Abstract
We generated influenza virus-like particles (VLPs) containing the wild type (WT) H5 hemagglutinin (HA) from A/Viet Nam/1203/04 virus or a mutant H5 HA with a deletion of the multibasic cleavage motif. VLPs containing mutant H5 HA were found to be as immunogenic as VLPs containing WT HA. A single intramuscular vaccination with either type of H5 VLPs provided complete protection against lethal challenge. In contrast, the recombinant H5 HA vaccine was less immunogenic and vaccination even with 5 fold high dose did not induce protective immunity. VLP vaccines were superior to the recombinant HA in inducing T helper type 1 immune responses, hemagglutination inhibition titers, and antibody secreting cells, which significantly contribute to inducing protective immunity after a single dose vaccination. This study provides insights into the potential mechanisms of improved immunogenicity by H5 VLP vaccines as an approach to improve the protective efficacy against potential pandemic viruses.
Keywords: Virus like particles, subunit vaccine, influenza virus, H5N1, protection, memory immune responses
Introduction
Wild aquatic birds are a continuous source of influenza viruses and provide a reservoir for each of the 16 known subtypes of hemagglutinin (HA) and 9 subtypes of neuraminidase (NA) (Fouchier et al., 2005; Rohm et al., 1996; Webster et al., 1992). The emergence or re-emergence of highly pathogenic avian influenza (HPAI) viruses in domestic poultry and the increasing numbers of direct transmission of avian viruses to humans underscore the persistent threat to public health. Six fatal cases of human infection with HPAI H5N1 virus were first reported during an outbreak in 1997 in Hong Kong (Claas et al., 1998; Subbarao et al., 1998). Since then, repeated outbreaks of HPAI H5N1 virus have emerged and spread to countries across Asia, Europe, the Middle East, Africa, and the Pacific. Human cases have increased with a high fatality rate of 60%, and some H5N1 isolates shown resistance to the antiviral drugs amantadine and rimantadine (Cheung et al., 2006; de Jong et al., 2005; Le et al., 2005). Currently, H5N1, H7N2, H7N3, H7N7 and H9N2 avian origin influenza viruses have been shown to cause human infections on multiple occasions (Fouchier et al., 2004; Peiris et al., 1999; Wong and Yuen, 2006). Most recently, the 2009 outbreak of a new H1N1 virus illustrates how fast a new pandemic virus can spread in the human population once it acquires the ability to transmit among humans (Nava et al., 2009; Solovyov et al., 2009). Vaccination is considered to be the most effective preventive measure against potential epidemic and pandemic influenza viruses.
Among the licensed vaccine formulations, split and subunit vaccines are most frequently used for immunization against the yearly influenza epidemics (Nicholson et al., 1979; Wright et al., 1977). In preclinical studies, two doses of inactivated split vaccine against H5 subtype provided protection against lethal challenges with homologous or heterologous H5N1 viruses in mice or ferrets (Govorkova et al., 2006; Lipatov et al., 2006; Nicolson et al., 2005; Subbarao et al., 2003; Webby et al., 2004). Phase I clinical trials indicate that inactivated split vaccines are not optimally immunogenic (Nicholson et al., 2001) and require multiple doses (Stephenson et al., 2003) or the inclusion of an adjuvant to induce a protective immune response (Atmar et al., 2006; Bresson et al., 2006; Hehme et al., 2002; Lin et al., 2006). A baculovirus-expressed recombinant H5 protein or subvirion H5 influenza vaccine was poorly immunogenic and two doses of 90 μg HA were required to develop a desired antibody response to the vaccine (Treanor et al., 2006; Treanor et al., 2001). Although live, attenuated virus vaccines are licensed in an intranasal spray form, there are some challenges for developing an effective and safe live attenuated vaccine against avian influenza viruses (Subbarao and Joseph, 2007). There is a risk of gene reassortment of the live vaccine virus with human influenza viruses.
The currently licensed influenza vaccines are produced in embryonated hen eggs, and the manufacturing process usually takes approximately 6 months when a new virus seed is required. If the pandemic virus causes widespread morbidity and mortality in poultry, the supply of embryonated eggs needed for producing seasonal and pandemic vaccines at the same time might be significantly compromised. Therefore, developing an alternative vaccine production system not reliant on egg substrates is highly desirable.
Most pandemic vaccine candidates such as inactivated split or live attenuated virus contain a modified HA with a deletion of the multibasic amino-acid motif that is involved in broadening the tropism and in enhancing the virulence for HPAI viruses (Steinhauer, 1999). The effect of the multibasic amino-acid motif on inducing protective immunity by pandemic vaccines has not been well studied. Also, we do not understand the basis for the low immunogenicity of split or subunit H5 vaccines in naïve individuals. In addition, protective immune correlates, doses of vaccines, and frequencies of vaccination required for inducing protection against pandemic viruses are not well understood. Therefore, we investigated some of these important issues in this study using the novel vaccine platform of non-infectious influenza VLPs.
Our laboratory and others have developed H5 influenza VLP vaccines produced in an egg-free cell culture system using recombinant technology (Bright et al., 2008; Haynes et al., 2009; Kang et al., 2009). Influenza VLP vaccines do not require handling of live influenza virus during the development, manufacturing, or administration. In this study, we have used a mouse model to investigate the immunogenicity of influenza VLPs containing the WT H5 HA or mutant H5 HA with a deletion of the multibasic amino-acid motif in the cleavage site as well as a recombinant H5 HA protein vaccine after a single dose intramuscular vaccination.
Materials and Methods
Cells, Virus, and Reagents
Spodoptera frugiperda sf9 insect cells were purchased from the American Type Culture Collection (ATCC, CRL-1711) and maintained in SF900-II SFM medium at 27° C incubator. Sf9 insect cells were used for production of recombinant baculoviruses (rBVs) and VLPs. Madin-Darby canine kidney (MDCK) cells for viral titration were purchased from ATCC and cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM) with 10% fetal bovine serum at 37°C, 5% CO2 conditioned incubator. The WT virus used for challenge studies was derived by reverse genetics from HPAI A/Viet Nam/1203/04 (VN/04) H5N1 virus isolated from a fatal human infection (Sui et al., 2009). Vaccine candidate virus rgΔH5N1 containing the multibasic amino acid deletion in HA gene and the wt NA gene from VN/04 and internal protein-coding genes from A/Puerto Rico/8/34 (PR/8/34) was reported previously (Donis, 2005; Wan et al., 2008). The virus was inactivated as described (Kang et al., 2009) and used as an H5 viral antigen for in vivo and in vitro antigenic challenge studies. All work with live H5N1 virus was performed in bio-safety level 3 facilities at the Centers for Disease Control and Prevention. Purified recombinant H5 HA protein that was produced using a baculovirus expression system was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository (NIAID, NIH) and used for vaccination. The VN/04 H5 HA protein expressed in mammalian cells (293T cells) by transfection was purified and used for an ELISA antigen.
Preparation of H5 VLPs
Influenza H5 VLPs were generated and purified by following a procedure previously described (Kang et al., 2009; Quan et al., 2007). To generate the rBVs expressing influenza proteins, M1, and WT or multi-basic amino acid deleted (del or Δ) HA, genes from influenza VN/04 virus were cloned into Bacmid (Invitrogen, Carlsbad, CA). Lipofectin mixed with 2 μg of Bacmid was used for plasmid transfection with sf9 insect cells and recombinant baculoviruses were generated according to the manufacturer’s instruction. rBvs expressing HA and M1 protein were co-infected into sf9 insect cells to produce H5 VLPs. At 3 days post-infection, the infected cell culture supernatants were clarified by centrifugation and then were concentrated by hollow fiber based filtration using Quixtand (GE Healthcare, Waukesha, WI). Sucrose gradient ultracentrifugation with layers of 20%, 30% and 60% (wt/vol) was carried for purification of VLPs at 28,000× g for 60 min. WT and del-H5 VLPs were mainly purified from a band between 30–60% sucrose gradient.
