Abstract
The elongator (ELP) complex consisting of Elp1-6p has been indicated to play roles in multiple cellular processes. In yeast, the ELP complex has been shown to genetically interact with Uba4p/Urm1p and Kti11-13p for a function in tRNA modification. Through a Caenorhabditis elegans genetic suppressor screen and positional cloning, we discovered that loss-of-function mutations of moc-3 and dph-3, orthologs of the yeast UBA4 and KTI11, respectively, effectively suppress the Multivulva (Muv) phenotype of the lin-1(e1275, R175Opal) mutation. These mutations do not suppress the Muv phenotype caused by other lin-1 alleles or by gain-of-function alleles of ras or raf that act upstream of lin-1. The suppression can also be reverted by RNA interference of lin-1. Furthermore, we showed that dph-3(lf) also suppressed the defect of lin-1(e1275) in promoting the expression of a downstream target (egl-17). These results indicate that suppression by the moc-3 and dph-3 mutations is due to the elevated activity of lin-1(e1275) itself rather than the altered activity of a factor downstream of lin-1. We further showed that loss-of-function mutations of urm-1 and elpc-1-4, the worm counterparts of URM1 and ELP complex components in yeast, also suppressed lin-1(e1275). We also confirmed that moc-3(lf) and dph-3(lf) have defects in tRNA modifications as do the mutants of their yeast orthologs. These results, together with the observation of a likely readthrough product from a lin-1(e1275)∷gfp fusion transgene indicate that the aberrant tRNA modification led to failed recognition of a premature stop codon in lin-1(e1275). Our genetic data suggest that the functional interaction of moc-3/urm-1 and dph-3 with the ELP complex is an evolutionarily conserved mechanism involved in tRNA functions that are important for accurate translation.
THE MOCS3 protein has been shown to be involved in two unlinked cellular processes: molybdenum cofactor (Moco) synthesis and tRNA modification (Schwarz and Mendel 2006; Leidel et al. 2009). Moco is a cofactor of several essential metabolic enzymes (sulfite oxidase, xanthine dehydrogenase, aldehyde oxidase, and nitrate reductase), and, thus, the Moco synthesis pathway is conserved from bacteria to humans (Schwarz and Mendel 2006). In the Moco synthesis pathway, MOCS3 transfers a sulfur atom to the MOCS2B protein, and this sulfur atom is further transferred to the Moco precursor (supporting information, Figure S1A) (Schwarz and Mendel 2006). The structures of these two proteins and the sulfur transfer during Moco biosynthesis share significant similarities with the structures and functions of ubiquitin-activating enzymes, leading to the suggestion that the ubiquitin-dependent protein conjugation system may have evolved from the evolutionarily older Moco synthesis pathway (Schwarz and Mendel 2006).
MOCS3 has recently been found to have another ubiquitin-like partner, URM1 (Ubiquitin-Related Modifier 1), in Saccharomyces cerevisiae (Furukawa et al. 2000; Goehring et al. 2003a,b; Rubio-Texeira 2007). Urm1p is conserved from yeast to humans (Furukawa et al. 2000) and known to be conjugated to proteins, as are other ubiquitin family members (Furukawa et al. 2000; Goehring et al. 2003a,b). A series of recent articles has demonstrated a novel function of the URM1/UBA4 (MOCS3 yeast ortholog) pathway in the modification of cytosolic tRNAs (Huang et al. 2008; Nakai et al. 2008; Schlieker et al. 2008; Leidel et al. 2009; Noma et al. 2009). The oxygen atom in position 2 of the wobble uridine, U34, of several tRNAs is replaced with a sulfur atom, s2U34, requiring the Urm1p/Uba4p module (Figure S1B) (Huang et al. 2008; Nakai et al. 2008; Schlieker et al. 2008; Leidel et al. 2009; Noma et al. 2009). In addition, U34 is modified to a 5-methoxy-carbonyl-methyl (mcm5) or 5-carbamoylmethyluridine (ncm5U), where an initial common step requires Kti11-13p proteins and the elongator protein (ELP) complex (Figure S1B) (Huang et al. 2005; Lu et al. 2005). The ELP complex had been indicated for roles in transcriptional elongation (Otero et al. 1999; Krogan and Greenblatt 2001; Kim et al. 2002), but it was also recently linked to tRNA modification (Huang et al. 2005; Lu et al. 2005; Esberg et al. 2006). It is noted that in the elp mutants of S. cerevisiae, lack of modification at position 5 will reduce thiolation at position 2 of U34 (Nakai et al. 2008; Leidel et al. 2009; Noma et al. 2009). A recent report, however, suggested that this hierarchy may not describe the situation in Caenorhabditis elegans (Chen et al. 2009). Furthermore, the Urm1p/Uba4p module and Kti11p have been shown to maintain Elp1p function by preventing post-translational modification of Elp1p (Fichtner et al. 2003).
C. elegans vulval development involves multiple steps during the four larval stages (L1–L4) (Sternberg 2005). The canonical RAS/RAF/MAPK pathway in C. elegans plays a critical role in inducing vulval differentiation during these stages (Sternberg and Han 1998; Sundaram 2005). Two key transcription factors have been shown to act downstream of MPK-1: LIN-1, an ETS family member protein, and LIN-31, a winged helix family member protein (Miller et al. 1993; Beitel et al. 1995; Kornfeld 1997). Loss of function of either transcription factor causes ectopic vulval induction [Multivulva (Muv) phenotype], indicating that these two genes act as negative regulators of vulval induction. A previous report also suggests that LIN-1 and LIN-31 form a functional heterodimer that represses vulval-specific functions (Tan et al. 1998). Upon activation of the RTK/RAS/MAPK pathway, MPK-1 phosphorylates both LIN-1 and LIN-31, breaking up the heterodimer and allowing for vulval induction (Figure S2). Currently, it is not clear how phosphorylation of LIN-1 triggers the cellular events related to vulval formation and what the direct regulatory targets of LIN-1 are.
In an attempt to identify factors that act downstream or with LIN-1 for its vulval function, we carried out genetic screens for mutations that can suppress the weak temperature-sensitive lin-1 allele, e1275. We identified and cloned two genes defined by two of these suppressor mutations in C. elegans: dph-3, encoding a homolog to yeast KTI11 and mammalian DPH3, and moc-3, encoding an ortholog of UBA4/MOCS3. We show that the suppressor effects of loss-of-function mutations (lf) in these two genes are highly specific to this single lin-1 allele. Subsequent analysis revealed that these two evolutionarily conserved genes are not specifically involved in vulval cell differentiation, but are involved in tRNA modifications, the disruption of which leads to translational inaccuracy and the observed suppressor effects.
MATERIALS AND METHODS
Culture methods and strains:
Maintenance, culturing, and genetic manipulations of C. elegans strains were carried out according to standard procedures (Brenner 1974) and conducted at 20°. The strains used or generated are as follows: lin-1 (e1275), lin-1(n1047), lin-1(e1275) unc-24(e138) dpy-20(e1282), lin-1(e1275) moc-3(ku300), lin-1(e1275) moc-3(tm3742), lin-1(e1275) dph-3(ku305), dph-3(ku432), lin-1(e1275) dph-3(ku432), lin-1(n1047) dph-3(ku432), lin-1(n176) dph-3(ku432), lin-1(n176) moc-3(tm3742), let-60(sy130), let-60(sy130) dph-3(ku432), lin-45(gf), lin-45(gf); moc-3(ku300), lin-31(n301), lin-31(n301); dph-3(ku432), lin-31(n1053), lin-31(n1053); moc-3(ku300), elpc-3(ok2452), lin-1(e1275); elpc-3(ok2452), lin-1(n176); elpc-3(ok2452), ayIs4[egl-17∷GFP, dpy-20(+)], Ex[pSK002(sur-5p∷lin-1(e1275)∷NLS∷gfp), psur-5p∷DsRed, unc-119(+)] (this article), and kuIs76[sur-5p∷gfp∷lin-1(e1275)∷flag, unc-119(+)] (this article). Opal stop mutants used dpy-5(e61), lin-1(n176), and lon-1(sp3). Information regarding each mutation can be found at http://www.wormbase.org.
