Abstract
Studies in all sensory modalities have demonstrated amplification of early brain responses to attended signals, but less is known about the processes by which listeners selectively ignore stimuli. Here we use MEG and a new paradigm to dissociate the effects of selectively attending, and ignoring in time. Two different tasks were performed successively on the same acoustic stimuli: triplets of tones (A, B, C) with noise-bursts interspersed between the triplets. In the COMPARE task subjects were instructed to respond when tones A and C were of same frequency. In the PASSIVE task they were instructed to respond as fast as possible to noise-bursts. COMPARE requires attending to A and C and actively ignoring tone B, but PASSIVE involves neither attending to nor ignoring the tones. The data were analyzed separately for frontal and auditory-cortical channels to independently address attentional effects on low-level sensory versus putative control processing. We observe the earliest attend/ignore effects as early as 100 ms post stimulus onset in auditory cortex. These appear to be generated by modulation of exogenous (stimulus-driven) sensory evoked activity. Specifically related to ignoring, we demonstrate that active-ignoring-induced input inhibition involves early selection. We identified a sequence of early (<200ms post onset) auditory cortical effects, comprised of onset response attenuation and the emergence of an inhibitory response, and provide new, direct evidence that listeners actively ignoring a sound can reduce their stimulus related activity in auditory cortex by 100 ms after onset when this is required to execute specific behavioral objectives.
Keywords: Attention, suppression, MEG, auditory evoked response, M100, auditory cortex, frontal cortex, gain modulation
Attention is often illustrated by the metaphor of an internal spotlight, a mechanism that enables an organism to select and preferentially process behaviorally relevant input. Brain-imaging studies in humans (see Hillyard et al., 1998; Kastner & Ungerleider, 2000; Herrmann & Knight, 2001 for reviews), mostly of the visual system (e.g. Corbetta et al., 1990; Luck et al., 1997; Shulman et al., 1997) but also in the auditory modality (e.g. Hillyard et al., 1973; Woldorff & Hillyard, 1991; Giard et al., 2000; Petkov et al., 2004; Fritz et al, 2007; Elhilali et al, 2009) have demonstrated that attending to a stimulus, can influence very early processing phases by boosting the sensitivity of low-level sensory processes and gating sensory input.
Another aspect of attention is the ability to ignore irrelevant or distracting stimuli. Ignoring may be viewed as a direct side effect of attending, caused by limited immediate processing resources when the scene is busy (e.g. Lavie, 2005). Indeed, increased activity in brain areas that process attended signals or internal cognitive tasks is often accompanied by suppression of activity in other regions (e.g. Kastner & Ungerleider, 2000; Johnson & Zatorre, 2005; Ghatan et al., 1998; Rees et al., 1997; Rees et al., 1999). However, in addition to being a consequence of focused attention, ignoring is frequently an active process - for instance, if the distracter is attention-grabbing and hinders the organism's ability to concentrate on task-relevant features (e.g. Ipata et al., 2006; Melara et al., 2002).
In EEG (Electroencephalography) or MEG (Magnetoencephalography) studies of auditory selective attention, brain responses to attended sounds usually show increased amplitudes relative to responses to the same sounds when they are not attended (e.g. Naatanen, 1992; Hillyard et al., 1973; Alain & Woods, 1994; Teder-Sälejärvi et al., 1999; Snyder et al., 2006; Ross et al., 2009; Bidet-Caulet et al., 2007). This difference could arise from amplification of responses to attended stimuli, attenuation of responses to non-attended stimuli, or both. A number of studies have demonstrated decreased responses to ignored tones relative to neutral conditions, suggesting that inhibition of processing of non-attended signals contributes to the measured difference (Berman et al., 1989; Rif et al., 1991; Michie et al., 1990; Alain & Woods, 1994; Alain et al., 1993; Michie et al., 1993; Bidet-Caulet et al., 2010). Such event-related potential (ERP) inhibition has usually been demonstrated in cases where the irrelevant stimuli can be perceptually suppressed based on the ongoing stimulus context ℃ situations when distracters and to-be-attended signals differ by some feature such as frequency (Alain et al., 1993; Alain & Woods, 1994; Rif et al.. 1991) or location (Rif et al., 1991; Melara et al., 2002; Michie et al., 1993; Alho et al., 1994b; Bidet-Caulet et al., 2010) that allows the segregation of the scene into task-relevant and task-irrelevant streams of stimuli. For example, Alain & Woods (1994) used tone-pips of three different frequencies presented in random order. Subjects were instructed to attend to one of the frequencies and detect deviant (longer duration) tones at that frequency. The data suggested that clustering the distracters to facilitate ignoring resulted in suppressed ERPs to distracter tones as well as increased responses to attended tones.
In most cases the effects of distracter suppression have been reported to occur substantially later than the enhancement of the response to attended signals (e.g. reviewed in Giard et al., 2000). While attentional facilitation effects commonly emerge from about 100 ms post tone onset – at the time of the N1/M100 auditory onset response (Hillyard et al., 1973; Hansen & Hillyard, 1988; Michie et al., 1993; Alho et al. 1994b; Fujiwara et al., 1998; Melara et al., 2002) or even earlier (Woldorff & Hillyard, 1991; Woldorff et al., 1993), suppression effects are usually reported to emerge later – from about 200 ms post onset (Alho et al., 1987; Michie et al., 1990; Rif et al., 1991; Michie et al., 1993; Alain & Woods, 1994; Melara et al., 2002; Degerman et al., 2008; Bidet-Caulet et al., 2010). These different temporal properties can be taken to suggest that attentional facilitation and inhibition may be two independent processes.
In the present MEG study we elaborate on these mechanisms and their temporal properties with a new stimulus/task paradigm which allows us to temporally dissociate the effects of active ignoring and active attending within the same ongoing auditory `scene': The stimuli consisted of a series of triplets of tones (A, B, C), with a number of noise bursts randomly interspersed between the triplets. Two tasks were performed successively on the same stimuli in a counterbalanced fashion. In the COMPARE task, subjects were instructed to disregard the noise bursts and respond when tones A and C were of the same frequency. In the PASSIVE task, subjects were instructed to respond as quickly as possible to the noise bursts. Since the stimuli were identical in both tasks, differences in response can be attributed to the perceptual state of the listeners induced by task demands. Whereas the PASSIVE task required no special effort to attend or ignore the tones, good performance in the COMPARE task could only be achieved if subjects ignored B tones while attending to A and C. Frequencies were roved to prevent listeners from attending to or suppressing a particular frequency range and the stimuli were optimized to encourage the suppression of tone B in time.
It is known that listeners are able to selectively orient attention in time (Griffin et al., 2001), and that such allocation of attention can affect very early processing stages (Lange et al., 2003; Sanders & Astheimer, 2008). Lange et al., (2003) demonstrated that auditory stimuli at attended, compared to un-attended moments in time elicited enhanced N1 onset responses, similarly to what has been demonstrated for spatial attention (e.g. Hillyard et al., 1973). Based on these findings we expect an enhanced response to C tones in the COMPARE relative to the PASSIVE listening task. However our study asks an additional question: what are the neural correlates of selectively ignoring the irrelevant B tone in the COMPARE task? Can listeners selectively ignore a moment in time? Does the perceptual state of attempting to ignore tone B affect the way tone B is processed and if so, how early in the possessing stream does this effect arise?
