Abstract
A vaccine comprising human papillomavirus type 16 (HPV16) L2, E6 and E7 in a single tandem fusion protein (termed TA-CIN) has the potential advantages of both broad cross-protection against HPV transmission through induction of L2 antibodies able to cross neutralize different HPV types and of therapy by stimulating T cell responses targeting HPV16 early proteins. However, patients vaccinated with TA-CIN alone develop weak HPV neutralizing antibody and E6/E7-specific T cell responses. Here we test TA-CIN formulated along with the adjuvant GPI-0100, a semi-synthetic quillaja saponin analog that was developed to promote both humoral and cellular immune responses. Subcutaneous administration to mice of TA-CIN (20 μg) with 50 μg GPI-0100, three times at biweekly intervals, elicited high titer HPV16 neutralizing serum antibody, robust neutralizing titers for other HPV16-related types, including HPV31 and HPV58, and neutralized to a lesser extent other genital mucosatropic papillomaviruses like HPV18, HPV45, HPV6 and HPV11. Notably, vaccination with TA-CIN in GPI-0100 protected mice from cutaneous HPV16 challenge as effectively as HPV16 L1 VLP without adjuvant. Formulation of TA-CIN with GPI-0100 enhanced the production of E7-specific, interferon γ producing CD8+ T cell precursors by 20-fold. Vaccination with TA-CIN in GPI-0100 also completely prevented tumor growth after challenge with 5 × 104 HPV16-transformed TC-1 tumor cells, whereas vaccination with TA-CIN alone delayed tumor growth. Furthermore, three monthly vaccinations with 125 μg of TA-CIN and 1000 μg GPI-0100 were well tolerated by pigtail macaques and induced both HPV16 E6/E7-specific T cell responses and serum antibodies that neutralized all HPV types tested.
Keywords: Human papillomavirus, Vaccine, GPI-0100
1. Introduction
Persistent with infection with certain high risk HPV types is a necessary but not sufficient cause of cervical cancer. Thus HPV-related cancers and their intraepithelial neoplasia precursors could be prevented by generating virus neutralizing antibodies and/or an appropriate virus-specific cellular immune response prior to infection. The newly licensed preventive HPV vaccines, Gardasil® and Cervarix®, are both based upon HPV L1 virus-like particles (VLPs) and provide type-restricted protection against new infection [1,2]. The licensed HPV vaccines target only two of the 15+ known high risk HPV types, namely HPV16 and HPV18 that together account for 70–80% of cervical cancer cases. Multivalent vaccines containing L1 VLP of more HPV types are therefore being tested [3]. Unfortunately, the L1 VLP vaccines do not alter the course of established HPV infections, and thus will not reduce rates of cervical cancer for a couple of decades [4]. A clear need for a virus-specific therapy able to trigger the clearance of established disease remains.
The minor capsid protein L2 is another possible target for vaccine development [5–7]. Importantly, vaccination with L2 induces widely cross-type neutralizing antibodies that mediate broader protection than L1 VLPs in an animal model [8–11]. However, L2 is less immunogenic than L1 VLP, and like L1 VLP, it fails to trigger regression of established HPV disease [8,11]. The failure to induce lesion regression by either capsid antigen probably reflects their expression only in the upper epithelial layers rather than the basal cells that harbor the infection. By contrast the early viral oncoproteins E6 and E7 are promising rejection antigens both being expressed throughout the viral life cycle and required to maintain the transformed state [12]. Indeed, natural immunity almost certainly requires the development of T cells targeting HPV early genes [13]. Consequently, a plethora of approaches to treat cervical cancer and its precursors by vaccination against E6 and E7 have been tested with limited success to date (reviewed in [14]).
TA-CIN is a single fusion protein comprising HPV16 L2, E7 and E6 that has potential as a candidate preventive and therapeutic HPV vaccine. A placebo-controlled, double-blinded phase I dose escalation study provided preliminary evidence that vaccination three times with 533 μg of TA-CIN in the absence of an adjuvant is safe, well tolerated and immunogenic in healthy volunteers [15]. However, vaccination produced only low titers of L2-specific cross-neutralizing antibodies and weak E6/E7-specific interferon γ and proliferative T cell responses following a TA-CIN dose response [15,16]. In phase II studies in 29 predominantly HPV16+ high grade vaginal or vulval intraepithelial neoplasia (VAIN or VIN) patients, vaccination with TA-CIN followed by a boost with recombinant vaccinia virus expressing E6 and E7 of both HPV16 and HPV18 (TA-HPV) failed to induce an improved rate of lesion regression as compared to earlier studies utilizing TA-HPV alone in 30 VIN patients [17]. The weak antibody and T cell responses to vaccination with TA-CIN protein alone strongly suggested the need to formulate TA-CIN with a potent adjuvant.
GPI-0100 is a semi-synthetic analog developed to retain the adjuvant properties of quillaja saponins, yet with less toxicity and greater stability in aqueous solution [18,19]. In several animal models GPI-0100 stimulates potent humoral as well as cytotoxic T lymphocyte responses targeting exogenous antigens. Vaccination of SKH-1 hairless mice with herpes simplex virus type-1 (HSV-1) glycoprotein D (gD) formulated in GPI-0100 demonstrated significantly reduced death rates, lesion severity and viral titers on challenge with HSV-1 [20]. Furthermore, the protective effects were greater for mice immunized with gD/GPI-0100 prior to cutaneous challenge with HSV-1 than those for those mice receiving gD in alum adjuvant [20]. GPI-0100 has also been tested as an adjuvant for candidate therapeutic cancer vaccines targeting glycolipid, carbohydrate or peptide epitopes on protein carriers [21–23]. GPI-0100 mixed with a bivalent vaccine containing the glycolipid Globo H and the glycosylated mucin MUC2 conjugated to keyhole limpet hemocyanin (KLH) was tested at GPI-0100 doses ranging from 100 to 5000 μg in a total of 34 prostate cancer patients who had no evidence of disease except for rising PSA levels. GPI-0100 was well tolerated and specific antibody titers increased with escalating doses [24]. A phase II trial utilizing GPI-0100 with KLH-FITC is currently ongoing in patients with progressive metastatic renal cancer (Clinical trial identifier NCT00485563).
Herein we test the influence of the adjuvant GPI-0100 upon L2-specific neutralizing antibody responses and E6/E7-specific T cell responses to TA-CIN vaccination in mice and macaques. The ability of this combination to protect against HPV infection and HPV-induced tumor challenge is tested.
