Abstract
In this review we present critical overview of some of the available literature on the fundamental biology of telomeres and telomerase in Metazoan. With the exception of Nematodes and Arthropods, the (TTAGGG)n sequence is conserved in most Metazoa. Available data shows that telomerase-based end maintenance is a very ancient mechanism in unicellular and multicellular organisms. In invertebrates, fish, amphibian, and reptiles persistent telomerase activity in somatic tissues might allow the maintenance of the extensive regenerative potentials of these species. Telomerase repression among birds and many mammals suggests that, as humans, they may use replicative aging as a tumor protection mechanism.
Keywords: telomeres, telomerase, senescence, Metazoa, vertebrates, invertebrates
1. Introduction
Normal human somatic cells display a limited capacity to proliferate, a phenomenon known as the “Hayflick limit” [1]. Telomeres provide the molecular clock that determines this replicative lifespan [2]. Each of the 92 human telomere ends is formed by thousand of repeats of the six nucleotide sequence TTAGGG bound by telomere-associated proteins such as the shelterin complex [3–5]. Human telomere length decreases both as a function of donor age in tissues and number of cell divisions in culture [2, 6–8].
Telomerase is a ribonucleoprotein cellular reverse transcriptase that uses its catalytic component (hTERT) to synthesize telomeric DNA (TTAGGG)n directly onto chromosome ends [9–10]. In humans, this enzyme is expressed in embryonic tissues and specific germline cells. Telomerase is detected in fetal and adult testis but is neither found in most normal somatic cells, nor in non-dividing oocytes and mature spermatozoa[11–12]. In adult humans many stem or stem-like cells activate telomerase activity when stimulated to divide. Among them are specific proliferative cells of renewal tissues (e.g. hematopoietic stem cells, activated lymphocytes, basal cells of the epidermis, proliferative endometrium, and intestinal crypt cells). Although the low levels of telomerase activity in these cells may be sufficient to slow telomeric loss, it is not able to prevent telomere shortening since these cells still undergo senescence [12].
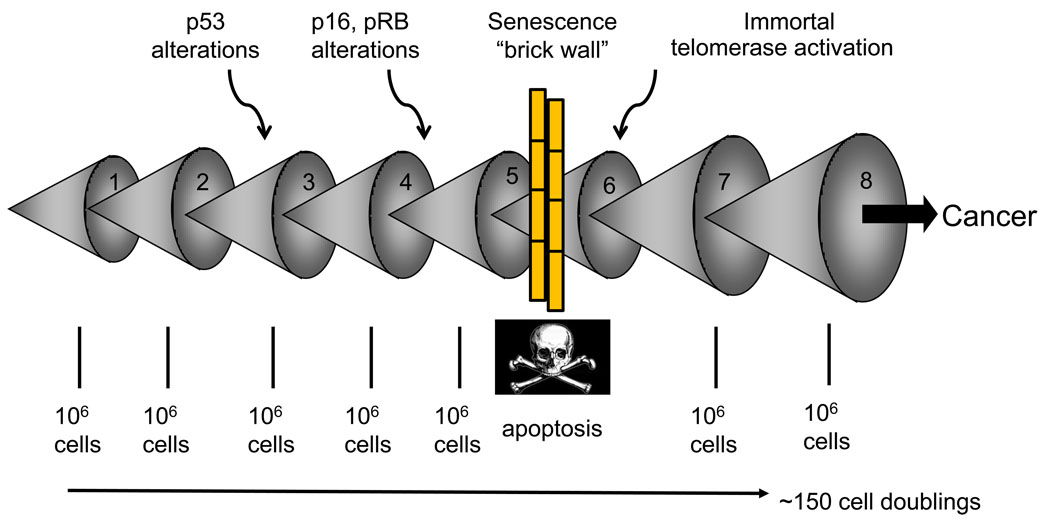
Telomere-based replicative senescence is thought to have evolved as a tumor protection mechanism in long-lived organisms such as humans, preventing the early development of cancer [13]. The reason human somatic fibroblasts rarely immortalize in culture is partly because at least three independent tumor prevention pathways (p53, p16INK4a/pRB, telomere shortening) have to be altered in order to allow cell immortalization [13]. Cancer cells must acquire several mutations before they become malignant [14]. Replicative aging blocks this progression by halting cell division before many mutations are able to accumulate within a single cell (Fig. 1). The cell containing an initial mutation must expand to a population size of perhaps one million cells before there is a reasonable probability for a second mutation to occur, so each mutation would require at least 20 divisions (220=106). Since most mutations are recessive, an additional clonal expansion would be required to eliminate the remaining wild-type allele (usually through loss of heterozygosity). By setting the number of possible replications below 100 the progression of pre-malignant cells would be blocked while they still had few mutations [12]. The fact that ~85% of human tumors have upregulated or reactivated telomerase activity and are able to maintain their telomeres supports this hypothesis. Immortalization may occur by gene(s) mutation in the telomerase repression pathway [15].
Figure 1. Replicative aging and cancer.
Multiple mutations are required before a cell can become malignant. This occurs as a series of clonal expansions. This uses a sufficient number of cell doublings so that senescence imposed by telomere shortening forms a barrier to the progression of tumor cells.
Another way telomeres can be maintained is through telomerase independent mechanisms known as alternative lengthening of telomeres (ALT) [16]. This ALT pathway is only detected in a few rarer cancers (e.g. sarcomas), but is low in the more frequent epithelial neoplasias (carcinomas). This may reflect tighter telomerase regulation in mesenchymal versus epithelial tissues [17]. The ALT pathway is characterized by an array of phenotypes such as a very heterogeneous distribution of telomere sizes and length fluctuations, ALT-associated PML bodies (APBs), higher levels of telomere sister chromatic exchanges (T-SCE), and raised levels of C-circles [17]. Recent studies in mice have suggested that telomerase-independent telomere elongation plays a role in normal development [11]. Mice oocyte telomere elongation following fertilization seems to be achieved through a recombination based mechanism characterized by extensive T-SCE. At the blastocyst stage, telomerase appears to take control of telomere maintenance [11]. Undifferentiated mouse ES cells expressing a gene cluster (Zscan4) undergo rapid telomere extension and long-term genomic stability, probably by telomere recombination or T-SCE. Unlike other cells that display T-SCE, such ALT tumor cells and survivors of telomerase knockout Terc2/2 ES cells, telomerase activity is detected in Zscan4 ES cells [18].
Replicative aging is believed to function as a potent antitumor mechanism in humans. Whether this mechanism is conserved in other species remains unclear. Telomerase-based end maintenance is likely to be a very ancient mechanism since it is found in widely divergent species that represent many of the major eukaryote lineages (ciliates, animals, fungi, green plants). Telomeres play a central role in preventing chromosome ends from being recognized as double-strand breaks. The loss of telomerase in some unicellular organisms is a catastrophic event unless there is immediate (within a few generations) replacement by an alternative system.
In this review we present an inter-species critical overview of some of the available literature on the fundamental biology of telomeres and telomerase in development, regeneration, cancer and aging during the evolution of Metazoans.
2. Metazoa
2.1 Invertebrates
2.1.1 Lower Metazoan
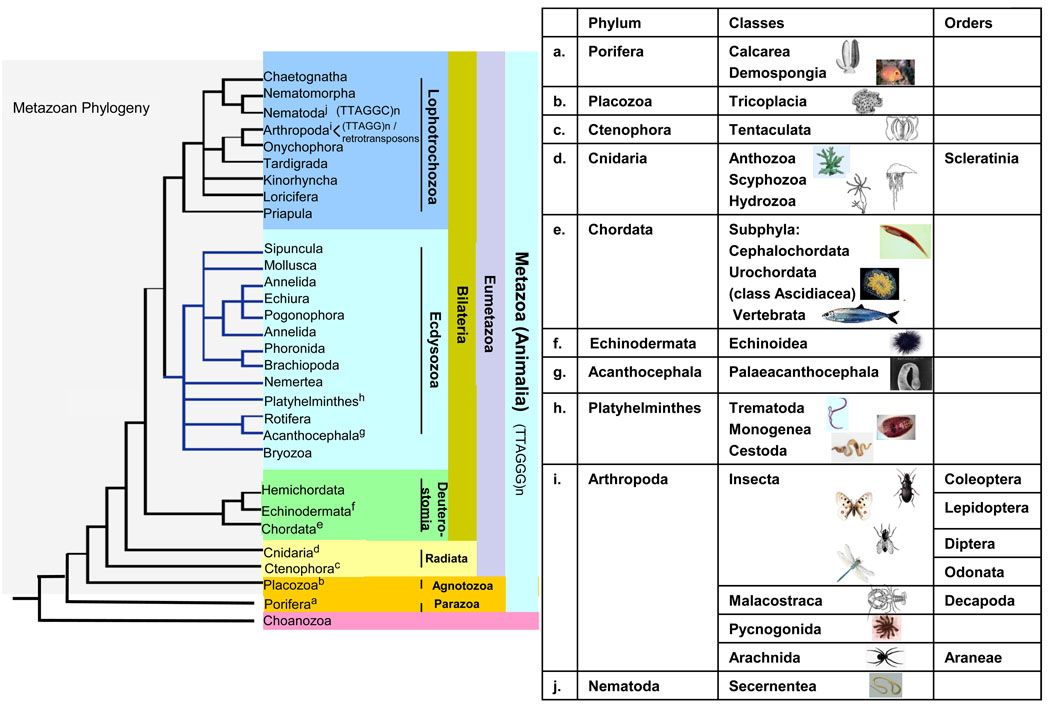
The Lower Metazoan includes the phyla Porifera (sponges), Placozoa (Trichoplax adhaerens), Cnidaria (corals and jellyfish) and Ctenophora (comb jellies) and are considered an evolutionary bridge between fungi and higher animals [19] (Fig. 2). All these phyla display the “vertebrate” telomeric motif, also found in the unicellular metazoan sister group Choanozoa [20].
Figure 2. Phylogenetic tree of metazoa (animalia).
The tree and chart shows the relationships of the different species whose telomere biology is discussed, keyed to superscript letters.
The lowest metazoan phylum is Porifera (Fig. 2.a) in which many species are reported to present negligible senescence [21]. Sponge species usually show continuous growth, long lifespans, and a highly flexible cell lineage determination [22]. Species from this phyla are known for their extensive regenerative capacity and use of both sexual and vegetative forms of reproduction [21]. In vivo and in vitro studies in marine demosponges Suberites domuncula and Geodia cydonium exhibit telomerase activity in their somatic and immortal germ tissues. After dissociation into single cell suspensions, isolated cells retain their proliferative capacity but lose telomerase activity, possibly due to lack of contact/adhesion factors. However, telomerase activity is recovered after aggregation of the cells to form primmorphs [22].
