
Cellular regulation of the transcriptome and proteome is complex and the relationship between gene expression and protein changes in vivo remain poorly understood in fishes [1]. We used the adult female goldfish hypothalamus as a model system to characterize the rapid transcriptomic and proteomic responses to injection of dopamine (DA) receptor agonists. DA is widely distributed in the vertebrate brain and is involved in motivation, cognition, movement, and endocrine responses. DA exerts its effects via the D1- and D2-classes of 7-transmembrane domain G-protein-coupled receptors [2]. In fish, it is well understood that DA, acting through the D1 and D2 receptor, stimulates growth hormone release and inhibits luteinizing hormone (LH) release, respectively [3]. Upon ligand binding, the D1-receptor stimulates adenylate cyclase (AC) activity whereas the D2-receptor inhibits AC activity [4], leading us to hypothesize that the specific receptor agonists would lead to distinct transcriptomic and proteomic profiles in the hypothalamus that reflect the mode of action of the distinct receptors. Both D1 and D2 receptors also modulate intracellular calcium levels [2]. We chose to characterize the response to DA because it is a major central nervous system (CNS) neurotransmitter with a fundamental inhibitory role in vertebrate reproduction [3], [5] and because of the importance of DA to neurological disorders in humans [6], [7], [8].
Our model organism of choice was the goldfish, Carassius auratus [3] because i) the role of DA as a central regulator of reproductive processes is best-described in the goldfish; ii) a goldfish EST project has been initiated; iii) transcriptomic analysis is possible because of the development of a goldfish-carp cDNA microarray; iv) it is a member of the Cyprinidae, one of the largest vertebrate classes with over 2,400 species; and because v) goldfish are more amenable to physiological and endocrine manipulations than smaller fish such as zebrafish and medaka. On the other hand, the paucity of genomic and proteomic data in goldfish, and in many other important animal models other than laboratory rodents and humans, presents a major challenge to evolutionary and comparative physiologists.
To address this challenge, we developed a method for transcriptomic and proteomic comparison and demonstrate its utility for use on a model species with value to physiology and endocrinology but having limited genomic information. We provide insights into the hypothalamic processes that are under the regulation of DA in relation to its potent inhibitory actions on pituitary luteinizing hormone (LH) release and thus vertebrate reproductive function [3], [5].
Materials and Methods
Ethics Statement
All procedures used were approved by the University of Ottawa Protocol Review Committee (permit BL-234) and followed standard Canadian Council on Animal Care guidelines on the use of animals in research.
Experimental animals and design
Common adult female goldfish were purchased from a commercial supplier (Aleong's International Inc., Mississauga, ON, Canada) and maintained at 18°C under a natural simulated photoperiod on standard flaked goldfish food. Goldfish were anaesthetized using 3-aminobenzoic acid ethylester (MS222) for all handling, injection, and dissection procedures. Sexually mature, pre-spawning (mid-May; GSI 4.5±1.3%) female goldfish (15–40 g) were injected intraperitoneally with either SKF 38393 (D1 agonist; SKF; 1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol; 40 µg/g) or LY 171555 (D2 agonist; LY; (-)-Quinpirole hydrochloride; 2 µg/g) purchased from Tocris (Ballwin, MO, USA). The experimental design and doses chosen were identical to Otto et al. [9] who showed rapid effects on goldfish brain somatostatin mRNAs. SKF was first dissolved in a minimal amount (0.099% final concentration) of dimethylsulfoxide (DMSO), and subsequently diluted with physiological fish saline (0.6% NaCl). Concentrations of DMSO up to 0.1% do not affect GH or LH levels [9]. LY was dissolved in saline. Control fish received 2 i.p. injections (5 µL/g body weight) of saline or the DMSO vehicle. SKF and LY-treated animals respectively received a second injection of either saline or DMSO to control for the 2 different drug vehicles.
After 5 hours, blood was sampled (400–600 µL) by puncture of the caudal vasculature via a 25-gauge needle attached to a 1-mL syringe. The fish were sacrificed by spinal transection and hypothalamic tissues were rapidly dissected and immediately frozen on dry ice. Hypothalami were pooled (3/tube) to increase RNA yield prior to RNA isolation. Serum was collected by centrifuging the blood at 4,000 g at 4°C for 10 minutes. Serum was stored at −80°C until used for the radioimmunoassay.
Radioimmunoassay for Luteinizing Hormone
The double antibody RIA protocol of Peter et al. [10] was used to analyze serum LH levels, with minor modifications described by Zhao et al. [11]. Data were tested for normality using SPSS v17.0 and determined not to be normally distributed. Data were therefore log-transformed, determined to be normally distributed, and a one-way ANOVA was performed to test for significant differences (p<0.05).
RNA isolation and quality and cDNA synthesis
RNA was isolated with the TRIzol method (Invitrogen, Burlington, ON, Canada) as per the manufacturer's protocol. Samples were treated with DNase on-column in an RNeasy Mini kit (Qiagen, Mississauga, ON, Canada). RNA quantity was evaluated using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). RNA quality was evaluated using the 2100 BioAnalyzer (Agilent); the RNA integrity number for all samples was >8.4.
Microarray hybridizations
We previously described and validated the production and use of our goldfish-carp cDNA microarray [12], [13], [14]. The array contains 8832 cDNAs printed in duplicate and a detailed description is published elsewhere [15]. Four microarray hybridizations were performed for hypothalamic tissue for both D1 and D2 agonists (total of 8 arrays) to screen for the effects of the agonists in the neuroendocrine brain. Three separate pools of RNA from treated fish were hybridized to the microarrays, and a fourth hybridization was a replicate dye-reversal of one of the three RNA pooled samples. Hybridizations were carried out relative to a common pool of control samples (∼30 control fish) for each tissue, which decreases technical variation as only one reference is utilized while maintaining biological variation of the treatment samples [16]. All cDNA synthesis, labeling, and hybridizations were performed using the Genisphere 3DNA Array 900MPX kit according to the manufacturer's protocol (Genisphere, Hatfield, PA). Hybridizations and scanning protocols were described previously [12], [13], [14]. Briefly, microarrays were scanned at full-speed 10-µm resolution with the ScanArray 5000 XL system (Packard Biosciences/PerkinElmer, Woodbridge, ON, Canada) using both red and blue lasers. Images were obtained with ScanArray Express software using automatic calibration sensitivity varying photomultiplier (PMT) gain (PMT starting at 65% for Cy5 and 70% for Cy3) with fixed laser power at 80% and the target intensity set for 90%. Microarray images were analyzed with QuantArray (Packard Biosciences/Perkin Elmer), and raw signal intensity values were obtained for duplicate spots of genes. Raw intensity values for all microarray data and microarray platform information have been deposited in the NCBI Gene Expression Omnibus database (Series accession no. GSE14607 (SKF) and GSE14610 (LY)) under MIAME compliance. Generalized Procrustes Analysis [17] was used for normalization of the array data and the Significance Analysis of Microarrays (SAM) method [18] was used to identify significantly regulated transcripts.