2. Materials and methods
2.1. Vaccine formulation
TA-CIN consists of recombinant HPV16 L2E7E6 (aa 725 comprising full length tandem fusions) that was isolated from solubilized E. coli inclusion bodies under reducing conditions and purified by chromatography. The 80 kDa L2E7E6 antigen was vialed as a discrete, 0.22 μm filterable, stable protein aggregate formulated in 5 mM phosphate, 5 mM glycine buffer (pH 8.0) containing 0.9 mM cysteine and stored at −80 °C until use [15]. Alum (AlHydroGel) was obtained from Sigma. GPI-0100 was provided by Hawaii Biotech Inc. (Aiea, HI). Vaccine formulation containing 20 μg of TA-CIN was mixed with 50 μg of GPI-0100 along with 1 μg of Tween 40 in 250 μl of PBS and incubated overnight on a gentle shaker at 4 °C prior to vaccination. Each mouse received 250 μl vaccine formulation. Gardasil® (Merck & Co.) was purchased from the Pharmacy. One dose of Gardasil® contains L1 VLP of HPV6 (20 μg), HPV11 (40 μg), HPV16 (40 μg) and HPV18 (20 μg) formulated in 0.5-ml. Each dose of the vaccine contains approximately 225 μg of aluminum as amorphous aluminum hydroxyphosphate sulfate adjuvant, 9.56 mg of sodium chloride, 0.78 mg of L-histidine, 50 μg of polysorbate 80, 35 μg of sodium borate, and water for injection.
2.2. Vaccination of mice with TA-CIN
Female Balb/c (six to eight weeks old) were purchased from the National Cancer Institute (Frederick, MD, USA) and kept in the animal facility of the Johns Hopkins School of Medicine (Baltimore, MD, USA). Mouse experiments were approved by the Johns Hopkins University Animal Care and Use Committee. All animal procedures were performed according to approved protocols and in accordance with the recommendations for the proper use and care of laboratory animals. Animals were vaccinated with TA-CIN (20 μg) in presence and absence of adjuvant GPI-0100 (50 μg) and Tween 40 (1 μg) at days 0, 15, and 30. Mice vaccinated with PBS or GPI-0100 (50 μg) alone were utilized as negative controls and mice vaccinated with HPV16 VLP (10 μg) prepared from 293TT cells or one fifth of a dose of Gardasil® were utilized as positive controls. After the third immunization with antigens the serum was collected and antibodies were determined by ELISA and HPV pseudoviron neutralization assays. Mice were challenged with HPV16 pseudovirus carrying the luciferase reporter gene to infect cutaneous epithelium.
2.3. Measurement of HPV16 E6- and E7-reactive antibody by ELISA
HPV16 E7- and E6-specific serum antibodies were individually measured by ELISA using proteins donated by Dr. T.-C. Wu [25]. HPV-16 E6 DNA was amplified by PCR using the primer set CCTCGAGATCTGATGCACCAAAAGAGAAC and CTTCGAATTCTTACAGCTGGGTTTCTCT and cloned into the BglII and EcoRI sites of pTrcHisC (Invitrogen). E6 protein was purified from bacteria by his-tag affinity chromatography according to the manufacturer’s instructions. Ninety-six well plates (Nunc Maxisorp) were coated separately with HPV16 E7 or E6 protein (800ng/well) in 100 mM carbonate buffer, pH 9.6, overnight at 4 °C. Wells were then blocked with 1% bovine serum albumin (BSA)-PBS for 1 h at room temperature and incubated with 2-fold dilutions of mouse/macaque sera for 1 h at room temperature. Following washing with PBS-T, peroxidase-labeled goat anti-mouse/monkey immunoglobulin G (KPL, Inc., Gaithersburg, MD) diluted 1:5000 in 1% BSA-PBS was added for 1 h. The plates were then washed with PBS-Tween and developed with ABTS reagent (Roche) for 10 min.
2.4. TC-1 tumor challenge studies in mice
TC-1 cells were cultured in RPMI1640 + 10% FCS. B6 mice were vaccinated subcutaneously with 20 μg TA-CIN plus adjuvant in 200 μl, PBS at day 0, 15, and day 30. Mice were sacrificed at day 37 for the analysis of splenocytes for HPV-specific cellular immunity, and the remainder of the mice from each group was challenged with 5 × 104 TC-1 tumor cells/mouse by subcutaneous injection in the right leg. Tumor growth was monitored by visual inspection and palpation twice a week as described previously [25]. Following TC-1 challenge, tumor development in mice was monitored for 90 days.
2.5. Intracellular cytokine staining and flow cytometry analysis of mouse splenocytes
Cell surface marker staining of CD8 and intracellular cytokine staining for IFN-γ as well as flow cytometric analysis was performed using conditions described previously [26]. Prior to FACScan, splenocytes from different vaccinated groups of mice were collected, pooled and incubated for 16 h with 1 μg/ml of HPV16 E7 peptide (aa 49–57) or HPV16 E6 peptide (aa 50–57) in the presence of GolgiPlug (1 μl/ml) for detecting E6- and E7-specific CD8+ T cell precursors [27,28]. The stimulated splenocytes were then washed twice with FACScan buffer. Cell surface marker staining for CD8 and intracellular cytokine staining for IFN-γ were performed using conditions described previously [26]. The number of IFN-γ secreting CD8+ T cells was analyzed using flow cytometry. Analysis was performed on a Becton–Dickinson FACSCalibur with CELLQuest software (Becton–Dickinson Immunocytometry System, Mountain View, CA, USA).
2.6. Neutralization assays
The HPV16, 18, 31, 58, 45, 11 and HPV6 pseudovirions with encapsulated secreted alkaline phosphatase (SEAP) were generated by co-transfection of 293TT cells with plasmids encoding HPV L1 and L2 and a SEAP reporter plasmid as described previously [29] and fully detailed protocols can be found at http://home.ccr.cancer.gov/lco/production.asp. Cells collected after transfection were treated overnight with Brij 58 (0.5%), Benzonase (0.5%) and purified by centrifugation on an Optiprep step gradient (27, 33, and 39%) at 40,000 rpm for 4.5 h. Pseudovirus neutralization assays were carried out as outlined previously [9,29]. Briefly, the pseudovirus and the pooled mouse immune sera were incubated for 1 h and the mixture was used to infect 293TT cells. 68–72 h post-infection, the supernatants were collected and SEAP activity in the supernatants was measured by colorimetric assay. Serum neutralization titers were defined as the highest dilution that caused at least a 50% reduction in SEAP activity, compared to control pre-immune serum samples. Sera from 10 patients vaccinated three times with 50 μg of HPV16 L1 VLP [30] were kindly provided by Dr J. Schiller and were utilized with the approval of the Johns Hopkins University Institutional Review Board.