Materials and Methods
Subjects
Fourteen paid subjects (Average age: 23.2 years, 7 female), took part in the experiment. All participants were right handed (Oldfield, 1971), reported normal hearing, and had no history of neurological disorder. The data from one subject were discarded from analysis due to excessive head movements during the experiment. The experimental procedures were approved by the University of Maryland institutional review board and written informed consent was obtained from each participant.
Stimuli
The stimuli were triplets of 100 ms pure tones (A, B and C, respectively) with a 600 ms inter-tone-interval (from offset to onset). Each tone was ramped on and off with 10 ms cosine-squared ramps. The initial and final tones (A and C) were identical in frequency or differed by plus or minus the subject's discrimination threshold (see `threshold determination' below). Tone B was an octave above or below tone A. In order to prevent the subjects from listening to a particular frequency range we used two frequency ranges for the A and C tones: 400 and 800 Hz, resulting in 4 types of triplets: [A ≈ 400 Hz, B ≈ 200 Hz, C ≈ 400 Hz ]; [A ≈ 400 Hz, B ≈ 800 Hz, C ≈ 400 Hz ]; [A ≈ 800 Hz, B ≈ 400 Hz, C ≈ 800 Hz ]; [A ≈ 800 Hz, B ≈ 1600 Hz, C ≈ 800 Hz]. In addition, the frequencies of A, C and B tones were roved in 3 steps, where each step equaled the subject's frequency discrimination threshold. All stimulus conditions appeared randomly and with identical probability, and tones A and C were equal in exactly half of the trials. Overall, listeners heard 640 triplets. The stimulus set also included a proportion (25%; 240) of 200 ms wide-band noise bursts (ramped on and off with 10 ms cosine-squared ramps), interspersed randomly between the triplets (but never within a triplet).
The stimuli were created off-line and saved in 16-bit stereo wave format at a sampling rate of 44kHz, delivered to the subjects' ears with a tubephone (E-A-RTONE 3A 50 ohm, Etymotic Research, Inc) attached to E-A-RLINK foam plugs inserted into the ear-canal and presented at a comfortable listening level.
Two tasks were performed on these stimuli. In the PASSIVE task subjects were instructed to respond as fast as possible (by pressing a response button held in the right hand) to the noise bursts. In the COMPARE task, subjects were instructed to disregard the noise bursts and respond when tones A and C were of the same frequency. This is a difficult task because A and C, when different, are close in frequency, and because tone B, one octave above or below the frequency region of A and C, distracts the listener away from the spectral region necessary for performing the discrimination task. In order to successfully perform the COMPARE task, listeners must attempt to ignore the B tone. To make sure that they do not solve the task by ignoring a particular frequency range throughout the experiment, we used several different frequency regions for the tones, as described above. In this way, stimulus and task parameters were optimized to encourage subjects to ignore tone B in time.
To control for order, habituation and fatigue effects, the task order was counter-balanced across subjects. Within each task-block the order of presentation was randomized, with the inter-stimulus interval (ISI; from offset to onset of a triplet) randomized between 1400–2100 ms.
Discrimination threshold estimation
Pure tone frequency discrimination thresholds were measured in a three-interval one-up three-down adaptive procedure in which subjects had to decide which of a series of three tones was different from the other two. Visual feedback was provided. Tone and inter-tone-interval durations were 100 ms and 1100 ms respectively. The frequency difference, initially 12%, was reduced by a factor of 2 after the first two correct responses, 1.4 after the next two and 1.2 after that. The estimation procedure ended after twelve reversals, and the threshold was taken as the average of the last 8 reversals. The nominal base frequency was either 400 Hz or 800 Hz, and was roved over a ± 6% range. Tracks for 400 Hz and 800 Hz were interleaved.
The average thresholds measured for this group of non-trained individuals was 7.2% or 28.8 Hz (stdev = 6.9%) for 400Hz tones and 5.2% or 41.6 Hz (stdev = 4.6%) for 800Hz tones. The threshold used to create the experimental stimuli for each subject was set to be the higher of the subject's two thresholds.
Procedure
Before the recording, individual discrimination thresholds were obtained for each participant, as described above, and stimuli were appropriately adjusted. The two task blocks were administered successively (order counter-balanced across subjects) during the same recording session. The experimental task was explained to the subjects only immediately before each task block. Immediately before the COMPARE task block, subjects received a training run (~100 triplets) with visual feedback. During the task-blocks proper, no feedback was provided.
Subjects lay supine inside a magnetically shielded room. In a pre-experiment just prior to the first task, they listened to 200 repetitions of a 1 kHz, 50 ms sinusoidal tone (ISI randomized between 750–1550 ms). Responses were used to verify that signals from auditory cortex had a satisfactory signal to noise ratio (SNR), that the subject was positioned properly in the machine, and to determine which MEG channels best respond to activity within auditory cortex.
In the experiment proper (about 1.5 hours), subjects listened to stimuli while performing the tasks (using a button held in their right hand) as described above. The instructions in the COMPARE task encouraged accuracy whereas instructions in the PASSIVE task condition emphasized speed. Stimulus presentation was divided into runs of 100 stimuli (8 runs per task-block). Between runs subjects were allowed a short rest but were required to stay still. Throughout, participants were asked to keep their eyes closed, among other reasons to avoid overt eye movement artifacts.
Five electromagnetic coils were attached to the listeners' heads prior to the MEG measurement. The locations of the coils were calculated with respect to anatomical landmarks on the scalp using 3D digitizer software (Source Signal Imaging, Inc) and digitizing hardware (Polhemus, Inc). To verify that no head movement occurred during the relatively long session, the coils were periodically (3 times during the session) localized with respect to the MEG sensors. Subjects with excessive head movements (1 subject in the present experiment) were excluded from analysis.
Neuromagnetic recording and data analysis
The magnetic signals were recorded using a 156-channel, whole-head axial gradiometer system (KIT, Kanazawa, Japan). Data for the pre-experiment were acquired with a sampling rate of 1kHz, filtered online between 1 Hz (hardware filter) and 58.8 Hz (17 ms moving average filter), stored in 500 ms stimulus-related epochs starting 100 ms pre-onset. Data for the main (PASSIVE and COMPARE tasks) experiment were acquired continuously with a sampling rate of 0.5 kHz, filtered in hardware between 1 and 200 Hz with a notch at 60 Hz (to remove line noise), and stored for later analysis. The data were noise-reduced using the Time-Shift Principle Component Analysis algorithm (de Cheveigné & Simon, 2007).
Channel Selection
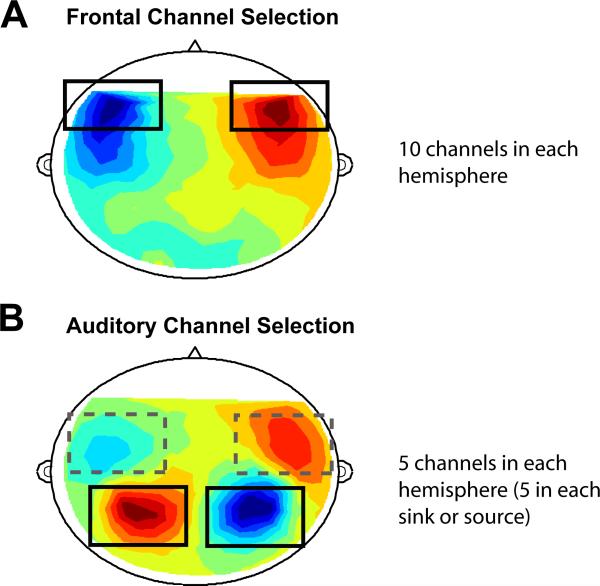
We analyze the raw signal, without applying a source model, by selecting subsets of channels that would best reflect frontal lobe and auditory cortex activation. Because the skull and brain tissue have uniform conductivity to magnetic fields, MEG (unlike EEG) allows gross separation of activity into topographic areas (e.g. the different cortices) without restrictive models. Figure 1A shows a typical magnetic field pattern over a listener's scalp resulting from frontal cortex activity (the specific pattern shown is taken from 500 ms post triplet onset in the PASSIVE task condition; see also Figure 3). We selected the 10 most frontal channels in the sensory array on each side of the head (black squares in Fig 1A; same for all subjects) to represent frontal activity. The larger number of frontal channels (relative to auditory channels, see below) was used to insure that frontal activity (which is less well characterized with MEG relative to auditory activity) is captured adequately for subjects with different head-sizes.