In Calcarea (Leucosolenia sp and Sycon sp.) (Fig. 2.a) telomere sizes seem to range from below 1 kb to over 20 kb. One study in Calcarea that also examined the demosponge Suberites failed to detect telomerase activity in either species [20]. This is unexpected and conflicts with the Suberites study cited above, so it is premature to conclude that Calcarea do not express telomerase.
Among Cnidarians (Fig. 2.d), the Anthozoans (Corals) are the most basal organism reported to exhibit the (TTAGGG)n telomeric sequence. This repeat is found in DNA from several Scleractian order corals: Acropora surculosa, Leptoria phrygia, Favia pallida and Goniastrea retiformis. Average telomere length of Acropora surculosa is 3.5 kb [19]. Reef corals display vegetative growth of hundreds of years, their rate of mortality decreases as coral body mass increases and several species show increasing fecundity as the colonies grow larger [21]. In spite of these properties, which are characteristic of negligible senescence, reef corals show signs of aging, with declining growth, calcification and reproduction before colony death in Stylophora pistillata [21, 23].
Cnidaria Scyphozoa species (Fig. 2.d) such as compass jellyfish (Chrysaora hysoscella) and blue jellyfish (Cyanea lamarckii) and Ctenophora (Pleurobrachia pileus) (Fig. 2.c) reported telomere sizes range from less than 1 kb to over 20 kb. In the Cnidaria Hydra vulgaris (Fig. 2.d) sizes seem to be around 20 kb. Telomerase activity has been found in gonad extracts of Cnidaria moon jelly (Aurelia aurita) and the ctenophore (Pleurobrachia pileus). However, similar studies in Cnidarians such as hydra or in Placozoan (Trichoplax) (Fig. 2.b) did not detect telomerase activity [20].
2.1.2 Bilateria invertebrates
Among Bilateria (Fig. 2), the phyla Onychophora, Platyhelminthes, most Annelida and Mollusca, Echinodermata and the subphylum Urochordata (Fig. 2.e), seem to share the “vertebrate” telomere motif (TTAGGG)n [24–31].
2.1.2.1 Ecdysozoa (Platyhelmintes and Acanthocephala)
In the Trematode Schistosoma mansoni (Fig. 2.h) chromosomes are also protected from degradation by telomeres [32]. A telomeric study of parasitic worms including the Platyhelminthes flatworm groups Monogenea and Cestoda, and thorny-headed worms (Syndermata: Acanthocephala) revealed conservation of the (TTAGGG)n sequence, in Monogenea (Paradiplozoon homoion) and Cestoda (Caryophyllaeus laticeps, Caryophyllaeides fennica, and Nippotaenia mogurndae. However neither this motif or the nematode motif were present in the parasitic Acanthocephala (Pomphorhynchus laevis and Pomphorhynchus tereticollis) (Fig. 2.g) suggesting the existence of an as yet unknown telomeric repeat sequence or an alternative mechanism of telomere maintenance [33].
2.1.2.2 Lophotrochoa (Nematodes and Arthropods)
The so called nematode motif (TTAGGC)n, is found in the Secernentea roundworms Ascaris lumbricoides, Ascaris sum and Parascaris univalens (Fig. 2.j) [34]. In Ascaris, chromatin fragmentation involves a complex molecular mechanism that includes site-specific chromosome breaks, telomeric synthesis, and degradation of DNA [20, 35]. In Parascaris univalens the haploid germline genome is contained in a single large chromosome and the somatic genome is surrounded by heterochromatin (HET) blocks constituted by segments of the repeats TTGCA and TTTGTGCGTG. However, in both species, the ends of the germline chromosomes are said to be capped by the same (TTAGGC)n tracts, which are added to all the new somatic ends after removal of the old ones during the complex chromatin diminution process [34]. Chromosome capping in the free-living nematode Caenorhabditis elegans, is achieved by the 4–9 kb telomeric repeats (TTAGGC)n [36].
All the major arthropod Subphyla (Chelicerata--except spiders, Myriapoda, Crustacea and most Hexapoda) (Fig. 2.i) have the (TTAGG)n telomere motif [20]. Unlike mammals that stop growing after adulthood, some invertebrates, such as the Decapoda crustacean lobster (Homarus americanus) grow continuously throughout life, although growth rates seem to decrease with age. Lobsters show asymptotic growth and can occasionally weigh over forty pounds, and seem to present negligible or very slow gradual senescence. Lobsters have very long lifespans of 50 to 100 year and neither sex exhibits a post-reproductive phase nor molting cessation. They are also able to regenerate their limbs even at advanced ages [21, 37]. Telomere analysis reveals the sequence (TTAGG)n and telomerase expression has been found in fully differentiated tissues of all organs, with high levels detected in the hepatopancreas and heart and moderate levels in skin and muscle tissues [37]. Tumors are rare in adult lobsters and do not seem to correlate with size or lifespan [21]. Another Decapoda crustacean, the green sea crab (Carcinus maenas) also has the pentameric (TTAGG)n telomere sequence and high telomerase activity in its tissues [38].
The low number of tumor reports in decapod crustaceans may represent a truly low incidence of neoplasia compared to other well studied animal groups rather than insufficient information. This is a large animal group of more than 10,000 species, many commercially important and well investigated, such as lobsters, crabs, shrimp and crayfish. Despite many of these species having long lifespans, some reaching almost 100 years, neoplasias are said to be extremely rare [39]. Furthermore, many of these species are benthic, and have an elevated exposure to carcinogens but the frequencies of tumors are remarkably different from mollusks, bottom feeding fish and other fish and even insects [39].
The reason for the low cancer incidences observed in this Phyla are unknown, but many mechanisms may play a role in this event. Decapod crustaceans exhibit some remarkable carcinogen detoxification pathways such as rapid elimination of PAH-related DNA adducts from the tissues. Their immune system includes only innate responses and is reported to be able to either phagocytose or melanize and encapsulate all kinds of foreign material. Arthropods use this rigid melanin barrier to isolate and, together with quinolone cellular toxicity, eliminate cancer cells and damaged tissue areas [39]. Stem cell maintenance until the end of life, for example by telomere protection due to high telomerase activity in tissues throughout life, has also been suggested as contributors for the virtual absence of age-related cancer in the Decapoda [39]. These species may provide excellent models for tumor protection mechanism studies.
With the exception of the heterogeneous Coleoptera, most insect orders can be divided into those that use the telomeric repeat (TTAGG)n (e.g. Lepidoptera) or the ones that do not (e.g. Diptera) [19, 40–43].
Telomerase activity has recently been detected in crickets, cockroaches, and species of Lepidoptera [44]. The telomerase reverse transcriptase (TERT) subunit has been identified and characterized in the domestic silkworm (Bombyx mori) and the flour beetle (Tribolium castaneum) [45]. In the group of insects with the largest number of species, the beetle (order Coleoptera), the telomerase-dependent (TTAGG)n motif has been repeatedly lost (5 to 6 times) in different phylogenetic branches and was likely replaced with the alternative mechanisms of telomere elongation [46]. The order Diptera seems to be an exception from the general pattern of having short G-rich repeats at their telomeres, and instead often has arrays of complex long satellite repeats at the ends of their chromosomes (e.g. Chironomus & Anopheles gambiae) [20, 47–48]. Elongation of telomeres in the mosquito (Anopheles) is done through gene conversion between complex terminal satellite repeats that are present at natural telomeres [48]. One hypothesis is that Diptera may have lost the telomerase gene and was forced to use alternative mechanisms of telomere elongation [48–49]. The fruit fly (Drosophila melanogaster) uses telomerase independent mechanisms such as chromosome end capping with non-LTR retrotransposons. Chromosome end-elongation is predominantly achieved by terminal insertion of two classes of telomere-specific LINE-like retrotransposable elements, HeT-A and TART [50]. However, Drosophila telomeres can also be extended by gene conversion [51] and perhaps by recombination between telomeric HeT-A elements [52]. The telomeric structure of Damselflies (Zygoptera) and spiders (Araneae) is still unclear [31, 43]. Sea spiders (Pycnogonida) also have the (TTAGG)n telomeric motif [20].
2.1.2.3 Deuterostomia
In Deuterostomia, which includes the phyla Chordata (Fig. 2.e and 3.a) and Echinodermata (e.g. sea urchins) (Fig. 2.f), many examples of long-lived species have been found. Longevities of a decade or more are found in many sea urchins, and in fact, mortality rates decrease with size in adults [21]. The Red Sea urchin (Strongylocentrotus franciscanus) (Fig. 2.f) grows indeterminately during a lifespan that can go beyond 100 years without evidence of age-related disease or decline in reproductive potential, while other species such as the green sea urchin (Lytechinus variegatus) are fast growing and short lived, with a maximum lifespan of 3 to 4 years. Telomere studies in the Red Sea urchin reveals telomerase activity in mature eggs, and also during early stages of development of L. variegatus and in tissues during adulthood in both species (Aristotle’s lantern muscle, ampullae, esophagus, intestine, tube feet, male and female gonads). Long telomere lengths (>20 kb) were found both in germ and somatic tissues of L. variegatus. The adult tissues of S. franciscanus have short telomere lengths (≈ 5 kb), similar to the California purple sea urchin (S. purpuratus) (6 kb), and no telomere shortening occurs throughout life of these species [53–54]. These results seem to indicate that neither short nor long-lived sea urchins use replicative aging as a tumor protective mechanism [53]. Furthermore, the number of reported cases of neoplasia in sea urchins, a very intensively studied model organism, is very low [55].
