Abstract
BH3-only BCL-2 family proteins are effectors of canonical mitochondrial apoptosis. They discharge their pro-apoptotic functions through BH1-3 pro-apoptotic proteins such as BAX and BAK, while their activity is suppressed by BH1-4 anti-apoptotic BCL-2 family members. The precise mechanism by which BH3-only proteins mediate apoptosis remains unresolved. The existing data are consistent with three mutually non-exclusive models 1) displacement of BH1-3 proteins from complexes with BH1-4 proteins; 2) direct interaction with and conformational activation of BH1-3 proteins; and 3) membrane insertion and membrane remodeling. The BH3-only proteins appear to play critical roles in restraining cancer and inflammatory diseases such as rheumatoid arthritis. Molecules that mimic the effect of BH3-only proteins are being used in treatments against these diseases. The cell death activity of a subclass of BH3-only members (BNIP3 and BNIP3L) is linked to cardiomyocyte loss during heart failure. In addition to their established role in apoptosis, several BH3-only members also regulate diverse cellular functions in cell cycle regulation, DNA repair and metabolism. Several members are implicated in the induction of autophagy and autophagic cell death, possibly through unleashing of the BH3-only autophagic effector Beclin 1 from complexes with BCL-2/BCL-xL. The Chapters included in the current Oncogene Review issues provide in-depth discussions on various aspects of major BH3-only proteins.
Keywords: BH3-only, Apoptosis, Autophagy, Non-apoptotic functions, Cancer
Introduction
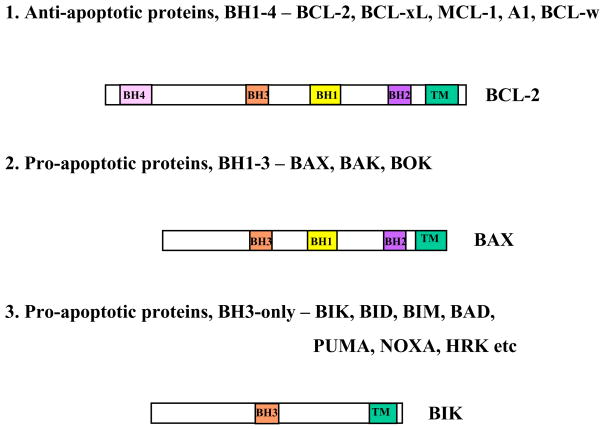
The BCL-2 family proteins are well-characterized regulators of apoptosis and are grouped into three sub-families based on the number of BH (BCL-2 Homology) domains they share (Fig. 1). The first sub-family includes the anti-apoptotic proteins BCL-2, BCL-xL, BCL-w, MCL-1, and A1/BFL-1. These proteins possess four BH domains -BH1-4. The next two groups are pro-apoptotic proteins possessing three BH (BH1-3) domains represented by BAX, BAK, and BOK and BH3-only proteins that, as their name implies, are characterized by the presence of only the BH3 domain. BCL-2 family proteins form a complex regulatory network that controls cell survival and death in response to different physiological and pathological stimuli. Our current understanding of the canonical apoptotic pathway in animal cells is that various cell death stimuli either transcriptionally or post-transcriptionally activate one or more of BH3-only effectors that integrate and transmit the death signal via the multi-domain BH1-3 pro-apoptotic proteins, BAX and BAK. The BH1-3 pro-apoptotic proteins undergo conformational activation leading to oligomerization and insertion in the outer mitochondrial membrane. This process is presumed to result in permeablization of outer mitochondrial membrane and egress of apoptogenic factors such as cytochrome c that activate the caspase cascade leading to cellular demise. This process is actively opposed by the BH1-4 anti-apoptotic members. The general mechanisms by which the BCL-2 family proteins regulate cell survival and death have been reviewed in a number of review articles. Since the discovery of the first member of the BH3-only family proteins (Boyd et al., 1995), a large number of this family’s members have been identified and their roles in physiological and pathological cell elimination have been documented. Surprisingly, several members of this family, in addition to their function as the effectors of cell death, also play other non-cell death functions. This article provides an overview of the known BH3-only proteins and their functions. Comprehensive reviews on major BH3-only members, viz., BIK, EGl-1, BIM, BMF, NOXA, BID, BAD, BNIP3 and Beclin-1 and their physiological and pathological functions are reviewed in the Chapters included in the present Oncogene Review Issue.
Figure 1.
BCL-2 family proteins. Domain structures of representative members of each subfamily are shown.
Discovery of the BH3 domain and the first BH3-only BCL-2 family member
In 1995, a collaborative study between Tom Chittenden and Bob Lutz at Immunogen Inc. and the laboratory of G. Chinnadurai (Saint Louis University School of Medicine) that involved functional dissection of BAK (Chittenden et al., 1995) and the identification and cloning of the pro-apoptotic protein BIK (Bcl-2 Interacting Killer) that shared a conserved domain with other BCL-2 family proteins (Boyd et al., 1995) resulted in the first identification of the functions of the BH3 domain and the BH3-only proteins. These studies showed that: 1) the cell death activities of BAK and BIK were dependent on the conserved BH3 domain; 2) the BH3 domain of BAK was required and sufficient for heterodimerization with BCL-xL; and 3) the BH3 domain of BIK mediated interaction with cellular (BCL-2, BCL-xL) and viral (E1B-19K and EBV-BHRF1) anti-apoptosis proteins. In subsequent publications, the function of the BH3 domain was also identified by mutational analysis of BAX (Hunter et al., 1996; Zha et al., 1996a). The physiological relevance of the BH3-only members as death effector molecules in whole animal models was demonstrated by genetic studies in C. elegans (Conradt and Horvitz, 1998). This study identified the worm BH3-only member EGL-1 and demonstrated that it acted at the point of the cell death pathway where signals of cell death were integrated to activate the process of programmed cell death. The egl-1 gene was shown to be required for most somatic and genotoxic stress-induced cell death in the worm (Gartner et al., 2000; Gumienny et al., 1999). The cell death activity of EGL-1 was linked to its interaction with CED-9, the worm ortholog of BCL-2 (Conradt and Horvitz, 1998) (see Chapter by Nehme and Conradt). The generation of a knockout mouse model that identified the function of BIM in mouse development and in cell death induced by external stimuli (Bouillet et al., 1999) further substantiated the role of a BH3-only member in a vertebrate. The genetic studies in C. elegans and in mouse suggested that the BH3-only proteins function as apical sentinels of different cell death signals and initiators of the canonical apoptotic program in animals (reviewed in (Bouillet and Strasser, 2002; Huang and Strasser, 2000)). The generation of mouse models deficient in several other BH3-only members such as Noxa, Puma, Bnip3 and Bnip3L has established their role in cell death in response to physiological and pathological stimuli (see accompanying Chapters).
The BH3-only subfamily and siblings
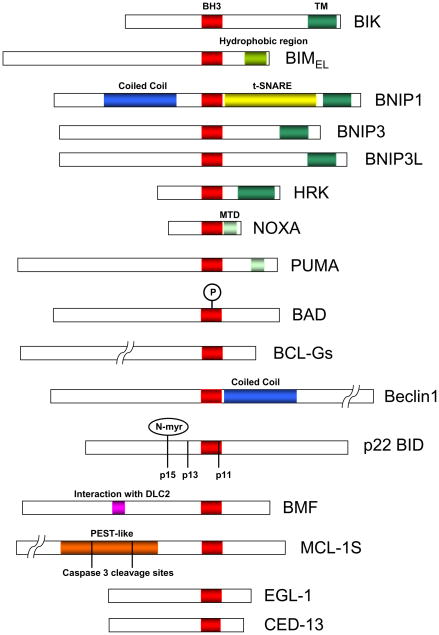
After the discovery of BIK, the list of BH3-only members has increased substantially (Fig. 2). The well known members of this family such as BID (Wang et al., 1996), HRK/DP5 (Imaizumi et al., 1997; Inohara et al., 1997) and BIM/BOD (Hsu et al., 1998; O’Connor et al., 1998) were discovered in interaction cloning with cellular anti-apoptosis proteins BCL-2, BCL-xL and MCL-1. BIK was also identified in interaction cloning with adenovirus E1B-19K (Han et al., 1996). Proteins such as BNIP1, BNIP3 (Boyd et al., 1994) and BAD (Yang et al., 1995) were subsequently included in the BH3-only list. Other members such as NOXA (Oda et al., 2000), PUMA/BBC3 (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001) and BMF (Puthalakath et al., 2001) were identified on the basis of differential mRNA expression in cells exposed to apoptotic stress or by interaction cloning. The autophagy effector Beclin 1 which was originally identified as a BCL-2 interacting protein (Liang et al., 1998) was also identified as a BH3-only member (Feng et al., 2007; Oberstein et al., 2007) (see Chapter by Sinha and Levine). Certain splice variants of multi-domain BCL-2 family members have also gained entry into this subfamily. They include MCL-1S (Bae et al., 2000; Bingle et al., 2000) and BCL-GS (Guo et al., 2001). In C. elegans, a BH3-only member CED-13 (that shares a substantial homology with EGL-1 in the BH3 region) induced by the cep-1/p53 gene in response to genotoxic stress has also been identified (Schumacher et al., 2005). Alternative splicing of the BH3-only members also appear to contribute to the diversity of these proteins and regulate their functions. For example, multiple splice isoforms were described for BIM (Marani et al., 2002; O’Connor et al., 1998; U et al., 2001), BID (Renshaw et al., 2004), NOXA (Wang and Sun, 2008), BNIP1 (Zhang et al., 1999), BMF (Morales et al., 2004). Some of these splice isoforms (like in BIM) retain the BH3-domain, while in other (like in BMF and NOXA) it is spliced out. In contrast to the worm and mammals, no BH3-only proteins have been identified in the annotation of Drosophila genome (Claveria and Torres, 2003). This raises an interesting question as to whether the functional niche of BH3-only proteins, if any, has been occupied by structurally different cell death regulators in Drosophila.
Figure 2.
Domain structure of major mammalian and worm BH3-only proteins. In addition to the BH3 domain, the C-terminal trans-membrane domain (TM), and other membrane targeting hydrophobic sequences are indicated. MTD, indicates mitochondrial targeting domain. The caspase cleavage sites in BID and MCL-1S and the phosphorylation site in the BH3 domain of BAD are indicated.
