Abstract
The cardiovascular complications reported to be associated with cyclooxygenase inhibitor use have shifted our focus toward prostaglandins and their respective receptors. Prostaglandin D2 and its DP1 receptor have been implicated in various normal and pathologic conditions, but their role in stroke is still poorly defined. Here, we tested whether DP1 deletion aggravates N-methyl-d-aspartic acid (NMDA)-induced acute toxicity and whether DP1 pharmacologic activation protects mice from acute excitotoxicity and transient cerebral ischemia. Moreover, since the elderly are more vulnerable to stroke-related damage than are younger patients, we tested the susceptibility of aged DP1 knockout (DP1−/−) mice to brain damage. We found that intrastriatal injection of 15 nmol NMDA caused significantly larger lesion volumes (27.2 ± 6.4%) in young adult DP1−/− mice than in their wild-type counterparts. Additionally, intracerebroventricular pretreatment of wild-type mice with 10, 25, and 50 nmol of the DP1-selective agonist BW245C significantly attenuated the NMDA-induced lesion size by 19.5 ± 5.0%, 39.6 ± 7.7%, and 28.9 ± 7.0%, respectively. The lowest tested dose of BW245C also was able to reduce middle cerebral artery occlusion-induced brain infarction size significantly (21.0 ± 5.7%). Interestingly, the aggravated NMDA-induced brain damage was persistent in older DP1−/− mice as well. We conclude that the DP1 receptor plays an important role in attenuating brain damage and that selective targeting of this receptor could be considered as an adjunct therapeutic tool to minimize stroke damage.
Keywords: BW245C, G-protein-coupled receptors, Mouse, Neurodegeneration, Neuroprotection, NMDA, Prostaglandins
Introduction
Stroke is one of the leading causes of neurologic dysfunction and death but has limited therapeutic options (Rosamond et al. 2008). Treatments that are available must be given within 3 h of onset. Delayed neuronal cell death follows ischemic–reperfusion injury mainly as a result of free radical damage and neuroinflammatory cascade (Moore and Traystman 1994; Chan 2001; Endres et al. 2008). Cyclooxygenase (COX), a proinflammatory mediator, has been implicated in the progression of stroke damage. During various pathologic events, COX-2 is highly upregulated; therefore, COX-2 inhibitors have been considered to be potential therapeutic remedies against stroke. Unfortunately, clinical trials associate COX-2 inhibitors with enhanced risk of cardiovascular complications (Bresalier et al. 2005; Graham et al. 2005). Therefore, focus has shifted toward the cascade of prostaglandins downstream of COX enzymes.
Prostaglandin D2 (PGD2) is formed by the action of PGD synthases on the COX product PGH2, and recently PGD synthases have been suggested to be protective in ischemic stroke (Saleem et al. 2009). PGD2 is widely distributed in rat and human brain (Abdel-Halim et al. 1977; Narumiya et al. 1982; Ogorochi et al. 1984). In peripheral tissues, PGD2 executes a wide range of functions, including vasodilatation, inhibition of platelet aggregation, glycogenolysis, vasoconstriction, allergic reaction mediation, and intraocular pressure reduction (Whittle et al. 1983; Narumiya and Toda 1985; Casteleijn et al. 1988; Sturzebecher et al. 1989; Darius et al. 1994; Matsugi et al. 1995; Matsuoka et al. 2000; Angeli et al. 2004). In the brain, PGD2 has been shown to contribute to sleep induction, modulation of body temperature, olfactory function, hormone release, nociception, and neuromodulation (Eguchi et al. 1999; Urade and Hayaishi 1999; Mizoguchi et al. 2001; Hayaishi 2002; Hayaishi and Urade 2002; Gelir et al. 2005).
PGD2 exerts its effects by binding with the membrane-bound G-protein-coupled DP1 receptor. The DP1 receptor is coupled to Gs protein and stimulates adenylyl cyclase, leading to increased levels of cyclic adenosine monophosphate (cAMP; Hata and Breyer 2004). A second type of DP receptor [DP2, also referred to as chemoattractant receptor-homologous molecule expressed on T helper cells (CRTH2)] has also been reported, but it has no significant homology with DP1 and has different biological functions and cellular distribution (Hirai et al. 2001; Sawyer et al. 2002), and some of its signaling is thought to be independent of PGD2 levels. Furthermore, it has been documented that its activation decreases cAMP and increases intracellular calcium levels (Bohm et al. 2004; Sandig et al. 2006). The in vivo role of DP1 in the peripheral nervous system has been well investigated (Angeli et al. 2004; Hata and Breyer 2004; Koch et al. 2005), but our knowledge of its role in the brain is still very limited (Campbell and Feinberg 1996; Urade and Hayaishi 1999; Hayaishi and Urade 2002; Obal and Krueger 2003).