Figure 1.
Channel selection. A: an axial view of a typical magnetic field pattern over a (flattened) listener's scalp resulting from frontal cortex activity (he specific pattern shown is taken from 500 ms post onset in the PASSIVE condition). We selected the 10 most frontal channels on each side of the sensory array (black squares) to sample frontal activity. B: an axial view of a typical magnetic field pattern over a listener's scalp resulting from auditory cortical activity. Auditory channels were selected for each subject individually based on the M100 auditory evoked response to the pure tones presented in the pre-experiment. To reduce overlap with frontal activity and otherwise non-auditory cortical activation, we chose the 5 most activated channels in the posterior sink and source (black squares). Red: source; Blue: sink.
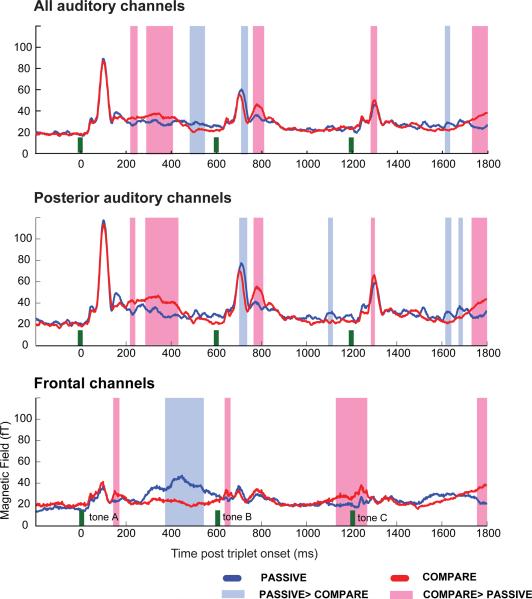
Figure 3.
The RMS time course of composite (averaged across triplet conditions and hemispheres) COMPARE and PASSIVE amplitudes recorded from all auditory (top), posterior auditory (middle) and frontal (bottom) channels. Tone onsets are marked with green bars. Statistically significant differences between COMPARE (red) and PASSIVE (blue) amplitudes are marked with pink (COMPARE > PASSIVE) or light-blue (PASSIVE > COMPARE) shading.
Auditory cortical activity was sampled with 5 channels on each side of the head, selected individually for each subject based on the M100 auditory evoked response to the pure tones presented in the pre-experiment. The M100 is a prominent and robust (across listeners and stimuli) deflection at about 100 ms post onset, and has been the most investigated auditory MEG response (see Roberts et al., 2000 for review). It was identified for each subject as a dipole-like pattern (i.e. a source/sink pair) in the magnetic field contour plots distributed over the temporal region of each hemisphere (Figure 1B). In previous studies, under the same conditions, the resulting M100 current source localized to the upper banks of the superior temporal gyrus in both hemispheres (Hari, 1990; Pantev et al., 1995; Lütkenhöner & Steinsträter, 1998).
In the absence of any hypothesis involving frontal activity, we would otherwise use the 20 strongest channels at the peak of the M100 (5 in each sink and source, yielding 10 in each hemisphere; solid and dashed squares in Figure 1B) to sample auditory activity (e.g. Chait et al., 2005; 2006). However, since the stimuli and task used in current study involve cognitive demands that are usually associated with significant frontal lobe activation (e.g. Duncan & Owen, 2000) and because in some subjects the rostral auditory channels (dashed squares in figure 1B) overlap with frontal channels (solid squares in Figure 1A) we chose the posterior auditory channels (solid squares in Figure 1B) for the analysis of auditory cortical activation. Note that even with these selection criteria the separation into “frontal” and “auditory” activity is not perfect because frontal channels still capture some auditory activity. The opposite, however, is not true – the caudal (posterior) auditory channels are unlikely to pick up frontal-lobe activity (though they may pick up some activity from parietal cortex).
Evoked responses
In this study we investigate the temporal characteristics of the brain responses evoked by our stimuli. One measure of cortical processing dynamics is the amplitude time course (e.g. increases and decreases in activation) as reflected in the root mean square (RMS) of the selected channels. For illustration purposes, we plot the grand-average (average over all subjects for each of the 156 channels) or the group-RMS (RMS of individual subjects' RMSs). However, statistical analysis is always performed on a subject-by-subject basis, using the RMS values of the channels chosen for each subject in each hemisphere. Data were not spectrally filtered beyond the online hardware filtering described above.
The COMPARE task was designed in such a way that the initial processing of A, B and C tones is the same for all triplets (targets and non-targets), diverging only after the onset of tone C. In the analysis, we therefore collapse across target (f(A)=f(C)) and non target (f(A)≠f(C)) stimuli to increase the response SNR. Epochs of 2000 ms duration (including 200 ms pre-onset) were constructed for each of the eight stimulus conditions (4 triplets × 2 tasks). Epochs with amplitudes larger than 3 pT (~5%) were considered artifactual and discarded, and the remaining epochs were averaged. The root mean square (RMS) of the field strength across the selected frontal and auditory channels was calculated at each time point. Thirty-two RMS time series, one for each condition (eight), area (frontal and auditory) and hemisphere (left and right), were thus created for each subject.
To evaluate congruity across subjects, the individual RMS time series were combined into group-RMS (RMS of individual RMSs) time series. Consistency of peaks in each group-RMS was automatically assessed with the Bootstrap method (500 iterations; balanced; Efron & Tibshirani, 1993). The consistency, across subjects, of magnetic field distributions at those peaks was assessed automatically as described in Chait et al. (2005).
Because of a stimulus delivery problem, for 20% of the triplets the ISI preceding a triplet was shorter for the PASSIVE than for COMPARE tasks. Data for those triplets were discarded and the results reported here are based on the remaining 80% of the PASSIVE stimuli. Analysis was also performed on the full data set; comparison of the analyses did not reveal any substantial differences.
Comparison across task conditions
To identify the time intervals where PASSIVE and COMPARE task conditions exhibit significant amplitude differences, for each subject we create a combined RMS (RMS of RMSs; over 4 triplet conditions × 2 hemispheres; these conditions were combined because we found no effects of triplet-type or hemisphere) for each task condition. This is a `conservative' measure for effects that are consistent across conditions and is relatively insensitive to spurious effects due to outliers. We also performed the same analysis using collapsed conditions (averaged over all triplet epochs) and comparison of the analyses did not reveal any substantive differences between analyses.