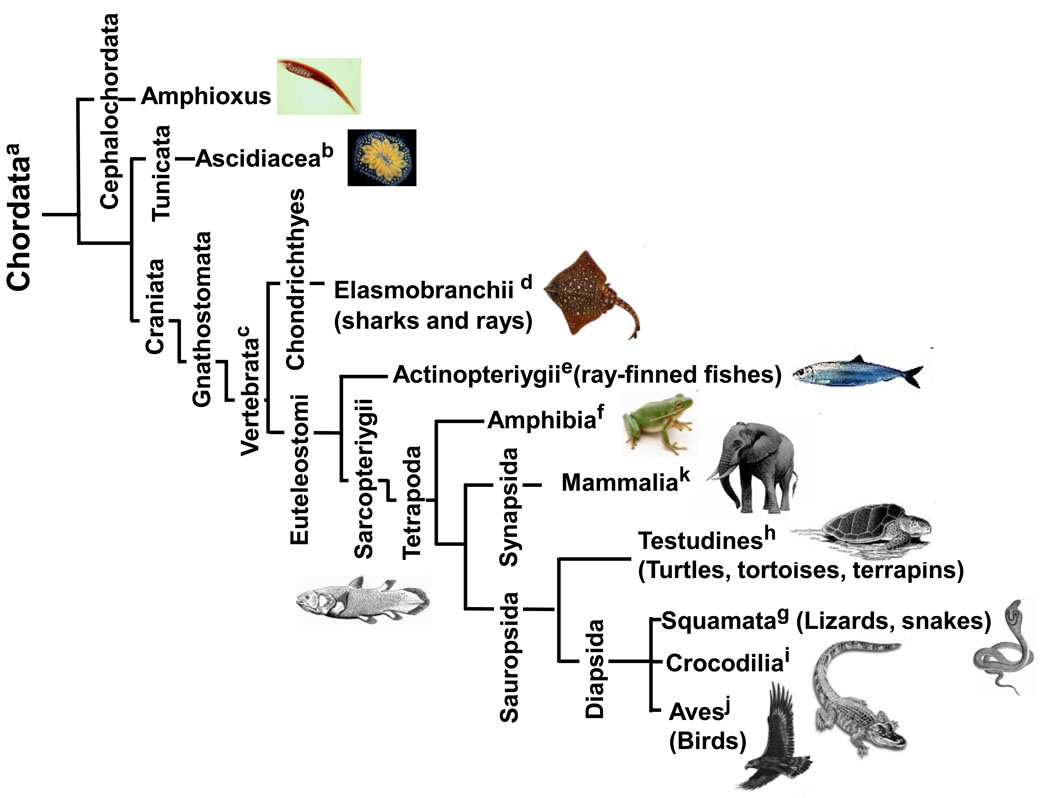
Figure 3. Phylogenetic tree of the phylum chordata.
The tree shows the relationships of the different chordates whose telomere biology is discussed, keyed to superscript letters.
The golden star tunicate (Botryllus schlosseri), the model Urochordate (Fig. 2.e and Fig. 3.b), is a colonial organism that propagates both asexually and sexually during the 2 to 5 years of colony life. Asexual budding occurs continually from the progenitor body wall and when the colony reaches a critical size sexual reproduction initiates with the production of gonads. It has been proposed that pools of stem cells assure renovation throughout the lifespan. Heterogeneous telomeres of 6–15 kb protect the chromosome ends and high levels of telomerase have been reported in germ and embryonic tissues [24]. Telomerase activity peaks in tissues containing bud rudiments, then decreases in buds that are going through organogenesis and drops to even lower levels in functional zooids, in individual organs and blood [24]. It has been hypothesized that telomerase activity needs to be retained in progenitor and stem cells, is downregulated during differentiation, and is not necessary to maintain the relatively short-lived somatic tissues of Botryllus [24].
Information about telomere sequences and telomerase TERT and TR/TERC sequences and structure in invertebrates is now readily available [56].
2.2 Vertebrates (Fig. 2.e and 3.c)
2.2.1 Fish
Several fish species can grow throughout life with high proliferative capacity displayed by all somatic cells [57]. In many other species, organs continue to grow and growth after the larval stage is dependent on both cellular hyperplasia and hypertrophy [58–59].
Among Elasmobranchs (Fig. 3.d), the dogfish shark (Squalus acanthias) is the longest lived (70 years) but the reported lifespan of most cartilagenous fish is much lower than 15 years in captured specimens [21]. Telomeric (TTAGGG)n sequences are present in cartilagenous fish [60]. Dogfish shark (Squalus acanthis) has human-like telomeres (10–15 kb) and high levels of telomerase expression [38, 61]. Telomere bands of 3 kb seem to be common to four species of Batoidea (Torpedo marmorata, Torpedo ocellata, Raja asterias, Raja montagui) and two species of Galeomorphii (Mustelus asterias, Scyliorhinus stellaris). In the little skate (Raja erinacea) telomeres ranged between 10–15 kb and in other rays, intense short telomeric bands varying in length from 0.5 to 2 kb, were observed [38, 60]. Telomeric sequences in the paracentromeric and/or interstitial regions was observed in chromosomes of two Batoidea, the blue-spotted stingray (Taeniura lymma) and the electric ray (Torpedo ocellata). This finding supports the hypothesis that in cartilaginous fish Robertsonian fusions involving telomeres could have led to an increase in bi-armed chromosomes and a decrease of the acrocentric ones, thus playing an important role in karyotype evolution [60, 62].
Teleosts (bony fish, within the class Actinoptergii) (Fig. 3.e) represent more than half of the forty to fifty thousand vertebrate species [21]. Many reports show that eels, sturgeons, and other teleosts can live 80 years or more. In teleosts the record lifespan of 152 years is held by the lake sturgeon (Acipenser fulvescens) and the beluga sturgeon (Huso) (118 years), reaching weights of over 3 tons [21, 63]. Teleost fishes exhibit different patterns of aging. The pacific salmon (Oncorhynchus) and eel (Anguilla anguilla) exhibit rapid senescence and death at first spawning, while other fish such as medaka (Oryzias latipes) and guppy (Poecilia reticulata) seem to display gradual “mammalian-like” senescence [64]. In Cyprinidae, species with very different lifespans such as carp (Cyprinus carpio, which may live more than 100 years) and zebrafish (Danio rerio), which has a lifespan of approximately 5 years) exhibit growth characteristics that imply very slow senescence [64]. The short life, short generation time (3–5 months), and seemingly unlimited capacity to regenerate their fins in 7–10 days of zebrafish place it in a privileged spot as a genetically tractable vertebrate model for studying functional aging, where genetic mutant screens could be used to study gradual senescence [64–65]. Small telomeric sizes have been reported in teleosts: zebrafish (Dana rerio) (2–10 kb); killifish (Fundulus heteroclitus) (2–10 kb), japanese medaka (Oryzias latipes) (3–12 kb) and american eel (Anguilla rostrata) (10–15 kb) [38]. In trout (Oncorhynchus mykiss), erythrocytes have larger average telomeric terminal restriction fragment (TRF) lengths of 20 kb [54].
Telomerase activity is detected in cells and tissues of several teleost fish (e.g. fugu, zebrafish, rainbow trout, Japanese medaka, flounder) [61, 64, 66–67]. The integral telomerase RNA (TR/TERC)) from five teleost fish, Danio rerio, Oryzias latipes, Gasterosteus aculeatus, Takifugu rubripes and Tetraodon nigroviridis has been characterized [68]. The gene encoding the TERT subunit of telomerase has been isolated and cloned in pufferfish (Fugu rubripes) and zebrafish [59, 69]. In Fugo, the fTERT mRNA is found at low levels in several tissues such as skin, stomach, spleen, heart, brain, and eye, with high expression in the gill, testis and ovary. fTERT expression is detected in an immortalized eye-derived cell line from Fugu. The level of expression is higher in actively dividing cells and is reduced at quiescence, suggesting cell cycle regulation of TERT [69]. In zebrafish, TERT mRNA expression and telomerase activity correlate closely and are detected in all somatic tissues, including retina and brain, with the highest activities found in gills and in the ovary, where the highly proliferative germ cells are found [59]. Telomerase activity is found in several somatic tissues of the American eel (Anguilla rostrata)[61].
Since significant levels of telomerase have been detected at both short and long lived aquatic species, it’s been suggested that the expression of telomerase in fish is likely related to tissue regeneration and not lifespan [38]. Telomere lengths in fish species are short ‘human-like’ and are maintained by an increase in telomerase activity during regeneration of injured tissues of killifish, Japanese medaka, and zebrafish [38].
The different patterns of senescence reported in fish make them unique models for studying the aging process. Most marine species with their high regenerative capacities and long lifespans seem to maintain telomerase in their tissues. The lack of telomerase repression in somatic tissues suggests that they do not use telomere shortening and replicative aging as a tumor-protection mechanism. Many of these species may prove excellent models for studies in regeneration, stem cells, DNA repair, cancer and aging.
2.2.2 Amphibians (Fig. 3.f)
Most data on the experimental model African clawed frog (Xenopus), which can live at least 15 years, suggests that senescence in amphibians is negligible or very slow [21]. Xenopus telomeres range from less than 10 kb to over 50 kb, in a polymorphic pattern between individuals [70]. Unusual inheritance patterns of some bands are observed when Xenopus telomeres from whole embryos are compared to telomeres in parent spleens. In some crossings the telomeres of the embryo or in the male testis are shorter than the telomeres of the parents’ spleen, consistent with a significant amount of DNA rearrangement at telomeres. Telomere length regulation of Xenopus may be different from that reported in mammals. Telomere data in Xenopus is also consistent with the occurrence of some degree of meiotic rearrangement [70]. A TERT gene from Xenopus, designated xTERT has been identified [71]. Telomerase activity is found in oocytes, embryos, and tissues from adult frogs (>1–2 years, Xenopus laevis). Telomerase activity is most abundant in testis, spleen, liver, and embryos [72–73]. In brain and muscle tissues telomerase activity is lower but still readily detectable. Furthermore, this activity does not seem to be limited to the polyploid members of the genus since telomerase activity is also found in somatic tissues of the diploid Xenopus tropicalis [73].
2.2.3 Reptiles (Fig. 3.g,h,i)
The sequence (TTAGGG)n has been documented in species from the Squamata orders Sauria and Serpentia (Fig. 3.g)[74]. Among adult garter snakes (Thamnophis elegans) telomeres range between 16–25 kb and decrease with age [75]. However, in water pythons (Liasis fuscus) telomere length did not change between 1 and 20 years of age. In adult pythons, reported telomere length was about 28 kb. Telomeres of hatchling pythons (about 7 kb) were significantly shorter than from one-year-old adults. It has been hypothesized that since hatchlings show high somatic cellular proliferation rates, the increase in telomeric length may have been caused by increased telomerase activity [76]. Some lizards are known to have excellent tissue regeneration capacity. Telomerase activity has been observed in all tissues of the six-lined racerunner (Cnemidophorus sexlineatus), a teiid lizard (lifespan 4 years) [77]. The same study found that skin fibroblasts of a juvenile blue racer (Columber constrictor) can undergo more than 124 population doublings (PD) with strong telomerase activity detected after 100 PD, which is suggestive of immortalization of the culture [78]. In another lizard, the Carolina anole (Anolis carolinensis), cellular proliferative capacities were greater than human diploid cells [79]. Telomere length (27–34 Kb) in erythrocytes from the Crocodilia American Alligator (Alligator mississippiensis) decreases with body length, and telomere lengths inversely correlate with age in the Chinese Alligator (Alligator sinensis) (Fig. 3.i)[80–81].