Activation of DP1 receptors is primarily associated with anti-inflammatory effects, but proinflammatory effects of DP1 also have been described (Matsuoka et al. 2000; Hammad et al. 2003; Angeli et al. 2004; Spik et al. 2005). It has been reported that PGD2 induces nerve growth factor and brain-derived neurotrophic factor (Toyomoto et al. 2004). In addition, a study by Andreasson’s group showed that the DP1 agonist BW245C increases the level of cAMP and protects hippocampal slice cultures against N-methyl-d-aspartic acid (NMDA)-induced excitotoxicity (Liang et al. 2005). Our data previously revealed that mice lacking DP1 are more susceptible to middle cerebral artery occlusion (MCAO)-induced brain damage than are wild-type (WT) mice (Saleem et al. 2007). Because excitotoxicity is a major component in propagating stroke damage, here we hypothesized that genetic deletion of the DP1 receptor would also augment NMDA-induced neurotoxicity and that activation of the DP1 receptor would attenuate the brain damage caused by excitotoxicity and MCAO. We also tested whether the NMDA-induced brain damage augmentation would be sustained in aged DP1−/− mice. To test this hypothesis, we compared the outcomes in young and old WT and DP1−/− mice subjected to an NMDA model of acute excitotoxicity. In addition, the pharmacological effect of the DP1 agonist BW245C on outcomes from NMDA-induced excitotoxicity and MCAO in young adult male WT mice was also determined. To the best of our knowledge, this is the first study to test the in vivo effect of BW245C against acute excitotoxicity and MCAO.
Materials and methods
Animals and drugs
Studies were carried out on 8–10-week-old male C57BL/6 mice (20–25 g) obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA). To determine the effect of the DP1 receptor in aged mice, 14–18-month-old WT and DP1−/− mice were used. Colonies of WT and DP1−/− mice (obtained from Dr. Shuh Narumiya (Matsuoka et al. 2000)) were maintained in our animal facility. These mice were back-crossed eight to ten generations, and genotype was determined by PCR before the experiment. The DP1−/− mice develop normally and have no gross abnormalities in behavior, macroscopic anatomy, or biochemical or hematologic indices (Matsuoka et al. 2000). All animal protocols were approved by the Johns Hopkins University Animal Care and Use Committee. The animals were allowed free access to water and food before and after surgery. BW245C [5-(6-carboxyhexyl)-1-(3-cyclohexyl-3-hydroxypropyl) hydantoin] was purchased from Cayman Chemicals, Ann Arbor, MI, USA. BW245C is a selective DP1 agonist with high binding affinity (Ki = 250 nM) toward mouse DP1 (Kiriyama et al. 1997; Narumiya et al. 1999); it has been shown to increase intracellular levels of cAMP and activate adenylyl cyclase (Ientile et al. 1983). Earlier studies proposed that BW245C had nonspecific effects through EP2 and EP4 receptors (Giles et al. 1989; Hamid-Bloomfield and Whittle 1989; Matsugi et al. 1995; Rangachari et al. 1995); however, these effects were reported to be species specific. Nevertheless, nonspecific effects of BW245C in neurological settings have not been reported in mice. The known affinity of BW245C toward mouse DP1 is more than seven times its affinity toward FP and more than 40 times its affinity toward other mouse prostanoid receptors. Correspondingly, the affinity of BW245C toward human DP1 is approximately 330 times higher than toward human EP4 and 547 times higher than toward human EP2 (Kiriyama et al. 1997; Wright et al. 1998; Abramovitz et al. 2000; Narumiya and FitzGerald 2001).
Pretreatment with BW245C
After their weight and rectal temperature were recorded, mice were anesthetized and placed on a stereotaxic stand for intracerebral microinjections as described previously (Ahmad et al. 2006a). Briefly, mice were given a single 0.2-µl injection containing 10 nmol (n = 9), 25 nmol (n = 7), or 50 nmol (n = 6) BW245C (for excitotoxicity model) or 10 nmol BW245C (for MCAO model; n = 8) in the right cerebral ventricle. After the injection, the hole was plugged with bone wax, and the skin overlying the skull was sutured. Mice were then prepared for either stereotaxic NMDA injection or the MCAO procedure.
Unilateral NMDA-induced acute excitotoxicity model
Twenty minutes after the BW245C treatment, 15 nmol NMDA in a volume of 0.3 µl was injected slowly into the right striatum; 5 min later, the needle was retracted slowly, the hole was blocked with bone wax, and the skin was sutured. Two control groups were used. One received only the 15-nmol intrastriatal NMDA injection (n = 7), whereas the other received a 0.2-µl intracerebroventricular (ICV) injection of vehicle (0.5% DMSO) 20 min before the NMDA injection (n = 6). To test the effect of NMDA-induced toxicity in young and old DP1−/− mice, 8–10-week-old WT (n = 7) and DP1−/− (n = 6) mice and 14–18-month-old WT (n = 8) and DP1−/− (n = 8) mice were given a single intrastriatal injection of NMDA. After the procedure, mice were placed in a thermo-regulated chamber to recover from anesthesia before being transferred to their home cages and allowed to survive for 48 h. Throughout the experimental procedure, rectal temperature of mice was monitored and maintained at 37.0 ± 0.5°C.
Quantification of the excitotoxic lesion volume
At 48 h after NMDA injection, weight and rectal temperature were recorded, and mice were deeply anesthetized with pentobarbital and transcardially perfused with cold PBS followed by 4% paraformaldehyde in PBS (pH 7.2). Brains were harvested quickly, postfixed for 24 h, and then equilibrated in 30% sucrose and frozen in precooled 2-methylbutane. Sequential brain sections of 25 µm obtained on a cryostat were stained with Cresyl Violet, and lesion volume was analyzed according to the protocol described previously (Ahmad et al. 2006b).
