Abstract
Membrane fusion and fission in intracellular trafficking is controlled by both intraluminal Ca2+ release and phosphoinositide signaling. However, the molecular identities of the Ca2+ release channels and the target proteins of phosphoinositides are elusive. Here, by direct patch-clamping of the endolysosomal membrane, we report that PI(3,5)P2, an endolysosome-specific phosphoinositide, binds and activates endolysosome-localized mucolipin TRP (TRPML) channels with specificity and potency. Both PI(3,5)P2-deficient cells and cells that lack TRPML1 exhibited enlarged endolysosomes/vacuoles and trafficking defects in the late endocytic pathway. We find that the enlarged vacuole phenotype observed in PI(3,5)P2-deficient mouse fibroblasts is suppressed by overexpression of TRPML1. Notably, this PI(3,5)P2-dependent regulation of TRPML1 is evolutionarily conserved. In budding yeast, hyperosmotic stress induces Ca2+ release from the vacuole. Here, we show that this release requires both PI(3,5)P2 production and a yeast functional TRPML homolog. We propose that TRPMLs regulate membrane trafficking by transducing information about PI(3,5)P2 levels into changes in juxtaorganellar Ca2+, thereby triggering membrane fusion/fission events.
Keywords: Whole-endolysosome recording; TRP channel; Ca2+ release channel; PIKfyve; Fab1; phosphoinositide; PI(3,5)P2; type IV Mucolipidosis; endosome; lysosome; membrane trafficking; vacuole
Introduction
Ca2+ is a key regulator of synaptic vesicle fusion during neurotransmission. Similarly, Ca2+ is thought to regulate other, more general membrane trafficking pathways 1. However, in these cases, the source of Ca2+ is unknown. For these general pathways, it has been postulated that Ca2+ is released through unidentified Ca2+ channels from the lumen of vesicles and organelles 1–3. Transient receptor potential (TRP) proteins are a superfamily of Ca2+-permeable cation channels that are localized at the plasma membrane and/or membranes of intracellular organelles 4. For example, mucolipin TRPs (TRPML1-3) are localized in the membranes of endosomes and lysosomes (collectively endolysosomes) 5–6. Mutations in the human TRPML1 gene cause mucolipidosis type IV (ML4) neurodegenerative disease 7–8. Cells that lack TRPML1 exhibit enlarged endolysosomes and trafficking defects in the late endocytic pathway (reviewed in Refs. 5–6). TRPMLs are, therefore, natural candidate channels for Ca2+ release in the endolysosome.
PI(3,5)P2 is a low-abundance endolysosome-specific phosphoinositide 9–12. PI(3,5)P2 can be generated from PI(3)P through PIKfyve/Fab1, a PI 5-kinase that is localized in the endolysosome of both yeast and mammalian cells 11,13–15. The activity of PIKfyve/Fab1 can be positively regulated by several associated proteins such as Fig4, Vac14, and Vac7 10–12,16. On the other hand, PI(3,5)P2 can be metabolized into PI(5)P through the myotubularin (MTM/MTMR)-family of PI-3 phosphatase 11,15,17. Human mutations in PI(3,5)P2-metabolizing enzymes and their regulators cause a variety of neurodegenerative diseases including amyotrophic lateral sclerosis (ALS) and Charcot-Marie-Tooth (CMT) disease 10–11,18. At the cellular level, PI(3,5)P2-deficient cells reportedly exhibit enlarged endolysosomes/vacuoles and trafficking defects in the endocytic pathways 10–12,16.
Because the cellular phenotypes in PI(3,5)P2-deficient cells are similar to those observed in cells lacking TRPML1, we hypothesized that TRPML1 may act as an endolysosomal Ca2+-release channel that is regulated by PI(3,5)P2. In this study, by direct patch-clamping of the endolysosomal membrane, we have found that PI(3,5)P2 activates TRPMLs with striking specificity and potency. Protein-lipid interaction and mutational analyses revealed that PI(3,5)P2 binds directly to the N-terminus of TRPML1. Overexpression of TRPML1 suppresses the enlarged vacuole phenotype observed in PI(3,5)P2-deficient cells. We conclude that PI(3,5)P2 controls endolysosomal membrane trafficking by regulating TRPML channels to change juxtaorganellar Ca2+ levels.
