Abstract
A catalyst based on a new biarylphosphine ligand (3) for the Pd-catalyzed cross-coupling reactions of amides and aryl chlorides is described. This system shows the highest turnover frequencies reported to date for these reactions, especially for aryl chloride substrates bearing an ortho substituent. An array of amides and aryl chlorides were successfully reacted in good to excellent yields.
Keywords: Palladium, Phosphine, Cross-Coupling, Amidation
1. Introduction
Metal-catalyzed amidation reactions of aryl halides or pseudo halides are an attractive method for synthesizing N-arylamides. These reactions were traditionally performed with aryl iodides under Goldberg-modified Ullman cross-coupling conditions using stoichiometric Cu and high reaction temperatures.1 Recent advances in this area have allowed for the reactions of amides and aryl iodides or aryl bromides to be performed using catalytic amounts of Cu under milder conditions.2 Pd-based catalyst systems using phosphine ligands have also been developed, which allow for the coupling of amides with aryl sulfonates,3 aryl bromides,4 and most recently, aryl chlorides.5 These methods have proven to be useful to synthetic chemists and have been widely used in both industrial and academic laboratories. 6
Of the aryl halides, aryl chlorides are generally the most attractive substrates for cross-coupling reactions because they are less expensive and more readily available. Our group previously reported a catalyst system, based on ligand 1, that effectively promoted the cross-coupling of amides and unhindered aryl chlorides,5a but was less effective with ortho-substituted aryl chlorides. Mechanistic studies and DFT calculations indicated to us that the methyl substituent ortho to the di-tert-butylphosphino group in ligand 1 prohibited rotation around the P-CAr bond, forcing the Pd(II) center of the resulting oxidative addition complex to position itself over the non-phosphine-containing ring (Figure 2). It was postulated that this confirmation inhibited the formation of the κ2- amidate complex, accelerating reductive elimination. On the other hand, DFT calculations have also indicated that the amine binding step of the catalytic cycle using biarylphosphine ligands is slower when the Pd(II) is positioned over the non-phosphine containing ring.7 Having an ortho substituent on the arene in these complexes compounds this effect by increasing the crowding around the Pd(II) center, hence, further slowing “transmetallation” (amide binding/deprotonation). Herein, we report a catalyst system, which displays high activity for the amidation of aryl chlorides, including those with ortho substitution, by facilitating transmetallation while still inhibiting the formation of a κ2-amidate intermediate.
Figure 2.
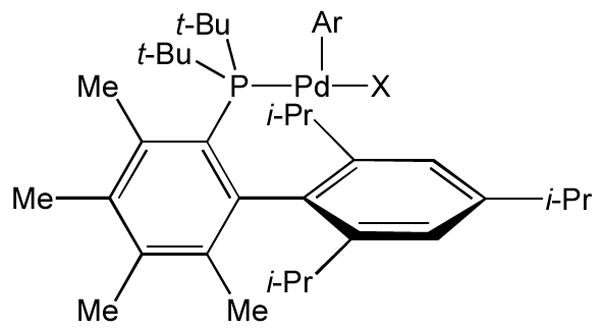
Pd(II) oxidative addition complex based on ligand 1.
2. Results and Discussion
2.1. Optimization of the Pd-catalyzed amidation reactions of aryl chlorides
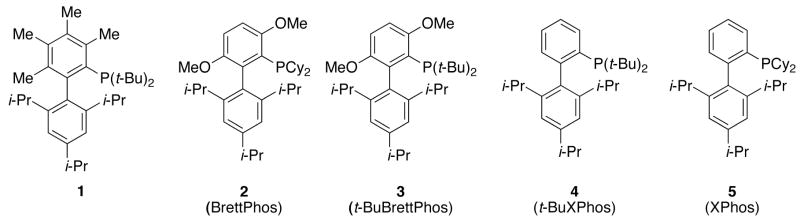
Our group recently disclosed a catalyst system, based on 2, that was highly active and selective for the monoarylation of primary amines.8 In this study we observed that, in solution, the oxidative addition complexes of 2 existed as two rotamers; for one of which the environment around the Pd center was less sterically demanding. We hypothesized that the substitution of a methoxy substituent ortho to the di-tert-butylphosphino group in 1 (see ligand 3) would allow for more freedom of rotation around the P-CAr bond and help promote transmetallation with relatively hindered substrates. This new ligand (3) was easily synthesized in 2 steps from commercially available starting materials (see experimental section)
Initial studies employing the supporting ligand 3 focused on the coupling of acetamide and 2-chlorotoluene in the presence of various Pd sources, bases, and solvents. We found that when the catalyst was formed using our recently reported water-mediated catalyst preactivation method9 with 3, Pd(OAc)2, and K3PO4 as the base in t-BuOH, the reaction gave a 99% GC yield after 40 minutes at 110 °C (Table 1, entry 1). This initial result was very promising as it represented the highest turnover frequency observed to date for the Pd-catalyzed amidation reactions of aryl chlorides bearing an ortho substituent.5a Switching the Pd source to Pd2(dba)3 or Pd(dba)2 resulted in a dramatic reduction in yield (Table 1, entries 2 and 3). This loss of activity is likely caused by coordination of the dba ligands to the Pd, which is a well known effect of dba in cross-coupling reactions.10 The use of Pd(II) salts (e.g., [(allyl)PdCl]2, (H3CCN)2PdCl2, or Pd(OAc)2) without preactivation, all of which need to be reduced quickly and efficiently in order to generate an active catalyst, leads to yields below 60%.11 These results demonstrated the importance of forming the active monoligated Pd(0) complex in these reactions. The use of carbonate bases showed similar results for this coupling reaction,12 but a substantial decline in yield was observed with all bases examined when other common solvents were used (Table 1, entries 9 and 10)
Table 1.