Among Testudines (Fig. 3.h), turtles have been reported to live more than 100 years, in captivity and have very high annual survival rates in natural conditions. Senescence has not been proven to occur in these species. Mortality does not seem to increase during aging, the reproductive capacity of females grows during their lifespan, and apart from carapace alteration from soil abrasion, no age-specific diseases are known [82]. Studies in mature breeding sea turtles (Chelonia mydas) have reported an absence of a decline in growth rate [83].
Population doublings of 100–130 have been observed in fibroblast cultures from young Galapagos tortoises (Geochelene nigra) [84]. Another turtle species (Pseudemys scripta) has been found to have long average telomere lengths (≈ 50 kb) [54]. However, in a study, cell culture senescence has been observed between PD 18–45 in yellow mud turtles (Kinosternon flavescens), which have a lifespan of 30–45 years. It has been shown that fibroblasts from hatchlings undergo about twice as many population doublings in culture as those from 25 year old mud turtles. Telomere shortening of about 30–50% was observed between hatchlings and adults, and apart from the gonad, no telomerase was found in tissues from these turtles [78]. More studies are needed to clarify if this cellular growth arrest is due to culture stress from inadequate growth conditions leading to stasis or from telomere-based replicative aging. In the same study, cells from the long-lived snapping turtle (Chelydra serpentina), (lifespan over 100 years) reportedly multiplied in culture for over 265 PD. In these snapping turtles, telomerase activity went from barely detectable at 157 PD to very strong at 191 PD. Telomerase activity was also detected in old painted turtles (Chrysemys picta) and cultured cells from this species were still dividing well at PD 120. Painted turtles present continuous growth during their lifespan of over 60 years and don’t show reproductive senescence with age. In this species, telomeres don’t seem to vary with age and range above 60 Kb [85]. Telomerase activity was found in gonads of two ornate box turtles (Terrapene ornata) hatchlings and in other organs of one of them [78]. Telomerase activity has therefore been found in two divergent families of turtle (Chelydridae and Emydidae).
Telomeres of about 20 kb were found in both embryos and adult erythrocytes in European freshwater turtles (Emys orbicularis). This species has a similar longevity to humans but is not known to display signs of senescence. Telomeric shortening did not occur in European freshwater turtles, but information about telomerase activity in the tissues of this species and many other species of Chelonian (and Reptilia in general) is not available. [82]. The available data suggests that telomerase is often found in adult somatic tissues of reptiles and telomere based replicative senescence is unlikely to occur in most of the species studied to date.
2.2.4 Birds (Fig. 3.j)
Birds (class Aves) and other homeothermic vertebrates exhibit gradual senescence with a definite lifespan [21]. Bird species are significantly longer lived than mammals of similar body weight [86].
In Galliformes, chicken (Gallus gallus) telomeric DNA represents at least 3 to 4% of the genomic DNA, about 10 times higher than what has been found in the human genome. Three overlapping sizes of telomere arrays are found in Southern blot analysis of chickens and classified as: Class I (0.5 to 10 kb), Class II (10 to 40 kb) and Class III (200 kb to 3 Mb) [87–88]. Class I bands do not exhibit age-related telomere shortening and are resistant to digestion by Bal 31 exonuclease, indicating that these arrays are located internally rather than at the end of the chromosomes [87, 89]. Chicken and primitive Palaeognathae birds commonly exhibit truly interstitial (non-centromeric and non-telomeric) (TTAGGG)n sites [90]. Chicken Class II telomeres seem to shorten with age, similarly to human telomeres [87]. Class III “mega-telomere” arrays are the largest reported in all vertebrates and are located at chromosome ends by Bal 31 exonuclease analysis. These highly polymorphic elements map to the 7 to 23 Mb microchromosomes [87]. These arrays were mapped to four autosomes and one sex chromosome (one array per chromosome). The female-specific array (2.8 Mb) was mapped to the q arm the female-specific sex chromosome [88]. Mega-telomere number and distribution is variable but two mega-telomere loci (GGA 9 and GGAW) are common among diverse chicken genetic lines. The same study reports that the DF-1 cell line contains the greatest amount of telomeric sequence per genome (17%), as compared to UCD 001 (5%) and DT40 (1.2%) [91]. Studies in inbred chicken lines showed a hyper-variable inheritance pattern suggestive of a high degree of recombination of these Class III arrays [88].
The chicken telomerase reverse transcriptase (chTERT) component has been well characterized [92]. Telomerase activity is high in early stage embryos and during organ development but is down-regulated during late embryogenesis or postnatally in most somatic tissues. Renewable tissues such as reproductive and immune organs seem to retain high levels of telomerase activity even in adults (4–5 years). Telomerase activity in chickens tends to correlate with the proliferative potential of the tissue. The telomere arrays of the somatic and germ tissues in the embryo display similar telomeric sizes, but telomeres in adult somatic tissues arrays are shorter, exhibiting an average decrease in size of 3.2 kb. Telomere shortening is detected in telomerase positive adult tissues (kidney, intestine, spleen), a pattern also reported in some human tissues [89, 93]. Primary cultures of embryonic chicken cells have telomerase activity which, after serial culture passages, is downregulated and cells growth arrest at about 35 PD. At senescence, these cells exhibit mean telomere sizes of about 5 kb [94]. This value is also similar to the one observed in human senescent cell cultures (5–6 kb). However, this growth arrest could have been driven by inadequate growth conditions leading to senescence so the critical experiment to establish a telomere-based senescence would be to verify that one could immortalize these cells through ectopic telomerase expression [95].
Adult blood cell average telomere lengths in two longlived seabirds, the European shag (Phalacrocorax aristotelis) and the wandering albatross (Diomedea exulans), were 8.4 kb and 9.9 kb respectively. Telomere length in blood cells shortened between the chick stage and adulthood in both species. However, among adults, telomere length was independent of age [96]. Other studies in lesser black-backed gulls (Larus fuscus) showed that larger hatchlings had shorter telomere lengths, suggesting that embryonic growth rate could have influenced telomere attrition. It was also observed that males had longer telomeres at hatching than females [97]. In two long-lived seabirds, the northern and southern giant petrels (Macronectes spp.) telomeres were shorter in adults than chicks, but there was also no trend for adult telomere length to decrease with age [97]. Telomere shortening in erythrocytes was reported in a variety of avian species by comparing erythrocyte and sperm telomere length [87, 98]. In a study of 18 species of birds, most displayed the Class I, II and III telomeric arrays [87]. Extremely long arrays, ranging from hundreds of kilobases to 1–2 Mb (Class III) were observed in all but two raptor species, the northern goshawk (Accipiter gentilis) and the American bald eagle (Haliaeetus leucocephalus). In erythrocytes of zebra finch (Taeniopygia guttata), common terns (Sterna hirundo), tree swallows (Tachycineta bicolor), and Adélie penguins (Pygoscelis adeliae) the average TRF length decreases with age [99]. Lifespans of these species range from 5–26 years. Surprisingly, in Leach’s storm-petrel (Oceanodroma leucorhoa) erythrocytes, TRF length did not decrease but actually increased with age. This species is long-lived, with observed lifespans of 36 years [99]. Higher telomerase activities are observed in the Leach’s storm-petrel in most tissues studied (intestine, liver, kidney, brain, bone marrow). Across these species and all age-classes, telomerase activity is generally higher in the proliferative tissues than in the postmitotic tissues. Telomeric shortening per year was higher in species of birds with shorter lifespans than in the species with longer lifespans [99]. The short-lived zebra finch and tree swallow sharply down-regulate bone marrow telomerase before adulthood, whereas the long-lived common tern and Leach’s storm-petrel express bone marrow telomerase at high levels throughout life that could produce the slower rates of erythrocyte telomere shortening observed. Post-natal telomerase activity is generally absent in the brain, skeletal muscle, kidney and liver in all species, although higher telomerase activity is observed in the skeletal muscle, kidney and brain of hatchling common terns and Leach’s storm-petrels than what is reported in chickens. Telomerase profiles in the bone marrow, gonads and intestine are elevated at all stages of life [99]. Few cancer rate studies in long-lived bird species are available but a low incidence of cancer has been reported in wild birds, and specifically in long-lived seabirds [100–101]. Damage susceptibility, repair abilities, shelterin proteins (which control the synthesis of telomeric DNA by telomerase) are also likely to be important in determining these telomeric shortening rates.
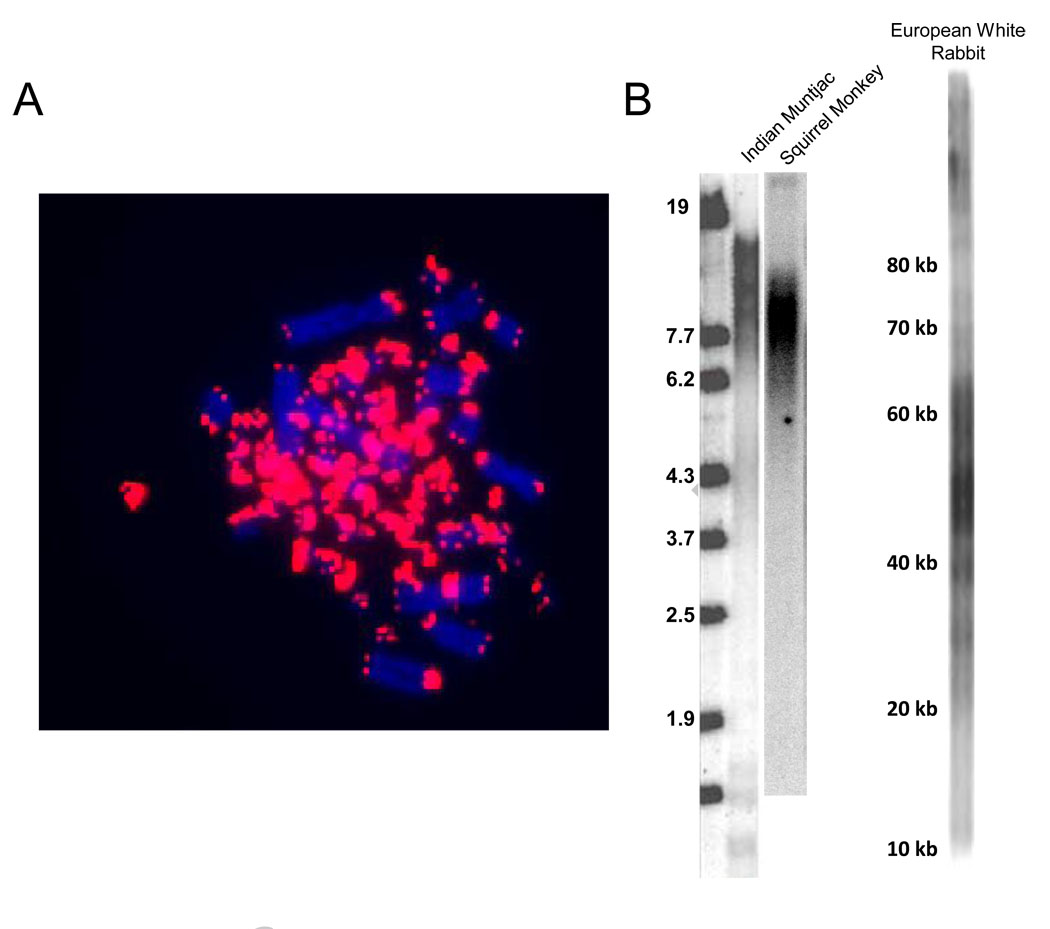
Telomeric (TTAGGG)n sequences are abundant in avian microchromosomes [90]. In studies of Japanese quail (Coturnix coturnix japonica) (unpublished results) telomeric repeats are preferentially localized to the 66 microchromosomes (2n=78) (Fig. 4A). A study of the chromosomal distribution of (TTAGGG)n sequences in 16 bird species representative of seven different orders, showed that several species, in particular the ratites, display (TTAGGG)n hybridization signals in interstitial and centromeric regions of their macrochromosomes. The microchromosomes of most species seem to be enriched with (TTAGGG)n sequences, displaying heterogeneous hybridization patterns, and it has been proposed that this high density of (TTAGGG)n repeats plays an important role in the exceptionally high meiotic recombination rates of avian microchromosomes [90]. However, other avian reports claim otherwise [102].
