Abstract
Objectives
The purpose of this study was to determine the normal volume ranges of cochlear duct, saccule, and utricle, and to assess endolymphatic hydrops in Ménière disease.
Study Design
Retrospective temporal bone study.
Methods
Three-dimensional (3-D) images of membranous labyrinth were reconstructed from 31 normal temporal bones, six temporal bones from three patients with bilateral Ménière disease, and 16 temporal bones from eight patients with unilateral Ménière disease. Volumes of each part of membranous labyrinth were measured in each temporal bone group after 3-D reconstruction.
Results
The mean volumes and upper normal volume limits (over the 95% confidence interval) of the cochlear duct, saccule, and utricle were 7.67 and 9.77 mm3, 2.42 and 3.68 mm3, and 10.65 and 16.45 mm3, respectively. All three patients with bilateral Ménière disease showed endolymphatic hydrops (excess of volume over normal limits) in both ears. Of eight patients with unilateral Ménière disease, five had no symptom in the contralateral ear, whereas three patients had histories of progression from uni-lateral to bilateral Ménière disease 13–21 years after the initial onset. All of the diseased and three of eight contralateral ears showed endolymphatic hydrops. In contrast, no hydrops was observed in any part of the membranous labyrinth in asymptomatic ears.
Conclusions
Our findings suggest that cochleosaccular hydrops is a sensitive finding in Ménière disease. In addition, the volume data obtained from this study could be useful as a standard value for the assessment of hydrops in diagnostic imaging of the inner ear in Ménière disease.
Keywords: Ménière disease, membranous labyrinth, volume measurement, endolymphatic hydrops, three-dimensional reconstruction, human temporal bone
INTRODUCTION
Endolymphatic hydrops is a typical finding of Ménière disease in histopathologic examination of temporal bones. Severe forms of hydrops in cochlear duct and saccule, and mild to moderate hydrops of utricle, have been reported in the temporal bones with Ménière disease.1 Endolymphatic hydrops has been thought to precede Ménière symptoms and be the cause of symptoms such as episodic vertigo and sensorineural hearing loss, but there are still controversies on this issue. Hydrops has been recently reported to be considered as a histologic marker for Ménière syndrome rather than being directly responsible for its symptoms in a human temporal bone study.2 Hydrops can be studied under light microscopy by observing the changes of Reisner's membrane, saccular membrane, and utricular membrane.3-5 However, the main drawback for two-dimensional (2-D) assessment of endolymphatic hydrops under light microscopy is that defining and grading hydrops is difficult and subjective, especially in the case of mild dilated membranous labyrinth. In addition, it is much better to evaluate hydrops in the whole membranous labyrinth, in all of the temporal bone sections. To overcome these shortcomings and study endolymphatic hydrops more precisely, it is important to reconstruct detailed 3-D images of the membranous labyrinth from serial temporal bone sections and to establish the normal limits of volumes in each part of the membranous labyrinth (cochlear duct, saccule, and utricle). With the increasing processing power of computers and availability of higher resolution imaging, endolymphatic hydrops of the inner ear can be detected in living patients using magnetic resonance imaging (MRI) with gadolinium enhancement.6,7 The impossibility of direct studies of the relationship between hydrops and Ménière symptoms in animal models and living Ménière disease patients makes diagnostic imaging very important in the study of Ménière disease. Establishing the normal volume range for membranous labyrinth in normal temporal bones would also be important, because it could be useful as a reference and gold standard for evaluating endolymphatic hydrops in these diagnostic imaging techniques.
We reconstructed 3-D images of the inner ear from serial temporal bone sections to establish the normal volume-ranges of cochlear duct, saccule, and utricle in normal temporal bones. We also reconstructed 3-D images of Ménière disease and measured the volumes of membranous labyrinth to clarify the localization and significance of hydrops (excess of volume over normal limits) in the temporal bones with Ménière disease.