Screen of reaction parameters for the cross-coupling of acetamide and 2-chlorotoluene.
 | ||||
|---|---|---|---|---|
| Entry | Pd Source | Base | Solvent | Yield |
| 1 | Pd(OAc)2/H2O Act. | K3PO4 | t-BuOH | 99% |
| 2 | Pd2(dba)3 | K3PO4 | t-BuOH | 27% |
| 3 | Pd(dba)2 | K3PO4 | t-BuOH | 25% |
| 4 | [(allyl)PdCI]2 | K3PO4 | t-BuOH | 48% |
| 5 | (H3CCN)2PdCI2 | K3PO4 | t-BuOH | 46% |
| 6 | Pd(OAc)2 | K3PO4 | t-BuOH | 57% |
| 7 | Pd(OAc)2/H2O Act. | Cs2CO3 | t-BuOH | 99% |
| 8 | Pd(OAc)2/H2O Act. | K2C03 | t-BuOH | 96% |
| 9 | Pd(OAc)2/H2O Act. | K3PO4 | Toluene | 10% |
| 10 | Pd(OAc)2/H20 Act. | K3PO4 | 1,4-Dioxane | 0% |
2.2. Comparison of ligands for the Pd-catalyzed amidation reactions of aryl chlorides
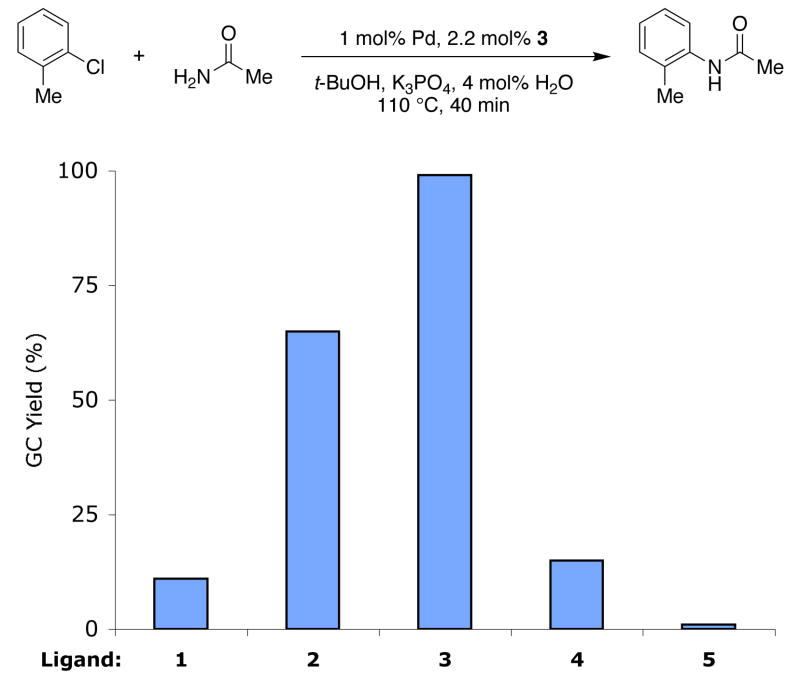
Encouraged by our initial results, we set out to compare reactions with ligand 3 directly with those employing other biarylphosphine ligands that had previously been used for Pd-catalyzed amidation reactions. Using a catalyst based on 1 for the cross-coupling of acetamide and 2-chlorotoluene resulted in a 12% yield (GC), whereas using a catalyst supported by 3 afforded the product in a 99% yield (Figure 3). This result demonstrates that changing the substituent ortho to the di-tert-butylphosphino group in 1 from a methyl to a methoxy group, as in 3, has a dramatic effect on the activity of these systems. We believe this increase in activity is due to the increased freedom of rotation around the P-CAr bond in 3, which may accelerate transmetallation. With 2, the dicyclohexylphosphino analogue of 3, a 65% yield of product was realized. With the ligand lacking the two methoxy substituents, t-BuXPhos (4), only a 15% yield of the desired product was formed.5a,13 These results reveal that both the methoxy group ortho to the di-tert-butylphosphino group and the di-tert-butyl groups on the phosphorus center in 3 are important to the high activity observed when this ligand is employed. Finally, with ligand 5 (XPhos), which had previously been reported to be suitable ligand for Pd-catalyzed amidation reactions of aryl tosylates, only a trace of product was observed.3e
Figure 3.
Results observed for the coupling of acetamide and 2-chlorotoluene with various ligands.
2.3. Substrate scope for the Pd-catalyzed amidation reactions of aryl chlorides using a catalyst system based on ligand 3
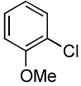
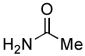
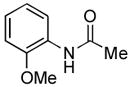
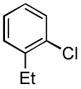
We next explored the scope of the Pd-catalyzed cross-coupling reactions of aryl chlorides and amides with our new catalyst system. We began by examining the effect of the ortho substituent on the aryl chloride. As we had already observed, 2-chlorotoluene was successfully coupled with acetamide in high yield (Table 2, entry 1). When the methyl substituent was replaced with the larger ethyl group the coupling still proceeded in good yield (Table 2, entry 2). When the ortho substituent was changed to an electron-donating methoxy group the rate of the coupling decreased slightly, the reaction took 3 hours to proceed to completion, but still gave 91% of the desired product (Table 2, entry 3). Aryl chlorides with ortho substitution were also coupled with benzamide in excellent yields (Table 2, entries 4 and 5). These examples show the superior reactivity of this system over previously reported catalysts. Unfortunately, the reactions of aryl chlorides with two ortho substituents failed to provide product under these conditions.
Table 2.
Pd-catalyzed cross-coupling of amides and aryl chlorides.a
| Entry | ArCl | Amide | Product | Time | Yieldb | Entry | ArCl | Amide | Product | Time | Yieldb |
|---|---|---|---|---|---|---|---|---|---|---|---|
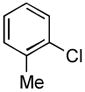
| 1 |
|
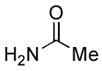
|
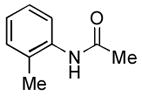
|
40 min | 83% | 9 |
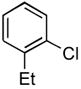
|
|
|
3 h | 85% |
| 2 |
|
|
|
1.5 h | 78% | 10 |
|
|
|
1.5 h | 90% |
| 3 |
|
|
|
3 h | 91% | 11 |
|
|
|
3 h | 84% |
| 4 |
|
|

|
3 h | 93% | 12 |
|
|
|
1.5 h | 96% |
| 5 |
|

|
|
1.5 h | 92% | 13 |
|
|
|
5 h | 90% |
| 6 |
|
|
|
2 h | 80% | 14 |
|
|
|
4 h | 70% |
| 7 |
|
|
|
5 h | 83% | 15 |
|
|
|
12 h | 61% |
| 8 |
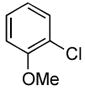
|
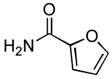
|
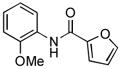
|
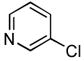
2 h | 98% | 16 |
|
|
|
1.5 h | 93% |
Reaction conditions: ArCl (1.0 equiv), amide (1.2 equiv), K3PO4 (1.4 equiv), Pd(OAc)2 (1 mol%), 3 (2.2 mol%), H2O (4 mol%), t-BuOH (2 mL/mmol), 110 °C.