To compare PASSIVE and COMPARE task conditions, we then used a repeated measures analysis where, for each subject, the squared RMS value of one condition is subtracted from the squared RMS value of the other condition and the individual difference time series are subjected to a bootstrap analysis (500 iterations; balanced; Efron & Tibshirani, 1993). At each time point, the proportion of iterations below the zero line was counted. If that proportion was less than 5%, or more than 95% for 10 adjacent samples (20 ms), and if the average absolute difference at that time was sufficiently large (exceeded a threshold of 200 fT2), the difference was judged to be significant. The temporal threshold (20 ms) was chosen to correspond to the length of the (a priori hypothesized) M100 effect at posterior auditor channels for tone C (Figure 3); the amplitude difference threshold was set to correspond to the mean absolute amplitude difference (difference of square RMS values) between conditions over the entire stimulus epoch. This value was not statistically different between `frontal' and `auditory' channels and the same threshold was used for both.
Results
Behavior
In the COMPARE task, subjects exhibited an average hit rate of 82.4% (stdev = 13.29%) and a false positive rate of 15.88% (stdev = 9%) resulting in an average d-prime (Macmillan & Creelman, 2008) of 2.05. These data indicate that the task was difficult but manageable. Average response time was 679 ms (stdev = 96ms) after the onset of tone C (1979 ms post triplet onset). In the PASSIVE task, subjects performed at ceiling, with an average hit rate of 98.7 % (stdev = 2.3%) and a false positive rate of 0.06% (stdev=0.24%). Average response time was 340 ms (stdev = 95 ms) post noise onset. There was no effect of task order on any of these measures.
Electrophysiological data
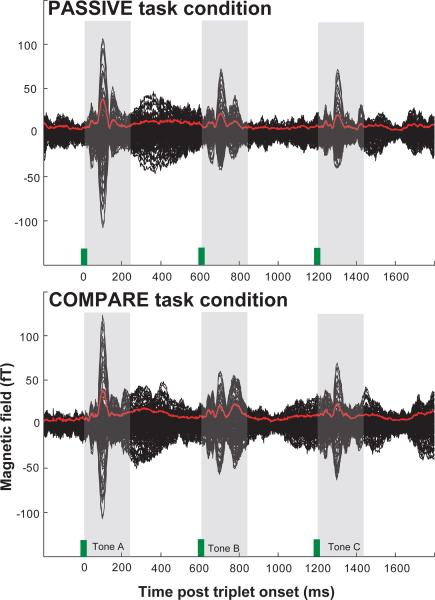
Figure 2 shows the time course of magnetic fields for one stimulus condition [A ≈ 400 Hz, B ≈ 800 Hz, C ≈ 400 Hz] in the PASSIVE (top) and COMPARE (bottom) tasks. Plotted in black are the data for each of the 156 channels averaged over subjects. The root mean square (RMS) over all channels is plotted in red. Responses to other stimulus conditions (not shown) are similar. We did not observe any hemispheric differences or consistent effects of stimulus condition and the data are therefore combined in subsequent analysis (see `Methods'). The M100 onset responses to A, B and C tones are clearly seen at 100, 700 and 1300 ms. Below we quantify and discuss the differences between COMPARE and PASSIVE tasks in the brain activity leading to, and following the appearance each tone.
Figure 2.
Illustration of response data. Grand-average (average over all subjects for each of the 160 channels; in black) of the evoked cortical responses to one stimulus condition [A ≈ 400 Hz, B ≈ 800 Hz, C ≈ 400 Hz] in the PASSIVE (top) and COMPARE (bottom) task conditions. The root mean square (RMS) over all channels is plotted in red. Tone onsets are marked with green bars. The M100 onset responses to A, B and C tones are clearly seen at 100, 700 and 1300 ms (shaded).
Task effects on brain responses to the triplet stimuli
Figure 3 presents the RMS time course of COMPARE and PASSIVE responses averaged across triplet conditions and hemispheres. Tones are marked as green bars. Significant differences between COMPARE and PASSIVE amplitudes (see methods) are marked with pink (COMPARE > PASSIVE) or blue (PASSIVE > COMPARE) shading. The top panel shows responses recorded from all auditory channels (see Figure 1 and methods). The middle panel shows responses recorded from posterior auditory channels only and the bottom plot shows responses recorded from frontal channels.
Comparison of the three panels in Figure 3 suggests that responses in the top panel (all auditory channels) are somewhat contaminated by activity from frontal and prefrontal channel groupings (bottom panel). We therefore focus on the posterior auditory channels (see Figure 1B) as indexes of auditory cortical activation. These will be referred to as “auditory channels” in the remainder of this report. The pre-triplet interval (baseline) did not differ between task conditions for the auditory channels. For the frontal channels it was somewhat higher for COMPARE than for PASSIVE (though not consistently across listeners). Because such differences are of potential interest, we did not apply baseline correction (subtraction from the response of mean pre-stimulus activity).
Peak M100 amplitudes in auditory channels did not differ between conditions for A tones. This may reflect the fact that both tasks require the subject to attend to the onset of each new triplet because it is relevant for both tasks (it must be distinguished from noise for PASSIVE, and its pitch recorded for the COMPARE task). However, the M100 responses for tones B and C exhibit small but significant task-related amplitude differences. Peak M100 amplitudes are significantly higher for COMPARE than PASSIVE for tone C (an average amplitude difference of about 7 fT or 10%), and lower for tone B (an average amplitude difference of about 7 fT or 9%). The sizes of these effects are comparable to those reported in other attention-related evoked response studies (e.g. Lange et al., 2003; Gutschalk et al., 2005; Gazzaley et al., 2005; Sanders & Astheimer, 2008). Although it appears from the average that there is a difference in latency between tone B M100 responses in PASSIVE and COMPARE, this was not significant across subjects.
In Figure 3 (auditory channels) the M100 response to tone B appears to be preceded by an ongoing difference between conditions (500 to 650 ms). One might speculate that the tone B M100 `rides' upon this ongoing baseline, and that this explains the amplitude differences at the M100 response. To investigate this possibility we reanalyzed the data by baseline-correcting the responses according to the 100 ms interval preceding the onset of tone B (presented in Supplementary Figure 1). The figure demonstrates that the difference at the M100 response survives this re-analysis and is therefore likely not due to an ongoing amplitude difference preceding tone B, but rather reflects a genuine sensory response to tone B. Supp Fig 1 also demonstrates that a difference at the time of the M50 response emerges after baseline correction, suggesting the possibility of even earlier effects of attention, but this effect is difficult to interpret and is not discussed further.
In both task conditions, M100 amplitudes progressively decline from tone A to tone B to tone C. This corresponds to the often-noted amplitude decrement for closely spaced stimuli which is known in the EEG/MEG literature as `response refractoriness' (e.g. Hari et al., 1982; Budd et al., 1998; See also Ahveninen et al., 2006), hypothesized to result from refractory effects within the neural generators which underlie the M100 response. Indeed, a repeated measures ANOVA on M100 peak amplitudes (defined for each subject as the maximum amplitude in a ±20 ms interval around the group-RMS peak) with task-condition and tone (A, B or C) as factors revealed a main effect of tone (p<0.001) and a task × tone interaction (p=0.006), resulting from the reversal in amplitude dominance between COMPARE and PASSIVE for tone B relative to tones A and C.
To examine potential latency effects, a repeated measured ANOVA on M100 peak-latencies, with task-condition and tone (A, B or C) as factors revealed only a main effect of tone (p=0.009), due to M100 latency being shortest for tone C and longest for tone B. We found no significant effects of task.