Characterization of H5 VLPs
The morphology of VLPs was examined by electron microscopy as described (Quan et al., 2007). Expression of influenza proteins in VLP were analyzed by SDS-PAGE using 12% polyacrylamide gels with Gel code blue staining solution (Thermo) and by western blotting using rabbit anti-HA polyclonal antibodies (ProSci, Poway, CA) or mouse anti-M1 antibody (Serotec, Raleigh, NC) followed by detection with horseradish peroxidase (HRP)-conjugated goat-anti rabbit or mouse IgG (Southernbiotech, Birmingham, AL). To quantitate the incorporating influenza proteins in VLP, the total proteins of VLP and purified recombinant HA were measured by DC protein assay kit (Bio-Rad, Irvine, CA) and normalized to 1 μg/μl of total protein concentration. Densitometer scanning of the HA protein band was performed using a Fluorchem FC2 imaging system (Alpha Innotech, San Leandro, CA) and the data analyzed using Alphaview software (Alpha Innotech). Conformational integrity and biological activity of HA protein on VLPs were determined by trypsin cleavability and hemagglutination assays as previously described (Kang et al., 2009).
Immunization and challenge
Six to eight-week-old female BALB/c mice (Charles River, Wilmington, MA) were housed in the animal facility of Emory University and all experiments were carried out following the approved IACUC protocol. Groups of mice (n=9) were given an intramuscular (i.m.) injection with 50 μl PBS solution containing 0.4 μg or 2.0 μg based on HA content of either recombinant HA, WT or del H5 VLPs. Immunized or PBS control mice were challenged with 50 fold of 50% mouse lethal doses (50 MLD50) of WT VN/04 virus. Challenged mice were monitored daily for disease signs and body weight changes for 2 weeks. All challenge experiments with VN/04 virus were performed under the enhanced animal bio-safety level 3 facilities in the Centers for Disease Control and Prevention.
To investigate plasma and memory response, additional mice were allotted into another 3 groups (n=6) and intramuscularly vaccinated with 2 μg of recombinant HA, WT or del H5 VLPs containing 2 μg HA.
Virus titration
Infected mice were euthanized at 4 days post challenge. Lung, brain and spleen were harvested and homogenized in 1 ml of PBS for virus titration using a plaque assay in MDCK cells as previously described (Kang et al., 2009). The limit of virus detection was 50 pfu (1.69 Log10) per 1ml of organ homogenate.
Serologic test for determination of antibody responses
Peripheral blood samples were collected before and after immunization at different time points. Influenza virus specific antibody titers were determined by ELISA as previously described (Quan et al., 2007). Briefly, 4 μg of inactivated influenza rg ΔH5N1 virus or recombinant H5 HA protein produced in mammalian cells were used as a coating antigen on the 96 well microtiter plates (Nunc, Rochester, NY) with overnight incubation at 4 °C. The wells were washed three times with PBS containing 0.05% Tween 20 (PBST) and blocked with PBST containing 3% BSA for 2h at 37 °C. Serially diluted serum samples were added and incubated for 1hr at 37 °C then HRP-conjugated goat anti-mouse IgG, IgG1, IgG2a and IgG2b were used as secondary antibodies and O-phenylenediamine (OPD) as a substrator. To determine the antibody titers, absorbance was read at 450 nm. Receptor destroying enzyme (RDE) treated serum samples were used for hemagglutination inhibition (HI) assay (Wakamiya et al., 1992). Briefly, two fold serial diluted serum samples (25 μl) were incubated with equal volumes of inactivated rgΔH5N1 virus (4HAU) at room temperature (RT) for 30 min. After incubation, equal volume of 1% horse erythrocytes was added and incubated at RT for 1 h. The HI titer was expressed as the reciprocal of the highest dilution of the samples and the detection limit was 20 HAI.
ELISPOT for determination of T cell responses
Capture antibodies against mouse IFN- γ and IL-4 cytokines (3 μg/ml in coating buffer, BD Biosciences, San Diego, CA) were coated on the Multi-screen 96 well plates (Millipore, Billerica, MA). Splenocytes (1.0 × 10° cells/well) isolated from different vaccinated or control mice were cultured on the plate with 100 μl of RPMI1640 media with 10% FBS with or without HA1 or HA2 subunit peptide pool stimulator (10 μg/ml) derived from A/Thailand/16/04 (H5N1) which has highly homologous HA with VN/04 (Kang et al., 2009). After 36 h incubation, the plates were washed with PBS and further incubated with biotinylated rat anti-mouse IFN- γ or IL-4 (1 μg/ml in PBST with 5% FBS). After three additional washes, HRP-conjugated streptavidin solution was added to each well for 3 h at RT. The plates were washed and developed with stable DAB (3, 3-Diaminobenzidine). The number of IFN- γ or IL-4 secreting T cells was counted using an ImmunoSpot ELISpot reader (Cellular Technology, Shaker Heights, OH.).
Antibody secreting cell assay
Bone marrow and spleen derived single cell suspensions were prepared at 6 months post vaccination with a single dose of VLPs or recombinant vaccine with or without antigen rechallenge 6 days before sacrifice. Bone marrow (0.5 × 106 cells/well) and spleen (1.0 × 106 cells/well) cells were placed in 96 well culture plate with or without VN/04 influenza inactivated virus coating for in vitro antigen stimulation and subjected to ex vivo cultures. Culture supernatants were harvested at day 1 and 5 and levels of H5 HA specific antibodies in the culture supernatants secreted by antibody secreting cells were determined by ELISA.
Statistical analysis
To determine the statistical significance, the Kaplan-Meier survival analysis was performed using Graphpad PRISM. All experimental data were recorded for individuals within groups unless specified. A two-tailed Student’s t-test was used when comparing two different conditions. A p value less than 0.05 was considered to be significant.
Results
Production and characterization of H5 influenza VLPs
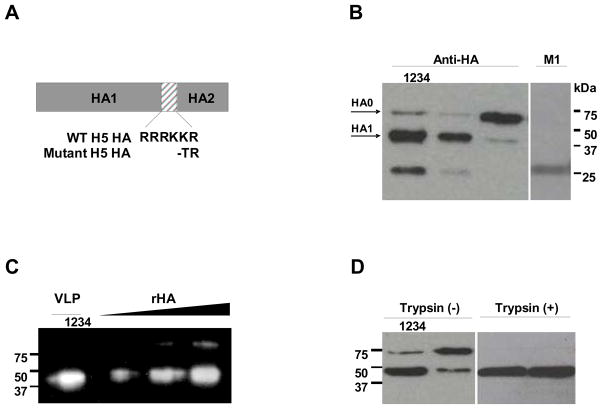
The highly virulent nature of HPAI viruses generates safety concerns for use in manufacturing. Therefore, viruses containing mutated HA have been previously generated for vaccine production purposes (Govorkova et al., 2006; Lipatov et al., 2006; Subbarao et al., 2003). Recent studies suggest that antibodies to the fusion peptide of the HA, adjacent to the deleted amino acids, may contribute to protective immunity (Sui et al., 2009). In this study, we compared the H5N1 influenza VLPs containing the WT or mutant HA with a deletion of multi-basic amino acids in the cleavage site (Fig. 1A) using the rBV expression system. Both WT and del H5 VLPs were found to exhibit hemagglutination titers (HAU) in the range of 2560 to 5120 (1 mg of VLPs in 1 ml PBS solution).
Figure 1. Characterization of influenza H5 VLPs.