Mutagenesis and phenotype scoring:
lin-1(e1275) animals were mutagenized with 50 mm ethyl methanesulfonate (Brenner 1974). Mutagenized adults were individually transferred to a petri plate and allowed to lay eggs. The F1 progeny were cloned to individual plates. The F2 generation was then scored under a dissecting microscope for suppression of the Muv phenotype. Approximately 20,000 haploid genomes (10,000 F1 plates) were screened, and 24 independent strains were isolated. A moc-3 allele, ku300, and a dph-3 allele, ku305, were mapped to LG IV. moc-3(ku300) and dph-3(ku305) were outcrossed more than five times. Muv percentage was scored under a dissecting scope as described previously (Ferguson and Horvitz 1985).
Genetic mapping and molecular cloning:
dph-3 and moc-3 were mapped relative to the cloned markers unc-24 and dpy-20 on LG IV. The strains lin-1(e1275) dph-3(ku305)/lin-1(e1275) unc-24(e138) dpy-20(e1282)and lin-1(e1275) moc-3(ku300)/lin-1(e1275) unc-24(e138) dpy-20(e1282) were constructed, and a standard three-factor recombination analysis was performed. Further mapping was done by SNP analysis using a Bristol (N2)/Hawaii (CB4856) hybrid strain. The lin-1(e1275) strain was crossed to CB4856 10 times to generate a lin-1 mutant strain with mostly Hawaii chromosomes. N2 lin-1(e1275) unc-24(e138) ku305dpy-20(e1282)/Hawaii lin-1(e1275) and N2 lin-1(e1275) unc-24(e138) ku300dpy-20(e1282)/Hawaii lin-1(e1275) were then constructed, and recombinants were isolated from them. Analysis of SNP sequence in the region pinpointed the genes to small genetic regions between cosmids W09C2 and K07F5 for dph-3 and between cosmids C49H3 and T09A12 for moc-3.
Combination or individual cosmids in this region were injected at 1–10 ng/μl into lin-1(e1275) dph-3(ku305) or lin-1(e1275) moc-3(ku300) mutant animals to locate the gene activity that can rescue the mutant defect of ku300 or ku305 [i.e., to recover the Muv phenotype associated with lin-1(e1275)]. The dominant marker SUR-5 TXN∷GFP was co-injected at 50 ng/μl (Yochem et al. 1998). Cosmid ZK1251, as well as a subsequent subclone pWJ043 containing a 3-kb HindIII fragment and the gene K01H12.1, could rescue the ku305 mutant phenotype. Cosmid F42G8, as well as an 8-kb SalI fragment containing the coding sequence of F42G8.6, could rescue the ku300 mutant phenotype.
Molecular analysis of the mutant lesion and RNA interference:
To identify molecular lesions in the ku300 and ku305 alleles, the coding regions of the genomic DNA were PCR amplified, followed by sequence analysis. For RNA interference (RNAi) analysis, moc-3, urm-1, dph-3, elpc-1, elpc-2, elpc-3, and elpc-4 cDNA were isolated from N2 worms and subcloned into the pPD129.36 RNAi feeding vector, and double-stranded-RNA-containing bacteria were fed to lin-1(e1275); rrf-3(pk1426) worms as described previously (Fire et al. 1998).
Targeted deletion mutation screen:
N2 worms were mutagenized with UV-trimethyl psoralen (TMP) to create deletions (Yandell et al. 1994). These worms were processed according to a protocol described by R. Barstead (http://www.mutantfactory.ouhsc.edu/protocols.asp) and frozen into 1120 aliquots. The library was screened with 1° (primary) and 2° (secondary) reactions using two sets of primers. For the 1° reaction, a poison primer was also used. One-degree primers amplify 1581 bp, and their sequences are 5′-tcatccaaaggatccgggtcg and 3′-aaacaaaatcctcaagcttcc. The sequence of the poison primer is atgtcagttttccacgacgaag (beginning at the ATG of dph-3). The 2° primers amplify 680 bp, and their sequences are 5′-cccaacctctctcgcccc and 3′-tttactcgcgaaacccgtatc.
Double-mutant analysis:
Double mutants in Tables 1–3 were constructed and analyzed by standard genetic method. To analyze the suppression effect of moc-3(ku300) on lin-1(n303), we generated lin-1(n303)/moc-3(ku300) dpy-20(e1282) animals and identified 23 L4 animals that were homozygous for both the lin-1 and dpy-20 alleles and derived from at least 12 independent recombination events. All of these animals became severely Muv, and all but three were lethal as adults without any progeny. Since the moc-3 gene is located much closer to dpy-20 (1.6 μm) than to lin-1 (12.3 μm), most of these recombinants are expected to be homozygous for moc-3(ku300), suggesting that the moc-3 allele failed to suppress the Muv phenotype of the lin-1 allele and that the double mutants were lethal.
TABLE 1.
Allele-specific suppression of the Muv phenotype of lin-1(e1275) by moc-3(lf) and dph-3(lf)
| Genotype | % multivulva (n)a |
|---|---|
| lin-1(e1275) | 81.9 (191) |
| dph-3(ku305) | 0.0 (100) |
| lin-1(e1275) dph-3(ku305) | 1.2 (169) |
| dph-3(ku432) | 1.0 (100) |
| lin-1(e1275) dph-3(ku432) | 7.6 (131) |
| moc-3(ku300) | 0.0 (100) |
| lin-1(e1275) moc-3(ku300) | 1.6 (122) |
| lin-1(e1275) moc-3(tm3742) | 6.6 (333) |
| lin-1(n176) | 100 (100) |
| lin-1(n1047) | 100 (100) |
| lin-1(n1047) dph-3(ku432) | 100 (100) |
| lin-1(n176) dph-3(ku432) | 100 (100) |
| lin-1(n176) moc-3(tm3742) | 100 (50) |
Percentage of Muv animals at adult stage. n, the number of animals scored.
TABLE 2.
Suppression of lin-1(e1275) Muv phenotype by loss-of-function of urm-1 and genes of the Elongator complex
| Genotype | % multivulva (n)a |
|---|---|
| GFP RNAi | 85 (93) |
| dph-3 RNAi | 16 (101) |
| moc-3 RNAi | 21 (185) |
| urm-1 RNAi | 50 (192) |
| elpc-1(Y110A7A.16) RNAi | 27 (195) |
| elpc-2(Y111B2A.17) RNAi | 44 (207) |
| elpc-3(ZK863.3) RNAi | 12 (82) |
| elpc-4(C26B2.6) RNAi | 42 (210) |
| lin-1(e1275);elpc-3(ok2452) | 12 (150) |
| lin-1(n176);elpc-3(ok2452) | 100 (69) |
L4 worms of lin-1(e1275);rrf-3(pk1426) were placed on RNAi plate and Muv phenotype of F1 were scored. n, the number of animals scored.
TABLE 3.
moc-3(lf) and dph-3(lf) did not block Ras/MAPK pathway
| Genotype | % multivulva (n)a |
|---|---|
| let-60(sy130) | 57 (100) |
| let-60(sy130) dph-3(ku432) | 65 (100) |
| lin-45(gf) | 48 (100) |
| lin-45(gf); moc-3(ku300) | 53 (100) |
| lin-45(gf); dph-3(ku432) | 55 (100) |
| lin-31(n301) | 78 (100) |
| lin-31(n301); dph-3(ku432) | 83 (100) |
| lin-31(n1053) | 76 (100) |
| lin-31(n1053); moc-3(ku300) | 71 (100) |
Percentage of Muv animals at adult stage. n, the number of animals scored.
Quantitative PCR:
RNAs of mixed-stage worms were isolated by Trizol (Sigma, St. Louis) extraction. Quantitative PCR (qPCR) was performed on the Rotor-gene RG-3000 (QIAGEN, Germantown, MD). The primers used were rpl-26 (5′-atgaaggtcaatccgttcgt and 3′-aggacacgtccagtgtttcc; 209 bp) and lin-1 (5′-ccacatttggcgtcccagtcac and 3′-tttgtggcctggaatgcggag; 152 bp). Amplification was done with SYBR Green JumpStart Taq Ready Mix (Sigma).
