Abstract
The modulation of voltage-dependent calcium channels by hormones and neurotransmitters has important implications for the control of many Ca2+-dependent cellular functions including exocytosis and contractility1–7. We made use of electrophysiological techniques, including whole-cell patch-clamp recordings from dorsal root ganglion (DRG) neurones, to demonstrate a role for GTP-binding proteins (G-proteins) as signal transducers in the noradrenaline- and γ-aminobutyric acid (GABA)-induced inhibition of voltage-dependent calcium channels8–11. This action of the transmitters was blocked by: (1) preincubation of the cells with pertussis toxin (a bacterial exotoxin catalysing ADP-ribosylation of G-proteins12); or (2) intracellular administration of guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), a non-hydrolysable analogue of GDP that competitively inhibits the binding of GTP to G-proteins13. Our findings provide the first direct demonstration of the G-protein-mediated inhibition of voltage-dependent calcium channels by neurotransmitters. This mode of transmitter action may explain the ability of noradrenaline and GABA to presynaptically inhibit Ca2+-dependent neurosecretion from DRG sensory neurones4,5.
Recordings were obtained from primary cultures of embryonic chick DRG cells (see Fig. 1). When bathed in solutions containing 1 mM Ba2+ and 2 mM Ca2+, these cells generate action potentials with a prominent calcium-dependent plateau phase. The plateau results from a regenerative inward current carried by Ba2+ and Ca2+ ions and is blocked by cobalt14. Previous studies have demonstrated that noradrenaline and GABA decrease the duration of these action potentials by inhibiting the depolarization-induced calcium current8,9. In the present study, a saturating concentration of noradrenaline (50 µM) reduced the action-potential duration in 75% of the cells tested by an average of 43 ± 4.7% (Fig. 1a, Table 1). The inhibitory action of noradrenaline was also observed as a decrease in the Ca current recorded from voltage-clamped DRG cells (Fig. 1b). Similar decreases in action-potential duration and Ca current were also observed during application of GABA (Table 1).
Fig. 1.
Noradrenaline and OAG decrease action-potential duration and Ca current in chick DRG cells. a, c, Current-clamp recordings of DRG cell action potentials; b, d, voltage-clamp recordings of Ca currents. Traces were recorded before (1), during (2) and after (3) treatment with either 50 µM noradrenaline (a, b) or 60 µM OAG (c, d). Action potentials were evoked by direct current injection through the recording electrode. Ca currents were evoked by a +50 mV step depolarization from a holding potential of −60 mV. At this holding potential the predominant inward current is through non-inactivating Ca channels. Scale bars: a, 20 mV, 5 ms; b, 4 nA, 40 ms; c, 20 mV, 10 ms; d, 2 nA, 40 ms. Methods. Primary cultures of chick DRG cells were prepared as previously described8,9. Briefly, ganglia from 11–12 day-old embryos were dissociated in a Ca2+- and Mg2+-free Pucks solution, suspended in MEM (supplemented with nerve growth factor, 5% chick embryo extract, 10% horse serum, 2 mM glutamine, penicillin (50 U ml−1) and streptomyocin (50 µg ml−1), γ-irradiated (5,000 rads) and plated in 35-mm collagen-coated tissue culture dishes. Action potentials were recorded using an amplifier with an active bridge circuit allowing current injection through the recording microelectrodes (40–80 MΩ, filled with 2 M KCl). The bathing solution contained (mM): 132 NaCl, 2.5 KCl, 2.0 CaCl2, 1.0 BaCl2, 0.8 MgCl2 and 25 HEPES (pH 7.4). Ca currents were recorded as described previously17, using the whole-cell configuration of the patch-clamp technique18. The pipette solution contained (mM): 150 CsCl, 5 bis(o-aminophenoxy)ethane-N,N,N′,N,-tetraacetic acid (BAPTA), 5 MgATP and 10 HEPES (pH 7.3); the external bathing solution contained 133 NaCl, 1 CaCl2, 10 tetraethylam-monium, 0.3 µM tetrodotoxin and 25 HEPES (pH 7.3). Little or no rundown of the Ca current was observed during the first 10–15 min of recording. Noradrenaline, GABA and OAG (Sigma) were dissolved in the appropriate bathing solution and pressure ejected from blunt-tipped pipettes as previously described9.
Table 1.