(A) Schematic diagram of the WT and mutant H5 HA genes used for preparation of H5 VLPs. The multi-basic amino acid motif was deleted in the mutant H5 HA. (B) Western blot of H5 VLPs. Recombinant H5 HA protein (lane 1, 0.2ug) and WT (lane 2) or mutant (lane 3 and 4) H5 VLPs purified by sucrose gradient ultracentrifugation were analyzed for incorporation of HA and NA (data not shown) by western blotting (1 μg/lane) using rabbit anti-HA antibody or mouse anti-M1 antibody. (C) Spot densitometry analysis of HA protein incorporation in VLPs. Three micrograms of WT H5 VLPs were loaded in lane 1 and three different amount of H5 rHA proteins (100, 200, 400 ng) were in lane 2, 3, and 4 respectively. The chemiluminescence signals were digitized by densitometer scanning of image intensities on western blot. VLPs and recombinant proteins were quantitated by Alphaview software (Alpha Innotech). (D) Trypsin cleavability of H5 HA incorporated into VLPs. WT (lane 1 and 3) and mutant (lane 2 and 4) H5 VLPs (1 μg/lane) were incubated without or with 0.1ug of TPCK-treated trypsin for 2 h at 37°C and cleaved form of proteins were confirmed by western blotting.
Incorporation of influenza virus proteins into VLPs was confirmed by western blotting (Fig. 1B). As we expected, a different cleavage pattern of HA protein was observed between the WT and mutant form of HA. Mutant del H5 VLPs lacking the polybasic amino acid in HA cleavage site showed the precursor HA0 as the major HA form incorporated (Fig. 1B Lane3). On the other hand, HA1, a cleaved HA product of approximately 42 kDa, was the dominant protein in the WT H5 VLPs (Fig. 1B Lane2) and recombinant H5 HA protein (Fig.1B Lane1), both of which were expressed in insect cells. The observation of the HA1 cleavage product in the mutant H5 VLPs even as a minor band indicates the presence of protease like furin in insect cells (Stieneke-Grober et al., 1992), which can cleave mutant H5 HA0 but much less efficiently compared to the wild type H5 HA0. To determine the amount of HA protein incorporated in H5 VLPs, we performed immunoblotting with H5 VLPs in comparison with H5 HA recombinant proteins (100, 200, 400 ng) as a reference standard (Fig 1C). The chemiluminescence intensity which represents the protein amount was measured by spot densitometry to calculate the protein content based on densitometry scanning (Fig 1C). As a result, the amount of HA incorporated in 3μg of VLPs was estimated to be equivalent to 341 ng of HA recombinant protein, representing approximately 11.4% of the total VLP proteins. Finally, we determined the accessibility of trypsin to the H5 HA protein in VLPs. As shown in Fig 1D, both the WT (Fig. 1D Lane1 and 3) and mutant H5 HA proteins (Fig. 1D Lane2 and 4) incorporated into VLPs were similarly susceptible to trypsin-mediated cleavage.
These results indicate that both WT and mutant H5 HA proteins incorporated into VLPs maintain conformational integrity. Also, the WT H5 VLPs incorporated a cleaved form of HA dominantly as compared to that of mutant H5 VLPs that contain predominantly a precursor form of HA. Thus, insect cells can effectively process the WT H5 HA proteins containing multiple basic amino acids at the cleavage site.
Kinetics of antibody responses after a single vaccination with HA protein or VLPs
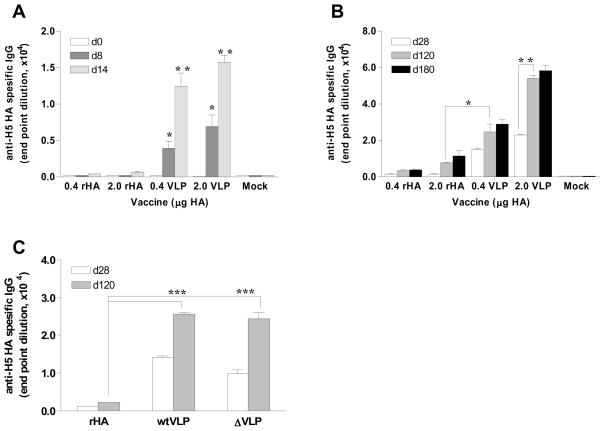
Conventional inactivated influenza vaccines are licensed for use by intramuscular injection. To determine the immunogenicity of H5 antigens in a recombinant HA (rHA) or particulate VLP formulation, groups of 9 mice were intramuscularly immunized with a low (0.4 μg H5 HA) or high (2.0 μg H5 HA) dose of rHA or WT H5 VLPs. Serum antibody responses induced after a single dose vaccine were evaluated at different time points (days 8, 14, 28, 120 and 180) by ELISA coated with the purified H5 HA protein antigen. As early as day 8 post immunization, low but significant levels of H5 HA specific antibodies were induced in the groups of VLP immunized mice (Fig. 2A, p <0.05 between same dose of rHA and VLP groups). The levels of antibodies gradually increased up to day 120 post vaccination for high dose VLP groups (Fig. 2B, p <0.01), and were maintained for at least 6 months.
Figure 2. Kinetics of antibody responses after a single dose vaccination.
Groups of mice (n=9) were immunized by intramuscular injections with WT H5 VLPs containing 0.4 or 2.0 μg HA, or with the 0.4 or 2.0 μg of H5 HA recombinant protein vaccine. (A) H5 HA specific IgG antibody titers by ELISA in sera at days 0, 8 and 14 (d0, d8, d14 respectively) post a single dose vaccination. (B) H5 HA specific IgG antibody titers at days 28, 120 and 180 (d28, d120, d180 respectively) post a single dose vaccination. (C) Titers of H5 HA specific IgG antibodies among different forms of vaccine. wtVLP: VLPs containing the 0.4 μg of WT H5 HA, ΔVLP: VLPs containing 0.4 μg of the mutant H5 HA. Titers are expressed as the highest fold dilution giving an optical density at 450nm greater than the mean value plus 3 standard deviations of naïve mouse serum samples. The asterisk indicates a significant difference between groups, *p < 0.05, ** p < 0.01, *** p < 0.001.
In contrast, after a single dose immunization with recombinant H5 HA protein, no significant levels of antibodies were observed until day 28 post immunization even in the high dose HA group (2.0 μg of HA). At days 120 and 180 post vaccination, antibodies were detected in rHA immunized groups but the levels were significantly lower than even low dose VLP immunized groups (Fig. 2B, p<0.05). Mutant VLP vaccinated group also showed significantly higher levels of antibodies than those in recombinant H5 HA groups (Fig. 2C, p<0.001) and the antibody induction kinetics were similar to those observed in the WT H5 VLPs (Fig. 2C). Therefore, the results indicate that both WT and mutant H5 VLP vaccines were similarly superior to the recombinant H5 HA vaccine in inducing higher antibody responses with faster kinetics.
Antibody isotypes and hemagglutination inhibition titers
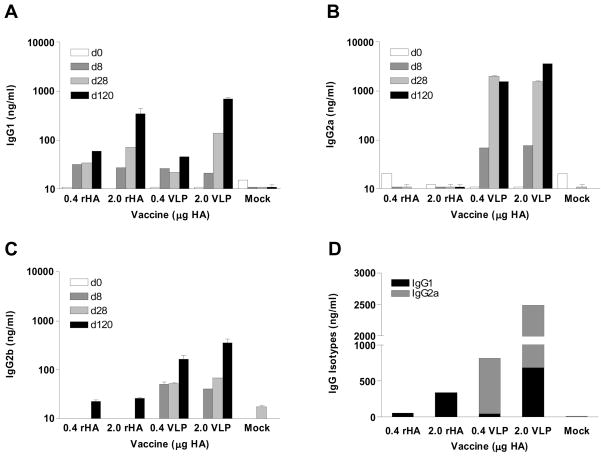
To further characterize the antibody responses, we determined the pattern of isotypes induced by VLP or recombinant protein vaccination. IgG1 antibody, which reflects a T helper type 2 (Th2) biased response, was found to be the dominant subclass in the high dose soluble protein vaccination group when analyzed at day 120 post vaccination (Fig. 3A). In contrast, the low dose VLP group induced IgG2a and IgG2b antibodies predominantly at days 28 and 120 post vaccination, indicating Th1 biased responses (Fig. 3B, C). The high dose VLP vaccination induced not only IgG2a and IgG2b antibodies but also the IgG1 subclass, representing both Th1 and Th2 immune responses (Fig. 3D). The kinetic analysis of isotypes showed that VLP vaccines induced IgG2a isotype antibody at an earlier time point than other subclasses (Fig. 3B). Taken together, the results show that VLP vaccines elicited robust and sustainable antibody responses. Also, the types of vaccines (VLP versus recombinant form) as well as doses of vaccines affected the pattern and kinetics of isotype antibody responses.