2.7. Vaccination of monkeys with TA-CIN
Three female Simian retrovirus (SRV) seropositive pigtail macaques of ages 21 (M746), 11 (M831) and 6 (M811) years were obtained from Pennsylvania State University (Hershey, PA) and housed in the animal facility of the Johns Hopkins School of Medicine (Baltimore, MD, USA). All animal procedures were performed by a veterinarian (RJA) according to approved protocols and in accordance with the recommendations for the proper use and care of laboratory animals, and with institutional approval. The pigtail macaques were vaccinated in the deltoid muscle with TA-CIN (125 μg) formulated in GPI-0100 (1 mg) at day 0, 30, and 60 while chemically restrained with ketamine hydrochloride. Prior to study, after every immunization, and monthly thereafter, serum (5cc), plasma and PBMCs (from 20cc peripheral blood collected in ACD and separated by Ficol gradient centrifugation) were collected for immunologic analysis.
2.8. Measurement of primate T cell responses
Pools of 30 HPV-16 E6 and 18 HPV-16 E7 15mer overlapping peptides (overlapped by 10 amino acids) spanning the full length of E6 and E7 proteins were synthesized by Sigma–Genosys (The Woodlands, Texas). The identities of the peptides were validated by mass spectrometric analysis and the purity of the peptides was confirmed by high-performance liquid chromatography. The antibodies used in this study were FITC-conjugated anti-IFN-γ (clone B27), PE-conjugated anti-CD8 (clone RPA-T8), PerCP-Cy5.5-conjugated anti-CD4 (clone L200), and purified anti-CD49d (clone 9F10). All the antibodies were purchased from BD Biosciences. Frozen PBMCs from the indicated monkey were thawed and rested at 37 °C in a 5% CO2 environment for 6 h. PBMCs were then stimulated with either HPV16 E6 15mer overlapping peptide pool or HPV16 E7 15mer overlapping peptide pool at the concentration of 5 μg/ml, or PHA (Remel Inc., Lenexa, KS) at 2 μg/ml as positive control, or left unstimulated as negative control for 24 h at the presence of 1 μl/ml of GolgiPlug (BD Biosciences) and 1 μg/ml anti-CD49d antibody. The cultured cells were then harvested and IFN-γ intra-cellular staining assay was performed to detect IFN-γ producing CD4+ and CD8+ T cells. The cells were acquired by FACSCalibur and analyzed using CellQuest Software (BD Biosciences). The data are presented as the percentage of either CD4+IFN-γ+ T cells/total CD4+ T cells or CD8+IFN-γ+ T cells/total CD8+ T cells.
3. Results
3.1. GPI-0100 greatly enhances HPV neutralizing antibody responses to vaccination of mice with TA-CIN
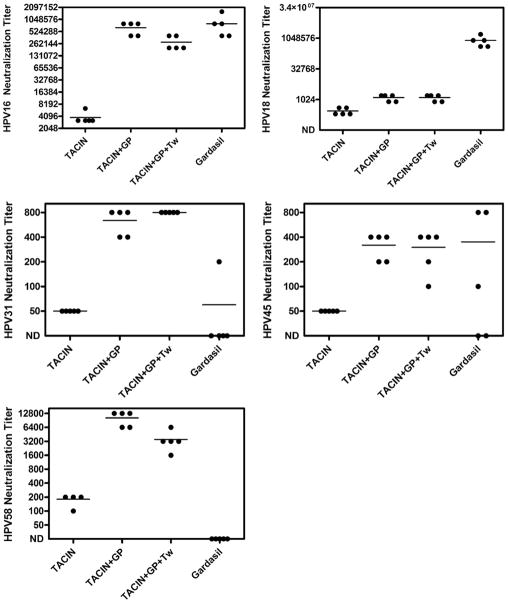
Since vaccination of patients with TA-CIN alone induces weak HPV neutralizing antibody responses [16], we determined the effectiveness of GPI-0100 as an adjuvant for TA-CIN in mice. Groups of five BALB/c female mice were immunized s.c. three times at two-week intervals with TA-CIN either alone, TA-CIN with GPI-0100, or TA-CIN with GPI-0100 and Tween 40. Sera from mice vaccinated with TA-CIN alone exhibited low level HPV16 neutralization titers, consistent with responses observed in patients after vaccination with TA-CIN alone [16]. By contrast, sera from mice vaccinated with TA-CIN mixed with GPI-0100 with or without Tween 40 exhibited dramatically elevated titers for neutralization of HPV16 (Fig. 1).
Fig. 1.
Induction of HPV neutralizing antibodies in the serum of mice by vaccination with TA-CIN and Gardasil®. Groups of Balb/c mice (n = 5) were vaccinated s.c. three times at two-week intervals with TA-CIN (20 μg) alone, or with GPI-0100 (50 μg) or with GPI-0100 and Tween 40 (1 μg), or one fifth of a human dose of Gardasil®. Sera were harvested two weeks after the final immunization and tested individually for in vitro neutralization titers for HPV pseudovirons using the genotypes indicated at 2-fold dilutions from 1:50. Samples that failed to neutralize at 1:50 are arbitrarily assigned a titer of 25. No neutralization antibody was detected in any assay when using sera from control mice, or those vaccinated with GPI-0100 adjuvant alone (not shown).
Gardasil®, which contains L1 VLP of HPV16 as well as HPV6, HPV11 and HPV18 formulated in alum, has proven highly effective in protecting young women against HPV16 infection [1]. Three immunizations using one fifth of a human dose of Gardasil® per mouse for each s.c. injection were administered to five mice on the same schedule as for TA-CIN vaccination and serum was obtained from each animal for testing at two weeks after the final immunization. The sera from mice vaccinated with Gardasil® exhibited high titers of neutralizing antibodies against HPV16 and HPV18 pseudovirions. The mean serum HPV16 neutralization titers of the five mice vaccinated with Gardasil® were far superior to the mice vaccinated with TA-CIN alone but similar to the mice vaccinated with TA-CIN in GPI-0100 with or without Tween 40 (Fig. 1). However, the mean serum HPV18 neutralization titer induced by vaccination with Gardasil® was dramatically higher than that produced with TA-CIN, even with GPI-0100 adjuvant.