MATERIALS AND METHODS
We selected 31 normal temporal bones (right, 15; left, 16) from 25 individuals with no history of ear diseases, ototoxic drugs, or systemic diseases that affect the inner ear. Temporal bones with histopathological findings other than presbycusis-associated changes were excluded. We also randomly selected six temporal bones from three cases with bilateral Ménière disease and 16 temporal bones from eight cases with unilateral Ménière disease. Clinical diagnosis of Ménière disease had been made based on the criteria of the committee on hearing and equilibrium, American Academy of Otolaryngology–Head and Neck Surgery.8
The temporal bones were obtained from the collection of the Otopathology Laboratory, University of Minnesota, Minneapolis, Minnesota, U.S.A. All of the temporal bones had been removed at autopsy, fixed in formalin solution, decalcified, and embedded in celloidin. Each temporal bone was serially sectioned in the horizontal plane at a thickness of 20 μm. Every 5–10th section was stained with hematoxylin and eosin and mounted on a glass slide. The reference marks, which allow the orientation of the sections to be maintained, were not inserted in these sections.
Entire serial sections of temporal bones were captured at a resolution of 150 pixels per inch, using a digital camera connected to a personal computer equipped with ACT-1 control software (Nikon Instech Co., Ltd., Kanagawa, Japan). A 2-mm scale bar was superimposed on these captured sections to calibrate the scale value of 3-D images.
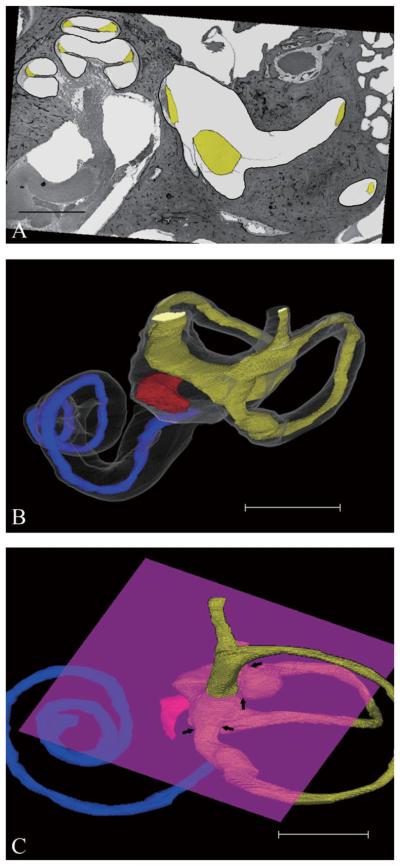
The following is the process of 3-D reconstruction that was performed using commercially available personal computer (PC) software, TRI/3D-SRF II (RATOC System Engineering Co., Ltd., Tokyo, Japan). The binary tint images were made from all the sections and aligned automatically. The results of alignment were checked, and the images were realigned in more detail. The membranes of cochlear duct, saccule, utricle, and lumina of semicircular canals were traced precisely on the PC monitor and the insides of these target objects were labeled in yellow (Fig. 1A). The rupture of the membranous labyrinth also could be traced with lines connecting both ends of ruptured membranes. Four additional intermediate target images were automatically generated from reading previous/next section images after the extraction of the target objects. The 3-D reconstruction images were generated from these extracted target images (Fig. 1B). The utricle and semicircular canals were separated using a clipping plane, which allowed clipping the images under 3-D view (Fig. 1C). Finally, the volumes of target objects were measured in each temporal bone.
Fig. 1.
(A) Horizontal histopathologic section of normal temporal bone. The target is traced precisely and filled with yellow. Scale bar indicates 3 mm. (B) Computer-generated three-dimensional images of normal temporal bone (anterior-medial view). The superior semicircular canal is not reconstructed. Blue, red, and yellow indicate cochlear duct, saccule, and utricle, respectively. Scale bar indicates 5 mm. (C) The utricle and semicircular canals are divided by the clipping plane at the root of nonampullated end of the semicircular canal or the boundary between the utricle and the ampulla of the semicircular canal (arrows). Scale bar indicates 4 mm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The Kolmogorov-Smirnov test (with Lilliefors's correction) was used to test data for normality of the estimated underlying population in the volumes of cochlear duct, saccule, and utricle of the normal temporal bones. Mann-Whitney rank sum test was used to detect the difference in the total volume of membranous labyrinth between two groups. Significant difference was defined as P < .05.