Protein quantification and database search using iTRAQ labeling
The iTRAQ labelling protocol has been previously described in detail in Martyniuk et al. [19]. Briefly, approximately 20 mg of hypothalamic tissue was collected and mechanically disrupted and homogenized in 500 µL RIPA (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% nonyl phenoxylpolyethoxylethanol-40, 1% sodium deoxycholate and 0.1% SDS) (Pierce, Thermo Fisher Scientific Inc. Rockford, IL., USA) and proteins were precipitated in 3 mL of acetone. After removal of acetone, proteins were resuspended in iTRAQ dissolution buffer (Applied Biosystems Inc, Foster City, CA) and vortexed. Using 100 µg total protein/sample, we performed three independent iTRAQ labeling experiments following the manufacturer's protocol (Applied Biosystems Inc,). For proteomics analysis, each labeling reaction consisted of a single hypothalamus for control (label 114), LY 171555 (D2 agonist; label 115), and SKF 38393 (D1 agonist; label 117) (total n = 9 samples used; n = 3 per iTRAQ experiment). After labelling the independent samples for each iTRAQ experiment, they were mixed together and processed through desalting via a macrospin column Vydac Silica C18 (The Nest Group Inc, Southboro, MA), each and then subjected to off-line SCX fractionation on a polysulfoethylA column. The following fractions were collected for each of the three iTRAQ experiments: 7 (iTRAQ 1), 11 (iTRAQ 2), and 10 (iTRAQ 3). LC-MS/MS analysis on each of these fractions was performed on a hybrid quadrupole-TOF mass spectrometer QSTAR XL (Applied Biosystems).
Peptides were searched against a ray-finned fish database (details in [19] using MS/MS data interpretation algorithms within Protein Pilot™ (Paragon™ algorithm, v 2.0, Applied Biosystems). The Paragon algorithm searched iTRAQ 4-plex samples as variable modifications with methyl methanethiosulfonate as a fixed modification [20]. The Protein Pilot™ algorithm was selected to search automatically for biological modifications such as homocysteines. The confidence level for protein identification was set up to 1.3 (95%), which is the default setting for the detected protein threshold in a Paragon™ method. Proteomics System Performance Evaluation Pipeline (ProteomicS PEP, Applied Biosystems) in Protein Pilot™ was used to create a reversed ray-finned fish database to calculate a false discovery rate (FDR). When searching the ProteomicS PEP reverse database, 621 proteins were identified with an FDR of 1%, thus there is high confidence (>99%) in the peptide-protein assignments in this study. Differential expression ratios for proteins were obtained from Protein Pilot™ which calculates protein ratios using only ratios from the spectra that are distinct to each protein, excluding the shared peptides of protein isoforms. Peptides with low spectral counts were also excluded from the calculation of averages by setting the intensity threshold for the sum of the signal-to-noise ratio for all the peak pairs at >9. A protein with three high quality peptide spectra used in quantitation is considered to be a confident quantitation. However, we also report proteins in which two spectra were used in the quantitation for comparison. To calculate differential expression ratios, all identified spectra from a protein were used to obtain an average protein ratio relative to the control label (i.e. fold change). The p-value was calculated using the confidence intervals from the error factor generated in Protein Pilot™.
Bioinformatics
Protein sequences from proteins identified by iTRAQ analysis as differentially expressed were downloaded from NCBI using extracted GI numbers with a BioPerl script (Fig. S1). The protein sequences were converted into a searchable database using formatdb. All of the nucleotide sequences identified as being differentially expressed (q<5%) from the agonist experiment were compared (blast-2.2.19) against the above database through Blast2GO [21]. A graphical depiction of the workflow is presented in Fig. S2.
Results and Discussion
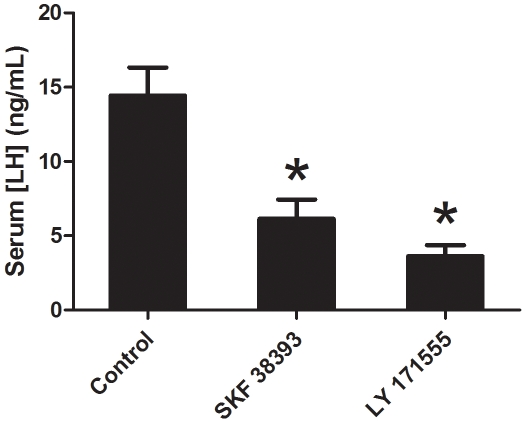
Our in vivo treatments both confirmed previous research and provided new hormone-regulatory data. Circulating serum LH was rapidly suppressed following DA agonist injections (Fig. 1). It is well known that DA, via the pituitary D2 receptor, is the primary inhibitor of LH release in goldfish and numerous other teleosts [5], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. Here we corroborate these findings and show that LY 171555 (LY) rapidly reduced circulating LH levels to 25% of control. Unexpectantly, we found that the DA-D1 agonist SKF 38393 (SKF) decreased LH by 43%, which is a novel finding for DA regulation of in vivo LH release in fish. It is known that activation of D1-receptors inhibits the release of gonadotropin-releasing hormone (GnRH) [32], and thus may have an impact on GnRH-stimulated LH release. We have subsequently begun further investigation the involvement of D1 receptors in LH release [33]. Most relevant here, however, is that our DA agonist treatments produced physiologically relevant changes in circulating hormone levels, so we proceeded to analyse transcriptomic and proteomic responses in the hypothalamus, the central integrator of external and endogenous signals. Compared to other vertebrates, fish have very high hypothalamic levels of DA due to a duplicated tyrosine hydroxylase gene (th2) [34]. Importantly, the goldfish posterior tuberculum (TPp; or nucleus posterior tuberis; NPT), a region with intense immunostaining for th1 but lacking immunostaining for dopamine β-hydroxylase, lies within the hypothalamus [35], [36], [37]. Thus, we proceeded to determine the effects of DA agonist injection on hypothalamic function.
Figure 1. Serum LH concentration following DA agonist injections.
Mean (± SEM) serum LH concentration in control and injected (40 ug/g SKF 38393 or 2 ug/g LY 171555) female goldfish (n = 23−26 each). Results presented are the average of 2 identical but independent experiments that showed similar results. Data was log-transformed to approximate normality and a one-way ANOVA was performed in SPSS v16 with significance considered at p<0.05, followed by Tukey's HSD multiple comparisons as data was homoscedastic. * signifies p<0.001 relative to control.
Transcripts identified in the goldfish hypothalamus as differentially regulated by dopamine agonists
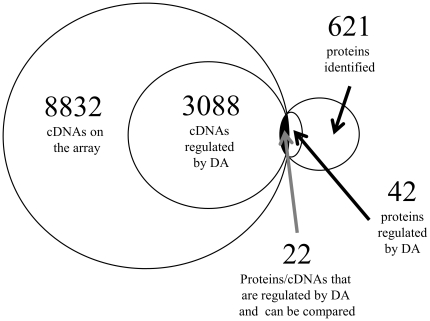
In total, 3088 ESTs were identified as being statistically (q<5%) significantly differentially expressed following either D1- or D2-receptor stimulation in the hypothalamus. Many of these are as yet uncharacterized (Fig. S3). Of the 1042 ESTs that are annotated, gene ontology (GO) classifications (Fig. S4) revealed that a large percentage are involved in the regulation of biological process (13%), signal transduction (10%) and nucleotide binding (19%). Furthermore, 29% of the cDNAs were localized to GO Cellular Component category of the protein complex, suggesting that many of the proteins are involved in macromolecular complexes, reflecting the receptor targets for the agonists.