Transgenic line:
unc-119(ed3);him-5(e1467) adults were injected with two different mixes: (1) 5 ng/μl pSK002, 30 ng/μl psur-5p∷DsRed, 50 ng/μl unc-119(+), 50 ng/μl pBluescript SK and (2) 5 ng/μl pSK016, 50 ng/μl unc-119(+), 50 ng/μl pBluescript SK. The latter established a stable extrachromosomal array line and was subjected to UV irradiation for the generation of the genomic integration line according to the modified version of the original protocol from S. Mitani (http://www.faculty.ucr.edu/∼mmaduro/int.html). Briefly, 200–300 L4 worms were washed with M9 buffer several times to remove the residual bacteria. Then the worms were put on NGM plates without bacteria and irradiated with 300 J/m2 of 254 nm UV using Stratalinker 2400 (Stratagene, La Jolla, CA). L4 worms were subsequently transferred to an OP50 plate at 10 animals/plate and incubated for 7–10 days until the animals on the plates were starved out. Two days after moving the animals to a new OP50 plate, the candidate integrant animals were singled to another plate to see if the progeny were all integrants. The resulting integration line was outcrossed three times against unc-119(ed3);him-5(e1467). To confirm the expression of pSK016, we performed Western blot from mixed-stage worms according to the standard procedure.
tRNA isolation and HPLC analysis:
tRNAs from C. elegans were extracted and analyzed by HPLC as described previously (Chen et al. 2009).
RESULTS
moc-3(lf) and dph-3(lf) mutations suppress the Muv phenotype of a lin-1 temperature-sensitive mutation:
We carried out a genetic screen for mutations that could suppress the Muv phenotype of a partial lf allele of lin-1, e1275. lin-1(e1275) has a nonsense mutation that causes variable Muv phenotypes at different temperatures (Beitel et al. 1995). We screened 20,000 mutagenized lin-1(e1275) haploid genomes at 20° and isolated 24 suppressors. Two of these suppressors, ku300 and ku305, reduced the Muv phenotype from 81.9% to 1.6% and 1.2%, respectively, at 20° (Table 1). After molecular cloning of the genes defined by these two mutations (see below), we named the gene defined by the ku300 allele as moc-3 (MOCo synthesis pathway gene 3, F42G8.6), and the gene defined by the ku305 allele as dph-3 (homolog of mammalian DPH3 proteins).
While the recessive nature of ku300 and ku305 suggests that they may be lf mutations, the molecular lesions (see below) do not provide the basis for such a conclusion. Large-deletion mutations of both genes suppressed the Muv phenotype of lin-1(e1275) to a similar extent as the two alleles isolated in the suppressor screen (Table 1), indicating that the suppressors are indeed lf mutations. The dph-3 deletion allele, ku432, was isolated by screening a UV-TMP-generated deletion mutation library (Figure 1A). The moc-3(tm3742) deletion allele was obtained from the National Bioresource Project (Mitani lab, Tokyo Women's Medical University) (Figure 2A). Furthermore, the lf nature is supported by the fact that RNAi of moc-3 and dph-3 also suppressed the Muv phenotype of lin-1(e1275) (Table 2).
Figure 1.—
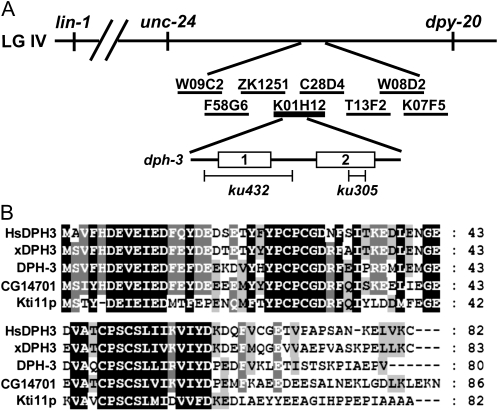
Molecular cloning and sequence analysis of dph-3 defined by a lin-1(e1275) suppressor mutation. (A) Cartoon of the dph-3 location relative to mapping marker and rescuing DNA clones and regions uncovered by the two deletion mutations. (B) Protein alignment of DPH-3 and its orthologs in other representative eukaryotes. The orthologs are Homo sapiens (HsDPH3), Xenopus laevis (xDPH3), Drosophila melanogaster (CG14701), and S. cerevisiae (Kti11p). Alignment of multiple protein sequences was produced by Clustalw (http://align.genome.jp/) and edited manually by using GENEDOC (http://www.nrbsc.org/gfx/genedoc/).
Figure 2.—
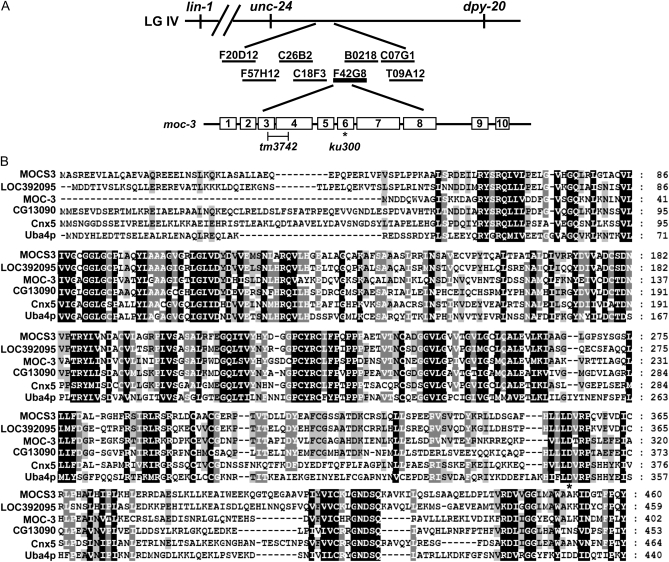
Molecular cloning and sequence analysis of moc-3 defined by a lin-1(e1275) suppressor. (A) Cartoon of the moc-3 location relative to mapping markers and rescuing DNAs and regions uncovered by deletion mutations. The asterisk indicates the site of ku300 mutation. (B) Alignment of MOC-3 and its orthologs in other species. The orthologs were chosen from the representative eukaryotic species of H. sapiens (MOCS3), Danio rerio (LOC393095), D. melanogaster (CG13090), Arabidopsis thaliana (Cnx5), and S. cerevisiae (UBA4). Alignment of multiple protein sequences was produced by Clustalw (http://align.genome.jp/) and edited manually by using GENEDOC (http://www.nrbsc.org/gfx/genedoc/).
moc-3(lf) and dph-3(lf) mutations do not suppress the Muv phenotype caused by other lin-1 alleles or by mutations in other components in the vulval induction pathway:
To investigate the mechanism of the observed suppression, we tested whether the suppression by the moc-3 and dph-3 alleles is specific to the lin-1(e1275) allele. We generated double mutants combining the moc-3(lf) or dph-3(lf) allele with three other stronger lin-1(lf) alleles: lin-1(n176), a premature opal stop codon at R255; lin-1(n1047), an Y126F missense mutation; and lin-1(n303), a R121K missense mutation (Beitel et al. 1995). Surprisingly, lin-1(n176) and lin-1(n1047) were not suppressed by moc-3(lf) or dph-3(lf) (Table 1), and lin-1(n303) also appeared not to be suppressed by moc-3(lf) (data not shown; materials and methods), suggesting that the suppression of lin-1(e1275) by moc-3(lf) and dph-3(lf) depended on either the relatively weak penetrance of the mutant phenotype or an unknown property of the e1275 allele.