RESULTS
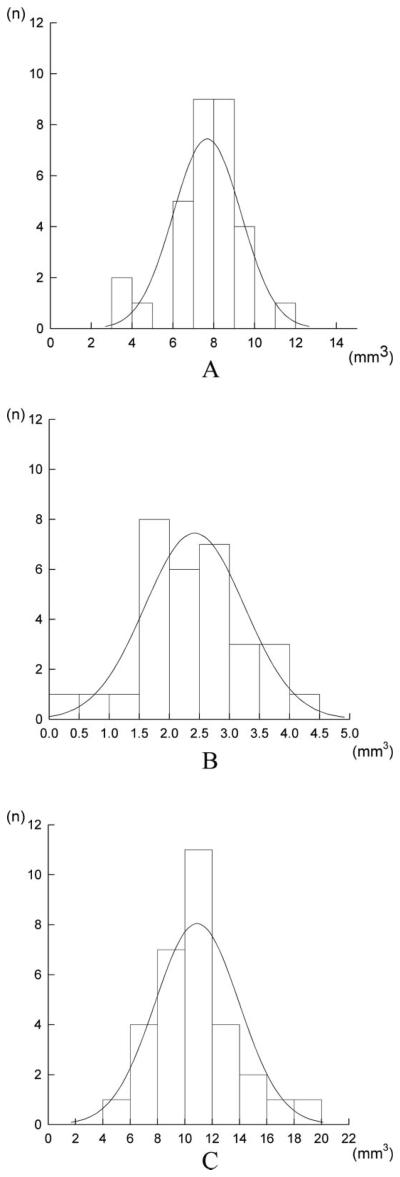
No rupture was seen in any part of membranous labyrinth in normal temporal bones. The volume data of the cochlear duct, saccule, and utricle in the normal temporal bones fitted the pattern with a normal distribution. The volumes (mean ± standard deviation) of the cochlear duct, saccule, and utricle were 7.67 ± 1.66, 2.42 ± 0.83, and 10.65 ± 2.80 mm3, respectively (Fig. 2). The 95% confidence interval values of the abovementioned structures' volumes were considered as upper normal limits. They were as follows: 9.77 mm3 in the cochlear duct, 3.68 mm3 in the saccule, and 16.45 mm3 in the utricle. The total volume of membranous labyrinth excluding semicircular canals was 20.75 ± 4.13 mm3.
Fig. 2.
(A) Histogram of the volumes of cochlear duct in normal temporal bones. The volumes are distributed in the range of 3.45–11.53 mm3. (B) Histogram of the volumes of saccule in normal temporal bones. The volumes are distributed in the range of 0.45–4.31 mm3. (C) Histogram of the volumes of utricle in normal temporal bones. The volumes are distributed in the range of 5.22–18.26 mm3.
The rupture was seen in 13 of 22 temporal bones with Ménière disease. The most common site of rupture was utricle (nine of 22), followed by ampullae of the semicircular canals (six of 22). Rupture of saccular membrane was seen in only two temporal bones.
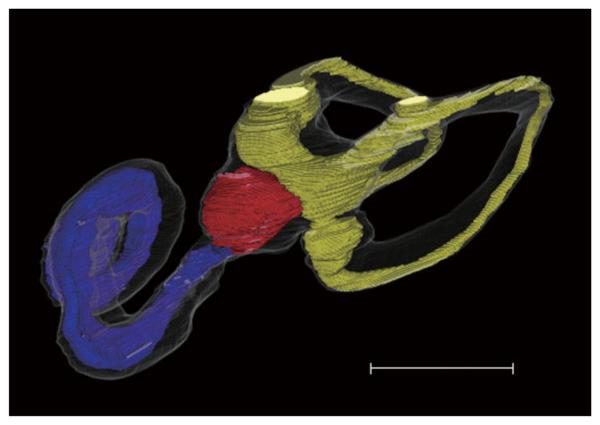
The volumes of the cochlear duct, saccule, and utricle were measured in each Ménière disease temporal bone (Fig. 3 and Table I). Cochleosaccular hydrops was detected in both sides in all of the cases with bilateral Ménière disease. Two of three cases also showed bilateral utricular hydrops. All cases with unilateral Ménière disease had saccular hydrops in the diseased sides of temporal bones. Cochlear hydrops was observed in all of the diseased sides of temporal bones except two. Utricular hydrops was seen in four of eight diseased sides. Contralateral sides of temporal bones in three of eight unilateral Ménière disease patients showed saccular hydrops. Cochlear and utricular hydrops was also seen in two and one of three contralateral sides in these patients, respectively. These three patients had shown fluctuating or sensorineural hearing loss in the symptomatic contralateral ears 13–21 years after initial onset.