A recent review by Altar et al. [38] summarized targets for the identification of CNS diseases using transcriptional profiling of human post-mortem brain, animal models, and cell culture studies. Many of the transcriptional targets reported by Altar et al. [38] were also differentially regulated by DA in the goldfish hypothalamus. For example, mRNAs for glutamic acid decarboxylase (GAD) 1, microtubule-associated protein tau, serpin A, malate dehydrogenase, regulator of G-protein signaling, transferrin, s100 calcium binding protein, glutathione-S-transferase, calmodulin, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors, glial fibrillary acidic protein, N-methyl-D-aspartic acid (NMDA) receptor 1, glutamate transporter, calbindin, alpha enolase, peroxiredoxin, fructose-bisphosphate aldolase c, glutamine synthetase, and DA receptors and a DA transporter were all identified as being differentially regulated (Table S1) and are linked to CNS diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), and schizophrenia, as well as brain aging [38], [39], [40].
Injection of SKF modulated hypothalamic mRNA levels for key transcripts in the glutamate and γ-aminobutyric acid (GABA) pathways (Table S1) in goldfish [3], [41]. In goldfish, GABA has a prominent stimulatory action on LH release by enhancing GnRH release and by reducing DA turnover in the hypothalamus [42], [43]. Our working hypothesis is that GABAergic systems transduce environmental (e.g. temperature) and endocrine (e.g. sex steroid) signals by rapid effects on both GnRH and DA to enhance LH release during seasonal gonadal redevelopment [41], [44], [45]. Results from the current study support this hypothesis.
Protein identification in the goldfish hypothalamus
For this experiment, we repeated DA agonist treatments on the same date the following year using an identical design. Serum LH was similarly decreased in both years and the data presented were combined (Fig. 1). The hypothalami from this experiment were subjected to iTRAQ proteomic analysis.
There were 621 proteins identified in this study using a ray-finned fish database previously constructed [19] (Table S2). The total number of peptide spectra detected was 8569, representing 4779 distinct peptides that are listed in Dataset S1. Of the peptides identified, 59.7% could be assigned to a protein, leaving approximately 40% of the spectra unidentified by homology searches against other ray-finned fishes.
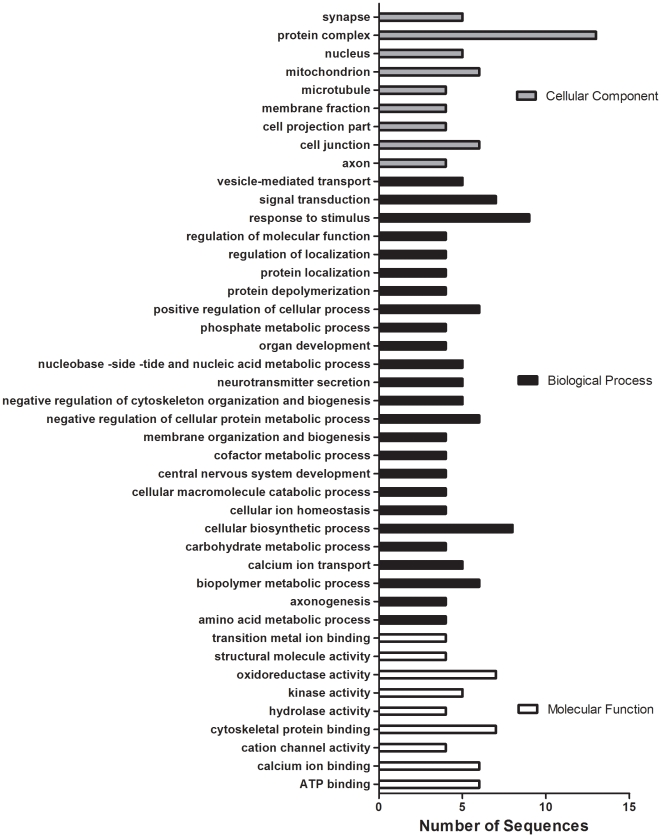
Of the 621 identifiable proteins, 42 were determined as being significantly (p<0.05) differentially regulated by either SKF or LY (Fig. 2). The protein dataset (for both D1 and D2 results combined) was analyzed using Blast2GO and binned into their corresponding GO terms (Fig. 3). Similarly to the mRNAs affected in this study, many of the proteins affected by the treatments are localized to the GO Cellular Component of the protein complex and are involved in a wide variety of biological processes, including signal transduction, response to stimulus, and both positive and negative regulation of cellular process. Of interest here are the proteins in the GO category of Biological Processes as related to neurotransmitter secretion and calcium ion transport. Calcium/calmodulin-dependent kinase II α subunit (CaMKIIα), calbindin 2, neuronal calcium-binding protein 2, plasma membrane calcium ATPase 4, and calmodulin (CaM) proteins were significantly affected by at least 1 of the DA agonists (Table 1).
Figure 2. Venn Diagram summarizing the number of cDNAs and proteins found in the hypothalamus of female goldfish.
The comparison of those regulated by DA was limited to cDNAs (q<5%) and proteins (FDR-adj p<0.05) identified as being statistically significant. Duplicate cDNAs were removed; cDNAs and proteins were counted once regardless if they were regulated by both agonists. The complete listing of cDNAs and proteins are listed in Tables S1 and S2, respectively.
Figure 3. GO categorization of differentially expressed proteins identified in the current study.
Proteins identified by iTRAQ (42; p<0.05) were binned into multilevel GO categorizations with a sequence cut-off of 3. Both treatments (D1 and D2) and both directions are included in this analysis but are counted only once if the protein is common to both treatments.
Table 1. Proteins and mRNAs identified and affected by DA agonists in the hypothalamus of goldfish.
