Abstract
Extracellular inorganic phosphate (Pi) concentrations are the highest in the growth plate just before the onset of mineralization. The study reported here demonstrates that Pi not only is required for hydroxyapatite mineral formation but also modulates terminal differentiation and apoptosis of growth plate chondrocytes. Extracellular Pi stimulated terminal differentiation marker gene expression, including the progressive ankylosis gene (ank), alkaline phosphatase (APase), matrix metalloproteinase-13 (MMP-13), osteocalcin, and runx2, mineralization, and apoptosis of growth plate chondrocytes. The stimulatory effect of extracellular Pi on terminal differentiation and apoptosis events of growth plate chondrocytes was dependent on the concentration, the expression levels of type III Na+/Pi co-transporters, and ultimately Pi uptake. A high extracellular Pi concentration was required for the stimulation of apoptosis, whereas lower Pi concentrations were required for the most effective stimulation of terminal differentiation events, including terminal differentiation marker gene expression and mineralization. Suppression of Pit-1 was sufficient to inhibit the stimulatory effects of extracellular Pi on terminal differentiation events. On the other hand, increasing the local extracellular Pi concentration by overexpressing ANK, a protein transporting intracellular PPi to the extracellular milieu where it is hydrolyzed to Pi in the presence of APase, resulted in marked increases of hypertrophic and early terminal differentiation marker mRNA levels, including APase, runx2 and type X collagen, and slight increase of MMP-13 mRNA levels, but decreased osteocalcin mRNA level, a late terminal differentiation markers. In the presence of levamisole, a specific APase inhibitor to prevent hydrolysis of extracellular PPi to Pi, ANK overexpression of growth plate chondrocytes resulted in decreased mRNA levels of hypertrophic and terminal differentiation markers but increased MMP-13 mRNA levels. In conclusion, with extracellular PPi inhibiting and extracellular Pi stimulating hypertrophic and terminal differentiation events, a precise regulation of PPi/Pi homeostasis is required for the spatial and temporal control of terminal differentiation events of growth plate chondrocytes.
Keywords: Apoptosis, progressive ankylosis protein (ANK), alkaline phosphatase, growth plate chondrocytes, phosphate, pyrophosphate
1. Introduction
Growth plate chondrocytes undergo a series of differentiation events, including proliferation, hypertrophy, terminal differentiation, and mineralization. Eventually, terminally differentiated growth plate chondrocytes undergo programmed cell death (apoptosis) [1]. Little is known about the mechanisms and factors that regulate terminal differentiation events and apoptosis of growth plate chondrocytes. Inorganic phosphate (Pi) is one of the two main ionic components required for hydroxyapatite formation during the mineralization of the extracellular matrix of skeletal tissue cells, including growth plate chondrocytes and osteoblasts. Pi levels markedly increase both in the extracellular matrix and in the cells from the proliferative to the hypertrophic region of the growth plate and reach their highest levels in the zone of terminally differentiated growth plate chondrocytes [2–4]. Disorders in Pi homeostasis lead to abnormal endochondral ossification. For example, hypophosphatemia in vitamin D receptor (VDR)-null mice, hypophosphatemia in Hyp mice, an animal model of X-linked hypophosphatemia, or feeding wild-type C57BL/6J mice a low-phosphorous/high-calcium diet resulted in an enlarged hypertrophic zone because of the reduced rate of apoptosis of terminally differentiated growth plate chondrocytes [5]. Feeding a high Pi diet to vitamin D receptor–deficient mice resulted in rescue of the growth plate phenotype. These findings suggest that extracellular Pi is a regulator of apoptosis of growth plate chondrocytes, and were supported by in vitro studies showing that increasing extracellular Pi concentrations resulted in apoptosis of growth plate chondrocytes [5–8]. Other studies have suggested that extracellular Pi not only affects apoptosis of growth plate chondrocytes but that it may affect growth plate chondrocyte differentiation. For example, extracellular Pi was shown to regulate transcription of type X collagen in the chondrocytic ATDC5 cell line [9]. In addition, loss of alkaline phosphatase (APase) function in mice, the enzyme that provides extracellular Pi in hypertrophic growth plate cartilage by hydrolyzing extracellular inorganic pyrophosphate (PPi), resulted in diminished hypertrophic growth plate zones [10]. However, the exact role of extracellular Pi homeostasis in growth plate chondrocyte biology remains to be established.
Extracellular Pi generation in the growth plate is partially controlled by extracellular PPi and the presence of APase, which hydrolyzes extracellular PPi into Pi. Extracellular PPi concentrations in the growth plate are mainly regulated by two proteins, the phosphodiesterase nucleotide pyrophosphatase family isoenzyme plasma cell membrane glycoprotein-1 (PC-1) and the progressive ankylosis protein (ANK) [11, 12]. PC-1 is an enzyme that hydrolyzes extracellular adenosine triphosphate, thereby producing PPi [12]. ANK is a transmembrane protein that transports intracellular PPi to the extracellular milieu [11]. Extracellular Pi–induced effects on skeletal tissue cells and other cells are dependent on Pi entry into cells. The primary mechanism for extracellular Pi entry through the cell membrane is via a family of Na+-dependent Pi transporters. This family of transporters is subdivided into three groups, based in part on tissue specificity [13]. Growth plate chondrocytes express mainly the type III (NPT3) transporters, which were first identified as receptors for the gibbon ape leukemia virus (Glvr-1, Pit-1) and amphotropic murine retrovirus (RAM, Pit-2) [14]. Blocking these transporters with phosphoformic acid (PFA) inhibited the effects of extracellular Pi on apoptosis of growth plate chondrocytes [8]. A recent study has shown that extracellular PPi directly affected osteopontin gene expression in osteoblasts [15]. Therefore, it is possible that extracellular PPi and Pi affect growth plate chondrocyte differentiation. To test this hypothesis, we determined the effects of extracellular Pi or PPi on growth plate chondrocyte terminal differentiation and apoptosis by culturing growth plate chondrocytes in the presence of extracellular Pi, or by overexpression of ANK in growth plate chondrocytes in the absence or presence of levamisole, a specific inhibitor of APase activity.
