Abstract
Background
The MDRD Study equation underestimates measured GFR at levels greater than 60 ml/min per 1.73 m2, with variable accuracy among subgroups; consequently estimated GFR (eGFR) ≥ 60 ml/min/1.73 m2 is not reported by clinical laboratories. Here, the performance of a more accurate GFR estimating equation, the CKD-EPI equation, is reported by level of GFR and clinical characteristics.
Study Design
Test of diagnostic accuracy
Setting and Participants
Pooled dataset of 3896 people from 16 studies with measured GFR (not used for development of either equation). Subgroups were defined by eGFR, age, sex, race, diabetes, prior solid organ transplant, and body mass index.
Index Tests
eGFR from the CKD-EPI and MDRD Study equations and standardized serum creatinine
Reference Test
Measured GFR using urinary or plasma clearance of exogenous filtration markers
Results
Mean (SD) measured GFR was 68 (36) ml/min/1.73 m2. For eGFR less than 30 ml/min/1.73 m2, both equations have similar bias (median difference compared to measured GFR). For eGFR between 30-59 ml/min/1.73 m2, bias was reduced from 4.9 to 2.1 ml/min/1.73 m2 (57% improvement). For eGFR between 60-89 ml/min/1.73 m2, bias was reduced from 11.9 to 4.2 ml/min/1.73 m2 (61 % improvement). For eGFR between 90-119 ml/min/1.73 m2, bias was reduced from 10.0 to 1.9 ml/min/1.73 m2 (75% improvement). Similar or improved performance was noted for most subgroups with eGFR < 90 ml/min/1.73 m2, other than BMI less than 20 kg/m2, with greater variation noted for some subgroups with eGFR ≥ 90 ml/min/1.73 m2.
Limitations
Limited number of elderly people and racial and ethnic minorities with measured GFR.
Conclusions
The CKD-EPI equation is more accurate than the MDRD Study equation overall and across most subgroups. In contrast to the MDRD Study equation, eGFR ≥ 60 ml/min/1.73 m2 can be reported using the CKD-EPI equation.
Index words: Estimating equations, glomerular filtration rate, performance
Accurate estimation of GFR is important for the detection of chronic kidney disease (CKD), evaluation of its severity and rate of progression, as well as the initiation of appropriate management. Currently, serum creatinine is ordered more than 281 million times annually in the United States 1, 2, and recent reports show that greater than 70% of laboratories now report estimated GFR (eGFR) using the IDMS-traceable 4-variable MDRD Study equation3. It has been previously demonstrated that the MDRD Study equation underestimates measured GFR (mGFR) at levels of eGFR greater than 60 ml/min per 1.73 m2, with variable accuracy among subgroups 4. Therefore, current recommendations are to censor numeric estimates greater than 60 ml/min per 1.73 m2, which limits the use of GFR estimates in clinical practice. For example, eGFR in patients at risk for CKD, such as Blacks or patients with type 1 diabetes, is not reported until it is substantially reduced.
A new equation, the CKD-EPI equation, uses the same four variables as the MDRD Study equation, and is more accurate for estimating GFR, especially at higher levels of GFR 5, 6. The improvement in accuracy is primarily due to a substantial reduction in systematic differences between measured and estimated GFR (bias) with a relatively smaller improvement in precision. Appropriate interpretation of eGFR using the CKD-EPI equation requires understanding of its performance. The purpose of this report is to describe bias according to clinical characteristics and level of eGFR and to discuss clinical implications of reporting eGFR greater than 60 ml/min per 1.73 m2.
Methods
Sources of Data and Measurements
The CKD Epidemiology Collaboration (CKD-EPI) is a research group funded by the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) to address challenges in the study and care of CKD, including development and validation of improved GFR estimating equations by pooling data from research studies and clinical populations (hereafter referred to as “studies”).7-26
The methods for selection of studies and pooling of studies have been previously described5, and are briefly reviewed here. Studies were first identified from the Medline database and through investigators’ and collaborators’ contacts. Inclusion criteria were GFR measured using exogenous filtration markers and serum samples available for calibration of the serum creatinine assay. New equations were developed and internally validated in a dataset developed from pooling of 10 studies (6 research studies and 4 clinical populations) with a total of 8254 participants, divided randomly into separate datasets for development (n=5504) and internal validation (n=2750). The equations were then externally validated in a separate pooled dataset of 16 studies with a total of 3896 participants, the dataset used for the current analysis. Methods for measurement of GFR and serum creatinine have been previously described 5, 27.
Development and validation
Methods for equation development and validation have been previously described in detail 5, 6. In brief, least squares linear regression was used to relate measured GFR to serum creatinine and clinical characteristics available in the development dataset. Predictor variables tested included serum creatinine, age, sex, and race (black vs. white and other), diabetes, transplant and weight, and the interactions among all variables. GFR was adjusted for body surface area (BSA) as ml/min/1.73 m2. 28 GFR and serum creatinine were transformed to natural logarithms to reflect their multiplicative (inverse) relationship and to stabilize variance across the range of GFR. Optimal transformation of log serum creatinine was shown to be a piecewise linear spline with a knot at 0.7 mg/dl in men and 0.9 mg/dl in women.
