Abstract
Neurodegenerative diseases encompass a large group of neurological disorders. Clinical symptoms can include memory loss, cognitive impairment, loss of movement or loss of control of movement, and loss of sensation. Symptoms are typically adult onset (although severe cases can occur in adolescents) and are reflective of neuronal and glial cell loss in the central nervous system. Neurodegenerative diseases also are considered progressive, with increased severity of symptoms over time, also reflective of increased neuronal cell death. However, various neurodegenerative diseases differentially affect certain brain regions or neuronal or glial cell types. As an example, Alzheimer disease (AD) primarily affects the temporal lobe, whereas neuronal loss in Parkinson disease (PD) is largely (although not exclusively) confined to the nigrostriatal system. Neuronal loss is almost invariably accompanied by abnormal insoluble aggregates, either intra- or extracellular. Thus, neurodegenerative diseases are categorized by (a) the composite of clinical symptoms, (b) the brain regions or types of brain cells primarily affected, and (c) the types of protein aggregates found in the brain. Here we review the methods by which Drosophila melanogaster has been used to model aspects of polyglutamine diseases, Parkinson disease, and amyotrophic lateral sclerosis and key insights into that have been gained from these models; Alzheimer disease and the tauopathies are covered elsewhere in this special issue.
Keywords: polyglutamine, synuclein, parkin, DJ-1, PINK1, LRRK2, SOD, VAPB, TDP-43, Drosophila, neurodegeneration
Introduction
The last 12 years have seen quite an increase in the number of laboratories using invertebrates to model human neurodegenerative diseases. The simple fruit fly Drosophila has been the subject of hundreds of manuscripts detailing phenotypes and defining genetic and chemical modifiers. Here we will discuss those approaches taken using mostly misexpression of human disease genes; Drosophila genes that cause neurodegeeneration when mutated have been the subject of an exhaustive recent review (Lessing and Bonini, 2009). Models of Alzheimer disease and other tauopathies are covered elsewhere in this special issue.
Why Flies?
Although fruit flies at first glance may seem far removed from human biology, fundamental cellular processes are very similar between humans and flies; these include regulation of gene expression, subcellular trafficking, synaptic transmission, synaptogenesis, and cell death. Additionally, many genes and signaling pathways are conserved between human and flies, including key regulatory pathways in mammals, such as Wnt, Ras/Extracellular Regulated Kinase (ERK), and Toll-like pathways; each of these was first identified in flies and later found to have homolog and conserved functions in mammals. Flies have simpler genetics (only 4 pairs of homologous chromosomes, as compared to 23 in humans; 12,000 gene,s as compared to 20,000 in humans) and simpler nervous systems (~200,000 neurons compared to ~100 billion neurons in humans); nonetheless, flies still are capable of performing complex motor behaviors, such as walking, climbing, and flying, and they can be trained using fear conditioning paradigms to test learning and memory. Drosophila behavior can be useful in parsing out the effects of a gene product on neuronal function and connectivity. The relative simplicity of Drosophila systems and genetics makes it ideal for creating animal models of complex disorders that can individually model a subset of phenotypes associated with a disease in order to simplify analysis of the disease.
Another advantage of Drosophila as a model organism is its short reproductive and developmental cycles (10 – 14 days from embryo to reproductively mature adults), as well as the ease of creating genetic deletions, insertions, knock-downs or transgenics as compared to mammals. Maintenance of stocks is simple and inexpensive, making Drosophila an ideal laboratory animal, as hundreds of different mutant and transgenic lines can all be kept within the confines of a single laboratory. A range of genetic manipulations have been developed in Drosophila that are impossible or impractical in mammals. Most of these manipulations have taken advantage of the naturally found transposable element in Drosophila, the P element. Although the natural P element originally consisted of inverted repeats surrounding a transposase gene to allow for hopping into endogenous chromosomes, the P element in the hands of researchers has since taken on a life of its own and has becomes the dominant tool in creating disease models in Drosophila. P elements insert semirandomly into the genome, although there are certain “hotspots” in chromatin integration, and preferentially insert into promoter regions, disrupting transcription of the downstream gene. The simplest application of this phenomenon has been libraries of “P element mutants,” which largely represent null alleles of the affected gene. Mapping the flanking regions around the insertion is a straightforward process, and allows for much more rapid mapping mutations as compared to classical chemically induced mutagens, e.g., ethyl methane sulfonate (EMS).
Taken a step further, P elements can be engineered to carry genes other than transposase while they maintain the inverted repeats, thus allowing for genomic insertion when another source of transposase is present. Fusion of a cell- or tissue-specific promoter and/or enhancer placed 5' to the gene within the P element allows for the creation of transgenic lines that express the gene of interest in a tissue-specific manner, taking advantage of the P element's ability to insert into the host genome without the need for homologous recombination. Further, multiple P-element-derived trangenes can be used in combination with each other to allow for tissue-specific misexpression of either other transgenes or endogenous genomic sequences. The GAL4/UAS system of expression (Brand and Perrimon, 1993) uses yeast-derived GAL4, a transcription factor that binds to the Upstream Activating Sequence (UAS) enhancer element, driving expression of the gene immediately downstream of the UAS. Thus two transgenes are required: (1) a transgene expressing GAL4 under control of tissue-specific enhancers, and (2) a transgene carrying a UAS placed just upstream of a gene of interest. There are several advantages to this binary expression system. One is the conditional nature of the expression, such that if expression of the gene is deleterious, the precursor lines can be maintained and crossed as needed to carry out experiments. A second advantage is that a single UAS transgenic line can be ectopically expressed in many different tissue types, depending on the tissue-specific enhancer line used to express the GAL4 gene (such as line is commonly referred to as a “driver”). This is highly advantageous especially in creating models of neurodegeneration, as different tissue types or even different cell types within the same tissue may manifest different phenotypes caused by the same misexpressed gene. Third, the GAL4/UAS system often yields much higher expression of the gene of interest than direct promoter-fused transgenes, and expression can be further augmented by adding more copies of either the driver or the UAS construct.
Together, these features of Drosophila make it an ideal organism to perform genetic and pharmacological screens for modifiers of neurodegeneration, once a phenotype has been established, be it morphological or behavioral. Such screens often take advantage of large collections of fly lines with mapped mutations, or mapped insertions with “empty” UAS constructs, termed “enhancer-promoter” or “EP” elements, which allow for the overexpression of an endogenous gene that is near the UAS insertion, in combination with a source of GAL4. These collections (Rorth, 1996; Bellen et al., 2004) can be obtained from the large stock centers found worldwide, such as the Bloomington Drosophila Stock Center maintained by Indiana University in Bloomington, Indiana. These lines and other similar collections can then be crossed to the disease model tester strain, allowing for the rapid identification of modifiers. The GAL4-UAS system can also be used to express siRNAs in cell-specific manner, and thus can be used to knock down gene targets as well. Pharmacological screens can similarly be carried out in a high throughput manner, as drugs and other chemical compounds can be readily mixed into fly food media, on which both developing larvae and adult flies feed. Such screens can yield novel genetic interactions in disease processes, and help to further refine proposed and established mechanisms of pathology.
Polyglutamine Diseases
Polyglutamine diseases are caused by mutations that lead to expansions of unstable CAG repeats, which are translated as glutamine (symbolized in biochemical short-hand by the letter Q) in normal functioning proteins. Huntington disease (HD) and the dominant spinocerebellar ataxias (SCA) are representative of this class of disease; these disorders show characteristic features in patients, such as (a) nuclear inclusions containing the mutant protein, (b) repeat length inversely correlated with age of onset, (c) motor impairment, and (d) age-dependent degeneration. Polyglutamine diseases are due to single gene defects and were the first neurodegenerative models successfully created in Drosophila that used human transgenes. Examples of some retinal phenotypes of human disease genes are shown in Figure 1. We created a model of HD using expression of truncated wild type and mutant forms of huntingtin/htt (Jackson et al., 1998), and Bonini and coworkers reported a model of SCA 3 or Machado-Joseph disease (SCA3/MJD) expressing truncated ataxin 3 (also referred to as MJD1), also using different glutamine repeat lengths (Warrick et al., 1998); both papers demonstrated that increased polyQ expansion led to more severe degeneration, age-dependent degeneration, and repeat length-dependent nuclear aggregation (Fig. 1B and D). These models provided a platform to demonstrate that (a) human disease genes can yield parallel neurodegenerative effects in Drosophila, and (b) the fly eye can serve as a model tissue to monitor neurodegeneration, serving as readout to identify genetic modifiers of neurotoxicity. A few investigators have also shown that polyQ expression in glia can cause lethality and neurodegeneration (Kretzschmar et al., 2005; Tamura et al., 2009). It is worth noting that most studies of polyQ diseases in Drosophila have focused on neurotoxic aspects of the proteins using the retina, rather than on the motor impairments also associated with the diseases; however, two studies using the same truncated ataxin-3 lines established by Bonini's group found significant motor impairments with increased polyQ repeat lengths in adult flies when expressed either pan-neuronally (Kim et al., 2004) or in glia (Kretzschmar et al., 2005). Thus, polyglutamine models in the fly have been helpful in demonstrating both neuronal and glial phenotypes.