2. Materials and Methods
2.1. Cell culture
Chondrocytes were isolated from the hypertrophic zone of 19-day embryonic chick tibial growth plate cartilage or rib cartilage of newborn C57BL/6J mice as described previously [16,17]. Cells were plated at a density of 3 × 106 into 100-mm-diameter tissue culture dishes and grown in monolayer cultures in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Gaithersburg, MD) containing 5% fetal calf serum (FCS; HyClone, Logan, Utah), 2mM L-glutamine (Invitrogen, Carlsbad, CA), and 50 U/ml of penicillin and streptomycin (Invitrogen) (complete medium). The various treatments were started after cells reached confluence. To evaluate the effect of extracellular Pi on terminal differentiation and apoptosis of growth plate chondrocytes, cells were cultured in the presence of phosphate (NaH2PO4) at concentrations varying from 1mM to 8mM in the absence or presence of 35nM retinoic acid (RA; Sigma Aldrich, St. Louis, MO) for up to 4 days. To determine the role of Pi uptake by growth plate chondrocytes, cells were cultured in the presence of 1mM PFA (Sigma Aldrich). To determine the role of extracellular PPi resulting from ANK transport on terminal differentiation, semiconfluent growth plate chondrocytes were transfected with empty pcDNA expression vector or pcDNA expression vector containing ank cDNA using FuGENE 6 transfection reagent per the manufacturer's protocol (Roche, Branchburg, NJ). After transfection, cells were cultured for 2 days in the absence or presence of 0.8mM levamisole (Sigma) to prevent hydrolysis of Pi. Freshly isolated mouse chondrocytes were transfected in suspension with 30μM control siRNA or Pit-1 specific siRNA (Ambion, Austin, TX) using the NeoFX transfection agent (Ambion) following the manufacturer's instruction. Transfected cells were seeded on monolayer as described above and cultured in the presence of 50μg/ml ascorbic acid and 1mM or 4mM Pi for 2 days.
2.2. Caspase-3 activity and cell viability assays
Caspase-3 activity was measured using the ApoAlert caspase fluorescent assay kit (Clontech, Mountain View, CA) as described previously [18]. Briefly, chondrocyte cultures were washed twice with ice-cold phosphate-buffered saline (PBS), scraped into tubes, and centrifuged at 1500 rpm for 10 min. Cell pellets were washed one more time with ice-cold PBS and centrifuged again. Air-dried cell pellets were resuspended in 60 μl of chilled cell lysis buffer and incubated on ice for 10 min. Cellular debris was removed by centrifugation, and 50 μl of 2× reaction buffer/dithiothreitol mixture and 5μl of 1mM caspase-3 substrate (DEVD-7-amino-4-trifluoromethylcoumarin) were added to 50 μl of each sample and incubated for 1 h at 37°C. Caspase-3 activity was measured in a fluorimeter (Berthold Instruments, Oak Ridge, TN) using the excitation wavelength of 400 nm and the emission wavelength of 505 nm. Caspase-3 activity was quantitated using 7-amino-4-trifluoromethylcoumarin standard and normalized to the protein content in each culture. Cell number was determined by using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville, MD) per the manufacturer's protocol.
2.3. In situ detection of apoptotic chondrocytes by TUNEL labeling
Apoptotic chondrocytes were detected using the ApopTag in situ apoptosis detection kit (Chemicon/Millipore, Billerica, MA) to label apoptotic cells by modifying genomic DNA utilizing terminal deoxynucleotidyltransferase and flow cytometry. Briefly, chondrocytes were washed twice with PBS and fixed with 1% paraformaldehyde/PBS solution (pH 7.4) for 10 min. Then fixed chondrocytes were incubated with 1% Triton/PBS solution, followed by incubation with a proteinase K solution (20 μg/ml) for 10 min at room temperature. Samples were incubated with a reaction mixture containing terminal deoxynucleotidyltransferase enzyme and fluorescein-labeled dNTPs at 37°C in a humidified chamber. After 1 h, the reaction was stopped and cells were centrifuged. As a negative control, terminal deoxynucleotidyltransferase enzyme was replaced with water. Cells were stained with propidium iodide and analyzed by flow cytometry using green fluorescence at 520 ± 20 nm and red fluorescence at 620 nm.
2.4. Pi uptake by growth plate chondrocytes
The intracellular Pi concentration of growth plate chondrocytes was measured by using the PiPer phosphate assay kit (Molecular Probes, Eugene, OR). Cells were washed, and the cytoplasmic fraction was obtained by ultracentrifugation as described previously [19]. Ten microliters of the cytoplasmic fraction was used, and the Pi concentration was determined per the manufacturer's instructions.
2.5. RT-PCR and real-time PCR analysis
Total RNA was isolated from growth plate chondrocyte cultures by using the RNeasy minikit (Qiagen, Valencia, CA). Gene expression of Pit-1 and Pit-2 was analyzed by RT-PCR, while gene expression of APase, bcl-2, matrix metalloproteinase-13 (MMP-13), osteocalcin, runx2, and type II and X collagen was quantified by real-time PCR as described previously [19]. Briefly, 1 μg of total RNA was reverse-transcribed by using an Omniscript RT kit (Qiagen). PCR was then performed with Pit-1 and Pit-2 primers generated from the mouse sequences. The primer sequences were as follows: Pit-1 forward primer, 5′GAT GAA ATG GAG ACG CTG AC-3′; Pit-1 reverse primer, 5′-AGG AAC TGG AAG AGA GAA GGG A-3′; Pit-2 forward primer, 5′-GGC TTC CTA TGG ACG GGC AC-3′; Pit-2 reverse primer, 5′-CAG CCA CTG CGT TGC AGT AG-3′. PCR was performed with an annealing temperature of 51°C, and the number of cycles was adjusted to 30. Actin was amplified at the same time and was used as an internal control.
A 1:100 dilution of the resulting cDNA was used as the template to quantitate the relative content of mRNA by real-time PCR (ABI Prism 7300 sequence detection system; Applied Biosystems, Foster City, CA) with the respective primers and SYBR Green. The following primers were used for real-time PCR analysis: ANK forward primer, 5'-GCC TCC ATC TCA GAT GTC ATA GC-3̀; ANK reverse primer, 5̀-GCT CCC TGC ACT CCA AGT GA-3̀; APase forward primer, 5′-CCC TGA CAT CGA GGT GAT CCT-3′; APase reverse primer, 5′-GGT ACT CCA CAT CGC TGG TGT T-3′; bcl-2 forward primers 5'-GGT GAC CCG AAG CAT CAAA-3'; bcl-2 reverse primer, 5'-AGC GAC ACG AAA AAC CCA AAC-3'; collagen type II forward primer, 5′-GGC CCT AGC AGG TTC ACG TAC A-3′; collagen type II reverse primer, 5′-CGA TAA CAG TCT TGC CCC ACT T-3′; collagen type X forward primer, 5′-AGT GCT GTC ATT GAT CTC ATG GA-3′; collagen type X reverse primer, 5′-TCA GAG GAA TAG AGA CCA TTG GAT T-3′; MMP-13 forward primer, 5'-TGG ATG GAC CCT CTG GAT TAC TG -3'; MMP-13 reverse primer, 5̀-CAA AAT GGG CAT CTC CTC CAT A-3'; runx2 forward primer, 5′-CGC GGA GCT GCG AAA T-3′; runx2 reverse primer, 5′-ACG AAT CGC AGG TCA TTG AAT-3′; osteocalcin forward primer, 5′-TCG CGG CGC TGC TCA CAT TCA-3′; osteocalcin reverse primer, 5′-TGG CGG TGG GAG ATG AAG GCT TTA-3′. RT-PCRs were performed with a TaqMan PCR Master Mix kit (Applied Biosystems), with 40 cycles of 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. The 18S RNA was amplified at the same time and used as an internal control. The cycle threshold values for 18S RNA and the samples were measured and calculated by computer software. Relative transcript levels were calculated as x = 2−ΔΔCt, in which ΔΔCt = ΔE − ΔC, ΔE = Ctexp − Ct18S, and ΔC = Ctctl − Ct18S.