Isolated yields, average of two runs.
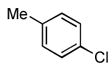
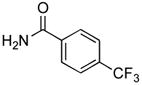
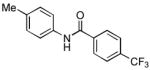
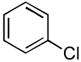
We next examined the reactions of aryl chlorides that lacked an ortho substituent. Utilizing 3 as the ligand, the reaction of 4-chlorotoluene with 4-trifluoromethylbenzamide gave the desired product in good yield with 1 mol% Pd in only 2 h (Table 2, entry 6). The reactions of similar substrates with the best previously reported catalyst system required times between 12 – 24 h.5a
Due to the ubiquity of heterocycles in many biologically active molecules, we next wanted to extend the scope of this system to heteroaryl substrates. Amides containing furans, pyridines, and thiophenes were all well tolerated in these reactions. These could be successfully coupled with a variety of aryl chlorides in good to excellent yields, all with reaction times that were <5 h using 1 mol% Pd (Table 2, entries 8 – 14). For example, the coupling of furan-2-carboxamide with 2-chloroanisole gave a 98% yield of the desired product in 2 h (Table 2, entry 8). Additonally, a heteroaryl chloride, 3-chloropyridine, was also an excellent coupling partner for this reaction (Table 2, entries 15 and 16).
3. Conclusion
In summary, a catalyst based on the new ligand 3 was developed for the Pd-catalyzed cross-coupling of amides and aryl chlorides. This system was much more active than previous catalyst systems that have been used for these reactions, particularly for the coupling of amides with aryl chlorides bearing an ortho substituent. An array of amides were successfully combined with aryl chlorides in good to excellent yields using 1 mol% Pd and with reaction times <5 h.
4. Experimental
4.1. General Reagent Information
All reactions were set up in the air and carried out under an atmosphere of argon. Flash chromatography was performed using a) silica gel from American International Chemical or b) a Biotage SP4 instrument with prepacked silica cartridges. The tert-butanol was purchased from Aldrich Chemical Co. in Sure-Seal bottles and was used as received. Pd(OAc)2 was a gift from BASF. Aryl halides and amides were purchased from Aldrich Chemical Co., Alfa Aesar, Acros Organics, or Oakwood Products and were used as purchased without further purification. Deionized water was degassed by brief (30 sec) sonication under vacuum and then evacuated and backfilled with argon (this procedure was repeated three times). Anhydrous tribasic potassium phosphate was purchased from Fluka Chemical Co., stored in a nitrogen filled glovebox and removed in small quantities and stored on the bench for up to two weeks. Ligands 1,5a 2,7 and 43e were synthesized using literature procedures and ligand 5 was purchased from Strem Chemicals Inc.
4.2. General Analytical Information
Yields refer to isolated yields of compounds greater than 95% purity as determined by gas chromatography (GC) and 1H NMR. All yields reported in Table 2 are for an average of two experiments. All compounds were characterized by 1H NMR, 13C NMR, IR spectroscopy, melting point, and in most cases, elemental analysis. Nuclear Magnetic Resonance spectra were recorded on a Varian 300 MHz instrument or a Bruker 400 MHz instruement. All 1H NMR experiments are reported in δ units, parts per million (ppm), and were measured relative to the solvent residual peak. All 13C NMR spectra are reported in ppm relative to solvent residual peak and were obtained with 1H decoupling. All IR spectra were made on a Perkin – Elmer 2000 FTIR. All GC analyses were performed on a Agilent 6890 gas chromatograph with an FID detector using a J & W DB-1 column (10 m, 0.1 mm I.D.). Elemental analyses were performed by Atlantic Microlabs Inc., Norcross, GA. HRMS spectra were performed on a Bruker APEXIV 4.7t FT-ICR mass spectrometer.
4.3. Synthesis of 3
An oven-dried 300 mL round bottom Schlenk flask, which was equipped with a magnetic stir bar, fitted with a rubber septum, and charged with 2-iodo-2′,4′,6′-triisopropyl-3,6-dimethoxybiphenyl7 (3.78 g, 8.11 mmol), was evacuated and backfilled with argon (this process was repeated a total of 3 times). THF (40 mL) was added via syringe, the reaction was cooled to −78 °C, and t-BuLi (1.7 M in Hexane, 9.54 mL, 16.22 mmol) was added in a dropwise fashion over a 20 min period. The solution was stirred for 30 min and then under a positive pressure of argon the septum was removed from the Schlenk flask and anhydrous CuCl (804 mg, 8.11 mmol), which was weighed out in nitrogen filled glovebox, was added rapidly. The flask was refitted with the rubber septum and ClP(t-Bu)2 (1.70 mL, 8.92 mmol) was added in a dropwise fashion over a 10 minute period. The reaction was warmed from −78 °C to room temperature at which point the flask was sealed with a Teflon screw cap and heated to 70 °C for 60 h. The solution was cooled to room temperature, diluted with ethyl acetate, washed with aqueous NH4OH (this process was repeated a total of three times), washed with brine, dried over MgSO4, and concentrated in vacuo. The crude material was recrystallized from hot methanol to yield the desired product as white crystals (2.081 g, 53%). 1H NMR (300 MHz, CDCl3) δ: 6.96 (s, 2H), 6.87 (d, J = 8.7 Hz, 1H), 6.83 (d, J = 9.0 Hz, 1H), 3.78 (s, 3H), 3.56 (s, 3H), 2.95 (septet, J = 6.9 Hz, 1H), 2.49 (septet, J = 6.6 Hz, 2H), 1.32 (d, J = 6.9 Hz, 6H), 1.21 (d, J = 6.6 Hz, 6H), 1.14 (s, 9H), 1.10 (s, 9H), 0.93 (d, J = 6.6 Hz, 6H) ppm. 13C NMR (75 MHz, CDCl3) δ: 155.9, 152.6, 152.5, 147.2, 146.7, 140.5, 140.0, 133.0, 127.6, 127.0, 120.1, 110.9, 108.2, 54.4, 54.0, 34.2, 34.1, 33.8, 32.1, 31.8, 31.3, 25.7, 24.3, 23.6 ppm (Observed complexity is due to P-C splitting). 31P NMR (121 MHz, CDCl3) δ: 34.78 ppm. IR (neat, cm−1): 2958, 2864, 1581, 1486, 1421, 1250, 1086, 1020, 799, 715. HRMS (ESI) Calcd. for C31H50O2P [M+H+]: 485.3543; Found: 485.3537.