Gardasil® does not contain L1 VLP of HPV31, HPV45 or HPV58, although studies to determine if vaccination with Gardasil® provides partial protective efficacy against one or more of these high risk types in patients are ongoing. Of the sera derived from five mice vaccinated with Gardasil®, 1/5 contained detectable (1:50 or greater) HPV31 neutralizing antibody, 3/5 contained detectable HPV45 neutralizing antibody, and 0/5 contained HPV58 neutralizing antibody. By contrast, 5/5 sera of mice vaccinated with TA-CIN in GPI-0100 either with or without Tween 40 effectively neutralized HPV31, HPV45 and HPV58 at 1:50 or greater (Fig. 1B). Immunizations with TA-CIN and GPI-0100 with or without Tween 40 generated modest neutralizing titers against low risk HPV types (median titers of 100 and 200 respectively for HPV6 and 200 and 400 respectively for HPV11) that cause the majority of external genital warts (not shown), but neutralizing antibody was not detected at a dilution of 50 for sera from mice vaccinated with TA-CIN alone. No neutralizing antibody was detected for any HPV type in the sera of mice vaccinated with PBS, or GPI-0100.
3.2. Vaccination with TA-CIN in GPI-0100 protects BALB/c mice against cutaneous challenge with HPV16 pseudovirus carrying a luciferase reporter
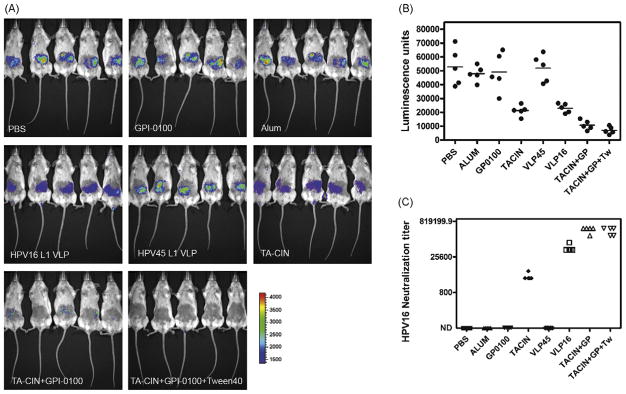
To test whether neutralizing antibody titers generated by vaccination with TA-CIN and GPI-0100 are sufficient to mediate protection in BALB/c mice, we applied a cutaneous challenge of HPV16 pseudovirus [31]. At six weeks after completing the vaccination schedule, a patch on the belly of each anesthetized BALB/c mouse was shaved with an electric razor. The mice were challenged with HPV pseudovirus by application to the shaved skin of 3 × 109 HPV16 pseudovirions (100ng) carrying an encapsidated luciferase reporter construct in 10 μl of 0.6% carboxymethylcellulose. Three days later, the mice were injected with luciferin (100 μl at 7 mg/ml), and their images were acquired for 10 min with a Xenogen IVIS200. Mice vaccinated with TA-CIN and GPI-0100 were protected against HPV16 viral challenges (Fig. 2). Vaccination with TA-CIN protein alone also provided some degree of protection, suggesting that the low titer neutralizing antibody response was partially protective. Vaccination on the same schedule with HPV16 L1 VLP (prepared in our laboratory) without adjuvant also generated good protection but HPV45 L1 VLP, GPI-0100 and PBS controls did not show any protective effect against HPV16 challenge as compared with PBS immunized control mice. It is noteworthy that the HPV16 neutralizing titers and degree of protection elicited by HPV16 L1 VLP without adjuvant was weaker than for Gardasil® that is formulated with an alum adjuvant and that 5/5 mice vaccinated with Gardasil® were protected against experimental challenge in this system (not shown). A direct relationship between in vitro HPV16 neutralization titer measured prior to challenge and the extent of protection from HPV16 pseudovirus challenge was apparent (Fig. 2).
Fig. 2.
Vaccination with TA-CIN and adjuvant GPI-0100 protects mice from cutaneous challenge with HPV16 pseudovirions. Groups of Balb/c mice (n = 5) were vaccinated s.c. three times at two-week intervals with PBS, GPI-0100 alone, TA-CIN (20 μg) alone, or TA-CIN with GPI-0100 (50 μg) or TA-CIN with GPI-0100 and Tween 40 (1 μg), or 10 μg of L1 VLP of HPV16 or HPV45 without adjuvant. (A) The vaccinated mice received a cutaneous challenge with HPV16 pseudovirus one month after the final vaccination. Three days later, the mice were anesthetized and injected with luciferin, and their images were acquired for 10 min with a Xenogen IVIS 200. (B) Equally sized areas encompassing the site of inoculation were analyzed using Living Image 2.20 software, and plotted after background subtraction. (C) Two weeks after the final immunization (and prior to challenge), sera were harvested from the immunized mice and tested for in vitro HPV16 neutralization titer.
3.3. The adjuvant GPI-0100 boosts HPV16 E6- and E7-specific B and T cell responses in mice vaccinated with TA-CIN
While neutralizing antibodies protect against initial infection, cell mediated immune responses to HPV16 E6 and E7 generated by TA-CIN vaccination might contribute to protection against HPV16-related disease by clearing HPV infected cells. Humoral responses to an antigen are often indicative of a concomitant cell mediated immune response. To measure HPV16 E6- and E7-specific humoral immune responses generated by vaccination of mice with TA-CIN, sera harvested from immunized BALB/c mice two weeks after the final immunization was tested by ELISA using either full length HPV16 E6 or E7 protein. The adjuvant effect of GPI-0100 upon E7-specific antibody titers was quite evident; serum from TA-CIN treated mice had a modest E7-specific antibody titer (Fig. 3), whereas the sera from mice vaccinated with TA-CIN and GPI-0100 (± Tween 40) showed significantly higher titers of 10,240. Humoral responses to E6 were strong in mice vaccinated with TA-CIN either with or without GPI-0100. Use of Tween 40 along with GPI-0100 further boosted E6-specific antibody titers. There was no E6 or E7-specific antibody response detected in mice vaccinated with either PBS or GPI-0100 alone (Fig. 3).
Fig. 3.