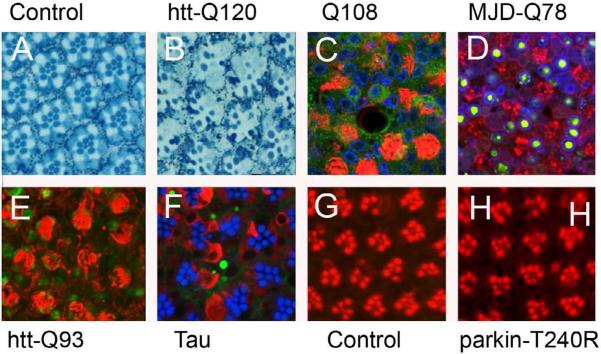
Fig. 1.
Retinal phenotypes (or lack thereof) or various human neurodegenerative disease genes (tangential views). A and B, toludine blue-stained plastic sections. A, control retina shows normal ommatidia with regular profiles of seven rhabdomeres. B, huntingtin GMR-Q120 (GMR-Q120/+)(Jackson et al., 1998) fly at ten days shows degeneration of photoreceptor cells. C, the “pure” polyglutamine construct under control of GMR-GAL4 (GMR-GAL4/UAS-Q108)(Marsh et al., 2000) shows severely abnormal rhadomeres. Red, TRITC-phalloidin; green, htt17 (Sang et al., 2005); blue, lamin. D, the truncated MJD protein Q78 (GMR-GAL4/UAS-MJD-Q78) (Warrick et al., 1998) shows degenerated rhadomeres with largely nuclear localization of HA tagged aggregates. Red, TRITC-phalloidin; green, ant-HA; blue, lamin. E, The huntingtin exon 1 Q93 phenotype (Steffan et al., 2001) shows severe rhabdomere abnormaities (GMR-GAL4/UAS-htt-Q93). Red, phalloidoin TRITC; blue, TUNEL staining. F, the tau eye phenotype (GMR or gl-Tau/+) (Jackson et al., 2002)(see accompanying article by Wentzell and Kretzschmar for further discussion) shows abnormal polarity and some loss of rhabodmeres, with both diffuse and aggregated patterns of tau staining. Blue, phalloidoin-Cy3; red, total tau (T14); blue Alz50 (a conformation dependent tau epitope). G and H, phalloidoin-TRITC. G, control GMR-GAL4 retina (GMR-GAL4/+) at 60 days shows some mild irregularities and rhadomere droupout. The familial PD gene T240R, which shows dominant toxicity to DA neurons (Sang et al., 2007), has no obvious effect in retina greater than that of driver alone even at 60 days (GMR-GAL4/UAS-parkinT240R). Thus, contrary to popular belief, it is not trivial to obtain retinal phenotypes in the fly by misexpressing human genes. Adapted from (Jackson et al., 1998; Jackson et al., 2002; Sang et al., 2007; Sang and Jackson, 2005; Sang et al., 2005).
Use of these models and other polyQ disease genes has already yielded valuable insights into polyglutamine pathogenesis and potential therapeutics. As might be expected with overexpression of aggregation-prone proteins, changes in genes that regulate degradation of misfolded proteins (e.g., chaperone proteins and ubiquitin ligases), strongly modify the degenerative effects of polyQ proteins (Al-Ramahi et al., 2006; Bilen and Bonini, 2007; Fernandez-Funez et al., 2000; Warrick et al., 1999). Interestingly, one of the E3 ligases that recognizes polyglutamine genes is CHIP (C terminus of Hsc-70 Interacting Protein) (Al-Ramahi et al., 2006), which also targets the microtubule binding protein tau for ubiquitination and degradation (Dickey et al., 2006; Petrucelli et al., 2004). Additionally, genes related to apoptosis (Bae et al., 2005; Higashiyama et al., 2002; Sang et al., 2005; Warrick et al., 1998) and some signaling pathways (Chen et al., 2003; Scappini et al., 2007) have been reported to modulate polyQ degeneration.
Engineered proteins containing only polyQ tracts, expressed outside the context of a disease-associated gene, were demonstrated to be toxic in and of themselves (Fig. 1C (Kazemi-Esfarjani and Benzer, 2000; Marsh et al., 2000), and can form nuclear and axonal inclusions in neurons (Gunawardena et al., 2003). However, the toxic effects of these polyQ tracts can be mitigated if additional, non-glutamine amino acids are added, even by as few as twenty-six amino acids that comprise an epitope tag (Marsh et al., 2000). In addition, engineered expansion of polyQ tracts in non-disease associated genes that contain functional polyQ tracts, such as Dishevelled (dsh) in Drosophila, demonstrate very little of the same degenerative phenotype that expression of the polyQ tract by itself exhibits; instead, they appear to disrupt the normal function of the host protein, yielding phenotypes similar to dsh loss-of-function mutations (Marsh et al., 2000). The presence of other proteins or peptides with polyQ tracts also appears to modulate polyQ degeneration, depending on the type of proteins/peptides expressed. Although expression of exon 1 of human huntingtin with a normal polyQ tract of 20 glutamines (Q20) has no toxic effect on its own, co-expression of htt-Q20 strongly enhances and accelerates degeneration and increases the number of nuclear inclusions induced by expression of htt-Q93, presumably acting in amyloidogenic fashion to enhance seeding for aggregation (Slepko et al., 2006). Additionally, two independent ivestigators found that increased expression of wild-type ataxin-2, a polyQ-containing protein associated with spinocerebellar ataxia 2 (SCA2), enhances both ataxin-1- (Al-Ramahi et al., 2007) and ataxin-3-induced toxicity (Lessing and Bonini, 2008) through a direct interaction with both proteins, and that both disease models can be mitigated by a reduction in ataxin-2 expression. The normal ataxin-2 appears to be recruited to the nucleus by the other mutant ataxin proteins, where it can produce toxic effects, even with a normal polyQ length (Al-Ramahi et al., 2007). However, co-expression of wild-type ataxin-3, a protein involved in ubiquitin-binding and the ubiquitin-proteasome cycle, can suppress the toxicity of several polyQ proteins, including pathogenic forms of ataxin-3, htt, and ataxin-1, presumably by helping to target these harmful proteins for degradation (Warrick et al., 2005). Thus, fly models have demonstrated that the protein context in which the deleterious polyQ tract is situated, and the presence of other wild-type polyQ proteins, help to determine the toxic effects of expanded polyQ tracts.
The use of Drosophila models followed work in mammalian models (Klement et al., 1998; Saudou et al., 1998) that suggested that translocation to the nucleus of the mutant polyQ protein was necessary to induce neurodegeneration, Furthermore, the use of Drosophila models demonstrated that the toxicity could be mitigated if the mutant protein was trapped outside of the nucleus (Takeyama et al., 2002). Additionally, transcription factors and other nuclear proteins have been found to strongly modify polyQ toxicity (Bilen and Bonini, 2007; Branco et al., 2008; Fernandez-Funez et al., 2000; Steffan et al., 2001; Taylor et al., 2003). These results suggest that dysregulation of transcription is an important event in polyQ-mediated toxicity, and addresses the prominence of inclusions localized to the nucleus. However, it is still unclear what function the nuclear inclusions play, as several studies have shown that toxicity can persist in the absence of inclusions, and conversely that toxicity can be mitigated with little effect on inclusion formation or stability (Bilen and Bonini, 2007; Klement et al., 1998; Saudou et al., 1998; Warrick et al., 1999).
Findings that certain polyglutamine proteins such as huntingtin, even with wild type repeat lengths, bind to and inhibit the acetyltransferase function of histone acetylases led to the testing of genetic and pharmacological interventions to maintain acetylation levels by reducing histone deacetylase (HDAC) activities; these have proven to be effective strategies in suppressing polyQ-induced toxicity in cellular and fly models (Agrawal et al., 2005; Pallos et al., 2008; Steffan et al., 2001). Specifically, the HDAC Rpd3 was found as a common modifier by two different groups (Fernandez-Funez et al., 2000; Pallos et al., 2008), and the HDAC co-activator Sin3a was also found as a common modifier by the Marsh and Botas groups as well as independently by a third group (Bilen and Bonini, 2007). Beyond histone acetylation, HDAC6 has been shown to promote autophagy, which can specifically degrade polyQ proteins (Pandey et al., 2007). Other regulators of autophagy, specifically phosphoinositide-dependent kinase-1 (PDK1), p70 ribosomal S6 kinase (S6K) (Nelson et al., 2005), and endosomal sorting complex required for transport (ESCRT) complexes (Rusten et al., 2007) have also been shown to modify polyQ toxicity, suggesting that autophagy, in addition to the UPS, has a significant role in degrading toxic proteins or aggregates.
Although most research has focused on toxicity due to polyQ proteins, some new and exciting work has shown that CUG or CAG codon expansions in mRNA can themselves confer neurotoxicity without the need for translation, and that increased repeat length correlates with increased neurodegeneration (Le Mee et al., 2008; Li et al., 2008; Mutsuddi et al., 2004); these observations provide a framework to understand why many groups have reported RNA binding proteins as strong modifiers of polyQ toxicity (Bilen and Bonini, 2007; Fernandez-Funez et al., 2000; Li et al., 2008; Murata et al., 2008; Mutsuddi et al., 2004; Satterfield and Pallanck, 2006) and to better understand the means by which microRNAs modify polyQ-mediated neurodegeneration (Bilen et al., 2006).