Figure 3. Kinetics of isotype antibody responses.
(A) IgG1, (B) IgG2a, (C), IgG2b, (D) sum of IgG2a/IgG1 at days 120. Groups of mice (n=9) immunized with a single dose are the same as described in the legend of Fig. 2. IgG2a and IgG1 isotype titers were determined by quantitative ELISA (average±S.D.) of 1:100 diluted serum samples.
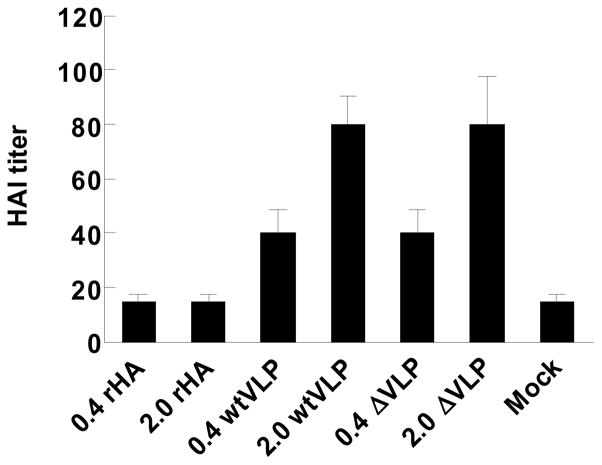
To assess the induction of functional antibodies by each vaccine, we determined the hemagglutination inhibition (HI) titers by measuring the ability of serum samples to inhibit agglutination of horse erythrocytes (Gillim-Ross and Subbarao, 2006). Sera from mice immunized with recombinant H5 HA vaccines regardless of doses showed only background levels of HI titers, similar to serum samples from naïve mice (Fig. 4). Low doses (0.4 μg HA) of both WT and del H5 VLP vaccines elicited meaningful HI titers of 40. High doses of H5 VLP vaccines elicited HI titers of 80, an average 2 fold increase. Therefore, presentation of H5 HA on VLPs was likely to have displayed HA in a more immunogenic form and induced higher levels of functional antibodies compared to the recombinant HA protein vaccine.
Figure 4. Hemagglutination inhibition (HI) titers.
Serum samples were collected at 4 months after a single dose vaccination and HI titers were determined by standard methods using 4 HA units of inactivated reassortant H5N1 virus (rg H5N1) and 1% horse erythrocyte suspension. rHA, recombinant H5 HA protein; WTVLP, influenza VLPs containing H5 WT HA; ΔVLP, influenza VLPs containing H5 mutant HA. Parenthesis indicates the amount of HA protein used per mouse. Mock, a mock control group immunized with PBS buffer.
Protective efficacy of the H5N1 influenza VLP vaccine in mice
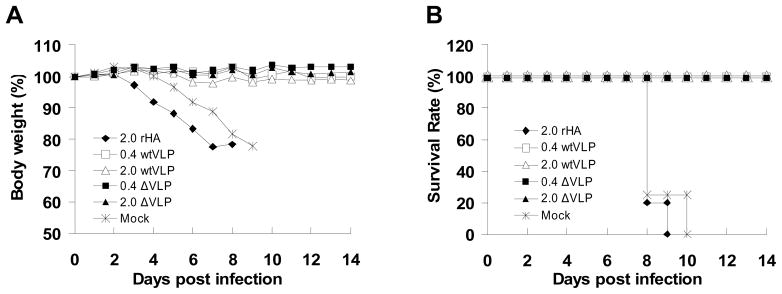
The protective efficacy of recombinant H5 HA and VLP vaccines was assessed at 6 months after a single dose vaccination. Immunized mice in groups of 6 were intranasally challenged with 50 MLD50 of WT H5N1 influenza virus (VN/04) and their health status monitored daily for 14 days. As shown in Fig 5A, all groups that received a single dose of either wild type or mutant H5 VLPs were 100% protected from lethal challenge. No significant body weight loss was observed in mice immunized with either WT or del VLPs even containing a low dose of HA (0.4 μg). On the other hand, mice vaccinated with the recombinant HA protein or naïve infected mice rapidly lost body weight and died or had to be euthanized because of their severe clinical symptoms on days 8 to 10 post challenge (Fig. 5A and B).
Figure 5. Protection against wild type influenza A/Viet Nam/1203/04 (H5N1) virus challenge by a single dose vaccination.
Groups of vaccinated mice and a mock control (PBS) were intranasally challenged with a lethal dose (50 x MLD50) of H5N1 influenza virus (n=6 per group). (A) Body weight changes. (B) Survival rate. The percent of body weight loss calculated from the initial body weight taken at day 0 was monitored for 14 days. Data are the average from 6 individual mice. rHA, recombinant H5 HA protein; wtVLP, influenza VLPs containing H5 WT HA; ΔVLP, influenza VLPs containing H5 mutant HA. Parenthesis indicates the amount of HA used per mouse. Mock, a mock control group injected with PBS buffer.
We also determined virus titers in lung, spleen and brain of the challenged mice on day 4 post infection. The lung viral titers of mice immunized with 2.0 μg of WT H5 VLPs were approximately 600-fold lower than that of the naïve control. Importantly, lung viral titers in mice immunized with the high dose of mutant H5 VLPs were below the detection limit (Fig. 6). The lung viral titers in mice immunized with the low dose of WT or mutant H5 VLPs were lower by 60 or 10 fold compared to the naïve control group, respectively. Challenge virus was detected in the brain in one of the recombinant HA vaccinated and control mice. However, there was no viral spread to any other organs in VLP immunized mice (Fig. 6).
Figure 6. Effective control of viral replication by a single dose VLP vaccination.
Groups of mice that received a single intramuscular vaccination with recombinant H5 HA protein or H5 VLPs were intranasally infected with influenza A/Viet Nam/1203/04 (H5N1) virus (50 x MLD50) and sacrificed on day 4 post challenge (n=3). Spleen, lung and brain tissue samples were harvested from individual mice and the homogenates were used for plaque assay to determine virus titers. Data are represented by the mean virus titer (plaque forming unit [pfu] per ml of homogenate) with standard error of mean (SEM). The dashed line indicates the limit of detection (1.7 log10 pfu/ml). * Virus was isolated from one out of three mice.