Pertussis toxin blocks DRG cell responses to noradrenaline and GABA
| Noradrenaline | GABA | |||
|---|---|---|---|---|
| Cells responding |
Mean decrease in action- potential duration (%) |
Cells responding |
Mean decrease in action- potential duration (%) |
|
| Control | 15/20 | 43 ± 4.7 | 16/19 | 44 ± 4.6 |
| PTX-treated | 2/22 | 24 ± 5.0 | 3/16 | 36 ± 2.5 |
| Vehicle-treated | 9/10 | 45 ± 6.0 | — | — |
DRG cell responses to 50 µM noradrenaline and 50 µM GABA were recorded as a decrease in action-potential duration measured at 1/2 peak spike amplitude. Only reversible and repeatable responses were included in the results. Under the recording conditions used, measurements of decreases in action-potential duration of <10% were considered unreliable and were therefore scored as no effect. PTX was stored at 4 °C as a stock suspension (1.4 mg ml−1) in saturated (NH4)2SO4. The stock suspension was diluted 1:1,000 in 10 mM sodium phosphate buffer (pH 7.2) containing 50 mM NaCl and 0.04% heat-inactivated bovine serum albumin. Freshly diluted PTX was then diluted 10-fold in MEM containing 0.1% glutamine (no horse serum, nerve growth factor or penicillin-streptomyocin added) to give a final dilution factor of 1:10,000 containing 140 ng ml−1 PTX. DRG cell cultures were incubated in 2 ml of 140 ng ml−1 PTX at 37 °C for 4–8 h. Control cultures were incubated at 37 °C for 4–8 h in MEM containing sodium phosphate buffer but with no added PTX or (NH4)2SO4. Vehicle-treated cultures were incubated at 37 °C for 4–8 h in MEM containing saturated (NH4)2SO4 diluted 1:10,000. All results were obtained from cultures of the same plating. Results are expressed as mean ±s.e.m. We did not observe any direct effects of PTX on the electrical properties of the neurones. The mean resting membrane potential, action-potential duration and action-potential amplitude of PTX-treated cells did not differ significantly from that of untreated cells. Furthermore, the mean amplitude of the Ca current recorded from voltage-clamped cells was similar for both PTX-treated and untreated cells.
Pertussis toxin (PTX) blocks G-protein-mediated responses to hormones and neurotransmitters in many cell types12. Specifically, PTX catalyses ADP-ribosylation of G-proteins, thereby preventing agonist-induced dissociation of the proteins into active subunits15,16. When applied to DRG cells, PTX inhibits the transmitter-induced decrease in action-potential duration (Table 1). Following exposure to PTX (140 ng ml−1), only 9 and 19% of the cells responded to noradrenaline and GABA, respectively. Note that the mean percentage decrease in action-potential duration for those PTX-treated cells which did respond to noradrenaline and GABA was also reduced relative to control. The responses recorded from PTX-treated cells were very slow in onset: the maximal decrease in action-potential duration was observed only after continued application of noradrenaline or GABA for 1–2 min. In contrast, noradrenaline and GABA responses recorded from cells untreated with PTX were rapid in onset, reaching a maximal decrease in action-potential duration after only 20–30 s of continual application.
Recordings from voltage-clamped DRG cells demonstrated that, as expected, PTX also blocked the transmitter-induced decrease in Ca current. Before PTX treatment, noradrenaline (10 µM) reduced the Ca current by 35 ± 6.0% in seven of 8 cells tested. After treatment with 140 ng ml−1 PTX, the fraction of cells responding to noradrenaline was reduced to two of eight, and the average decrease in Ca current was only 13 ± 2.4%. The action of PTX seems to be selective for receptor-mediated alterations in Ca channel function: PTX did not block responses to the diacylglycerol analogue 1,2-oleoyl acetylglycerol (OAG), an activator of protein kinase C that mimics the effects of noradrenaline and GABA on DRG cells17. A saturating concentration of OAG (60 µM) decreased the action-potential duration by an average of 37 ± 2.9% in 74% of the cells tested (n = 19) and decreased the Ca current by 38 ± 5.3% in five of five cells tested (Fig. 1c, d). In cultures treated with PTX (140 ng ml−1), the responses to OAG were not attenuated.