Developed models were evaluated in the internal database. The development and internal validation datasets were then combined and validated equations were refit to yield more precise final coefficients to be used in subsequent analyses.
Models were then evaluated in the external validation dataset and a final model was selected using a pre-specified series of steps. The final model included serum creatinine, age, sex and race and is listed in Table S1 (provided as online supplementary material available with this article at www.ajkd.org) 5, 6, 29.
Statistical analyses
Bias was measured as the median difference (mGFR − eGFR) and median percent difference (100*[ mGFR − eGFR] / mGFR) between measured and estimated GFR, with positive values indicating lower eGFR than measured GFR (under-estimation). Subgroups for analyses were defined by eGFR and clinical characteristics. Level of eGFR was categorized as greater than 120, 90-119, 60-89, 30-59, 15-29, or less than 15 ml/min per 1.73m2, as used for staging the severity of CKD30, and by < 60, 60-89 and > 90 ml/min per 1.73 m2. eGFR ≥ 90 ml/min/1.73 m2 was subdivided at 120 ml/min/1.73 m2 because of extreme overestimation of measured GFR by the MDRD Study equation at this range. eGFR, rather than measured GFR, was used to categorize kidney function subgroups because GFR is measured with error and use of eGFR provides information that will be available to the clinician. Subgroups of clinical characteristics were described as age (< 40, 40-64, > 65 years); sex; race (Black, White or other); diabetes (yes, no), prior organ transplant (yes, no); body mass index (BMI, < 20, 20 to 25, 26 to 30 and > 30 kg/m2).
Confidence intervals were calculated by bootstrap methods (2000 bootstraps) for difference and percent difference. Elementary nonparametric bootstrap analyses were used for simple random samples assuming a normal sampling distribution. Analyses used R (Version 2.7, Free Software Foundation, Inc., http://www.r-project.org/) and SAS software (version, 9.1, http://www.sas.com/). Smooth estimates of the mean in the figures were created using the lowess function in R.
The institutional review boards of all participating institutions approved the study.
Results
Table 1 shows clinical characteristics of subjects in the external validation dataset (not used for development of either equation) according to eGFR computed using the CKD-EPI equation30. Briefly, approximately 49% of people had an eGFR less than 60, 25 % had eGFR between 60 and 89, and 26% had eGFR greater than 90 ml/min per 1.73 m2. Approximately 15% of subjects were older than 65 years of age. The median age of the subjects older than 65 (25th-75th percentile and 99th percentile) was 71 (68 to 75 and 85) years. Subjects with higher levels of eGFR were younger, more likely to be White, have a lower weight, not be a transplant recipient.
Table 1.
Clinical characteristics of the external validation cohort
| Group | Total | eGFR categories* | |||
|---|---|---|---|---|---|
| <60 | 60-89 | >90 | p-value† | ||
| No. | 3896 | 1902 (49) | 990 (25) | 1004 (26) | |
| Age, years | 50 +/- 15 | 56 +/- 13 | 49 +/- 12 | 37 +/- 12 | <0.001 |
| < 40 | 1136 +/- 29 | 256 +/- 13 | 260 +/- 26 | 620 +/- 62 | } <0.001 |
| 40-65 | 2192 +/- 56 | 1170 +/- 62 | 645 +/- 65 | 377 +/- 38 | |
| > 65 | 568 +/- 15 | 476 +/- 25 | 85 +/- 9 | 7 +/- 0 | |
| Sex | <0.001 | ||||
| Female | 1767 (45) | 780 (41) | 509 (51) | 526 (52) | |
| Male | 2129 (55) | 1122 (59) | 481 (49) | 478 (48) | |
| Race | <0.001 | ||||
| Black | 384 (10) | 220 (12) | 69 (7) | 95 (9) | |
| White or other | 3512 (90) | 1682 (88) | 921 (93) | 909 (91) | |
| Weight, kg | 79 +/- 18 | 80 +/- 19 | 77.64 +/- 17.89 | 76 +/- 18 | <0.001 |
| <60 | 588 +/- 15 | 258 +/- 14 | 168 +/- 17 | 162 +/- 16 | }<0.001 |
| 60-90 | 2403 +/- 62 | 1152 +/- 61 | 592 +/- 60 | 659 +/- 66 | |
| >90 | 905 +/- 23 | 492 +/- 26 | 230 +/- 23 | 183 +/- 18 | |
| Diabetes | <0.001 | ||||
| Yes | 1089 (28) | 553 (29) | 204 (21) | 332 (23) | |
| No | 2807 (72) | 1349 (71) | 786 (79) | 672 (67) | |
| Transplant | <0.001 | ||||
| Yes | 1134 (29) | 731 (38) | 311 (31) | 92 (9) | |
| No | 2762 (71) | 1171 (62) | 679 (69) | 912 (91) | |
| BSA, 1.73 m2 | 1.90+/- 0.23 | 1.91+/- 0.23 | 1.88+/- 0.23 | 1.88+/- 0.23 | <0.001 |
| SCr, mg/dL | 1.52+/- 1.00 | 2.15+/- 1.11 | 1.05+/- 0.18 | 0.79+/- 0.15 | <0.001 |
| BMI, kg/m2 | 27 +/- 6 | 28 +/- 7 | 27 +/- 6 | 26 +/- 5 | <0.001 |
| <20 | 249 +/- 6 | 120 +/- 6 | 73 +/- 7 | 56 +/- 6 | } <0.001 |
| 20-25 | 1357 +/- 35 | 589 +/- 31 | 341 +/- 35 | 427 +/- 43 | |
| 26-30 | 1339 +/- 35 | 643 +/- 34 | 350 +/- 35 | 346 +/- 34 | |
| >30 | 951 +/- 24 | 550 +/- 29 | 226 +/- 23 | 175 +/- 17 | |
Note: except where otherwise indicated, values are given as no. (%) or mean +/- SD. Unit for conversion of SCr in mg/dL to micromol/L, x88.4.