4.4. General procedure for Table 2
An oven-dried test tube, which was equipped with a magnetic stir bar and fitted with a teflon screwcap septum, was charged with Pd(OAc)2 (1 mol%) and 3 (2.2 mol%). The vessel was evacuated and backfilled with argon (this process was repeated a total of 3 times) and t-BuOH (2.0 mL) and degassed H2O (4 mol%) were added via syringe. After addition of the water, the solution was heated to 110 °C for 1.5 min. A second oven-dried test tube equipped with a magnetic stir bar and fitted with a Teflon screwcap septum, was charged with amide (1.2 mmol) and K3PO4 (1.4 mmol) (aryl chlorides that were solids at room temperature were added with the base). The vessel was evacuated and backfilled with argon (this process was repeated a total of 3 times) and then the aryl chloride (1.0 mmol) was added via syringe and the activated catalyst solution was transferred from the first reaction vessel into the second via cannula. The solution was heated to 110 °C until the aryl chloride had been completely consumed as judged by GC analysis. The reaction mixture was then cooled to room temperature, diluted with ethyl acetate and washed with water. The layers were separated and the organic layer was dried over anhydrous MgSO4, filtered and concentrated in vacuo. The crude product was purified via flash chromatography on silica gel.
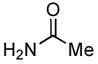
4.5. N-o-Tolylacetamide5a (Table 2, entry 1)
Following the general procedure, a mixture of 2-chlorotoluene (117μL, 1.0 mmol), acetamide (71 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 40 minutes. The crude product was purified by flash chromatography on silica gel (40% hexanes in EtOAc) to provide the title compound as a white solid (119 mg, 80%). Mp = 109 – 109 °C (lit. 108 – 109). 1H NMR (300 MHz, CDCl3) δ: 7.68 (d, J = 7.8 Hz, 1H), 7.23 – 7.15 (m, 3H) 7.07 (t, J = 7.5 Hz, 1H), 2.23 (s, 3H), 2.17 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 168.8, 135.7, 130.5, 130.1, 126.6, 125.5, 124.0, 24.1, 17.9 ppm. IR (neat, cm−1): 3290, 3028, 2981, 1654, 1588, 1531, 1486, 1460, 1369, 1288, 1272, 1118, 1039, 1018, 756, 714, 700, 653, 608, 562, 534, 446. Anal. Calcd. for C9H11NO: C, 72.46; H, 7.43. Found: C, 72.25, H, 7.59.
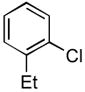
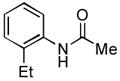
4.6. N-(2-Ethylphenyl)acetamide14 (Table 2, entry 2)
Following the general procedure, a mixture of 1-chloro-2-ethylbenzene (132μL, 1.0 mmol), acetamide (71 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 1.5 h. The crude product was purified by flash chromatography on silica gel (50% EtOAc in hexanes) to provide the title compound as a white solid (129 mg, 79%). Mp = 115 – 116 °C (lit. 111 – 111.8 °C). 1H NMR (300 MHz, CDCl3) δ: 7.63 (d, J = 7.2 Hz, 1H), 7.33 (bs, 1H), 7.21 – 7.10 (m, 3H), 2.57 (q, J = 7.5 Hz, 2H), 2.15 (s, 3H), 1.20 (t, J = 7.8 Hz, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 168.9, 136.0, 135.0, 128.6, 126.6, 125.9, 124.6, 24.27, 24.19, 14.0 ppm. IR (neat, cm−1): 3271, 3037, 2964, 2929, 2869, 1653, 1588, 1540, 1448, 1373, 1296, 1053, 1018, 971, 749, 717, 610, 487. HRMS (ESI) Calcd. for C10H14NO [M+H+]: 164.1070; Found: 164.1077.
4.7. N-(2-Methoxyphenyl)acetamide5a (Table 2, entry 3)
Following the general procedure, a mixture of 2-chloroanisole (127 μL, 1.0 mmol), acetamide (71 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 3 h. The crude product was purified by flash chromatography on silica gel (40% EtOAc in hexanes) to provide the title compound as a white solid (150 mg, 91%). Mp = 87 – 88 °C (lit. 87 – 88). 1H NMR (300 MHz, CDCl3) δ: 8.35 (dd, J = 8.1, 1.7 Hz, 1H), 7.78 (bs, 1H), 7.03 (td, J = 7.5, 1.8 Hz, 1H), 6.95 (td, J = 7.8, 1.5 Hz, 1H), 6.86 (dd, J = 8.1, 1.5 Hz, 1H), 3.87 (s, 3H), 2.19 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 168.3, 147.7, 127.8, 123.7, 121.1, 119.8, 109.9, 55.7, 25.0. ppm. IR (neat, cm−1): 3249, 3195, 3137, 3063, 3021, 2964, 1659, 1596, 1544, 1496, 1468, 1459, 1436, 1368, 1323, 1291, 1272, 1252, 1025, 751. Anal. Calcd. for C9H11NO2: C, 65.44; H, 6.71. Found: C, 65.89, H, 6.90.
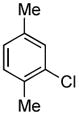
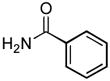
4.8. N-(2,5-Dimethylphenyl)benzamide (Table 2, entry 4)
Following the general procedure, a mixture of 2-chloro-p-xylene (133 μL, 1.0 mmol), benzamide (145 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 3 h. The crude product was purified by flash chromatography on silica gel (20% EtOAc in hexanes) to provide the title compound as a white solid (215 mg, 95%). Mp = 151 – 152 °C. 1H NMR (300 MHz, CDCl3) δ: 7.88 (dt, J = 6.6, 1.5 Hz, 2H), 7.78 (s, 1H), 7.71 (bs, 1H), 7.56 (tt, J = 6.9, 1.8 Hz, 1H), 7.49 (tt, J = 6.6, 1.5 Hz, 2H), 7.11 (d, J = 7.8 Hz, 1H), 6.94 (dd, J = 7.5, 0.9 Hz, 1H), 2.34 (s, 3H), 2.28 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 136.7, 135.6, 135.1, 131.9, 130.43, 130.41, 128.9, 127.1, 126.2, 123.8, 21.3, 17.5 ppm. IR (neat, cm−1): 3253, 2922, 1644, 1618, 1580, 1530, 1489, 1501, 1308, 1290, 1029, 875, 808, 708, 602, 450. Anal. Calcd. for C15H15NO: C, 79.97; H, 6.71. Found: C, 79.81; H, 6.78.