The establishment of these Drosophila models of polyglutamine diseases has also created fertile ground for testing pharmacological therapeutics. Many chemical compounds targeted to reducing polyQ protein aggregation have been identified that also alleviate neurodegenerative phenotypes in Drosophila (Kazantsev, 2002; Nagai et al., 2003; Pollitt et al., 2003; Zhang et al., 2005). Also, the use of HDAC inhibitors, either alone or in combination with aggregation inhibitors, has shown great promise (Agrawal et al., 2005; Ehrnhoefer et al., 2006; Pallos et al., 2008; Steffan et al., 2001). Other novel therapeutics have also been identified in Drosophila models, such as the use of intracellular antibodies (or “intrabodies”) that target protein aggregates and have been shown to strongly ameliorate toxicity and significantly lengthen lifespan (Wolfgang et al., 2005).
Parkinson disease
PD is the second most common neurodegenerative disorder of the central nervous system, affecting an estimated 1 in 50 people over the age of 60 years. PD is the result of loss of a majority of DA neurons in the midbrain substantia nigra. Another pathological hallmark of PD is the presence of cytoplasmic inclusions called Lewy bodies, comprised primarily of α-synuclein, in the surviving DA neurons. Rare familial cases have been attributed to mutations in genes including SNCA (Polymeropoulos et al., 1997), PARK2 (Kitada et al., 1998), DJ-1 (Bonifati et al., 2003) PINK1, (Valente et al., 2004) and LRRK2 (Paisan-Ruiz et al., 2004). The six familial Parkinson disease genes that have been cloned are α-synuclein, parkin, ubiquitin C-terminal hydrolase–1 (Uchl-1), DJ-1, phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1), and leucine-rich repeat kinase 2 (LRRK2). Of these, α-synuclein and parkin have been most extensively studied.
α-Synuclein
Synucleins make up a group of small soluble proteins predominantly found in neural tissues in addition to certain forms of tumors. The group is comprised of three known proteins: α-, β- and γ-. Amongst these, α-synuclein has been shown to be involved in PD pathogenesis. Missense mutations A53T (Polymeropoulos et al., 1997) and A30P (Kruger et al., 1998), as well as genomic duplication and triplication of α-synuclein gene (Ibanez et al., 2004; Singleton et al., 2003) have been identified as causes of autosomal dominant familial PD; E46K is likely associated with dementia with Lewy bodies and parkinsonism (Zarranz et al., 2004). In vitro studies have shown that α-synuclein can self aggregate and form fibrils, similar to observations with Aβ in AD. All these findings provided impetus for the development of reliable animal models that recapitulate pathophysiological features of PD and thus contribute to deciphering of underlying molecular mechanisms.
The first fly PD model was reported by Feany, who expressed a transgene encoding human α-synuclein in all Drosophila neurons (Feany and Bender, 2000). This resulted in an age-dependent loss of DA neurons; other neuronal polulations, e.g., serotoninergic, remained largely unaffected. Death of DA neurons was observed upon expression of both wild type and mutant α–synuclein. The authors also showed that some of the DA neurons accumulated intracellular aggregates closely resembling the Lewy bodies found in the Parkinson's disease patients. The inclusions were comprised of α– synuclein filaments 7–10 nm in diameter, comparable to those in human Lewy bodies. In order to identify motor impairments that might mirror those seen in PD, the authors examined negative geotaxis. Young flies overexpressing α-synuclein performed well in this test, but aged transgenic flies frequently fell back to the bottom of the tube. Flies expressing wild-type α-synuclein or the A53T mutant performed similarly to one another in this test, but climbing defects were more severe in flies expressing the A30P mutant. Thus, the fly model clearly replicated many of the characteristic features of PD. The Bonini lab also reported on modifers of α-synuclein in the fly (Auluck and Bonini, 2002; Auluck et al., 2002).
Mardon and coworkers did not find robust phenotypes with alpha-synuclein misexpression (Pesah et al., 2005). One possible explanation for this contradictory result could be that these authors used whole mount immuno analysis with confocal microscopy of fly brain with an antibody against tyrosine hydroxylase (TH), a marker for DA neurons, whereas earlier studies used peroxidase staining in paraffin sections. Whole mount immunohistochemistry coupled with confocal microscopy may yield more reproducible results than paraffin sections, since all DA neurons in the targeted cluster can be viewed simultaneously in a single preparation. Another potential explanation for this contradictory result is that α-synuclein overexpression does not lead to neuronal death per se but rather causes DA dysfunction and thus reduces TH expression. In addition, Mardon and colleagues failed to show any defects in negative geotaxis in animals with pan neuronal α-synuclein expression. These data suggest that DA cell loss or climbing defects observed with the misexpression of α-synuclein may not be fully penetrant under all experimental conditions.
However, work reported by Pallanck and coworkers was able to increase the expression of α-synuclein and demonstrate age-dependent loss of DA neurons in the Drosophila brain (Trinh et al., 2008). Based on observation that several familial forms of parkinsonism result from increased gene dosage of α-synuclein (Ibanez et al., 2004; Singleton et al., 2003), this group tried to augment expression levels of the α-synuclein transgene. First, they generated an α-synuclein expression construct bearing sequence alterations designed to improve the translational efficiency of this cDNA in Drosophila. Second, they maximized α-synuclein protein expression in the fly brain by making use of flies bearing two copies each of the UAS-α-synuclein transgene and the TH-GAL4 driver; these manipulations approximately doubled the abundance of α-synuclein protein relative to flies bearing a single copy of each of these transgenes. This work appears to have succeeded in generating a more robust Drosophila model for studying synucleinopathies.
Various molecular mechanisms have been proposed to underlie the neuropathological effects of α-synuclein. These include posttranslational modifications, mutations that lead to formation of toxic aggregates, ability of the protein to induce oxidative stress, effects on DA homeostasis, and indirect effects via interaction with various other molecules known to cause PD. Fly models have been beneficial in deciphering and validating some of these basic molecular mechanisms and may prove to be useful for designing therapeutic strategies in the near future. Abnormalities of protein aggregation have been implicated as one of the key features underlying pathogenesis of many neurodegenerative diseases, including Parkinson disease (Cookson, 2005; Trojanowski and Lee, 2000). Since α-synuclein aggregates are a major constituent of Lewy bodies, considerable effort has been directed toward investigating the mechanisms that regulate this aggregate formation. Feany and colleagues (Periquet et al., 2007) have shown that the central hydrophobic region of α-synuclein is essential for aggregation of the protein. On the other hand, the carboxy-terminal region acts as an inhibitor or negative regulator of α-synuclein aggregation. The authors reported that misexpressing a truncated form of α-synuclein lacking amino acid residues between position 71–82 lead to neither formation of large aggregates or oligomeric species of α-synuclein nor loss of tyrosine hydroxylase-positive neurons. However, when the carboxy-terminal truncated form of α-synuclein was misexpressed, aggregation into large inclusion bodies, increased accumulation of high molecular weight α-synuclein species, and enhancement of neurotoxicity were observed. The authors speculated that oligomeric α-synuclein aggregates are potentially toxic and do not confer neuroprotection, unlike their larger counterparts.
Another posttranslational modification of α-synuclein implicated in its toxicity is proteolytic cleavage of the protein. Calpain I has been highlighted as a possible player in this arena. Calpain I is a calcium-dependent protease implicated in numerous pathological states including Alzheimer disease and stroke (Vanderklish and Bahr, 2000). α-synuclein is a substrate for calpain cleavage (Mishizen-Eberz et al., 2003; Mishizen-Eberz et al., 2005), and calpain-cleaved α-synuclein species are similar in molecular weight to truncated α-synuclein fragments that promote its aggregation and enhance cellular toxicity (Li et al., 2005; Murray et al., 2003; Serpell et al., 2000). Rohn, Feany, and coworkers (Dufty et al., 2007) have shown the presence of calpain-cleaved α-synuclein in the fly brain by using site-directed calpain-cleavage antibodies to α- synuclein.
Several post-translational modifications of α-synuclein are prevalent in Lewy bodies, including phosphorylation at serine 129 (Fujiwara et al., 2002), nitrosylation at tyrosine residues (Giasson et al., 2000), and carboxy terminal truncation (Li et al., 2005). These post-translational modifications have been speculated to regulate the aggregation and toxicity of α-synuclein. To assess the role of phosphorylation of serine129 in α-synuclein toxicity and inclusion formation, Chen and Feany (Chen and Feany, 2005) performed mutagenesis studies in their Drosophila α-synuclein model. Substitution of serine 129 to nonphosphorylatable alanine completely suppressed DA neuronal loss caused by human α-synuclein misexpression, whereas mutating serine 129 to the phosphomimetic aspartate enhanced α-synuclein toxicity. The authors additionally showed that G protein–coupled receptor kinase 2 (Gprk2) phosphorylated Ser129 and enhanced α-synuclein toxicity in vivo. Furthermore, blocking phosphorylation at Ser129 increased aggregate formation. The authors concluded that Ser129 phosphorylation regulates α-synuclein neurotoxicity and inclusion formation (Chen and Feany, 2005).