We next tested whether moc-3(lf) and dph-3(lf) genetically interact with mutations in ras and raf genes that also cause an incompletely penetrant Muv phenotype like lin-1(e1275) (Beitel et al. 1990; Han and Sternberg 1990; Yoder et al. 2004). Strikingly, no suppression was observed in dph-3(ku432) let-60/ras(sy130gf), lin-45/raf(gf); moc-3(ku300), or lin-45/raf(gf); dph-3(ku432) (Table 3). Furthermore, moc-3(lf) and dph-3(lf) did not suppress the Muv phenotype of a lin-31(lf) allele, which also acts in the Ras/MAPK pathway as described (Table 3). Since the Muv phenotypes of the ras, raf, and lin-31 alleles are not stronger than the lin-1(e1275) allele, these results indicate that moc-3(lf) and dph-3(lf) specifically alter the gene activity of lin-1(e1275) rather than reduce the output of the signaling pathway.
To further support a role for moc-3(lf) and dph-3(lf) in elevating lin-1(e1275) gene activity that leads to the suppression, we applied lin-1 RNAi on lin-1(e1275) moc-3(ku300) and lin-1(e1275) dph-3(ku432) strains and found that the treatment restored the Muv phenotype in both strains (Table S1).
dph-3 encodes a conserved protein homologous to DPH3/KTI11:
We mapped dph-3(ku305) using genetic and SNP markers and cloned it by microinjection transformation (materials and methods). Sequencing DNA from lin-1(e1275) dph-3(ku305) worms revealed a 28-bp deletion within exon 2 of the predicted open reading frame K01H12.1 (Figure 1A). This deletion eliminates the 19 C-terminal amino acids and replaces them with 13 alternate amino acids. RT–PCR data indicated that this altered transcript was produced in ku305 worms (data not shown). This mutation did not remove any of the conserved cysteine residues necessary for Zn2+ binding (Sun et al. 2005). We also determined that ku432 contains a 298-bp deletion that eliminated 57 bp of the promoter, the first exon (132 bp), and 109 bp of intronic DNA (Figure 1A). Given the small size of the gene, this deletion almost certainly eliminates the gene function (null allele). BLAST search indicated that dph-3 encodes an 80-amino-acid protein that is highly conserved from yeast to humans (Figure 1B). The S. cerevisiae homolog, Kti11p, is associated with the ELP/Toxin Target (TOT) protein complex that has been proposed to be involved in transcriptional elongation as described above (Fichtner and Schaffrath 2002) (see below for additional discussion). Kti11p has been thought to function within the elongator complex by inhibiting the post-translational modification of Elp1p (Fichtner et al. 2003). In yeast and mammalian cells, KTI11 was independently identified as DPH3/DESR1 (Liu and Leppla 2003; Liu et al. 2004). DPH3 is a component of the diphthamide synthesis protein complex that was suggested to play a role in a certain translation processes and has been shown to be essential in mouse development (Liu et al. 2006).
moc-3 encodes a sulfur transferase in the MOCO synthesis pathway:
moc-3(ku300) was also genetically mapped and identified to be a candidate allele of moc-3 (F42G8.6) through microinjection transformation. Sequencing DNA from lin-1(e1275) moc-3(ku300) worms revealed a G-to-A point mutation within the coding region, which is expected to result in a D310N missense mutation in the Rhodanese domain that is important for the sulfur transferase activity of this family of proteins (Figure 2). Protein sequence alignment of moc-3 orthologs showed that D310 is highly conserved in eukaryotes, implicating a critical role of this amino acid in MOC-3 function (Figure 2B). The tm3742 deletion allele of moc-3 (Mitani Lab) lacks 321 C-terminal amino acids after V81, with an addition of 24 unrelated amino acids (Figure 2A), strongly suggesting that it is also a null allele. As ku300 showed similar suppression of lin-1(e1275) to tm3742, we concluded that the D310N mutation of ku300 also abrogated the function of MOC-3 protein.
dph-3(lf) also suppresses the defect of lin-1(e1275) in promoting egl-17 expression:
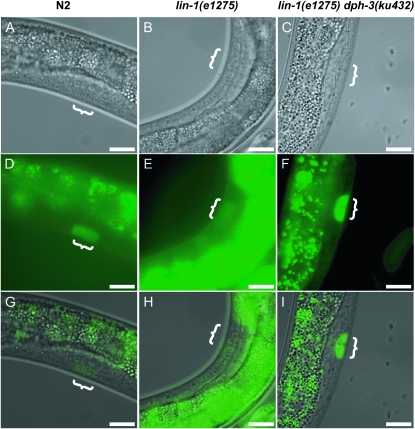
In addition to the negative role in repressing vulval induction, LIN-1 also has a positive role in vulval development, as it is required for the expression of egl-17 in vulval cells (Tiensuu et al. 2005). An egl-17∷gfp reporter is expressed in P6.p during the first two rounds of vulval cell division, and then its expression shifts to descendants of P5.p and P7.p (Figure 3, A, D, and G) (Burdine et al. 1998; Cui and Han 2003). In lin-1(e1275) worms, egl-17∷gfp was not expressed in P6.p (Figure 3, B, E, and H), whereas, in lin-1(e1275) dph-3(ku432) worms, egl-17∷gfp expression in P6.p was restored to wild type (Figure 3, C, F, and I). The simplest interpretation of these results is that there was an increase in LIN-1 activity in the presence of the dph-3(ku432) mutation.
Figure 3.—
The reduced expression of the egl-17∷GFP reporter in lin-1(e1275) was restored by dph-3(ku432). Nomarski (A, B, and C), GFP fluorescence (D, E, and F), and merged (G, H, and I) images of L3 larvae of the genotypes indicated. Exposure time of E and H was about 5 times longer than others to show the reduced expression of the reporter gene. White braces indicate the progeny of P6.p that express egl-17∷GFP in wild-type animals but not in lin-1(lf) mutants (Burdine et al. 1998). Bars, 20 μm.
Mutating ELP complex components also suppressed the Muv phenotype of lin-1(e1275):
As we mentioned above, the ELP complex has been shown to physically or functionally interact with Kti11p, a yeast ortholog of DPH-3, and Uba4p, a yeast counterpart of MOC-3 (Fichtner et al. 2003). The yeast ELP complex is composed of six genes (ELP1–6) (Krogan and Greenblatt 2001; Winkler et al. 2001) and, among them, ELP1–4 are conserved in C. elegans on the basis of protein sequence homology. These worm genes have thus been named as elpc-1, elpc-2, elpc-3, and elpc-4. elpc-1 and elpc-3 have also been recently shown to be functionally conserved with respect to the tRNA modification activity in C. elegans (Chen et al. 2009). To determine if the functions of moc-3 and dph-3, indicated by the ability of their mutations to suppress lin-1(e1275), reflect the functions of the ELP protein complex, we performed RNAi analysis on the C. elegans orthologs of the subunits of the yeast ELP complex. RNAi of elpc-1, -2, -3, and -4 was able to significantly suppress the Muv phenotype (12–44% Muv; Table 2) of lin-1(e1275), which was comparable to the effect of RNAi on moc-3 and dph-3 (Table 2). In addition, a null allele of elpc-3, ok2452, also showed strong suppression of the Muv phenotype of lin-1(e1275) (Table 2). By contrast, lin-1(n176); elpc-3(ok2452) still displayed the fully penetrant Muv phenotype, as did lin-1(n176) alone (Table 2). The allele-specific suppression by elpc-3(ok2452) suggests that the functional interactions of the ELP complex with moc-3 and dph-3 are evolutionarily conserved. We also analyzed the RNAi effects of the moc-3, urm-1, and dph-3 genes on lin-1(e1275); elpc-3(ok2452) to further examine the functional relationship between elpc-3 and the other three genes. As shown in Table S2, RNAi of any of the three genes did not enhance the suppression of the Muv phenotype by elpc-3(ok2452), supporting the theory that moc-3, urm-1, dph-3, and the ELP complex act in the same pathway for the function related to suppression.