2.6. SDS-polyacrylamide gel electrophoresis and immunoblotting
To determine the degree of Pit-1, and Pit-2 protein expression in chondrocyte cultures, cells were collected and incubated in 200 μl of lysis solution (50mM Tris-HCl [pH 8.0], 150mM NaCl, 1mM EDTA, 1.2% Triton X-100) on ice for 20 min as described elsewhere [19]. After centrifugation, the supernatant was collected and equal amounts of protein were dissolved in 4× NuPAGE sodium dodecyl sulfate (SDS) sample buffer containing a reducing agent (Invitrogen), denatured at 70°C for 10 min, and analyzed by electrophoresis in 10% bis-Tris polyacrylamide gels. Samples were electroblotted onto nitrocellulose filters after electrophoresis. After blocking with a solution of low-fat milk protein, blotted proteins were immunostained with primary antibodies specific for Pit-1 (LifeSpan Biosciences, Seattle, WA), or Pit-2 (LifeSpan Biosciences), and then peroxidase-conjugated secondary antibody, and the signal was detected by enhanced chemiluminescence (Pierce Chemical, Rockford, Ill.) as previously described [19].
2.7. Statistical analysis
Student t-tests were performed to evaluate differences between two groups, analysis of variance for three or more groups. Tukey's multiple comparison test was applied as a post hoc test. Statistical significance was defined as p < 0.05.
3. Results
3.1. Extracellular Pi stimulates terminal differentiation of growth plate chondrocytes
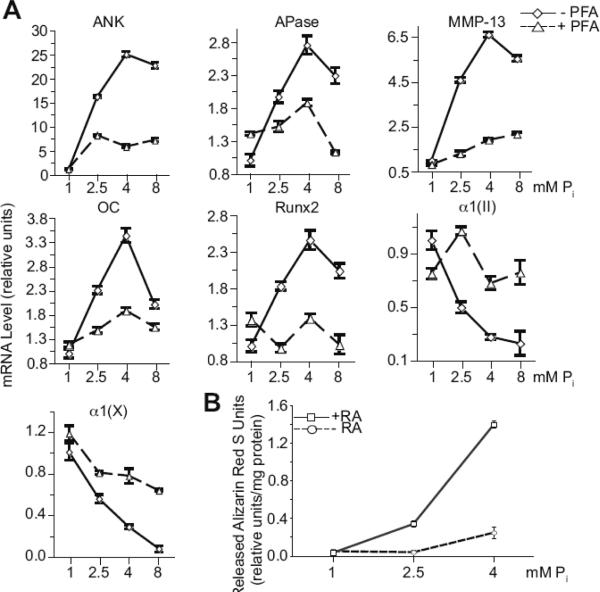
To examine the role of extracellular Pi in terminal differentiation events of growth plate chondrocytes, we cultured d-19 embryonic chick growth plate chondrocytes in the presence of 1mM, 2.5mM, 4mM, and 8mM Pi for 2 days. mRNA levels of terminal differentiation marker genes, including APase, MMP-13, osteocalcin, and runx2, increased with increasing concentrations of extracellular Pi with 4mM Pi being the most effective concentration in stimulating the mRNA levels of these marker genes (Fig. 1A). We have previously shown that type II and X collagen expression levels decreased during the terminal differentiation of growth plate chondrocytes [20]. Consequently, increasing concentrations of extracellular Pi resulted in further decreases in the mRNA levels of type II and X collagen with 8mM Pi being the most effective in decreasing mRNA levels of type II and X collagen (Fig. 1A).
Figure 1.
(A) mRNA levels of hypertrophic and terminal differentiation markers, including ANK, APase, MMP-13, osteocalcin (OC), runx2 and type X collagen α1(X)), and type II collagen (α1(II)), and (B) mineralization of growth plate chondrocytes cultured in the presence of various concentrations of extracellular Pi(1, 2.5, 4, and 8mM) and in the absence (−PFA) or the presence of PFA (+PFA). (A) The levels of these hypertrophic and terminal differentiation marker and type II collagen mRNAs were determined after 2-day treatment with Pi by real-time PCR and SYBR Green and normalized to the 18S RNA levels. Data are means of triplicate PCRs using RNA from three different cultures; error bars represent standard deviations. (B) The degree of mineralization of growth plate chondrocyte cultures treated for 4 days with various concentrations of Pi(1, 2.5, 4mM) in the absence (−RA) or presence of 35nM RA (+RA) was determined using alizarin red S staining. To quantitate the alizarin red S stain each dish was incubated with cetylpyridinium chloride for 1h. The optical density of alizarin red S stain released into solution was measured at 570 nm, and normalized to the total amount of protein. Data are means of four experiments; error bars represent standard deviations.
Next, we determined whether inhibition of Pi uptake via Na+-Pi co-transporters inhibits the stimulation of terminal differentiation by increasing concentrations of extracellular Pi. Growth plate chondrocytes were treated with PFA, which inhibits Na+-Pi co-transporters. PFA treatment resulted in decreased mRNA levels of APase, MMP-13, osteocalcin, and runx2 compared to the mRNA levels of these genes in growth plate chondrocytes treated with various concentrations of extracellular Pi in the absence of PFA, whereas mRNA levels of type II and X collagen increased (Fig. 1A). Interestingly, increasing concentrations of extracellular Pi resulted in increased mRNA levels of ANK, a major regulator of extracellular PPi/Pi homeostasis in growth plate chondrocytes [19], with 4mM Pi being the most effective concentration (Fig. 1A). The increases of ANK mRNA levels in the presence of extracellular Pi were inhibited by PFA (Fig. 1A). Increasing extracellular Pi concentrations also resulted in increased mineralization of growth plate chondrocytes as indicated by increased alizarin red S staining (Fig. 1B). Treatment of growth plate chondrocytes with 4mM Pi for 4 days resulted in a marked increase of mineralization compared to the degree of mineralization in cultures treated with 1 or 2.5mM Pi (Fig. 1B). These findings show that extracellular Pi stimulates terminal differentiation events of growth plate chondrocytes and that the effect of Pi on terminal differentiation is mediated through Pi uptake via Na+-Pi co-transporters. In addition, extracellular Pi stimulates the expression levels of ANK and APase, resulting in further increases of extracellular PPi and extracellular Pi via hydrolysis of PPi by APase.