Results
Activation of endolysosomal TRPML channels by PI(3,5)P2
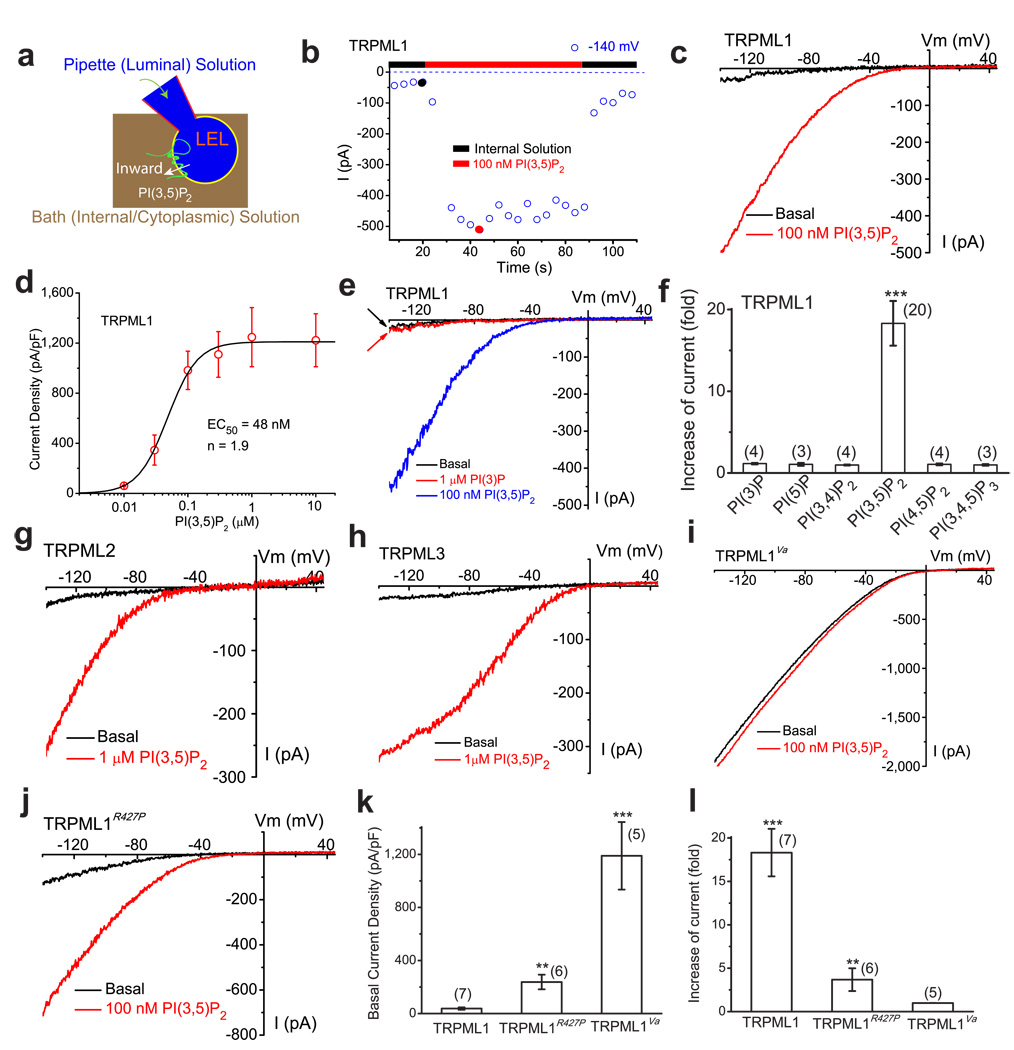
TRPML1 is primarily localized on membranes of late endosomes and lysosomes (LELs) 5,19 (see Supplementary Fig. S1), which are inaccessible to conventional electrophysiological approaches. Using our recently-established modified patch-clamp method 4,20, we performed recordings directly on native LEL membranes. HEK293T or Cos-1 cells were transfected with either EGFP-TRPML1 alone, or co-transfected with mCherry-TRPML1 and EGFP-Lamp1 (a marker for LEL). Whole-endolysosome recordings (see Fig. 1a; note that the inward current indicates cations flowing out of the endolysosome) were performed on enlarged vacuoles manually isolated from cells pre-treated with vacuolin-1 21, which caused an increase in the diameter of the vacuoles from < 0.5 µm to up to 5 µm (mean capacitance = 0.68 ± 0.05 pF, N = 44 vacuoles). The majority (>85%) of mCherry-TRPML1-positive vacuoles were also EGFP-Lamp1-positive, confirming that the TRPML1-positive vacuoles were enlarged LELs 21. In the TRPML1-positive enlarged LELs, small basal inwardly rectifying currents (72 ± 12 pA/pF at −140 mV, N = 65 vacuoles) were seen under the whole-endolysosome configuration (Fig. 1b, 1c). Bath application of 100 nM PI(3,5)P2 in a water-soluble diC8 form, rapidly and dramatically activated TRPML1-mediated current (ITRPML1; τ= 15 ± 4 s at −140 mV, N = 8 vacuoles; 18.3 ± 2.7-fold increase of basal activity, N = 20 vacuoles) (Fig. 1b–f ). PI(3,5)P2-dependent activation was dose-dependent (EC50= 48 ± 14 nM, Hill slope (n) = 1.9, N = 7 vacuoles). On average, ITRPML1 in the presence of 100 nM diC8 PI(3,5)P2 was 982 ± 150 pA/pF at −140 mV (N = 23 vacuoles). Full-length diC16 PI(3,5)P2 appeared to be less effective, presumably because of its low aqueous solubility (Supplementary Fig. S2). Therefore, diC8 PI(3,5)P2 was used for electrophysiological studies hereafter.