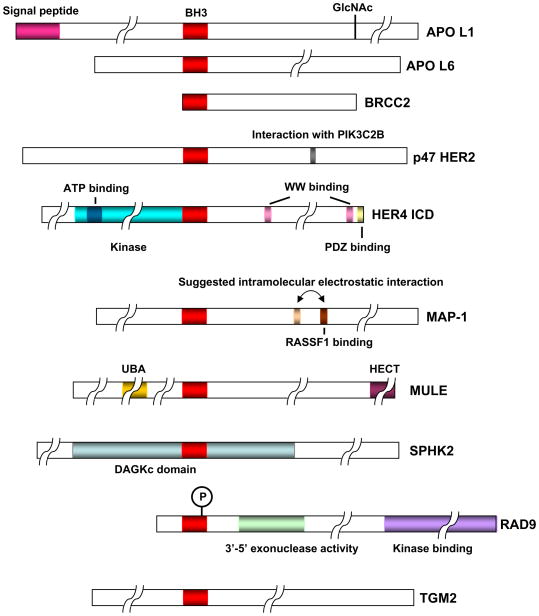
Several other proteins were reported to contain BH3-like domains and possess death-inducing activity and/or ability to interact with anti-apoptotic BCL-2 family proteins (Fig. 3). They include: p193 (Tsai et al., 2000); MAP-1 (Tan et al., 2001); Spike (Mund et al., 2003); SphK2 (Liu et al., 2003a); BRCC2 (Broustas et al., 2004); TG2 (Rodolfo et al., 2004); MULE/ARF-BP1 (Zhong et al., 2005); ApoL6 (Liu et al., 2005); ApoL1 (Wan et al., 2008); ERBB4/HER4 (Naresh et al., 2006); and ERBB2/HER-2 (Strohecker et al., 2008). Other proteins such as murine ITM2BS were reported to contain BH3-like domain and were shown to induce apoptosis (Fleischer et al., 2002). Relatively little is known about integration of these proteins in the apoptosis signaling pathways.
Figure 3.
Domain structure of some BH3-only-like proteins. The BH3 domain and other functional domain(s) in the respective proteins are indicated.
BH3 domain of BH3-only proteins – engagement with pro-survival proteins
It is well established that the BH3 domain of BH3-only proteins is essential for protein-protein interactions with BH1-4 BCL-2 family proteins. The interactions of BH3-only proteins with anti-apoptotic BCL-2 family proteins were readily detected by commonly used methods such as coimmunoprecipitation and two-hybrid interactions in yeast. The interactions were abolished by mutations within the BH3 domain. These results were substantiated by detailed structural studies. The first of such structural studies involved the analysis of a complex between a 16-amino acid peptide derived from the BH3 region of BAK and BCL-xL (Sattler et al., 1997). This study revealed that insertion of the BH3 α-helix of BAK into the hydrophobic cleft of BCL-xL (formed by its BH1, BH2 and BH3 domains) was a key event in heterodimerization between BCL-2-like cell death agonists and antagonists. Similar structural information has been gleaned from studies involving the following complexes between peptides containing the BH3 domain helixes of BH3-only proteins and different anti-apoptosis proteins: BAD:BCL-xL (Petros et al., 2000); BIM:BCL-xL (Liu et al., 2003b); BID:BCL-w (Denisov et al., 2006); Beclin 1:BCL-xL (Feng et al., 2007; Oberstein et al., 2007); hBIM:hMCL-1 (a truncated version containing BCL-2-like region); and mNOXA-B:hMCL1 (Czabotar et al., 2007). The structures of NOXA BH3 and PUMA BH3 in complex with MCL-1 (Day et al., 2008) were also demonstrated using different experimental approaches. Computer-aided modeling was used for the following: BID:BCL-xL complex (Chou et al., 1999; McDonnell et al., 1999), BAD:BCLxL and BIM:BCLxL complexes (Lama and Sankararamakrishnan, 2008). A rigorous structural, biochemical and functional study of EGL-1 interaction with CED-9 also confirmed the critical aspects of such interactions (Yan et al., 2004).
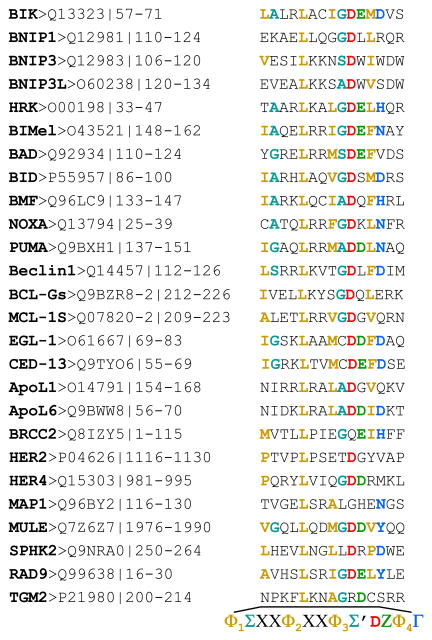
The BH3 domain consists of 9–16 amino acids (Fig. 3). A phylogenic analysis of BH3 domain-containing proteins suggested that the core of BH3 domain is formed by a seven amino acid sequence motif LXXXGDE, where X is any amino acid (Lanave et al., 2004), although neither the Gly nor the Glu residues are strictly conserved. More recently, based on structural studies and sequence analyses a 13-residue consensus motif (Fig. 4) has been proposed (Day et al., 2008). Again, there is no strict conservation. The most conserved leucine and aspartic acid residues of the core BH3 domain appear to be more important for interactions with the pro-survival proteins. The leucine residue is buried in the protein-protein interface and packs against conserved residues of pro-survival protein, while the solvent-exposed aspartate forms ionic interaction with the conserved arginine residue in the BH1 domain of pro-survival protein (reviewed in (Hinds and Day, 2005). Additional important features of the BH3 domain: BH1-4 binding interface were recently reported to include the network of van der Waals, hydrophobic and hydrophilic interactions (Day et al., 2008; Lama and Sankararamakrishnan, 2008).
Figure 4.
Amino acid sequences of the BH3 domains. The sequences of various BH3-only and BH3-only-like proteins, their accession numbers and amino acid coordinates are indicated. The consensus sequence of BH3 domain is shown in the bottom, Φ represents a hydrophobic residue (Φ2 is usually leucine); Σ is a small residue (G, A, S), Ζ is usually an acidic residue; and Γ is a hydrophilic residue (N, H, D or Y) (Day et al., 2008).
Based on the diversity of BH3-only proteins it would be important to know whether these proteins would differ in their binding selectivity to anti-apoptotic proteins. Indeed, initial in vitro binding assays demonstrated some preferences for interaction of the BH3 peptides from different BH3-only proteins with various anti-apoptotic proteins (Kuwana et al., 2005; Letai et al., 2002). A comprehensive in vitro analysis using recombinant anti-apoptotic proteins (human BCL-2 ΔC22, BCL-w C29S, A128EΔC29, BCL-xLΔC24 and mouse MCL-1ΔN151ΔC23 and A1ΔC20) and synthetic BH3-peptides of BH3-only proteins revealed a marked difference in binding selectivity among them (Chen et al., 2005). Among eight BH3-peptides derived from human BAD, BID, BIK, BIM, HRK, NOXA, PUMA and mouse BMF proteins, only BIM and PUMA peptides had comparable strong affinities to all anti-apoptotic proteins tested. BAD and BMF peptides preferably bound to BCL-2, BCL-xL and BCL-w. NOXA peptide bound strongly to MCL-1 and A1, but not to others. BID, BIK, and HRK peptides interacted more strongly with BCL-xL, BCL-w and A1. It was likely that the observed selectivity relied on the differences in amino acids sequence within the BH3 domains, because mutations in BH3 domain were able to change the binding preferences. Thus, these binding studies demonstrated additional functional diversity among the BH3-only proteins and provided a mechanistic explanation for the differences in apoptotic signaling induced by BH3-only proteins (see below).
In contrast to the near universal and well documented feature of the BH3-only proteins - their ability to bind to anti-apoptotic multidomain BH1-4 proteins, the ability to interact with pro-apoptotic multidomain BH1-3 proteins is limited to only a few of them. For example, direct binding of BID to BAX and BAK (Wang et al., 1996) and binding of BIM to BAX (Harada et al., 2004) have been reported. Another example appears to be the BH3-only-like protein tissue or type 2 transglutaminase (TGM2). TGM2 possesses a functional BH3 domain through which it was shown to interact with pro-apoptotic proteins BAX and BAK but not with anti-apoptotic BCL-2 and BCL-xL. Upon induction of cell death, mitochondrially localized TGM2 was shown to complex with proteins, including BAX (Rodolfo et al., 2004). Yeast two-hybrid and co-immunoprecipitation analyses revealed that the binding of BID and PUMA to BAX was via their BH3-domain and the firstα helix of BAX (Cartron et al., 2004). Recently, an NMR spectroscopic analysis revealed a novel BH3-interaction interaction site (comprising of helices α1 andα6) on BAX (Gavathiotis et al., 2008). A peptide containing the BH3 domain of BIM (in the form of ‘stabilized α-helix of BCL-2 domain’, SAHB) bound to the α1-α6 binding site BAX (Gavathiotis et al., 2008). Importantly, conformational activation of BAX was initiated at this novel site by BIM SAHB binding. Interestingly, the predicted structure of the BIM-BH3-BAX complex showed an interaction topography that was similar to that of BIM BH3 with anti-apoptotic BCL-xL, MCL1 and A1 that were previously determined by X-ray crystallography. Thus, the work of Gavathiotis et al. was the first direct structural study of a complex between the BH3 domain of a BH3-only protein and BAX. However, it is still uncertain whether the same interaction occurs in the context of full length proteins, and whether binding to BAK proceeds through a similar mechanism. Therefore, the functional significance of interaction of certain BH3-only proteins with BH1-3 pro-apoptotic proteins remains to be fully addressed.
Diversity in BH3 functions
After the identification and functional characterization of the BH3 domain in BIK (Boyd et al., 1995) and BAK (Chittenden et al., 1995), as well as in multiple BH3-only proteins in subsequent studies, it became clear that the BH3 domain represented the minimal “death domain” (reviewed in (Kelekar and Thompson, 1998)). Importantly, numerous studies have shown that mutations in the BH3 domain of BH3-only proteins resulted in simultaneous abrogation of their binding to pro-survival BCL-2 family proteins and their pro-apoptotic activity (Boyd et al., 1995; Inohara et al., 1997; O’Connor et al., 1998; Puthalakath et al., 2001; Wang et al., 1996; Zha et al., 1997). Thus, the interaction of the BH3-domain with anti-apoptotic multi-BH-domain proteins is crucial for cell death activity of BH3-only proteins. Is this sufficient for entire pro-apoptotic activity of all BH3-only proteins? It appears that the answer may vary depending on the context of the individual BH3-only member.