Abbreviations: BSA, Body surface area; BMI, Body mass index; eGFR, estimated glomerular filtration rate; SCr, Serum creatinine
The estimated GFR categories were determined based on the National Kidney Foundation’s KDOQI clinical practice guidelines for chronic kidney disease evaluation, classification, and stratification30. Units are ml/min/1.73 m2; factor for conversion to ml/s/1.73 m2, x0.01667.
The p-values are derived from the Tukey’s test (for the continuous variables) and Chi-square tests (for the categorical variables) for comparison of the means across the three eGFR categories
Table 2 compares bias of the CKD-EPI and MDRD Study equations according to level of eGFR. At levels of eGFR less than 30 ml/min per 1.73 m2, both equations have similar bias; above this level the CKD-EPI equation has improved bias. For levels of eGFR between 30-59 ml/min per 1.73 m2, bias was reduced from 4.9 (95% confidence intervals, (4.4, 5.5) to 2.1 (1.6, 2.7) ml/min per 1.73 m2, a 57% improvement. For levels of eGFR between 60-89 ml/min per 1.73 m2, bias was reduced from 11.9 (95% confidence intervals, 11.0, 13.0) to 4.2 (3.2, 5.5) ml/min per 1.73 m2, a 65% improvement. For levels of eGFR between 90-119 ml/min per 1.73 m2, bias was reduced from 10.0 (6.9, 11.3) to 1.9 (0.2, 4.0) ml/min per 1.73 m2, a 81% improvement. Performance was also improved for eGFR of 120 ml/min per 1.73 m2 and higher, with a substantial decrease in the over-estimation of measured GFR, although the number of subjects was small.
Table 2.
Comparison of the Performance of the CKD-EPI and MDRD Study Equations by eGFR
| eGFR (mL/min/1.73 m2) | Equation | No. | Median Bias (95% CI ) | Median % Bias (95% CI) |
|---|---|---|---|---|
| Overall | CKD-EPI | 3896 | 2.2 (1.8,2.6) | 4.4 (3.7,5.1) |
| MDRD | 3896 | 5.4 (5.0, 5.9) | 10.3 (9.7, 11.1) | |
| >120 | CKD-EPI | 220 | -2.9 (-5.1,-0.1) | -2.4 (-4.2,-0.1) |
| MDRD | 159 | -8.0 (-9.8, -2.7) | -6.7 (-7.9, -2.0) | |
| 90-119 | CKD-EPI | 784 | 1.9 (0.2,4.0) | 1.9 (0.3,3.9) |
| MDRD | 513 | 10.0 (6.9, 11.3) | 8.6 (6.3, 10.2) | |
| 60-89 | CKD-EPI | 990 | 4.2 (3.2,5.5) | 5.6 (4.1,6.9) |
| MDRD | 1124 | 11.9 (11.0, 13.0) | 14.3 (12.9, 11.3) | |
| 30-59 | CKD-EPI | 1293 | 2.1 (1.6,2.7) | 4.8 (3.6,6.0) |
| MDRD | 1450 | 4.9 (4.4, 5.5) | 10.2 (9.2, 11.3) | |
| 16-29 | CKD-EPI | 469 | 1.9 (1.1,2.5) | 7.6 (4.8,9.8) |
| MDRD | 509 | 2.0 (1.3, 2.6) | 8.1 (5.2, 10.2) | |
| < 15 | CKD-EPI | 140 | 0.9 (0.3,1.7) | 8.9 (2.6,13.9) |
| MDRD | 141 | 0.8 (0.3, 1.4) | 6.7 (2.7, 12.0) |
Note: Bias is calculated as mGFR − eGFR, where mGFR is measured GFR. Percent bias (% bias) is calculated as 100 × (mGFR − eGFR)/mGFR.