| Protein | Corresponding mRNA | ||||||
| Accession | Name | Fold Change | Accession | Blast2GO-annotated mRNA (NCBI) | Fold Change | ||
| SKF | LY | SKF | LY | ||||
| AAW82445 | 14 kDa apolipoprotein* | −1.3 | CA967592 | 14 kda apolipoprotein | −1.6 | ||
| AAW82445 | 14 kDa apolipoprotein* | −1.5 | CF662502 | 14 kda apolipoprotein | 1.9 | ||
| CAG00145 | 25 kDa synaptosomal-associated protein | −1.5 | CA969142 | synaptosomal-associated protein 25 | 1.4 | ||
| NP_956213 | adaptor-related protein complex 2, beta 1 subunit | 1.3 | |||||
| AAH83251 | Atp2b4 protein | 1.4 | |||||
| AAZ38450 | beta thymosin-like protein | −1.3 | |||||
| AAF79948 | brain-type fatty-acid binding protein; B-Fabp | −1.4 | |||||
| CAK04737 | calbindin 2, like | −1.2 | |||||
| NP_001017741 | calcium/calmodulin-dependent protein kinase II alpha | 2.3 | 1.8 | ||||
| Q71UH6 | Calmodulin | −1.2 | CA969795 | calmodulin variant 1 | 1.4 | ||
| CAF92971 | Creatine kinase, brain | −1.4 | |||||
| NP_942096 | creatine kinase, mitochondrial 1 | 1.2 | |||||
| CAK10905 | cytochrome c oxidase subunit IV isoform 1 | 1.2 | |||||
| AAV52802 | glutamine synthetase | 1.4 | −1.3 | FG393017 | glutamine synthetase | 1.9 | |
| ABD67511 | glutathione S-transferase rho | −1.3 | −1.2 | CA964231 | glutathione S-transferase rho | −1.6 | |
| AAV52803 | glyceraldehyde-3-phosphate dehydrogenase | −1.4 | DY231775 | GAPDH | 1.6 | ||
| AAA21578 | kainate receptor α subunit | 1.8 | 1.3 | FG392717 | kainate receptor α subunit | 1.4 | |
| AAH63955 | Krt5 protein | 2.6 | |||||
| AAM21708 | liver-basic fatty acid binding protein* | 3.6 | CA968596 | fatty acid binding protein liver basic | 1.4 | ||
| AAM21708 | liver-basic fatty acid binding protein* | 8.6 | CA970443 | fatty acid binding protein liver basic | −1.5 | ||
| NP_956241 | malate dehydrogenase 1a, NAD (soluble) | 1.2 | CA964750 | malate dehydrogenase nad | 2.0 | 1.3 | |
| ABC69306 | myoglobin isoform 2 | −1.4 | CA968088 | myoglobin | 1.4 | ||
| NP_958898 | N-ethylmaleimide-sensitive factor | 1.3 | FG392958 | n-ethylmaleimide-sensitive factor | 1.4 | ||
| CAN88379 | novel protein sim to vert EF hand calcium binding protein 2 (EFCBP2) | −1.1 | |||||
| CAK05381 | parvalbumin | −1.1 | CA969705 | parvalbumin | 1.5 | ||
| ABF57553 | Pi-class glutathione S-transferase | −1.7 | −1.6 | ||||
| BAA78376 | polypeptide elongation factor 1 alpha | 1.3 | CA967511 | eukaryotic translation elongation factor 1 alpha 1 | −1.8 | ||
| XP_001340376 | PREDICTED: myelin basic protein isoform 3 | −3.9 | CA967910 | myelin basic protein | 2.0 | ||
| CAF98839 | PREDICTED: similar to germinal histone H4 gene | −1.1 | |||||
| XP_001338014 | PREDICTED: similar to microtubule-associated protein 1 A | 1.4 | |||||
| XP_696230 | PREDICTED: similar to microtubule-associated protein tau | −1.1 | CA967834 | microtubule-associated protein tau | 1.9 | ||
| XP_001335551 | PREDICTED: similar to Myelin basic protein | −1.5 | −1.5 | ||||
| XP_691535 | PREDICTED: similar to Nj-synaphin 2 | −2.2 | |||||
| CAF95822 | Putative histone cluster 1, H2bb | −1.6 | −1.5 | ||||
| AAG14350 | putative oncoprotein nm23 | −1.3 | CA964203 | non-metastatic cells 2, protein (NM23B) | −1.6 | ||
| AAI14255 | short chain dehydrogenase/reductase | −1.9 | −1.5 | CA968680 | dehydrogenase reductase sdr family member 12 | 1.3 | |
| NP_001091958 | spectrin alpha 2 | 1.3 | FG392760 | spectrin alpha 2 | 1.4 | ||
| NP_001017850 | stathmin 1/oncoprotein 18* | −1.3 | CA969799 | stathmin 1 oncoprotein 18 variant 8 | 1.4 | ||
| NP_001017850 | stathmin 1/oncoprotein 18* | −1.5 | CA966170 | stathmin 1 oncoprotein 18 variant 8 | 1.5 | ||
| NP_001018488 | synuclein, gamma b (breast cancer-specific protein 1) | −1.5 | |||||
| AAM90972 | transferrin variant A1 | 1.5 | CA968595 | transferrin variant c | 1.9 | ||
| AAM90973 | transferrin variant B1 | −1.9 | |||||
| NP_705954 | triosephosphate isomerase 1b | −1.3 | −1.2 | CA968504 | triosephosphate isomerase 1b | 1.8 | |
| NP_997770 | tyrosine 3-/tryptophan 5-monooxygenase activation protein, epsilon polypeptide | 1.1 | |||||
| AAQ94569 | ubiquitin C | −1.3 | |||||
Proteins were determined by iTRAQ as being significantly (FDR-adj p<0.05) differentially regulated in the hypothalamus of female goldfish treated with either SKF 38393 (SKF) or LY 171555 (LY) agonists. This table also show the cDNAs corresponding to the proteins identified by microarray analysis as significantly (q<5%) affected by the same treatments. Negative values indicate a decrease relative to control. Absent values indicate either that no significant change was detected, or, in the case of the mRNAs, that the corresponding cDNA was not present on the array. mRNAs were annotated using Blast2GO's Blast Descriptor Annotator with default values except the Blast ExpectValue was changed from 1.0E-3 to 1.0E-5. Following the Mapping step, the Annotation Configuration E-Value-Hit-Filter was changed from 1.0E-6 (default) to 1.0E-8 to increase the likelihood of proper GO annotation. Duplicates were assessed on the basis of sequence comparison and removed if a similar expression was observed. In the case where different expression profiles were seen (*), both ESTs were included, as it is possible that the sequences correspond to separate genes.
Calmodulin protein was decreased by LY, but not SKF, suggesting that in hypothalamic CaM expression is D2-, rather than D1-, receptor-regulated. Previous research demonstrated that CaM is expressed in the hypothalamus and the pituitary of goldfish and LY, but not SKF, decreased CaM mRNA levels in goldfish pituitary cells [46]. Together the data indicate CaM is under the regulation of the D2 receptor in the goldfish hypothalamo-pituitary system. We have also identified CaM as being important and regulated in the hypothalamus using a meta-type analysis of data from multiple goldfish microarray experiments performed across the seasonal breeding cycle. The mRNA for CaM was relatively highly expressed in the hypothalamus of sexually mature females in May, compared to both sexually regressed (August) or recrudescing animals in the gonadal redevelopment phase (December) [47]. This information, coupled with the changes in mRNA and protein levels of CaM (this study), suggests that CaM may be important for DA inhibition on LH release and thus inhibitory control of reproduction.
CaMKIIα protein levels, whose transcript levels follow the same seasonal profile as CaM (high in May, low in August and December) [47], were increased in both D1- and D2-agonist treated fish suggesting that, as for CaM, CaMKIIα may be important in hypothalamic signalling. CaMKII phosphorylates cAMP response binding element (CREB) protein, thereby inhibiting its function [48], which may lead to downstream transcriptional repression of genes involved in reproduction. Furthermore, CaMKII positively regulates the D2 receptor promoter in rats [49], suggestive of a feedback mechanism of DAergic action.
We observed a decrease in hypothalamic Apo-14 protein expression with both D1- and D2- receptor agonists. Apo-14 appears to be specific to teleost fish [50], although a recent phylogenetic analysis revealed that Apo-14 is the homologue to mammalian ApoA-II [51]. Apo-14 is mainly expressed in liver and brain of adult orange-spotted groupers and has been suggested to play a role in neuronal growth and repair [52], similar to ApoE [53]. Vitale and Carbajal [54] demonstrated that DA induces substantial cytoskeletal remodelling in rat lactotrophs in vitro. The decreases in Apo-14, stathmin 1, microtubule-associated protein tau, along with an increase in microtubule-associated protein 1A and spectrin alpha 2 (Table 1) suggests that DA may also have remodelling effects on the cytoskeleton of cells in the goldfish hypothalamus. The likely high energetic demands for such remodelling is supported by the observed increase of mitochondrial creatine kinase, cytochrome c oxidase subunit IV, and malate dehydrogenase protein levels (Table 1).