Figure 4. Telomeres in vertebrates.
A. Quail microchromosomes contain abundant telomeric sequences. Approximately 66 of the 78 quail chromosomes are 7–23 Mb microcromosomes. In situ hybridization using a probe for telomeric repeats reveals that most of the telomeric signal is coming from the small microchromosomes.
B. Diversity of mammalian telomere sizes. Primates (squirrel monkey) and Artiodactyls (Indian Munjac, a small barking deer) have small human-like telomeres of less than 20 kb while lagomorphs (European white rabbit) have much longer ~50 kb telomeres.
2.2.5 Mammals (Fig. 3.k)
Telomere-based replicative senescence is thought to function as a potent mechanism of tumor protection in humans [103]. It is becoming increasingly evident that many other mammalian species do not use telomeres in this way. For example, there is very good evidence suggesting that mice do not use telomere shortening to produce replicative aging.
Laboratory rodents have extremely long and polymorphic telomeres (25–150 Kb) and shortening was not observed during aging [104–105]. An In situ hybridization study estimated that mice telomeres are long (10–80 kb), but smaller than measured by TRF (possibly due to long subtelomeric regions) [106]. Other studies in rat (Rattus norvegicus) and mouse (Mus musculus) hepatocytes also revealed very long average TRF lengths (50 and 40 kb respectively) [54]. Telomere shortening observed in the postmitotic heart and brain telomeres of mice and rats has been attributed to oxidative stress [95, 107–108]. Telomerase activity has been detected in most murine tissues. In two mouse strains telomerase activity was reported in adult testes, ovary, breast, colon and liver, but was absent in brain, heart, stomach, muscle and skin [95, 109–110]. mTERT protein is only found in telomerase positive tissues, but the finding of mTERT mRNA in all tissues (including telomerase negative ones), suggests a quiescent state rather than lack of telomerase competency [111]. Mechanisms of alternate splicing triggered by quiescence may be responsible for the lack of telomerase expression [95, 111].
The growth crisis that occurs in mouse cultures after 10–15 doublings has been called senescence and was long considered equivalent to the replicative senescence observed in human cultures. Studies of the telomerase negative mTR−/− mouse demonstrate that this growth arrest is not due to telomere shortening and does not limit tumor growth [112]. mTR−/− mouse cells reportedly escape from this growth arrest as frequently as wild-type mice and can continue to divide for at least 200–300 PD [112]. Inadequate culture conditions and diverse environmental stresses can activate growth inhibitory genes due to a process termed stasis (stress or aberrant signaling induced senescence) [12]. MEFs (mouse embryo fibroblasts) from mice defective in DNA repair factors such as Ku80, ATM (mutated in Ataxia Telangiectasis) or BrCA2 (mutated in some breast cancers) growth arrest after only 3–4 PD and exhibit high levels of p53 and p21Cipl [13, 113–115]. Since these cells divide adequately in vivo, the premature growth arrest observed in vitro suggests that conventional culture conditions are probably inducing DNA damage which these mutants are unable to efficiently repair. Ambient oxygen is a major contributor to DNA damage, and one major cause of stasis in mouse cells is oxygen since mouse cells grown under reduced oxygen atmosphere do not seem to exhibit cellular senescence [116].
If the stochastic nature of mutations is taken into account, their number will be the result of the product of both time and pool size. Multiplying the weight and lifespan of humans versus mice, humans may need to be about 100,000 times more resistant to the formation of tumors than rodents. The telomerase knockout mouse mTR−/− still displays a normal frequency of neoplasias suggesting that escaping replicative aging by telomerase activation is not a requirement for murine tumorigenesis and that the involvement of other mechanisms of tumor protection such as cell cycle checkpoints, immune surveillance and cellular/DNA repair are sufficient for tumor protection during the short life of these small sized rodents [13, 95, 112]. Together, these results show that the senescence of mouse cells in culture is not due to telomere shortening, and that telomere based replicative aging is not used as a tumor protection mechanism in laboratory mice. There are examples of wild rodents such as the Algerian mouse (Mus spretus) that display “human sized” telomeres [109], and it has not yet been specifically established whether or not they might the use telomere shortening as a tumor protection mechanism.
Telomerase activity was detected in somatic tissues of 15 rodent species, and long telomere lengths (>30 kb) were observed in most species. The lowest levels of telomerase activity were seen in the largest species tested, beaver and capybara, which (together with guinea-pig and deer mouse) displayed shorter “human-like” telomeres [117]. These authors suggested that telomerase activity co-evolves inversely with body mass, not lifespan, with larger rodents displaying lower telomerase activities, and that telomere length did not show any correlation with size or lifespan [117].
Our current knowledge of the consequences of inadequate growth conditions leads us to conclude that studies claiming that the replicative potential of fibroblasts positively correlates with body mass or longevity need to be re-evaluated [118–119]. The studies of Lorenzini (2005) included the early growth arrest in culture of fibroblasts from smaller, shorter lived species such as rodents (half of the species) and carnivores as an example of telomere-based replicative aging[118]. In fact, studies show that fibroblasts from many of these species, given adequate media and more physiological (2% O2) growth conditions, can grow for over 100 PD [120–121].
Telomere biology has been studied in a few domestic and farm animals. In horse (Equus equus), telomere shortening was observed in fibroblasts cultured to senescence. No telomerase activity was detected in primary cell cultures, in normal equine tissues or equine benign tumor samples of the sarcoid or papilloma type. In adult donkeys (Equus asinus) blood samples, telomeres ranged from 7 to 21 kb and telomere lengths was showed to decrease with lifespan [122]. Sheep (Ovis aries) dermal and lung fibroblasts have a finite lifespan in culture, after which the cells growth arrest. Terminal restriction fragment lengths from sheep tissues reveal “human-like” telomere lengths (9–23 kb). Telomerase activity is found in the testis but suppressed in somatic tissues. Similarly to humans, senescent sheep skin fibroblasts have increased levels of p53 and p21WAF1 compared to young cells [123]. Pigs (Sus scrofa) also seem to display replicative aging [124]. Among Carnivores, several breeds of dog (Canis lupus familiaris) show heterogeneity in telomere lengths in their somatic tissues. Average telomeres range between 12 and 23 kb. Telomerase activity was low or absent in normal somatic tissues and was detected in testis and tumor tissues. Canine soft tissue sarcomas with mean TRFs of 22 and 18 kb have been reported [125]. In tissues obtained from 2 domestic shorthair cats (Felis catus) mean TRF values ranged 5 to 26 kb and there was significant telomeric attrition with increasing age of cats. The same study did not detect telomerase activity in normal tissues [126]. Another report in cats showed that average telomere lengths of lymphocytes and granulocytes, analyzed by fluorescence in situ hybridization and flow cytometry (Flow FISH), are 5- to 10- fold longer than in humans. However, much higher telomeric shortening rates are observed both in vivo and in vitro (500 bp/PD in T cells), suggesting that this shortening might not be caused by the end replication problem but by other mechanisms [127].
During the last several years we have been investigating the role of telomeres and replicative aging in most orders of the mammalian radiation. We have shown that, as humans, other primates also exhibit replicative aging [128]. In skin fibroblasts from the “Old World” primates [118] and “New World” primates telomere shortening limits replicative capacity. Human telomerase expression in anthropoid fibroblasts is able to produce telomere elongation and the extension of their in vitro lifespan [128]. A longitudinal study in leukocytes from outbred newborn baboons (Papio hamadryas cynocephalus) reveals heterogeneity in telomere length, with two animals having longer telomeres at birth (≈25–28 kb) compared to two other animals (≈13–15 kb) [129]. The same Flow-FISH study reported a fast telomere length shortening of about 2–3 kb during the first year of life. However, after the first 50–70 weeks, telomere length appeared to stabilize, leading to the hypothesis that baboons hematopoietic stem cells switch from a fast expansion stage to a phase with significantly lower turnover rate [129]. In contrast to the rigorous control of replicative aging by telomere shortening conserved among anthropoid primates, barriers to immortalization were reduced in the prosimian ring-tailed lemur (Lemur catta). Lemur cells have both long and short telomeres. Following ~150 days of senescence a subset of lemur cells showing reduced chromosome number overgrew the cultures without activation of telomerase and displayed increased apoptosis [128]. The lack of telomerase and the presence of large amounts of extrachromosomal telomere sequences indicates that they had spontaneously activated the ALT mechanism, something that essentially never happens in human cells.