MCAO and reperfusion
During the MCAO procedure, mouse rectal temperature was monitored and maintained at 37.0 ± 0.5°C by a heating pad, and anesthesia was maintained with continuous flow of halothane in oxygen-enriched air via a nose cone. Relative cerebral blood flow was monitored with laser Doppler flowmetry (Moor Instruments, Devon, England) by a flexible fiber-optic probe affixed to the skull over the parietal cortex supplied by the middle cerebral artery (2 mm posterior and 6 mm lateral to the bregma). MCAO was carried out under aseptic conditions with a silicone-coated nylon monofilament as described previously (Ahmad et al. 2006c). The filament was left in position for 90 min, during which the incision was closed with sutures, anesthesia was discontinued, and the animals were transferred to a temperature-controlled chamber to maintain their body temperature at 37.0 ± 0.5°C. At 90 min of occlusion, the mice were briefly reanesthetized with halothane, and reperfusion was achieved by withdrawing the filament. After their incision was sutured, the mice were returned to the temperature-controlled chamber for 2 h before being transferred to their home cages for 4 days.
Analysis of physiologic parameters
Physiologic parameters were measured in a group of mice separate from that used to assess infarct volume. Mice were injected with either vehicle (n = 6) or 10 nmol BW245C (n = 6) before being subjected to MCAO. Arterial blood samples collected via femoral catheter were analyzed for pH, PaO2, and PaCO2 before the occlusion, during occlusion, and 1 h after the occlusion. Mean arterial blood pressure (MABP) was measured at the same time points by a pressure transducer connected to the femoral catheter.
Temperature regulation
A third cohort of mice was implanted with intra-abdominal radiofrequency probes [IPTT-200, Bio Medic Data System (BMDS), Seaford, DE] 7 days before injection of BW245C or vehicle (n = 6 per group). Core temperature was sampled at 10-min intervals from before the mice were anesthetized until 90 min after injection and then once daily for 4 days at room temperature via receivers (DAS-5002 Notebook System™; BMDS). This telemetry system minimizes stress and allows temperature control and monitoring in freely moving animals.
Quantification of infarct volume
Four days after surgery, mice were deeply anesthetized, and their brains were harvested and sliced coronally into 2-mm-thick sections. The sections were incubated with 1% 2,3,5-triphenyl-tetrazolium chloride (TTC) in saline for 20 min at 37°C. The area of infarcted brain, identified by the lack of TTC staining, was measured on the rostral and caudal surfaces of each slice and numerically integrated across the thickness of the slice to obtain an estimate of infarct volume in each slice (SigmaScan Pro, SPSS, Port Richmond, CA, USA). Volumes from all five slices were summed to calculate total infarct volume over the entire hemisphere and expressed as a percentage of the volume of the contralateral structure. Infarct volume was corrected for swelling by comparing the volumes in the ipsilateral and contralateral hemispheres. The corrected infarct volume was calculated as: volume of contralateral hemisphere − (volume of ipsilateral hemisphere − volume of infarct).
Statistical analysis
The brain sections were imaged and analyzed with SigmaScan Pro 5.0 software (Systat, Inc., Point Richmond, CA, USA). Statistical analysis was performed by one-way ANOVA followed by Bonferroni multiple comparison test; P values <0.05 were considered to be significant. All values are expressed as mean ± standard error of the mean (SEM).
Results
Genetic deletion of DP1 receptor aggravates excitotoxic brain damage
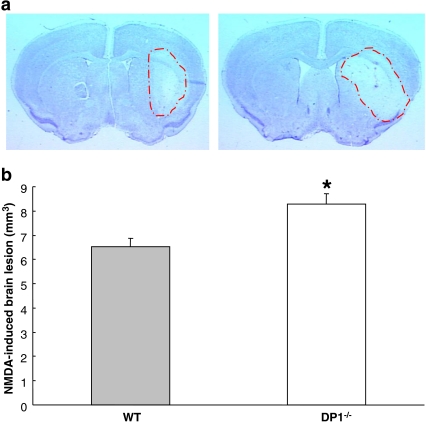
DP1−/− mice were found to be more vulnerable to NMDA-induced toxicity as compared to the WT mice (Fig. 1a). Quantification showed that NMDA-treated DP1−/− mice had a mean lesion volume of 8.29 mm3, which was significantly greater than that of the WT mice, which had a mean lesion volume of 6.51 mm3 (P < 0.05; Fig. 1b).
Fig. 1.
NMDA-induced toxicity is aggravated in DP1−/− mice. A single dose of 15 nmol NMDA was injected stereotactically into the striatum of C57BL/6 WT (n = 7) and DP1−/− mice (n = 6). a Representative photographs of coronal sections of the brains of WT (left panel) and DP1−/− (right panel) mice show aggravated lesion in the latter. b Analysis of the Cresyl-Violet-stained brain sections shows that the NMDA-induced lesion size was significantly greater in the DP1−/− mice. *P < 0.05 when compared with the WT group
NMDA-induced brain damage is decreased by BW245C
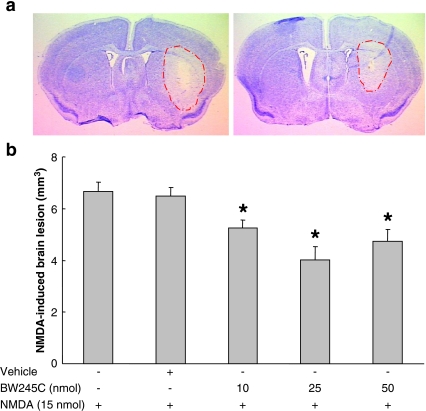
NMDA-induced brain damage in the ipsilateral striatum appeared to be attenuated by pretreatment with BW245C (Fig. 2a). Analysis of the stained sections revealed a significant decrease in the lesion volume of BW245C-pretreated groups compared with the vehicle-treated control group (Fig. 2b). The lesion volume decreased significantly (P < 0.05) by 19.5 ± 5.0%, 39.6 ± 7.6%, and 28.9 ± 7.0% in the 10-, 25-, and 50-nmol-treated groups, respectively. Pretreatment with vehicle did not affect the level of NMDA-induced toxicity.