Fig. 3.
Computer-generated three-dimensional images of Ménière disease (anterior-medial view). The superior semicircular canal is not reconstructed. Blue, red, and yellow indicate cochlear duct, saccule, and utricle, respectively. Note cochlear and saccular hydrops. Scale bar indicates 5 mm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE I.
Volume of Cochlear Duct, Saccule, and Utricle in Ménière Disease.
| Side | Case No. | Volume (mm3) |
||
|---|---|---|---|---|
| Cochlear Duct | Saccule | Utricle | ||
| Bilateral MD | ||||
| Right | 1 | 13.79* | 23.69* | 9.86 |
| 2 | 34.28* | 23.02* | 16.54* | |
| 3 | 36.89* | 28.60* | 17.97* | |
| Left | 1 | 12.75* | 20.10* | 10.60 |
| 2 | 34.35* | 26.57* | 21.84* | |
| 3 | 17.88* | 19.92* | 17.02* | |
| Unilateral MD | ||||
| Diseased | 4 | 34.53* | 20.96* | 27.78* |
| 5 | 5.67 | 16.16* | 16.84* | |
| 6 | 29.72* | 11.28* | 16.65* | |
| 7 | 20.53* | 30.25* | 15.84 | |
| 8 | 41.85* | 3.90* | 12.49 | |
| 9 | 19.66* | 10.82* | 13.66 | |
| 10 | 7.79 | 21.26* | 14.62 | |
| 11 | 34.84* | 28.37* | 41.60* | |
| Contralateral | 4 | 8.59 | 3.58 | 11.33 |
| 5 | 4.06 | 1.95 | 15.86 | |
| 6 | 4.87 | 3.08 | 14.49 | |
| 7 | 6.78 | 2.75 | 13.52 | |
| 8 | 7.19 | 2.76 | 9.91 | |
| 9 | 30.14* | 23.13* | 10.58 | |
| 10 | 8.72 | 22.08* | 10.86 | |
| 11 | 37.39* | 9.15* | 57.75* | |
Indicates endolymphatic hydrops (excess of volume over normal limits).
MD = Ménière disease.
The mean total volumes of membranous labyrinth in bilateral and diseased sides of unilateral Ménière disease were significantly larger than in normal temporal bones (64.28 mm3, P < .001; and 62.13 mm3 vs. 20.75 mm3, P < .001).
DISCUSSION
The mean volume of saccule (2.42 mm3) in our study corresponded to the results of Igarashi et al.9 In that study, the mean volume of the saccule was 2.10 mm3 in 30 normal temporal bones. However, the mean volume of utricle (10.65 mm3) in this study was larger than that study, in which it was 8.19 mm3 in 31 temporal bones. This variation may be partly explained by the difference in the method of delineating the utricle from semicircular canals, rather than the use of different 3-D reconstruction software. Because the utricle consists of two parts (the superior and inferior portion) and is continuous with the semicircular canals, it is difficult to define the boundary between the utricle and semicircular canal precisely under the 2-D view. Therefore, we set the boundary between the utricle and semicircular canals using a clipping plane under the 3-D view. A large deviation was confirmed between the measurements of volumes in each part of membranous labyrinth in this study. Possible explanations for a large deviation are as follows: 1) there are differences among individuals in the size of membranous labyrinth by nature; and 2) some of the normal temporal bones have an increase in volume in membranous labyrinth, but it is insufficient for the onset of Meniere's symptoms.
In the present study, endolymphatic hydrops was seen in both temporal bones in all patients with bilateral Ménière disease. In the patients with unilateral Ménière disease, hydrops was also seen in all of the diseased ears and three of eight symptomatic contralateral ears. Hydrops was mainly observed in the pars inferior, especially in the saccule. On the other hand, no hydrops was observed in any part of the membranous labyrinth in the remaining contralateral ears of unilateral Ménière disease patients who had no symptoms in contralateral ears. This finding is in line with the report of Carfrae et al.6 regarding evaluating hydrops by 3-Tesla magnetic resonance imaging with gadolinium enhancement. In that study, endolymphatic hydrops was noted only in the affected ears, whereas the unaffected ears exhibited a normal architecture in the patients with unilateral Ménière disease.