Vaccination of mice and macaques with TA-CIN in GPI-0100 adjuvant induces HPV16 E6- and E7-specific antibody. (A) Groups of Balb/c mice (n = 5) were vaccinated s.c. three times at two-week intervals with TA-CIN (20 μg) alone, or with GPI-0100 (50 μg) or with GPI-0100 and Tween 40 (1 μg). ELISA was performed using HPV16 E6 or HPV16 E7 protein to detect specific serum antibody at one month after the third immunization using sera pooled from each group. ELISA was performed using HPV16 E6 (B) or HPV16 E7 (C) protein to detect specific serum antibody obtained from macaques 746, 811 and 831 at one month after 1, 2 or 3 immunizations with 125 μg of TA-CIN with 1000 μg GPI-0100 that were initiated at week 0.
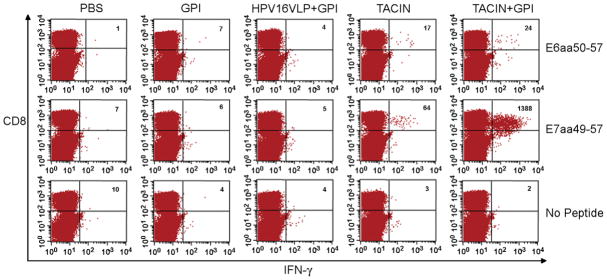
To assess E6- and E7-specific cytotoxic T cell immune responses generated by TA-CIN vaccination, we utilized another strain of mice (C57BL/6) for which the relevant MHC class I epitopes are well characterized and the E6/E7-transformed TC-1 tumor-challenge model is available. C57BL/6 mice (ten per group) were immunized subcutaneously with the TA-CIN vaccine, both with and without adjuvants, and splenocytes were harvested one week after the third immunization. Intracellular cytokine staining and flow cytometry analysis was performed to determine the number of E6- and E7-specific IFN-γ secreting CD8+ T cells generated in response to overnight stimulation with E6 (aa 50–57) or E7 (aa 49–57) peptide respectively (Fig. 4). Significant numbers of E6-specific IFN-γ secreting CD8+ T cells were observed after stimulation of splenocytes derived from mice immunized with TA-CIN alone and with GPI-0100. These responses were absent from splenocytes harvested from mice immunized with HPV16 L1 VLP, GPI-0100 or PBS. Likewise, no IFN-γ secreting CD8+ T cells were detected without appropriate peptide stimulation.
Fig. 4.
Vaccination of mice with TA-CIN induces HPV16 E6- and E7-specific CD8+ T cells and the latter are dramatically enhanced by GP0-0100. C57BL/6 mice (ten per group) were immunized with PBS, GPI-0100 alone, HPV16 L1 VLP (10 μg) in GPI-0100, TA-CIN (20 μg) alone, or TA-CIN with adjuvant GPI-0100 (50 μg) on days 0, 15, and 30. Splenocytes from vaccinated mice were harvested one week after the last vaccination and characterized for HPV16 E6- and E7-specific CD8+ T cells, using intracellular IFN-γ staining and flow cytometry analysis after overnight stimulation with the immunodominant T cell epitope shown. Numbers in the upper right hand corners represent the number of E6- and E7-specific IFN-γ secreting CD8+ T cells per 3 × 105 splenocytes.
In contrast to the E6-specific IFN-γ secreting CD8+ T cell response, the E7-related response was dramatically boosted by use of the GPI-0100 adjuvant with TA-CIN (Fig. 4). While significant numbers of E7-specific IFN-γ secreting CD8+ T cells were observed in the mice immunized with TA-CIN, its administration with GPI-0100 generated a ~20-fold higher level of E7-specific IFN-γ secreting CD8+ T cells. Again no responses were seen in mice vaccinated with PBS, GPI-0100 alone or HPV16 L1 VLP in GPI-0100. The boosting of E7 but not E6 by inclusion of GPI-0100 with the TA-CIN vaccine likely reflects the previously observed immunodominance of the E7 epitope in this strain of mice.
3.4. GPI-0100 enhances immunity against TC-1 tumor challenge in mice vaccinated with TA-CIN
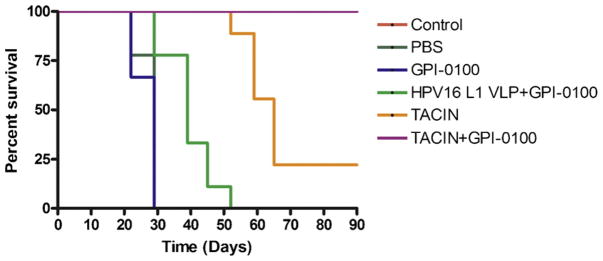
Vaccination against the early viral antigens E6 and E7 will not protect against initial infection but may prevent disease by elimination of transcriptionally active infected cells before the development of clinically apparent HPV lesions (thereby potentially enhancing the prophylactic efficacy of TA-CIN). In order to assess the anti-tumor immunity generated by TA-CIN vaccines, we performed an in vivo tumor challenge with TC-1 cells that express HPV16 E6 and E7, but not L2. One week after the third immunization TA-CIN and adjuvant combinations, nine mice/group were challenged subcutaneously with 5 × 104/mouse of TC-1 tumor cells (Fig. 5). Tumor growth was monitored by visual inspection and palpation twice a week. Immunization with TA-CIN in GPI-0100 completely protected mice from the development of TC-1 tumors, whereas TA-CIN only delayed tumor development and provided partial protection (Logrank test p < 0.0001). Vaccination with GPI-0100 alone had no effect upon TC-1 tumor growth and mouse survival, whereas vaccination with HPV16 L1 VLPs had a minimal effect (Fig. 5) similar to that observed previously [32].
Fig. 5.
Vaccination of mice with TA-CIN in GPI-0100 adjuvant completely protects against TC-1 tumor challenge. C57BL/6 mice (nine per group) were immunized with PBS, GPI-0100 alone, HPV16 L1 VLP (10 μg) in GPI-0100, TA-CIN (20 μg) alone, or TA-CIN with adjuvant GPI-0100 (50 μg) on days 0, 15, and 30. Seven days after the last vaccination the mice were challenged by subcutaneous injection of 5 × 104 TC-1 tumor cells/mouse. The mice were monitored for tumor growth by inspection and palpitation twice per week for 90 days. Tumor volume was measured starting from day 7 after tumor challenge and until euthanasia was required. A Kaplan–Meier survival analysis of mice challenged with TC-1 cells is shown.