Fig. 2.
Pretreatment with BW245C mitigates NMDA-induced brain damage. Mice were given a single ICV injection of vehicle (n = 6) or 10 nmol (n = 9), 25 nmol (n = 7), or 50 nmol (n = 6) BW245C 20 min before an injection of 15 nmol NMDA. Forty-eight hours later, mice were euthanized and brain sections were stained with Cresyl Violet to estimate the lesion volume. a Representative photographs of brain coronal sections from mice pretreated with vehicle (left panel) or 10 nmol BW245C (right panel). b Analysis of the brain sections reveals a significant reduction in the mean lesion volume of BW245C-treated mice as compared with that of the vehicle-treated control group. Vehicle injection had no effect on NMDA-induced toxicity. *P < 0.05, when compared with the vehicle-treated control group
BW245C attenuates infarct volume in WT mice
We chose the lowest dose of BW245C that was effective in the NMDA model to determine whether it had the potential to minimize MCAO outcome. We found that mice given an ICV injection of 10 nmol BW245C immediately before MCAO had hemispheric infarct volumes that were 21.0 ± 5.7% (P < 0.05) smaller than those of the vehicle-treated group (Fig. 3).
Fig. 3.
Pretreatment with BW245C attenuates MCAO-induced brain infarction. Mice (n = 8 per group) were injected with either vehicle or 10.0 nmol BW245C in the lateral ventricle before being subjected to 90-min MCAO and 4 days of reperfusion. a Representative photographs of TTC-stained brain slices of mice treated with vehicle (left panel) or 10 nmol BW245C (right panel) show smaller infarct size in the BW245C-treated slice. b Analysis of the brain slices reveals that the hemispheric infarct volume was significantly attenuated in mice treated with 10 nmol BW245C compared with vehicle-treated mice. *P < 0.05 compared with the vehicle-treated group
BW245C does not affect physiologic parameters
The physiologic parameters that we measured (pH, PaCO2, PaO2, and MABP) were within normal physiologic ranges for all mice, and none showed any noteworthy differences between the vehicle-treated group and the group given 10.0 nmol BW245C (Table 1). Core body temperature was unchanged during occlusion and early reperfusion within each group.
Table 1.
Effect of BW245C on physiologic parameters
| Parameter | Vehicle | 10.0 nmol BW245C |
|---|---|---|
| Preischemia | ||
| MABP | 79 ± 2 | 77 ± 2 |
| pH | 7.23 ± 0.03 | 7.25 ± 0.03 |
| PaCO2 | 43.7 ± 1.5 | 49.2 ± 2.5 |
| PaO2 | 140 ± 4 | 144 ± 7 |
| Ischemia | ||
| MABP | 74 ± 2 | 77 ± 2 |
| pH | 7.24 ± 0.02 | 7.29 ± 0.03 |
| PaCO2 | 44.0 ± 1.0 | 47.8 ± 1.2 |
| PaO2 | 135 ± 4 | 143 ± 7 |
| Postischemia | ||
| MABP | 75 ± 1 | 76 ± 2 |
| pH | 7.27 ± 0.02 | 7.23 ± 0.03 |
| PaCO2 | 45.2 ± 1.2 | 46.8 ± 2.4 |
| PaO2 | 142 ± 4 | 139 ± 6 |
BW245C does not significantly alter core body temperature in mice
Because PGD2 has been implicated in temperature regulation, we wanted to determine whether it affected the temperature of the mice in our experiments. We injected DP1 agonist BW245C (10 nmol) ICV in a cohort of mice that did not undergo MCAO and monitored body temperature for 96 h. Core body temperature did not significantly differ between BW245C-treated mice and vehicle-treated mice at any time during the 96-h period (Fig. 4).
Fig. 4.
Pretreatment with BW245C does not affect mouse core body temperature. BW245C (10 nmol) was injected ICV, and the body temperatures of the mice (n = 6 per group) were recorded with an intra-abdominal radiofrequency probe every 10 min during the first 120 min after injection and then once daily for 4 days while the mice were housed at room temperature. No significant differences in core body temperature were observed between the saline-treated and BW245C-treated groups
Augmentation of NMDA-induced brain damage in young DP1−/− is sustained in aged DP1−/− mice
Similar to our observations in young (8–10 weeks old) mice, aged (14–18 months old) DP1−/− mice were also more vulnerable than aged WT mice to NMDA-induced excitoxicity, as noted by the greater lesion size in the knockout mice (Fig. 5a). Analysis of the sections revealed that NMDA-treated aged DP1−/− mice had a significantly (P < 0.05) larger mean lesion volume of 4.98 mm3 than did the WT mice, which had a mean lesion size of 3.24 mm3 (Fig. 5b).