3.2. RA enhances Pi-mediated stimulation of terminal differentiation events
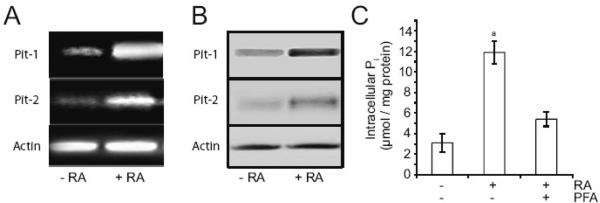
We and others have previously shown that RA stimulates hypertrophic and terminal differentiation events [6, 20–22]. Mineralization of growth plate chondrocytes in the presence of 2.5mM Pi or 4mM Pi was greatly enhanced in the presence of RA. RA in the presence of 1mM Pi did not enhance mineralization (Fig. 1B). Interestingly, both Pit-1 and Pit-2, the two major Na+/Pi co-transporters expressed by growth plate chondrocytes [14], were upregulated following RA treatment. After 2 days of RA treatment, expression of both Pit-1 and Pit-2 was markedly upregulated, as indicated by levels of mRNA (Fig. 2A) and protein (Fig. 2B). In addition, intracellular Pi concentration was markedly increased after 2-day treatment with RA compared to that of untreated cells (Fig. 2C). PFA resulted in a decrease of intracellular Pi concentration of RA-treated cells (Fig. 2C). These findings reveal that RA treatment causes upregulation of Pit-1 and Pit-2 expression and increase of Pi uptake in growth plate chondrocytes.
Figure 2.
mRNA (A) and protein levels (B) of Pit-1 and Pit-2, and intracellular Pi concentration (C) of growth plate chondrocytes cultured in the absence or presence of RA and/or PFA. (A, B, C) Growth plate chondrocytes were cultured for 2 days in the presence of 1mM Pi and in the absence or presence of 35nM RA and/or 1mM PFA. (A) Pit-1 and Pit-2 mRNA levels as determined by PCR using primers (described in “Materials and methods”) encoding Pit-1, Pit-2, or actin. (B) Immunostaining of cell extracts for Pit-1, Pit-2 and actin was performed using antibodies specific for Pit-1, Pit-2, and actin. (C) Intracellular Pi concentrations of growth plate chondrocytes as determined per the method described in “Materials and methods.” Data are means of four experiments; error bars represent standard deviations. ap < 0.01 vs. untreated cells.
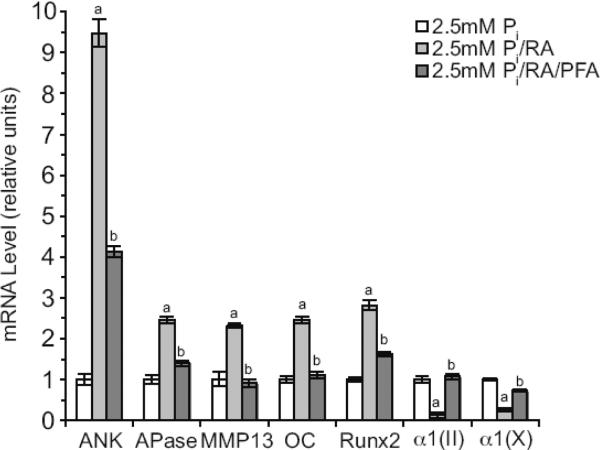
RA treatment markedly increased the mRNA levels of terminal differentiation markers, including ANK, APase, MMP-13, osteocalcin and runx2, of growth plate chondrocytes cultured in the presence of 2.5mM Pi for 2 days compared to cells cultured in the presence of 2.5mM Pi and the absence of RA (Fig. 3). The mRNA levels of type II and type X collagen were further decreased in growth plate chondrocytes treated with RA and 2.5mM Pi compared to the mRNA levels of 2.5mM Pi-treated cells (Fig. 3). PFA treatment inhibited the increases of mRNA levels of terminal differentiation marker genes and the decreases of mRNA levels of type II and X collagen in RA/2.5mM Pi-treated growth plate chondrocytes (Fig. 3). These findings reveal that Pit-mediated Pi uptake are key regulators of RA-mediated stimulation of terminal differentiation events of growth plate chondrocytes.
Figure 3.
(A) mRNA levels of ANK, APase, MMP-13, osteocalcin (OC), runx2, type II collagen (α1(II)), and type X collagen α1(X)) of growth plate chondrocytes cultured in the presence of 2.5mM extracellular Pi and in the absence or the presence of RA and PFA. The levels of hypertrophic and terminal differentiation marker and type II collagen mRNAs were determined after 2-day treatment by real-time PCR and SYBR Green and normalized to the 18S RNA levels. Data are means of triplicate PCRs using RNA from three different cultures; error bars represent standard deviations (ap < 0.01 vs. 2.5mM Pi-treated cells; bp < 0.01 vs. 2.5mM Pi/RA-treated cells).
3.3. Suppression of Pit-1 expression inhibits the stimulatory effects of extracellular Pi on terminal differentiation events
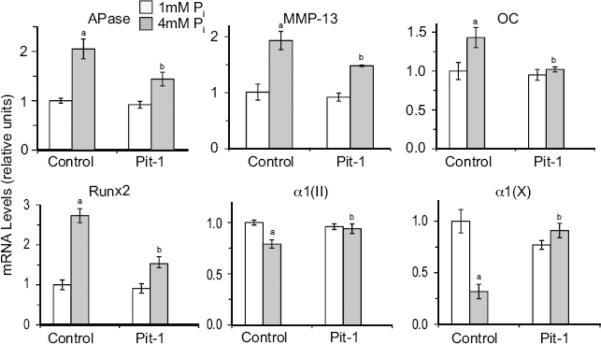
Using commercially available Pit-1 specific siRNA (Ambion) we were able to suppress Pit-1 expression in rib chondrocytes isolated from newborn mice by ~60% (data not shown). Similar to the results shown in Fig. 1A, 4mM Pi treatment resulted in a marked increase in APase, MMP-13, osteocalcin, and runx2 mRNA levels compared to the mRNA levels of these genes in cells cultured in the presence of 1mM Pi, whereas the mRNA levels of type II and type X collagen decreased (Fig. 4). Suppression of Pit-1 expression did not affect the mRNA levels of these genes in 1mM Pi-treated cells. Suppression of Pit-1 expression, however, markedly reduced the mRNA levels of the hypertrophic and terminal differentiation marker genes in 4mM Pi-treated cells compared to the levels of cells treated with 4mM Pi and transfected with control siRNA, while increasing the mRNA levels of type II and type X collagen (Fig. 4). These findings demonstrate that suppression of Pit-1 expression is sufficient to inhibit the stimulatory effect of 4mM Pi on terminal differentiation of mouse rib chondrocytes.