A comparison of the differentially expressed transcriptome to the differentially expressed proteome in response to DA agonists
The protein dataset was further compared to the microarray dataset by extracting the GI numbers from the protein results. A BioPerl script (Fig. S1) was used to obtain the corresponding amino acid sequences from GenBank, which were converted into a database that can be queried using the BLAST algorithm. This step was necessary in order to obtain the longest possible protein sequence data for the comparison. The nucleotide sequences represented on the microarray were compared (BLASTx) to this differentially-expressed protein database. The results (Table 1) show directional correlation for some mRNAs and proteins (for example, kainate receptor α subunit and 14 kDa apolipoprotein for D1 and liver-basic fatty acid binding protein for D2), while others are inversely correlated (for example, stathmin 1/oncoprotein 18 with either agonist). The mRNAs and their respective proteins exhibiting discordant directional change following agonist treatments nevertheless indicate that particular pathways and processes are DA-regulated. Differences in the direction of change between transcript and protein is likely related to our single sampling time-point, as the time-series relationship between changes in transcript versus protein in vivo are poorly understood in fish [1]. Furthermore, the regulatory mechanisms of the genome and proteome are complex and both turnover and stability of mRNA levels are important for translation of mRNA into protein [55]. For example, if the mRNA is decreased, but the protein is increased, it is possible that the mRNA has already begun to be degraded. Conversely, if the mRNA is increased, but the protein is decreased, there may be regulation of translational pathways, or increased protein degradation leading to induced transcription. These are good candidates for temporal (i.e., 1–3 hr time-course), biochemical (with/without cycloheximide) and pulse-chase analysis to better understand the differences. The interest here, however, lies with those mRNAs and proteins that share a common direction. In the D1-agonist-treated fish, 8 out of the 14 common mRNAs/proteins (57%) share a common directional change, whereas only 1 out of 7 of the common mRNAs/proteins (14%) for the D2-agonist-treated fish that change in parallel (Table 1). These results are comparable to what has been shown in the rat colon mucosa in vivo where only 16% direction identity between the transcriptome and the proteome was found [56]. Furthermore, that study included the development of a TRIzol®-based method to analyze both the transcriptome and the proteome from the same sample, which should reduce disagreements in the gene-protein correlation. Our results show that a comparable directional correlation from independent animals and experiments can also be achieved. This is significant, as it shows that the technique is applicable to a species with limited genomic information.
While some of the proteins identified as differentially expressed had corresponding changes in mRNA levels, many other cDNAs representing coding sequences for other proteins that were regulated were not printed on our array. For example, the Nj-synaphin 1 (also known as complexin 1) protein was identified as being down-regulated 2.2-fold in response to the D2-agonist (Table 1) but the complexin cDNA was not on the array.
Interestingly, in addition to complexin 1, proteins for both N-ethylmaleimide-sensitive factor (NSF) and soluble NSF attachment protein- (SNAP-) 25, all of which are major players in the exocytosis of neurosecretory vesicles [57], were affected by the D2 agonist. Current evidence [58], [59] indicates that complexin holds the vesicle in a “ready-to-release” state near the membrane, while preventing the spontaneous zippering of the t- and v-SNAREs and thus spontaneous fusion of the vesicle to the membrane. Upon introduction of Ca2+, which binds to synaptotagmin, complexin is removed and the membranes fuse, resulting in exocytosis. Since complexin expression is decreased, our results suggest that DA is stimulating some aspects of exocytosis in the hypothalamus via the D2 receptor. However, we found that SNAP-25 protein levels were reduced by the D2-agonist, suggesting that DA may also be inhibiting some of the exocytotic machinery. Perhaps this is a homeostatic mechanism to prevent or reduce the release of neurotransmitters and neurohormones in response to acute DAergic overstimulation.
NSF protein levels were increased in response to the D2 agonist. NSF transcript levels were initially found to be decreased in schizophrenic patients [60] but this was not observed in subsequent studies [38]. Similarly to CaM, the meta-analysis by Zhang et al. [47] identified NSF transcripts as being relatively highly expressed in May when goldfish are sexually mature. This information, coupled with the D1-mediated increase in NSF mRNA or D2-mediated increase in NSF protein found in this study, suggest that the DAergic inhibition of LH release involves hypothalamic NSF-dependent mechanisms.
Glutamine synthetase (GlnS) mRNA and protein levels were increased in response to SKF (Table 1). GlnS converts glutamate (Glu) to glutamine (Gln) and thus may limit the available pool of Glu, which is an excitatory neurotransmitter stimulating LH release in vertebrates including goldfish [41]. Increased GlnS could also potentially limit the Glu available to be converted by GAD to GABA. The observation in this study that GlnS is increased in response to a D1-specific agonist supports this hypothesis and suggests a possible mechanism of decreased LH secretion via D1-receptor stimulation. In contrast to SKF, GlnS protein levels were decreased in response to LY, with no observable effect on GlnS mRNA levels (Table 1). This differential response to the 2 DA agonists is likely due to responses in adenylate cyclase (AC) [61] since both receptors act via this second messenger system [2]. Generally, D1-class receptors, through interactions with Gs proteins, stimulate AC, whereas D2-class receptors, through interactions with Gi proteins, inhibit AC [2]. Glutamate can also be converted to glutathione-conjugated products through multiple enzymatic steps with the final step being mediated by glutathione S-transferase (GST). GST rho and Pi-class GST (GSTp) protein levels were reduced by both DA receptor agonists in the current study. It is not clear at this time whether the reduced GST protein levels are the result of DA receptor stimulation or rather a consequence of reduced substrate flux through that pathway initiated by limited pool of available Glu, as discussed above. However, it is likely not the latter case, as GST protein levels were reduced by both DA agonists, but GlnS protein levels were affected in different directions.
Glutathione is an antioxidant and helps to protect cells against damage from reactive oxygen species [62]. DA has been shown to induce apoptotic cell death in a CNS-derived catecholaminergic cell line [63] and Ishisaki et al. [64] identified GSTp as a candidate that protects against cell death in PC12 cells. Furthermore, inhibition of GSTp increased DAergic neuronal cell death in Swiss-Webster rats treated with MPTP [65], a specific DAergic neurotoxin. Interestingly, Shi et al. [66] recently reported increased GSTp protein levels in synaptosomal fractions from the frontal cortices of patients with pathologically-verified PD and suggest that GSTp may be important in the progression of the disease. In studies with GSTp-null mice, Henderson et al. [67] demonstrated that GSTp may enhance the hepatotoxicity of acetaminophen. While speculative, the observation of decreased GSTp protein levels in response to either D1- or D2-specific agonists in the current study suggests that DA may also modulate hypothalamic neuronal cell death in fish. This hypothesis is further supported by predominant increases observed in mRNAs for multiple heat shock proteins, ubiquitination enzymes, and proteasomal subunits by both D1- and D2-specific agonists (Table S1).
Several other proteins identified as differentially regulated by DA in goldfish have also been reported to be involved in human neurological disorders. For example, malate dehydrogenase, CaM, transferrin, tyrosine-3-monooxygenase/tryptophan-3-monooxygenase activation protein epsilon (YWHAE), microtubule-associated protein tau and beta-synuclein are among proteins identified that are known to be involved in neurodegenerative and/or psychiatric diseases [38].