moc-3 and dph-3 mutations do not increase the level of lin-1(e1275) mRNA:
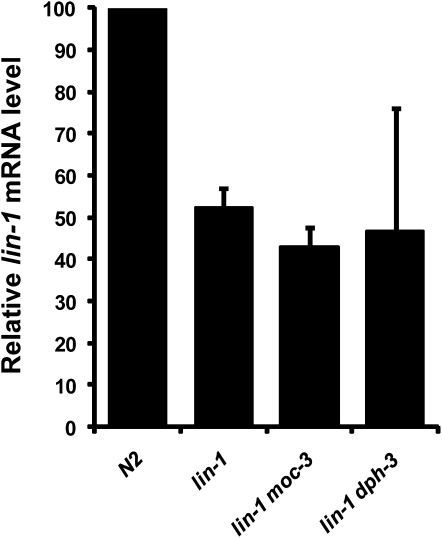
Given that the ELP complex has been known to regulate transcriptional elongation as mentioned earlier, a possible hypothesis is that moc-3 and dph-3 regulate the transcription of lin-1. To test this possibility, we performed quantitative RT–PCR to measure the lin-1 transcript level. The mRNA level of lin-1 in the lin-1(e1275) background was ∼50% of the wild-type level, consistent with the theory that nonsense-mediated mRNA decay is involved in degrading the lin-1(e1275) transcript (Figure 4, bars 1 and 2). Neither lin-1(e1275) moc-3(ku300) nor lin-1(e1275) dph-3(ku432), however, showed a statistically significant difference in lin-1 mRNA level compared to lin-1(e1275) alone (Figure 4, bars 3 and 4; P > 0.05). These results suggested that moc-3 and dph-3 are unlikely to play a significant role in lin-1 transcriptional regulation or mRNA stability.
Figure 4.—
lin-1 mRNA level was not affected by mutating either dph-3 or moc-3. Bar graph indicates the results of qRT–PCR analysis of mRNA levels of lin-1 in the strains listed. rpl-26 was used as an internal control during the qRT–PCR experiments. Error bar indicates ±SD. Statistical analysis is described in the results.
tRNA modification leading to stop-codon readthrough is the likely mechanism for suppression of lin-1(e1275) by mutations in moc-3, dph-3, and the ELP complex:
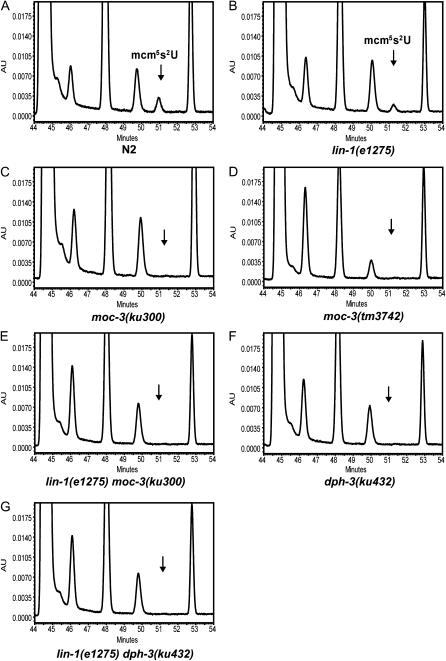
As we described above, Uba4p has a tRNA modification function with its partner, Urm1p (Huang et al. 2008; Nakai et al. 2008; Schlieker et al. 2008; Leidel et al. 2009; Noma et al. 2009). To confirm that MOC-3 and DPH-3 are involved in tRNA modification as their yeast counterparts, we examined wobble uridine tRNA modification by HPLC analysis. Whereas the mcm5s2U nucleoside at the wobble position of tRNA was seen as a prominent peak in samples from N2 and lin-1(e1275) control worms, this peak was missing in moc-3(lf) and dph-3(lf) mutants (Figure 5 and Table S3). Furthermore, in dph-3(lf) background, the s2U nucleoside arose due to the failure of the addition of the mcm5 side chain (Table S3). These data confirm that, like their yeast orthologs, DPH-3 and MOC-3 are required for the formation of the mcm5 and s2 side chains, respectively. For an unknown reason, the modified wobble uridine was observed to be slightly less abundant in lin-1(e1275) mutants than in wild type (Figure 5 and Table S3). Given that the formation of this modified nucleoside has been shown to be complicated, involving multiple enzymatic activities in yeast (Huang et al. 2008), this difference may reflect an unknown LIN-1 function in the Elongator complex and thereby the formation of this nucleoside.
Figure 5.—
moc-3 and dph-3 are required for the formation of the wobble uridine nucleoside mcm5s2U. Chromatograms constructed on the basis of HPLC analysis of modified tRNA nucleosides from strains of indicated genotypes are shown. Chromatograms were monitored at 254 nm, and only the graphs between retention time 44 and 54 are shown. The arrows in A–G indicate the expected retention time of mcm5s2U.
To determine whether the suppression of lin-1(e1275) by moc-3 is mediated by this conserved Uba4p/Urm1p module, urm-1(RNAi) of lin-1(e1275) was tested. Interestingly, RNAi of urm-1 significantly suppressed the Muv phenotype of lin-1(e1275) (Table 2). This result led us to hypothesize that a tRNA modification defect in moc-3(lf) and dph-3(lf) animals leads to an increase in translational readthrough of the opal stop codon in lin-1(e1275) transcripts.
This readthrough hypothesis is also supported by additional circumstantial evidence. As described earlier, the inability of the moc-3 and dph-3 mutations to suppress the Muv phenotype of other lin-1(lf) mutants and ras/raf gf mutants, as well as their loss of suppression by lin-1(RNAi), indicates that moc-3(lf) and dph-3(lf) alter the activity of lin-1 itself. We have also shown that the mRNA level of lin-1 is not raised in the moc-3 or dph-3 mutants. Causing readthrough of the opal stop codon of the lin-1(e1275) allele appears to be the most logical explanation because the truncated protein is highly unlikely to be functional. The truncation is expected to delete the LIN-1 transactivation domain, presumably disrupting the basic function of this transcription factor. This assumption is also consistent with the fact that the lin-1(n176) allele, which is characterized as a strong lf or null allele on the basis of its completely penetrant phenotype, contains a premature stop (R255Opal) that is predicted to produce a truncated protein larger than the e1275 truncated protein (R175Opal).
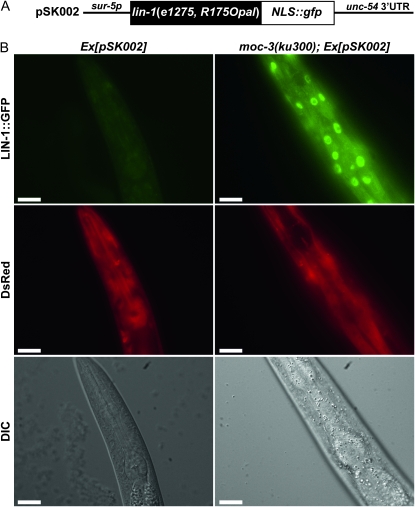
To observe the readthrough protein product from the lin-1(e1275 R175Opal) allele, we generated transgenic worms containing a sur-5p∷lin-1(e1275)∷gfp fusion gene (Figure 6A) and a sur-5∷DsRed marker on the same extrachromosomal array. The sur-5 promoter drives near-ubiquitous expression in C. elegans (Yochem et al. 1998), with GFP expression in this strain expected to depend on the translation reading through the premature opal stop codon. We observed prominent GFP expression in a fraction of cells in the moc-3(ku300) mutant animals but not in the wild-type background (Figure 6B). The GFP expression was not detectable in a number of cell types including vulval cells, even in moc-3(ku300), likely due to a low-level expression of the transgene in these cells. We have not been able to directly detect the readthrough protein product using antibodies from commercial sources, nor those generated in our own lab; all immunochemical tests failed to detect LIN-1 protein on a Western blot from whole-worm extracts (data not shown). We also generated an integrated transgenic line, kuIs76, which ubiquitously expressed GFP-LIN-1(e1275)-FLAG proteins under the sur-5 promoter (Figure S3) (Gu et al. 1998). kuIs76 worms showed a high level of stable GFP expression throughout all stages and in most tissues, including the vulva (Figure S3B). We were also able to detect the truncated LIN-1(e1275) protein from mixed-stage worm lysates by Western blot (Figure S3C). However, we failed to detect the full-length GFP-LIN-1-FLAG proteins expressed from this transgene in the moc-3(lf) or dph-3(lf) background by Western blot following immunoprecipitation using anti-GFP or FLAG antibodies. A likely explanation for this outcome is that the readthrough product is only a very small fraction of the total LIN-1 protein and/or the readthrough occurred in only a small fraction of tissues. Presumably, this low-level expression is sufficient for its function in vulval cells but eluded detection by Western blot. To test if there is a small amount of LIN-1 full-length protein from kuIs76, we generated a series of double or triple mutants as depicted in Table 4. We found that kuIs76 significantly suppressed the fully penetrant Muv phenotype of lin-1(n176) moc-3(tm3742) and lin-1(n176) dph-3(ku432) (Table 4). Furthermore, the Muv phenotype of lin-1(n176) was slightly suppressed by kuIs76 alone (Table 4), consistent with the incomplete penetrance phenotype and temperature-sensitive nature of lin-1(e1275). These genetic data are consistent with the theory that the opal stop codon of e1275 causes leaky translation and thus favors the hypothesis that the defects in tRNA modification in moc-3(lf) or dph-3(lf) mutants lead to the translational readthrough of lin-1(e1275).