More recently, Feany and colleagues reported that α-synuclein also is phosphorylated at tyrosine 125 in transgenic Drosophila expressing wild type human α-synuclein (Chen et al., 2009), and that this tyrosine phosphorylation protects from α-synuclein neurotoxicity. In addition, immunoblots showed that levels of soluble oligomeric species of α-synuclein were increased by phosphorylation at serine 129 and decreased by tyrosine 125 phosphorylation. Tyrosine 125 phosphorylation diminished with aging in both humans and flies. Furthermore, the authors found that cortical tissue from patients with the PD–related synucleinopathy, dementia with Lewy bodies, showed less phosphorylation at tyrosine 125 (Chen et al., 2009). These data suggest that α-synuclein neurotoxicity in PD may result from an imbalance between the detrimental, oligomer-promoting effect of serine 129 phosphorylation and a neuroprotective action of tyrosine 125 phosphorylation that inhibits toxic oligomer formation. The fly alpha-synuclein models, then, have been helpful in recapitulating various aspects of alpha- synuclein pathology and elucidating its underlying mechanisms.
Parkin
Mutations in parkin are the second most common genetic cause of PD, after LRRK2/dardarin (Foroud et al., 2003; Kitada et al., 1998; Klein et al., 2003) (Gilks et al., 2005; Hedrich et al., 2004; Hernandez et al., 2005; Lincoln et al., 2003). Parkin mutations originally were identified in families with autosomal recessive juvenile parkinsonism (AR-JP) (Kitada et al., 1998). Cell culture and in vitro studies have indicated that parkin functions as an E3 ligase (Giasson and Lee, 2001; Giasson and Lee, 2003; Hattori and Mizuno, 2004; Moore et al., 2005), although additional roles in microtubule-based transport (Ren et al., 2003) and regulation of DA transporter activity have been suggested (Jiang et al., 2004). It is generally thought that the loss of E3 ligase activity is involved in the pathogenesis of parkin-linked PD.
In order to understand the role of the Drosophila parkin gene, parkin null mutant flies were generated by various groups (Cha et al., 2005; Greene et al., 2003; Pesah et al., 2004). The characteristic features of these mutant flies were mitochondrial pathology and degeneration of indirect flight muscle (Figure 2). Mutants also displayed reduced life span, male sterility, and hypersensitivity to oxidative stress. Initial analysis failed to find loss of DA neurons. However, in a subsequent study, whole mount confocal analysis reported DA neuron loss (Whitworth et al., 2005).
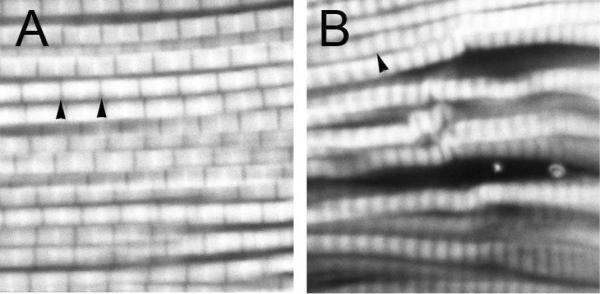
Fig. 2.
Indirect flight muscle degeneration in dparkin mutants. Confocal images of phalloidin-stained muscle preps. A, control muscle shows normal organization of sarcomeres. Arrowheads, normal Z bands. B, parkin null mutant shows irregularly sized and spaced sarcomere. Adapted from (Sang et al., 2007).
Lu and colleagues used a different approach to create a parkin loss of function phenotype (Yang et al., 2003). They created transgenic RNAi against parkin, which also enabled them to attain tissue and cell specific gene knockdown. Targeted expression of parkin double-stranded RNA in Drosophila DA neurons also did not result in significant loss of DA neurons. Lu and coworkers did find that targeted overexpression of human parkin-associated endothelin like receptor (Pael-R), a parkin substrate (Imai et al., 2001), resulted in a reduction of TH immunoreactive DA neurons; this phenotype was exacerbated in the parkin RNAi background (Yang et al., 2003). Conversely, overexpression of human parkin suppressed Pael-R-induced toxicity. These in vivo genetic interaction studies confirmed the biochemical relationship between parkin and Pael-R and indicated that accumulation of abnormal Pael-R protein in parkin-deficient DA neurons might be one of the causes of neuronal death. These results provided the basis for other work in mice, where it was shown that Pael-R induces degeneration of DA neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity; Pael-R toxicity is enhanced under parkin inactivation conditions (Kitao et al., 2007). Hence, Drosophila models can obtain results similar to those derived from vertebrate systems and point the way toward appropriate mouse work to elucidate molecular mechanisms underlying PD.
Our laboratory generated another fly PD model by showing that expression of mutant but not wild type human parkin in Drosophila causes age-dependent, selective degeneration of DA neurons accompanied by a progressive motor impairment (Sang et al., 2007). We generated transgenic lines expressing two mutant forms of human parkin derived from familial PD, Gln311Stop (Q311X) and Thr240Arg (T240R) (Shimura et al., 2000). Both mutant forms showed age-dependent neurodegeneration and neuronal dysfunction in younger animals. These data suggested a possible dominant mechanism underlying the pathological phenotypes caused by mutant parkin in Drosophila and argued against any dominant negative effect. We also have shown that overexpression or knockdown of the Drosophila vesicular monoamine transporter, which regulates cytosolic DA homeostasis, partially rescues or exacerbates, respectively, the degenerative phenotypes caused by mutant human parkin. These results support a model in which the vulnerability of DA neurons to parkin-induced neurotoxicity results from the interaction of mutant parkin with cytoplasmic dopamine. Lim and colleagues reported that the R275W but not the G328E mutant also produced dominant toxicity in the fly (Wang et al., 2007). Recently we also demonstrated dominant toxicity of truncated parkin using a BAC mouse model (Lu et al., 2009a).
There are examples of protective roles of parkin in PD models. Parkin and α- synuclein may functionally interact in the disease process. Parkin has been reported to colocalize with α-synuclein in Lewy bodies, and an O-glycosylated form of α-synuclein has been claimed to be serve as a substrate of parkin (Shimura et al., 2001). In cell culture, overexpression of mutant α-synuclein decreases proteasome function, which can be counteracted by overexpression of parkin (Petrucelli et al., 2002). In a rat lentiviral model of PD, overexpression of wild-type rat parkin protected against the toxicity of disease-associated human α-synuclein (Lo Bianco et al., 2004). Animals expressing parkin show significant reductions in α-synuclein-induced neuropathology, leading to preservation of TH-positive cell bodies in the substantia nigra and to sparing of TH-positive nerve terminals in the striatum (Lo Bianco et al., 2004). Additionally, parkin-mediated neuroprotection has been associated with an increase of hyperphosphorylated α-synuclein inclusions, supporting a role for parkin in the genesis of Lewy bodies and implicating an intriguing neuroprotective role for α-synuclein inclusion formation in the disease process (Lo Bianco et al., 2004). In Drosophila, Lu and coworkers reported that overexpression of parkin has reported to suppress α-synuclein induced degeneration of DA neurons (Yang et al., 2003). This suppression was not associated with significant changes in total cellular α-synuclein levels. The authors speculated that the toxic species of α-synuclein, if targeted by parkin, may represent only a small portion of total α-synuclein protein. Alternatively, they suggest that parkin suppression of α-synuclein toxicity may be mediated through a mechanism unrelated to degradation by the ubiquitin-proteasome pathway. Another group also reported protective effects of parkin against α-synuclein toxicity in Drosophila using other techniques, i.e., negative geotaxis and retinal phenotypes (Haywood and Staveley, 2004). In summary, then both loss of function and gain of function studies in the fly have supplied some valuable and surprising information regarding potential disease mechanisms.
PINK1
One of the candidates targeted to understand mechanisms underlying mitochondrial dysfunction in pathological hallmarks of PD is PINK1. PINK1 is a putative serine/threonine kinase with a mitochondrial targeting sequence (Valente et al., 2004). The Drosophila PINK1 gene encodes a polypeptide of 721 amino acids with two characteristic motifs: a mitochondrial targeting motif and a serine/threonine kinase domain, like its human PINK1 counterpart (Clark et al., 2006; Park et al., 2006; Yang et al., 2006). The kinase domain exhibits 60% similarity (42% identity) to that of human PINK1. Consistent with the localization of human PINK1, Drosophila PINK1 also is localized in mitochondria.
Two different approaches were used to generate Drosophila PINK1 models: 1) transposon-mediated mutagenesis, and 2) RNAi. Guo and coworkers (Clark et al., 2006) observed phenotypes inclduing male sterility, apoptotic muscle degeneration, increased sensitivity to oxidative stress and fragmentation of mitochondrial cristae in the dPINK1 mutants. In addition to these phenotypes, another group found that dPink1 mutants undergo DA neuronal degeneration accompanied by defects in locomotion (Park et al., 2006). Furthermore, transmission electron microscopy analysis and a rescue experiment with Drosophila Bcl-2 suggested that mitochondrial dysfunction accounts for the degenerative phenotypes of dPINK1 mutants (Park et al., 2006). Using transgenic RNAi, Lu and coworkers demonstrated that inhibition of dPINK1 function results in energy depletion, shortened life span, and degeneration of both indirect flight muscles and select DA neurons (Yang et al., 2006). The muscle pathology was preceded by mitochondrial enlargement and disintegration. These phenotypes were rescued by wild type but not by a truncated form of human PINK1, indicating functional conservation between fly and human PINK1 (Yang et al., 2006). Another independent study (Wang et al., 2006) using the RNAi approach showed that inactivation of dPINK1 resulted in progressive loss of DA and photoreceptor neurons, which could be rescued by expression of human PINK1. They also showed that expression of human SOD1 and treatment with the antioxidants SOD and vitamin E could significantly inhibit photoreceptor degeneration in dPINK1 RNAi flies (Wang et al., 2006). All these studies strongly implicated mitochondrial dysfunction and oxidative stress in PINK1 pathogenesis.