Abbreviations: eGFR, estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Diet in Renal Disease; CI, confidence interval.
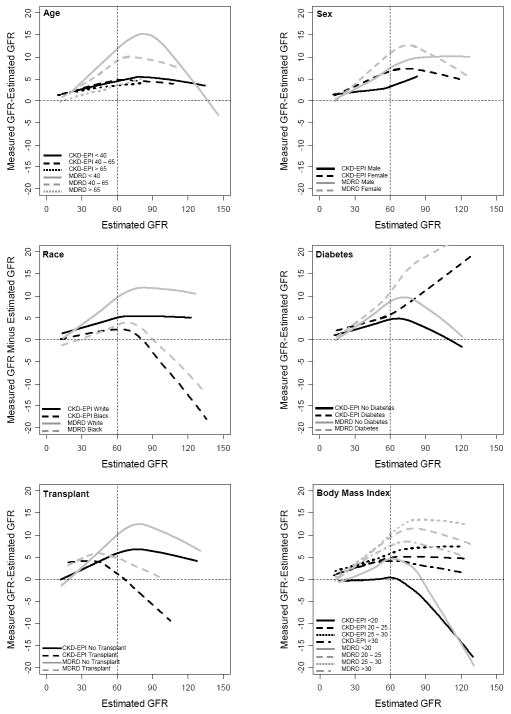
Table 3 and Figure 1 compare the CKD-EPI and MDRD Study equations in subgroups defined by clinical characteristics overall and by level of eGFR. In Table 3, data were combined for less than 30 and 30-60 ml/min per 1.73 m2, as the primary focus is performance at eGFR greater than 60 ml/min/1.73 m2. Overall, with the CKD-EPI equation compared to the MDRD Study equation, bias was improved substantially in people less than 65 years of age, both men and women, Whites, people with or without diabetes, organ transplants, and BMI greater than 20 kg/ m2 across the range of eGFR. There was no substantial change in performance for people older than 65 years of age [1.4 (0.5, 2.0) vs. 1.3 (0.6, 2.0) ml/min per 1.73 m2] and Blacks [-1.1 (-2.3, 0.5) vs. 0.3 (-1.1, 1.6) ml/min per 1.73 m2], while bias was larger for people with BMI less than 20 kg/m2 [-3.5 (-4.7, -1.6) vs. 0.6 (-1.5, 2.1) ml/min per 1.73 m2].
Table 3.
Comparison of the Performance of the CKD-EPI and MDRD Study Equations by Clinical Characteristics
| Group | Equation | Overall | eGFR < 60 | eGFR 60 - 89 | eGFR > 90 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Bias | %Bias | No. | Bias | %Bias | No. | Bias | %Bias | No. | Bias | %Bias | |||
| Overall | CKD-EPI | 3896 | 2.2 | 4.4 | 1902 | 1.9 | 5.6 | 990 | 4.2 | 5.6 | 1004 | 0.9 | 0.8 | |
| MDRD | 3896 | 5.4 | 10.3 | 2100 | 3.4 | 9.6 | 1124 | 11.9 | 14.3 | 672 | 6.4 | 5.7 | ||
| Age | <40 | } CKD-EPI | 1136 | 2.1 | 3.4 | 256 | 1.6 | 4.1 | 260 | 4.9 | 6.4 | 620 | 0.9 | 0.8 |
| 40-65 | 2192 | 2.5 | 5.1 | 1170 | 2.2 | 6.3 | 645 | 4.5 | 5.9 | 377 | 1.0 | 1.0 | ||
| >65 | 568 | 1.3 | 3.7 | 476 | 1.3 | 4.3 | 85 | 0.7 | 1.0 | 7 | 0.6 | 0.6 | ||
| <40 | } MDRD | 1136 | 9.7 | 12.5 | 309 | 4.9 | 13.0 | 379 | 15.4 | 17.1 | 44 | 9.7 | 7.8 | |
| 40-65 | 2192 | 5.6 | 11.1 | 1310 | 4.2 | 11.1 | 665 | 11.2 | 13.1 | 217 | 2.2 | 2.2 | ||
| >65 | 568 | 1.4 | 3.8 | 481 | 0.9 | 3.3 | 80 | 4.5 | 5.9 | 7 | -16.3 | -19.8 | ||
| Female | Yes | }CKD-EPI | 1767 | 2.2 | 4.3 | 780 | 1.6 | 5.0 | 509 | 5.8 | 7.3 | 478 | 0.4 | 0.4 |
| No | 2129 | 2.2 | 4.4 | 1122 | 2.1 | 5.9 | 481 | 3.1 | 4.0 | 526 | 1.6 | 1.6 | ||
| Yes | } MDRD | 1767 | 6.4 | 11.6 | 880 | 4.2 | 11.4 | 562 | 13.4 | 15.4 | 325 | 6.9 | 5.7 | |