Fig. 5.
Aggravated NMDA-induced toxicity is sustained in aged DP1−/− mice. One 15-nmol dose of NMDA was injected stereotactically into the striatum of 14–18-month-old WT and DP1−/− mice (n = 8 per group). a Representative photographs of coronal sections of the brains of aged WT (left panel) and DP1−/− (right panel) mice show aggravated lesion in the latter. b Analysis of the Cresyl-Violet-stained brain sections shows that the NMDA-induced lesion size was significantly larger in the aged DP1−/− mice than in the aged WT mice. *P < 0.05 when compared with the WT group
Discussion
Prostaglandin D2, the most abundant prostanoid of mammalian brain, has been shown to have roles in a variety of functions, from regulation of sleep and temperature to inflammatory disorders. However, its activity in neurodegeneration and neuroprotection has not been fully investigated. Our study was designed to evaluate the role of the PGD2 DP1 receptor in cerebral ischemia and excitotoxicity. We found that genetic deletion of DP1 made mice more vulnerable to NMDA-induced toxicity. Interestingly, pharmacological activation of DP1 by BW245C attenuated the lesion volume induced by NMDA. After establishing an effective dose curve, we wanted to know if the minimum effective dose of 10 nmol could lead to neuroprotection in MCAO. Indeed, 10 nmol of BW245C significantly reduced the size of the infarction following transient ischemia without affecting the monitored physiologic variables. Moreover, given the contribution of aging in stroke, we determined whether the neurotoxic effect of DP1 deletion was sustained in older DP1−/− mice. Our data suggest that, indeed, augmented NMDA-induced brain damage is persistent in aged DP1−/− mice. To our knowledge, this is the first in vivo study to show that NMDA-induced brain damage is augmented in DP1−/− mice in both young and aged animals and that DP1 receptor stimulation attenuates ischemic and excitotoxic brain damage.
We have previously shown that genetic deletion of the DP1 receptor leads to aggravated brain infarction after MCAO (Saleem et al. 2007). Because excitotoxicity contributes substantially to the propagation of stroke damage, we went further in this study to determine the effect of DP1 deletion on NMDA-induced acute excitotoxicity. Figure 1 illustrates that the DP1 receptor is capable of minimizing NMDA-induced brain damage. This is the first study to determine the role of DP1 receptor activation by the agonist BW245C in acute excitotoxicity and MCAO paradigm in vivo. One other group has essentially shown that the DP1 agonist BW245C provides protection in rat E18 hippocampal cultures and in rat hippocampal slices (Liang et al. 2005). While the affinity of BW245C has been well characterized for mice and humans but not in rats, our in vivo studies using both DP1−/− mice and the DP1 selective agonist in WT mice suggest that pharmacologic DP1 stimulation by BW245C is neuroprotective against excitotoxicity and stroke.
Furthermore, based on the susceptibility of the aged population to stroke, we hypothesized that the aggravated brain damage in young DP1−/− would be sustained in older DP1−/− mice, and our data very well supported this hypothesis. However, it should be noted that older WT mice had smaller lesion volumes than did young WT mice, and the same was true for DP1−/− mice. This finding can be explained by the fact that, with age, the population of glutamatergic receptors, such as the NMDA receptor, as well as glutamate content, decreases in corticostriatal and hippocampal areas (Banay-Schwartz et al. 1989; Saransaari and Oja 1995). Other studies also have shown that older animals have decreased NMDA receptor sensitivity in normal and excitotoxic conditions (Peterson and Cotman 1989; Wenk et al. 1991; Cohen and Muller 1992; Magnusson and Cotman 1993; see also reviews by Segovia et al. 2001; Dickstein et al. 2007). Our study supports these previous reports suggesting that age affects ischemic and excitotoxic brain damage outcomes. In our study, the decrease in excitotoxic injury in aged mice could have various causes, including a decrease in glutamatergic receptors, a decrease in glutamate content, and loss of dendritic spines. Thus, it is the first report that the neuroprotective role of the DP1 receptor is maintained in aged mice.
Few studies have shown a potential role for PGD2 in regulation of vasodilatation. PGD2 was shown to induce long-duration vasodilatation of the ascaris-sensitized pig airways without having major effects on the systemic arterial blood pressure (Alving et al. 1991); it was also shown to decrease relaxation of isolated rat mesenteric arteries (Shirahase et al. 2000). Activation of the DP1 receptor induced dilatation in contracted human pulmonary venous preparations (Walch et al. 1999) and enhanced nicotinic-acid-induced vasodilatation in mouse ear and nicotinic-acid-induced dermal flushing in humans (Cheng et al. 2006). Here, we did not observe any significant changes in the MABP or relative cerebral blood flow after ICV injection of BW245C in mice. We do not conclude that DP1 does not mediate vasodilatation; rather, we attribute our findings to the different testing paradigms and doses used in our study. Moreover, we did not observe any significant differences in other physiologic variables tested (Table 1).