Lin et al.5 reported that six of 17 (35%) temporal bone cases with unilateral Ménière disease (11 definite, three probable, and three possible) have saccular hydrops in the asymptomatic ear, and 27% of 82 patients with unilateral Ménière disease have “Ménière-like” vestibular-evoked myogenic potential (VEMP) changes of both threshold and tuning in the asymptomatic ears. It was concluded that saccular hydrops precedes clinical symptoms in Ménière disease, and VEMP may detect asymptomatic or presymptomatic saccular hydrops.5 However, the presence of endolymphatic hydrops in asymptomatic contralateral ears is questionable in that study, as saccular hydrops was not clinically visualized using diagnostic imaging in the patients who showed VEMP changes in the asymptomatic ear. In our previous study, the contralateral ear in patients with unilateral Ménière disease showed significantly more damage in the inner ear structures compared with the age-matched normal control temporal bones,10 which may be a reason for minor changes in the VEMP test observed in the asymptomatic ear of unilateral Ménière patients.
Slight hydropic changes had been also observed in the cochlear duct and saccule in the asymptomatic contralateral ear in unilateral Ménière disease.10 Although there were some slight hydropic changes in the contra-lateral saccule under 2-D view, apparently the hydrops is not sufficient to be statistically significant in 3-D reconstruction, which is much more accurate.
The small rupture, which could be traced with lines connecting both ends of ruptured membranes, was seen in many temporal bones with Ménière disease. Reconstruction of the membranous labyrinth must be difficult in the case of membranes with large rupture, especially when accompanied by a collapse. Moreover, it is impossible to reconstruct 3-D images when the membranous labyrinth is broken. All temporal bones of Ménière disease showed neither large rupture nor collapse in any part of membranous labyrinth in this study.
Decreasing hydropic pressure has been shown to improve Ménière symptoms as seen in endolymphatic sac surgery.11,12 These findings indicate that the increase of endolymphatic pressure is a possible mechanism for the onset of Ménière symptoms in the affected ear. As the changes of pressure in the membranous labyrinth are impossible to evaluate in a temporal bone study, the participation of endolymphatic pressure in hydrops and the onset of Ménière symptom is unclear. However, our finding that endolymphatic hydrops was only seen in the affected ears of Ménière disease and symptomatic contralateral ears of Ménière disease suggests that the presence of endolymphatic hydrops is an essential finding for symptomatic ears in Ménière disease. Further prospective clinical studies are needed to clarify the relationships between the progression of hydrops and the onset of symptoms in the patients with Ménière disease using functional tests and diagnostic imaging.
We could not clarify a precise threshold value of endolymphatic hydrops that results in Ménière symptoms because lack of information regarding whether all volunteers and patients showed symptoms at the time of death. We confirmed that the volumes of membranous labyrinth were within 20.75 mm3 in total volume in all asymptomatic ears with Ménière disease. Therefore, temporal bones showing membranous labyrinth volume in excess of that determined to be normal in this study can develop Meniere's symptoms. However, this value should be confirmed by measuring and comparing the volumes of endolymphatic spaces before and just after Ménière attacks in living patients with Ménière disease using MRI. Although separate measurements of the volumes of each part of the membranous labyrinth are still impossible using diagnostic imaging, higher-resolution T2-weighted MRI has made it possible to reconstruct 3-D images of the inner ear,6,7,13 to measure and calculate the total volumes of the inner ear clinically; several authors have reported the volumes.14,15 In the future, it is expected that the volumes of cochlear duct, saccule, and utricle can be separately measured with increases in the resolving power of MRI. To this point, the normal volume ranges of membranous labyrinth established by our study and the volume data in Ménière disease would be useful as a standard and an abnormal value for the assessment of endolymphatic hydrops in patients with Ménière disease.