3.5. Vaccination of pigtail macaques with TA-CIN in GPI-0100 generates a broadly neutralizing serum antibody response
In human patients receiving three monthly immunizations with TA-CIN without adjuvant, the 500 μg dose generated a superior neutralizing antibody response to the 125 μg dose [15,16]. Doses of 100–5000 μg of GPI-0100 have been tested as adjuvants in small numbers of patients [24]. To address the immunogencitiy of vaccination at intermediate dosing levels of the combination, three female pigtail macaques (Macaca nemestrina) were immunized subcutaneously with 125 μg of TA-CIN in 1000 μg of GPI-0100 three times at monthly intervals while sedated with ketamine hydrochloride. Serum samples were collected monthly starting one month prior to vaccination. Macaque CBC and body weights were monitored at monthly intervals. During the study period macaque 811’s body weight went from 16.6 to 19.8 lbs, macaque 831 went from 19.2 to 17.2 lbs, and macaque 746 went from 22.8 to 23.2 lbs. No unusual clinical signs were noted during the study.
The in vitro neutralizing antibody titers for five high risk HPV pseudoviruses and two low risk types were determined for the sera obtained from each macaque at one month after the final immunization with TA-CIN + GPI-0100 (Table 1A). All three macaques exhibited robust neutralizing antibody titers against HPV16, with lesser responses against all of the heterologous HPV types tested. These neutralizing antibody titers were compared with sera from patients who had been vaccinated three times with 50 μg of HPV16 L1 VLP without adjuvant from an earlier study [30] (Table 1B). Although some cross-neutralization was observed for closely related HPV types (HPV31 and surprisingly HPV58) from within species A9 [33], the sera of patients vaccinated with L1 VLP did not neutralize HPV types (HPV18, HPV45, HPV6 and HPV11) from the other species tested (A7 and A10).
Table 1.
Vaccination of macaques with TA-CIN induced broadly neutralizing antibody responses.
| In vitro neutralization assay | Macaque 746 | Macaque 811 | Macaque 831 |
|---|---|---|---|
| TA-CIN + GPI-0100 | TA-CIN + GPI-0100 | TA-CIN + GPI-0100 | |
| A | |||
| HPV16 | 12,800 | 12,800 | 12,800 |
| HPV31 | 100 | 200 | 100 |
| HPV58 | 3,200 | 6,400 | 3,200 |
| HPV18 | 200 | 400 | 200 |
| HPV45 | 100 | 200 | 100 |
| HPV11 | 100 | 100 | 100 |
| HPV6 | 50 | 50 | 50 |
| In vitro neutralization assay | Human (n = 7) HPV16 L1 VLP | ||
| B | |||
| HPV16 | 12,800 (1600–102,400) | ||
| HPV31 | 150 (100–200) | ||
| HPV58 | 400 (200–3200) | ||
| HPV18 | <25 (<25–<25) | ||
| HPV45 | <25 (<25–<25) | ||
| HPV11 | <25 (<25–<25) | ||
| HPV6 | <25 (<25–<25) | ||
In vitro neutralization assays were performed using HPV6, HPV11, HPV16, HPV18, HPV45 and HPV58 for sera obtained one month after 3 immunizations with 125 μg of TA-CIN with 1000 μg GPI-0100 individually for each of three macaques. Sera were diluted 2-fold from 1:50. Samples that failed to neutralize at 1:25 are indicated as <25. B. In vitro neutralization titers are shown for sera obtained one month after the final vaccination with HPV16 L1 VLP (50 μg, no adjuvant, months 0, 1 and 4) from a separate published phase I clinical trial [30]. Titers measured in sera of 7 patients are presented as median (range) values. No neutralizing antibodies to any HPV type tested were detected in the sera of a patient who received the placebo vaccination.
3.6. Macaques vaccinated with TA-CIN and adjuvant GPI-0100 generate HPV16 E6 and E7 specific B and T cell responses
The vaccine-specific antibody titer in the monkey serum samples was estimated by HPV16 E6 and E7 ELISAs. HPV16 E6- and E7-specific antibody was detected in all three macaques after immunization. In no cases were there significant antibody titers specific to HPV16 E6 or E7 prior to vaccination. While there was considerable variation in the accumulation of E6 and E7 antibodies over the course of immunization, a modest antibody titer generally was observed one month after the first vaccination, followed by a general upward trend in titer following subsequent immunizations (Fig. 3).
In order to assess the level of E6- and E7-specific T cell immune responses generated by immunization with TA-CIN + GPI-0100, PBMCs harvested from each macaque at one month after the third immunization were stimulated with pooled peptides spanning HPV16 E6 or E7 or as a positive control PHA. The number of E6- and E7-specific IFN-γ secreting and CD4+ and CD8+ T cells generated in response to vaccination was examined by intracellular cytokine staining and flow cytometry analysis (Table 2). Responses to E6 and E7 were detected in macaques 811 and 831 after immunization, but not in macaque 746 (Table 2). Notably macaque 746 was much older (22 years of age) than the two responding animals (811 was age 6, 831 was age 12) and all three were SRV positive by serology prior to the study.
Table 2.
Vaccination of macaques with TA-CIN in GPI-0100 adjuvant induces HPV16 E6- and E7-specific CD4+ and CD8+ T cell responses.
| % of T cells IFN-γ+ | No stimulation | PHA | HPV16 E6 peptide 1–30 pool | HPV16 E7 peptide 1–18 pool |
|---|---|---|---|---|
| CD4+ | ||||
| Macaque 746 | 0.002 | 0.53 | 0.009 | 0.013 |
| Macaque 811 | 0.027 | 0.406 | 0.238 | 0.093 |
| Macaque 831 | 0.001 | 0.128 | 0.099 | 0.134 |
| CD8+ | ||||
| Macaque 746 | 0.019 | 6.33 | 0.026 | 0.014 |
| Macaque 811 | 0.052 | 5.060 | 0.278 | 0.091 |
| Macaque 831 | 0.013 | 1.537 | 0.058 | 0.114 |
Pigtail macaques (numbers 746, 811 and 831) were immunized with 125 μg of TA-CIN with 1000 μg GPI-0100 at monthly intervals. PBMCs were harvested one month after the last vaccination and characterized for HPV16 E6- and E7-specific CD4+ (A) and CD8+ (B) T cells using intracellular IFN-γ staining and flow cytometric analysis after stimulation with overlapping peptide pools or as a positive control, PHA. The percentage of CD4 (A) or CD8 (B) positive PBMCs secreting IFN-γ are presented.