Similarity between the dPINK1 and the dParkin phenotypes with regard to defects in male fertility, mitochondrial morphology, indirect flight muscle, and DA neuron survival led investigators to investigate the genetic epistatic relationship between PINK1 and parkin (Clark et al., 2006; Park et al., 2006). Overexpression of parkin rescued the male sterility and mitochondrial morphology defects of dPINK1 mutants, whereas double mutants lacking both Pink1 and Parkin function showed phenotypes identical to those observed in either mutant alone, i.e., mutant phenotypes were not additive. Overexpression of PINK1 had no effect on dParkin mutant phenotypes. These observations suggested that PINK1 and parkin function in the same pathway, with PINK1 acting upstream of parkin.
The presence of prominent mitochondrial morphological defects in dPINK1 and dParkin mutants prompted investigators to examine the mitochondrial fission/fusion pathway. This pathway is a conserved mitochondria remodeling process that controls the dynamic distribution and morphology of mitochondria in all eukaryotes (Chan, 2006). Mitochondrial fission/fusion has been shown to be important for regulating synaptic structure and plasticity (Li et al., 2004), and imbalance of mitochondrial fission/fusion can lead to neurodegeneration (Bossy-Wetzel et al., 2003). Pallanck and colleagues have suggested that the PINK1/Parkin pathway promotes mitochondrial fission and that the loss of mitochondrial and tissue integrity in PINK1 and parkin mutants results from reduced mitochondrial fission (Poole et al., 2008). They have shown that heterozygous loss of function mutations of drp1, which encodes the Dynamin related protein I, a key mitochondrial fission-promoting component (Frank et al., 2001; Hoppins et al., 2007), are largely lethal in a PINK1 or parkin mutant background (Poole et al., 2008). Conversely, the flight muscle degeneration and mitochondrial morphological alterations that result from mutations in PINK1 and parkin are strongly suppressed by increased drp1 gene dosage and by heterozygous loss-of-function mutations affecting the mitochondrial fusion-promoting factors OPA1 (Optic atrophy 1, a GTPase) and Mfn2 (Mitofusin 2). Finally, they found that the eye morphology phenotype associated with misexpression of PINK1 is suppressed by perturbations that reduce mitochondrial fission and enhanced by perturbations that reduce mitochondrial fusion (Poole et al., 2008).
Additionally, Yang and coworkers (Yang et al., 2008) reported that the PINK1/parkin interaction with mitochondrial fusion and fission occurs not only in non-neuronal tissues but also in dopaminergic neurons. Together, these findings illustrate a role of the PINK1/parkin pathway promoting fission and/or inhibiting fusion in Drosophila muscle and neuronal mitochondria. However, these findings also point out discrepancies between studies in flies and some mammalian cell models. siRNA-knockdown of PINK1 in HeLa cells was observed to increase mitochondrial fragmentation, or presumably promote fission (Exner et al., 2007). Similar results were observed in neuroblastoma cells with siRNA knockdown of PINK1 and parkin (Park et al., 2009) and in primary cells cultures from patients carrying PINK1 mutations (Exner et al., 2007; Wood-Kaczmar et al., 2008). Similarly, Chu and coworkers reported that PINK1 expression in SH-SY5Y cells increased interconnectivity of mitochondria, while PINK1 RNAi knockdown or expression of kinase-inactive PINK1 increased mitochondrial fragmentation (Dagda et al., 2009). These data suggest that the PINK1/parkin pathway can promote fusion and/or inhibit fission in mammalian cells, the opposite of the findings from Drosophila models. In contrast, Cookson, Bandmann, and coworkers demonstrated that cultured fibroblasts from patients carrying parkin mutations exhibited mitochondrial morphological abnormalities, being longer and more branched than controls (Mortiboys et al., 2008). In addition, Lu and colleagues found that overexpression of PINK1 in COS-7 cells resulted in punctate mitochondria, whereas suppression of PINK1 with shRNA resulted in long, tubular mitochondria, a phenotype inhibited by the overexpression of the fission proteins hFis1 or Drp1 (Yang et al., 2008). Thus, these mammalian cell models generally though not in all cases support the findings from the Drosophila models. Although mitochondrial morphology alone does not necessarily predict the expected effects on fission and fusion (Berman et al., 2009), there is a need of further investigation to resolve these conflicts. The possibility that PINK1/parkin effects may differ depending on the cell type and/or cellular conditions cannot be ruled out considering the fact that mitochondrial fission-fusion is a dynamic and interrelated regulatory process.
Additional work by de Strooper and colleagues suggests that in Drosophila neurons, PINK1 deficiency affects synaptic function, as the reserve pool of synaptic vesicles is not mobilized during rapid stimulation (Morais et al., 2009). This deficit could be rescued by adding ATP to the synapse and henceforth suggested a possible role of PINK1 in energy maintenance under severe conditions. In parallel work in a mouse model, this group reported that human wild type PINK1, but not PINK1 containing disease-associated mutations, can rescue complex I deficiency. Finally, they concluded that PD mutations in PINK1 result in decreased complex I activity and deficient synaptic function and that this may be yet another mechanism underlying PINK1-mediated mitochondrial dysfunction in PD (Morais et al., 2009).
DJ-1
Mutations in the DJ-1 gene are a rare cause of PD (Bonifati et al., 2003). Investigators have tried to develop model organisms that recapitulate DJ-1-linked parkinsonism by generating homozygous null alleles for DJ-1. There are two DJ-1 homologs in Drosophila, DJ-1A and DJ-1B. DJ-1A is a closer homolog of human DJ-1 than DJ-1B. Bonini, Pallanck, and coworkers developed DJ-1A and DJ-1B double-knockout flies that displayed normal viability and life span; however, they displayed a selective sensitivity to environmental toxins such as paraquat and rotenone. This sensitivity was speculated to result primarily from loss of DJ-1B protein, which becomes modified upon oxidative stress (Meulener et al., 2005). Min and colleagues reported that DJ-1B loss-of-function mutants were found to have an extended survival of DA neurons and resistance to paraquat; however, they also had an acute sensitivity to hydrogen peroxide treatment. There was also a compensatory upregulation of DJ-1A expression in the brain of the DJ-1B mutant, suggesting that overexpression of DJ-1A in DA neurons may be sufficient to confer protection against paraquat (Menzies et al., 2005). The differential sensitivity to paraquat by the DJ-1B-mutant flies in these two studies was speculated to be due to different genomic deletions or genetic backgrounds.
Using the RNAi technique, Lu and colleagues group showed that knocking down DJ-1A in a cell type specific manner resulted in the accumulation of reactive oxygen species (ROS), hypersensitivity to oxidative stress, and dysfunction and degeneration of DA and photoreceptor neurons (Yang et al., 2005). Their data also suggest that DJ- 1A RNAi animals exhibit apparently stronger phenotypes than the DJ-1A or DJ-1B genetic mutants, since they suffer DA and photoreceptor neuron loss and early mortality. The authors speculated possible off-target effects associated with the RNAi approach or the existence of certain compensatory mechanisms associated with genomic deletions. With an objective in mind to decipher genes and pathways that could act as modifiers of the DJ-1-associated neurodegeneration, this group used the DJ-1A RNAi-induced photoreceptor degeneration assay in a candidate screen. Their search led to the isolation of components of the PI3K/Akt signaling pathway as specific genetic modifiers. Reduction of PI3K/Akt signaling enhanced DJ-1A RNAi phenotypes, whereas activation of PI3K/Akt signaling significantly rescued DJ-1A RNAi phenotypes. The modifying effects of PI3K/Akt signaling on DJ-1A RNAi phenotypes were also shown to manifest in DA neurons (Yang et al., 2005).
LRRK2
LRRK2 mutations are likely the most common genetic cause of PD (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). Amongst the large number of LRRK2 mutations that have been identified, the G2019S (Lesage et al., 2006) and the G2385R (Tan and Schapira, 2008) variants appears to be more prevalent. LRRK2 is a large protein (2527 amino acids) comprised of multiple domains, including a GTPase domain and a kinase domain capable of exhibiting GTP-dependent phosphorylation activity (West et al., 2005). In general, disease-associated mutations of LRRK2 have been shown to increase its kinase activity and thereby its toxicity (Smith et al., 2006; West et al., 2007). However, significant variations have been observed in many cases; hence, the exact mechanism by which LRRK2 mutations cause disease is not entirely clear. For example, the I2012T LRRK2 mutant appears to have reduced kinase activity (West et al., 2007), whereas several LRRK2 mutants also exhibit a propensity to aggregate when expressed in cultured cells (Greggio et al., 2006).