| No | 2129 | 4.8 | 9.6 | 1220 | 2.9 | 8.5 | 562 | 10.8 | 12.7 | 347 | 6.0 | 5.7 | ||
| Race | Black | } CKD-EPI | 384 | -1.1 | -3.0 | 220 | 0.5 | 1.7 | 69 | 3.4 | 4.5 | 95 | -13.2 | -12.5 |
| White/other | 3512 | 2.5 | 4.9 | 1682 | 2.0 | 6.0 | 921 | 4.4 | 5.8 | 909 | 2.4 | 2.4 | ||
| Black | } MDRD | 384 | 0.3 | 0.6 | 222 | 0.2 | 0.6 | 83 | 7.9 | 9.4 | 79 | -8.3 | -6.9 | |
| White/other | 3512 | 6.0 | 11.1 | 1878 | 3.9 | 10.2 | 1041 | 12.2 | 14.8 | 593 | 8.8 | 7.6 | ||
| Diabetes | Yes | } CKD-EPI | 1089 | 4.6 | 4.6 | 553 | 2.6 | 7.1 | 204 | 4.0 | 5.2 | 332 | 12.3 | 10.3 |
| No | 2807 | 1.3 | 2.7 | 1349 | 1.6 | 4.8 | 786 | 4.4 | 5.7 | 672 | -3.3 | -3.3 | ||
| Yes | } MDRD | 1089 | 8.1 | 13.0 | 601 | 4.6 | 11.0 | 235 | 15.3 | 17.4 | 253 | 19.1 | 14.9 | |
| No | 2807 | 4.6 | 9.2 | 1499 | 3.0 | 9.0 | 889 | 11.5 | 13.4 | 419 | 0.4 | 0.4 | ||
| Transplant | Yes | } CKD-EPI | 1134 | 1.5 | 3.1 | 731 | 2.6 | 6.7 | 311 | -1.2 | -1.8 | 92 | -10.0 | -11.8 |
| No | 2762 | 2.5 | 4.9 | 1171 | 1.4 | 4.8 | 679 | 7.6 | 8.8 | 912 | 2.2 | 2.1 | ||
| Yes | }MDRD | 1134 | 4.8 | 9.4 | 840 | 5.1 | 11.7 | 241 | 3.9 | 5.5 | 53 | -5.6 | -6.1 | |
| No | 2762 | 5.8 | 10.7 | 1260 | 2.6 | 8.4 | 883 | 14.5 | 16.0 | 619 | 7.2 | 6.4 | ||
| BMI (kg/m2) | <20 | }CKD-EPI | 249 | -3.5 | -6.8 | 120 | -1.7 | -6.3 | 73 | -1.2 | -1.9 | 56 | -16.6 | -18.1 |
| 20-25 | 1357 | 2.2 | 4.2 | 589 | 1.7 | 4.9 | 341 | 4.5 | 5.7 | 427 | 1.5 | 1.5 | ||
| 26-30 | 1339 | 3.2 | 6.3 | 643 | 2.3 | 7.1 | 350 | 4.9 | 6.7 | 346 | 5.2 | 4.4 | ||
| >30 | 951 | 2.1 | 4.4 | 550 | 2.1 | 6.1 | 226 | 3.9 | 5.1 | 175 | -0.9 | -0.7 | ||
| <20 | }MDRD | 249 | 0.6 | 1.0 | 139 | 0.1 | 0.3 | 74 | 7.4 | 8.9 | 36 | -13.7 | -14.0 | |
| 20-25 | 1357 | 5.9 | 10.7 | 656 | 4.1 | 10.6 | 408 | 12.2 | 14.2 | 293 | 6.8 | 6.4 | ||
| 26-30 | 1339 | 6.7 | 12.0 | 705 | 3.7 | 9.9 | 412 | 13.4 | 15.4 | 222 | 9.9 | 8.1 | ||
| >30 | 951 | 4.4 | 9.6 | 600 | 3.2 | 9.6 | 230 | 10.3 | 12.4 | 121 | 6.3 | 5.3 | ||
Note: Bias is calculated as mGFR − eGFR, where mGFR is measured GFR. Percent bias (%Bias) is calculated as 100 × (mGFR − eGFR)/mGFR. GFR expressed in ml/min/1.73 m2; factor for conversion to ml/s/1.73 m2, x0.01667.
Abbreviations: CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Renal Disease; BMI = Body mass index; eGFR, estimated glomerular filtration rate.
Figure 1. Performance of the CKD-EPI and MDRD Study equations by Clinical Characteristics Subgroups.
CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Diet in Renal Disease
At levels of eGFR less than 60 ml/min per 1.73 m2 and particularly for eGFR between 60 to 89 ml/min per 1.73 m2, there was an improvement or no change in bias for every subgroup with the CKD-EPI equation compared to the MDRD Study equation, except for people with BMI less than 20 ml/min per 1.73 m2 [-1.7 (-4.1, 0.0) vs. 0.1 (-1.7, 1.3) ml/min per 1.73 m2]. At eGFR greater than 90 ml/min per 1.73 m2, the CKD-EPI equation demonstrated less bias than the MDRD Study equation for subgroups defined by age, sex and diabetes, but greater bias in Blacks [-13.2 (-20.2, -7.6) vs. -8.3 (-15.5, -1.9) ml/min per 1.73 m2], patients without diabetes [-3.3 (- 5.1, -2.1) vs.0.4 (-2.2, 2.6) ml/min per 1.73 m2], transplant recipients [-10.0 (-13.5, -3.7) vs. -5.6 (-14.3, 3.4) ml/min per 1.73 m2], and people with BMI less than 20 kg/m2 [-16.6 (-22.9, -8.4) vs. -13.7 (-21.8, -8.4) ml/min per 1.73 m2]. For many of the subgroups where the CKD-EPI equation resulted in worse bias, the groups are of small size (n less than 100 for each).
Discussion
There are numerous studies attempting to identify a replacement for serum creatinine as a filtration marker, but no one marker has thus been definitely established 18, 31-34. Thus, in spite of acknowledged weaknesses,35, 36 GFR estimates based on serum creatinine will remain the mainstay of clinical assessment of kidney function for the foreseeable future, and use of equations that improve the accuracy of GFR estimated from serum creatinine is an important goal. A new creatinine-based estimating equation, the CKD-EPI equation, which is more accurate than the MDRD Study equation, was recently reported. Using the CKD-EPI equation, bias is substantially decreased bias compared to measured GFR, especially among people with eGFR greater than 60 ml/min/1.73m2, and classification according to the presence or absence of CKD and the stage of CKD is more often correct5. In a separate publication, it was demonstrated that the addition of diabetes, transplant and weight as predictor variables did not improve performance of the CKD-EPI equation6. In this report, the bias across the range of eGFR for subgroups defined by demographic and clinical characteristics was described. This has important clinical implications for reporting of eGFR by clinical laboratories and interpretation in practice.
The current analyses demonstrate that compared to the MDRD Study equation, the CKD-EPI equation reduced bias in almost all subgroups. In particular, there was improvement in bias in subgroups at low risk for CKD where underestimation of measured GFR may have led to overestimation of CKD prevalence, including age less than 65 years, women, and whites. For eGFR up to 90 ml/min/1.73 m2, bias is small and consistent across subgroups. Importantly, at this range of eGFR, there is unbiased GFR estimation in groups at increased risk of CKD, including the elderly, blacks, patients with diabetes and organ transplant recipients and the overweight and obese. For eGFR greater than 90 ml/min per 1.73 m2, the overall bias is low, but heterogeneity is observed among subgroups. Despite this heterogeneity, median bias using the CKD-EPI equation is lower than the median bias for eGFR less than 60 ml/min/1.73 m2 using the MDRD Study equation.
The improvement in the performance of the CKD-EPI equation is due in part to the inclusion of a diverse population in the development of the equation. However, the dataset did not include all people in whom GFR will be estimated and therefore the ability to comment on some groups is limited by the available data. Key populations not adequately represented are people with eGFR greater than 60 ml/min/1.73 m2, particularly those older than 65 years of age and racial and ethnic minorities. The study population is also limited by possible selection bias for recruitment in the studies included in the pooled database. Studies in representative populations will be required to overcome this limitation.
The large overestimate observed in transplant recipients at eGFR greater than 90 ml/min/1.73 m2 may be an artifact as few transplant recipients have measured GFR this high. Possibly, the large overestimate that was observed reflects reduced creatinine production and low serum creatinine due to muscle wasting associated with comorbid conditions in transplant recipients selected for GFR measurement. Similarly, the large overestimate in subjects with low BMI and eGFR greater than 90 ml/min/1.73 m2 may also reflect muscle wasting. Endogenous filtration markers other than serum creatinine are likely to be necessary to improve GFR estimation in patients with muscle wasting. Furthermore, it was previously shown that inclusion of terms in the estimating equation for transplant status and body weight did not improve overall performance of the equations in the validation dataset, suggesting this large bias is not related to transplant status or weight per se.6
More accurate estimates has important implications in public health and clinical care. The CKD-EPI equation leads to lower estimated prevalence of CKD in NHANES (the National Health and Nutrition Examination Survey), with reclassification to higher CKD Stages particularly in groups at low risk for CKD. 5 Recent studies in the ARIC (Atherosclerosis Research in Communities) and Aus-Diab (Australian Diabetes, Obesity and Lifestyle) studies have confirmed these findings37, 38. These studies also showed that participants reclassified to higher GFR stages had lower risk for subsequent adverse events. In addition to improved prognosis, reclassification to a higher GFR stage would lead to benefit across the range of GFR for better detection, evaluation and management of CKD. For example, reclassification would improve clinicians’ ability to adjust drug dosages and identify individuals who may be at increased risk for side effects of medications or diagnostic procedures such as contrast media for imaging or oral phosphate-based solutions in preparation for colonoscopy, and as such would improve patient safety39, 40. In addition, reporting of eGFR using the CKD-EPI equation would allow reporting of numeric results for eGFR values greater than 60 ml/min/1.73 m2. The clinical impact of availability of accurate eGFR values above 60 ml/min per 1.73 m2 has not been evaluated because until now there has been no simple clinical tool for accurate estimation. Potential applications include monitoring eGFR decline from normal to mild to moderate reduction, particularly in patients with an increased risk for development and progression of CKD, such as Blacks or patients with diabetes. 41-43 Implementation of reporting of eGFR > 60 ml/min per 1.73 m2 should be performed with adequate education tools such that clinicians do not falsely diagnosis CKD at eGFR ≥ 60 ml/min/1.73 m2 in the absence of persistent markers of kidney damage.