Figure 4.
The effect of suppression of Pit-1 expression on extracellular Pi-mediated stimulation of terminal differentiation markers, including APase, MMP-13, osteocalcin (OC), runx2, type X collagen (α1(X)), and type II collagen (α1(II)). Pit-1 expression was suppressed by specific siRNA (Pit-1). Control cells were transfected with a control siRNA (Control). After 2 days of transfection, cells were cultured in the presence of 1mM or 4mM Pi. The levels of hypertrophic and terminal differentiation marker mRNAs were determined by real-time PCR and SYBR Green and normalized to the 18S RNA levels. Data are means of triplicate PCRs using RNA from three different cultures; error bars represent standard deviations (ap < 0.01 vs. 1mM Pi-treated/control siRNA-transfected-cells; bp < 0.01 vs. 4mM Pi-treated/control siRNA-transfected cells)
3.4. ANK together with APase via regulating extracellular PPi and Pi concentrations control terminal differentiation events
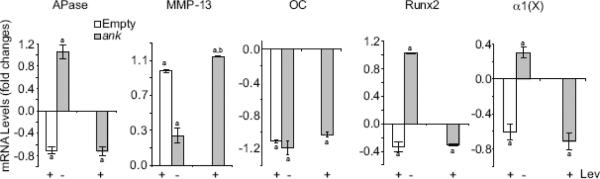
Since ANK together with APase play a major role in regulating extracellular PPi concentration and ultimately extracellular Pi concentration in growth plate cartilage, we determined how overexpression of ANK affected terminal differentiation of growth plate chondrocytes. We overexpressed ANK in chicken growth plate chondrocytes using the pcDNA expression vector. We obtained a 2- to 3-fold increase in ANK protein expression in growth plate chondrocytes transfected with pcDNA vector containing cDNA encoding full-length ank compared to cells transfected with empty pcDNA vector (data not shown). Overexpression of ANK led to increases of APase, MMP-13, runx2, and type X collagen mRNA levels compared to mRNA levels of these genes in growth plate chondrocytes transfected with empty pcDNA vector, whereas osteocalcin mRNA levels decreased in ANK-overexpressing growth plate chondrocytes compared to the levels of empty vector-transfected cells (Fig. 5).
Figure 5.
The effect of elevated extracellular PPi and/or Pi levels on mRNA levels of hypertrophic and terminal differentiation markers, including APase, MMP-13, osteocalcin (OC), runx2, and type X collagen (α1(X)). To elevate extracellular PPi levels and prevent extracellular PPi hydrolysis to Pi, empty vector-transfected growth plate chondrocytes were cultured in the presence of levamisole (+ Lev) to inhibit APase activity. In addition, growth plate chondrocytes were transfected with pcDNA expression vector containing full-length ank cDNA (ank) and cultured in the presence of levamisole (+ Lev) for 2 days after transfection. To increase local extracellular Pi concentrations generated by ANK and APase, growth plate chondrocytes were transfected with pcDNA expression vector containing full-length ank cDNA (ank) in the absence of levamisole (− Lev). The levels of hypertrophic and terminal differentiation marker mRNAs, including APase, MMP-13, osteocalcin (OC), runx2, and type X collagen (α1(X)), were determined by real-time PCR and SYBR Green and normalized to the 18S RNA levels. Data are means of triplicate PCRs using RNA from three different cultures, and expressed as fold changes compared to untreated growth plate chondrocytes transfected with empty vector (Empty); error bars represent standard deviations (ap < 0.01 vs. cells transfected with empty vector; bp < 0.01 vs. cells transfected with empty vector and treated with levamisole).
Since extracellular PPi resulting from ANK transport is easily hydrolyzed to Pi in the presence of APase [18], we prevented hydrolysis of extracellular PPi by treatment of cells with the specific APase inhibitor levamisole [23]. Levamisole treatment of empty vector–transfected growth plate chondrocytes decreased the mRNA levels of hypertrophic and terminal differentiation marker genes, including APase, osteocalcin, runx2, and type X collagen compared to the levels of untreated, empty vector-transfected cells, whereas MMP-13 mRNA levels increased (Fig. 5). Levamisole treatment of ANK-overexpressing growth plate chondrocytes decreased APase, osteocalcin, runx2, and type X collagen mRNA levels to levels similar to the ones of levamisole-treated, empty vector-transfected growth plate chondrocytes (Fig. 5). MMP-13 mRNA level increased to a higher level than the level of levamisole-treated, empty vector-transfected cells (Fig. 5). These findings reveal that extracellular PPi resulting from ANK transport directly stimulates MMP-13 gene expression, while decreasing the expression levels of other hypertrophic and terminal differentiation marker genes. Contrary, extracellular Pi stimulates hypertrophic and early terminal differentiation events in a concentration-dependent manner, suggesting that the precise control of extracellular PPi/Pi homeostasis plays a critical role in the regulation of terminal differentiation events of growth plate chondrocytes.