Among Artiodactyla, we studied the small Asian barking deer, the Indian Muntjac (Muntiacus muntjak). This species is an ideal model to study telomere biology since it has the fewest number of diploid chromosomes of all mammals with only six chromosomes (1, 2, 3) in the female and seven in the male (1, 2, 3 + X) [130]. We observed that Indian muntjac skin fibroblasts growth arrested at PD 89 and that human TERT expression can immortalize them [131]. Approaching senescence, the telomeric ends gradually became FISH signal-free and chromosome abnormalities increased dramatically. This species is an excellent candidate as a telomere-based replicative senescence model for human cells [131]. In Indian Muntjac we also observed that interstitial telomere sequences coincided with fragile sites, suggesting that these remnants of chromosome fusion events might play a role in genome instability [131]. These intrachromosomal TTAGGG sequence sites are known to be fragile “hot spots” prone to breakage and recombination in the Armenian hamster (Cricetulus migratorius) and Chinese hamster (Cricetulus griseus) [131–133] and are thought to be involved in the process of karyotype evolution during speciation due to Robertsonian fusions [60, 134].
Our studies in mammals are showing that both the telomere-based tumor protection mechanism and the mouse-like “long telomere” phenotype are widely distributed in the mammalian evolutionary tree. We find that Lagomorpha cells, although mostly telomerase negative, do not growth arrest in culture due to their extremely long telomeric arrays (Fig. 4B). Endogenous telomerase activity is present in the North American pika (Ochotona princes). These data suggest it is unlikely that lagomorphs use telomere shortening and replicative senescence as a tumor protective mechanism [135]. There may be trade-offs between the advantages of repressing telomerase/having short telomeres to count cell divisions/tumor protection and the advantages of maintaining telomerase activity and having very long telomeres. We also observe that species using replicative aging tend to have longer lifespans and higher adult body weights, and that telomeric patterns tend to be conserved within evolutionary blocks (e.g. the bulk of rodents and nearby species have very long telomeres, although individual species such as the deer mouse can have short telomeres). It is hoped that an explanation for these patterns and their implications for telomere biology will become more clear as additional studies are completed.
3. Conclusion
Telomerase-based end maintenance is likely to be a very ancient mechanism that plays a key role in chromosomal maintenance and stability in unicellular and multicellular organisms. In invertebrates, fish, amphibian, and reptiles persistent telomerase activity in somatic tissues also allows the maintenance of the extensive regenerative potentials of these species. A higher efficiency in telomerase repression among birds and many mammals suggests that, as humans, they use replicative aging as a tumor protection mechanism. Rodents and other small short lived mammalian clades appear to have adopted another telomere strategy that has abandoned replicative aging, potentially allowing a reduction in the energy invested to protect their telomeres and increasing their regeneration capacity. The lack of telomerase repression in poikylotherms suggests that these animals do not use replicative aging, and that replicative aging may have evolved as an early adaptation to the increased cancer risk associated with elevated body temperatures to provide a barrier to tumor protection under the additional mutational load that occurs in eutherians.
Telomere dysfunction and telomerase gene mutations have both been implicated in a variety of human age-related pathologies but the link between replicative senescence and aging remains controversial [136–138]. Recent advances in embryonic and adult stem cells have revived interest in the role of telomeres and telomerase regulation. The addition of more species to the genome and protein databases will allow an emergence of more in depth studies on the role of the shelterin proteins in telomeric regulation during development and aging in many Metazoans.
Acknowledgments
Supported by the European Union Programs POCI 2010 & FSE (N.M.V.G.),national funds from the Portuguese Ministry for Science, Technology and Superior Education (N.M.V.G) and the Keck Foundation (WEW).
List of abbreviations
- ALT
Alternative Lengthening of Telomeres
- BJ
Human Foreskin Fibroblast Cell line
- bp
Base Pair(s)
- CDK4
Cyclin-dependent Kinase 4
- chTERT
Chicken Telomerase Reverse Transcriptase
- DNA
Deoxyribonucleic Acid
- FISH
Fluorescence in situ Hybridization
- fTERT
Fugo Telomerase Reverse Transcriptase (Protein Component)
- hTERT
Human Telomerase Reverse Transcriptase (Protein Component)
- TR/TERC
Telomerase RNA (template RNA Component)
- mTR
Mouse Telomerase RNA (template RNA Component)
- kb
Kilobase Pair(s)
- Mb
Megabase Pair(s)
- MEFS
Mouse Embryo Fibroblasts
- mRNA
Messenger RNA
- mTERT
Mouse Telomerase Reverse Transcriptase (Protein Component)
- PD
Population Doublings
- stasis
stress or aberrant signaling induced senescence
- TRF
Telomere Restriction Fragment
- xTERT
Xenopus Telomerase Reverse Transcriptase (Protein Component)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell. Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;245:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J.Mol.Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 4.Moyzis RK, et al. A highly conservative repetitive DNA sequence (TTAGGG)npresent at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deLange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 6.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc. Natl. Acad. Sci. USA. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Nati. Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastie ND, et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura TM, et al. Telomerase catalytic subunit homologs from fission yeast and humans. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, et al. Telomere lengthening early in development. Nature Cell Biology. 2007;9:1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 12.Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2004;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 13.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention:fundamental differences in man and mouse telomere biology. Nat. Md. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 14.Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Horikawa I, Barrett JC, M O. Evidence for inactivation of distinct telomerase repressor genes in different types of human cancers. Int J Cancer. 2005;115:653–657. doi: 10.1002/ijc.20879. [DOI] [PubMed] [Google Scholar]
- 16.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nature Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 17.Henson JD, RR R. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010 doi: 10.1016/j.febslet.2010.06.009. doi:1016/j.febslet.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Zalzman M, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464:858–863. doi: 10.1038/nature08882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinclair CS, Richmond RH, Ostrander GK. Characterization of the telomere regions of scleractian coral. Acropora surculosa. Genetica. 2007;129:227–233. doi: 10.1007/s10709-006-0001-x. [DOI] [PubMed] [Google Scholar]
- 20.Traut W, et al. The telomere repeat motif of basal Metazoa. Chromosome Research. 2007;15:371–382. doi: 10.1007/s10577-007-1132-3. [DOI] [PubMed] [Google Scholar]
- 21.Finch CE. Longevity, Senescence and the Genome. Chicago: The University of Chicago Press; 1990. p. 922. [Google Scholar]
- 22.Koziol C, Borojevic R, Steffen R, Muller WEG. Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: the telomerase activity in somatic cells. Mechanisms of Ageing and Development. 1998;100:107–120. doi: 10.1016/s0047-6374(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 23.Rinkevich B, Loya Y. Senescence and dying signals in a reef-building coral. Experientia. 1986;42:320–322. [Google Scholar]
- 24.Laird DJ, Weissman L. Telomerase maintained in self-renewing tissues during serial regeneration of the urochordate. Bothryllus schosserii. Dev Biol. 2004;273:185–194. doi: 10.1016/j.ydbio.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Castro LFC, Holand P. Fluorescent in situ hybridisation to amphioxus chromosomes. Zoo Sci. 2002;19:1349–1353. doi: 10.2108/zsj.19.1349. [DOI] [PubMed] [Google Scholar]
- 26.Plohl M, et al. Telomeric localization of the vertebrate type hexamer repeat (TTAGGG)n in the wedgeshell clam Donax trunculus and other marine invertebrate genomes. Jour Biol Chem. 2002;277:19839–19846. doi: 10.1074/jbc.M201032200. [DOI] [PubMed] [Google Scholar]
- 27.Joffe BI, Solovei I, Macgregor HC. Ends of chromosomes in Polycelis tenuis (Platyhelminthes)have telomeric epeat TTAGGG. Chromosome Res. 1998;4:323–324. doi: 10.1007/BF02263686. [DOI] [PubMed] [Google Scholar]
- 28.Jha AN, et al. Localization of a vertebrate telomeric sequence in the chromosomes of two marine worms (phylum Annelida: class polychaeta) Chromosome Res. 1995;3:507–508. doi: 10.1007/BF00713966. [DOI] [PubMed] [Google Scholar]
- 29.Vitturi R, Colomba MS, Gianguzza P, Pirrone AM. Chromosomal location of ribosomal DNA (rDNA), (GATA)n and (TTAGGG)n telomeric repeats in the neogastropod Fasciolaria lignaria (Mollusca: Prosobranchia) Genetica. 2000;108:253–257. doi: 10.1023/a:1004151513129. [DOI] [PubMed] [Google Scholar]
- 30.Wang YP, Guo XM. Chromosomal mapping of the vertebrate telomeric sequence (TTAGGG)n in four bivalve molluscs by fluorescence in situ hybridization. J Shellfish Res. 2001;20:1187–1190. [Google Scholar]
- 31.Vitkova M, Kral J, Traut W, Zrzavy J, Marec F. The Evolutionary origin of insect telomeric repeats, (TTAGG)n. Chromosome Res. 2005;13:145–156. doi: 10.1007/s10577-005-7721-0. [DOI] [PubMed] [Google Scholar]
- 32.Hirai H, LoVerde PT. Identification of the Telomeres on Schistosoma mansoni chromosomes by FISH. J Parasit. 1996;82:511–512. [PubMed] [Google Scholar]
- 33.Bombarová M, Vítková M, Spakulová M, Koubková B. Telomere analysis of platyhelminths and acanthocephalans by FISH and Southern hybridization. Genome. 2009;52:897–903. doi: 10.1139/g09-063. [DOI] [PubMed] [Google Scholar]