There is a discrepancy between the number of mRNAs and proteins that were identified as differentially regulated that must be addressed. Many mRNAs for abundant ribosomal proteins were induced, and some of the corresponding proteins were detected, but did not change. This is not unexpected since it would be difficult to observe a change in protein concentration above the background of these proteins found in ribosomes in eukaryotic tissues. This is similar to what has been demonstrated in yeast [68]. However, for other mRNAs/proteins, there may be other factors restricting concordant changes. For example, our use of a “snapshot” time frame study is a likely limitation that does not allow us to take into account differences in mRNA versus protein half-lives. Furthermore, steady-state mRNA levels for many ESTs were increased by 5 h following agonist treatments, but the translational machinery may require additional time to efficiently produce the corresponding proteins. Importantly, despite the recognized limitations we outline, the level of concordance in the mRNA and protein changes in the goldfish brain are well within the ranges seen with better characterized vertebrate systems [56], [69], [70]. Future studies aimed at examining the temporal correlation between the hypothalamic transcriptome and proteome should reveal further relationships and critical pathways regulated by the neurotransmitter DA, and provide insights into the neural processes governing reproduction.
In conclusion, we have demonstrated the applicability of advanced high-through genomic and proteomic analyses in an amenable well-studied teleost model species whose genome has yet to be sequenced. Furthermore, we demonstrate the first evidence of D1-receptor involvement in the inhibition of LH release and suggest a mechanism through the potential modulation of other stimulatory neurotransmitters, namely glutamate and/or GABA. Refinement of the bioinformatic methods for time-course analysis should further reveal the importance of DA in regulating hypothalamic function.
Supporting Information
Perl script used to extract amino sequences from GenBank.
(0.24 MB TIF)
Information workflow diagram for comparing mRNAs to proteins.
(0.42 MB TIF)
Number of ESTs identified by microarray analysis as being statistically (q<5%) differentially regulated by dopamine agonists in the hypothalamus of female goldfish 5 h post-i.p.-injection. The data distribution is shown as output from Blast2GO. Duplicates were removed. Overlapping ESTs (i.e. ESTs regulated by more than 1 agonist) are indicated as “Shared between…”.
(0.44 MB TIF)
Multilevel Gene Ontology categorization of the 1042 annotated ESTs into a) Biological Process, b) Molecular Function, and c) Cellular Component. Annotations were first converted to GO-Slim annotations (goslim_generic.obo) and the multilevel chart was constructed using a sequence convergence cutoff of 30 to reduce the complexity of the chart. Both agonists and both up- and down-regulated genes (q<5%) are included in this analysis.
(3.21 MB TIF)
Complete list of cDNAs identified as significantly (q<5%) differentially regulated by SKF 38393 (SKF) or LY 171555 (LY). Negative fold changes indicate a decrease in the mRNA level.
(1.07 MB DOC)
All goldfish proteins identified in the hypothalamus in this study. Proteins in which a single peptide was used in identification are also presented in this table. % Cov is the amount of amino acid coverage (%) by peptides. Ratios (e.g. 115∶114) are each treatment (tag 115 or 117) divided by control (tag 114) to obtain relative fold change. Pval is the p-value after all peptides for a protein were used for quantitation. The Error Factor (EF) expresses the 95% uncertainty range for a reported ratio. The true protein ratio is expected to be found between the (reported ratio)*(EF) and the (reported ratio)/(EF) 95% of the time. Peptides used in quantification also included all peptides with post-translational modifications and all charge states (Dataset S1). Peptides that do not have a Ratio or P-value were not quantified because 1) peptide signal was too low; 2) peptide did not meet standard for quantitation; or 3) peptide belonged to more than one unique protein.
(0.75 MB DOC)
The total number of peptide spectra detected in the goldfish hypothalamus
(2.92 MB XLS)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported financially by an Ontario Graduate Scholarship (JTP), the Parkinson's Research Consortium (JTP, VLT), NSERC Grant 203152 (VLT) and National Institute of Environmental Health Sciences Superfund Research Program RO1 ES015449 (NDD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Martyniuk CJ, Denslow ND. Towards functional genomics in fish using quantitative proteomics. Gen Comp Endocrinol. 2009;164:135–141. doi: 10.1016/j.ygcen.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Popesku JT, Martyniuk CJ, Mennigen J, Xiong H, Zhang D, et al. The goldfish (Carassius auratus) as a model for neuroendocrine signaling. Mol Cell Endocrinol. 2008;293:43–56. doi: 10.1016/j.mce.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 5.Dufour S, Weltzien FA, Sebert ME, N LEB, Vidal B, et al. Dopaminergic inhibition of reproduction in teleost fishes: ecophysiological and evolutionary implications. Ann N Y Acad Sci. 2005;1040:9–21. doi: 10.1196/annals.1327.002. [DOI] [PubMed] [Google Scholar]
- 6.Barbeau A. Dopamine and disease. Can Med Assoc J. 1970;103:824–832. [PMC free article] [PubMed] [Google Scholar]
- 7.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 9.Otto CJ, Lin X, Peter RE. Dopaminergic regulation of three somatostatin mRNAs in goldfish brain. Regul Pept. 1999;83:97–104. doi: 10.1016/s0167-0115(99)00052-x. [DOI] [PubMed] [Google Scholar]
- 10.Peter RE, Nahorniak CS, Chang JP, Crim LW. Gonadotropin release from the pars distalis of goldfish, Carassius auratus, transplanted beside the brain or into the brain ventricles: additional evidence for gonadotropin-release-inhibitory factor. Gen Comp Endocrinol. 1984;55:337–346. doi: 10.1016/0016-6480(84)90001-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhao E, Basak A, Trudeau VL. Secretoneurin stimulates goldfish pituitary luteinizing hormone production. Neuropeptides. 2006;40:275–282. doi: 10.1016/j.npep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Marlatt VL, Martyniuk CJ, Zhang D, Xiong H, Watt J, et al. Auto-regulation of estrogen receptor subtypes and gene expression profiling of 17beta-estradiol action in the neuroendocrine axis of male goldfish. Mol Cell Endocrinol. 2008;283:38–48. doi: 10.1016/j.mce.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Martyniuk CJ, Xiong H, Crump K, Chiu S, Sardana R, et al. Gene expression profiling in the neuroendocrine brain of male goldfish (Carassius auratus) exposed to 17alpha-ethinylestradiol. Physiol Genomics. 2006;27:328–336. doi: 10.1152/physiolgenomics.00090.2006. [DOI] [PubMed] [Google Scholar]
- 14.Mennigen JA, Martyniuk CJ, Crump K, Xiong H, Zhao E, et al. Effects of fluoxetine on the reproductive axis of female goldfish (Carassius auratus). Physiol Genomics. 2008;35:273–282. doi: 10.1152/physiolgenomics.90263.2008. [DOI] [PubMed] [Google Scholar]