3.5. Extracellular Pi stimulates apoptosis of terminally differentiated growth plate chondrocytes
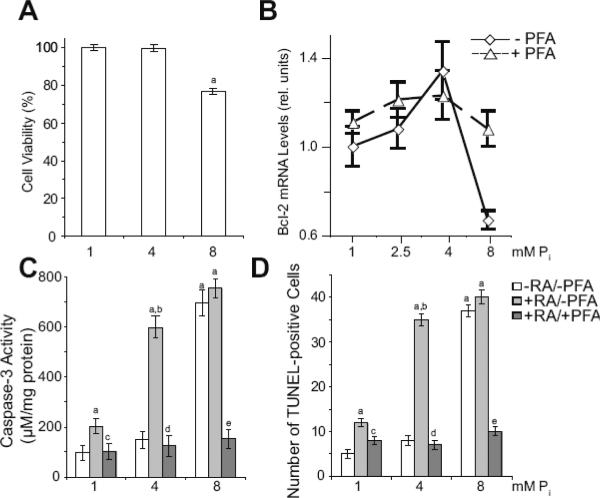
Increasing concentrations of Pi have been shown in previous studies to induce apoptosis of growth plate chondrocytes [5–8]. In most of these studies, however, growth plate chondrocytes were cultured in serum-free conditions, which have been shown to induce apoptosis of growth plate chondrocytes independent of their differentiation stage, and in the presence of factors (such as RA) known to induce terminal differentiation events in growth plate chondrocytes, including apoptosis [6, 20–22]. When growth plate chondrocytes were cultured in serum-containing conditions in the presence of various concentrations of Pi for 4 days, cell number was not reduced in the presence of 4mM Pi compared to 1mM Pi-treated cultures, whereas in the presence of 8mM Pi cell number was reduced by ~23% (Fig. 6A). The anti-apoptotic bcl-2 mRNA levels slightly but not statistically significant increased in the presence of 2.5mM and 4mM Pi compared to the bcl-2 mRNA levels of 1mM Pi-treated cells, whereas the bcl-2 mRNA levels sharply decreased in the presence of 8mM Pi (Fig. 6B). Bcl-2 mRNA levels of 8mM Pi-treated growth plate chondrocytes increased in the presence of PFA (Fig. 6B). Loss of cell number of growth plate chondrocytes in the presence of increasing concentrations of Pi was caused by apoptosis, as revealed by increased caspase-3 activity and increased number of TUNEL-positive cells in growth plate chondrocyte cultures treated with 8mM Pi compared to caspase-3 activity and TUNEL-positive cells in 1mM and 4mM Pi-treated cells (Fig. 6C,D). In the presence of RA, which stimulated the expression of Pit-1 and Pit-2 and consequently Pi uptake (see Fig. 2), caspase-3 activity and the number of TUNEL-positive cells increased in 4mM Pi-treated growth plate chondrocyte cultures to levels similar to the levels of 8mM Pi-treated cultures. RA treatment slightly but not statistically significant increased caspase-3 activity and TUNEL-positive cells in 8mM Pi-treated growth plate chondrocyte cultures (Fig. 6C,D). PFA inhibited the increases of caspase-3 activity and the number of TUNEL-positive growth plate chondrocytes treated with 1mM Pi/RA, 4mM Pi/RA, or 8mM Pi (Fig. 6C,D). These findings demonstrate that extracellular Pi stimulates terminal differentiation and apoptotic events in a concentration-dependent manner.
Figure 6.
Apoptosis of growth plate chondrocytes treated with various concentrations of extracellular Pi in the absence or presence of RA and PFA. (A) Cell number of growth plate chondrocytes cultured in the presence of 1, 4, and 8mM extracellular Pi for 4 days. Cell number was determined using the CCK-8 assay; cell number of cells cultured in the presence of 1mM Pi was set to 100%. Data are means of four experiments; error bars represent standard deviations (ap < 0.01 vs. 1mM Pi-treated cells). (B) The levels of bcl-2 mRNA were determined after 2-day treatment with various Pi concentrations (1, 2.5, 4, 8mM) in the absence (− PFA) or presence of PFA (+ PFA) by real-time PCR and SYBR Green and normalized to the 18S RNA levels. Data are means of triplicate PCRs using RNA from three different cultures; error bars represent standard deviations. (C) Caspase-3 activity of growth plate chondrocytes cultured in the presence of various concentrations of extracellular Pi (1, 4, 8mM) and in the absence of RA and PFA (−RA/−PFA), presence of RA and absence of PFA (+RA/−PFA), or presence of RA and PFA (+RA/+PFA) for 4 days. Caspase-3 activity was measured and normalized to the total protein concentration. Data are means of four experiments; error bars represent standard deviations (ap < 0.01 vs. 1mM Pi-treated cells; bp < 0.01 vs. 4mM Pi-treated cells; cp < 0.01 vs. 1mM Pi/RA-treated cells; dp < 0.01 vs. 4mM Pi/RA-treated cells; ep < 0.01 vs. 8mM Pi/RA-treated cells). (D) Percent TUNEL-positive cells among growth plate chondrocytes cultured as in (C) as determined by flow cytometric analysis. Data are means of four experiments; error bars represent standard deviations (ap < 0.01 vs. 1mM Pi-treated cells; bp < 0.01 vs. 4mM Pi-treated cells; cp < 0.01 vs. 1mM Pi/RA-treated cells; dp < 0.01 vs. 4mM Pi/RA-treated cells; ep < 0.01 vs. 8mM Pi/RA-treated cells).
4. Discussion
Extracellular Pi concentrations in the growth plate increase when growth plate chondrocytes undergo hypertrophic differentiation and reach the highest levels just before mineralization of the extracellular matrix starts [2–4]. Previous studies have implicated extracellular Pi as a modulator of chondrocyte apoptosis. [5–8]. Our data demonstrates that extracellular Pi not only modulates chondrocyte apoptosis but the entire terminal differentiation process. As shown in this study, extracellular Pi modulates the expression of hypertrophic and terminal differentiation marker genes, mineralization and apoptosis of growth plate chondrocytes. The modulator effects of extracellular Pi on terminal differentiation events is ultimately dependent on the extracellular Pi concentration and the uptake of extracellular Pi via Na+/Pi co-transporters by growth plate chondrocytes. In addition, our findings suggest that the stimulatory effects of RA on terminal differentiation of growth plate chondrocytes are at least partially due to increasing Na+/Pi co-transporter expression and Pi uptake. A previous study showed increased type X collagen expression in articular chondrocytes by interleukin-8 treatment was also due to increases of Pit -1 expression and Pi uptake [25]. These and our findings reveal that extracellular Pi and Pi uptake by chondrocytes play an important role in hypertrophic and terminal differentiation events during development and pathology, and that factors, which play major roles in the regulation of these events, including RA [22, 26], may regulate these events by affecting extracellular Pi concentrations and/or the expression of Pi transporters.