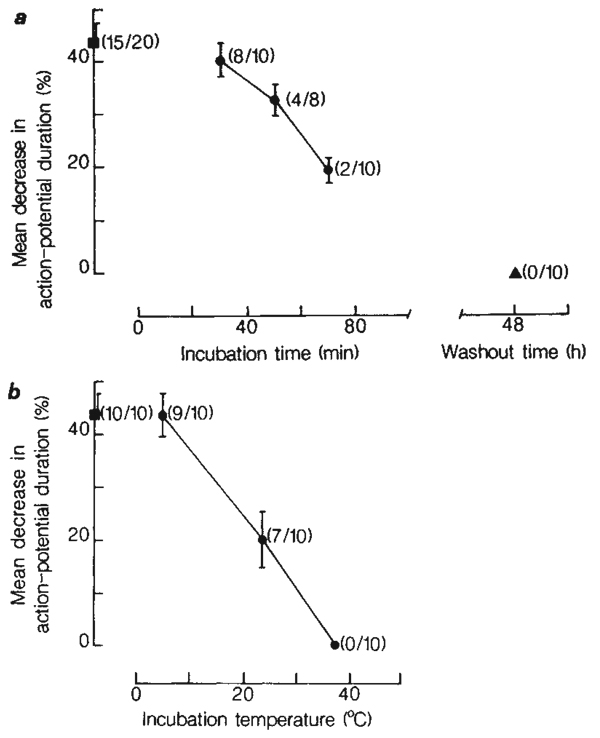
ADP-ribosylation of G-proteins by PTX requires prior internalization and activation of the toxin12. The effects of PTX, therefore, occur with some delay. When PTX was tested on DRG cells, we found its action to be slow in onset and prolonged in duration (Fig. 2a). A progressive decrease in the number of cells responding to noradrenaline was observed after treatment with PTX for 30, 50 and 70 min. A progressive decrease in the magnitude of the response to noradrenaline was also observed. A lag time of ~30 min preceded the inhibitory actions of PTX. To test for recovery, cultures were exposed to 140 ng ml−1 PTX for 4h, washed repeatedly with minimal essential medium (MEM) and re-incubated in culture medium. Even after 48 h of recovery, a total blockade of transmitter action was observed (Fig. 2a).
Fig. 2.
Time- and temperature-dependent blockade by PTX of DRG cell responses to noradrenaline. a, Time dependence of the action of PTX. DRG cell cultures were incubated in MEM containing 140 ng ml−1 PTX at 37 °C for 30, 50 and 70 min (circles). Control cultures (square) were incubated in MEM (without added PTX or (NH4)2SO4) at 37 °C for 1 h. Recovery from PTX-treatment was tested at 48 h following washout of PTX (triangle). The results are expressed as the mean (±s.c.m.) percentage decrease in action-potential duration in response to 50 µM noradrenaline. In parentheses is indicated the fraction of cells tested that responded to noradrenaline. b, Temperature dependence of the action of PTX. Cultures were incubated in MEM containing 140 ng ml−1 PTX for 4 h at 5, 23 and 37 °C (circles). Control cultures were incubated in MEM for 4 h at 5 °C (square). The fraction of cells responding to noradrenaline is also indicated in parentheses. The medium was HEPES-buffered and equilibrated with air when incubating at 5 and 23 °C. All results in a and b were obtained from cultures of the same plating.
As expected for a process of internalization, the action of PTX was temperature-dependent. Incubation of cultures in MEM containing 140 ng ml−1 PTX for 4 h at 5 °C did not diminish the response to noradrenaline (Fig. 2b). When cultures were incubated in PTX for 4 h at 23 °C, the number of cells responding to noradrenaline was reduced, and the magnitude of the response decreased relative to the control response. With an increase in incubation temperature to 37 °C, a total blockade of the action of noradrenaline was observed.
The blockade of noradrenaline and GABA responses by PTX suggests that G-proteins mediate the inhibitory actions of transmitters on neuronal Ca channels. To substantiate such a role for G-proteins, DRG cells were voltage-clamped and loaded with GDP-β-S by the whole-cell recording variation of the patch-clamp technique18. GDP-β-S competes with GTP for the guanine nucleotide binding site on G-proteins, thereby blocking GTP-dependent activation of the proteins by hormones and neurotransmitters19–21. Intracellular dialysis of DRG cells with GDP-β-S (100–500 µM) blocked the noradrenaline-induced decrease in Ca current in a dose-dependent manner (Fig. 3). The blockade of noradrenaline responses was observed using concentrations of GDP-β-S similar to that reported to block adrenergic receptor-mediated inhibition of adenylate cyclase in human platelets20. Additional experiments demonstrated that GDP-β-S does not interfere with the action of OAG on DRG cells. Before exposure to GDP-β-S, OAG reduced the Ca current by 38 ± 5.3% (n = 5), whereas after treatment with 250 µM GDP-β-S, OAG decreased the Ca current by 35 ± 3.0% (n = 5).