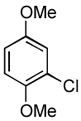
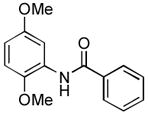
4.9. N-(2,5-Dimethoxyphenyl)benzamide15 (Table 2, entry 5)
Following the general procedure, a mixture of 2,5-dimethoxychlorobenzene (143μL, 1.0 mmol), benzamide (145 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 1.5 h. The crude product was purified by flash chromatography on silica gel (10% EtOAc in hexanes) to provide the title compound as a white-tan solid (242 mg, 94%). Mp = 87 – 88 °C (lit. 82 – 84 °C). 1H NMR (300 MHz, CDCl3) δ: 8.60 (bs, 1H), 8.30 (d, J = 3.0 Hz, 1H), 7.89 (dt, J = 6.6, 1.8 Hz, 2H), 7.58 – 7.46 (m, 3H), 6.83 (d, J = 9.0 Hz, 1H), 6.61 (dd, J = 9.0, 3.0 Hz, 1H), 3.87 (s, 3H), 3.82 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 165.3, 154.0, 142.4, 135.2, 131.9, 128.9, 128.5, 127.1, 110.7, 108.9, 105.9, 56.3, 55.9 ppm. IR (neat, cm−1): 3427, 3335, 3057, 3000, 2937, 2955, 2833, 1668, 1601, 1532, 1498, 1478, 1464, 1423, 1268, 1220, 1202, 1179, 1164, 1047, 1024, 862, 797, 704, 679. Anal. Calcd. for C15H15NO3: C, 70.02; H, 5.88. Found: C, 69.89; H, 6.03.
4.10. N-p-Tolyl-4-(trifluoromethyl)benzamide16 (Table 2, entry 6)
Following the general procedure, a mixture of 4-chlorotoluene (118μL, 1.0 mmol), 4-trifluoromethylbenzamide (227 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 2 h. The crude product was dissolved in acetone, evaporated on to a small amount of silica gel, and purified by flash chromatography on silica gel (gradient eluent: 10 – 30% EtOAc in hexanes) to provide the title compound as a white solid (222 mg, 80%). Mp = 235 – 236 °C (lit. 236 – 238 °C). 1H NMR (300 MHz, (CD3)2SO) δ: 10.4 (s, 1H), 8.14 (d, J = 7.8 Hz, 2H), 7.90 (d, J = 8.4 Hz, 2H), 7.66 (dd, J = 8.4 Hz, 2H), 7.17 (d, J = 8.1 Hz, 2H), 2.28 (s, 3H) ppm. 13C NMR (75 MHz, (CD3)2SO) δ: 164.2, 138.9, 136.4, 133.1, 131.5, 131.1, 129.5, 129.1, 128.6, 125.8, 125.4, 125.4, 120.5, 20.5 ppm (complexity is due to CF3-group). IR (neat, cm−1): 3333, 2919, 1649, 1599, 1580, 1530, 1407, 1160, 1125, 861, 815, 770. Anal. Calcd. for C15H12F3NO: C, 64.51; H, 4.33. Found: C, 65.00; H, 4.68.
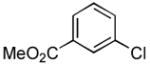
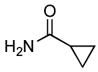
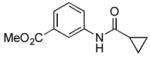
4.11. Methyl 3-(cyclopropanecarboxamido)benzoate(Table 2, entry 7)
Following the general procedure, a mixture of methyl 3-chlorobenzoate (139 μL, 1.0 mmol), cyclopropanecarboxamide (102 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 5 h. The crude product was purified by flash chromatography on silica gel (gradient eluent: 20 – 30% EtOAc in hexanes) to provide the title compound as a white solid (169 mg, 77%). Mp = 129 – 130 °C. 1H NMR (300 MHz, CDCl3) δ: 8.10 – 8.07 (m, 2H), 7.86 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.35 (t, J = 8.1 Hz, 1H), 3.87 (s, 3H), 1.55 (sep, J = 3.9 Hz, 1H), 1.08 (dt, J = 6.9, 3.9 Hz, 2H), 0.85 – 0.79 (m, 2H) ppm. 13C NMR (75 MHz, CDCl3) δ: 172.6, 167.0, 138.5, 130.8, 129.2, 125.1, 124.4, 120.7, 52.4, 15.7, 8.3 ppm. IR (neat, cm−1): 3294, 3013, 2952, 1724, 1663, 1596, 1548, 1488, 1431, 1395, 1302, 1273, 1241, 1197, 1178, 1107, 1082, 956, 755, 688. Anal. Calcd. for C12H13NO3: C, 65.74; H, 5.98. Found: C, 65.47; H, 6.10.
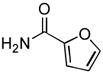
4.12. N-(2-Methoxyphenyl)furan-2-carboxamide(Table 2, entry 8)
Following general procedure A, a mixture of 2-chloroanisole (127 μL, 1.0 mmol), 2-furamide (133 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 2 h. The crude product was purified by flash chromatography on silica gel (10% EtOAc in hexanes) to provide the title compound as a white solid (208 mg, 96%). Mp = 123 – 124 °C. 1H NMR (300 MHz, CDCl3) δ: 8.78 (bs, 1H), 8.50 (dd, J = 7.8, 1.8 Hz, 1H), 7.52 (dd, J = 1.5, 0.6 Hz, 1H), 7.22 (dd, J = 3.6, 0.9 Hz, 1H), 7.08 (td, J = 7.8, 1.8 Hz, 1H), 7.00 (tdd, J = 7.7, 1.5, 0.3 Hz, 1H), 6.91 (dd, J = 8.1, 1.5 Hz, 1H), 6.54 (dd, J = 3.6, 1.8 Hz, 1H) 3.93 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 156.0, 148.3, 148.1, 144.28, 144.26, 127.3, 124.0, 121.2, 119.8, 115.03, 114.98, 112.57, 112.53, 110.0, 55.9 ppm (complexity of 13C NMR data is due to detection of rotamers). IR (neat, cm−1): 3410, 3141, 3118, 3019, 2979, 2943, 2843, 1672, 1606, 1584, 1534, 1524, 1462, 1437, 1251, 1226, 1216, 1105, 1020, 1012, 765, 750, 653, 604, 593. Anal. Calcd. for C12H11NO3: C, 66.35; H, 5.10,. Found: C, 66.16; H, 5.19.