Our findings show that the extracellular PPi/Pi homeostasis controlled by extracellular local PPi resulting from ANK transport, and local and circulating Pi play key roles in the control of hypertrophic and terminal differentiation events of growth plate chondrocytes. Increasing extracellular Pi concentration indirectly by increasing extracellular PPi via overexpression of ANK in growth plate chondrocytes was sufficient for the stimulation of hypertrophic and early terminal differentiation marker genes, including APase, runx2, and type X collagen, but not for the stimulation of osteocalcin, a late terminal differentiation marker. Four mM extracellular Pi resulted in the most effective stimulation of early and late terminal differentiation marker genes, whereas 8mM extracellular Pi resulted in the stimulation of apoptotic events in growth plate chondrocytes. In addition, our and other findings have demonstrated that during terminal differentiation the expression levels of Pit-1 and Pit-2 and ultimately extracellular Pi uptake increase during terminal differentiation of growth plate chondrocytes [19, 25, 27]. Furthermore, extracellular Pi itself upregulates the expression of Pit-1 and Pit-2, and as shown in this study the expression of ANK, thereby creating a positive feed back loop further increasing local extracellular PPi and Pi concentrations and the uptake of extracellular Pi [28]. Contrary, suppression of Pit-1 expression in chondrocytes was sufficient to inhibit the increase of the expression levels of early and late terminal differentiation marker genes mediated by 4mM extracellular Pi. These findings suggest that the precise regulation of extracellular Pi concentrations and the expression levels of Na+-Pi co-transporters controls Pi uptake into growth plate chondrocytes and ultimately plays an important role in the temporal and spatial regulation of terminal differentiation, mineralization and apoptosis of growth plate chondrocytes.
Our findings showing that only 8mM Pi resulted in marked increases in caspase-3 activity and number of TUNEL-positive cells, reveals that extracellular Pi-mediated growth plate chondrocyte apoptosis requires higher extracellular Pi concentrations than required for the stimulation of hypertrophic and terminal differentiation events. Therefore, it is plausible that a precise regulation of a concentration gradient of extracellular Pi formed by local and circulating extracellular Pi is required to allow the spatial and temporal regulation of terminal differentiation and apoptosis events of growth plate chondrocytes. Since vascularization of the growth plate occurs in mineralized growth plate cartilage where fully terminally differentiated chondrocytes release the angiogenic factor vascular endothelia growth factor [29], circulating Pi levels are expected to mostly increase the extracellular Pi levels to levels sufficient to stimulate apoptotic events in terminally differentiated growth plate chondrocytes, whereas increasing ANK, APase, PC-1, and Pit-1 and Pit-2 expression levels during terminal differentiation are mostly expected to control extracellular Pi levels to levels sufficient to appropriately stimulate hypertrophic and terminal differentiation events. This model proposing that a extracellular Pi gradient regulates terminal differentiation and apoptosis events in growth plate chondrocytes is supported by recent findings showing that the reduction of Pi serum levels in the vitamin D receptor-deficient mice or the Hyp mice affected apoptosis of growth plate chondrocytes, but not their hypertrophic and terminal differentiation and mineralization, whereas APase null-mice showed decreased mineralization and a significant reduction in the hypertrophic zone [5, 10].
Our study shows that not only extracellular Pi but also extracellular PPi directly affects growth plate chondrocyte hypertrophic and terminal differentiation events. Extracellular PPi stimulated the expression of MMP-13 expression, whereas extracellular PPi reduced the expression of other hypertrophic and terminal differentiation markers, including APase, osteocalcin, runx2, and type X collagen. MMP-13 expression occurs late in the growth plate, and therefore it was concluded that MMP-13 is a late terminal differentiation marker [30, 31]. In addition, runx2 has been shown to regulate the expression of MMP-13 in growth plate and osteoarthritic cartilage [32–34]. However, MMP-13 expression is highly activated in articular chondrocytes already early in the disease [35]. Our results suggest that the stimulation of MMP-13 expression by extracellular PPi is independent of runx2 and chondrocyte hypertrophy and/or terminal differentiation. These findings together with findings showing a marked upregulation of ANK expression in osteoarthritic cartilage suggest that extracellular PPi may play an important role in the regulation of MMP-13 expression in osteoarthritis [19, 36, 37]. Furthermore, our findings suggest that extracellular PPi acts as a negative regulator of terminal differentiation and mineralization events. Extracellular PPi has been previously shown to directly inhibit mineralization by binding to hydroxyapatite and preventing its growth [15]. Therefore, extracellular PPi may play an important role in controlling terminal differentiation and mineralization of growth plate chondrocytes to prevent uncontrolled terminal differentiation and excessive mineralization.
In conclusion, our study demonstrates that extracellular PPi/Pi homeostasis plays a crucial role in the regulation of hypertrophic and terminal differentiation of growth plate chondrocytes. The effect of extracellular Pi on growth plate chondrocyte terminal differentiation and apoptosis is dependent on the concentration of extracellular Pi, the expression of Pi-transporters, and ultimately the uptake of extracellular Pi. Finally, not only extracellular Pi but also extracellular PPi directly independent of its hydrolysis to Pi regulates terminal differentiation events of growth plate chondrocytes. Our and other findings suggest that that extracellular PPi controls extracellular Pi-mediated stimulation of hypertrophic, terminal differentiation, and mineralization events of growth plate chondrocytes to prevent uncontrolled and excessive terminal differentiation and mineralization [15,38]. Therefore, a precise regulation of PPi/Pi homeostasis in growth plate cartilage is required for the spatial and temporal regulation of terminal differentiation and apoptosis of growth plate chondrocytes.
Acknowledgments
This study was funded by NIAMS/NIH grants R01AR046245 and R01AR049074 to T.K.