- 15.Williams DR, Li W, Hughes MA, Gonzalez SF, Vernon C, et al. Genomic resources and microarrays for the common carp Cyprinus carpio L. Journal of Fish Biology. 2008;72:2095–2117. [Google Scholar]
- 16.Churchill GA. Fundamentals of experimental design for cDNA microarrays. Nat Genet. 2002;32(Suppl):490–495. doi: 10.1038/ng1031. [DOI] [PubMed] [Google Scholar]
- 17.Xiong H, Zhang D, Martyniuk CJ, Trudeau VL, Xia X. Using generalized procrustes analysis (GPA) for normalization of cDNA microarray data. BMC Bioinformatics. 2008;9:25. doi: 10.1186/1471-2105-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martyniuk CJ, Alvarez S, McClung S, Villeneuve DL, Ankley GT, et al. Quantitative proteomic profiles of androgen receptor signaling in the liver of fathead minnows (Pimephales promelas). J Proteome Res. 2009;8:2186–2200. doi: 10.1021/pr800627n. [DOI] [PubMed] [Google Scholar]
- 20.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 22.Aizen J, Meiri I, Tzchori I, Levavi-Sivan B, Rosenfeld H. Enhancing spawning in the grey mullet (Mugil cephalus) by removal of dopaminergic inhibition. Gen Comp Endocrinol. 2005;142:212–221. doi: 10.1016/j.ygcen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Chang JP, Cook AF, Peter RE. Influence of catecholamines on gonadotropin secretion in goldfish, Carassius auratus. Gen Comp Endocrinol. 1983;49:22–31. doi: 10.1016/0016-6480(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 24.Chang JP, Peter RE. Effects of dopamine on gonadotropin release in female goldfish, Carassius auratus. Neuroendocrinology. 1983;36:351–357. doi: 10.1159/000123480. [DOI] [PubMed] [Google Scholar]
- 25.Chang JP, Peter RE, Nahorniak CS, Sokolowska M. Effects of catecholaminergic agonists and antagonists on serum gonadotropin concentrations and ovulation in goldfish: evidence for specificity of dopamine inhibition of gonadotropin secretion. Gen Comp Endocrinol. 1984;55:351–360. doi: 10.1016/0016-6480(84)90003-0. [DOI] [PubMed] [Google Scholar]
- 26.de Leeuw R, Goos HJ, van Oordt PG. The dopaminergic inhibition of the gonadotropin-releasing hormone-induced gonadotropin release: an in vitro study with fragments and cell suspensions from pituitaries of the African catfish, Clarias gariepinus (Burchell). Gen Comp Endocrinol. 1986;63:171–177. doi: 10.1016/0016-6480(86)90153-x. [DOI] [PubMed] [Google Scholar]
- 27.De Leeuw R, Van 't Veer C, Goos HJ, Van Oordt PG. The dopaminergic regulation of gonadotropin-releasing hormone receptor binding in the pituitary of the African catfish, Clarias gariepinus. Gen Comp Endocrinol. 1988;72:408–415. doi: 10.1016/0016-6480(88)90163-3. [DOI] [PubMed] [Google Scholar]
- 28.Dufour S, Lopez E, Le Menn F, Le Belle N, Baloche S, et al. Stimulation of gonadotropin release and of ovarian development, by the administration of a gonadoliberin agonist and of dopamine antagonists, in female silver eel pretreated with estradiol. Gen Comp Endocrinol. 1988;70:20–30. doi: 10.1016/0016-6480(88)90090-1. [DOI] [PubMed] [Google Scholar]
- 29.Peter RE, Paulencu CR. Involvement of the preoptic region in gonadotropin release-inhibition in goldfish, Carassius auratus. Neuroendocrinology. 1980;31:133–141. doi: 10.1159/000123064. [DOI] [PubMed] [Google Scholar]
- 30.Saligaut C, Linard B, Mananos EL, Kah O, Breton B, et al. Release of pituitary gonadotrophins GtH I and GtH II in the rainbow trout (Oncorhynchus mykiss): modulation by estradiol and catecholamines. Gen Comp Endocrinol. 1998;109:302–309. doi: 10.1006/gcen.1997.7033. [DOI] [PubMed] [Google Scholar]
- 31.Levavi-Sivan B, Biran J, Fireman E. Sex steroids are involved in the regulation of gonadotropin-releasing hormone and dopamine D2 receptors in female tilapia pituitary. Biol Reprod. 2006;75:642–650. doi: 10.1095/biolreprod.106.051540. [DOI] [PubMed] [Google Scholar]
- 32.Chang JP, Johnson JD, Sawisky GR, Grey CL, Mitchell G, et al. Signal transduction in multifactorial neuroendocrine control of gonadotropin secretion and synthesis in teleosts-studies on the goldfish model. Gen Comp Endocrinol. 2009;161:42–52. doi: 10.1016/j.ygcen.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Popesku JT, Trudeau VL. Dopamine D1 receptor blockage potentiates AMPA-stimulated LH release in the goldfish (Carassius auratus). Biol Reprod. 2008;78:53. doi: 10.1111/j.1365-2826.2011.02114.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P. Two tyrosine hydroxylase genes in vertebrates New dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci. 2010;43:394–402. doi: 10.1016/j.mcn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Goping G, Pollard HB, Adeyemo OM, Kuijpers GA. Effect of MPTP on dopaminergic neurons in the goldfish brain: a light and electron microscope study. Brain Res. 1995;687:35–52. doi: 10.1016/0006-8993(95)00391-3. [DOI] [PubMed] [Google Scholar]
- 36.Hornby PJ, Piekut DT. Distribution of catecholamine-synthesizing enzymes in goldfish brains: presumptive dopamine and norepinephrine neuronal organization. Brain Behav Evol. 1990;35:49–64. doi: 10.1159/000115856. [DOI] [PubMed] [Google Scholar]
- 37.Peter RE, Gill VE. A stereotaxic atlas and technique for forebrain nuclei of the goldfish, Carassius auratus. J Comp Neurol. 1975;159:69–101. doi: 10.1002/cne.901590106. [DOI] [PubMed] [Google Scholar]
- 38.Altar CA, Vawter MP, Ginsberg SD. Target identification for CNS diseases by transcriptional profiling. Neuropsychopharmacology. 2009;34:18–54. doi: 10.1038/npp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Curr Protein Pept Sci. 2008;9:325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- 40.Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24(Suppl 1):S123–127; discussion S131. doi: 10.1016/s0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 41.Trudeau VL, Spanswick D, Fraser EJ, Lariviere K, Crump D, et al. The role of amino acid neurotransmitters in the regulation of pituitary gonadotropin release in fish. Biochem Cell Biol. 2000;78:241–259. [PubMed] [Google Scholar]
- 42.Trudeau VL, Sloley BD, Peter RE. GABA stimulation of gonadotropin-II release in goldfish: involvement of GABAA receptors, dopamine, and sex steroids. Am J Physiol. 1993;265:R348–355. doi: 10.1152/ajpregu.1993.265.2.R348. [DOI] [PubMed] [Google Scholar]
- 43.Kah O, Trudeau VL, Sloley BD, Chang JP, Dubourg P, et al. Influence of GABA on gonadotrophin release in the goldfish. Neuroendocrinology. 1992;55:396–404. doi: 10.1159/000126150. [DOI] [PubMed] [Google Scholar]
- 44.Trudeau VL. Neuroendocrine regulation of gonadotrophin II release and gonadal growth in the goldfish, Carassius auratus. Rev Reprod. 1997;2:55–68. doi: 10.1530/ror.0.0020055. [DOI] [PubMed] [Google Scholar]