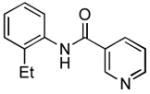
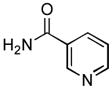
4.13. N-(2-Ethylphenyl)nicotinamide(Table 2, entry 9)
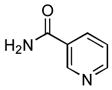
Following the general procedure, a mixture of 1-chloro-2-ethylbenzene (132μL, 1.0 mmol), nicotinamide (147 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 3 h. The crude product was purified by flash chromatography on silica gel (80% EtOAc in hexanes) to provide the title compound as a white solid (189 mg, 83%). Mp = 101 – 102.5 °C. 1H NMR (300 MHz, (CD3)2SO) δ: 10.1 (s, 1H), 9.13 (d, J = 2.1 Hz, 1H), 8.77 (dd, J = 4.8, 1.2 Hz, 1H), 8.31 (d, J = 7.8 Hz, 1H), 7.58 (dd, J = 8.1, 4.8 Hz, 1H), 7.33 – 7.23 (m, 4H), 2.63 (q, J = 7.8 Hz, 2H), 1.13 (t, J = 7.8 Hz, 3H) ppm. 13C NMR (75 MHz, (CD3)2SO) δ: 164.3, 152.2, 148.7, 139.9, 135.4, 130.1, 128.6, 127.6, 126.8, 126.2, 123.6, 24.0, 14.2 ppm. IR (neat, cm−1): 3262, 3035, 2968, 2934, 2875, 1651, 1591, 1525, 1491, 1473, 1452, 1418, 1306, 1275, 1026, 759, 749, 706. HRMS (ESI) Calcd. for C14H15N2O [M+H+]: 227.1179; Found: 227.1176.
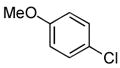
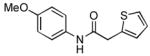
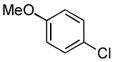
4.14. N-(4-Methoxyphenyl)-2-(thiophen-2-yl)acetamide(Table 2, entry 10)
Following the general procedure, a mixture of 4-chloroanisole (122μL, 1.0 mmol), 2- thiopheneacetamide (169 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 1.5 h. The crude product was purified via the Biotage SP4 (silica-packed 25+M; 0 – 100% EtOAc/hexanes) to provide the title compound as a pale yellow solid (223 mg, 90%). Mp = 122 – 123 °C. 1H NMR (400 MHz, CD3CN) δ: 8.35 (bs, 1H), 7.43 (dt, J = 9.0, 3.4 Hz, 2H), 7.29 (dd, J = 4.4, 2.0 Hz, 1H), 6.99 – 6.97 (m, 2H), 6.86 (dt, J = 9.0, 3.3 Hz, 2H), 3.82 (s, 2H), 3.75 (s, 3H) ppm. 13C NMR (100 MHz, CD3CN) δ: 168.9, 157.1, 138.1, 132.7, 127.7, 127.6, 125.9, 122.3, 114.8, 55.9, 38.4. IR (neat, cm−1): 3329, 3106, 2960, 2834, 1659, 1609, 1544, 1511, 1454, 1407, 1310, 1242, 1173, 1025, 831, 702. HRMS (ESI) Calcd. for C13H14NO2S [M+H+]: 248.0740; Found: 248,0740.
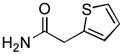
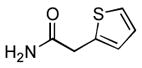
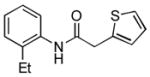
4.15. N-(2-Ethylphenyl)-2-(thiophen-2-yl)acetamide(Table 2, entry 11)
Following the general procedure, a mixture of 1-chloro-2-ethylbenzene (132μL, 1.0 mmol), 2- thiopheneacetamide (169 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 3 h. The crude product was purified by flash chromatography on silica gel (20% EtOAc in hexanes) to provide the title compound as a white solid (207 mg, 84%). Mp = 120.5 – 121.5 °C. 1H NMR (300 MHz, CDCl3) δ: 7.92 (d, J = 7.8 Hz, 1H), 7.35 – 7.33 (m, 1H), 7.20 (td, J = 8.1, 2.1 Hz, 1H), 7.14 – 7.05 (m, 4H), 3.99 (s, 3H), 2.31 (q, J = 7.5 Hz, 2H), 0.99 (t, J = 7.5 Hz, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 168.0, 136.0, 134.8, 134.3, 128.8, 128.2, 127.8, 126.9, 126.4, 125.4, 122.6, 38.5, 24.5, 14.1 ppm. IR (neat, cm−1): 3257, 3034, 2963, 2930, 2871, 1655, 1585, 1532, 1447, 1405, 1342, 1256, 967, 752, 689. HRMS (ESI) Calcd. for C14H16NOS [M+H+]: 246.0947; Found: 246.0949.
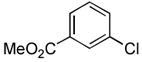
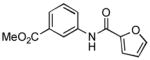
4.16. Methyl 3-(furan-2-carboxamido)benzoate(Table 2, entry 12)
Following the general procedure, a mixture of methyl 3-chlorobenzoate (139μL, 1.0 mmol), 2-furamide (133 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 1.5 h. The crude product was purified by flash chromatography on silica gel (30% EtOAc in hexanes) to provide the title compound as a white solid (237 mg, 97%). Mp = 83 – 85 °C. 1H NMR (300 MHz, CDCl3) δ: 8.39 (bs, 1H), 8.19 (t, J = 1.8 Hz, 1H), 8.02 (ddd, J = 8.1, 2.4, 1.2 Hz, 1H), 7.78 (ddd, J = 7.8, 1.5, 0.9 Hz, 1H), 7.45 (dd, J = 1.8, 0.8 Hz, 1H), 7.39 (t, J = 8.1 Hz, 1H), 7.21 (dd, J = 4.2, 0.8 Hz, 1H), 6.51 (dd, J = 3.6, 1.8 Hz, 1H), 3.87 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ: 166.7, 156.3, 147.5, 144.54, 144.51, 137.7, 130.9, 129.3, 125.5, 124.5, 120.9, 115.7, 115.6, 112.71, 112.67, 52.3 ppm (complexity of 13C NMR data is due to detection of rotamers). IR (neat, cm−1): 3325, 3130, 2952, 2845, 1722, 1668, 1598, 1583, 1542, 1489, 1473, 1441, 1323, 1294, 1265, 1230, 1165, 1118, 1083, 1012, 884, 755, 684, 594. HRMS (ESI) Calcd. for C13H12NO4 [M+H+]: 246.0761; Found: 246.0771.