To understand the role of LRRK2 mutations in vivo, a number of groups have recently generated Drosophila models of LRRK2 mutant-induced PD. Smith and coworkers showed that over expression of human wild type LRRK2 or the G2019S mutant in flies triggered photoreceptor and DA neuron degeneration (Liu et al., 2008). Similarly, Lu and colleagues observed neurodegeneration in flies expressing Drosophila orthologs of human LRRK2 Y1699C or I2020T mutants, although the degeneration in this case was confined to certain DA neuronal clusters and failed to affect the eye (Imai et al., 2008). In contrast, Chung and colleagues reported that transgenic flies over expressing human wild type or R1441C mutant LRRK2 did not exhibit any significant defects in the tissues examined, including DA neurons and muscles (Lee et al., 2007). Lim and coworkers reported that transgenic flies harboring G2019S, Y1699C, or G2385R LRRK2, but not the wild-type protein, exhibited late onset loss of DA neurons in selected clusters that is accompanied by locomotion defects (Ng et al., 2009). Furthermore, LRRK2 mutant flies also displayed reduced lifespan and increased sensitivity to rotenone, a mitochondrial complex I inhibitor. They also found that coexpression of human parkin in LRRK2 G2019S-expressing flies provided significant protection against DA neurodegeneration that occurred with age or in response to rotenone. Taken together, their results suggested a possible link between LRRK2, parkin, and mitochondria in the pathogenesis of LRRK2-related Parkinsonism.
In summary, the fly models of Parkinson Disease have provided vital information regarding several molecular mechanisms underlying the pathophysiologies of the disease. Posttransaltional modifications like phosphorylation, C-terminal truncation of alpha synuclein and mutations that promote its aggregation have been successfully demonstrated as some of the molecular mechanisms leading to neurotoxicity in the Drosophila model system. Additionally the role of Parkin (E3 Ubiquitin Ligase) in PD has been modeled in the flies. The findings that Parkin could interact with one of its substrate, PaelR (parkin-associated endothelin like receptor) in mediating neuroprotection has paved roads for the design of therapeutic strategies to control the onset of the disease. Studies in the flies, supporting a model in which the vulnerability of DA neurons to parkin induced neurotoxicity resulted from the interaction of mutant parkin with cytoplasmic dopamine directs researchers to design therapies to regulate the dopamine homeostatic machinery to control the disease. The neuroprotective role of Parkin against alpha synuclein mediated neurotoxicity can be further exploited for therapeutic interventions. Studies in Drosophila, strongly implicated mitochondrial dysfunction and oxidative stress in PINK1 pathogenesis. Additionally, the involvement of Pink1- Parkin interaction in regulating mitochondrial fission-fussion process further lay foundations of designing ways to regulate the mitochondrial dysfunction as a means to cure the disease. The modifying effects of PI3K/Akt signaling on DJ-1A RNAi phenotypes in dopaminergic neurons also suggests that modulating certaining signaling cascades could aid in ameliorating the neurotoxicity. Finally studies in flies have also suggested a possible link between LRRK2, parkin, and mitochondria in the pathogenesis of LRRK2-related Parkinsonism thereby establishing a multifactorial cascade in triggering the disease. This aids in directing researchers to design therapeutics which can target multiple factors to increase efficacy of the drugs to combat the onset of the disease.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) primarily affects upper motor neurons and is the prototypical motoneuron disease. About 10% of cases are familial. Superoxide dismutase (SOD) mutations were one of the earliest genetic factors found causing neurodegenerative disease, and neuronal inclusions of SOD can be found in ALS cases, suggesting oxidative stress and blockage of axonal transport as major factors in the disease (Wood et al., 2003). Although much research has been done on SOD function, oxidative stress, and longevity assays, much less work has been done on the neuronal (or glial) specific toxic action of SOD gene and protein on motor neuron diseases. Early studies with null alleles of the Drosophila homologue showed reduced longevity and increased susceptibility to oxidative stress (Phillips et al., 1989 PNAS). Later studies expressing either mutant or wild-type Drosophila or human SOD also showed neuronal toxicity and increased aggregation (Phillips et al., 1989; Watson et al., 2008). An intriguing finding from both studies was that aberrant forms of SOD were able to sequester wild type protein into aggregates, and that even functional orthologous but foreign SOD (i.e., human SOD) may negatively sequester endogenous wild type protein, suggesting a high sensitivity of SOD to conformational change.
A more recent mutation linked to ALS was identified in the locus ALS8, which encodes “vesicle-associated membrane protein/synaptobrevin-associated membrane protein B,” more conveniently referred to as “VAPB”. Three independent models of VAPB-mediated neurodegeneration in Drosophila were established and reported within months of one other (Chai et al., 2007; Ratnaparkhi et al., 2008; Tsuda et al., 2008). All three studies employed wild-type and disease-associated mutant VAPB, and all three demonstrated an increased propensity of the mutant VAPB to aggregate or form inclusions. The mutant VAPB recruits wild-type protein. Bellen and coworkers reported that VAPB is cleaved and secreted; however, mutant VAPB fails to be secreted and induces ER stress, initiating the upfolded protein reponse (UPR) (Tsuda et al., 2008). Synaptic bouton morphology defects with impairment of transmission have been identified (Chai et al., 2007; Ratnaparkhi et al., 2008), and signaling pathways involving bone morphogenetic protein (BMP)(Ratnaparkhi et al., 2008) or ephrin (Tsuda et al., 2008) have also been implicated in pathological phenotypes in the models. These studies provide novel insights into VAPB function and possible mechanisms that can lead to motor neuronal degeneration. We found that increased expression of wild type VAPB in sensory neurons lead to loss of notal bristles on the dorsal-posterior thorax, and that bristles could be restored when wild-type VAPB was inhibited (Ratnaparkhi et al., 2008). The establishment of an external, neuronal-associated phenotype may aid in genetic or pharmacological screens to further identify modifiers of VAPB-mediated neurodegeneration.
In 2008, several independent studies identified TARDBP as a novel gene linked to both familial and sporadic ALS. This gene encodes for the heterogeneous nuclear ribonuclear protein (hnRNP) Transactive Response-DNA Binding Protein, with a molecular weight of approximately 43 kDa, otherwise known as “TDP-43” (Gitcho et al., 2008; Kabashi et al., 2008; Sreedharan et al., 2008; Van Deerlin et al., 2008). This was a remarkable discovery, as just two years earlier in 2006, ubiquitin-positive/tau-negative inclusions in neurons from patients with frontotemporal lobar degeneration (FTLD-U) were shown be TDP-43 immunoreactive in both familial and sporadic cases (Neumann et al., 2006). These findings have led to an explosion of research since they were initially reported, with the majority of studies characterizing pathologically suspected protein modifications, such as cleavage, phosphorylation, aggregation, and translocation from nucleus to cytosol (Gendron et al.,; Kwong et al., 2007). TDP-43 is highly conserved between humans, mice, Drosophila, and Caenorhabditis elegans, with orthologous function in DNA binding and regulation of splicing (Ayala et al., 2005; Wang et al., 2004). The first genetic models of TDP-43 mediated toxicity were first reported in yeast (Johnson et al., 2008), and in transfected mouse primary neuronal hippocampal cultures and human embryonic kidney cells (Winton et al., 2008), with both reports demonstrating cytosolic translocation from the nucleus and increased propensity of mutant TDP-43 to aggregate. Genetic models with Drosophila followed shortly (Feiguin et al., 2009; Hanson et al.,; Li et al.,; Lu et al., 2009b), along with the first mouse models reported in late 2009/early 2010 (Kraemer et al.,; Sephton et al.,; Wegorzewska et al., 2009; Wils et al.).
In Drosophila, investigators have examined phenotypes associated with manipulation of both endogenous Drosophila TDP-43 and transgenic expression of human TDP-43 (hTDP-43)/TBPH. dTDP and hTDP-43 both have two RNA recognition motifs (RRMs) and a glycine rich region (GRR) toward the amino-terminus, but the carboxy terminal domain is longer in dTDP than in full length wild type hTDP-43. Thus, dTDP has 531 amino acids versus 414 amino acids for hTDP-43, and so runs at a higher molecular weight. Independent antibodies for dTDP targeted toward the amino-terminal region (Feiguin et al., 2009; Lu et al., 2009b) showed strong signals at a molecular weight just above 55 kDa; signals were not present or minimal as compared with dTDP null alleles created by these groups (genomic deletions created by imprecise P-element excisions used by Feiguin and coworkers, and a point mutation introducing a stop codon – Q367X - used by Gao et al. RNAi lines available from the Vienna Drosophila RNAi Center were also used (Feiguin et al., 2009; Li et al.,; Lu et al., 2009b). The major findings of these four studies were largely consistent with one other. Homozygous null dTDP flies, or depletion by ubiquitous RNAi expression, led to larval lethality. Flies heterozygous for the mutation appear functionally similar to controls. Thus, dTDP is essential for development to adulthood and survival. Expression of wild-type hTDP-43 in a dTDP mutant background was sufficient to restore survival to adulthood and locomotor functions, and hTDP-43 and dTDP overexpression yielded equivalent phenotypes, demonstrating functional conservation between human and Drosophila TDP-43. However, overexpression of hTDP-43 in a dTDP wild-type background led to larval lethality and motor impairments, similar to mutant dTDP null phenotypes. Additionally, loss-of-function alleles of dTDP and hTDP-43 overexpression yielded similar phenotypes with regard to (a) reduced number of axonal branches, (b) number of synaptic boutons, and (c) calibre of neuritic processes (axons and dendrites). Interestingly, overexpression of dTDP or hTDP-43 promote dendritic branching in sensory neurons (Lu et al., 2009b), suggesting sub-cellular differences in TDP-43 function in axons and dendrites.