PGD2 also plays a role in radiation-induced hyperthermia temperature responses in rats (Kandasamy and Hunt 1990). Microinjection of low-dose PGD2 into the preoptic area or ICV injection caused hypothermia in rats but not in rabbits, whereas high-dose microinjection caused hyperthermia in rabbits but not in rats (Ito et al. 1989). However, we were unable to find a systematic study of the role of PGD2 in temperature in mice. Considering the role that PGD2 plays in the regulation of temperature, we monitored mouse core body temperature for 96 h after a 10 nmol BW245C injection. We did not observe any difference between the vehicle-treated and the drug-treated groups. Lower doses of BW245C also failed to produce significant temperature differences (data not shown). Although we did not investigate the role of DP1 in temperature regulation extensively, our data suggest that DP1 activation does not affect core body temperature in our experimental paradigm. It is noteworthy that a rat study predicted that despite its abundance in brain (Siren 1982), PGD2 might not have an important role to play in central cardiovascular and thermal regulation. Moreover, Brus et al. (1980) reported no changes in behavior, body temperature, or blood pressure of Wistar rats after ICV injection of PGD2.
Various studies related to the protective role of prostaglandin E2 receptors in paradigms of neuronal toxicity or animal models of neurodegeneration have indicated a potential role for increasing intracellular levels of cAMP and its downstream MAP kinase or protein kinase signaling pathways (McCullough et al. 2004; Echeverria et al. 2005; Ahmad et al. 2006c). In parallel with these reports, Liang et al. (2005) showed that DP1 receptor agonist BW245C protects against glutamate toxicity in cultured hippocampal neurons and organotypic slices in a cAMP-dependent fashion; they also showed that neurotoxic effects produced by glutamate in dispersed hippocampal neurons were abolished by protein kinase inhibitors. It is generally accepted that an increase in the level of intracellular cAMP leads to the activation of PKA, which subsequently leads to neuroprotection (Mattson et al. 1988; Hartikka et al. 1992; Sklair-Tavron and Segal 1993). Therefore, we believe that the protection observed in our studies could be partly mediated by an increase in intracellular cAMP concentration and activation of PKA signaling. As a proof of concept, this study involving in vivo experiments shows for the first time that DP1 activation is neuroprotective, and it establishes the first step toward defining the neuroprotective effects of DP1. However, more effort is needed to fully elucidate the detailed neuroprotective mechanism of action of this receptor.
In conclusion, we have demonstrated that the DP1 receptor is vital to preventing brain damage. Both young and aged DP1−/− mice were more vulnerable than controls to acute excitotoxicity, and activation of the DP1 receptor attenuated both acute and chronic brain damage in mice. The results provide evidence that the DP1 receptor is beneficial against stroke and excitotoxicity. Accordingly, selective targeting of the DP1 receptor could be considered as a potential therapeutic tool against stroke and excitotoxic brain damage.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health NS046400 and AG022971 (SD) and the American Heart Association 0830172N (ASA). We thank Claire Levine for assistance in the preparation of the manuscript and all members of the Doré lab team for assistance in this project.
Conflict of interest None of the authors have any conflict of interest associated with this work.
Footnotes
A. S. Ahmad and M. Ahmad contributed equally to this work.
References
- Abdel-Halim MS, Hamberg M, Sjoquist B, Anggard E. Identification of prostaglandin D2 as a major prostaglandin in homogenates of rat brain. Prostaglandins. 1977;14:633–643. doi: 10.1016/0090-6980(77)90190-3. [DOI] [PubMed] [Google Scholar]
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Saleem S, Ahmad M, Doré S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006;89:265–270. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Zhuang H, Echeverria V, Doré S. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. J Neurotrauma. 2006;23:1895–1903. doi: 10.1089/neu.2006.23.1895. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Saleem S, Zhuang H, Ahmad AS, Echeverria V, Sapirstein A, Doré S. 1-HydroxyPGE1 reduces infarction volume in mouse transient cerebral ischemia. Eur J Neurosci. 2006;23:35–42. doi: 10.1111/j.1460-9568.2005.04540.x. [DOI] [PubMed] [Google Scholar]
- Alving K, Matran R, Lundberg JM. The possible role of prostaglandin D2 in the long-lasting airways vasodilatation induced by allergen in the sensitized pig. Acta Physiol Scand. 1991;143:93–103. doi: 10.1111/j.1748-1716.1991.tb09204.x. [DOI] [PubMed] [Google Scholar]
- Angeli V, Staumont D, Charbonnier AS, Hammad H, Gosset P, Pichavant M, Lambrecht BN, Capron M, Dombrowicz D, Trottein F. Activation of the D prostanoid receptor 1 regulates immune and skin allergic responses. J Immunol. 2004;172:3822–3829. doi: 10.4049/jimmunol.172.6.3822. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. I. Glutamate and related amino acids. Neurochem Res. 1989;14:555–562. doi: 10.1007/BF00964918. [DOI] [PubMed] [Google Scholar]