The first evidence for functional diversity within the BH3 domain came from an in vitro functional analysis of the BH3-domain-containing peptides derived from different BH3-only proteins to induce mitochondrial release of cytochrome c in purified mouse mitochondria (Letai et al., 2002). This study provided evidence for two distinct subclasses of BH3 peptides: one called “activators”, which included the BH3 domain of BID and BIM, that can directly activate BAK to oligomerize and induce mitochondrial release of cytochrome c; the other subclass called “sensitizers”, and is represented by BID, BIK and NOXA. This subclass did not directly activate BAK nor induce cytochrome c release, but instead bound to anti-apoptotic BCL-2 to displace BID-like peptides. Importantly, this study demonstrated a level of cooperation between BH3-only peptides. Transduction of the sensitizer BH3-peptide (BAD-BH3) and the activator peptide (BID-BH3) had a synergistic effect in killing cancer cells (Letai et al., 2002). Similar results were obtained using synthetic liposomes loaded with an expanded set of BH3 peptides and recombinant BAX protein (Kuwana et al., 2005). Taken together, the in vitro studies based on binding of BH3 peptides to multidomain proteins provided the experimental basis for different models on the mode of action of BH3-only proteins (see below). However, the limitation of such models is that they are based on BH3-peptides rather than native BH3-only proteins. Some aspects of these models based on BH3 peptides appear to contradict with certain results obtained with expression of BH3-only proteins from expression vectors. A detailed analysis of the pro-apoptotic activity of a panel of BIK mutants revealed that a C-terminal region (aa 120–134) was required in addition to the BH3-domain for efficient cell death activity of BIK (Elangovan and Chinnadurai, 1997). A recombinant version of BIK was shown to efficiently induce cytochrome c release in isolated mitochondria (Shimizu and Tsujimoto, 2000). The killing activity of NOXA BH3 domain was functional only when targeted to mitochondria via the C-terminal domain of the protein (Seo et al., 2003). It is possible that intact BIK and NOXA proteins might be potentially as effective as BID and BIM in inducing permeabilization of the outer mitochondrial membrane (MOMP). Thus, additional sequence elements in various BH3-only members may enhance the activity of BH3 domain and the BH3 domain-directed activity may be dependent on the whole protein.
Another important question is whether BH3-only proteins are the only proteins involved in binding to the anti-apoptotic BCL-2 family members and thereby inducing apoptosis. Several reports argue that some cellular proteins lacking a BH3-domain are capable of binding to anti-apoptotic proteins and inducing apoptosis. The orphan nuclear receptor Nur77 was shown to translocate from the nucleus to the cytosol and interact with BCL-2 to induce apoptosis (Cao et al., 2004; Lin et al., 2004). Phosphorylation of K-RAS by protein kinase C promoted its rapid dissociation from the plasma membrane and association with mitochondria, where phospho-K-RAS bound to BCL-xL and induced apoptosis (Bivona et al., 2006). Calpain-mediated cleavage of Atg5 (an autophagy effector) resulted in translocation of truncated Atg5 from the cytosol to mitochondria, association with BCL-xL and apoptotic cell death (Yousefi et al., 2006). A transcription-independent pathway of p53-dependent apoptosis was shown to include physical interaction between mitochondrial p53 and anti- (BCL-2, BCL-xL) and pro-apoptotic (BAX, BAK) members of the BCL-2 family proteins (Chipuk et al., 2004; Leu et al., 2004; Mihara et al., 2003). It was recently suggested that mitochondrial p53 functionally resembled a ‘super’ BH3-only protein because it acted both as an ‘enabler’ and as an ‘activator’ BH3-only protein (Wolff et al., 2008). Therefore, there are probably diverse groups of proteins that are structurally different from BH3-only proteins that can execute the same or equivalent functions.
Multiple modes of regulation of the pro-apoptotic activity of BH3-only proteins
Transcriptional activation and silencing
The activity of BH3-only proteins is stringently regulated at multiple levels. The steady-state levels of BH3-only proteins are regulated both at transcriptional and at post-transcriptional levels. Under normal unstressed conditions, the level of many BH3-only proteins is very low or undetectable by common methods. Different apoptotic stimuli were reported to activate transcription of several BH3-only members through specific transcription factors. The transcription factor E2F-1 was shown to directly up-regulate expression of Puma, Noxa, Bim, and Hrk/Dp5 (Hershko and Ginsberg, 2004) and Bik (Real et al., 2006; Subramanian et al., 2007). Puma and Noxa were shown to be transcriptionally activated by p53 in response to DNA damage and other cell death signals (Han et al., 2001; Michalak et al., 2008; Nakano and Vousden, 2001; Oda et al., 2000; Shibue et al., 2006; Villunger et al., 2003; Yu et al., 2001). Transcriptional activation of Bik by estrogen starvation or anti-estrogen exposure in estrogen-dependent breast cancer cell lines was also reported to depend on an activity of p53 that did not involve DNA binding to canonical p53-dinding sites (Hur et al., 2006; Hur et al., 2004). The forkhead box transcription factor FOXO3a (FKHRL1) was shown to up-regulate Bim expression in hematopoetic cells (Dijkers et al., 2000), neurons (Gilley et al., 2003) and in paclitaxel-treated breast cancer cells (Sunters et al., 2003). FOXO3a also up-regulated Puma expression in response to cytokine/growth factors withdrawal (You et al., 2006). Dp5/Hrk was shown to be a direct target for c-Jun during potassium deprivation-induced apoptosis in cerebellar granule neurons (Ma et al., 2007). Noxa (Kim et al., 2004) and Bnip3 (Bruick, 2000; Sowter et al., 2001) were activated by HIF-1α in response to hypoxia. In contrast to the apoptotic stimuli, pro-survival signals were shown to promote transcriptional silencing of BH3-only genes. The transcriptional repressor DREAM that is activated by IL-3 was shown to bind to a silencer sequence in the 3′ untranslated region of the Hrk gene and abrogate its expression in interleukin-3 (IL-3)-dependent hematopoietic progenitor cells (Sanz et al., 2001). Under non-stress conditions, the expression of Bnip3 was shown to be transcriptionally repressed by the p65 subunit of NF- B in cardiomyocytes (Shaw et al., 2008) or by the repressive form of E2F, E2F4, in fetal liver cells (Tracy et al., 2007). As described in the accompanying Chapters, the transcription BH3-only members such as Bnip3 (Okami et al., 2004), Noxa (Yamashita et al., 2008) and Bik (Dai et al., 2006; Pompeia et al., 2004) were shown to be epigenetically silenced by promoter methylation and histone acetylation in different cellular contexts.
Stability and translation of mRNA
The posttranscriptional regulation of mRNA levels of the BH3-only members is the least studied of the regulatory mechanisms that control their activity. An example of such a regulation was noted in the control of stability of Hrk mRNA (Inohara et al., 1997). An AUUUA sequence motif in the 3′ untranslated region of Hrk was shown to contribute to mRNA destabilization. The stability of Bim mRNA was also reported to be regulated by an AU-rich element in the 3′ untranslated region (Matsui et al., 2007). The micro RNA (miR) cluster miR-17-92 was reported to inhibit translation of Bim mRNA (Xiao et al., 2008). Transgenic expression of the miR-17-92 resulted in phenotypes similar to those seen in Bim−/− mice (see Chapter by Penon, Egle and Villunger).
Protein stability
A number of studies that examined the effects of various proteasome inhibitors reported substantial increases in the levels of BH3-only proteins such as BIK (Hur et al., 2004; Marshansky et al., 2001; Zhu et al., 2005), BIM (Nikrad et al., 2005) and NOXA (Fernandez et al., 2005). Direct evidence linking the proteasomal pathway in down-regulation of the steady-state levels of BH3-only proteins is limited. A direct role for ubiquitilation-dependent regulation of BIM came from studies on apoptosis regulation in osteoclsts (Akiyama et al., 2003). It was shown that following growth factors withdrawal, reduced ubiquitylation and proteasomal degradation of BIM resulted in a rapid and sustained increase in the level of BIM. Importantly, c-Cbl family E3 ubiquitin ligases were involved in ubiquitilation and proteasomel degradation of BIM in osteoclasts. It appears that the regulation at the level of protein stability is required to prevent unscheduled apoptosis in cells where the BH3-only members are constitutively transcribed. For example, in some breast cancer cell lines that expressed relatively high levels of Bik mRNA, BIK protein was undetectable and treatment with proteasome inhibitors increased BIK accumulation (Hur et al., 2004).
Post-translational modifications
Phosphorylation of BH3-only proteins has been reported to either positively or negatively influence their apoptotic activity. Phosphorylation of BAD was shown to abolish its pro-apoptotic activity (Zha et al., 1996b; Zhou et al., 2000), and dephosphorylation of BAD by Ca2+-sensitive phosphatases such as calcineurin led to apoptosis (Wang et al., 1999). Analysis of the regulation of BIM activity by phosphorylation (reviewed in (Ley et al., 2005)) revealed opposing effects of phosphorylation on its activity depending on the site(s) of modification and the kinase(s) involved. A more recent study using transgenic mice constructed with a series of mutant alleles with phosphorylation-defective BIM proteins clearly demonstrated that phosphorylation of BIM on different sites contributed to the sensitivity of cellular apoptotic responses (Hubner et al., 2008). Mutation of Thr112 caused decreased binding of BIM to BCL-2 and increased cell survival. In contrast, mutation of the Ser55, 65, and 73 sites caused increased apoptosis due to reduced proteasomal degradation of BIM. Phosphorylation of human BIK was shown to increase the pro-apoptotic activity through enhanced interaction with anti-apoptotic proteins (Verma et al., 2001). A phosphomimetic version of BIK (designated BikDD) was used to as an anti-cancer therapeutic gene to kill pancreatic cancer cells in an orthotopic mouse model (Xie et al., 2007).
The cell-cycle-checkpoint and DNA damage repair protein RAD9 was shown to interact with BCL-2 and BCL-xL through its BH3 domain and promote apoptosis after DNA damage (Komatsu et al., 2000). Exposure to DNA-damaging agents was shown to activate c-Abl tyrosine kinase that bound to the C-terminal region of RAD9 via the SH3 domain and phosphorylated the RAD9 BH3 domain at Tyr28. Phosphorylation of RAD9 induced binding to BCL-xL and apoptosis (Yoshida et al., 2002). Mutation of the phosphorylation site (Tyr28→Phe) completely inhibited c-Abl-mediated phosphorylation, abrogated binding to BCL-xL and attenuated the apoptotic activity (Yoshida et al., 2002).
Conformational activation
As most BH3-only proteins appear to be constitutively pro-apoptotic when overexpressed, there are only limited examples of conformational activation of these proteins. An interesting example is the BH3-only-like protein, modulator of apoptosis-1 (MAP-1) that is involved in receptor-mediated apoptosis (Baksh et al., 2005). The BH3 domain of MAP-1 appeared to be hidden inside the inactive molecule through an intramolecular interaction. The death receptor signaling was shown to promote intermolecular electrostatic interactions involving the C-terminus of the tumor suppressor RASSF1A and a basic stretch within MAP-1 resulting in the opening of MAP-1. The ‘open’ form of MAP-1 was show to associate with BAX and induce BAX conformational change (Baksh et al., 2005). There are several BH3-only-like proteins without significant cell death activity under normal assay conditions. It is possible that some of these proteins may need appropriate conformational activation to unleash their apoptotic activity.