- 45.Martyniuk CJ, Chang JP, Trudeau VL. The effects of GABA agonists on glutamic acid decarboxylase, GABA-transaminase, activin, salmon gonadotrophin-releasing hormone and tyrosine hydroxylase mRNA in the goldfish (Carassius auratus) neuroendocrine brain. J Neuroendocrinol. 2007;19:390–396. doi: 10.1111/j.1365-2826.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 46.Huo L, Lee EK, Leung PC, Wong AO. Goldfish calmodulin: molecular cloning, tissue distribution, and regulation of transcript expression in goldfish pituitary cells. Endocrinology. 2004;145:5056–5067. doi: 10.1210/en.2004-0584. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D, Xiong H, Mennigen JA, Popesku JT, Marlatt VL, et al. Defining global neuroendocrine gene expression patterns associated with reproductive seasonality in fish. PLoS ONE. 2009;4:e5816. doi: 10.1371/journal.pone.0005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, et al. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeuchi Y, Miyamoto E, Fukunaga K. Activation of the rat dopamine D2 receptor promoter by mitogen-activated protein kinase and Ca2+/calmodulin-dependent protein kinase II pathways. J Neurochem. 2002;83:784–796. doi: 10.1046/j.1471-4159.2002.01180.x. [DOI] [PubMed] [Google Scholar]
- 50.Kondo H, Morinaga K, Misaki R, Nakaya M, Watabe S. Characterization of the pufferfish Takifugu rubripes apolipoprotein multigene family. Gene. 2005;346:257–266. doi: 10.1016/j.gene.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Choudhury M, Yamada S, Komatsu M, Kishimura H, Ando S. Homologue of mammalian apolipoprotein A-II in non-mammalian vertebrates. Acta Biochim Biophys Sin (Shanghai) 2009;41:370–378. doi: 10.1093/abbs/gmp015. [DOI] [PubMed] [Google Scholar]
- 52.Zhou L, Wang Y, Yao B, Li CJ, Ji GD, et al. Molecular cloning and expression pattern of 14 kDa apolipoprotein in orange-spotted grouper, Epinephelus coioides. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:432–437. doi: 10.1016/j.cbpb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Babin PJ, Thisse C, Durliat M, Andre M, Akimenko MA, et al. Both apolipoprotein E and A-I genes are present in a nonmammalian vertebrate and are highly expressed during embryonic development. Proc Natl Acad Sci U S A. 1997;94:8622–8627. doi: 10.1073/pnas.94.16.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitale ML, Carbajal ME. Involvement of myosin II in dopamine-induced reorganization of the lactotroph cell's actin cytoskeleton. J Histochem Cytochem. 2004;52:517–527. doi: 10.1177/002215540405200410. [DOI] [PubMed] [Google Scholar]
- 55.Bolognani F, Perrone-Bizzozero NI. RNA-protein interactions and control of mRNA stability in neurons. J Neurosci Res. 2008;86:481–489. doi: 10.1002/jnr.21473. [DOI] [PubMed] [Google Scholar]
- 56.Dihal AA, van der Woude H, Hendriksen PJ, Charif H, Dekker LJ, et al. Transcriptome and proteome profiling of colon mucosa from quercetin fed F344 rats point to tumor preventive mechanisms, increased mitochondrial fatty acid degradation and decreased glycolysis. Proteomics. 2008;8:45–61. doi: 10.1002/pmic.200700364. [DOI] [PubMed] [Google Scholar]
- 57.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, et al. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 61.Bhandari B, Beckwith KD, Miller RE. Cloning, nucleotide sequence, and potential regulatory elements of the glutamine synthetase gene from murine 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1988;85:5789–5793. doi: 10.1073/pnas.85.16.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 63.Masserano JM, Gong L, Kulaga H, Baker I, Wyatt RJ. Dopamine induces apoptotic cell death of a catecholaminergic cell line derived from the central nervous system. Mol Pharmacol. 1996;50:1309–1315. [PubMed] [Google Scholar]
- 64.Ishisaki A, Hayashi H, Suzuki S, Ozawa K, Mizukoshi E, et al. Glutathione S-transferase Pi is a dopamine-inducible suppressor of dopamine-induced apoptosis in PC12 cells. J Neurochem. 2001;77:1362–1371. doi: 10.1046/j.1471-4159.2001.00351.x. [DOI] [PubMed] [Google Scholar]
- 65.Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, et al. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci U S A. 2007;104:1977–1982. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi M, Bradner J, Bammler TK, Eaton DL, Zhang J, et al. Identification of glutathione S-transferase pi as a protein involved in Parkinson disease progression. Am J Pathol. 2009;175:54–65. doi: 10.2353/ajpath.2009.081019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, et al. Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci U S A. 2000;97:12741–12745. doi: 10.1073/pnas.220176997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, et al. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- 69.Maiya R, Ponomarev I, Linse KD, Harris RA, Mayfield RD. Defining the dopamine transporter proteome by convergent biochemical and in silico analyses. Genes Brain Behav. 2007;6:97–106. doi: 10.1111/j.1601-183X.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- 70.Hack CJ. Integrated transcriptome and proteome data: the challenges ahead. Brief Funct Genomic Proteomic. 2004;3:212–219. doi: 10.1093/bfgp/3.3.212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perl script used to extract amino sequences from GenBank.
(0.24 MB TIF)
Information workflow diagram for comparing mRNAs to proteins.
(0.42 MB TIF)
Number of ESTs identified by microarray analysis as being statistically (q<5%) differentially regulated by dopamine agonists in the hypothalamus of female goldfish 5 h post-i.p.-injection. The data distribution is shown as output from Blast2GO. Duplicates were removed. Overlapping ESTs (i.e. ESTs regulated by more than 1 agonist) are indicated as “Shared between…”.
(0.44 MB TIF)
Multilevel Gene Ontology categorization of the 1042 annotated ESTs into a) Biological Process, b) Molecular Function, and c) Cellular Component. Annotations were first converted to GO-Slim annotations (goslim_generic.obo) and the multilevel chart was constructed using a sequence convergence cutoff of 30 to reduce the complexity of the chart. Both agonists and both up- and down-regulated genes (q<5%) are included in this analysis.
(3.21 MB TIF)
Complete list of cDNAs identified as significantly (q<5%) differentially regulated by SKF 38393 (SKF) or LY 171555 (LY). Negative fold changes indicate a decrease in the mRNA level.
(1.07 MB DOC)
All goldfish proteins identified in the hypothalamus in this study. Proteins in which a single peptide was used in identification are also presented in this table. % Cov is the amount of amino acid coverage (%) by peptides. Ratios (e.g. 115∶114) are each treatment (tag 115 or 117) divided by control (tag 114) to obtain relative fold change. Pval is the p-value after all peptides for a protein were used for quantitation. The Error Factor (EF) expresses the 95% uncertainty range for a reported ratio. The true protein ratio is expected to be found between the (reported ratio)*(EF) and the (reported ratio)/(EF) 95% of the time. Peptides used in quantification also included all peptides with post-translational modifications and all charge states (Dataset S1). Peptides that do not have a Ratio or P-value were not quantified because 1) peptide signal was too low; 2) peptide did not meet standard for quantitation; or 3) peptide belonged to more than one unique protein.
(0.75 MB DOC)
The total number of peptide spectra detected in the goldfish hypothalamus
(2.92 MB XLS)