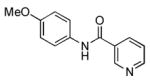
4.17. N-(4-Methoxyphenyl)nicotinamide(Table 2, entry 13)
Following the general procedure, a mixture of 4-chloroanisole (122 μL, 1.0 mmol), nicotinamide (147 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 5 h. The crude product was purified by flash chromatography on silica gel with EtOAc as eluent to provide the title compound as a white solid (192 mg, 84%). Mp = 152 – 154 °C. 1H NMR (300 MHz, (CD3)2SO) δ: 10.3 (s, 1H), 9.11 (dd, J = 2.1, 0.8 Hz, 1H), 8.75 (dd, J = 4.8, 1.5 Hz, 1H), 8.28 (ddd, J = 7.8, 2.1, 1.5 Hz, 1H), 7.69 (d, J = 9.0 Hz, 2H), 7.55 (ddd, J = 7.8, 4.8, 0.9 Hz, 1H), 6.95 (d, J = 9.0 Hz, 2H), 3.75 (s, 3H) ppm. 13C NMR (75 MHz, (CD3)2SO) δ: 163.6, 155.8, 152.0, 148.7, 135.4, 131.9, 130.7, 123.5, 122.0, 113.8, 55.2 ppm. IR (neat, cm−1): 3321, 3010, 2952, 2838, 1672, 1643, 1614, 1546, 1510, 1485, 1423, 1409, 1276, 1248, 1177, 1124, 1030, 824, 706. Anal. Calcd. for C13H12N2O2: C, 68.41; H, 5.30. Found: C, 67.86; H, 5.26.
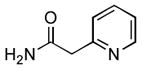
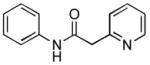
4.18. N-Phenyl-2-(pyridin-2-yl)acetamide(Table 2, entry 14)
Following the general procedure, a mixture of chlorobenzene (102μL, 1.0 mmol), pyridine-2-acetamide (163 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 4 h. The crude product was purified via the Biotage SP4 (silica-packed 25+M; 0 – 100% EtOAc/hexanes) to provide the title compound as a white solid (178 mg, 84%). Mp = 135 – 136 °C. 1H NMR (400 MHz, (CD3)2SO) δ: 10.3 (s, 1H), 8.45 (d, J = 4.4 Hz, 1H), 7.75 (td, J = 7.6, 1.8 Hz, 1H), 7.62 (d, J = 7.7 Hz, 2H), 7.40 (d, J = 7.8 Hz, 1H), 7.32 – 7.25 (m, 3H), 7.04 (t, J = 7.4 Hz, 1H), 3.85 (s, 2H) ppm. 13C NMR (100 MHz, (CD3)2SO) δ: 168.2, 156.1, 149.0, 139.2, 136.6, 128.7, 124.0, 123.2, 121.9, 119.1, 45.9. IR (neat, cm−1): 3228, 3185, 3011, 2973, 1676, 1593, 1545, 1496, 1476, 1444, 1343, 1326, 1274, 1209, 1150, 1002, 780, 747, 685. HRMS (ESI) Calcd. for C13H13N2O [M+H+]: 213.1022; Found: 213.1025.
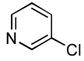
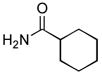
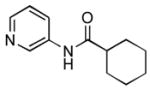
4.19. N-(Pyridin-3-yl)cyclohexanecarboxamide17(Table 2, entry 15)
Following the general procedure, a mixture of 3-chloropyridine (95 μL, 1.0 mmol), cyclohexanecarboxamide (153 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 12 h. The crude product was purified by flash chromatography on silica gel with EtOAc as eluent followed by a second purification via the Biotage SP4 (silica-packed 25+M; 70 – 100% EtOAc in hexanes) to provide the title compound as a white solid (133 mg, 65%). Mp = 128.5 – 130.5 °C (lit. 134 – 135 °C). 1H NMR (300 MHz, (CD3)2SO) δ: 10.0 (s, 1H), 8.75 (d, J = 2.4 Hz, 1H), 8.22 (dd, J = 4.8, 1.5 Hz, 1H), 8.05 (dt, J = 8.4, 1.8 Hz, 1H), 7.30 (dd, J = 8.1, 1.5 Hz, 1H), 2.34 (tt, J = 11.4, 3.3 Hz, 1H), 1.82 – 1.62 (m, 5H), 1.46 – 1.14 (m, 5H) ppm. 13C NMR (75 MHz, CDCl3) δ: 174.9, 143.9, 140.7, 136.1, 125.9, 123.5, 44.8, 29.1, 25.4, 25.2 ppm. IR (neat, cm−1): 3299, 3180, 3121, 3047, 2931, 2855, 1667, 1600, 1587, 1543, 1483, 1450, 1422, 1335, 1278, 1196, 1173, 1131, 949, 896, 804, 761, 707, 635. HRMS (ESI) Calcd. for C12H17N2O [M+H+]: 205.1335; Found: 205.1326.
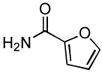
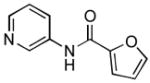
4.20. N-(Pyridin-3-yl)furan-2-carboxamide(Table 2, entry 16)
Following general procedure A, a mixture of 3-chloropyridine (95μL, 1.0 mmol), 2-furamide (133 mg, 1.2 mmol), K3PO4 (297 mg, 1.4 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), 3 (11 mg, 0.022 mmol), H2O (0.7μL, 0.04 mmol) and t-BuOH (2.0 mL) was heated to 110 °C for 1.5 h. The crude product was purified by flash chromatography on silica gel (gradient eluent: 50 – 100% EtOAc in CH2Cl2) to provide the title compound as a white-tan solid (181 mg, 96%). Mp = 142 – 143 °C. 1H NMR (300 MHz, CD3OD) δ: 8.88 (dd, J = 2.4, 0.8 Hz, 1H), 8.27 (dd, J = 4.5, 1.4 Hz, 1H), 8.22 (ddd, J = 8.4, 2.4, 1.5 Hz, 1H), 7.74 (dd, J = 1.8, 0.9 Hz, 1H), 7.41 (ddd, J = 8.4, 4.8, 0.6 Hz, 1H), 7.29 (dd, J = 3.6, 0.8 Hz, 1H), 6.63 (dd, J = 3.5, 1.7 Hz, 1H) ppm. 13C NMR (75 MHz, CD3OD) δ: 159.0, 148.5, 147.0, 145.5, 142.6, 137.0, 129.8, 125.2, 116.8, 113.4 ppm. IR (neat, cm−1): 3248, 3118, 3044, 1670, 1601, 1578, 1538, 1483, 1470, 1423, 1332, 1298, 1229, 1167, 1012, 884, 802, 756, 706, 595. HRMS (ESI) Calcd. for C10H9N2O2 [M+H+]: 189.0659; Found: 189.0661.
Supplementary Material
Figure 1.
Biarylphosphine ligands.