The BH3-only protein BID (which appears to be structurally related to BAX, see Chapter by Billen, Shamas-Din and Andrews) was shown to be non-functional as a full-length protein. Upon death factor receptor signaling, BID was shown to be cleaved by caspase-8 resulting in a N-terminally truncated form (tBID) (Li et al., 1998; Luo et al., 1998). tBID was shown to be targeted to mitochondria and amplify death receptor-induced cell death via the mitochondrial pathway. Thus, the activation of BID appears to represent a unique mode of post-translational activation.
Subcellular localization and sequestration
Several BH3-only proteins, including BIK, BIM, BNIP3, and HRK have predicted transmembrane domains (Fig. 2), that are implicated in targeting them to intracellular membranes (including the ER and/or mitochondria). PUMA was shown to be targeted to mitochondria by a C-terminal hydrophobic domain (Nakano and Vousden, 2001; Yu et al., 2003). Other BH3-only members such as BAD, BID and NOXA do not contain obvious membrane targeting sequences. NOXA was shown to possess a unique mitochondrial targeting domain in the C-terminal region (Seo et al., 2003). The mitochondrial targeting of tBID was shown to be facilitated by post-translational (rather than co-translational) N-myristoylation upon exposure of a glycine residue after cleavage with caspase-8 (Zha et al., 2000) as well as by two α helices (Garcia-Saez et al., 2004). Several of the BH3-only proteins have been reported to mediate efficient apoptosis independent of such membrane targeting sequence, at least under in vitro assay conditions that involved transfection of expression vectors. This raises a question whether interaction with multidomain BCL-2 family proteins may also play a role in membrane localization. Mutants of BIM that either lacked the entire BH3-domain (O’Connor et al., 1998) or point mutants that carry mutations rendering them unable to bind to BCL-2 (Weber et al., 2007) were found to localize in intracellular membranes, suggesting that BH3-only proteins may autonomously localize to membranes. Thus, it appears that the membrane localization activity of BH3-only members is an intrinsic property intended to increase their local concentration in intra-cytoplasmic membranes.
In some instances the BH3-only proteins have been shown to be sequestered in inactive protein complexes. In healthy cells BIM was shown to be sequestered in the microtubule-associated dynein motor complex (Puthalakath et al., 1999). Apoptotic signals induced BIM dissociation from the complex and interaction with BCL-2. In a number of lung cancers with the worst prognosis, BNIP3 was reported to be localized in the nucleus (Giatromanolaki et al., 2004). Similar nuclear localization was also reported in the majority of primary glioblastoma multiforme samples (Burton et al., 2006) suggesting that functional sequestration of BNIP3 may play a role in the progression of solid tumors.
The above examples suggest that the activity of BH3-only proteins is strictly regulated in time and in place by multiple mechanisms to assure proper response to cell death/survival signals. Additional examples and discussion may be found in previous reviews on this subject (Fernandez-Luna, 2008; Puthalakath and Strasser, 2002; Shibue and Taniguchi, 2006; Willis and Adams, 2005).
Role in apoptosis: bind to neutralize or activate, insert to disturb
In the canonical mitochondrial apoptotic pathway, BH3-only proteins are considered to be apical sentinels that connect the various death stimuli with the multidomain BCL-2 family proteins to mediate apoptosis. But the precise mode of action of BH3-only proteins remains an intensely debated issue. At present there are two models – ‘direct binding and activation of BAX/BAK’ and ‘neutralization of anti-apoptotic BCL-2 family proteins and displacement of BAX/BAK’. These models have been discussed in several reviews (Chipuk and Green, 2008; Hacker and Weber, 2007; Shibue and Taniguchi, 2006; Willis and Adams, 2005) and in the accompanying Chapter by Giam, Huang and Bouillet (also see Fig. 5; indicated by bold arrows 1 and 2). Both of these models are based on the assumption that the key role of BH3-only proteins in apoptosis is the interaction through their BH3-domains with multidomain BCL-2 family proteins. In the first model, the priority and significance in mediating the irreversible decision to die is dependent on binding to multidomain pro-apoptotic proteins BAX and BAK. The second model is based on the near universal property of BH3-only proteins to interact with the anti-apoptotic BCL-2 family members. Both models agree that BAX and BAK are activated as the result of binding. According to the direct activation model, BAX and BAK are postulated to be conformationally activated as a result of binding with activator BH3-only proteins (such as tBID and BIM). In the second model, BAK is postulated to be released from complexes with BCL-xL and MCL-1. However, the displacement model does not adequately address the mode of BAX activation (see below). The two models predict that BH3-only proteins differ in their apoptotic potential. Moreover, according to both models BIM is a more potent killer than BAD. However, the rationale behind such supposition is different for the two models. According to the first model, BIM is more apoptotic because it is able to directly bind and activate BAX/BAK whereas the “sensitizer” BAD cannot bind to BAX/BAK and requires an additional “activator” BH3-only protein for apoptosis to proceed. By the second model, BIM is more active because it binds to all five anti-apoptotic BCL-2 proteins (BCL-2, BCL-xL, BCL-w, MCL-1 and A1), where as BAD is able to bind and neutralize only three of them (BCL-2, BCL-xL and BCL-w).
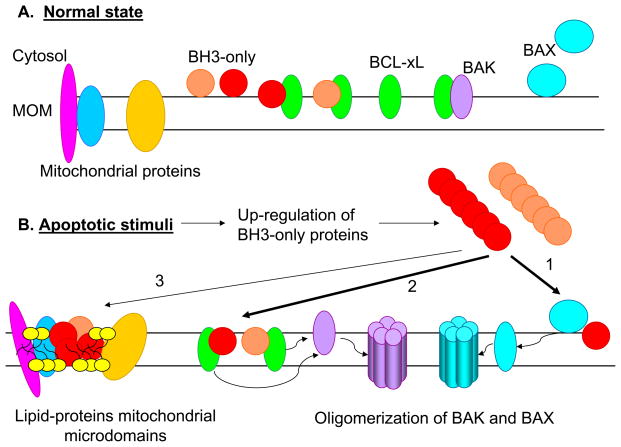
Figure 5.
Models for the apoptotic activity of BH3-only proteins. A. In normal state, BH3-only proteins are present in very low levels in the cytosol or in mitochondrial membrane, most likely in complex with anti-apoptotic BCL-2 family proteins. BAK is shown to be located in mitochondrial membrane in inactive complexes with BH1-4 anti-apoptotic proteins, where as BAX is shown to be in the cytosol or loosely attached to the mitochondrial membrane. B. Different apoptotic stimuli induce significant up-regulation of the BH3-only proteins through transcriptional and post-transcriptional mechanisms. BH3-only proteins then transmit the apoptotic signals to BAX and BAK proteins either through “direct binding/and activation” (1), or “neutralization of BH1-4 proteins and displacement of BAK/BAX” (2). In addition, BH3-only proteins is also shown to induce mitochondrial membrane remodeling/formation of altered microdomains through mitochondrial outer membrane (MOM) insertion and interaction with membrane lipids (e.g., cardiolipin – yellow heads) and/or with other mitochondrial proteins (3). Two classes of BH3-only proteins (that bind with multi domain anti-apoptotic and pro-apoptotic proteins or only with anti-apoptotic proteins) are indicated in red and orange.
Both of these models have limitations. The major concern about the direct activation model is that at present there is no direct structural evidence for complex formation between native activator BH3-only proteins and BAX/BAK. The second model cannot explain activation of BAX, because in normal cells BAX is presumed to exist mainly as a monomeric cytosolic protein and not in complex with anti-apoptotic proteins. Hence it is believed that BAX may not require displacement for activation. It appears that this issue may need further investigation to determine whether a fraction BAX is in complex with BCL-2/BCL-xL. It should be noted that BAX was originally discovered in complex with BCL-2 (Oltvai et al., 1993). Recently, BIK (which mediates its pro-apoptotic solely through BAX in human epithelial cells) was shown to activate BAX by a displacement mechanism (Gillissen et al., 2007). A caveat to both models is that they assume all BH3-only members function identically in different cell types. There is evidence to suggest that cell death activity of some BH3-only members is dependent on the cell type. Therefore, caution should be exercised in universal interpretation of the data on protein interactions and cell death activities.
In this context, it is necessary to consider additional features of BH3-only proteins that might be important for understanding their role in apoptosis – ability to interact with lipids (see Fig. 5, indicated by thin arrow, 3). Such an activity may contribute to the potency of activator BH3-only members BID and BIM. It has been suggested that the hydrophobic cleft at the protein surface of BID could be involved in binding to amphipathic molecules (Chou et al., 1999). Interestingly, the homologous cleft in BCL-xL was shown to bind with lysophosphatidylcholine (Losonczi et al., 2000). It was suggested that such binding mimicked natural interactions with membranes and their lipids, suggesting the natural ligand for the exposed hydrophobic cleft in BID and BCL-xL might be a lipid-like molecule (Cristea and Degli Esposti, 2004). Dynamic interactions of BID with membrane lipids, especially cardiolipin (reviewed in (Cristea and Degli Esposti, 2004)) and a channel-forming activity of BID in synthetic lipid bilayers have been reported (Schendel et al., 1999). A BH3-independent membrane destabilizing property of tBID has also been described (Kluck et al., 1999; Kudla et al., 2000). By analogy with BAX, recombinant tBID was reported to form oligomers in the presence of non-ionic detergents and mitochondrial lipids (Grinberg et al., 2002). tBID was reported to mediate remodeling of mitochondrial cristae resulting in relocation of cytochrome c from cristae stores to the intermembrane space, independent of interaction with BAX (Scorrano et al., 2002). Although the mechanism of cristae remodeling by tBID is unknown, it was speculated that tBID might participate in lipid-modifying multiprotein complexes that regulate changes of mitochondrial ultrastructure (Karbowski and Youle, 2003). Recent data on BIM also highlight the importance of mitochondrial membrane insertion for the cell-death activity (Weber et al., 2007). First, an analysis of interaction of BIM with anti-apoptotic BCL-2 proteins showed that the amount of BCL-2, BCL-xL, and MCL-1 co-precipitating with BIM-S appeared to be similar in healthy cells and in apoptotic cells with induced BIM-S expression. Second, examination of BIM-S mutants revealed that apoptosis induction correlated with mitochondrial localization but not with the ability to bind BCL-2. These results suggested that the pro-apoptotic activity of BIM-S was dependent more on insertion into mitochondrial membrane rather than on interaction with anti-apoptotic proteins. In the case of BNIP3, the trans-membrane domain (which can form autonomous stable detergent-resistant dimers) was reported to be the primary determinant for its cell death activity through mitochondrial membrane insertion and induction of mitochondrial membrane potential loss (Vande Velde et al., 2000) (see accompanying Chapter by Chinnadurai, Vijayalingam and Gibson)
Thus, it appears that the majority of the published data support the notion that anti-apoptotic BCL-2 family members block the apoptotic activity of BH3-only proteins by direct binding and sequestering them. But these data do not mean that the opposite is true i.e., the BH3-only proteins induce apoptosis primarily through binding to anti-apoptotic BCL-2-like proteins. Similarly, activation of BAX/BAK by direct binding with BH3-only proteins appears to be limited to BH3-only proteins such as BID and BIM. Since BID appears to be structurally similar to BAX, interpretations that it may function like other BH3-only members should also be tempered. Issues such as the potential interactions of BH3-only proteins with intracellular membranes/organelles (mainly mitochondria) should be considered.