- 34.Niedermaier J, Moritz KB. Organization and dynamics of satellite and telomere DNAs in Ascaris: implications for formation and programmed breakdown of compound chromosomes. Chromosoma. 2000;109:439–452. doi: 10.1007/s004120000104. [DOI] [PubMed] [Google Scholar]
- 35.Muller F, Wicky C, Spicker A, Tobler H. New telomere formation after developmentally regulated chromosomal breakage during the process of chromatin diminution in Ascaris lumbricoides. Cell. 1991;67:815–822. doi: 10.1016/0092-8674(91)90076-b. [DOI] [PubMed] [Google Scholar]
- 36.Wicky C, et al. Telomeric repeats (TTAGGC)n are sufficient for chromosome capping function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1996;93:8983–8988. doi: 10.1073/pnas.93.17.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klapper W, et al. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Letters. 1998;439:143–146. doi: 10.1016/s0014-5793(98)01357-x. [DOI] [PubMed] [Google Scholar]
- 38.Elmore LW, et al. Upregulation of Telomerase Function During Tissue Regeneration. Exp Biol Med. 2008;233:958–967. doi: 10.3181/0712-RM-345. [DOI] [PubMed] [Google Scholar]
- 39.Vogt G. How to minimize formation and growth of tumours: Potential benefits of decapod crustaceans for cancer research. Int. J. Cancer. 2008;123:2727–2734. doi: 10.1002/ijc.23947. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki S, Tsuchida K, Mackawa H, Fugiwara H. Identification of a pentanucleotide telomere sequence (TTAGG)n in the silkworm Bombyx mori and in other insects. Mol Cell Biol. 1993;13:1424–1432. doi: 10.1128/mcb.13.3.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyne JH, Imai HT. FISH analysis of the telomere sequences of bulldog ants (Myrmecia: Formicidae) Chromosoma. 1995;104:14–18. doi: 10.1007/BF00352221. [DOI] [PubMed] [Google Scholar]
- 42.Sahara KF, Marec F, Traut W. TTAGG telomeric repeats in Chromosomes of some insects and other arthropods. Chromosome Res. 1999;7:449–460. doi: 10.1023/a:1009297729547. [DOI] [PubMed] [Google Scholar]
- 43.Frydrychova R, Grossmann P, Trubac P, Vitkova M, Marec F. Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome. 2004;47:163–178. doi: 10.1139/g03-100. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki T, Fujiwara H. Detection and distribution patterns of telomerase activity in insects. Eur J Biochem. 2000;267:3025–3031. doi: 10.1046/j.1432-1033.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 45.Osanai M, Kojima KK, Futahashi R, Yaguchi S, Fujiwara H. Identification and characterization of the telomerase reverse transcriptase of Bombyx mori (silkworm) and Tribolium castaneum (flour beetle) Gene. 2006;376:281–289. doi: 10.1016/j.gene.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 46.Frydrychova R, Marec F. Repeated losses of TTAGG telomere repeats in evolution of beetles (Coleoptera) Genetica. 2002;115:179–187. doi: 10.1023/a:1020175912128. [DOI] [PubMed] [Google Scholar]
- 47.Rosen M, Edstrom J. DNA structures common for chironomid telomeres terminating with complex repeats. Insect Mol Biol. 2000;9:341–347. doi: 10.1046/j.1365-2583.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- 48.Walter MF, Bozorgnia L, Mahesshwari A, Biessmann H. The rate of terminal nucleotide loss from a telomere of the mosquito. Anopheles gambiae. Insect Mol Biol. 200;10:105–110. doi: 10.1046/j.1365-2583.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 49.Biessmann H, Mason JM. Telomere maintenance without telomerase. Chromosoma. 1997;106:63–69. doi: 10.1007/s004120050225. [DOI] [PubMed] [Google Scholar]
- 50.Mason JM, Biessmann H. The unusual telomeres of Drosophila. Trends Genet. 1995;11:58–62. doi: 10.1016/s0168-9525(00)88998-2. [DOI] [PubMed] [Google Scholar]
- 51.Mikhailovsky S, Belenkaya T, Georgiev P. Broken chromosomal ends can be elongated by conversion in Drosophila melanogaster. Chromosoma. 1999;108:114–120. doi: 10.1007/s004120050358. [DOI] [PubMed] [Google Scholar]
- 52.Kahn T, Savitsky M, Georgiev P. Attachment of HeTA sequences to chromosomal termini in Drosophila may occur by different mechanisms. Mol Cell Biol. 2000;20:7634–7642. doi: 10.1128/mcb.20.20.7634-7642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francis N, Gregg T, Owen R, Ebert T, Bodnar A. Lack of age-associated telomere shortening in long- and short lived species of sea urchins. FEBS Letters. 2006;580:4713–4717. doi: 10.1016/j.febslet.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 54.Lejnine S, Makarov VL, Langmore JP. Conserved nucleoprotein structurear the ends of vertebrate and invertebrate chromosomes. Proc. Natl. Acad.Sci.USA. 1995;94:2393–2397. doi: 10.1073/pnas.92.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. www.pathology-registry.org(
- 56.Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen J. the telomerase database. Nucleic Acids Res. 2007:D339–D343. doi: 10.1093/nar/gkm700. http://telomerase.asu.edu/ [DOI] [PMC free article] [PubMed]
- 57.Patbaik B, Mahapatro N, Jena B. Ageing in Fishes. Gerontology. 1994;40:113–132. doi: 10.1159/000213582. [DOI] [PubMed] [Google Scholar]
- 58.Mommsen TP. Paradigms of growth in fish.Comparative Biochemistry and Physiology. Part B, Biochemistry and Molecular Biology. 2001;129:207–219. doi: 10.1016/s1096-4959(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 59.Lau BW-M, Wong AO-L, Tsao GS-W, So K-F, Yip HK-F. Molecular Cloning and Characterization of the Zebrafish (Danio rerio) Telomerase Catalytic Subunit (Telomerase Reverse Transcriptase, TERT) J Mol Neurosci. 2008;34:63–75. doi: 10.1007/s12031-007-0072-x. [DOI] [PubMed] [Google Scholar]
- 60.Rocco L, Costagliola D, Stingo V. (TTAGG)n telomeric sequence in selachian chromosomes. Heredity. 2001;87:583–588. doi: 10.1046/j.1365-2540.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- 61.McChesney PA, Elmore LW, HOLT SE. Vertebrate Marine Species as Model Systems for Studying Telomeres and Telomerase. Zebrafish. 2004/2005;1:349–355. doi: 10.1089/zeb.2005.1.349. [DOI] [PubMed] [Google Scholar]
- 62.Rocco L, Morescalchi MA, Costagliola D, Stingo V. Karyotype and genome characterization in four cartilaginous fishes. Gene. 2002;295:289–298. doi: 10.1016/s0378-1119(02)00730-8. [DOI] [PubMed] [Google Scholar]
- 63.Tsepkin YA, Sokolov LI. The maximum size and age of some sturgeons. J.Ichthyol. 1971;11:444–446. [Google Scholar]
- 64.Kishi S, et al. The zebrafish as a vertebrate model of funcional aging and very gradual senescence. Experimental Gerontology. 2003;38:777–786. doi: 10.1016/s0531-5565(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 65.Johnson SL, Bennet P. Growth control in the ontogenic and regenerating zebrafish fin. Meth Cell Biol. 1999;59:301–311. doi: 10.1016/s0091-679x(08)61831-2. [DOI] [PubMed] [Google Scholar]
- 66.Bradford CS, et al. Characterization of cell cultures derived fromFugu, the Japanese pufferfish. Mol. Mar. Biol. Biotechnol. 1997;6:279–288. [PubMed] [Google Scholar]
- 67.Klapper W, Heidorn K, Kuhne K, Parwaresch R, Krupp G. Telomerase activity in immortal_ fish. FEBS Lett. 1998;434:409–412. doi: 10.1016/s0014-5793(98)01020-5. [DOI] [PubMed] [Google Scholar]
- 68.Xie M, et al. Structure and Function of the Smallest Vertebrate Telomerase RNA from Teleost Fish. THE JOURNAL OF BIOLOGICAL CHEMISTRY. 2008;283:2049–2059. doi: 10.1074/jbc.M708032200. [DOI] [PubMed] [Google Scholar]
- 69.Yap WH, Yeoh E, Brennert S, Venkatesh B. Cloning and expression of the reverse transcriptase component of pufferfish (Fugu rubripes) telomerase. Gene. 2005;353:207–217. doi: 10.1016/j.gene.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 70.Bassham S, Beam A, Shampay J. Telomere variation in Xenopus laevis. Molecular and Cell Biology. 1998;18:269–275. doi: 10.1128/mcb.18.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuramoto M, Ohsumi K, Kishimoto T, Ishikawa F. Identification and analyses of the Xenopus TERT gene that encodes the catalytic subunit of telomerase. Gene. 2001;277:101–110. doi: 10.1016/s0378-1119(01)00684-9. [DOI] [PubMed] [Google Scholar]
- 72.Mantell LL, Greider CW. Telomerase activity in germline and embryonic cells of Xenopus. EMBO J. 1994;13:3211–3217. doi: 10.1002/j.1460-2075.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bousman S, Schneider G, Shampay J. Telomerase Activity Is Widespread in Adult Somatic Tissues of Xenopus. Journal of Experimental Zoology (Mol Dev Evol) 2003;295B:82–86. doi: 10.1002/jez.b.7. [DOI] [PubMed] [Google Scholar]
- 74.Meyne J, Ratliff RL, Moyzis RK. Conservation of the Human Telomere Sequence (TTAGGG)n among Vertebrates. Proc. Natl. Acad. Sci. USA. 1989;86:7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bronikowski AM. The evolution of aging phenotypes in snakes: a review and synthesis with new data. AGE. 2008;30:169–176. doi: 10.1007/s11357-008-9060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ujvari B, Madsen T. Short Telomeres in Hatchling Snakes: Erythrocyte Telomere Dynamics and Longevity in Tropical Pythons. PLoS ONE. 2009;4:e7493. doi: 10.1371/journal.pone.0007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christiansen JL, et al. A final report of studies of the Hayflick limit in Reptiles, a test of potential immortality. Proceedings of the Iowa Space Grant Consortium. 2001 [Google Scholar]
- 78.Christiansen J, Johnson J, Henderson ER, Budke B, Lynch M. The relationship between telomeres, telomerase, reptilian lifespan, and reptilian tissue regeneration. Proceedings of the Iowa Space Grant Consortium. 2001:1–10. [Google Scholar]
- 79.Simpson SB, Rauch DM. Cells from the lizard Anolis do not exhibit senescence. Gerontologist, Special Issue. 1989;29:284A. (Abstract) [Google Scholar]
- 80.Scott NM, Haussmann MF, Elsey RM, Trosclair PL, III, Vleck CM. Telomere Length Shortens with Body Length in Alligator Mississippiensis. Southeastern Naturalist. 2006;5:685–692. [Google Scholar]
- 81.Min X, Xiao-Bing W, Peng Y, Hai-tao Z. Telomere Length Shortens With Age in Chinese Alligators (Alligator sinensis) Journal of Applied Animal Research. 2009:36. [Google Scholar]
- 82.Girondot M, Garcia J. In: Guyetant CMaR., editor. Senescence and longevity in turtles: what telomeres tell us; Proceedings of the 9th Ordinary General Meeting of the Societas Europaea Herpetologica In Current Studies in Herpetology:; France: Le Bourget du Lac; 1999. pp. 133–137. [Google Scholar]
- 83.Carr A, Goodman D. Ecologic implications of size and growth in Chelonia. Copeia. 1970;4:783–786. [Google Scholar]