- Bohm E, Sturm GJ, Weiglhofer I, Sandig H, Shichijo M, McNamee A, Pease JE, Kollroser M, Peskar BA, Heinemann A. 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J Biol Chem. 2004;279:7663–7670. doi: 10.1074/jbc.M310270200. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Brus R, Herman ZS, Szklinik R. Central effects of prostaglandin D2. Pol J Pharmacol Pharm. 1980;32:681–684. [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Noncompetitive NMDA channel blockade during waking intensely stimulates NREM delta. J Pharmacol Exp Ther. 1996;276:737–742. [PubMed] [Google Scholar]
- Casteleijn E, Kuiper J, Rooij HC, Kamps JA, Koster JF, Berkel TJ. Prostaglandin D2 mediates the stimulation of glycogenolysis in the liver by phorbol ester. Biochem J. 1988;250:77–80. doi: 10.1042/bj2500077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, Wright SD, Wang Z, O'Neill G, Lai E, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006;103:6682–6687. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SA, Muller WE. Age-related alterations of NMDA-receptor properties in the mouse forebrain: partial restoration by chronic phosphatidylserine treatment. Brain Res. 1992;584:174–180. doi: 10.1016/0006-8993(92)90892-D. [DOI] [PubMed] [Google Scholar]
- Darius H, Michael-Hepp J, Thierauch KH, Fisch A. Inhibition of human platelets and polymorphonuclear neutrophils by the potent and metabolically stable prostaglandin D2 analog ZK 118.182. Eur J Pharmacol. 1994;258:207–213. doi: 10.1016/0014-2999(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Clerman A, Doré S. Stimulation of PGE2 receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following β-amyloid exposure. Eur J Neurosci. 2005;22:2199–2206. doi: 10.1111/j.1460-9568.2005.04427.x. [DOI] [PubMed] [Google Scholar]
- Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, Yoshida N, Urade Y, Ito S, Hayaishi O. Lack of tactile pain (allodynia) in lipocalin-type prostaglandin D synthase-deficient mice. Proc Natl Acad Sci U S A. 1999;96:726–730. doi: 10.1073/pnas.96.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M, Schwab ME, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- Gelir E, Arslan SO, Sayan H, Pinar L. Effect of rapid-eye-movement sleep deprivation on rat hypothalamic prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 2005;73:391–396. doi: 10.1016/j.plefa.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Giles H, Leff P, Bolofo ML, Kelly MG, Robertson AD. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- Hamid-Bloomfield S, Whittle BJ. Antagonism of PGD2 vasodepressor responses in the rat in vivo by the novel, selective antagonist, BW A868C. Br J Pharmacol. 1989;96:307–312. doi: 10.1111/j.1476-5381.1989.tb11818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol. 2003;171:3936–3940. doi: 10.4049/jimmunol.171.8.3936. [DOI] [PubMed] [Google Scholar]
- Hartikka J, Staufenbiel M, Lubbert H. Cyclic AMP, but not basic FGF, increases the in vitro survival of mesencephalic dopaminergic neurons and protects them from MPP(+)-induced degeneration. J Neurosci Res. 1992;32:190–201. doi: 10.1002/jnr.490320208. [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hayaishi O. Molecular genetic studies on sleep–wake regulation, with special emphasis on the prostaglandin D2 system. J Appl Physiol. 2002;92:863–868. doi: 10.1152/japplphysiol.00766.2001. [DOI] [PubMed] [Google Scholar]
- Hayaishi O, Urade Y. Prostaglandin D2 in sleep–wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–15. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ientile R, Sarro A, Rotiroti D, Sarro GB, Nistico G. Stimulation of rat caudate nucleus adenylate cyclase activity by BW 245 C, a prostaglandin analogue with prostacyclin-like activity. J Pharm Pharmacol. 1983;35:62–64. doi: 10.1111/j.2042-7158.1983.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Narumiya S, Hayaishi O. Prostaglandin D2: a biochemical perspective. Prostaglandins Leukot Essent Fatty Acids. 1989;37:219–234. doi: 10.1016/0952-3278(89)90033-1. [DOI] [PubMed] [Google Scholar]
- Kandasamy SB, Hunt WA. Involvement of prostaglandins and histamine in radiation-induced temperature responses in rats. Radiat Res. 1990;121:84–90. doi: 10.2307/3577568. [DOI] [PubMed] [Google Scholar]
- Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KA, Wessale JL, Moreland R, Reinhart GA, Cox BF. Effects of BW245C, a prostaglandin DP receptor agonist, on systemic and regional haemodynamics in the anaesthetized rat. Clin Exp Pharmacol Physiol. 2005;32:931–935. doi: 10.1111/j.1440-1681.2005.04287.x. [DOI] [PubMed] [Google Scholar]
- Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Cotman CW. Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol Aging. 1993;14:197–206. doi: 10.1016/0197-4580(93)90001-R. [DOI] [PubMed] [Google Scholar]
- Matsugi T, Kageyama M, Nishimura K, Giles H, Shirasawa E. Selective prostaglandin D2 receptor stimulation elicits ocular hypotensive effects in rabbits and cats. Eur J Pharmacol. 1995;275:245–250. doi: 10.1016/0014-2999(94)00788-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. Intracellular messengers in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci Res. 1988;21:447–464. doi: 10.1002/jnr.490210236. [DOI] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, Mochizuki T, Lazarus M, Kobayashi T, Kaneko T, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LE, Traystman RJ. Role of oxygen free radicals and lipid peroxidation in cerebral reperfusion injury. In: Bosnjak ZJ, August JT, editors. Adv Pharmacol. San Diego: Academic; 1994. pp. 565–576. [DOI] [PubMed] [Google Scholar]
- Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Toda N. Different responsiveness of prostaglandin D2-sensitive systems to prostaglandin D2 and its analogues. Br J Pharmacol. 1985;85:367–375. doi: 10.1111/j.1476-5381.1985.tb08870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Ogorochi T, Nakao K, Hayaishi O. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci. 1982;31:2093–2103. doi: 10.1016/0024-3205(82)90101-1. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–d550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Ogorochi T, Narumiya S, Mizuno N, Yamashita K, Miyazaki H, Hayaishi O. Regional distribution of prostaglandins D2, E2, and F2 alpha and related enzymes in postmortem human brain. J Neurochem. 1984;43:71–82. doi: 10.1111/j.1471-4159.1984.tb06680.x. [DOI] [PubMed] [Google Scholar]
- Peterson C, Cotman CW. Strain-dependent decrease in glutamate binding to the N-methyl-d-aspartic acid receptor during aging. Neurosci Lett. 1989;104:309–313. doi: 10.1016/0304-3940(89)90594-6. [DOI] [PubMed] [Google Scholar]
- Rangachari P, Betti P, Prior E, Ln R. Effects of a selective DP receptor agonist (BW 245C) and antagonist (BW A868C) on the canine colonic epithelium: an argument for a different DP receptor? J Pharmacol Exp Ther. 1995;275:611–617. [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Saleem S, Zhuang H, Brum-Fernandes AJ, Maruyama T, Narumiya S, Doré S. PGD2 DP1 receptor protects brain from ischemia–reperfusion injury. Eur J Neurosci. 2007;26:73–78. doi: 10.1111/j.1460-9568.2007.05627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S, Shah ZA, Urade Y, Doré S. Lipocalin-prostaglandin D synthase is a critical beneficial factor in transient and permanent focal cerebral ischemia. Neuroscience. 2009;160:248–254. doi: 10.1016/j.neuroscience.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandig H, Andrew D, Barnes AA, Sabroe I, Pease J. 9α, 11β-PGF2 and its stereoisomer PGF2α are novel agonists of the chemoattractant receptor, CRTH2. FEBS Lett. 2006;580:373–379. doi: 10.1016/j.febslet.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Age-related changes in the uptake and release of glutamate and aspartate in the mouse brain. Mech Ageing Dev. 1995;81:61–71. doi: 10.1016/0047-6374(95)01583-L. [DOI] [PubMed] [Google Scholar]
- Sawyer N, Cauchon E, Chateauneuf A, Cruz RPG, Nicholson DW, Metters KM, O'Neill GP, Gervais FG. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137:1163–1172. doi: 10.1038/sj.bjp.0704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Porras A, Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/S0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Shirahase H, Kanda M, Nakamura S, Tarumi T, Uehara Y, Ichikawa A. Inhibitory effects of PGD2, PGJ2 and 15-deoxy-Δ12, 14-PGJ2 on iNOS induction in rat mesenteric artery. Life Sci. 2000;66:2173–2182. doi: 10.1016/S0024-3205(00)00544-0. [DOI] [PubMed] [Google Scholar]
- Siren AL. Central cardiovascular and thermal effects of prostaglandin D2 in rats. Prostaglandins Leukot Med. 1982;8:349–359. doi: 10.1016/0262-1746(82)90058-0. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Segal M. Neurotrophic effects of cAMP generating systems on central noradrenergic neurons. Brain Res. 1993;614:257–269. doi: 10.1016/0006-8993(93)91043-R. [DOI] [PubMed] [Google Scholar]
- Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, Trottein F, Dombrowicz D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- Sturzebecher S, Nieuweboer B, Matthes S, Schillinger E. Effects of PGD2, PGE1, and PGI2-analogues on PGDF-release and aggregation of human gel filtered platelets. Prog Clin Biol Res. 1989;301:365–369. [PubMed] [Google Scholar]
- Toyomoto M, Ohta M, Okumura K, Yano H, Matsumoto K, Inoue S, Hayashi K, Ikeda K. Prostaglandins are powerful inducers of NGF and BDNF production in mouse astrocyte cultures. FEBS Lett. 2004;562:211–215. doi: 10.1016/S0014-5793(04)00246-7. [DOI] [PubMed] [Google Scholar]
- Urade Y, Hayaishi O. Prostaglandin D2 and sleep regulation. Biochim Biophys Acta. 1999;1436:606–615. doi: 10.1016/s0005-2760(98)00163-5. [DOI] [PubMed] [Google Scholar]
- Walch L, Labat C, Gascard JP, Montpreville V, Brink C, Norel X. Prostanoid receptors involved in the relaxation of human pulmonary vessels. Br J Pharmacol. 1999;126:859–866. doi: 10.1038/sj.bjp.0702393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol Aging. 1991;12:93–98. doi: 10.1016/0197-4580(91)90047-N. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Moncada S, Mullane K, Vane JR. Platelet and cardiovascular activity of the hydantoin BW245C, a potent prostaglandin analogue. Prostaglandins. 1983;25:205–223. doi: 10.1016/0090-6980(83)90105-3. [DOI] [PubMed] [Google Scholar]
- Wright DH, Metters KM, Abramovitz M, Ford-Hutchinson AW. Characterization of the recombinant human prostanoid DP receptor and identification of L-644, 698, a novel selective DP agonist. Br J Pharmacol. 1998;123:1317–1324. doi: 10.1038/sj.bjp.0701708. [DOI] [PMC free article] [PubMed] [Google Scholar]