CONCLUSION
The cochlear duct, saccule, and utricle of the normal temporal bones were reconstructed three-dimensionally, and normal volume ranges were determined. Based on the results in normal temporal bones, the presence of hydrops was examined in the temporal bones with Ménière disease. Endolymphatic hydrops was observed in the affected ears of Ménière disease and symptomatic contralateral ears of Ménière disease, mainly in the pars inferior, whereas the asymptomatic contralateral ear of unilateral Ménière disease did not show hydrops in any parts of membranous labyrinth.
Acknowledgment
We thank Patricia A. Schachern and Carolyn Sutherland for helpful suggestions and technical assistance.
This research was supported by NIDCD/3U24DC008559-03S109, the International Hearing Foundation, the Starkey Foundation, and the Ministry of Education, Culture, Sports, Science and Technology, Japan (S.K.; 19591970).
REFERENCES
- 1.Schuknecht H. Pathology of the Ear. 2nd ed. Lea & Febiger; Philadelphia, PA: 1993. [Google Scholar]
- 2.Merchant SN, Adams JC, Nadol JB., Jr Pathophysiology of Ménière's syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Rauch SD, Merchant SN, Thedinger BA. Meniere's syndrome and endolymphatic hydrops. Double-blind temporal bone study. Ann Otol Rhinol Laryngol. 1989;98:873–883. doi: 10.1177/000348948909801108. [DOI] [PubMed] [Google Scholar]
- 4.Cureoglu S, Schachern PA, Paul S, Paparella MM, Singh RK. Cellular changes of Reissner's membrane in Meniere's disease: human temporal bone study. Otolaryngol Head Neck Surg. 2004;130:113–119. doi: 10.1016/j.otohns.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Lin MY, Timmer FC, Oriel BS, et al. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope. 2006;116:987–992. doi: 10.1097/01.mlg.0000216815.75512.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfrae MJ, Holtzman A, Eames F, Parnes SM, Lupinetti A. 3 Tesla delayed contrast magnetic resonance imaging evaluation of Ménière's disease. Laryngoscope. 2008;118:501–505. doi: 10.1097/MLG.0b013e31815c1a61. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima T, Naganawa S, Sugiura M, et al. Visualization of endolymphatic hydrops in patients with Meniere's disease. Laryngoscope. 2007;117:415–420. doi: 10.1097/MLG.0b013e31802c300c. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Otolaryngology- Head and Neck Foundation, Inc Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Ménière's disease. Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi M, O-Uchi T, Isago H, Wright WK. Utricular and saccular volumetry in human temporal bones. Acta Otolaryngol. 1983;95:75–80. doi: 10.3109/00016488309130918. [DOI] [PubMed] [Google Scholar]
- 10.Kariya S, Cureoglu S, Fukushima H, et al. Histopathologic changes of contralateral human temporal bone in unilateral Ménière's disease. Otol Neurotol. 2007;28:1063–1068. doi: 10.1097/MAO.0b013e31815a8433. [DOI] [PubMed] [Google Scholar]
- 11.Paparella MM. Endolymphatic sac revision for recurrent intractable Ménière's disease. Otolaryngol Clin North Am. 2006;39:713–721. doi: 10.1016/j.otc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Ulubil SA, Eshraghi AA, Telischi FF, Angeli SI, Balkany TJ, Joy JJ. Caloric function after endolymphatic sac surgery. Laryngoscope. 2008;118:295–299. doi: 10.1097/MLG.0b013e31815a05be. [DOI] [PubMed] [Google Scholar]
- 13.Krombach GA, Martiny S, Di Martino E, et al. T2-weighted turbo spin-echo images, maximum-intensity projections, and three-dimensional volume-rendering for delineation of pathologies and anatomic details of the inner ear. Invest Radiol. 2004;39:756–766. doi: 10.1097/00004424-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Melhem ER, Shakir H, Bakthavachalam S, et al. Inner ear volumetric measurements using high-resolution 3D T2-weighted fast spin-echo MR imaging: initial experience in healthy subjects. AJNR Am J Neuroradiol. 1998;19:1819–1822. [PMC free article] [PubMed] [Google Scholar]
- 15.Kendi TK, Arikan OK, Koc C. Volume of components of labyrinth: magnetic resonance imaging study. Otol Neurotol. 2005;26:778–781. doi: 10.1097/01.mao.0000169635.25322.9e. [DOI] [PubMed] [Google Scholar]