4. Discussion
Immunity to infection after vaccination with L2 is mediated by neutralizing antibodies [10,11,31]. Vaccination of mice as well as macaques with TA-CIN formulated in the adjuvant GPI-0100 induces L2-specific neutralizing antibodies. These antibodies neutralize both homologous type virus HPV16, and heterologous types HPV18, HPV31, HPV45, HPV58, HPV11 and HPV6 that are member of multiple phylogenetic species (A7, A9 and A10) with distinct biological properties (low risk and high risk genital types). While it remains unclear whether the neutralizing antibody titers generated by vaccination with TA-CIN with or without GPI-0100 are sufficient to protect against natural HPV infection, studies in animal models of papillomavirus infection have proven predictive of the outcome in patients in the case of L1 VLP vaccines. Several groups have shown that vaccination with full length L2 or peptides thereof is protective in cows and rabbits against homologous type BPV4 or CRPV challenge respectively [5–7,11,34–36], the same models utilized in the preclinical development of L1 VLP vaccines [37–39]. Indeed the titers of HPV16 neutralizing antibodies generated by vaccination with TA-CIN + GPI-0100 approached those obtained in mice vaccinated with Gardasil®, a vaccine that is highly effective in protecting women against natural HPV16 infection [1]. Furthermore, using a more recently developed pseudovirion challenge model [31,40], here we show that vaccination with TA-CIN + GPI-0100 protects mice from cutaneous challenge with HPV16, whereas vaccination with TA-CIN is less protective. Nonetheless, the data suggest that even low titers of neutralizing antibody offer significant, but perhaps incomplete protection. Furthermore, similar responses were observed in three macaques immunized with TA-CIN + GPI-0100, suggesting that these observations are not confined to mice. Finally, studies in healthy volunteers demonstrate that vaccination with TA-CIN alone generates low titers of neutralizing antibodies [15,16], and our findings herein suggest that these titers could be significantly boosted by the use of an adjuvant, GPI-0100.
While the induction of high titer VLP-specific antibodies upon vaccination with Gardasil® or Cervarix® is correlated with protection from type specific HPV infection, the minimal threshold titer of neutralizing antibodies to afford protection of patients from natural HPV transmission is currently not known [41]. A key question is whether broad cross-protection might be possible with TA-CIN vaccination. Several observations suggest that low titers of neutralizing antibodies are sufficient for protection. Although there was a decline over time in titer among women vaccinated with Gardasil®, notably for HPV18, however there were no breakthrough cases of HPV18-related CIN in patients over a 5-year period [42,43]. Another argument for a low threshold for protection against natural HPV infection is the partial protection against related HPV types observed in women vaccinated with L1 VLP vaccines [2], since neutralization titers against related heterologous types are at least 1–2 orders of magnitude lower than for the homologous type [44]. Finally, rabbits vaccinated with HPV16 L2 polypeptides were protected when challenged with CRPV and ROPV showing that cross-protection can occur between very divergent HPV types, and suggesting that low titers of neutralizing antibodies are sufficient for protection at cutaneous and mucosal sites [11]. It is likely that inclusion of an adjuvant like GPI-0100 with TA-CIN will extend the longevity of the HPV neutralizing antibodies, and may also reduce the dose of antigen required.
Studies to date generally suggest that vaccination with L1 VLPs or L2 does not influence pre-existing infection [10,11,37,45,46], although there are exceptions [47–49]. The lack of an obvious therapeutic effect likely reflects the absence of detectable capsid gene expression in HPV-infected basal cells. However, these cells do express E6 and E7 and thus E6- and E7-specific T cell responses could potentially eliminate HPV infection and disease. Vaccination of mice twice at three week intervals with TA-CIN in Novasome adjuvant beginning on the day of TC-1 challenge did prevent the growth of tumor in 7/10 animals [50]. However, vaccination of women with persistent HPV16+ high grade VIN/VAIN with TA-CIN (without adjuvant) in prime boost combinations with recombinant vaccinia virus expressing HPV16/18 E6 and E7 (TA-HPV) failed to enhance the rate of disease clearance [17,51,52]. Similarly, vaccination with HPV6 L2E7 in ASO2 adjuvant had no impact on pre-existing genital warts [46]. These outcomes may reflect induction of the incorrect type of immune response, a failure of the observed E6/E7-specific T cell responses to traffic to the site of the lesion, or their suppression within the lesion microenvironment, or some combination of these issues [16,53].
Although vaccination using TA-CIN without adjuvant has not been demonstrated to impact the clinical course of pre-existing lesions to date, it is possible that the induction of E6/E7-specific T cells responses prior to HPV infection could prevent the onset of disease from new infections or possibly upon reactivation of previously subclinical disease. In this situation issues of tolerance and suppression by the lesion microenvironment might be avoided. Indeed, prophylactic vaccination with TA-CIN + GPI-0100 completely protected mice from TC-1 tumor challenge, whereas vaccination with TA-CIN alone was poorly effective. Furthermore, the GPI-0100 adjuvant dramatically boosted the E7-specific IFN-γ secreting CD8+ T cell response in mice as compared to vaccination with TA-CIN alone. Spontaneous clearance of warts in patients is associated with robust CD4+ and to a lesser extent CD8+ T cell infiltrates [54]. Vaccination of macaques induced systemic E6- and E7-specific CD4+ and CD8+ T cell responses. Thus it is possible that any protective efficacy against HPV16-related disease by TA-CIN vaccination via the induction of L2-specific neutralizing antibody could potentially be augmented by HPV16 E6/E7-specific T cell responses. This might also be relevant in patients who test positive for HPV16 DNA but lack clinically or histologically apparent disease. It is currently unclear whether HPV E6/E7-targeted cell mediated immune responses are cross-reactive to any degree. Given the importance of HPV18 in causing cervical cancer, and the relatively weak cross-neutralization of HPV18 and HPV45 by TA-CIN antisera, it might be reasonable to consider a bivalent vaccine containing L2E6E7 protein derived from HPV16 and HPV18.