Non-apoptotic functions of BH3-only proteins
Growing evidence suggests that BH3-only proteins are also involved in other vital cellular functions besides their role in apoptosis (Hetz and Glimcher, 2008). The best studied example is BAD (see accompanying Chapter by Danial). BAD was reported to play a role in regulation of cell cycle progression through dimerization with BCL-xL to bypass G0/G1 arrest (Chattopadhyay et al., 2001). In a different study, overexpression of BAD was found to enhance cell cycle progression and interleukin 2 production after T cell activation as a consequence of Akt/BAD regulation in primary T cells (Mok et al., 1999). BAD was also shown to reside in a functional holoenzyme complex containing glucokinase involved in glucose-driven mitochondrial respiration (Danial et al., 2003). In addition, BAD was shown to play an important role in glucose-stimulated insulin secretion by pancreatic β cells (Danial et al., 2008). This function of BAD was specifically dependent upon the phosphorylation of its BH3 domain.
Although conflicting results on the role of BID in DNA-damage response have been reported (Kamer et al., 2005; Zinkel et al., 2005), others have reported no such effect (Kaufmann et al., 2007). As pointed out earlier, BID has been implicated in lipid transfer between mitochondria and other cellular membranes, suggesting that BID may be involved in the transport and recycling of mitochondrial phospholipids (Esposti et al., 2001). BNIP1 (also known as vesicle transport protein SEC20) was shown to participate in the organization and biogenesis of the ER by mediating membrane fusion as a component of the SNARE complex (Nakajima et al., 2004). Interestingly, the BH3 domain of BNIP1 was important for the binding to alpha-SNAP, an adaptor that serves as a link between the chaperone ATPase NSF and SNAREs (Nakajima et al., 2004). The role of RAD9 (which possesses a 3′→5′ double stranded DNA exonuclease activity (Bessho and Sancar, 2000)) as a component of the 9-1-1 cell-cycle checkpoint response complex in DNA repair is well established (Lieberman et al., 1996; St Onge et al., 1999). TGM2 (Tissue or type 2 transglutaminase) is a multifunctional enzyme belonging to the transglutaminase family (Lorand and Graham, 2003). Its primary enzymatic activity involves post-translational modification of proteins by establishing ε(γ-glutamyl)lysine and N, N-bis(γ-glutamyl)polyamine isopeptide linkages. This activity mediates cross-linking of proteins and conjugation of polyamines to proteins. Missense mutations in the Tgm2 gene encoding transglutaminase 2 were found in patients with early-onset type 2 diabetes (Porzio et al., 2007).
Role in autophagy and autophagic death
Beclin 1 was identified as a BCL-2 interacting protein (Liang et al., 1998). It is also known that Beclin 1 is a critical effector of autophagy in mammalian cells (Kihara et al., 2001; Zeng et al., 2006). The structure of BCL-xL – Beclin 1 complex demonstrated that Beclin 1 contained a BH3 domain through which it interacted with BCL-xL (Feng et al., 2007; Oberstein et al., 2007) and vBCL-2s (Ku et al., 2008; Sinha et al., 2008) (see Chapter by Sinha and Levine). Although these findings raised the possibility that Beclin 1 might have a pro-apoptotic role, no such activity has yet been associated with Beclin 1. However, interaction of Beclin 1 with cellular and viral BCL-2s was shown to inhibit autophagy (Pattingre et al., 2005). The BH3-only protein BNIP3 was associated with autophagic cell death induced by certain anticancer agents (Daido et al., 2004). Other BH3-only proteins BAD, EGL-1 and BIK as well as the BH3 mimetic ABT737 were also reported to induce autophagic death (Maiuri et al., 2007b; Rashmi et al., 2008). This activity of BAD, EGL-1, ABT737 (Maiuri et al., 2007b) or BNIP3 (Zhang et al., 2008) was linked to their ability to dislodge Beclin 1 from a complex with BCL-xL or BCL-2. Further studies are required to elucidate the role of BH3-only members in autophagy under normal physiological conditions. Studies with Bnip3L−/− mice revealed that BNIP3L was required for normal maturation erythrocytes (Diwan et al., 2007a). This activity of BNIP3L was linked to targeted autophagic elimination of mitochondria (Sandoval et al., 2008; Schweers et al., 2007). Knock-down of BAD in mammalian cells reduced starvation-induced autophagy while overexpression increased the autophagic response (Maiuri et al., 2007a). In C. elegans, a gain-of-function mutant of egl-1 induced autophagy, while a deletion diminished such a response (Maiuri et al., 2007a). Thus, the BH1-4 and BH3-only proteins have emerged as important regulators of autophagy and autophagic cell death in mammalian cells. It is possible that several BH3-only proteins may serve as sensors and initiators of autophagy.
Role in tumorigenesis
As BH3-only proteins play pivotal role in apoptosis following cellular damage, it is likely that these proteins are critical components of the tumor suppression pathways. Loss of expression of BH3-only genes may promote tumorigenesis due to lack of apoptosis and accumulation of cells with tumorigenic potential. There is strong evidence that several BH3-only members function as tumor suppressors (reviewed by (Fernandez-Luna, 2008; Karst and Li, 2007)). Gene disruption in mice revealed the role for some BH3-only proteins in suppressing tumor development, but not for others. Puma−/− and Noxa−/− mice exhibited no developmental abnormalities (Shibue et al., 2003; Villunger et al., 2003). Bid-deficient mice did not display a phenotype initially (Yin et al., 1999), but subsequently developed a myeloid hyperplasia that progressed to a malignancy resembling chronic myelomonocytic leukemia (Zinkel et al., 2003). These results suggested that BID might function as a tumor suppressor in myeloid cells. Bad−/− mice spontaneously developed diffuse B cell lymphoma (Ranger et al., 2003). Loss of a single or both alleles of Bim in mice was shown to promote B cell lymphoma development under conditions of oncogenic c-Myc expression (Egle et al., 2004). Thus, studies with mutant mouse models have suggested tumor suppressive functions for some of the BH3-only members.
Additional insights about the role of BH3-only proteins in tumorigenesis arise from genetic studies of human cancers. Different studies have indicated that loss of expression of several BH3-only proteins might contribute to human cancer. Frequent deletions encompassing the Puma locus in chromosome 19q13.3 were reported in gliomas, neuroblastomas, and in certain B cell lymphomas (reviewed in (Karst and Li, 2007)). The chromosomal locus containing Bmf (15q14/15) was also proposed to harbor a tumor suppressor gene, since loss of this region was associated with advanced carcinomas of the breast, lung and colon (Schmutte et al., 1999; Wick et al., 1996). Loss of BIK expression, associated with allelic loss and/or methylation, was reported in renal cell carcinomas (Sturm et al., 2006). Chromosomal deletions in 22q13 harboring the Bik gene were reported in gliomas (Bredel et al., 2005), colorectal cancers (Castells et al., 1999) and in head and neck cancers (Reis et al., 2002). Mutations in the Bik gene were found in B-cell lymphomas (Arena et al., 2003). Among a large number apoptosis regulating genes examined in a gene expression profiling study, only expression of Bik mRNA was highly activated in human lung, prostate and renal carcinoma cell lines that were treated with inhibitors of DNA methyltransferase and histone deacetylases, suggesting silencing of Bik expression in these cells (Dai et al., 2006). Inactivation of the Hrk gene by methylation was reported in gastric and colorectal cancers (Obata et al., 2003) and glioblastomas (Nakamura et al., 2005). It was also suggested that reduced expression of HRK may serve as one important molecular mechanism in the progression to secondary glioblastoma (Nakamura et al., 2005).
Low rates of inactivating Bid mutations were observed in gastric cancers (Lee et al., 2004). Bim mutations in human cancers have not been reported (Karst and Li, 2007). But, loss of the chromosomal region 2q13, containing the Bim gene, was observed in mantle cell lymphomas (Tagawa et al., 2005). Analysis of major subtypes of human B-cell lymphomas found no mutation in the Bad gene, indicating that mutational inactivation of BAD plays no role in the pathogenesis of these malignances (Schmitz et al., 2006). However, suppressed expression of Bad through methylation was found in a multiple myeloma cell line (Pompeia et al., 2004). The Noxa gene was rarely mutated in human carcinomas (Lee et al., 2003), but Noxa was found to be mutated and preferentially silenced in diffuse large B cell lymphomas (Mestre-Escorihuela et al., 2007). Together, these studies suggest that loss of expression of many BH3-only proteins through gene deletion or epigenetic silencing may contribute to tumor formation. Interestingly, inactivating mutations in BH3-only genes were rarely observed.
In contrast to the results summarized above, there are several reports that seem to suggest that some BH3-only proteins may not fit the tumor suppressor mold. A large study of BID protein expression in prostate, ovarian, colorectal, and brain cancers, and B cell non-Hodgkin’s lymphomas demonstrated increased expression in tumor tissues (Krajewska et al., 2002). An immunohistochemical analysis of BID expression in hepatocellular carcinomas revealed that BID expression was often stronger in nuclei of the epithelial cells in tissues from liver metastases than in the normal liver tissues, and the nuclei of metastatic cells showed considerable mitotic activity, indicating that they were in active proliferation (Chen et al., 2001). Tumorigenesis induced by diethylnitrosamine was considerably retarded in Bid-null mice (Bai et al., 2005). Further analysis demonstrated that there were significantly fewer proliferating cells in diethylnitrosamine-treated Bid−/− livers. Although the mechanism underlying such an activity of BID in cell proliferation is not known, it is possible that apoptosis incompetent full-length BID may play such a role. As pointed in the accompanying Chapter on BIK, overexpression of Bik in sporadic breast cancers (Garcia et al., 2005) and in non-small cell lung tumors from patients with high-risk cancer recurrences was reported (Lu et al., 2006). Since overexpression of Bik also accompanied other genes such as Bcl-2 and pro-inflammatory genes, it is possible that inflammatory response to chronic Bik/Bcl-2 expression may promote tumorigenesis. Similarly, high expression of Bnip3 was also reported in breast and lung cancers with poor prognosis. Since Bnip3 expression was enhanced in the necrotic areas of the tumor, tumor necrosis resulting from Bnip3 overexpression may promote tumor progression. Initial overexpression of BH3-only members may facilitate selection of aggressive tumor cells during tumor progression, ultimately leading to silencing of these genes in advanced cancers.