When comparing response amplitudes between tasks, the most striking effect of ignoring (or trying to ignore) tone B in the COMPARE task is the emergence of an enlarged peak, with M150 dipolar distribution, about 180 ms after the onset of tone B (just before 800 ms after the onset of the triplet). The fact that the frontal channels show no significant activation at this time range indicates that these effects are generated primarily in auditory cortex.
In addition to these main effects of interest which occur shortly after tone onset, the data also demonstrate response dynamics related to the anticipation of tone arrival and the subsequent encoding of tone information in memory. In auditory cortex, in the interval between tone A and tone B, auditory cortical activity in the COMPARE condition first increases and then (at about 400 ms post triplet onset) drops sharply, whereas PASSIVE activity maintains relatively constant amplitude. Higher sustained activity in the COMPARE condition may be related to encoding tone A in short term memory, a process which has been shown to involve sensory cortex (Grimault et al., 2009; Shulze et al., 2009; Gaab & Schlaug, 2003; Luo et al., 2005). Following the onset response to tones B and C, we observe no major differences between the two conditions (differences between targets and non-targets are washed out in the present analysis because we collapse over all triplet types). At about 1600 ms post onset response dynamics begin to reflect motor preparation and the eventual motor response, occurring at 1900 ms post triplet onset.
The pattern of activation in frontal channels reveals an initial difference between conditions after the onset response to tone A, at about 150 ms post triplet onset, where an increase in COMPARE vs. PASSIVE amplitude is observed. This activation is consistent with encoding tone A in memory, a process which has been shown to involve both sensory and frontal cortexes (Grimault et al., 2009; Schulze et al., 2009). The large difference between conditions in the time interval after tone A, where the PASSIVE condition exhibits higher amplitudes than COMPARE, likely reflects relaxation of expectation in case of COMPARE (subjects know they can relax for the duration of the triplet before waiting for the possible occurrence of the next noise burst). The lack of significance around 300 ms post onset suggests high inter-subject variability for this process.
Just before the onset of tones B and C, frontal channel activity in the COMPARE condition begins to rise, reflecting expectation of tone arrival (Lange et al., 2003) For tone B, the difference between conditions only becomes significant at 650 ms post triplet onset, but this is likely because of the large amplitude increase in the PASSIVE condition. For both tones, the dominance of COMPARE over PASSIVE continues until 80 ms post tone onset.
The frontal activation pattern observed in the present experiment is sustained over time and, as the statistical analysis suggests, is extremely consistent across subjects. This, in combination with the artifact rejection routine (see methods) strongly supports a brain source rather than eye movement artifacts. It is important to note, however, that because our data were high pass filtered (at 1 Hz) in hardware (due to technical limitations at the time of recording) it is possible that additional aspects of the dynamics of slow anticipatory activity (Brunia & van Boxtel, 2004; Ohgami et al., 2004) are not visible in this data set.
Comparing tones B and C
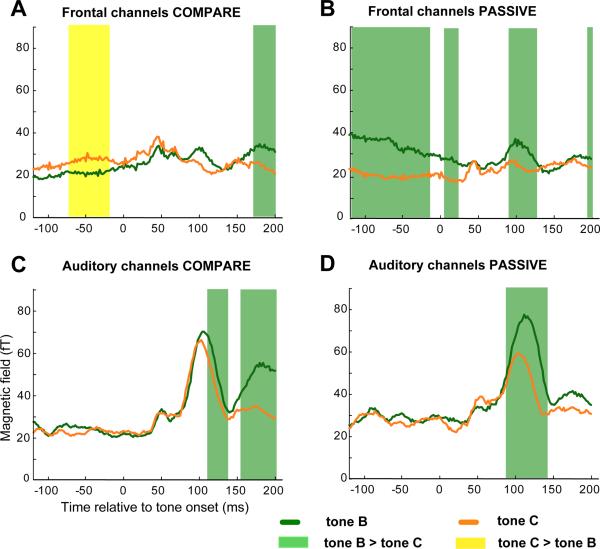
Another means of examining the effect of attending vs. ignoring is to compare activation of tone B relative to tone C in COMPARE and PASSIVE tasks. The temporal intervals involved are identical, and onset responses of B and C are expected to be similar, but with differences due to position within the triplet (B vs. C) and task (COMPARE vs. PASSIVE). In Fig. 4 we plot, for each task and each set of channels, the response to the onsets of B (green) and C (orange). In each plot, green shading indicates intervals for which activity for B is greater than for C, and yellow shading those for which activity for C is greater than for B.
Figure 4.
Comparison of tone B and tone C activation. The RMS time course of composite (averaged across triplet conditions and hemispheres) tone B (green) and C (orange) amplitudes recorded from frontal (top) and auditory (bottom) channels in the COMPARE (left) and PASSIVE (right) tasks. Tone onsets are at 0 ms on the x axis. Statistically significant differences between B and C amplitudes are marked with green (B > C) or yellow (C > B) shading.
Differences between B and C related activation in the PASSIVE task condition (right) are attributable to general order effects and aspects of the stimulus unrelated to attention. In order to identify effects that are specifically related to attention we therefore look for differences between C and B that emerge in the COMPARE task condition (left) relative to the PASSIVE condition (right). An inspection of frontal channel activity (top) reveals that whereas B tone-related activity dominates C tone-related activity in the PASSIVE condition (right), this largely reverses in the COMPARE condition (left). Specifically, in the COMPARE condition, before the onset of tone C, there is an increase in frontal channel amplitudes relative to the same time period before tone B (yellow shading in Figure 4A). This effect is unlikely to reflect motor preparation since it disappears at tone onset, and because it is restricted to frontal channels (activity due to motor preparation, reflected by an in increase activation in all channels, can be seen in Figure 3 after about 1600 ms post triplet onset). Instead, it is likely that this activation reflects preparatory process to optimize the processing of tone C.
The auditory channel data (Figure 4, bottom) demonstrates the differences in M100 refractoriness between PASSIVE and COMPARE conditions. The difference between B and C M100 amplitudes is larger in the PASSIVE condition (an average difference of about 18 fT which is 23% of the M100 for tone B) than in the COMPARE task condition (an average difference of about 4fT which is 5% of the M100 for tone B; compare amplitudes at 100 ms post tone onset in 4C and 4D). That is, whereas in the PASSIVE condition M100 amplitudes progressively decline from tone A to tone B to tone C, this effect is much smaller in the COMPARE task condition, where M100 amplitudes for tones B and C are not very different. This is due to the fact that M100 for tone B is attenuated relative to PASSIVE and M100 to tone C is amplified relative to the PASSIVE condition (Fig 3; see also Ahveninen et al., 2006).
The amplitude difference between the green and orange lines that emerges after 150 ms post onset in the COMPARE condition is due to increased M150 response after the M100 for tone B discussed above (Figure 3).
Discussion
The present study focuses on the temporal dynamics of attentional processes in auditory cortex – the neural processes underlying the amplification of responses to attended stimuli and suppression of responses to distracters within an acoustic scene that unfolds over time. We demonstrated that priming listeners to selectively attend to certain moments in time while ignoring other moments can modulate early onset responses in auditory cortex by attenuating responses to signals which listeners are attempting to ignore and boosting responses to task-relevant sounds.