Acknowledgments
The authors gratefully acknowledge Xenova Ltd, UK and CR UK for providing the TA-CIN, and Hawaii Biotech for the GPI-0100, and Saveria Campo (University of Glasgow, Scotland) for critical reading of this manuscript. We also thank John Schiller and Doug Lowy (NCI, NIH) for reagents and sera of patients vaccinated with HPV16 L1 VLP, Martin Muller (DKFZ, Germany) for codon-modified HPV16 L1 and L2, Tadahito Kanda (National Institute of Infectious Diseases, Japan) for codon-modified HPV31, and HPV58 L1 and L2. This research was supported by grants to RBSR from the PHS (National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252 and CA118790) and the Mary Kay Ash Foundation. Finally, we apologize to the many investigators whose pertinent work could not be cited herein due to space constraints.
Footnotes
Conflict of interest statement: RBSR is a paid consultant of Merck & Co, and Knobbe, Martens, Olson and Bear LLC. SJ and RBSR have received grant funding from GSK. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005 May;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007 June;369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004 August;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 4.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006 October;6(10):753–63. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181(2):572–9. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- 6.Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187(2):612–9. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 7.Chandrachud LM, Grindlay GJ, McGarvie GM, O’Neil BW, Wagner ER, Jarrett WF, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211(1):204–8. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- 8.Roden RB, Yutzy WI, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 9.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005 July;337(2):365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of l2, the minor capsid protein. J Virol. 2002 October;76(19):9798–805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N-terminus of HPV16 minor capsid antigen L2. J Virol. 2007 August;81(21):13927–31. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002 May;2(5):342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 13.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004 August;64(15):5449–55. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 14.Hung CF, Wu TC, Monie A, Roden R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol Rev. 2008 April;222:43–69. doi: 10.1111/j.1600-065X.2008.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong A, O’Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002 October;20(29–30):3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 16.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006 December;66(23):11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 17.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006 May–June;16(3):1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 18.Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak V, Gundy LE, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000 July;18(27):3141–51. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 19.Marciani DJ, Reynolds RC, Pathak AK, Finley-Woodman K, May RD. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine. 2003 September;21(25–26):3961–71. doi: 10.1016/s0264-410x(03)00298-6. [DOI] [PubMed] [Google Scholar]
- 20.Quenelle DC, Collins DJ, Marciani DJ, Kern ER. Effect of immunization with herpes simplex virus type-1 (HSV-1) glycoprotein D (gD) plus the immune enhancer GPI-0100 on infection with HSV-1 or HSV-2. Vaccine. 2006 March;24(10):1515–22. doi: 10.1016/j.vaccine.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, et al. On the power of chemical synthesis: immunological evaluation of models for multiantigenic carbohydrate-based cancer vaccines. Proc Natl Acad Sci U S A. 2002 October;99(21):13699–704. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragupathi G, Gathuru J, Livingston P. Antibody inducing polyvalent cancer vaccines. Cancer Treat Res. 2005;123:157–80. doi: 10.1007/0-387-27545-2_7. [DOI] [PubMed] [Google Scholar]
- 23.Gathuru JK, Koide F, Ragupathi G, Adams JL, Kerns RT, Coleman TP, et al. Identification of DHBcAg as a potent carrier protein comparable to KLH for augmenting MUC1 antigenicity. Vaccine. 2005 September;23(39):4727–33. doi: 10.1016/j.vaccine.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Slovin SF, Ragupathi G, Fernandez C, Jefferson MP, Diani M, Wilton AS, et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine. 2005 May;23(24):3114–22. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 25.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001 September;108(5):669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001 May;61(9):3698–703. [PubMed] [Google Scholar]
- 27.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23(9):2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 28.Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004 August;78(16):8468–76. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004 April;321(2):205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 31.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, et al. A protective and broadly cross-neutralizing epitope of Human Papillomavirus L2. J Virol Dec. 2007;81(24):13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenstone HL, Nieland JD, de Visser KE, De Bruijn ML, Kirnbauer R, Roden RB, et al. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci U S A. 1998;95(4):1800–5. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004 June;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Gaukroger JM, Chandrachud LM, O’Neil BW, Grindlay GJ, Knowles G, Campo MS. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J Gen Virol. 1996;77(Pt 7):1577–83. doi: 10.1099/0022-1317-77-7-1577. [DOI] [PubMed] [Google Scholar]
- 35.Campo MS, O’Neil BW, Grindlay GJ, Curtis F, Knowles G, Chandrachud L. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology. 1997;234(2):261–6. doi: 10.1006/viro.1997.8649. [DOI] [PubMed] [Google Scholar]
- 36.Palmer KE, Benko A, Doucette SA, Cameron TI, Foster T, Hanley KM, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006 June;24(26):5516–25. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 37.Kirnbauer R, Chandrachud LM, O’Neil BW, Wagner ER, Grindlay GJ, Armstrong A, et al. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219(1):37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 38.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70(2):960–5. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007 July;13(7):857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 41.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171(6):1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 42.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007 June;25(26):4931–9. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 43.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006 December;95(11):1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roden RB, Greenstone HL, Kirnbauer R, Booy FP, Jessie J, Lowy DR, et al. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70(9):5875–83. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007 August;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 46.Vandepapeliere P, Barrasso R, Meijer CJ, Walboomers JM, Wettendorff M, Stanberry LR, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005 December;192(12):2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LF, Zhou J, Chen S, Cai LL, Bao QY, Zheng FY, et al. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine. 2000;18(11–12):1051–8. doi: 10.1016/s0264-410x(99)00351-5. [DOI] [PubMed] [Google Scholar]
- 48.Jarrett WF, Smith KT, O’Neil BW, Gaukroger JM, Chandrachud LM, Grindlay GJ, et al. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- 49.Govan VA, Christensen ND, Berkower C, Jacobs WR, Jr, Williamson AL. Immunisation with recombinant BCG expressing the cottontail rabbit papillomavirus (CRPV) L1 gene provides protection from CRPV challenge. Vaccine. 2006 March;24(12):2087–93. doi: 10.1016/j.vaccine.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 50.van der Burg SH, Kwappenberg KM, O’Neill T, Brandt RM, Melief CJ, Hickling JK, et al. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine. 2001 June;19(27):3652–60. doi: 10.1016/s0264-410x(01)00086-x. [DOI] [PubMed] [Google Scholar]
- 51.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004 May;10(9):2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 52.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003 September;63(18):6032–41. [PubMed] [Google Scholar]
- 53.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007 July;104(29):12087–92. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102(6):768–74. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]