The role of aggregation, cellular localization, and differential effects of mutant hTDP-43 isoforms in neurotoxicty, however, is still unclear. Gao and coworkers (Lu et al., 2009b) overexpressed disease-associated mutant hTDP-43 isoforms (Q331K and M337V), but found increased dendritic branching similar to those seen with wild type hTDP-43; however, no other measurements such as longevity, locomotive behaviors, or protein localization or aggregation were reported; thus, the effect of such mutations in vivo remain to be fully elucidated. Two of these studies (Li et al.,; Lu et al., 2009b) used carboxy terminal fragments that maintained the glycine rich region (GGR) but lacked the RNA recognition motifs (RRMs); neither found toxicity due to this fragment. Thus, although the cleaved carboxy terminal fragments of TDP-43 are the most abundant in TDP-43-positive inclusions, it is the RRMs that are required for neurotoxicity.
Of the four studies discussed, only two specifically reported aggregation and localization experiments (Hanson et al.,; Li et al.) from neurons that expressed hTDP-43 in vivo, and these obtained different results. Although both groups reported robust degenerative phenotypes, Tibbets and colleagues (Hanson et al.) found most TDP-43 in motor neurons was localized to the nucleus, with minor cytosolic or aggregated/insoluble levels hTDP-43, while Wu and cowrokers (Li et al.) reported axonal inclusions in motor neurons with coincident axonal swelling, as well as appearance of TDP-43 in sarkosyl insoluble material; although most TDP-43 does also appear to be soluble and localized to the nucleus. However, cells expressing TDP-43 have abnormal amounts of highly condensed chromatin within the nucleus (Hanson et al.); this can also be seen in the images from Wu et al. but are not commented upon in that report. Furthermore, clearance of cytosolic and insoluble TDP-43 by the proteosome or autophagy (by co-expression of ubiquilin) failed to rescue degenerative effects, and in fact worsened the degenerative phenotype (Hanson et al.). Together, these data suggest a novel mechanism of early toxicity in the cell that possibly involves chromatin assembly, regulation of transcription and/or splicing. It may be that the nuclear exclusion of TDP-43 seen in the neurons of patients with ALS is due to the cell's defensive mechanism to remove TDP-43 away from its more toxic locus of action in the nucleus.
Concluding remarks
The last decade has seen a dramatic increase in the number of groups using invertebrates to model human neurodegenerative diseases. It is not possible in a review such as this one to provide a truly comprehensive review of this field, but it is hoped that by highlighting key papers on Parkinson disease, polyglutamine disorders, or ALS, this review may inspire those new to this area to use techniques such as those described to begin asking new questions. Some twelve years after we and others first demonstrated that human disease genes could elicit neurodegenerative phenotypes in the fly it is easy to forget that many predicted such efforts would never work; nonetheless, they did. The utility of such work has not turned out to be in monumental discoveries of novel pathways that have led to cures, but rather in incremental steps that have uncovered interactors or suggested ways of examining genetic interactions that have then been examined further in other systems. In this way, invertebrates have proved their worth as part of the neurodegenerative researcher's portfolio.
Acknowledgements
We apologize for not being able to cite all of the excellent work being done in this field. Supported by the NIH and American Health Assistance Foundation. Thanks to Nancy Bonini for the MJD flies, Leslie Thompson and Larry Marsh for Q93 and Q108 flies, and Leo Pallanck for the parkin flies used in the Figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal N, et al. Identification of combinatorial drug regimens for treatment of Huntington's disease using Drosophila. Proc Natl Acad Sci U S A. 2005;102:3777–81. doi: 10.1073/pnas.0500055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ramahi I, et al. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J Biol Chem. 2006;281:26714–24. doi: 10.1074/jbc.M601603200. [DOI] [PubMed] [Google Scholar]
- Al-Ramahi I, et al. dAtaxin-2 mediates expanded Ataxin-1-induced neurodegeneration in a Drosophila model of SCA1. PLoS Genet. 2007;3:e234. doi: 10.1371/journal.pgen.0030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–6. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- Auluck PK, et al. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–8. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Ayala YM, et al. Human, Drosophila, and C.elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–88. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Bae BI, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Berman SB, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–19. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J, Bonini NM. Genome-wide screen for modifiers of ataxin-3 neurodegeneration in Drosophila. PLoS Genet. 2007;3:1950–64. doi: 10.1371/journal.pgen.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J, et al. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006;24:157–63. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–9. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, et al. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–16. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Branco J, et al. Comparative analysis of genetic modifiers in Drosophila points to common and distinct mechanisms of pathogenesis among polyglutamine diseases. Hum Mol Genet. 2008;17:376–90. doi: 10.1093/hmg/ddm315. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cha GH, et al. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc Natl Acad Sci U S A. 2005;102:10345–50. doi: 10.1073/pnas.0500346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai A, et al. hVAPB, the causative gene of a heterogeneous group of motor neuron diseases in humans, is functionally interchangeable with its Drosophila homologue DVAP-33A at the Neuromuscular Junction. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen HK, et al. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–68. doi: 10.1016/s0092-8674(03)00349-0. [DOI] [PubMed] [Google Scholar]
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–63. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Chen L, et al. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119:3257–65. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–6. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Dagda RK, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–55. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci. 2006;26:6985–96. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufty BM, et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–38. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer DE, et al. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum Mol Genet. 2006;15:2743–51. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- Exner N, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–8. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–8. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Feiguin F, et al. Depletion of TDP-43 affects Drosophila motoneurons terminal synapsis and locomotive behavior. FEBS Lett. 2009;583:1586–92. doi: 10.1016/j.febslet.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Fernandez-Funez P, et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408:101–6. doi: 10.1038/35040584. [DOI] [PubMed] [Google Scholar]
- Foroud T, et al. Heterozygosity for a mutation in the parkin gene leads to later onset Parkinson disease. Neurology. 2003;60:796–801. doi: 10.1212/01.wnl.0000049470.00180.07. [DOI] [PubMed] [Google Scholar]
- Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gendron TF, et al. TDP-43: Mechanisms of neurodegeneration. Neuropathol Appl Neurobiol. doi: 10.1111/j.1365-2990.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–9. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Lee VM. Parkin and the molecular pathways of Parkinson's disease. Neuron. 2001;31:885–8. doi: 10.1016/s0896-6273(01)00439-1. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Lee VM. Are ubiquitination pathways central to Parkinson's disease? Cell. 2003;114:1–8. doi: 10.1016/s0092-8674(03)00509-9. [DOI] [PubMed] [Google Scholar]
- Gilks WP, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–6. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- Gitcho MA, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–8. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JC, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–41. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gunawardena S, et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 2003;40:25–40. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- Hanson KA, et al. Ubiquilin modifies toxicity of the 43 kilodalton TAR-DNA binding protein (TDP-43) in a Drosophila model of amyotrophic lateral sclerosis (ALS) J Biol Chem. 2010 doi: 10.1074/jbc.C109.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Mizuno Y. Pathogenetic mechanisms of parkin in Parkinson's disease. Lancet. 2004;364:722–4. doi: 10.1016/S0140-6736(04)16901-8. [DOI] [PubMed] [Google Scholar]
- Haywood AF, Staveley BE. Parkin counteracts symptoms in a Drosophila model of Parkinson's disease. BMC Neurosci. 2004;5:14. doi: 10.1186/1471-2202-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich K, et al. Distribution, type, and origin of Parkin mutations: review and case studies. Mov Disord. 2004;19:1146–57. doi: 10.1002/mds.20234. [DOI] [PubMed] [Google Scholar]
- Hernandez D, et al. The dardarin G 2019 S mutation is a common cause of Parkinson's disease but not other neurodegenerative diseases. Neurosci Lett. 2005;389:137–9. doi: 10.1016/j.neulet.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Higashiyama H, et al. Identification of ter94, Drosophila VCP, as a modulator of polyglutamine-induced neurodegeneration. Cell Death Differ. 2002;9:264–73. doi: 10.1038/sj.cdd.4400955. [DOI] [PubMed] [Google Scholar]
- Hoppins S, et al. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–80. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Ibanez P, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–71. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Imai Y, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–43. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, et al. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Jackson GR, et al. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron. 1998;21:633–42. doi: 10.1016/s0896-6273(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Jackson GR, et al. Human wild-type tau Interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34:509–19. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, et al. Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem. 2004;279:54380–6. doi: 10.1074/jbc.M409282200. [DOI] [PubMed] [Google Scholar]
- Johnson BS, et al. A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2008;105:6439–44. doi: 10.1073/pnas.0802082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–4. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kazantsev A, Walker HA, Slepko N, Bear JE, Preisinger E, Steffan JS, Zhu Y-Z, Gertler FB, Housman DE, Marsh JL, Thompson LM. A bivalent Huntingtin binding peptide suppresses polyglutamine aggregation and pathogenesis in Drosophila. Nature Genetics. 2002 doi: 10.1038/ng864. [DOI] [PubMed] [Google Scholar]
- Kazemi-Esfarjani P, Benzer S. Genetic suppression of polyglutamine toxicity in Drosophila. Science. 2000;287:1837–40. doi: 10.1126/science.287.5459.1837. [DOI] [PubMed] [Google Scholar]
- Kim YT, et al. Expression of expanded polyglutamine protein induces behavioral changes in Drosophila (polyglutamine-induced changes in Drosophila) Cell Mol Neurobiol. 2004;24:109–22. doi: 10.1023/B:CEMN.0000012716.14075.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitao Y, et al. Pael receptor induces death of dopaminergic neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity, which is enhanced under condition of parkin inactivation. Hum Mol Genet. 2007;16:50–60. doi: 10.1093/hmg/ddl439. [DOI] [PubMed] [Google Scholar]
- Klein C, et al. Frequency of parkin mutations in late-onset Parkinson's disease. Ann Neurol. 2003;54:415–6. doi: 10.1002/ana.10737. author reply 416–7. [DOI] [PubMed] [Google Scholar]
- Klement IA, et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice [see comments] Cell. 1998;95:41–53. doi: 10.1016/s0092-8674(00)81781-x. [DOI] [PubMed] [Google Scholar]