- 84.Goldstein S. Growth of Cultured cells from the Galapagos tortoise. Exp. Cell. Res. 1974;83:279–302. doi: 10.1016/0014-4827(74)90342-5. [DOI] [PubMed] [Google Scholar]
- 85.Paitz RT, Haussmann MF, Bowden RM, Janzen FJ, C V. Long telomeres may minimize the effect of aging in the Painted Turtle. Integr Comp Biol. 2004;44:617. [Google Scholar]
- 86.Holmes DJ, Fluckiger R, Austad SN. Comparative biology of aging in birds: an update. Experimental Gerontology. 2001;36:869–883. doi: 10.1016/s0531-5565(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 87.Delany ME, Krupkin AB, Miller MM. Organization of telomere sequences in birds: evidence for arrays of extreme length and for in vivo shortening. Cytogenet Cell Genet. 2000;90:139–145. doi: 10.1159/000015649. [DOI] [PubMed] [Google Scholar]
- 88.Rodrigue KL, May BP, Famula TR, ME D. Meiotic instability of chicken ultra-long telomeres and mapping of a 2.8 megabase array to the W-sex chromosome. Chromosome Res. 2005;13:581–591. doi: 10.1007/s10577-005-0984-7. [DOI] [PubMed] [Google Scholar]
- 89.Delany ME, Daniels LM, Swanberg SE, Taylor HA. Telomeres in the Chicken: Genome Stability and Chromosome Ends. Poultry Science. 2003;82:917–926. doi: 10.1093/ps/82.6.917. [DOI] [PubMed] [Google Scholar]
- 90.Nanda I, et al. Distribution of telomeric (TTAGGG)n sequences in avian chromosomes. Chromosoma. 2002;111:215–227. doi: 10.1007/s00412-002-0206-4. [DOI] [PubMed] [Google Scholar]
- 91.O’Hare TH, Delany ME. Genetic variation exists for telomeric array organization within and among the genomes of normal, immortalized, and transformed chicken systems. Chromosome Research. 2009;17:947–964. doi: 10.1007/s10577-009-9082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delany ME, Daniels LM. The chicken telomerase reverse transcriptase (chTERT): molecular and cytogenetic characterization with a comparative analysis. Gene. 2004;339:61–69. doi: 10.1016/j.gene.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 93.Hiyama E, et al. Telomerase activity in human intestine. Int. J. Oncol. 1996;9:453–458. doi: 10.3892/ijo.9.3.453. [DOI] [PubMed] [Google Scholar]
- 94.Venkatesan RN, Price C. Telomerase expression in chickens: constitutive activity in somatic tissues and downregulation in culture. Proc. Natl. Acad. Sci. USA. 1998;95:14763–14768. doi: 10.1073/pnas.95.25.14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Forsyth NR, Wright WE, Shay JW. Telomerase nd differentiation in multicellular organisms: Turn it off, turn it on, and turn it off again. Differentiation. 2002;69:188–197. doi: 10.1046/j.1432-0436.2002.690412.x. [DOI] [PubMed] [Google Scholar]
- 96.Hall ME, et al. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. Lond. B. 2004;271:1571–1576. doi: 10.1098/rspb.2004.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foote CG. Avian telomere dynamics. PhD. Glasgow: University of Glasgow; 2009. [Google Scholar]
- 98.Taylor HA, Delany ME. Ontogeny of telomerase in chicken: Impact of downregulation on pre-and postnatal telomere length in vivo. Develop. Growth Differ. 2000;42:613–621. doi: 10.1046/j.1440-169x.2000.00540.x. [DOI] [PubMed] [Google Scholar]
- 99.Haussmann MF, et al. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc. R. Soc. Lond. B. 2003;270:1387–1392. doi: 10.1098/rspb.2003.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siegfried M. Neoplasms identified in free-living birds. Avian Dis. 1983;27:86–99. [PubMed] [Google Scholar]
- 101.Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, Vleck CM. Telomerase activity is maintained throughout the lifespan of long-lived birds. Experimental Gerontology. 2007;42:610–618. doi: 10.1016/j.exger.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 102.Galkina S, Lukina N, Zakharova K, Rodionov AV. Interstitial (TTAGGG)n sequences are not hot spots of recombination in the chicken lampbrush macrochromosomes 1–3. Chromosome Research. 2005;13:551–557. doi: 10.1007/s10577-005-0980-y. [DOI] [PubMed] [Google Scholar]
- 103.Lansdorp PM. Telomeres and disease. The EMBO Journal. 2009;28:2532–2540. doi: 10.1038/emboj.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 105.Starling JA, Maule J, Hastie ND, Allshire RC. Extensive telomere repeat arrays in mouse are hypervarible. Nucl. Acids Res. 1990;18:6881–6888. doi: 10.1093/nar/18.23.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zijlmans JMJM, et al. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc. Natl. Acad. Sci. USA. 1997;94:7423–7428. doi: 10.1073/pnas.94.14.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oh H, et al. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy and survival. Proc. Natl. Acad. Sci. USA. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann NY Acad Sci. 2000;908:99–110. doi: 10.1111/j.1749-6632.2000.tb06639.x. [DOI] [PubMed] [Google Scholar]
- 109.Coviello-McLaughlin GM, Prowse KR. Telomere length regulation during postnatal development and ageing in Mus spretus. Nuc Acids Res. 1997;25:3051–3058. doi: 10.1093/nar/25.15.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prowse KR, Greider CW. Developmental and tissue specific regulation of mouse telomerase and telomere length. Proc. Natl. Acad. Sci. USA. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin-Rivera L, Herrera E, Albar JP, Blasco MA. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl. Acad. Sci. USA. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 113.Nussenzweig A, et al. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 114.Barlow C, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 115.Connor F, et al. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nature Genet. 1997;17:423–430. doi: 10.1038/ng1297-423. [DOI] [PubMed] [Google Scholar]
- 116.Parrinello S, et al. Oxygen sensitivity severely limit the replicative lifespan of murine fibroblasts. Nature Cell Biology. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seluanov A, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;1(6):45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lorenzini A, Tresini M, Austad SN, Cristofalo VJ. Cellular replicative capacity correlates primarily with species body mass not longevity. Mech Ageing Dev. 2005;126:1130–1133. doi: 10.1016/j.mad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 119.Rohme D. Evidence for a relationship between longevity of mammalian species and lifespans of normal fibroblasts in vitro and erytrocytes in vivo. Proc. Natl. Acad. Sci. USA. 1981;78:5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guyton AC, Hall E. Transport of oxygen and carbon dioxide in the blood and bodily fluids in: Textbook of Medical Phisiology. Philadelphia: 1966. [Google Scholar]
- 121.Wright WE, Shay JW. Inexpensive low-oxygen incubators. Nature Protocols. 2006:1. doi: 10.1038/nprot.2006.374. [DOI] [PubMed] [Google Scholar]
- 122.Argyle DJ, Ellsmore V, Gault EA, Munro AF, Nasir L. Equine telomeres and telomerase in cellular immortalization and ageing. Mechanisms of Ageing and Development. 2003;124:759–764. doi: 10.1016/s0047-6374(03)00104-0. [DOI] [PubMed] [Google Scholar]
- 123.Davisa T, Skinnera JW, Faragherb RGA, Jonesa CJ, Kipling D. Replicative senescence in sheep fibroblasts is a p53 dependent process. Experimental Gerontology. 2005;40:17–26. doi: 10.1016/j.exger.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 124.Pathak S, Multani AS, McConkey DJ, Imam AS, Amoss MS. Spontaneous regression of cutaneous melanoma in sinclair swine is associated with defective telomerase activity and extensive telomere erosion. Int J Oncol. 2000;17:1219–1224. doi: 10.3892/ijo.17.6.1219. [DOI] [PubMed] [Google Scholar]
- 125.Nasir L, Devliny P, Mckevitt T, Ruttemanz G, Argyle DJ. Telomere Lengths and Telomerase Activity in Dog Tissues: A Potential Model System to Study Human Telomere and Telomerase Biology. Neoplasia. 2001;3:351–359. doi: 10.1038/sj.neo.7900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McKevitt TP, Nasir L, Wallis CV, Argyle DJ. A cohort study of telomere and telomerase biology in cats. Am J Vet res. 2003;64:1496–1499. doi: 10.2460/ajvr.2003.64.1496. [DOI] [PubMed] [Google Scholar]
- 127.Brummendorf TH, et al. Longitudinal studies of telomere length in feline blood cells. Experimental Hematology. 2002;30:1147–1152. doi: 10.1016/s0301-472x(02)00888-3. [DOI] [PubMed] [Google Scholar]
- 128.Steinert S, White DM, Zou Y, Shay JW, Wright WE. Telomere Biology and Cellular Aging in Nonhuman Primate Cells. Experimental Cell Research. 2002;272:146–152. doi: 10.1006/excr.2001.5409. [DOI] [PubMed] [Google Scholar]
- 129.Baerlocher GM, Rice K, Vulto I, Lansdorp PM. Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell. 2007;6:121–123. doi: 10.1111/j.1474-9726.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 130.Wurster DH, Benirschke K. Indian muntjac, Muntiacus munjak: a deer with a low diploid chromosome number. Science. 1970;168:1364–1366. doi: 10.1126/science.168.3937.1364. [DOI] [PubMed] [Google Scholar]
- 131.Zou Y, Yi X, Wright WE, Shay JW. Human Telomerase Can Immortalize Indian Muntjac Cells. Experimental Cell Research. 2002;281:63–76. doi: 10.1006/excr.2002.5645. [DOI] [PubMed] [Google Scholar]
- 132.Baxter SM, Greizerstein MB, Kushlan DM, Ashley GW. Conformational Propertiesof DNA Hairpins with TTT and TTTT loops. Biochemistry. 1993;32:8702–8711. doi: 10.1021/bi00084a042. [DOI] [PubMed] [Google Scholar]
- 133.Day JP, Limoli CL, Morgan WF. Recombination involving interstitial telomere repeat-like sequences promotes chromosomal instability in Chinese Hamster cells. Carcinogenesis. 1998;19:259–265. doi: 10.1093/carcin/19.2.259. [DOI] [PubMed] [Google Scholar]
- 134.Meyne J, et al. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma. 1990;99:3–10. doi: 10.1007/BF01737283. [DOI] [PubMed] [Google Scholar]
- 135.Forsyth NR, Elder FFB, Shay JW, Wright WE. Lagomorphs (rabbits, pikas and hares) do not use telomere-directed replicative aging in vitro. Mechanisms of Ageing and Development. 2005;126:685–691. doi: 10.1016/j.mad.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 136.Blasco MA. Telomere length, stem cells and aging. Nature Chemical Biology. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 137.Crabbe L, Jauch A, Naeger CM, Holt-Grez H, Karlseder J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:2205–2210. doi: 10.1073/pnas.0609410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Eng J Med. 2007;356:1370–1372. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]