Figure 6.—
A product of translational readthrough of lin-1(e1275) may be observed in the moc-3(ku300) background. (A) Schematic of the pSK002 construct. lin-1(e1275) cDNA was fused to GFP. NLS, nuclear localization signal. (B) The readthrough at R175Opal in lin-1(e1275) likely resulted in the expression of the LIN-1∷GFP full-length protein in the nucleus. Shown here is the head region of representative L4 larvae. A sur-5 promoter-driven DsRed construct was used as a marker for transgenic worms. Full genotypes are Ex[pSK002], unc-119(ed3); him-5(e1467); Ex[pSK002], moc-3(ku300); Ex[pSK002], unc-119(ed3); moc-3(ku300); Ex[pSK002]. Bars, 20 μm.
TABLE 4.
Overexpression of GFP-LIN-1(e1275) protein suppresses the Muv phenotype of lin-1(n176) in moc-3(lf) and dph-3(lf) backgrounds
| Genotype | % multivulva (n)a |
|---|---|
| lin-1(n176) moc-3(tm3742) | 100 (50) |
| lin-1(n176) dph-3(ku432) | 100 (100) |
| kuIs76b | 0 (100) |
| lin-1(n176) | 100 (100) |
| lin-1(n176);kuIs76 | 95 (228) |
| lin-1(n176) moc-3(tm3742);kuIs76 | 21 (243) |
| lin-1(n176) dph-3(ku432);kuIs76 | 13 (47) |
Percentage of Muv animals at adult stage. n, the number of animals scored.
The full genotype is unc-119(ed3);him-5(e1467);kuIs76.
DISCUSSION
We have identified novel mutant phenotypes of moc-3 and dph-3 genes in suppressing the developmental defects of a temperature-sensitive allele (e1275) of lin-1 that encodes an ETS domain transcription factor. The lin-1(e1275) allele harbors a premature opal stop codon that is expected to produce a truncated LIN-1 protein without its regulatory domain (Beitel et al. 1995). Results of a series of genetic analyses provide strong evidence that the suppression conferred by moc-3 and dph-3 mutations is due to a specific increase in the activity of the lin-1(e1275) mutant gene. We also provide evidence for the idea that aberrant tRNA modification leads to failed recognition of the premature stop codon in lin-1(e1275). Our results suggest that the functional interaction of moc-3/urm-1 and dph-3 with the ELP complex is an evolutionarily conserved mechanism involved in tRNA functions that are important for accurate translation.
Our proposal that the moc-3 and dph-3 mutations affect LIN-1(e1275) protein translation via tRNA modification defects is based on the following pieces of data. First, moc-3(lf) and dph-3(lf) each suppresses the Muv phenotype of the opal allele e1275, but not of other lin-1(lf) alleles (Table 1). They also failed to suppress the incompletely penetrant Muv phenotypes of gain-of-function alleles of ras and raf that act upstream of LIN-1 in the vulval induction pathway (Table 3). Therefore, loss of moc-3 or dph-3 function may simply result in an increase in the level of functional LIN-1 protein. This conclusion is supported by lin-1 RNAi data showing a reversed suppression of the Muv phenotype in lin-1(e1275) moc-3(ku300) and lin-1(e1275) dph-3(ku432) (Table S1). Second, the decreased expression of egl-17∷GFP in the lin-1(e1275) mutant has been restored by the dph-3(lf) mutation (Figure 3). Because promoting egl-17 expression represents a positive role of LIN-1 as opposed to its negative role on vulval induction reflected by the Muv phenotype of the lin-1(lf) alleles, this result also supports the theory that dph-3(lf) specifically elevates lin-1(e1275) gene activity. Third, the overexpressed GFP-LIN-1(e1275) protein did not substantially suppress the Muv phenotype of lin-1(e1275) mutants under conditions where both moc-3 and dph-3 are functional (Table 4). This supports the idea that the truncated LIN-1(e1275) protein has little or no intrinsic activity and that suppression conferred by the mutants of moc-3 and dph-3 is due to increased readthrough of the opal stop codon in lin-1(e1275) mRNA (Table 4). The notion that the truncated LIN-1 protein produced by the e1275 allele lacks the wild-type function is also consistent with the fact that the strong loss-of-function lin-1(n176, R255Opal) allele is expected to produce a protein longer than that from lin-1(e1275, R175Opal). Fourth, RNAi or deletion mutants of components of the recently discovered tRNA modification modules, urm-1 (Leidel et al. 2009) and the ELP complex (Chen et al. 2009), also displayed similar suppression of lin-1(e1275) as did moc-3 and dph-3 mutations (Table 2). Notably, a recent study proved that elpc-1 and elpc-3 play an evolutionarily conserved role in tRNA modification and that their mutants displayed specific neurological and developmental defects (Chen et al. 2009), suggesting that tRNA modification defects appear to be restricted to certain specific cellular processes. Fifth, RNAi of moc-3, dph-3, and urm-1 did not enhance the suppression of the Muv phenotype of lin-1(e1275) by elpc-3(ok2452), suggesting that they belong to the same biochemical pathway (Table S2). Notably, the Moco synthesis pathway, another functional partner of moc-3, did not affect the Muv phenotype of lin-1(e1275) via RNAi or null mutation (data not shown), indicating that the tRNA modification function of the moc-3/urm-1 module, not the Moco synthesis function, is responsible for this suppression ability. Sixth, we were able to detect the expression of a GFP fusion protein that is expected to be the readthrough translation product from a lin-1(e1275)∷gfp transgene in the moc-3(ku300) mutants (Figure 6). Finally, biochemical analysis indicates that moc-3 and dph-3 play roles in tRNA modification; the mcm5s2U species of the tRNA wobble uridine were missing in samples from moc-3(lf) and dph-3(lf) mutants. While these data strongly support the idea that defects in tRNA modification lead to increased readthrough of the opal stop codon in lin-1(e1275), we have not been able to directly detect the readthrough protein product by biochemical methods (see results).
Studies in yeast indicate that the ELP complex interacts with the C-terminal tail of RNA polymerase II (RNAP II) in its hyperphosphorylated state to augment transcriptional elongation of specific genes (Otero et al. 1999; Gilbert et al. 2004). Inconsistent with the positive role of the ELP complex in transcriptional elongation, the suppression of the Muv phenotype of lin-1(e1275) by moc-3(lf) and dph-3(lf) would implicate only a negative regulatory role of MOC-3 and DPH-3 on lin-1 expression (Table 1). In addition, our data indicate that the regulation of lin-1 by MOC-3 and DPH-3 is not likely to be transcriptional (Figure 4). Therefore, our genetic and molecular analyses of moc-3, dph-3, urm-1, and the genes encoding other ELP complex components suggest a function of the ELP complex unrelated to transcription elongation.