Acknowledgments
We thank the National Institutes of Health (NIH) for financial support of this project (Grant GM-58160). We thank Merck, BASF (Pd compounds), Chemetall (Cs2CO3), and Nippon Chemical for additional support. BPF thanks the William Asbornsen Albert Memorial Fund for a fellowship. KD thanks the Department of Chemistry and the Interdisciplinary Nanoscience Center, University of Aarhus for funding. QZ thanks the China Scholarship Council and the National Science Foundation of China (Grant No. 20672088) for financial support. The Varian NMR instrument used was supported by the NSF (Grants CHE 9808061 and DBI 9729592).
Footnotes
Dedication
This paper is dedicated to Larry Overman on the occasion of his receipt of the 2008 Tetrahedron Prize in Organic Chemistry.
References and Notes
- 1.(a) Lindley J. Tetrahedron. 1984;40:1433. [Google Scholar]; (b) Goldberg I. Chem Ber. 1906;39:1691. [Google Scholar]
- 2.(a) Strieter ER, Bhayana B, Buchwald SL. J Am Chem Soc. 2009;131:78. doi: 10.1021/ja0781893. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ma D, Cai Q. Acc Chem Res. 2008;41:1450. doi: 10.1021/ar8000298. [DOI] [PubMed] [Google Scholar]; (c) Ley SV, Thomas AW. Angew Chem Int Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; (d) Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428. [Google Scholar]; (e) Klapars A, Huang X, Buchwald SL. J Am Chem Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]; (f) Klapars A, Antilla JC, Huang X, Buchwald SL. J Am Chem Soc. 2001;123:7727. doi: 10.1021/ja016226z. [DOI] [PubMed] [Google Scholar]
- 3.(a) Imbriglio JE, DiRocco D, Raghavan S, Ball RG, Tsou N, Mosley RT, Tata TR, Colletti SL. Tetrahedron Lett. 2008;49:4897. [Google Scholar]; (b) Klapars A, Campos KR, Chen C, Volante RP. Org Lett. 2005;7:1185. doi: 10.1021/ol050117y. [DOI] [PubMed] [Google Scholar]; (c) Willis MC, Brace GN, Holmes IP. Synlett. 2005:3229. doi: 10.1002/anie.200461598. [DOI] [PubMed] [Google Scholar]; (d) Wallace JW, Klauber DJ, Chen C, Volante RP. Org Lett. 2003;5:4749. doi: 10.1021/ol035959g. [DOI] [PubMed] [Google Scholar]; (e) Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am Chem Soc. 2003;125:6653. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]
- 4.(a) Shen Q, Hartwig JF. J Am Chem Soc. 2007;129:7734. doi: 10.1021/ja0722473. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McLaughlin M, Palucki M, Davies IW. Org Lett. 2006;8:3311. doi: 10.1021/ol061233j. [DOI] [PubMed] [Google Scholar]; (c) Shi F, Smith MR, Maleczka RE. Org Lett. 2006;8:1411. doi: 10.1021/ol060207i. [DOI] [PubMed] [Google Scholar]; (d) Yin J, Buchwald SL. J Am Chem Soc. 2002;124:6043. doi: 10.1021/ja012610k. [DOI] [PubMed] [Google Scholar]; (e) Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM. J Org Chem. 1999;64:5575. doi: 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ikawa T, Barder TE, Biscoe MR, Buchwald SL. J Am Chem Soc. 2007;129:13001. doi: 10.1021/ja0717414. [DOI] [PubMed] [Google Scholar]; (b) Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Angew Chem Int Ed. 2005;44:1371. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]; (c) Ghosh A, Sieser JE, Riou M, Cai W, Rivera-Ruiz L. Org Lett. 2003;5:2207. doi: 10.1021/ol034428p. [DOI] [PubMed] [Google Scholar]
- 6.(a) Nodwell M, Pereira A, Riffell JL, Zimmerman C, Patrick BO, Roberge M, Anderson RJ. J Org Chem. 2009;74:995. doi: 10.1021/jo802322s. [DOI] [PubMed] [Google Scholar]; (b) Surry DS, Buchwald SL. Angew Chem Int Ed. 2008;47:6338. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li X, Vince R. Bioorg Med Chem. 2006;14:5742. doi: 10.1016/j.bmc.2006.04.011. [DOI] [PubMed] [Google Scholar]; (d) Bringmann G, Gulder T, Reichert M, Meyer F. Org Lett. 2006;8:1037. doi: 10.1021/ol052946p. [DOI] [PubMed] [Google Scholar]
- 7.Barder TE, Buchwald SL. J Am Chem Soc. 2007;129:12003. doi: 10.1021/ja073747z. [DOI] [PubMed] [Google Scholar]
- 8.Fors BP, Watson DW, Biscoe MR, Buchwald SL. J Am Chem Soc. 2008;130:13552. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fors BP, Krattiger P, Strieter E, Buchwald SL. Org Lett. 2009;10:3505. doi: 10.1021/ol801285g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Fairlamb IJS, Kapdi AR, Lee AF, McGlacken GP, Weissburger F, de Vries AHM, de Vondervoort LS. Chem Eur J. 2006;12:8750. doi: 10.1002/chem.200600473. [DOI] [PubMed] [Google Scholar]; (b) Mace Y, Kapdi AR, Fairlamb IJS, Jutand A. Organometallics. 2006;14:1818. [Google Scholar]; (c) Amatore C, Jutand A. Coord Chem Rev. 1998;178–180:511. [Google Scholar]
- 11.Pd(II) salts must be reduced in situ to form the active Pd(0) catalyst. For examples see: Denmark SE, Smith RC. Synlett. 2006:2921.Viciu MS, Germaneau RF, Navarro-Fernandez O, Stevens ED, Nolan SP. Organometallics. 2002;21:5470.Trzeciak AM, Ciunik Z, Ziolowski JJ. Organometallics. 2002;21:132.Amatore C, Jutand A, M’Barki MA. Organometallics. 1992;11:3009.
- 12.K3PO4 was used for the remainder of the paper due to its lower cost than carbonate bases.
- 13.Anderson KW, Tundel RE, Ikawa T, Altman RA, Buchwald SL. Angew Chem Int Ed. 2006;45:6523. doi: 10.1002/anie.200601612. [DOI] [PubMed] [Google Scholar]
- 14.Hansch C. J Org Chem. 1955;20:10266. [Google Scholar]
- 15.Lyon MA, Lawrence S, Williams DJ, Jackson YA. J Chem Soc, Perkin Trans 1. 1999;4:437. [Google Scholar]
- 16.Zhang Z, Yu Y, Liebeskind LS. Org Lett. 2008;10:3005. doi: 10.1021/ol8009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barton DHR, Ozbalik N, Vacher B. Tetrahedron. 1988;44:3501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.