Abbreviations
- ank
progressive ankylosis gene
- ANK
progressive ankylosis protein
- APase
alkaline phosphatase
- CCK-8
Cell Counting Kit-8
- DMEM
Dulbecco's modified Eagle's medium
- FCS
fetal calf serum
- Glvr-1 or Pit-1
gibbon ape leukemia virus receptor-1
- MMP-13
matrix metalloproteinase-13
- PBS
phosphate-buffered saline
- PC-1
phosphodiesterase nucleotide pyrophosphatase family isoenzyme plasma cell membrane glycoprotein-1
- PFA
phosphoformic acid
- Pi
inorganic phosphate
- PPi
inorganic pyrophosphate
- RA
retinoic acid
- RAM or Pit-2
receptor for the amphotropic murine retrovirus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- [1].Adams CS, Shapiro IM. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit. Rev. Oral Biol. Med. 2002;13:465–473. doi: 10.1177/154411130201300604. [DOI] [PubMed] [Google Scholar]
- [2].Boyde A, Shapiro IM. Energy dispersive X-ray elemental analysis of isolated epiphyseal growth plate chondrocyte fragments. Histochemistry. 1980;69:85–94. doi: 10.1007/BF00508369. [DOI] [PubMed] [Google Scholar]
- [3].Shapiro IM, Boyde A. Microdissection--elemental analysis of the mineralizing growth cartilage of the normal and rachitic chick. Metab. Bone Dis. Relat. Res. 1984;5:317–326. doi: 10.1016/0221-8747(84)90019-5. [DOI] [PubMed] [Google Scholar]
- [4].Wu LN, Ishikawa Y, Sauer GR, Genge BR, Mwale F, Mishima H, Wuthier RE. Morphological and biochemical characterization of mineralizing primary cultures of avian growth plate chondrocytes: evidence for cellular processing of Ca2+ and Pi prior to matrix mineralization. J. Cell. Biochem. 1995;57:218–237. doi: 10.1002/jcb.240570206. [DOI] [PubMed] [Google Scholar]
- [5].Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl. Acad. Sci. U S A. 2005;102:9637–9642. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mansfield K, Pucci B, Adams CS, Shapiro IM. Induction of apoptosis in skeletal tissues: phosphate-mediated chick chondrocyte apoptosis is calcium dependent. Calcif. Tissue Int. 2003;73:161–172. doi: 10.1007/s00223-002-1056-z. [DOI] [PubMed] [Google Scholar]
- [7].Mansfield K, Rajpurohit R, Shapiro IM. Extracellular phosphate ions cause apoptosis of terminally differentiated epiphyseal chondrocytes. J. Cell. Physiol. 1999;179:276–286. doi: 10.1002/(SICI)1097-4652(199906)179:3<276::AID-JCP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [8].Mansfield K, Teixeira CC, Adams CS, Shapiro IM. Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism. Bone. 2001;28:1–8. doi: 10.1016/s8756-3282(00)00409-9. [DOI] [PubMed] [Google Scholar]
- [9].Magne D, Bluteau G, Faucheux C, Palmer G, Vignes-Colombeix C, Pilet P, Rouillon T, Caverzasio J, Weiss P, Daculsi G, Guicheux J. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J. Bone Miner. Res. 2003;18:1430–1442. doi: 10.1359/jbmr.2003.18.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millan JL, MacGregor GR, Whyte MP. Alkaline phosphatase knockout mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- [12].Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell. Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- [13].Takeda E, Taketani Y, Morita K, Tatsumi S, Katai K, Nii T, Yamamoto H, Miyamoto K. Molecular mechanisms of mammalian inorganic phosphate homeostasis. Adv. Enzyme Regul. 2000;40:285–302. doi: 10.1016/s0065-2571(99)00036-9. [DOI] [PubMed] [Google Scholar]
- [14].Caverzasio J, Bonjour JP. Characteristics and regulation of Pi transport in osteogenic cells for bone metabolism. Kidney Int. 1996;49:975–980. doi: 10.1038/ki.1996.138. [DOI] [PubMed] [Google Scholar]
- [15].Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007;282:15872–15883. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- [16].Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J. Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lefebvre V, Garofalo S, Zhou G, Metsaranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14:329–335. doi: 10.1016/0945-053x(94)90199-6. [DOI] [PubMed] [Google Scholar]
- [18].Wang W, Kirsch T. Annexin V/beta5 integrin interactions regulate apoptosis of growth plate chondrocytes. J. Biol. Chem. 2006;281:30848–30856. doi: 10.1074/jbc.M605937200. [DOI] [PubMed] [Google Scholar]
- [19].Wang W, Xu J, Du B, Kirsch T. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol. Cell. Biol. 2005;25:312–323. doi: 10.1128/MCB.25.1.312-323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J. Cell Biol. 2002;157:1061–1069. doi: 10.1083/jcb.200203014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pucci B, Adams CS, Fertala J, Snyder BC, Mansfield KD, Tafani M, Freeman T, Shapiro IM. Development of the terminally differentiated state sensitizes epiphyseal chondrocytes to apoptosis through caspase-3 activation. J. Cell. Physiol. 2007;210:609–615. doi: 10.1002/jcp.20857. [DOI] [PubMed] [Google Scholar]
- [22].Iwamoto M, Shapiro IM, Yagami K, Boskey AL, Leboy PS, Adams SL, Pacifici M. Retinoic acid induces rapid mineralization and expression of mineralization-related genes in chondrocytes. Exp.Cell Res. 1993;207:413–420. doi: 10.1006/excr.1993.1209. [DOI] [PubMed] [Google Scholar]
- [23].Van Belle H. Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin. Chem. 1976;22:972–976. [PubMed] [Google Scholar]
- [24].Kolettas E, Muir HI, Barrett JC, Hardingham TE. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology. 2001;40:1146–1156. doi: 10.1093/rheumatology/40.10.1146. [DOI] [PubMed] [Google Scholar]
- [25].Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005;52:144–154. doi: 10.1002/art.20748. [DOI] [PubMed] [Google Scholar]
- [26].Koyama E, Golden EB, Kirsch T, Adams SL, Chandraratna RA, Michaille JJ, Pacifici M. Retinoid signaling is required for chondrocyte maturation and endochondral bone formation during limb skeletogenesis. Dev. Biol. 1999;208:375–391. doi: 10.1006/dbio.1999.9207. [DOI] [PubMed] [Google Scholar]
- [27].Wuthier RE. Involvement of cellular metabolism of calcium and phosphate in calcification of avian growth plate cartilage. J. Nutr. 1993;123:301–309. doi: 10.1093/jn/123.suppl_2.301. [DOI] [PubMed] [Google Scholar]
- [28].Beck GR, Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp. Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- [29].Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- [30].Johansson N, Saarialhokere U, Airola K, Herva R, Nissinen L, Westermarck J, Vuorio E, Heino J, Kahari VM. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev. Dyn. 1997;208:387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [31].Tuckermann JP, Pittois K, Partridge NC, Merregaert J, Angel P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J. Bone Miner. Res. 2000;15:1257–1265. doi: 10.1359/jbmr.2000.15.7.1257. [DOI] [PubMed] [Google Scholar]
- [32].Zheng Q, Sebald E, Zhou G, Chen Y, Wilcox W, Lee B, Krakow D. Dysregulation of chondrogenesis in human cleidocranial dysplasia. Am. J. Hum. Genet. 2005;77:305–312. doi: 10.1086/432261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- [34].Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- [35].Takaishi H, Kimura T, Dalal S, Okada Y, D'Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm. Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- [36].Hirose J, Ryan LM, Masuda I. Up-regulated expression of cartilage intermediate-layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2002;46:3218–3229. doi: 10.1002/art.10632. [DOI] [PubMed] [Google Scholar]
- [37].Johnson K, Terkeltaub R. Upregulated ank expression in osteoarthritis can promote both chondrocyte MMP-13 expression and calcification via chondrocyte extracellular PPi excess. Osteoarthritis Cartilage. 2004;12:321–335. doi: 10.1016/j.joca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [38].Thouverey C, Bechkoff G, Pikula S, Buchet R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthritis Cartilage. 2009;17:64–72. doi: 10.1016/j.joca.2008.05.020. [DOI] [PubMed] [Google Scholar]