Recently, from the screening for novel proteasomal pathway components, several yeast mutants affecting uba4, elp2, and elp6 exhibited defects in proteasomal function (Hoyt et al. 2008). This raised the possibility that the mutations of moc-3 and the ELP complex led to the stabilization of the readthrough protein products from lin-1(e1275). In the same study, however, a yeast urm1 mutant did not show any proteasomal defect, whereas RNAi of urm-1 in C. elegans suppressed the Muv phenotype of lin-1(e1275), suggesting that the potential proteasomal function of Uba4p and the ELP complex may not affect the stability of the LIN-1(e1275)∷GFP protein. In addition, as we mentioned above, Kti11p/DPH3 has another function, diphthamide synthesis, in yeast and humans (Liu and Leppla 2003; Liu et al. 2004). Diphthamide is a modified histidine residue in translation elongation factor 2 (eEF2) and might have a function in protein translation in eukaryotes, although its exact role remains elusive. Moreover, Uba4p and the ELP complex in yeast and mammals have not been shown to be involved in diphthamide synthesis. Therefore, further discussion may be suspended until we know more about the role of this modified histidine in protein translation. In theory, the suppression that we observed could also be caused by stabilization of the low level of full-length LIN-1 protein as a readthrough product from the e1275 allele, and such stabilization could be the consequence of a novel response, such as a protein-folding response, to the production of the aberrant translation product. However, there is no evidence thus far to link Uba4p or the ELP complex to either of these possibilities. It is important to mention that the experimental evidence provided by a previous report (Esberg et al. 2006) raised the possibility that the Elongator complex is primarily involved in tRNA modification and that many of the other cellular defects observed in the mutants could be secondary effects caused by defects in translation.
Our data raise one intriguing question: What underlies the allele specificity of the suppression by mutating the genes in the ELP complex? The moc-3 and dph-3 mutations did not suppress unc-54(r308), dpy-5(e61), or lon-1(sp3), which also harbor premature opal stop codons (Table S4), indicating that moc-3(lf) and dph-3(lf) are not general informational suppressors. Potentially, this difference may be due to the difference in the impact of disrupting the tRNA modification function in different tissues. However, this difference would not explain why lin-1(n176), a strong loss-of-function allele of lin-1 that has a premature opal stop codon, was not suppressed by moc-3(lf) or dph-3(lf) (Table 1). One possibility is that the n176 transcript is subject to a stronger regulation by the nonsense-mediated mRNA decay (NMD) than is the e1275 transcript, leading to a very low level of mRNA as the template for the readthrough activity. However, the fact that the opal stop codon of n176(R225Opal) is 50 codons after that of the n1275 (R175Opal) does not seem to favor this idea because the efficiency of NMD usually decreases when the position of premature stop codon is farther downstream (Longman et al. 2007). Supporting this notion, our qPCR data clearly showed that lin-1(n176) worms maintain a level of lin-1 mRNA that is more than half that of wild type and similar to that of lin-1(e1275) (Figure S4). Alternatively, the difference between the two alleles could be due to the different sequence contexts flanking the stop codons.
Another issue is that we do not currently understand the spectrum of codon misreading caused by disrupting the tRNA modification function of the ELP complex. It has been shown that unmodified U34 of tRNA can bind to essentially any of the four nucleotides (Söll and RajBhandary 1995), leaving a possibility that the mutations in the ELP complex may cause misreading of a broad spectrum of different codons, including all three stop codons, even though we have identified misreading in only one opal codon in vivo. Additional genetic and biochemical analysis may be required to have a thorough understanding of the question.
It is important to point out that disrupting this complex has a more profound effect than just suppressing the lin-1(e1275) allele, as dph-3 and moc-3 mutants are associated with slow growth, partial lethality, and a weak high-incident-male (Him) phenotype. This is partly consistent with the previous data that a mutation affecting thiolation of tRNA causes a Him phenotype and genome instability (Dewez et al. 2008). These developmental defects may reflect codon misreading in many genes. Therefore, tRNA modification function of the ELP complex is expected to be one of the mechanisms that play important roles in accurate translation.
It is worth noting that lin-1(e1275) is a temperature-sensitive allele. The temperature sensitivity of lin-1(e1275) is unique among lin-1(lf) alleles harboring premature stop codons (Beitel et al. 1995; Miley et al. 2004). In addition, the temperature sensitivity indicates that there may be a low level of translational readthrough bypassing the opal stop codon at the lower temperature. In other words, moc-3(lf) and dph-3(lf) may have facilitated the readthrough of the opal stop via aberrant tRNA modification in lin-1(e1275), which is already prone to stop codon recognition failure. This is supported by the result that lin-1(n176);kuIs76 showed a slightly suppressed Muv phenotype (95%), while lin-1(n176) moc-3(tm3742);kuIs76 and lin-1(n176) dph-3(ku432);kuIs76 displayed a substantially suppressed Muv phenotype (21% and 13%, respectively) (Table 4). Furthermore, we showed a potential readthrough protein product using the lin-1(e1275)∷gfp transgene in the moc-3(lf) mutant (Figure 6). In that sense, the previous work on the exocytosis function of the ELP complex might need to be re-evaluated. It was reported that a null allele of ELP1 could suppress the exocytosis defect of a mutant in the Rab guanine nucleotide exchange factor Sec2p (Rahl et al. 2005). Intriguingly, the authors of this report used one single mutant of the sec2 gene, sec2-59, which has a temperature sensitivity and a premature opal stop codon that is similar to lin-1(e1275) (Nair et al. 1990). This potential cause of allele-specific suppression has also been pointed out previously in another article (Svejstrup 2007).
In conclusion, we have identified the roles of two genes, moc-3 and dph-3, involved in protein translation in C. elegans through isolating novel genetic suppressors of lin-1(e1275). We provide evidence that the e1275-specific suppression by moc-3(lf) and dph-3(lf) is independent of the RAS/MAPK pathway and may involve the tRNA modification modules, including urm-1 and the ELP complex. The phenotypes of these mutants (which demonstrate the suppression effect) likely reflect the fundamental functions of this complex in maintaining accurate translation. Further study would be required to elucidate how and to what extent this protein complex is involved in translation in C. elegans.
Acknowledgments
We thank S. Mitani for the moc-3(tm3742) strain and Haibo Liu, Amara Seng, and members of our laboratory for helpful discussion. We also thank Gunilla Jäger for performing the HPLC analysis of tRNA. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This work was supported by the Korea Research Foundation Grant funded by the Korean Government (Ministry of Education and Human Resources Development) (KRF-2006-352-C00062) to S.K., the Swedish Cancer Foundation (07 0637 to A.S.B.), the Swedish Science Research Council (621-2006-4269 to A.S.B.), the Bernhard and Signe Bäckström Foundation (223-438-07) to A.S.B., and the Howard Hughes Medical Institute, of which S.K. and W.J. were associates, A.K.S. is a lab manager, and M.H. is an investigator.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.118406/DC1.