Several previous studies have tried to isolate effects of attending and ignoring by comparing selective attention conditions with a `baseline' neutral condition, hypothesized to require neither attending nor ignoring. For instance, Michie et al. (1990) used a dichotic presentation task - signals were presented to left and right ears and listeners were instructed to attend to one ear while ignoring the other. As a control, subjects performed an unrelated visual task while passively listening to the auditory stimuli (Michie et al., 1990; 1993). Comparing brain responses to auditory signals when they were attended or unattended with responses to the same stimuli when subjects were focusing their attention on a visual task, revealed changes in both the attended and unattended ERPs relative to the control condition. Specifically, attended ERPs were enhanced over the interval from about 100 to 200 ms post onset, while unattended ERPs were suppressed over the interval from 200 to 500 ms post onset (see also Alho et al., 1994b; Bidet-Caulet et al., 2010).
Alain & Woods (1994; see also, Alain et al., 1993) attempted to isolate effects specific to attending and ignoring by presenting listeners with sequences of tone-pips of three different frequencies presented in random order. Listeners were instructed to attend to one of the tones while ignoring the others. In different conditions the three tones were either evenly spaced (`evenly spaced; ES condition), the attended tone was distinct, with the distracter tones clustered together (`clustered easy'; CE condition), or else the attended tone was grouped with one of the distracters (`clustered hard'; CH condition). The data indicated that distracter grouping enhanced the difference between the responses to task-relevant and task-irrelevant tones. Comparing the different conditions suggested that this effect was due both to increased negativity to attended tones from 150 to 170 ms post onset (CE vs. ES conditions) and decreased responses to non-attended tones from 190 to 450 ms post onset (CH vs. ES conditions).
While these previous studies examined attentional facilitation vs. suppression in the context of spatial attention or feature-based (pitch-based) attention, we investigated them from the perspective of attention in time. It has been shown that listeners can take advantage of the knowledge of when signals are expected to appear to optimize processing of task-relevant signals (Lange et al., 2003; Sanders & Astheimer, 2008). The novel aspect of the present work concerns the extent to which temporal information can facilitate ignoring a sound. Indeed, the demonstration of attentional modulation here is particularly compelling as it reveals the interplay between temporal response dynamics associated with both attending and ignoring within a single stimulus epoch (Figure 3).
Temporal dynamics of attending
Effects of attention in the present study were manifested as anticipatory activity in frontal channels and sensory response modulation in auditory cortex. The increase in frontal channel power, observed when listeners were preparing to attend to an expected stimulus (e.g. before tone B or C) is similar to the Stimulus Preceding Negativity slow brain potential (SPN; Birbaumer et al., 1990; van Boxtel & Böcker, 2004) which is interpreted in the literature as a cortical priming to preferentially process the expected stimulus (Walter et al., 1964; Brunia & van Boxtel, 2001). Previous studies have demonstrated an increase in power in pre-frontal cortex prior to auditory feedback stimuli (Ohgami et al., 2004; Brunia & van Boxtel, 2004; Lange et al., 2003; but see Engdahl et al., 2007). These responses are hypothesized to reflect voluntary orienting in a top-down (`goal directed') frontal cortex system which mediates bottom-up perceptual processes in sensory cortices, for example by modulating the excitability of the relevant sensory neurons (Corbetta & Shulman, 2002; Brunia & van Boxtel, 2004). In the present experiment, it is likely that the responses we observed in frontal channels originated from mechanisms that contributed to the pre-activation of sensory-specific systems in auditory cortex. It is noteworthy that both for tone B and C this anticipatory activity in frontal cortex immediately preceded, but did not overlap with, activity in auditory cortex (Figure 2).
In auditory cortex, the first effect of selective attention emerged at about 100 ms post tone onset, at the peak of the M100 response which originates from non-primary auditory cortex (Lütkenhöner & Steinsträter, 1998). This finding is consistent with similar results from auditory spatial attention studies (Hillyard et al., 1973; Woldorff & Hillyard, 1991; Woldorff et al., 1993; Alho et al. 1994; Fujiwara et al., 1998). These findings also replicate the results of Lange et al. (2003) who had participants listen to short (600ms) and long (1200 ms) temporal intervals marked by short noise bursts. The task was to attend either to the short or long intervals and respond to rarely occurring offset markers that differed in intensity. Stimuli presented at the attended, relative to the un-attended moments in time elicited an enhanced N1 onset response (see also Sanders & Astheimer, 2008). Notably, while Lange et al.'s task specifically involved time estimation, we demonstrate similar effects in the context of a pitch matching task which did not involve explicit time processing.
In contrast to previous reports (Woldorff & Hillyard, 1991; Woldorff et al., 1993), we did not find an effect during the earlier, M50 auditory evoked response time window, (see also Fujiwara et al., 1998). Early task-related effects were observed exclusively in frontal channels.
An enduring debate in the literature concerns whether the attentional effects observed at stimulus onset result from bottom-up, exogenous (stimulus-driven), modulation of sensory processing (Hillyard et al., 1973; Giard et al., 1988; Mangun & Hillyard, 1995; Giard et al., 2000) or from an overlap of the onset responses with an endogenous `processing negativity' component elicited by attention (Näätänen et al., 1978; Alho et al., 1994a). For instance, Näätänen (1992) has suggested that the processing negativity is generated by a matching process between incoming stimuli and an `attentional trace' – a neuronal representation of the attended stimulus.
The attentional effects we observed in auditory cortex were very narrowly tuned in time and coincided with the peak of the M100 response. While it is very likely that the amplitude differences between COMPARE and PASSIVE seen in the M100 time window are related to preparatory control activity in frontal cortex, this narrow temporal tuning strongly suggests they are due to genuine gating of sensory systems primed to expect input at a particular point in time (see e.g. Lange et al., 2003; Hillyard et al., 1998 for review) and not caused by changes in base-line firing rates or endogenous attention related auditory evoked components.
Temporal dynamics of ignoring
In the context of spatial or feature-based selective attention, several studies have demonstrated altered responses to distracter tones (Berman et al., 1989; Rif et al., 1991; Michie et al., 1990; Alain & Woods, 1994; Alain et al., 1993; Michie et al., 1993: Alho et al., 1994b; Bidet-Caulet et al., 2010). These effects have usually been reported to emerge around 200 ms from stimulus onset, overlapping with the P2 response.
This effect is clearly visible in our data (Fig. 3), as an increased M150 response in the COMPARE condition relative to PASSIVE, occurring after the M100 response for tone B. Based on its magnetic field distribution, which indicates an auditory cortical origin, and comparison of frontal and auditory activity, it appears that this effect is primarily generated in auditory cortex. Indeed, Rif et al. (1991) who also report an increased M150 response to unattended stimuli (although they could not attribute it specifically to ignoring due to the lack of an appropriate control) report its source to lie about 1 cm anterior to that of the M100 consistent with a locus in supra-temporal auditory cortex.
This increase in activation may underlie active inhibition of sensory processing of the to-be-ignored stimulus (Theeuwes & Chen, 2005) or resistance to stimulus-driven attentional capture (Ipata et al., 2006). Our findings are also consistent with results from Melara et al. (2002), who trained participants to selectively ignore distracter stimuli in a dichotic listening task. Training resulted in improved behavioral performance (greater sensitivity to targets, less response bias and faster responses) and accompanied by amplified P2 responses to distracters. These findings motivated the authors to argue that distracter positivity created by training reflects an active inhibitory process during auditory selective attention. The fact that we observe similar modulation, with a completely different task, provides strong support for this conclusion.