Despite the substantial reduction in bias with the CKD-EPI equation, GFR estimates remain imprecise 5, 6. Both the MDRD Study and CKD-EPI equations are based on serum creatinine; like all other creatinine-based estimation equations, they suffer the same irremediable limitations of creatinine as a filtration marker 35, 44-46. The terms for age, sex and race in both equations only capture some of the non-GFR determinants of creatinine, and the coefficients represent average effects observed in the development sample. For patients at the extremes of muscle mass, those with unusual diets, and those with conditions associated with reduced secretion or extra-renal elimination of creatinine, all estimates of GFR based on serum creatinine may be inaccurate. This is particularly relevant for populations who are most likely to require medications, such as the frail elderly, critically ill, or cancer patients 47. Clinicians should be mindful of muscle mass in interpretation of estimated GFR. Confirmatory tests with exogenous measured GFR or measured creatinine clearance should be performed for people in whom the estimates are thought to be inaccurate or when a highly accurate level is needed such as for toxic medications with a narrow therapeutic index or for some clinical trials looking at change in GFR over time48, although studies need to be performed to establish reference ranges for creatinine clearance using IDMS traceable creatinine methods.
The strengths of the study include the large diverse study population of people with and without kidney diseases; calibration of the creatinine assays in each study to standardized values; and rigorous statistical techniques for equation development and validation. Comparison of equations in a separate validation dataset overcomes some of the limitations of differences among studies in patient characteristics and methods for measurement of GFR and serum creatinine.
There are limitations to this study. As discussed above, studies were pooled from different populations to develop and validate the CKD-EPI equation. The selection of these study populations according to presence or absence of kidney disease may bias equation performance. This may affect the assessment of performance of either equation, but it would not affect their comparison. Complete data on ethnicity, diabetes type, immunosuppressive agents for transplantation, measures of muscle mass, and other clinical conditions and medications that might affect serum creatinine independently from GFR were not available. These variables may identify particular groups of people who are likely to have large errors in the estimates. However, the variables that were evaluated are the most readily available and easy to ascertain for widespread clinical application. Finally, in the validation dataset, GFR was measured in some individuals using a different exogenous marker than was used in the development of the equations. However, this would affect the performance of both equations, and therefore would not affect the relative performance of the equations.
In summary, the CKD-EPI equation is less biased than the MDRD Study equation in most subgroups defined by demographic and clinical characteristics and level of GFR. Implementation of eGFR reporting using the CKD-EPI equation across the entire range of eGFR will allow a better clinical assessment of kidney function than is now available. The CKD-EPI equation should replace the MDRD Study equation for general clinical use and can be reported throughout the GFR range.
Supplementary Material
Acknowledgments
Aghogho Okparavero, MBBS, MPH provided assistance in communications and manuscript preparation.
The membership of the CKD-EPI collaboration, including investigators, research staff, collaborators and the Scientific Advisory Committee have been previously published.5
Support: This study was supported by grants UO1 DK 053869, UO1 DK 067651, UO1 DK 35073 and K23-DK081017.