Influenza virus specific antibody secreting cell (ASC) responses in bone marrow
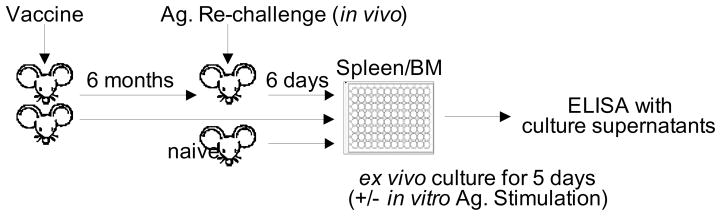
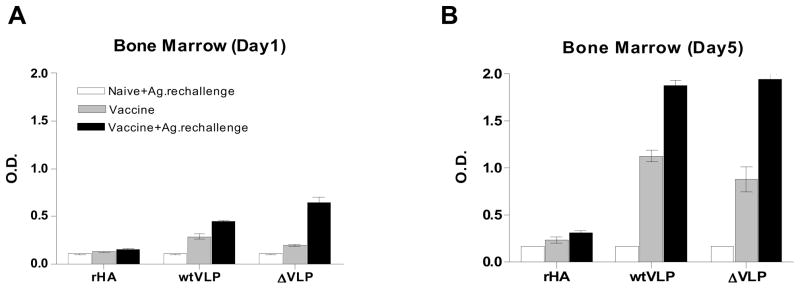
Since a single intramuscular vaccination with influenza VLP vaccines induced long term protective immunity, we wanted to determine the levels of antibody secreting cells in the bone marrow where long-lasting plasma cells reside. To study antibody responses of the plasma and memory B cells generated by vaccination as diagrammed in Fig. 7, mice (n=3) immunized intramuscularly once with recombinant H5 HA or VLPs 6 month earlier were sacrificed or were exposed to the corresponding vaccine (2 μg recombinant HA or 0.4 μg mutant or wt H5 HA VLPs) via the same route of delivery 6 days earlier prior to sacrifice. Bone marrow cells were cultured for 1 to 5 days in the absence of in vitro antigenic stimulation (Fig. 8). Ex vivo culture supernatants were harvested at days 1 and 5, and the H5 HA specific IgG levels were measured by ELISA using the recombinant H5 HA as a coating antigen. As shown in Fig. 8A, IgG antibodies secreted into the 1-day bone marrow cell culture media were detected even 6 month after a single VLP vaccination, but not with recombinant H5 HA vaccination (stripe bar and Table 1). Significant increases were observed in the levels of secreted antibodies from the group of VLP-vaccinated mice that received in vivo the VLP antigenic challenge 6 days prior to sacrifice (Table 1). In contrast, after treatment with in vivo recombinant HA antigenic challenge, the recombinant H5 HA immunized mice did not show such increases in the ex vivo antibody production.
Figure 7. Experimental design to determine antibody secreting plasma cells and recall immune responses.
To evaluate long-lived plasma and memory B cells, groups of mice were intramuscularly vaccinated with a single dose. Six months later a set of the same groups was intramuscularly re-immunized with the same vaccine antigen (Ag) 6 days earlier before sacrifice to determine in vivo recall immune responses (vaccine +/− Ag. rechallenge). In vitro Ag stimulation indicates in vitro antigen exposure with inactivated virus to activate pre-existing memory B cells.
Figure 8. In vitro antibody production during bone marrow cell culture.
Bone marrow cells isolated from vaccinated mice with or without in vivo vaccine Ag. re-challenge were placed on a 96-well plate in quadruplicate and incubated for 5 days without in vitro stimulation. Culture supernatants were harvested on day 1 (A) and day 5 (B) post culture, and influenza specific IgG levels in culture supernatants (2 x diluted) were determined by ELISA. Groups of mice (n=3) were intramuscularly vaccinated with recombinant H5 HA (rHA, 2 μg HA), influenza VLPs containing WT H5 HA (wtVLP, 2 μg HA), or influenza VLPs containing mutant H5 HA (ΔVLP, 2 μg HA) 6 months ago. Control (no pre vaccination), groups of naïve mice (n=3) intramuscularly injected with the corresponding vaccine indicated (2 μg of rHA, wtVLP or ΔVLP containing 0.4 μg HA) 6 days earlier prior to sacrifice. Vaccine: Vaccinated mice (n=3) without in vivo antigenic challenge. Vaccine+Ag.rechallenge, groups of vaccinated mice (n=3) intramuscularly 6 months earlier were re-immunized with the same vaccine (2 μg of rHA, WTVLP or delVLP containing 0.4 μg HA) 6 days prior to sacrifice.
Table 1.
IgG titers induced by plasma cell in BM during ex-vivo culturea
| Days of incubation | Recombinant HA | wtVLP | ΔVLP | none | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Vaccine | Vacc.+Ag. rechallenge | Control | Vaccine | Vacc.+Ag. rechallenge | Control | Vaccine | Vacc.+Ag. rechallenge | naïve | |
| day 1 | b106±26.3 | 84.2±24.6 | 122±31.1 | 56.1±19.9 | 364±98.3 | 1290±253 | 50.7±16.3 | 183±73.1 | 1670±402 | 40.0±8.5 |
| day 5 | 165±32.9 | 334±189 | 576±194 | 56.8±5.60 | 1070±286 | 3810±576 | 193±29.5 | 808±433 | 4150±651 | 84.7±6.1 |
IgG titers induced by plasma cell determined with quantitative ELISA.
Values are means ± S.D. (ng/ml) of four independent ex-vivo culture plate wells.
Control: Naïve mice (n=3) with in vivo antigenic challenge 6 days earlier before sacrifice. Vaccine: Vaccinated mice (n=3) without in vivo antigenic challenge. Vacc.+Ag. rechallenge: Vaccinated mice (n=3) with in vivo antigenic challenge 6 days earlier before sacrifice.
To determine the continued production of antibodies by plasma cells, the cultures were extended to 5 days. The IgG antibodies accumulated significantly in culture supernatants in both WT and mutant H5 VLP vaccination groups but only negligible amounts of antibodies were secreted into the culture supernatants in the recombinant H5 HA vaccinated group during the 5 days of culture (Fig. 8B stripe and closed bar and Table 1). Also, neither detectable levels nor cumulative effects were in the unvaccinated control group with an exposure to the in vivo VLP antigenic challenge 6 days earlier (Fig. 8 open bar, Table 1). These results provide evidence that VLPs are superior to the recombinant HA vaccine in generating long-lived antibody secreting plasma cells in bone marrow. Also, these results indicate that VLP vaccination can induce rapid increases in antibody secreting plasma cells in bone marrow within a time frame of 6 days post in vivo antigen exposure.
Antibody secreting and recall B cell responses in spleen induced by VLP vaccination
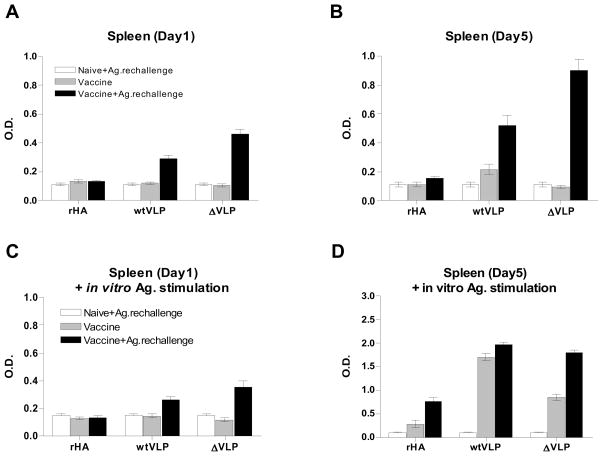
In order to evaluate the influenza virus specific memory B cell responses induced at 6 months after a single dose of VLP intramuscular vaccination, we performed a plasma and memory B cell assay with splenocytes with or without exposure to in vivo antigenic challenge followed by the presence or absence of in vitro exposure to inactivated H5 virus (Fig. 7). During the first day of ex vivo culture of splenocytes collected from mice vaccinated with recombinant HA or VLP vaccine without in vivo antigenic challenge, the levels of antibodies in the culture supernatants were negligible regardless of antigenic stimulation during in vitro cultures (Fig. 9A and C stripe bar). Even after 5 days of extended culture without the antigenic stimulation, the levels of antibody production ex vivo were not significant except for slight increases in the WT VLP immunized group (Table 2).
Figure 9. In vitro antibody production by spleen cell culture.
Groups of mice are the same as described in the legend of Fig. 8. Splenocytes were incubated in the absence (A, B) or in the presence (C, D) of inactivated A/VN/04 virus coated on the 96 well culture plates for in vitro stimulation and incubated for 5 days. Culture supernatants were harvested at day 1 (A, C) and day 5 culture (B, D), and influenza specific IgG levels were determined by ELISA.
Table 2.