Fig. 3.
Blockade by GDP-β-S of DRG cell responses to noradrenaline. DRG cells were dialysed for 5 min with 100 (a), 250 (b) and 500 (c) µM GDP-β-S (trilithium salt, Boehringer Mannheim) by inclusion of the GDP analogue in the patch pipette solution, which allows diffusional exchange between the intracellular fluid contents and the pipette solution. The Ca current was evoked from a holding potential of −60 mV in response to depolarizing test pulses of 50–60 mV. The amplitude of the Ca current was monitored at 10-s intervals. The mean decrease in Ca current in response to noradrenaline (10 µM) is indicated for untreated control cells (unshaded columns) and cells dialysed with GDP-β-S (shaded columns). The responses to noradrenaline obtained from cells treated with GDP-β-S were compared with responses recorded from untreated cells in the same culture dish. All results shown were obtained from cultures of the same plating. Error bars indicate s.e.m. (number of cells is indicated in parentheses). GDP-β-S did not directly inhibit the Ca current, nor did it accelerate run-down of the current: the mean amplitude of the current recorded from 250 µM GDP-β-S-treated and untreated cells was similar after dialysis for 5 min (treated, 2.7 ± 0.3 nA, n = 10; untreated, 3.0 ± 0.4 nA, n = 11). Dialysis of DRG cells with 1.5 mM LiCl (n = 9) did not reduce the response to noradrenaline relative to the control response.
The noradrenaline and GABA receptors mediating inhibition of DRG cell Ca channels are similar to α-2 adrenergic22 and GABA-B23 receptors, two receptor subtypes known to be negatively coupled to adenylate cyclase24,25. The actions of GDP-β-S and PTX on chick DRG cells may therefore result from their ability to block noradrenaline and GABA receptor-mediated activation of the G-protein (Ni) promoting transmitter inhibition of adenylate cyclase15,20,26,27. In this manner, noradrenaline and GABA would inhibit Ca channel function by lowering intracellular concentrations of cyclic AMP. To test this hypothesis, we dialysed DRG cells with solutions containing 5 mM cAMP and 250 µM 3-isobutyl-1-methylxanthine (a phosphodiesterase inhibitor). As previously reported28, cAMP did not attenuate the noradrenaline-induced inhibition of the Ca current (N = 5 cells). These findings indicate that in DRG cells the PTX substrate mediating transmitter inhibition is a G-protein structurally related to Ni, but not directly coupled to adenylate cyclase. One possibility is that this DRG cell G-protein corresponds to the PTX-sensitive N0 α-protein of relative molecular mass 39,000 isolated from bovine brain29,30.
Previous studies support a role for G-proteins in the receptor-mediated activation of membrane phospholipases31–35. It remains to be determined whether a similar G-protein-mediated stimulation of phospholipase activity underlies the inhibitory actions of noradrenaline and GABA on neuronal Ca channels. The best evidence implicating phospholipases in the inhibition of neuronal Ca channels is the demonstration that the protein kinase C activator OAG36 blocks the DRG cell Ca current in a manner similar to that of noradrenaline and GABA17. Alternatively, G-proteins may directly couple transmitter receptors to the Ca channel. Although these points remain to be resolved, our findings clearly indicate that cellular processes controlling Ca homeostasis are influenced by alterations in the structure and function of G-proteins. In terms of neuronal function, therefore, G-proteins are likely to serve as important intermediaries in processes governing receptor-mediated inhibition or facilitation of Ca2+-dependent neurosecretion.
Acknowledgments
We thank Dr Ron Sekura for the gift of PTX and Drs Paul Brehm and Michael Goy for their advice. This work was supported by grants to K.D. from the Klingenstein Foundation and the American Heart Association.