Footnotes
Portions of this report were presented at the American Society of Nephrology annual conference held November 6 to 9, 2008, in Philadelphia, PA
Financial Disclosure: The authors declare that they have no relevant financial interests.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steindel SJ, Rauch WJ, Simon MK, Handsfield J. National Inventory of Clinical Laboratory Testing Services (NICLTS). Development and test distribution for 1996. Archives of pathology & laboratory medicine. 2000;124(8):1201–1208. doi: 10.5858/2000-124-1201-NIOCLT. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA. Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am J Kidney Dis. 2010 Apr;55(4):622–7. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47(9):1017–1019. doi: 10.1515/CCLM.2009.264. [DOI] [PubMed] [Google Scholar]
- 4.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens LA, Schmid CH, Zhang Y, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25(2):449–457. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lewis JB, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African-Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim H, Mondress M, Tello A, Fan Y, Koopmeiners J, Thomas W. An alternative formula to the Cockcroft-Gault and the modification of diet in renal diseases formulas in predicting GFR in individuals with type 1 diabetes. J Am Soc Nephrol. 2005;16(4):1051–1060. doi: 10.1681/ASN.2004080692. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335(22):1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EJ, Kunsicker LG, Bain RP, Rohde RD Collaborative Study Group. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol. 2003;14(7 (suppl 2)):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 13.Greene T, Li L, Coresh J, et al. A statistical explanation for different relationships of serum creatinine (Scr) vs. GFR across populations: preliminary results [ASN abstract 912] J Am Soc Nephrol. 2005;16:319A. [Google Scholar]
- 14.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 15.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 16.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64(3):1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 17.Mauer M, Drummond K. The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes. 2002;51(5):1572–1579. doi: 10.2337/diabetes.51.5.1572. [DOI] [PubMed] [Google Scholar]
- 18.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51(8):1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 19.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Zinman B, Gardiner R, et al. The Relationship of Diabetic Retinopathy to Preclinical Diabetic Glomerulopathy Lesions in Type I Diabetic Patients. Diabetes. 2005;54:527–533. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- 21.Bosma RJ, Doorenbos CR, Stegeman CA, van der Heide JJ, Navis G. Predictive performance of renal function equations in renal transplant recipients: an analysis of patient factors in bias. Am J Transplant. 2005;5(9):2193–2203. doi: 10.1111/j.1600-6143.2005.00982.x. [DOI] [PubMed] [Google Scholar]
- 22.Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002;62(1):220–228. doi: 10.1046/j.1523-1755.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 23.Tarnow L, Rossing P, Jensen C, Hansen BV, Parving HH. Long-term renoprotective effect of nisoldipine and lisinopril in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2000;23(12):1725–1730. doi: 10.2337/diacare.23.12.1725. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen P, Andersen S, Rossing K, Hansen BV, Parving HH. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial Transplant. 2002;17(6):1019–1024. doi: 10.1093/ndt/17.6.1019. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the rennin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63(5):1874–1880. doi: 10.1046/j.1523-1755.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 26.Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. Brit Med J. 1991;303(6794):81–87. doi: 10.1136/bmj.303.6794.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50(1):21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. Abstract. [DOI] [PubMed] [Google Scholar]
- 29.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. Journal of nephrology. 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 30.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 31.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Current opinion in nephrology and hypertension. 2006;15(6):610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 32.White CA, Akbari A, Doucette S, et al. A novel equation to estimate glomerular filtration rate using beta-trace protein. Clin Chem. 2007;53(11):1965–1968. doi: 10.1373/clinchem.2007.090126. [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White C, Akbari A, Hussain N, et al. Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol. 2005;16(12):3763–3770. doi: 10.1681/ASN.2005050512. [DOI] [PubMed] [Google Scholar]
- 35.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 36.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38(1):167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk Implications of the New CKD Epidemiology Collaboration (CKD-EPI) Equation Compared With the MDRD Study Equation for Estimated GFR: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010 Apr;55(4):648–59. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the Prevalence and Mortality Risk of CKD in Australia Using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR Estimating Equations: The AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010 Apr;55(4):660–70. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Hurst FP, Bohen EM, Osgard EM, et al. Association of oral sodium phosphate purgative use with acute kidney injury. J Am Soc Nephrol. 2007;18(12):3192–3198. doi: 10.1681/ASN.2007030349. [DOI] [PubMed] [Google Scholar]
- 40.Khurana A, McLean L, Atkinson S, Foulks CJ. The effect of oral sodium phosphate drug products on renal function in adults undergoing bowel endoscopy. Arch Intern Med. 2008;168(6):593–597. doi: 10.1001/archinte.168.6.593. [DOI] [PubMed] [Google Scholar]
- 41.Kotchen TA, Piering AW, Cowley AW, et al. Glomerular hyperfiltration in hypertensive African Americans. Hypertension. 2000;35(3):822–826. doi: 10.1161/01.hyp.35.3.822. [DOI] [PubMed] [Google Scholar]
- 42.Parmer RJ, Stone RA, Cervenka JH. Renal hemodynamics in essential hypertension. Racial differences in response to changes in dietary sodium. Hypertension. 1994;24(6):752–757. doi: 10.1161/01.hyp.24.6.752. [DOI] [PubMed] [Google Scholar]
- 43.El-Gharbawy AH, Kotchen JM, Grim CE, et al. Predictors of target organ damage in hypertensive blacks and whites. Hypertension. 2001;38(4):761–766. doi: 10.1161/hy1001.092613. [DOI] [PubMed] [Google Scholar]
- 44.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28(5):830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 45.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ., 3rd For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75(10):1071–1078. doi: 10.1038/ki.2008.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference? Am J Kidney Dis. 2009;53(6):932–935. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poggio ED, Nef PC, Wang X, et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis. 2005;46(2):242–252. doi: 10.1053/j.ajkd.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20(11):2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.