IgG titers induced by ASC in spleen during ex-vivo culturea
| Days of incubation | Recombinant HA | wtVLP | ΔVLP | none | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Vaccine | Vacc.+Ag. rechallenge | Control | Vaccine | Vacc.+Ag. rechallenge | Control | Vaccine | Vacc.+Ag. rechallenge | naïve | |
| day 1 | b88.9±33.2 | 98.0±24.2 | 95.1±19.4 | 70.7±21.4 | 83.3±25.1 | 352±139 | 58.4±11.9 | 67.8±19.7 | 680±378 | 76.2±16.1 |
| day 5 | 103±27.3 | 76.8±28.1 | 129±43.4 | 99.1±64.9 | 269±165 | 535±199 | 61.3±30.8 | 63.0±29.6 | 973±132 | 76.1±39.0 |
IgG titers induced by plasma cell determined with quantitative ELISA.
Values are means ± S.D. (ng/ml) of four independent ex-vivo culture plate wells.
Control: Naïve mice (n=3) with in vivo antigenic challenge 6 days earlier before sacrifice. Vaccine: Vaccinated mice (n=3) without in vivo antigenic challenge. Vacc.+Ag. rechallenge: Vaccinated mice (n=3) with in vivo antigenic challenge 6 days earlier before sacrifice.
Importantly, after antigenic stimulation in vitro with inactivated H5 virus during the 5-day extended culture, the levels of antibodies secreted into the culture supernatants were significantly increased up to 16 to 18 fold in the groups vaccinated with mutant or WT VLPs compared to those during the first day culture (Fig. 9D stripe bar and Table 3). The WT VLP group was moderately higher than the mutant VLP group (Fig. 9D stripe bar, Table 3). These results provide evidence for the generation of long-lived memory B cells with VLP vaccination, which can differentiate into antibody secreting plasma cells by in vitro antigenic stimulation.
Table 3.
IgG titers induced by ASC in spleen during ex-vivo culture with in vitro antigen stimulationa
| Days of incubation | Recombinant HA | wtVLP | ΔVLP | none | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Vaccine | Vacc.+Ag. rechallenge | Control | Vaccine | Vacc.+Ag. rechallenge | Control | Vaccine | Vacc.+Ag. rechallenge | naïve | |
| day 1 | b157±41.2 | 92.6±22.2 | 101±38.1 | 78.2±30.4 | 116±41.6 | 374±147 | 73.6±33.0 | 83.0±37.2 | 475±224 | 117±32.5 |
| day 5 | 74.8±42.8 | 134±120 | 915±531 | 60.5±20.1 | 2170±500 | 3100±370 | 84.2±50.6 | 1320±224 | 2470±311 | 67.5±11.1 |
IgG titers induced by in vitro antigen stimulated ASC cell determined with quantitative ELISA.
Values are means ± S.D. (ng/ml) of four independent ex-vivo culture plate wells.
Control: Naïve mice (n=3) with in vivo antigenic challenge 6 days earlier before sacrifice. Vaccine: Vaccinated mice (n=3) without in vivo antigenic challenge. Vacc.+Ag. rechallenge: Vaccinated mice (n=3) with in vivo antigenic challenge 6 days earlier before sacrifice.
To further characterize the memory B cells generated by vaccination, recombinant HA or VLP vaccinated mice were antigenically re-stimulated in vivo with the corresponding type of vaccines (vaccine+Ag.rechallenge). Significant levels of antibodies secreted into the culture supernatants were detected in VLP vaccinated groups, with increases of 6 to 10 fold after 1 day ex vivo culture (Fig. 9A and Table 2, stripe versus closed bar), but not in the recombinant HA vaccine group (Fig. 9A). Noticeably, after in vitro antigenic stimulation with inactivated H5 virus, the levels of IgG antibodies secreted into the culture supernatants were increased by approximately 2.5 to 4 fold compared to those without the in vitro antigenic stimulation during 5 days ex-vivo culture (Fig. 9B and D closed bar and Tables 2 and 3). It was noted that the mutant H5 VLP group showed a trend of higher responses after in vivo antigenic challenge (Fig. 9B, Table 2), which might have contributed to more efficient lung viral control in this mutant H5 VLP group as observed in Fig. 6. These results suggest that influenza memory B cells induced by VLP vaccination, but not by the recombinant HA vaccine, can rapidly respond to antigenic challenge in vivo, differentiating to antibody secreting plasma cells in the spleen and eventually trafficking to the bone marrow.
Cellular immune responses induced by VLP vaccination
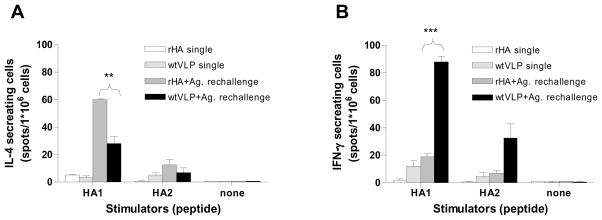
To investigate the cellular immune responses induced by VLP vaccination, we determined IL4 and IFN- γ secreting splenocytes by ELISPOT. Splenocytes were isolated from immunized mice after 6 months vaccination and stimulated with a peptide pool of HA1 or HA2 subunit derived from A/Thailand/16/04 (H5N1) influenza virus. Without the in vivo antigenic exposure, cytokine secreting splenocytes were detected at higher levels than those in naïve controls, but their levels were low (Fig. 10). After in vivo antigenic challenge with the corresponding vaccine antigen 6 days prior to sacrifice, higher levels of IL4 secreting cells were observed upon the stimulation with the HA1 peptide pool in the recombinant protein immunized group than those in the VLP (Fig. 10A). In contrast to IL4, the levels of IFN- γ secreting splenocytes in the VLP group were significantly higher than those in the recombinant H5 HA group (Fig. 10B). Taken together, these results indicate that the recombinant HA vaccines are likely to induce Th2 type responses as represented by the IL-4 cytokine production. The VLP vaccines induce both Th1 and Th2 type immune responses with preferences of the Th1 type immune responses as evidenced by higher levels of IFN- γ production.
Figure 10. Cellular recall immune responses after VLP vaccination.
The cellular immune responses were assessed by cytokine ELISPOT assay with splenocytes isolated from intramuscularly immunized mice (n=3) with a single dose of vaccine 6 months earlier (single) or a single dose vaccination plus in vivo antigenic challenge with the same type of vaccine 6 days prior to sacrifice (Ag. rechallenge). Splenocytes were incubated on the IL4 or IFN- γ capture antibody coated plates in the presence of an HA1 and HA2 peptide pool, as a stimulator, derived from influenza A/Thailand/16/04 (H5N1) virus which has over 99 % homology to A/VN/04 HA. After 36 hours incubation, the plates were developed with substrate, and IL4 and IFN- γ cytokine producing cells were counted. VLP single, Single vaccination with WT H5 VLPs; rHA single, single vaccination with the recombinant H5 HA protein; VLP+Ag. rechallenge, after 6 months mice received a boost immunization with WT H5 VLPs 6 days earlier prior to sacrifice; rHA+Ag. rechallenge, after 6 months mice received a boost immunization with the recombinant H5 HA protein 6 days earlier prior to sacrifice. Statistical significance **p < 0.01, ***p < 0.001.
Discussion
Two doses of influenza vaccine given four weeks apart are recommended to induce protective immunity particularly for children, who are more likely to be immunologically naïve. The majority of the population is also immunologically naïve to the HA glycoprotein antigens from pandemic potential H5N1 influenza viruses. It would therefore be highly desirable to develop an effective single dose vaccination. In this study, for the first time, we have investigated protective immune correlates including the detailed kinetics of antibody induction, protective potential, and the generation of long-lived plasma and memory B cell immune responses after a single dose intramuscular vaccination with H5 VLPs containing the wild type or mutant H5 HA, or a subunit vaccine consisting of recombinant H5 HA protein. H5 HA VLPs were found to be superior to the recombinant H5 HA protein in inducing binding and functional antibodies, and in providing protective immunity as well as in generating plasma and memory B cell responses.