References
- 1.Kupferman I. A. Rev. Neurosci. 1979;2:447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- 2.Shain W, Carpenter DO. Int. Rev. Neurobiol. 1981;22:205–250. doi: 10.1016/s0074-7742(08)60294-9. [DOI] [PubMed] [Google Scholar]
- 3.Dunlap K. In: The Mechanism of Gated Calcium Transport across Biological Membranes. Ohnishi ST, Endo M, editors. New York: Academic; 1981. pp. 87–97. [Google Scholar]
- 4.Fischbach GD, Dunlap K, Mudge AW, Leeman S. In: Neurosecretion and Brain Peptides. Martin JB, Reichlin S, Bick KL, editors. New York: Raven; 1981. pp. 175–188. [Google Scholar]
- 5.Holz GG, Kream RM, Dunlap K. Soc. Neurosci Abstr. 1985;11:126. [Google Scholar]
- 6.Holz GG, Shefner SA, Anderson EG. J. Neurosci. (in the press) [Google Scholar]
- 7.Reuter H, Scholz H. J. Physiol. Lond. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlap K, Fischbach G. J. Physiol., Lond. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlap K, Fischbach G. Nature. 1978;276:837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- 10.Houslay MD. Trends biochem. Sci. 1984;9:39–40. [Google Scholar]
- 11.Gilman AG. Cell. 1984;36:577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- 12.Ui M. Trends pharmac. Sci. 1984;5:277–279. [Google Scholar]
- 13.Eckstein F, Cassel D, Levkovitz H, Lowe M, Selinger Z. J. biol. Chem. 1979;254:9829–9834. [PubMed] [Google Scholar]
- 14.Dichter M, Fischbach GD. J. Physiol. Lond. 1977;267:281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katada T, Bokoch GM, Northup JK, Ui M, Gilman AG. J. biol. Chem. 1984;259:3568–3577. [PubMed] [Google Scholar]
- 16.Jakobs KH, Aktories K, Schultz G. Adv. Cyclic Nucleotide Res. Protein Phosphor. 1984;17:135–143. [PubMed] [Google Scholar]
- 17.Rane SG, Dunlap K. Proc. natn. Acad. Sci. U.S.A. 1986;83:184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamill O, Marty A, Neher E, Sakman B, Sigworth FJ. Pflügers Arch. ges. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 19.Cassel D, Eckstein F, Lowe M, Selinger Z. J. biol. Chem. 1979;254:9835–9838. [PubMed] [Google Scholar]
- 20.Jakobs KH. Eur. J. Biochem. 1983;132:125–130. doi: 10.1111/j.1432-1033.1983.tb07336.x. [DOI] [PubMed] [Google Scholar]
- 21.Lemos JR, Levitan IB. J. gen. Physiol. 1984;83:269–285. doi: 10.1085/jgp.83.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canfield DR, Dunlap K. Br. J. Pharmac. 1984;82:557–563. doi: 10.1111/j.1476-5381.1984.tb10794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlap K. Br. J. Pharmac. 1981;74:579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabol SL, Nirenberg M. J. biol. Chem. 1979;254:1913–1920. [PubMed] [Google Scholar]
- 25.Wojcik WJ, Neff NH. Molec. Pharmac. 1984;25:24–28. [PubMed] [Google Scholar]
- 26.Hazeki O, Ui M. J. biol. Chem. 1981;256:2856–2862. [PubMed] [Google Scholar]
- 27.Cote TE, Frey EA, Sekura RD. J. biol. Chem. 1984;259:8693–8698. [PubMed] [Google Scholar]
- 28.Forscher P, Oxford GS. J. gen. Physiol. 1985;85:743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neer E, Lok J, Wolf L. J. biol. Chem. 1984;259:14222–14229. [PubMed] [Google Scholar]
- 30.Sternweis PC, Robishaw JD. J. biol. Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 31.Nakamura T, Ui M. J. biol. Chem. 1985;260:3584–3593. [PubMed] [Google Scholar]
- 32.Bradford PG, Rubin RP. FEBS Lett. 1985;183:317–320. doi: 10.1016/0014-5793(85)80801-2. [DOI] [PubMed] [Google Scholar]
- 33.Volpi M, et al. Proc. natn. Acad. Sci. U.S.A. 1985;82:2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cockcroft S, Comperts B. Nature. 1985;314:534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- 35.Wallace MA, Fain JN. J. biol. Chem. 1985;260:9527–9530. [PubMed] [Google Scholar]
- 36.Nishizuka Y. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]