References
- Beitel, G. J., S. G. Clark and H. R. Horvitz, 1990. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348 503–509. [DOI] [PubMed] [Google Scholar]
- Beitel, G. J., S. Tuck, I. Greenwald and H. R. Horvitz, 1995. The Caenorhabditis elegans gene lin-1 encodes an ETS-domain protein and defines a branch of the vulval induction pathway. Genes Dev. 9 3149–3162. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine, R. D., C. S. Branda and M. J. Stern, 1998. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125 1083–1093. [DOI] [PubMed] [Google Scholar]
- Chen, C., S. Tuck and A. S. Bystrom, 2009. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 5 e1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, M., and M. Han, 2003. Cis regulatory requirements for vulval cell-specific expression of the Caenorhabditis elegans fibroblast growth factor gene egl-17. Dev. Biol. 257 104–116. [DOI] [PubMed] [Google Scholar]
- Dewez, M., F. Bauer, M. Dieu, M. Raes, J. Vandenhaute et al., 2008. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc. Natl. Acad. Sci. USA 105 5459–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esberg, A., B. Huang, M. J. Johansson and A. S. Bystrom, 2006. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol. Cell 24 139–148. [DOI] [PubMed] [Google Scholar]
- Ferguson, E. L., and H. R. Horvitz, 1985. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics 110 17–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner, L., and R. Schaffrath, 2002. KTI11 and KTI13, Saccharomyces cerevisiae genes controlling sensitivity to G1 arrest induced by Kluyveromyces lactis zymocin. Mol. Microbiol. 44 865–875. [DOI] [PubMed] [Google Scholar]
- Fichtner, L., D. Jablonowski, A. Schierhorn, H. K. Kitamoto, M. J. Stark et al., 2003. Elongator's toxin-target (TOT) function is nuclear localization sequence dependent and suppressed by post-translational modification. Mol. Microbiol. 49 1297–1307. [DOI] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806–811. [DOI] [PubMed] [Google Scholar]
- Furukawa, K., N. Mizushima, T. Noda and Y. Ohsumi, 2000. A protein conjugation system in yeast with homology to biosynthetic enzyme reaction of prokaryotes. J. Biol. Chem. 275 7462–7465. [DOI] [PubMed] [Google Scholar]
- Gilbert, C., A. Kristjuhan, G. S. Winkler and J. Q. Svejstrup, 2004. Elongator interactions with nascent mRNA revealed by RNA immunoprecipitation. Mol. Cell 14 457–464. [DOI] [PubMed] [Google Scholar]
- Goehring, A. S., D. M. Rivers and G. F. Sprague, Jr., 2003. a Attachment of the ubiquitin-related protein Urm1p to the antioxidant protein Ahp1p. Eukaryot. Cell 2 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring, A. S., D. M. Rivers and G. F. Sprague, Jr., 2003. b Urmylation: a ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol. Biol. Cell 14 4329–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, T., S. Orita and M. Han, 1998. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol. Cell. Biol. 18 4556–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, M., and P. W. Sternberg, 1990. let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell 63 921–931. [DOI] [PubMed] [Google Scholar]
- Hoyt, M. A., S. McDonough, S. A. Pimpl, H. Scheel, K. Hofmann et al., 2008. A genetic screen for Saccharomyces cerevisiae mutants affecting proteasome function, using a ubiquitin-independent substrate. Yeast 25 199–217. [DOI] [PubMed] [Google Scholar]
- Huang, B., M. J. Johansson and A. S. Bystrom, 2005. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, B., J. Lu and A. S. Bystrom, 2008. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA 14 2183–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H., W. S. Lane and D. Reinberg, 2002. Human Elongator facilitates RNA polymerase II transcription through chromatin. Proc. Natl. Acad. Sci. USA 99 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld, K., 1997. Vulval development in Caenorhabditis elegans. Trends Genet. 13 55–61. [DOI] [PubMed] [Google Scholar]
- Krogan, N. J., and J. F. Greenblatt, 2001. Characterization of a six-subunit holo-elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 21 8203–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel, S., P. G. Pedrioli, T. Bucher, R. Brost, M. Costanzo et al., 2009. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 458 228–232. [DOI] [PubMed] [Google Scholar]
- Liu, S., and S. H. Leppla, 2003. Retroviral insertional mutagenesis identifies a small protein required for synthesis of diphthamide, the target of bacterial ADP-ribosylating toxins. Mol. Cell 12 603–613. [DOI] [PubMed] [Google Scholar]
- Liu, S., G. T. Milne, J. G. Kuremsky, G. R. Fink and S. H. Leppla, 2004. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol. Cell. Biol. 24 9487–9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., J. F. Wiggins, T. Sreenath, A. B. Kulkarni, J. M. Ward et al., 2006. Dph3, a small protein required for diphthamide biosynthesis, is essential in mouse development. Mol. Cell. Biol. 26 3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman, D., R. H. Plasterk, I. L. Johnstone and J. F. Caceres, 2007. Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway. Genes Dev. 21 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J., B. Huang, A. Esberg, M. J. Johansson and A. S. Bystrom, 2005. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA 11 1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miley, G. R., D. Fantz, D. Glossip, X. Lu, R. M. Saito et al., 2004. Identification of residues of the Caenorhabditis elegans LIN-1 ETS domain that are necessary for DNA binding and regulation of vulval cell fates. Genetics 167 1697–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, L. M., M. E. Gallegos, B. A. Morisseau and S. K. Kim, 1993. lin-31, a Caenorhabditis elegans HNF-3/fork head transcription factor homolog, specifies three alternative cell fates in vulval development. Genes Dev. 7 933–947. [DOI] [PubMed] [Google Scholar]
- Nair, J., H. Muller, M. Peterson and P. Novick, 1990. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J. Cell Biol. 110 1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai, Y., M. Nakai and H. Hayashi, 2008. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J. Biol. Chem. 283 27469–27476. [DOI] [PubMed] [Google Scholar]
- Noma, A., Y. Sakaguchi and T. Suzuki, 2009. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 37 1335–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, G., J. Fellows, Y. Li, T. de Bizemont, A. M. Dirac et al., 1999. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3 109–118. [DOI] [PubMed] [Google Scholar]
- Rahl, P. B., C. Z. Chen and R. N. Collins, 2005. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol. Cell 17 841–853. [DOI] [PubMed] [Google Scholar]
- Rubio-Texeira, M., 2007. Urmylation controls Nil1p and Gln3p-dependent expression of nitrogen-catabolite repressed genes in Saccharomyces cerevisiae. FEBS Lett. 581 541–550. [DOI] [PubMed] [Google Scholar]
- Schlieker, C. D., A. G. Van der Veen, J. R. Damon, E. Spooner and H. L. Ploegh, 2008. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc. Natl. Acad. Sci. USA 105 18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, G., and R. R. Mendel, 2006. Molybdenum cofactor biosynthesis and molybdenum enzymes. Annu. Rev. Plant Biol. 57 623–647. [DOI] [PubMed] [Google Scholar]
- Söll, D., and U. Rajbhandary, 1995. tRNA: Structure, Biosynthesis, and Function. ASM Press, Washington, DC.
- Sternberg, P. W., 2005. Vulval development (June 25, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.6.1, http://www.wormbook.org.
- Sternberg, P. W., and M. Han, 1998. Genetics of RAS signaling in C. elegans. Trends Genet. 14 466–472. [DOI] [PubMed] [Google Scholar]
- Sun, J., J. Zhang, F. Wu, C. Xu, S. Li et al., 2005. Solution structure of Kti11p from Saccharomyces cerevisiae reveals a novel zinc-binding module. Biochemistry 44 8801–8809. [DOI] [PubMed] [Google Scholar]
- Sundaram, M., 2005. RTKRas/MAP kinase signaling (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.80.1, http://www.wormbook.org.
- Svejstrup, J. Q., 2007. Elongator complex: How many roles does it play? Curr. Opin. Cell Biol. 19 331–336. [DOI] [PubMed] [Google Scholar]
- Tan, P. B., M. R. Lackner and S. K. Kim, 1998. MAP kinase signaling specificity mediated by the LIN-1 Ets/LIN-31 WH transcription factor complex during C. elegans vulval induction. Cell 93 569–580. [DOI] [PubMed] [Google Scholar]
- Tiensuu, T., M. K. Larsen, E. Vernersson and S. Tuck, 2005. lin-1 has both positive and negative functions in specifying multiple cell fates induced by Ras/MAP kinase signaling in C. elegans. Dev. Biol. 286 338–351. [DOI] [PubMed] [Google Scholar]
- Winkler, G. S., T. G. Petrakis, S. Ethelberg, M. Tokunaga, H. Erdjument-Bromage et al., 2001. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J. Biol. Chem. 276 32743–32749. [DOI] [PubMed] [Google Scholar]
- Yandell, M. D., L. G. Edgar and W. B. Wood, 1994. Trimethylpsoralen induces small deletion mutations in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 91 1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem, J., T. Gu and M. Han, 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder, J. H., H. Chong, K. L. Guan and M. Han, 2004. Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 23 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]