Many influenza vaccine strains especially for vaccines against highly pathogenic avian influenza viruses are generated as reassortants using reverse genetics technology (Govorkova et al., 2006; Lipatov et al., 2006; Nicolson et al., 2005; Subbarao et al., 2003; Webby et al., 2004). The HA genes in the HPAI vaccines are modified to remove the highly cleavable multibasic amino acid motif, a known virulence determinant. However, the effect of the presence of this multibasic amino acid motif in the WT H5 HA on inducing protective immune responses has not been well studied. We produced VLPs containing either WT HA or a deletion mutant HA, lacking the multibasic sequence, and compared their immunogenicities. The mutant form of HA presented in an uncleaved precursor conformation on VLPs induced comparable immune responses as the WT form of HA presented in an processed conformation on VLPs and possibly provided superior protection as evidenced by effective virus clearance. Thus, this study provides first evidence supporting the rationale for generating pandemic vaccines with a mutated HA cleavage site.
Vaccination consisting of formalin-inactivated whole virus particles that were first licensed in 1945 in the U.S. was often associated with a relatively high incidence of adverse events particularly among children (Nicholson et al., 1979; Wright et al., 1977). Since then, detergent split or ether-disrupted vaccines or purified viral subunit HA and NA proteins have been more commonly used for intramuscular vaccination. Influenza VLPs have been suggested as a promising alternative candidate to influenza vaccines against highly pathogenic avian strains (Bright et al., 2008; Haynes et al., 2009; Kang et al., 2009). Also, Bright et al demonstrated that seasonal influenza VLPs induced broader immune responses compared to inactivated whole influenza virus vaccine (Bright et al., 2007). In these previous studies, prime and boost immunizations were applied for inducing protective immune responses against avian influenza viruses, which is a common vaccination regimen in using animal models. Considering that most individuals in the population are naïve to avian or pandemic potential strains, protection with a single dose vaccination in a naïve immune condition would have significant implications for public health. The present study provides insight into the kinetics and types of immune responses contributing to protection after a single dose VLP vaccination as well as potential mechanisms explaining low immunogenicity of soluble subunit vaccine.
Compared to the recombinant H5 HA vaccine, the H5 VLPs induced significantly higher levels of IgG antibody responses with faster kinetics. Vaccination with the recombinant H5 HA protein showed a delayed type of antibody responses until day 28 post vaccination. Also, the IgG1 antibody was found to be a major isotype, which was observed in the high dose H5 HA group (2 μg HA) only at a later time point. In contrast, both low and high doses of H5 VLP vaccines showed induction of IgG2a and IgG2b isotypes as early as day 8 post-vaccination. These observations suggest that the VLP vaccines stimulate the host immune system to initiate adaptive immunity in a different mechanism compared to that of the recombinant antigens. In supporting this idea, we recently observed that influenza VLPs were immunogenic even in CD4 T cell deficient mice, whereas, the recombinant HA protein was not able to induce antibody responses in the absence of CD4 T cells (Kang et al., unpublished data). Therefore, vaccines designed to mimic the virion structure as is the case with VLPs provide an approach to improve vaccine efficacy while retaining the safety of a recombinant protein vaccine.
The type of the immune response induced by vaccines is also important for protection. Infection by influenza virus or other viruses induces IgG2a dominantly in mice (IgG2c in C57BL/6 mice) (Coutelier et al., 1987; Fazekas et al., 1994). IgG2a antibody interacts efficiently with complement and Fc receptors by virtue of its Fc domain properties (Gessner et al., 1998; Heusser, Anderson, and Grey, 1977; Huber et al., 2006; Neuberger and Rajewsky, 1981 ). Therefore, induction of IgG2a antibody by VLP vaccination is considered to significantly contribute to viral clearance, probably via activation of the complement system, stimulation of antibody-dependent cellular cytoxicity and clearance of opsonized virus by macrophages (Huber et al., 2001; Jayasekera, Moseman, and Carroll, 2007; Mozdzanowska et al., 2006). The protective role of IgG2a antibody is further supported by a recent study demonstrating that IgG2a induced by vaccination with viral replicon particles was significantly more effective in protection with enhanced viral clearance against lethal challenge than IgG1 antibodies induced by DNA vaccination (Huber et al., 2006). Consistent with these previous studies, the recombinant H5 HA vaccine group which induced predominantly an IgG1 isotype response at a similar level to the high dose VLP group was not effective in controlling viral replication as this group showed lung viral titers as high as the naïve control challenge group. In contrast, H5 VLP immunized mice that were capable of inducing mainly IgG2a isotype had significantly lower lung viral titers by more than one hundred fold to below the detection limit.
Regarding the T cell responses, previous studies provided evidence that Th1 cells are superior to Th2 cells in providing protection against viral infection by secreting IFN- γ, stimulating B cells, and directing CD8+ T cell mediated cytolysis (Bot, Bot, and Bona, 1998; Moran et al., 1999; Swain et al., 2006; Thomas et al., 2006). VLP vaccination was found to be more effective in generating T cells secreting INF- γ indicating Th1 responses. In contrast, recombinant HA vaccination induced T cells secreting IL-4 cytokine representing Th2 type responses, which was not a protective response since all mice in this group died after challenge. One or two immunizations with the H5 HA protein (3 μg HA, A/Indonesia/05/2005) were reported to induce protection against lethal infection although mice showed a loss in body weight and protective immune correlates were not identified (Bright et al., 2008). There are many factors which could be involved in different results including the vaccine strain (A/Vietnam vs A/Indonesia) and dose, challenge virus strain (wild type vs reassortant) and dose (50LD50 vs 10LD50), and timing of challenge infection (6 months vs 5 weeks post vaccination). Nevertheless, the current study clearly indicates that influenza VLPs are an advantageous format of vaccine inducing predominantly IgG2a isotype antibodies as well as Th1 type cytokine such as IFN- γ, both of which are contributing to inducing protective immunity.
Long-term serum antibodies are maintained by plasma cells residing in the bone marrow and spontaneously secreting antibodies into the bloodstream (Slifka and Ahmed, 1996; Slifka et al., 1998; Slifka, Matloubian, and Ahmed, 1995). During the differentiation and development of B cells in the germinal centers upon antigen exposure, memory B cells are generated (Crotty et al., 2003; Kalia et al., 2006). Previously, prime and boost intranasal immunizations with H5 VLPs were shown to induce plasma and memory B cells in mice (Kang et al., 2009). In the present study to better understand the long-lasting protective immunity after a single intramuscular vaccination, the recombinant HA protein and VLP vaccines were compared. We observed that the recombinant H5 protein vaccine exhibited a significant limitation in generating both plasma cells in bone marrow and rapidly responding memory B cells in spleen compared to the VLP vaccine. In clinical studies, high dose and prime-boost immunizations with recombinant or conventional H5 vaccines were required, indicating their low immunogenicity (Bresson et al., 2006; Lin et al., 2006; Nicholson et al., 2001; Treanor et al., 2006; Treanor et al., 2001). Our study provides evidence for superior immunogenicity of influenza VLPs to recombinant H5 protein based subunit vaccine. It is speculated that influenza VLPs are an effective immunogen in stimulating innate immune cells particularly dendritic cells, a potent antigen presenting cells. A vaccine form capable of strongly activating pathogen recognition receptors such as Toll-like receptors on dendritic cells has been suggested to effectively induce adaptive immunity including Th1 type immune responses (Pulendran, 2004; Pulendran and Ahmed, 2006). Viruses themselves are known to induce strong B cell and cytotoxic T cell responses, while purified recombinant antigens require an adjuvant to induce cellular immune responses (Bachmann, Zinkernagel, and Oxenius, 1998). Thus, alternatively, VLPs mimic viruses in structure and morphology, and present HA molecules in a highly structured repetitive array, which might allow efficient cross-linking of B cell receptors (Bachmann and Zinkernagel, 1996) However, the underlying mechanisms remain to be determined. Therefore, VLPs appear to be a promising approach for developing a vaccine that effectively generates memory B cells that rapidly differentiate into antibody secreting plasma cells upon antigenic stimulation, thus, contributing to long-term protective immunity.