- Kraemer BC, et al. Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 119:409–19. doi: 10.1007/s00401-010-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, et al. Glial and neuronal expression of polyglutamine proteins induce behavioral changes and aggregate formation in Drosophila. Glia. 2005;49:59–72. doi: 10.1002/glia.20098. [DOI] [PubMed] [Google Scholar]
- Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kwong LK, et al. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114:63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- Le Mee G, et al. Repeat length and RNA expression level are not primary determinants in CUG expansion toxicity in Drosophila models. PLoS One. 2008;3:e1466. doi: 10.1371/journal.pone.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, et al. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem Biophys Res Commun. 2007;358:534–9. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- Lesage S, et al. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N Engl J Med. 2006;354:422–3. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- Lessing D, Bonini NM. Polyglutamine genes interact to modulate the severity and progression of neurodegeneration in Drosophila. PLoS Biol. 2008;6:e29. doi: 10.1371/journal.pbio.0060029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet. 2009;10:359–70. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, et al. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008;453:1107–11. doi: 10.1038/nature06909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–7. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci U S A. 2010;107:3169–74. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lincoln SJ, et al. Parkin variants in North American Parkinson's disease: cases and controls. Mov Disord. 2003;18:1306–11. doi: 10.1002/mds.10601. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2693–8. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:17510–5. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XH, et al. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci. 2009a;29:1962–76. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, et al. Frontotemporal dementia and amyotrophic lateral sclerosis-associated disease protein TDP-43 promotes dendritic branching. Mol Brain. 2009b;2:30. doi: 10.1186/1756-6606-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JL, et al. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet. 2000;9:13–25. doi: 10.1093/hmg/9.1.13. [DOI] [PubMed] [Google Scholar]
- Menzies FM, et al. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–82. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Meulener M, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–7. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Mishizen-Eberz AJ, et al. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J Neurochem. 2003;86:836–47. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- Mishizen-Eberz AJ, et al. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005;44:7818–29. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- Moore DJ, et al. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum Mol Genet. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- Morais VA, et al. Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiboys H, et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–65. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, et al. RNA-binding protein hoip accelerates polyQ-induced neurodegeneration in Drosophila. Biosci Biotechnol Biochem. 2008;72:2255–61. doi: 10.1271/bbb.70829. [DOI] [PubMed] [Google Scholar]
- Murray IV, et al. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–40. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- Mutsuddi M, et al. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14:302–8. doi: 10.1016/j.cub.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Nagai Y, et al. Prevention of polyglutamine oligomerization and neurodegeneration by the peptide inhibitor QBP1 in Drosophila. Hum Mol Genet. 2003;12:1253–9. doi: 10.1093/hmg/ddg144. [DOI] [PubMed] [Google Scholar]
- Nelson B, et al. Isolation of gene sets affected specifically by polyglutamine expression: implication of the TOR signaling pathway in neurodegeneration. Cell Death Differ. 2005;12:1115–23. doi: 10.1038/sj.cdd.4401635. [DOI] [PubMed] [Google Scholar]
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Ng CH, et al. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci. 2009;29:11257–62. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Pallos J, et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington's disease. Hum Mol Genet. 2008;17:3767–75. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey UB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Park J, et al. The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem Biophys Res Commun. 2009;378:518–23. doi: 10.1016/j.bbrc.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–61. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Periquet M, et al. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–46. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesah Y, et al. Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of alpha-Synuclein in Drosophila. Genesis. 2005;41:154–9. doi: 10.1002/gene.20106. [DOI] [PubMed] [Google Scholar]
- Pesah Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–94. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13:703–14. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–19. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Phillips JP, et al. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989;86:2761–5. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt SK, et al. A rapid cellular FRET assay of polyglutamine aggregation identifies a novel inhibitor. Neuron. 2003;40:685–94. doi: 10.1016/s0896-6273(03)00697-4. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaparkhi A, et al. A Drosophila model of ALS: human ALS-associated mutation in VAP33A suggests a dominant negative mechanism. PLoS ONE. 2008;3:e2334. doi: 10.1371/journal.pone.0002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, et al. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–24. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, et al. ESCRTing autophagic clearance of aggregating proteins. Autophagy. 2007;4 [Google Scholar]
- Sang TK, et al. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27:981–92. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang TK, Jackson GR. Drosophila models of neurodegenerative disease. NeuroRx. 2005;2:438–46. doi: 10.1602/neurorx.2.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang TK, et al. Inactivation of Drosophila Apaf-1 related killer suppresses formation of polyglutamine aggregates and blocks polyglutamine pathogenesis. Hum Mol Genet. 2005;14:357–72. doi: 10.1093/hmg/ddi032. [DOI] [PubMed] [Google Scholar]
- Satterfield TF, Pallanck LJ. Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Hum Mol Genet. 2006;15:2523–32. doi: 10.1093/hmg/ddl173. [DOI] [PubMed] [Google Scholar]
- Saudou F, et al. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- Scappini E, et al. Intersectin enhances huntingtin aggregation and neurodegeneration through activation of c-Jun-NH2-terminal kinase. Hum Mol Genet. 2007;16:1862–71. doi: 10.1093/hmg/ddm134. [DOI] [PubMed] [Google Scholar]
- Sephton CF, et al. TDP-43 is a developmentally regulated protein essential for early embryonic development. J Biol Chem. 285:6826–34. doi: 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell LC, et al. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci U S A. 2000;97:4897–902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–5. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Shimura H, et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293:263–9. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Slepko N, et al. Normal-repeat-length polyglutamine peptides accelerate aggregation nucleation and cytotoxicity of expanded polyglutamine proteins. Proc Natl Acad Sci U S A. 2006;103:14367–72. doi: 10.1073/pnas.0602348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WW, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–3. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–43. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- Takeyama K, et al. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron. 2002;35:855–64. doi: 10.1016/s0896-6273(02)00875-9. [DOI] [PubMed] [Google Scholar]
- Tamura T, et al. Glial cell lineage expression of mutant ataxin-1 and huntingtin induces developmental and late-onset neuronal pathologies in Drosophila models. PLoS One. 2009;4:e4262. doi: 10.1371/journal.pone.0004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EK, Schapira AH. Uniting Chinese across Asia: the LRRK2 Gly2385Arg risk variant. Eur J Neurol. 2008;15:203–4. doi: 10.1111/j.1468-1331.2007.02053.x. [DOI] [PubMed] [Google Scholar]
- Taylor JP, et al. Aberrant histone acetylation, altered transcription, and retinal degeneration in a Drosophila model of polyglutamine disease are rescued by CREB-binding protein. Genes Dev. 2003;17:1463–8. doi: 10.1101/gad.1087503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K, et al. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson's disease. J Neurosci. 2008;28:465–72. doi: 10.1523/JNEUROSCI.4778-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. “Fatal attractions” of proteins. A comprehensive hypothetical mechanism underlying Alzheimer's disease and other neurodegenerative disorders. Ann N Y Acad Sci. 2000;924:62–7. doi: 10.1111/j.1749-6632.2000.tb05561.x. [DOI] [PubMed] [Google Scholar]
- Tsuda H, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–77. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–60. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–16. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish PW, Bahr BA. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. Int J Exp Pathol. 2000;81:323–39. doi: 10.1111/j.1365-2613.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, et al. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J Neurosci. 2007;27:8563–70. doi: 10.1523/JNEUROSCI.0218-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, et al. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci U S A. 2006;103:13520–5. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, et al. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–9. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- Warrick JM, et al. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- Warrick JM, et al. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol Cell. 2005;18:37–48. doi: 10.1016/j.molcel.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Warrick JM, et al. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell. 1998;93:939–49. doi: 10.1016/s0092-8674(00)81200-3. [DOI] [PubMed] [Google Scholar]
- Watson MR, et al. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J Biol Chem. 2008;283:24972–81. doi: 10.1074/jbc.M804817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegorzewska I, et al. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2009;106:18809–14. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–7. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, et al. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–32. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, et al. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci U S A. 2005;102:8024–9. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils H, et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 107:3858–63. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton MJ, et al. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283:13302–9. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang WJ, et al. Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies. Proc Natl Acad Sci U S A. 2005;102:11563–8. doi: 10.1073/pnas.0505321102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood-Kaczmar A, et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS One. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, et al. Protein aggregation in motor neurone disorders. Neuropathol Appl Neurobiol. 2003;29:529–45. doi: 10.1046/j.0305-1846.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–5. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–8. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–24. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–5. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. A potent small molecule inhibits polyglutamine aggregation in Huntington's disease neurons and suppresses neurodegeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:892–7. doi: 10.1073/pnas.0408936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]