Role in cytotoxicity to anticancer drugs and in the development of cancer therapeutics
Multiple studies have demonstrated the pivotal role of BH3-only proteins in the cytotoxic response to anticancer agents. In addition, chemoresistance of many cancers was attributed to the loss or lower level of expression of BH3-only proteins. PUMA expression in many cancer cell lines was strongly and rapidly upregulated in response to DNA-damaging agents, such as adriamycin, 5-fluorouracil, etoposide, and ionizing radiation (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001). In addition, thymocytes and myeloid progenitors from Puma−/− mice were resistant to apoptosis induced by various cytotoxic stimuli, including anticancer drugs (Jeffers et al., 2003; Villunger et al., 2003). Dramatic up-regulation of NOXA, BIM, and BIK was reported in cancer cells treated with the proteasome inhibitor bortezomib (Velcade) (Fernandez et al., 2005; Nikrad et al., 2005; Qin et al., 2005; Zhu et al., 2005). Rapid accumulation of BIM was also noted in cancer cells treated with the anti-microtubule drug paclitaxel and Bim−/− cells were resistant to the drug (Tan et al., 2005). Cytotoxicity of glucocorticoid to lymphoid tumor cell lines was also linked to induction of BIM expression (Bachmann et al., 2005; Wang et al., 2003).
Based on the well-established feature of BH3-peptides of BH3-only proteins to bind to anti-apoptotic BCL-2 family proteins to induce apoptosis, it was hypothesized that BH3 peptides and non-peptide small-molecules that mimic BH3-peptide could be exploited as cancer drugs. Indeed, a search for non-peptide inhibitors of BCL-2, BCL-xL and MCL-1 resulted in the identification of several candidates (Enyedy et al., 2001; Kitada et al., 2003; Ponassi et al., 2008; Tang et al., 2007; Tzung et al., 2001). Intensive investigations on one of the compounds, ABT-737, demonstrated that as a single agent, ABT-737 effectively killed cells from several tumor types and caused regression of certain tumors in mice (Oltersdorf et al., 2005). Molecular analysis revealed that ABT-737 was similar to the BAD BH3-peptide in the binding preferences to BCL-2 family members - it targeted BCL-2, BCL-xL and BCL-w but not MCL-1 or A1 (Oltersdorf et al., 2005; van Delft et al., 2006). Another BH3 mimetic, GX15-070 bound to all five anti-apoptotic proteins (Nguyen et al., 2007; Shore and Viallet, 2005). GX15-070 is currently in multiple phase I and phase II clinical trials against solid tumors and hematologic malignancies (Fernandez-Luna, 2008; O’Brien et al., 2008). Importantly, design of new BH3-mimetics is being intensively pursued as a novel strategy for the development of new anti-cancer drugs. More comprehensive discussion on various BH3 peptidomimetics could be found in recent reviews (Fennell and Chacko, 2008; Vogler et al., 2008) and in the accompanying Chapter by Ni Chonghaile and Letai.
Role in rheumatoid arthritis and in heart failure
Rheumatoid arthritis (RA) is characterized by the excessive accumulation of inflammatory cells in joints. The increase in cellularity in RA joints appears to be results of reduced apoptosis. The fibroblast-like synoviocytes were shown to exhibit up-regulation of BCL-2 family anti-apoptosis proteins (Liu et al., 2006). BH3-only members BIM and BID were shown to protect against RA in the mouse model (Scatizzi et al., 2006; Scatizzi et al., 2007). BNIP3 was also up-regulated in synoviocytes from patients with RA, but its cell death activity appeared to be blocked (Kammouni et al., 2007). Considering the protective role of BIM and BID, in an accompanying Chapter Hutcheson and Perlman propose the use of BH3-mimetics for treatment of RA.
Heart failure as a result of ischemic and non-ischemic injuries is known to result in cardiomyocyte death. Based on studies with rodent models, two BH3-only members, BNIP3 and BNIP3L have been shown to play prominent roles in cardiomyocyte death during these injuries (reviewed by (Dorn and Diwan, 2008; Shaw and Kirshenbaum, 2008)). Expression of the dominant negative forms of BNIP3 and BNIP3L (lacking the respective trans-membrane domain) inhibited myocyte death (Regula et al., 2002; Yussman et al., 2002). Transduction of a cell permeable version of dominant negative BNIP3 (tagged with the HIV-1 Tat peptide) into whole heart conferred protection against ischemia/perfusion injury and improved cardiac function (Hamacher-Brady et al., 2007). Studies with the Bnip3−/− mouse model indicated that myocyte death following ischemia/perfusion was a delayed response (Diwan et al., 2007b). In an accompanying Chapter, Dorn and Kirshenbaum suggest that this delay could be exploited for rescue of myocytes from death using strategies to inhibit BNIP3 and BNIP3L.
Conclusions
Intense investigations on BH3-only family proteins during the past decade have been highly rewarding. This work has established their critical role in the initiation of apoptosis in animal cells. Although there has been a better understanding of their role in the apoptotic process, substantial aspects of their mechanism of action remain unresolved. In addition to their primary role as initiators of apoptosis, emerging evidence indicates that many of them also have a multitude of non-apoptotic functions. How their apoptotic vs non-apoptotic functions are differentially regulated and integrated will be an important area of future investigation. Studies on human cancers have revealed that they exert a restraining role during tumor progression. Anti-cancer drugs that stabilize the BH3-only proteins or transcriptionally activate their expression have shown significant promise against human cancers. Similarly, several small molecules that mimic the effect BH3-only proteins are showing promise as anti-cancer agents. It is anticipated that a second generation of such drugs will be forthcoming. These drugs may also find applications in treating inflammatory diseases such as arthritis. In contrast to these two classes of drugs that are intended to stimulate the activities of BH3-only molecules or mimic their effect to promote apoptosis, it could be envisioned that a third category of drugs that inhibit the activity of specific BH3-only molecules such as BNIP3 might be of great value in preventing pathological cell death as in heart failure. Such drugs might find applications in inhibiting BH3-only molecules in certain cancers where their expression is linked to poor prognosis. The availability of animal models for many BH3-only members offers excellent opportunity for further investigations on these important molecules.
Acknowledgments
G.C. received grant support from the National Cancer Institute grants CA-33616, CA-116262 and CA-73803. We thank John Tavis, Ling-jun Zhao and Carolyn Mulhall for reading the manuscript.
References
- Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. Embo J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena V, Martini M, Luongo M, Capelli A, Larocca LM. Mutations of the BIK gene in human peripheral B-cell lymphomas. Genes Chromosomes Cancer. 2003;38:91–96. doi: 10.1002/gcc.10245. [DOI] [PubMed] [Google Scholar]
- Bachmann PS, Gorman R, Mackenzie KL, Lutze-Mann L, Lock RB. Dexamethasone resistance in B-cell precursor childhood acute lymphoblastic leukemia occurs downstream of ligand-induced nuclear translocation of the glucocorticoid receptor. Blood. 2005;105:2519–2526. doi: 10.1182/blood-2004-05-2023. [DOI] [PubMed] [Google Scholar]
- Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- Bai L, Ni HM, Chen X, DiFrancesca D, Yin XM. Deletion of Bid impedes cell proliferation and hepatic carcinogenesis. Am J Pathol. 2005;166:1523–1532. doi: 10.1016/S0002-9440(10)62368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP, et al. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell. 2005;18:637–650. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Bessho T, Sancar A. Human DNA damage checkpoint protein hRAD9 is a 3′ to 5′ exonuclease. J Biol Chem. 2000;275:7451–7454. doi: 10.1074/jbc.275.11.7451. [DOI] [PubMed] [Google Scholar]
- Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P, Whyte MK. Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only gene product that promotes cell death. J Biol Chem. 2000;275:22136–22146. doi: 10.1074/jbc.M909572199. [DOI] [PubMed] [Google Scholar]
- Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Strasser A. BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- Boyd JM, Malstrom S, Subramanian T, Venkatesh LK, Schaeper U, Elangovan B, et al. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994;79:341–351. doi: 10.1016/0092-8674(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, et al. High-resolution genome-wide mapping of genetic alterations in human glial brain tumors. Cancer Res. 2005;65:4088–4096. doi: 10.1158/0008-5472.CAN-04-4229. [DOI] [PubMed] [Google Scholar]
- Broustas CG, Gokhale PC, Rahman A, Dritschilo A, Ahmad I, Kasid U. BRCC2, a novel BH3-like domain-containing protein, induces apoptosis in a caspase-dependent manner. J Biol Chem. 2004;279:26780–26788. doi: 10.1074/jbc.M400159200. [DOI] [PubMed] [Google Scholar]
- Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton TR, Henson ES, Baijal P, Eisenstat DD, Gibson SB. The pro-cell death Bcl-2 family member, BNIP3, is localized to the nucleus of human glial cells: Implications for glioblastoma multiforme tumor cell survival under hypoxia. Int J Cancer. 2006;118:1660–1669. doi: 10.1002/ijc.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, et al. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–9725. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Castells A, Ino Y, Louis DN, Ramesh V, Gusella JF, Rustgi AK. Mapping of a target region of allelic loss to a 0.5-cM interval on chromosome 22q13 in human colorectal cancer. Gastroenterology. 1999;117:831–837. doi: 10.1016/s0016-5085(99)70341-0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Chiang CW, Yang E. BAD/BCL-[X(L)] heterodimerization leads to bypass of G0/G1 arrest. Oncogene. 2001;20:4507–4518. doi: 10.1038/sj.onc.1204584. [DOI] [PubMed] [Google Scholar]
- Chen GG, Lai PB, Chak EC, Xu H, Lee KM, Lau WY. Immunohistochemical analysis of pro-apoptotic Bid level in chronic hepatitis, hepatocellular carcinoma and liver metastases. Cancer Lett. 2001;172:75–82. doi: 10.1016/s0304-3835(01)00630-9. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. Embo J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Claveria C, Torres M. Mitochondrial apoptotic pathways induced by Drosophila programmed cell death regulators. Biochem Biophys Res Commun. 2003;304:531–537. doi: 10.1016/s0006-291x(03)00626-0. [DOI] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Cristea IM, Degli Esposti M. Membrane lipids and cell death: an overview. Chem Phys Lipids. 2004;129:133–160. doi: 10.1016/j.chemphyslip.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Liu S, Marcucci G, Sadee W. 5-Aza-2′-deoxycytidine and depsipeptide synergistically induce expression of BIK (BCL2-interacting killer) Biochem Biophys Res Commun. 2006;351:455–461. doi: 10.1016/j.bbrc.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Denisov AY, Chen G, Sprules T, Moldoveanu T, Beauparlant P, Gehring K. Structural model of the BCL-w-BID peptide complex and its interactions with phospholipid micelles. Biochemistry. 2006;45:2250–2256. doi: 10.1021/bi052332s. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Diwan A, Koesters AG, Odley AM, Pushkaran S, Baines CP, Spike BT, et al. Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc Natl Acad Sci U S A. 2007a;104:6794–6799. doi: 10.1073/pnas.0610666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007b;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Diwan A. The rationale for cardiomyocyte resuscitation in myocardial salvage. J Mol Med. 2008;86:1085–1095. doi: 10.1007/s00109-008-0362-y. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan B, Chinnadurai G. Functional dissection of the pro-apoptotic protein Bik. Heterodimerization with anti-apoptosis proteins is insufficient for induction of cell death. J Biol Chem. 1997;272:24494–24498. doi: 10.1074/jbc.272.39.24494. [DOI] [PubMed] [Google Scholar]
- Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44:4313–4324. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- Esposti MD, Erler JT, Hickman JA, Dive C. Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Mol Cell Biol. 2001;21:7268–7276. doi: 10.1128/MCB.21.21.7268-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Huang S, Wu H, Zhang M. Molecular basis of Bcl-xL’s target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin-1. J Mol Biol. 2007;372:223–235. doi: 10.1016/j.jmb.2007.06.069. [DOI] [PubMed] [Google Scholar]
- Fennell DA, Chacko A. Exploiting BH3 only protein function for effective cancer therapy. Front Biosci. 2008;13:6682–6692. doi: 10.2741/3181. [DOI] [PubMed] [Google Scholar]
- Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- Fernandez-Luna JL. Regulation of pro-apoptotic BH3-only proteins and its contribution to cancer progression and chemoresistance. Cell Signal. 2008;20:1921–1926. doi: 10.1016/j.cellsig.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Fleischer A, Ayllon V, Dumoutier L, Renauld JC, Rebollo A. Proapoptotic activity of ITM2B(s), a BH3-only protein induced upon IL-2-deprivation which interacts with Bcl-2. Oncogene. 2002;21:3181–3189. doi: 10.1038/sj.onc.1205464. [DOI] [PubMed] [Google Scholar]
- Garcia N, Salamanca F, Astudillo-de la Vega H, Curiel-Quesada E, Alvarado I, Penaloza R, et al. A molecular analysis by gene expression profiling reveals Bik/NBK overexpression in sporadic breast tumor samples of Mexican females. BMC Cancer. 2005;5:93. doi: 10.1186/1471-2407-5-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Mingarro I, Perez-Paya E, Salgado J. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry. 2004;43:10930–10943. doi: 10.1021/bi036044c. [DOI] [PubMed] [Google Scholar]
- Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage--induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5:435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC, et al. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2004;10:5566–5571. doi: 10.1158/1078-0432.CCR-04-0076. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen B, Essmann F, Hemmati PG, Richter A, Oztop I, Chinnadurai G, et al. Mcl-1 determines the Bax dependency of Nbk/Bik-induced apoptosis. J Cell Biol. 2007;179:701–715. doi: 10.1083/jcb.200703040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg M, Sarig R, Zaltsman Y, Frumkin D, Grammatikakis N, Reuveny E, et al. tBID Homooligomerizes in the mitochondrial membrane to induce apoptosis. J Biol Chem. 2002;277:12237–12245. doi: 10.1074/jbc.M104893200. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Guo B, Godzik A, Reed JC. Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J Biol Chem. 2001;276:2780–2785. doi: 10.1074/jbc.M005889200. [DOI] [PubMed] [Google Scholar]
- Hacker G, Weber A. BH3-only proteins trigger cytochrome c release, but how? Arch Biochem Biophys. 2007;462:150–155. doi: 10.1016/j.abb.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Sabbatini P, White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol Cell Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- Hetz C, Glimcher L. The daily job of night killers: alternative roles of the BCL-2 family in organelle physiology. Trends Cell Biol. 2008;18:38–44. doi: 10.1016/j.tcb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Day CL. Regulation of apoptosis: uncovering the binding determinants. Curr Opin Struct Biol. 2005;15:690–699. doi: 10.1016/j.sbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Lin P, Hsueh AJ. BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members. Mol Endocrinol. 1998;12:1432–1440. doi: 10.1210/mend.12.9.0166. [DOI] [PubMed] [Google Scholar]
- Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JJ, Bond BL, Parslow TG. Functional dissection of the human Bc12 protein: sequence requirements for inhibition of apoptosis. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Bell DW, Dean KL, Coser KR, Hilario PC, Okimoto RA, et al. Regulation of expression of BIK proapoptotic protein in human breast cancer cells: p53-dependent induction of BIK mRNA by fulvestrant and proteasomal degradation of BIK protein. Cancer Res. 2006;66:10153–10161. doi: 10.1158/0008-5472.CAN-05-3696. [DOI] [PubMed] [Google Scholar]
- Hur J, Chesnes J, Coser KR, Lee RS, Geck P, Isselbacher KJ, et al. The Bik BH3-only protein is induced in estrogen-starved and antiestrogen-exposed breast cancer cells and provokes apoptosis. Proc Natl Acad Sci U S A. 2004;101:2351–2356. doi: 10.1073/pnas.0307337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K, Tsuda M, Imai Y, Wanaka A, Takagi T, Tohyama M. Molecular cloning of a novel polypeptide, DP5, induced during programmed neuronal death. J Biol Chem. 1997;272:18842–18848. doi: 10.1074/jbc.272.30.18842. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ding L, Chen S, Nunez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L) Embo J. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kammouni W, Wong K, Ma G, Firestein GS, Gibson SB, El-Gabalawy HS. Regulation of apoptosis in fibroblast-like synoviocytes by the hypoxia-induced Bcl-2 family member Bcl-2/adenovirus E1B 19-kd protein-interacting protein 3. Arthritis Rheum. 2007;56:2854–2863. doi: 10.1002/art.22853. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- Karst AM, Li G. BH3-only proteins in tumorigenesis and malignant melanoma. Cell Mol Life Sci. 2007;64:318–330. doi: 10.1007/s00018-006-6364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199:113–124. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, et al. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J Cell Biol. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K, Miyashita T, Hang H, Hopkins KM, Zheng W, Cuddeback S, et al. Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis. Nat Cell Biol. 2000;2:1–6. doi: 10.1038/71316. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Zapata JM, Meinhold-Heerlein I, Hedayat H, Monks A, Bettendorf H, et al. Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia. 2002;4:129–140. doi: 10.1038/sj.neo.7900222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku B, Woo JS, Liang C, Lee KH, Hong HS, EX, et al. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008;4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla G, Montessuit S, Eskes R, Berrier C, Martinou JC, Ghazi A, et al. The destabilization of lipid membranes induced by the C-terminal fragment of caspase 8-cleaved bid is inhibited by the N-terminal fragment. J Biol Chem. 2000;275:22713–22718. doi: 10.1074/jbc.M003807200. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lama D, Sankararamakrishnan R. Anti-apoptotic Bcl-X(L) protein in complex with BH3 peptides of pro-apoptotic Bak, Bad, and Bim proteins: Comparative molecular dynamics simulations. Proteins. 2008 doi: 10.1002/prot.22075. [DOI] [PubMed] [Google Scholar]
- Lanave C, Santamaria M, Saccone C. Comparative genomics: the evolutionary history of the Bcl-2 family. Gene. 2004;333:71–79. doi: 10.1016/j.gene.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Lee JH, Soung YH, Lee JW, Park WS, Kim SY, Cho YG, et al. Inactivating mutation of the pro-apoptotic gene BID in gastric cancer. J Pathol. 2004;202:439–445. doi: 10.1002/path.1532. [DOI] [PubMed] [Google Scholar]
- Lee SH, Soung YH, Lee JW, Kim HS, Lee JH, Park JY, et al. Mutational analysis of Noxa gene in human cancers. Apmis. 2003;111:599–604. doi: 10.1034/j.1600-0463.2003.1110602.x. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman HB, Hopkins KM, Nass M, Demetrick D, Davey S. A human homolog of the Schizosaccharomyces pombe rad9+ checkpoint control gene. Proc Natl Acad Sci U S A. 1996;93:13890–13895. doi: 10.1073/pnas.93.24.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Huang Q, Shi B, Eksarko P, Temkin V, Pope RM. Regulation of Mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006;54:3174–3181. doi: 10.1002/art.22132. [DOI] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003a;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity. 2003b;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lu H, Jiang Z, Pastuszyn A, Hu CA. Apolipoprotein l6, a novel proapoptotic Bcl-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol Cancer Res. 2005;3:21–31. [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Losonczi JA, Olejniczak ET, Betz SF, Harlan JE, Mack J, Fesik SW. NMR studies of the anti-apoptotic protein Bcl-xL in micelles. Biochemistry. 2000;39:11024–11033. doi: 10.1021/bi000919v. [DOI] [PubMed] [Google Scholar]
- Lu Y, Lemon W, Liu PY, Yi Y, Morrison C, Yang P, et al. A gene expression signature predicts survival of patients with stage I non-small cell lung cancer. PLoS Med. 2006;3:e467. doi: 10.1371/journal.pmed.0030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Ma C, Ying C, Yuan Z, Song B, Li D, Liu Y, et al. dp5/HRK is a c-Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. J Biol Chem. 2007;282:30901–30909. doi: 10.1074/jbc.M608694200. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007a;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. Embo J. 2007b;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V, Wang X, Bertrand R, Luo H, Duguid W, Chinnadurai G, et al. Proteasomes modulate balance among proapoptotic and antiapoptotic Bcl-2 family members and compromise functioning of the electron transport chain in leukemic cells. J Immunol. 2001;166:3130–3142. doi: 10.4049/jimmunol.166.5.3130. [DOI] [PubMed] [Google Scholar]
- Matsui H, Asou H, Inaba T. Cytokines direct the regulation of Bim mRNA stability by heat-shock cognate protein 70. Mol Cell. 2007;25:99–112. doi: 10.1016/j.molcel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Mestre-Escorihuela C, Rubio-Moscardo F, Richter JA, Siebert R, Climent J, Fresquet V, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2007;109:271–280. doi: 10.1182/blood-2006-06-026500. [DOI] [PubMed] [Google Scholar]
- Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 2008;15:1019–1029. doi: 10.1038/cdd.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Mok CL, Gil-Gomez G, Williams O, Coles M, Taga S, Tolaini M, et al. Bad can act as a key regulator of T cell apoptosis and T cell development. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. Expression and transcriptional regulation of functionally distinct Bmf isoforms in B-chronic lymphocytic leukemia cells. Leukemia. 2004;18:41–47. doi: 10.1038/sj.leu.2403183. [DOI] [PubMed] [Google Scholar]