Importantly, in contrast to previous studies where effects of distracter suppression were manifested only from about 200 ms post stimulus onset (Alho et al., 1987; Michie et al., 1990; Rif et al., 1991; Michie et al., 1993; Alain & Woods, 1994; Melara et al., 2002; Degerman et al., 2008; Bidet-Caulet et al., 2010), the present results show that ignoring can affect responses as early as 100 ms after stimulus onset. Our data demonstrate that, at least in situations where the timing of a distracter is known, ignoring (or attempting to ignore) a stimulus results in decreased M100 response in auditory cortex.
In the visual literature, a decrease in amplitude of the P1 (~80 ms post stimulus onset) component has been documented in ERP studies of spatial attention and hypothesized to be related to suppression of processing at unattended locations (Luck et al., 1994; Luck & Hillyard, 1995). However Gazzaley et al. (2005), in a visual ERP study that more closely resembles our auditory task, did not observe any amplitude suppression effects. The difference may stem from the fact that explicitly ignoring (as opposed to not actively attending to) a stimulus is costly in terms of energy, and subjects would not do so unless absolutely necessary for achieving their behavioral goals. The present data thus provide strong evidence that listeners are able to selectively reduce stimulus related activity in auditory cortex as early as 100 ms after onset, when this is required to execute their behavioral objectives.
One may argue that that the observed effect (decrease in M100, increase in M150) around the onset of tone B could be explained by an exogenous positivity (a dipolar source with opposite magnetic distribution to the M100) that overlaps with both the M100 and M150 time ranges, suggesting that the effect on tone B stems from passively allocating less attention to B during the COMPARE relative to the PASSIVE task-conditions. However several aspects of the data rule out such an explanation (see also discussion of the same point in Melara et al., 2002): Since the PASSIVE task does not involve any need specifically to attend or ignore any of the tones there is no reason to believe that different `amounts' of attention should be allocated to B and C tones in PASSIVE. At the same time, the COMPARE task, is designed in such a way that more attention should be allocated to C than to B. If the observed effect simply reflects decreased tone excitation, than we should expect to also see an enlarged M150 peak for C in PASSIVE relative to COMPARE – an effect which is clearly absent from the data (Fig 3). We therefore believe the dynamics of the response around tone B strongly suggest that the M150 effect reflects genuine voluntary inhibition of an interfering stimulus.
In summary, our data suggest that, auditory attention can, at least in certain situations, selectively down-regulate cortical responses known to encode perceptual analysis of auditory events such as the M100. This early-selection (Triesman, 1969), possibly better referred to as `early rejection', effect is supplemented by an additional, inhibitory process at about 200 ms post onset, (but still in auditory cortex) that sub-serves active distracter suppression.
Supplementary Material
Acknowledgements
We are grateful to Jeff Walker for excellent technical support and to Daniel Pressnitzer for providing the threshold estimation script. The research was supported by NIH grant R01DC008342 to JZS and NIH grant 2R01DC05660 to DP. MC is supported by a Deafness Research UK fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hamalainen M, Levanen S, Lin FH, Sams M, Shinn-Cunningham BG, Witzel T, Belliveau JW. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci U S A. 2006;103:14608–13. doi: 10.1073/pnas.0510480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Woods DL. Signal clustering modulates auditory cortical activity in humans. Percept Psychophys. 1994;56:501–16. doi: 10.3758/bf03206947. [DOI] [PubMed] [Google Scholar]
- Alain C, Achim A, Richer F. Perceptual context and the selective attention effect on auditory event-related brain potentials. Psychophysiology. 1993;30:572–80. doi: 10.1111/j.1469-8986.1993.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Alho K, Woods DL, Algazi A. Processing of auditory stimuli during auditory and visual attention as revealed by event-related potentials. Psychophysiology. 1994b;31:469–79. doi: 10.1111/j.1469-8986.1994.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Alho K, Teder W, Lavikainen J, Naatanen R. Strongly focused attention and auditory event-related potentials. Biol Psychol. 1994a;38:73–90. doi: 10.1016/0301-0511(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Alho K, Töttölä K, Reinikainen K, Sams M, Näätänen R. Brain mechanism of selective listening reflected by event-related potentials. Electroencephalogr Clin Neurophysiol. 1987;68:458–70. doi: 10.1016/0168-5597(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Berman SM, Heilweil R, Ritter W, Rosen J. Channel probability and Nd: an event-related potential sign of attention strategies. Biol Psychol. 1989;29:107–24. doi: 10.1016/0301-0511(89)90033-1. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A, Mikyska C, Knight RT. Load effects in auditory selective attention: evidence for distinct facilitation and inhibition mechanisms. Neuroimage. 2010;50:277–84. doi: 10.1016/j.neuroimage.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MH, Bertrand O. Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. J Neurosci. 2007;27:9252–61. doi: 10.1523/JNEUROSCI.1402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Anticipatory attention to verbal and non-verbal stimuli is reflected in a modality-specific SPN. Exp Brain Res. 2004;156:231–9. doi: 10.1007/s00221-003-1780-2. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. Int J Psychophysiol. 2001;43:59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Budd TW, Barry RJ, Gordon E, Rennie C, Michie PT. Decrement of the N1 auditory event-related potential with stimulus repetition: habituation vs. refractoriness. Int J Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Chait M, Poeppel D, Simon JZ. Neural response correlates of detection of monaurally and binaurally created pitches in humans. Cereb Cortex. 2006;16:835–48. doi: 10.1093/cercor/bhj027. [DOI] [PubMed] [Google Scholar]
- Chait M, Poeppel D, de Cheveigne A, Simon JZ. Human auditory cortical processing of changes in interaural correlation. J Neurosci. 2005;25:8518–27. doi: 10.1523/JNEUROSCI.1266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven Attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990;248:1556–9. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- de Cheveigné A, Simon JZ. Denoising based on Time-Shift PCA. J Neurosci. Methods. 2007;165:297–305. doi: 10.1016/j.jneumeth.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degerman A, Rinne T, Särkkä AK, Salmi J, Alho K. Selective attention to sound location or pitch studied with event-related brain potentials and magnetic fields. Eur J Neurosci. 2008;27:3329–41. doi: 10.1111/j.1460-9568.2008.06286.x. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the Bootstrap. Chapman and Hall; NY: 1993. [Google Scholar]
- Elhilali M, Xiang J, Shamma SA, Simon JZ. Interaction between attention and bottom-up saliency mediates the representation of foreground and background in an auditory scene. PLoS Biol. 2009;7:e1000129. doi: 10.1371/journal.pbio.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl L, Bjerre VK, Christoffersen GR. Contributions from eye movement potentials to stimulus preceding negativity during anticipation of auditory stimulation. Psychophysiology. 2007;44:918–26. doi: 10.1111/j.1469-8986.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention--focusing the searchlight on sound. Curr Opin Neurobiol. 2007;17:437–55. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Nagamine T, Imai M, Tanaka T, Shibasaki H. Role of the Primary auditory cortex in auditory selective attention studied by whole-head neuromagnetometer. Brain Res Cogn Brain Res. 1998;7:99–109. doi: 10.1016/s0926-6410(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Gaab N, Schlaug G. The effect of musicianship on pitch memory in performance matched groups. Neuroreport. 2003;14:2291–5. doi: 10.1097/00001756-200312190-00001. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–17. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Giard MH, Fort A, Mouchetant-Rostaing Y, Pernier J. Neurophysiological mechanisms of auditory selective attention in humans. Front Biosci. 2000;5:D84–94. doi: 10.2741/giard. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Peronnet F. Several attention-related wave forms in auditory areas: a topographic study. Electroencephalogr Clin Neurophysiol. 1988;69:371–84. doi: 10.1016/0013-4694(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Hsieh JC, Petersson KM, Stone-Elander S, Ingvar M. Coexistence of attention-based facilitation and inhibition in the human cortex. Neuroimage. 1998;7:23–9. doi: 10.1006/nimg.1997.0307. [DOI] [PubMed] [Google Scholar]