In conclusion, a single intramuscular administration of H5 VLPs but not of a recombinant H5 HA protein, provided complete protection against lethal challenge infection. The H5 VLP vaccine was found to be more effective in inducing Th1 type immune responses including IgG2a and IFN- γ cytokine-secreting T cells as well as in generating plasma and memory B cells contributing to protection after a single intramuscular vaccination. Therefore, influenza VLPs will offer significant advantages for vaccination particularly if a pandemic occurs. Considering the current platforms of approved vaccines, it is important to investigate detailed comparative immunogenicity and protective efficacy of H5 VLPs in comparison with inactivated whole virus and attenuated live H5N1 vaccines.
Acknowledgments
This work was supported by NIH/NIAID grant AI0680003 (R.W.C.) and partially by funds from the Georgia Research Alliance (S.M.K) and the Korea Research Foundation Grant KRF-2007-357-C00088 (J.M.S). We thank Dr. Yumiko Matsuoka for the inactivated reassortant H5N1 virus. A/Thailand/16/05 (H5N1) HA peptide pools were provided by the Biodefense and Emerging Infections Research Resources Repository. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
Footnotes
RWC and SMK are inventors on patents on VLP technology, some of which have been licensed to Zetra Biologicals developing VLP technology under license from Emory University. The VLP system reported here is different from VLP vaccine products under development and the information in this manuscript is not related to those products.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, Pompey J. Clin Infect Dis. 43(9):1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Zinkernagel RM. The influence of virus structure on antibody responses and virus serotype formation. Immunol Today. 1996;17(12):553–8. doi: 10.1016/s0167-5699(96)10066-9. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Zinkernagel RM, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J Immunol. 1998;161(11):5791–4. [PubMed] [Google Scholar]
- Bot A, Bot S, Bona C, Cate TR, Couch RB. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. A. (1998). Protective role of gamma interferon during the recall response to influenza virus. J Virol. 2006;72(8):6637–45. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Hoschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367(9523):1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, Ross TM. Cross-Clade Protective Immune Responses to Influenza Viruses with H5N1 HA and NA Elicited by an Influenza Virus-Like Particle. PLoS ONE. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25(19):3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- Cheung CL, Rayner JM, Smith GJ, Wang P, Naipospos TS, Zhang J, Yuen KY, Webster RG, Peiris JS, Guan Y, Chen H. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J Infect Dis. 2006;193(12):1626–9. doi: 10.1086/504723. [DOI] [PubMed] [Google Scholar]
- Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351(9101):472–7. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165(1):64–9. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, Bach VC, Phan TQ, Do QH, Guan Y, Peiris JS, Tran TH, Farrar J. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- Donis RO. Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11 (10):1515–21. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas G, Rosenwirth B, Dukor P, Gergely J, Rajnavolgyi E. IgG isotype distribution of local and systemic immune responses induced by influenza virus infection. Eur J Immunol. 1994;24(12):3063–7. doi: 10.1002/eji.1830241222. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79(5):2814–22. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101(5):1356–61. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76(6):231–48. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev. 2006;19(4):614–36. doi: 10.1128/CMR.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis. 2006;194(2):159–67. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, Harmsen AG, Richardson C. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27(4):530–41. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Hehme N, Engelmann H, Kunzel W, Neumeier E, Sanger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol. 2002;191(3–4):203–8. doi: 10.1007/s00430-002-0147-9. [DOI] [PubMed] [Google Scholar]
- Heusser CH, Anderson CL, Grey HM. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977;145(5):1316–27. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166(12):7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13(9):981–90. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81(7):3487–94. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18(3):255–64. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, Chen LM, Donis RO, Compans RW. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS ONE. 2009;4(3):e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, Pham ND, Ngyen HH, Yamada S, Muramoto Y, Horimoto T, Takada A, Goto H, Suzuki T, Suzuki Y, Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z, Yang M, Sun R, Li C, Lin S, Ji M, Liu Y, Wang X, Wood J, Feng Z, Wang Y, Yin W. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368(9540):991–7. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis. 2006;194(8):1040–3. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180(3):579–85. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352(2):418–26. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Nava GM, Attene-Ramos MS, Ang JK, Escorcia M. Origins of the new influenza A(H1N1) virus: time to take action. Euro Surveill. 2009;14(22) doi: 10.2807/ese.14.22.19228-en. [DOI] [PubMed] [Google Scholar]
- Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11(12):1012–6. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357(9272):1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Tyrrell DA, Harrison P, Potter CW, Jennings R, Clark A, Schild GC, Wood JM, Yetts R, Seagroatt V, Huggins A, Anderson SG. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J Biol Stand. 1979;7(2):123–36. doi: 10.1016/s0092-1157(79)80044-x. [DOI] [PubMed] [Google Scholar]
- Nicolson C, Major D, Wood JM, Robertson JS. Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system. Vaccine. 2005;23(22):2943–52. doi: 10.1016/j.vaccine.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. Human infection with influenza H9N2. Lancet. 1999;354(9182):916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124(4):849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81(7):3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm C, Zhou N, Suss J, Mackenzie J, Webster RG. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996;217(2):508–16. doi: 10.1006/viro.1996.0145. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Ahmed R. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J Immunol Methods. 1996;199(1):37–46. doi: 10.1016/s0022-1759(96)00146-9. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69(3):1895–902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyov A, Palacios G, Briese T, Lipkin WI, Rabadan R. Cluster analysis of the origins of the new influenza A(H1N1) virus. Euro Surveill. 2009;14(21) doi: 10.2807/ese.14.21.19224-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258(1):1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, Verlander NQ, Warburton F, Wood JM, Zambon MC. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362(9400):1959–66. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- Stieneke-Grober A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk HD, Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. Embo J. 1992;11(7):2407–14. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Chen H, Swayne D, Mingay L, Fodor E, Brownlee G, Xu X, Lu X, Katz J, Cox N, Matsuoka Y. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305(1):192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007;7(4):267–78. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279(5349):393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, Jones SC, Kamperschroer C, Lee WH, McKinstry KK, Roman E, Strutt T, Weng NP. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, Brown SA, Yue W, So J, Webby RJ, Doherty PC. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006;103(8):2764–9. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O'Brien D, Wolff M, Rabinovich G, Blackwelder W, Katz JM. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19(13–14):1732–7. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- Wakamiya N, Okuno Y, Sasao F, Ueda S, Yoshimatsu K, Naiki M, Kurimura T. Isolation and characterization of conglutinin as an influenza A virus inhibitor. Biochem Biophys Res Commun. 1992;187(3):1270–8. doi: 10.1016/0006-291x(92)90440-v. [DOI] [PubMed] [Google Scholar]
- Wan XF, Nguyen T, Davis CT, Smith CB, Zhao ZM, Carrel M, Inui K, Do HT, Mai DT, Jadhao S, Balish A, Shu B, Luo F, Emch M, Matsuoka Y, Lindstrom SE, Cox NJ, Nguyen CV, Klimov A, Donis RO. Evolution of highly pathogenic H5N1 avian influenza viruses in Vietnam between 2001 and 2007. PLoS One. 2008;3(10):e3462. doi: 10.1371/journal.pone.0003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, Govorkova EA, McClain-Moss LR, Peiris JS, Rehg JE, Tuomanen EI, Webster RG. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363(9415):1099–103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SS, Yuen KY. Avian influenza virus infections in humans. Chest. 2006;129(1):156–68. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PF, Thompson J, Vaughn WK, Folland DS, Sell SH, Karzon DT. Trials of influenza A/New Jersey/76 virus vaccine in normal children: an overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977;136(Suppl):S731–41. doi: 10.1093/infdis/136.supplement_3.s731. [DOI] [PubMed] [Google Scholar]