- Mund T, Gewies A, Schoenfeld N, Bauer MK, Grimm S. Spike, a novel BH3-only protein, regulates apoptosis at the endoplasmic reticulum. Faseb J. 2003;17:696–698. doi: 10.1096/fj.02-0657fje. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Hirose H, Taniguchi M, Kurashina H, Arasaki K, Nagahama M, et al. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. Embo J. 2004;23:3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Ishida E, Shimada K, Nakase H, Sakaki T, Konishi N. Frequent HRK inactivation associated with low apoptotic index in secondary glioblastomas. Acta Neuropathol. 2005;110:402–410. doi: 10.1007/s00401-005-1065-x. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, et al. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15–070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- Obata T, Toyota M, Satoh A, Sasaki Y, Ogi K, Akino K, et al. Identification of HRK as a target of epigenetic inactivation in colorectal and gastric cancer. Clin Cancer Res. 2003;9:6410–6418. [PubMed] [Google Scholar]
- Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15–070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2008 doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. Embo J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Okami J, Simeone DM, Logsdon CD. Silencing of the hypoxia-inducible cell death protein BNIP3 in pancreatic cancer. Cancer Res. 2004;64:5338–5346. doi: 10.1158/0008-5472.CAN-04-0089. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeia C, Hodge DR, Plass C, Wu YZ, Marquez VE, Kelley JA, et al. Microarray analysis of epigenetic silencing of gene expression in the KAS-6/1 multiple myeloma cell line. Cancer Res. 2004;64:3465–3473. doi: 10.1158/0008-5472.CAN-03-3970. [DOI] [PubMed] [Google Scholar]
- Ponassi R, Biasotti B, Tomati V, Bruno S, Poggi A, Malacarne D, et al. A novel Bim-BH3-derived Bcl-XL inhibitor: biochemical characterization, in vitro, in vivo and ex-vivo anti-leukemic activity. Cell Cycle. 2008;7:3211–3224. doi: 10.4161/cc.7.20.6830. [DOI] [PubMed] [Google Scholar]
- Porzio O, Massa O, Cunsolo V, Colombo C, Malaponti M, Bertuzzi F, et al. Missense mutations in the TGM2 gene encoding transglutaminase 2 are found in patients with early-onset type 2 diabetes. Mutation in brief no. 982. Online. Hum Mutat. 2007;28:1150. doi: 10.1002/humu.9511. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashmi R, Pillai SG, Vijayalingam S, Ryerse J, Chinnadurai G. BH3-only protein BIK induces caspase-independent cell death with autophagic features in Bcl-2 null cells. Oncogene. 2008;27:1366–1375. doi: 10.1038/sj.onc.1210783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real PJ, Sanz C, Gutierrez O, Pipaon C, Zubiaga AM, Fernandez-Luna JL. Transcriptional activation of the proapoptotic bik gene by E2F proteins in cancer cells. FEBS Lett. 2006;580:5905–5909. doi: 10.1016/j.febslet.2006.08.088. [DOI] [PubMed] [Google Scholar]
- Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- Reis PP, Rogatto SR, Kowalski LP, Nishimoto IN, Montovani JC, Corpus G, et al. Quantitative real-time PCR identifies a critical region of deletion on 22q13 related to prognosis in oral cancer. Oncogene. 2002;21:6480–6487. doi: 10.1038/sj.onc.1205864. [DOI] [PubMed] [Google Scholar]
- Renshaw SA, Dempsey CE, Barnes FA, Bagstaff SM, Dower SK, Bingle CD, et al. Three novel Bid proteins generated by alternative splicing of the human Bid gene. J Biol Chem. 2004;279:2846–2855. doi: 10.1074/jbc.M309769200. [DOI] [PubMed] [Google Scholar]
- Rodolfo C, Mormone E, Matarrese P, Ciccosanti F, Farrace MG, Garofano E, et al. Tissue transglutaminase is a multifunctional BH3-only protein. J Biol Chem. 2004;279:54783–54792. doi: 10.1074/jbc.M410938200. [DOI] [PubMed] [Google Scholar]
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz C, Mellstrom B, Link WA, Naranjo JR, Fernandez-Luna JL. Interleukin 3-dependent activation of DREAM is involved in transcriptional silencing of the apoptotic Hrk gene in hematopoietic progenitor cells. Embo J. 2001;20:2286–2292. doi: 10.1093/emboj/20.9.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Scatizzi JC, Bickel E, Hutcheson J, Haines GK, 3rd, Perlman H. Bim deficiency leads to exacerbation and prolongation of joint inflammation in experimental arthritis. Arthritis Rheum. 2006;54:3182–3193. doi: 10.1002/art.22133. [DOI] [PubMed] [Google Scholar]
- Scatizzi JC, Hutcheson J, Bickel E, Haines GK, 3rd, Perlman H. Pro-apoptotic Bid is required for the resolution of the effector phase of inflammatory arthritis. Arthritis Res Ther. 2007;9:R49. doi: 10.1186/ar2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- Schmitz R, Thomas RK, Harttrampf AC, Wickenhauser C, Schultze JL, Hansmann ML, et al. The major subtypes of human B-cell lymphomas lack mutations in BCL-2 family member BAD. Int J Cancer. 2006;119:1738–1740. doi: 10.1002/ijc.22010. [DOI] [PubMed] [Google Scholar]
- Schmutte C, Tombline G, Rhiem K, Sadoff MM, Schmutzler R, von Deimling A, et al. Characterization of the human Rad51 genomic locus and examination of tumors with 15q14–15 loss of heterozygosity (LOH) Cancer Res. 1999;59:4564–4569. [PubMed] [Google Scholar]
- Schumacher B, Schertel C, Wittenburg N, Tuck S, Mitani S, Gartner A, et al. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ. 2005;12:153–161. doi: 10.1038/sj.cdd.4401539. [DOI] [PubMed] [Google Scholar]
- Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Seo YW, Shin JN, Ko KH, Cha JH, Park JY, Lee BR, et al. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J Biol Chem. 2003;278:48292–48299. doi: 10.1074/jbc.M308785200. [DOI] [PubMed] [Google Scholar]
- Shaw J, Kirshenbaum LA. Molecular regulation of autophagy and apoptosis during ischemic and non-ischemic cardiomyopathy. Autophagy. 2008;4:427–434. doi: 10.4161/auto.5901. [DOI] [PubMed] [Google Scholar]
- Shaw J, Yurkova N, Zhang T, Gang H, Aguilar F, Weidman D, et al. Antagonism of E2F-1 regulated Bnip3 transcription by NF-kappaB is essential for basal cell survival. Proc Natl Acad Sci U S A. 2008;105:20734–20739. doi: 10.1073/pnas.0807735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, et al. Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. Embo J. 2006;25:4952–4962. doi: 10.1038/sj.emboj.7601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Taniguchi T. BH3-only proteins: integrated control point of apoptosis. Int J Cancer. 2006;119:2036–2043. doi: 10.1002/ijc.21751. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Tsujimoto Y. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc Natl Acad Sci U S A. 2000;97:577–582. doi: 10.1073/pnas.97.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematology Am Soc Hematol Educ Program. 2005:226–230. doi: 10.1182/asheducation-2005.1.226. [DOI] [PubMed] [Google Scholar]
- Sinha S, Colbert CL, Becker N, Wei Y, Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–6673. [PubMed] [Google Scholar]
- St Onge RP, Udell CM, Casselman R, Davey S. The human G2 checkpoint control protein hRAD9 is a nuclear phosphoprotein that forms complexes with hRAD1 and hHUS1. Mol Biol Cell. 1999;10:1985–1995. doi: 10.1091/mbc.10.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohecker AM, Yehiely F, Chen F, Cryns VL. Caspase cleavage of HER-2 releases a Bad-like cell death effector. J Biol Chem. 2008;283:18269–18282. doi: 10.1074/jbc.M802156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm I, Stephan C, Gillissen B, Siebert R, Janz M, Radetzki S, et al. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 2006;13:619–627. doi: 10.1038/sj.cdd.4401782. [DOI] [PubMed] [Google Scholar]
- Subramanian T, Vijayalingam S, Lomonosova E, Zhao LJ, Chinnadurai G. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J Virol. 2007;81:10486–10495. doi: 10.1128/JVI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–1358. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- Tan KO, Tan KM, Chan SL, Yee KS, Bevort M, Ang KC, et al. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J Biol Chem. 2001;276:2802–2807. doi: 10.1074/jbc.M008955200. [DOI] [PubMed] [Google Scholar]
- Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Tang G, Ding K, Nikolovska-Coleska Z, Yang CY, Qiu S, Shangary S, et al. Structure-based design of flavonoid compounds as a new class of small-molecule inhibitors of the anti-apoptotic Bcl-2 proteins. J Med Chem. 2007;50:3163–3166. doi: 10.1021/jm070383c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Boil. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SC, Pasumarthi KB, Pajak L, Franklin M, Patton B, Wang H, et al. Simian virus 40 large T antigen binds a novel Bcl-2 homology domain 3-containing proapoptosis protein in the cytoplasm. J Biol Chem. 2000;275:3239–3246. doi: 10.1074/jbc.275.5.3239. [DOI] [PubMed] [Google Scholar]
- Tzung SP, Kim KM, Basanez G, Giedt CD, Simon J, Zimmerberg J, et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- UM, Miyashita T, Shikama Y, Tadokoro K, Yamada M. Molecular cloning and characterization of six novel isoforms of human Bim, a member of the proapoptotic Bcl-2 family. FEBS Lett. 2001;509:135–141. doi: 10.1016/s0014-5793(01)03145-3. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Zhao LJ, Chinnadurai G. Phosphorylation of the pro-apoptotic protein BIK: mapping of phosphorylation sites and effect on apoptosis. J Biol Chem. 2001;276:4671–4676. doi: 10.1074/jbc.M008983200. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a Novel Bcl-2 Homology Domain 3-only Lipid-binding Protein, Induces Autophagic Cell Death. J Biol Chem. 2008;283:21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278:23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sun Y. Identification and characterization of two splicing variants of human Noxa. Anticancer Res. 2008;28:1667–1674. [PubMed] [Google Scholar]
- Weber A, Paschen SA, Heger K, Wilfling F, Frankenberg T, Bauerschmitt H, et al. BimS-induced apoptosis requires mitochondrial localization but not interaction with anti-apoptotic Bcl-2 proteins. J Cell Biol. 2007;177:625–636. doi: 10.1083/jcb.200610148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E, et al. Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene. 1996;12:973–978. [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S, Erster S, Palacios G, Moll UM. p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008;18:733–744. doi: 10.1038/cr.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Xia W, Li Z, Kuo HP, Liu Y, Ding Q, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Kuwahara M, Suzuki A, Hirahara K, Shinnaksu R, Hosokawa H, et al. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J Exp Med. 2008;205:1109–1120. doi: 10.1084/jem.20072000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Gu L, Kokel D, Chai J, Li W, Han A, et al. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Mol Cell. 2004;15:999–1006. doi: 10.1016/j.molcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Komatsu K, Wang HG, Kufe D. c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage. Mol Cell Biol. 2002;22:3292–3300. doi: 10.1128/MCB.22.10.3292-3300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996a;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996b;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang H, Heim J, Meyhack B. Novel BNIP1 variants and their interaction with BCL2 family members. FEBS Lett. 1999;448:23–27. doi: 10.1016/s0014-5793(99)00335-x. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. 2000;275:25046–25051. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhang L, Dong F, Guo W, Wu S, Teraishi F, et al. Bik/NBK accumulation correlates with apoptosis-induction by bortezomib (PS-341, Velcade) and other proteasome inhibitors. Oncogene. 2005;24:4993–4999. doi: 10.1038/sj.onc.1208683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Zinkel SS, Ong CC, Ferguson DO, Iwasaki H, Akashi K, Bronson RT, et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]