- Griffin IC, Miniussi C, Nobre AC. Orienting attention in time. Front Biosci. 2001;6:D660–71. doi: 10.2741/griffin. [DOI] [PubMed] [Google Scholar]
- Grimault S, Lefebvre C, Vachon F, Peretz I, Zatorre R, Robitaille N, Jolicoeur P. Load-dependent brain activity related to acoustic short-term memory for pitch: magneto encephalography and fMRI. Ann N Y Acad Sci. 2009;1169:273–7. doi: 10.1111/j.1749-6632.2009.04844.x. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Micheyl C, Melcher JR, Rupp A, Scherg M, Oxenham AJ. Neuromagnetic correlates of streaming in human auditory cortex. J Neurosci. 2005;25:5382–8. doi: 10.1523/JNEUROSCI.0347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JC, Hillyard SA. Temporal dynamics of human auditory selective attention. Psychophysiology. 1988;25:316–29. doi: 10.1111/j.1469-8986.1988.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Hari R, Kaila K, Katila T, Tuomisto T, Varpula T. Interstimulus interval dependence of the auditory vertex response and its magnetic counterpart: implications for their neural generation. Electroencephalogr Clin Neurophysiol. 1982;54:561–9. doi: 10.1016/0013-4694(82)90041-4. [DOI] [PubMed] [Google Scholar]
- Hari R. The neuromagnetic method in the study of the human auditory cortex. In: Grandori F, et al., editors. Auditory evoked magnetic fields and the electric potentials. Krager-Verlag; Basel: 1990. pp. 222–282. [Google Scholar]
- Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25:465–76. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos T.rans R Soc Lond B Biol Sci. 1998;353:1257–70. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–80. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Gottlieb J, Bisley JW, Goldberg ME. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat Neurosci. 2006;9:1071–6. doi: 10.1038/nn1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ. Attention to simultaneous unrelated auditory and Visual events: behavioral and neural correlates. Cereb Cortex. 2005;15:609–20. doi: 10.1093/cercor/bhi039. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Lange K, Rosler F, Roder B. Early processing stages are modulated when Auditory stimuli are presented at an attended moment in time: an event-related potential study. Psychophysiology. 2003;40:806–17. doi: 10.1111/1469-8986.00081. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. The role of attention in feature detection and conjunction discrimination: an electrophysiological analysis. Int J Neurosci. 1995;80:281–97. doi: 10.3109/00207459508986105. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Lütkenhöner B, Steinsträter O. High-precision neuromagnetic study of the functional organization of the human auditory cortex. Audiol. Neurootol. 1998;3:191–213. doi: 10.1159/000013790. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory – a user's guide. Psychology press; NY: 2008. [Google Scholar]
- Mangun GR, Hillyard SA. Mechanisms and models of selective attention. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind –Event-Related Brain Potentials and Cognition. Oxford University Press; Oxford: 1995. pp. 41–85. [Google Scholar]
- Melara RD, Rao A, Tong Y. The duality of selection: excitatory and inhibitory processes in auditory selective attention. J Exp Psychol Hum Percept Perform. 2002;28:279–306. [PubMed] [Google Scholar]
- Michie PT, Solowij N, Crawford JM, Glue LC. The effects of between-source discriminability on attended and unattended auditory ERPs. Psychophysiology. 1993;30:205–20. doi: 10.1111/j.1469-8986.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Michie PT, Bearpark HM, Crawford JM, Glue LC. The nature of selective attention effects on auditory event-related potentials. Biol Psychol. 1990;30:219–50. doi: 10.1016/0301-0511(90)90141-i. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Attention and brain function. Lawrence Erlbaum Associates; Hillsdale, NJ, USA: 1992. [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potentials reinterpreted. Acta Psychol (Amst) 1978;42:313–29. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Kotani Y, Hiraku S, Aihara Y, Ishii M. Effects of reward and stimulus modality on stimulus-preceding negativity. Psychophysiology. 2004;41:729–38. doi: 10.1111/j.1469-8986.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuierer G, Elbert T. Specific tonotopic organizations of different areas of the human auditory cortex revealed by simultaneous magnetic and electric recordings. Electroencephalogr Clin Neurophysiol. 1995;94:26–40. doi: 10.1016/0013-4694(94)00209-4. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Kang X, Alho K, Bertrand O, Yund EW, Woods DL. Attentional modulation of human auditory cortex. Nat Neurosci. 2004;7:658–63. doi: 10.1038/nn1256. [DOI] [PubMed] [Google Scholar]
- Rees G, Russell C, Frith CD, Driver J. Inattentional blindness versus inattentional amnesia for fixated but ignored words. Science. 1999;286:2504–7. doi: 10.1126/science.286.5449.2504. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–9. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Rif J, Hari R, Hämäläinen MS, Sams M. Auditory attention affects two different areas in the human supratemporal cortex. Electroencephalogr Clin Neurophysiol. 1991;79:464–72. doi: 10.1016/0013-4694(91)90166-2. [DOI] [PubMed] [Google Scholar]
- Roberts T, Ferrari P, Stufflebean S, Poeppel D. Latency of the auditory evoked neuromagnetic field components: Stimulus dependence and insights towards perception. J Clin Exp Neuropsychol. 2000;17:114–129. doi: 10.1097/00004691-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Sanders LD, Astheimer LB. Temporally selective attention modulates early perceptual processing: event-related potential evidence. Percept Psychophys. 2008;70:732–42. doi: 10.3758/pp.70.4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K, Gaab N, Schlaug G. Perceiving pitch absolutely: comparing absolute and relative pitch possessors in a pitch memory task. BMC Neurosci. 2009;10:106. doi: 10.1186/1471-2202-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE. Top-down modulation of early sensory cortex. Cereb Cortex. 1997;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Alain C, Picton TW. Effects of attention on neuroelectric correlates of auditory stream segregation. J Cogn Neurosci. 2006;18:1–13. doi: 10.1162/089892906775250021. [DOI] [PubMed] [Google Scholar]
- Teder-Sälejärvi WA, Hillyard SA, Röder B, Neville HJ. Spatial attention to central and peripheral auditory stimuli as indexed by event-related potentials. Brain Res Cogn Brain Res. 1999;8:213–27. doi: 10.1016/s0926-6410(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Chen CY. Attentional capture and inhibition (of return): the effect on perceptual sensitivity. Percept Psychophys. 2005;67:1305–12. doi: 10.3758/bf03193636. [DOI] [PubMed] [Google Scholar]
- Triesman A. Strategies and models of selective attention. Psychological Review. 1969;76:282–99. doi: 10.1037/h0027242. [DOI] [PubMed] [Google Scholar]
- Van Boxtel GJM, Böcker KBE. Cortical measures of anticipation. J Psychophysiol. 2004;18:61–76. [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCalum WC, Winter AL. Contingent negative variation: An electric sign of sensory motor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci U S A. 1993;90:8722–6. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalogr Clin Neurophysiol. 1991;79:170–91. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.