Figure 1. PI(3,5)P2 activates recombinant TRPML channels in the endolysosomal membranes.
a) Illustration of a whole-endolysosome recording configuration. Pipette (luminal) solution was a standard external (Tyrode’s) solution adjusted to pH 4.6 to mimic the acidic environment of the lysosome lumen. Bath (internal/cytoplasmic) solution was a K+-based solution (140 mM K+-gluconate). Putative membrane topology of TRPML channels is illustrated in the late endosome and lysosome (LEL). Note that the inward current indicates cations flowing out of the endolysosome (see red arrow for the direction). b) Bath application of PI(3,5)P2 (diC8, 100 nM) activated inwardly rectifying whole-endolysosome TRPML1-mediated current (ITRPML1) in an enlarged endolysosome/vacuole from a TRPML1-EGFP-expressing Cos-1 cell that was pre-treated with vacuolin-1. ITRPML1 was elicited by repeated voltage ramps (−140 to +140 mV; 400 ms) with a 4-s interval between ramps. ITRPML1 exhibited a small basal current prior to PI(3,5)P2 application; bath application of PI(3,5)P2 to the cytoplasmic side of the endolysosome resulted in maximal activation of 18-fold of baseline within a minute, measured at −140 mV of ITRPML1. c) Representative traces of ITRPML1 before (black) and after (red) PI(3,5)P2 at two time points, as shown in a (black and red circles). Only a portion of the voltage protocol is shown; holding potential = 0 mV. d) Dose-dependence of PI(3,5)P2-dependent activation (EC50 = 48 nM, n = 1.9). e) PI(3)P (1 µM) failed to activate ITRPML1. f) Specific activation of TRPML1 by PI(3,5)P2 ( in 100 nM), but not other diC8 PIPs (all in 1 µM). g) Activation of whole-endolysosome ITRPML2 by PI(3,5)P2 (1 µM). h) Activation of whole-endolysosome ITRPML3 by PI(3,5)P2 (1 µM). i) Whole-endolysosome ITRPML1-Va exhibited high basal activity but was insensitive to PI(3,5)P2 (100 nM). j) Whole-endolysosome ITRPML1-R427P was weakly activated by PI(3,5)P2. k) Basal current amplitudes of whole-endolysosome ITRPML1, ITRPML1-R427P, and ITRPML1-Va. l) Effects of PI(3,5)P2 on ITRPML1, ITRPML1-R427P, and ITRPML1-Va. For histogram graphs of all figures including panels (f, k, l) of this figure, data are presented as the mean ± standard error of the mean (SEM); the n numbers are in parentheses. Statistical comparisons were made using analysis of variance (ANOVA): P value < 0.05 was considered statistically significant and indicated with asterisks (*, 0.01< P < 0.05; **, P < 0.01; ***, P < 0.001).
In yeast, PI(3,5)P2 is exclusively produced from PI(3)P by the PIKfyve/Fab1 PI 5-kinase (see Supplementary Fig. S1) 9,22–23. PI(3,5)P2 can be quickly metabolized into PI(3)P by Fig4, or to PI(5)P by MTMR-family phosphatases 11,13,17,24. Neither PI(3)P (1 µM; 1.05 ± 0.17 fold increase of basal, N = 4; Fig. 1e, 1f) nor PI(5)P (1 µM; 0.98 ± 0.05 fold increase of basal, N=3; Fig. 1f) activated ITRPML1. PI(3)P and PI(3,5)P2 are localized in the endolysosome system. Other PIPs such as PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3, are localized in the plasma membrane, or in other intracellular organelles, and are sequestered from endolysosomes 15. ITRPML1 was not activated by these other PIPs (Fig. 1f & Supplementary Fig. S3). Thus, PI(3,5)P2 activated ITRPML1 with a striking specificity. TRPML2 and TRPML3 are also localized in the endolysosome 5. PI(3,5)P2, but not PI(3)P (data not shown) activated whole-endolysosome ITRPML2 (Fig. 1g) and ITRPML3 (Fig. 1h). Since PI(3,5)P2 and TRPMLs are both primarily localized in the LEL (Refs. 5, 11; see Supplementary Fig. S4), the insensitivity of ITRPML1 to PI(3)P or PI(5)P, and its robust activation by PI(3,5)P2 suggested that TRPML1 might be acutely regulated by the activities of PIKfyve/Fab1, or by Fig4 or MTMR phosphatases in the LEL.
PI(3,5)P2-mediated activation gating of TRPML1
To explore the mechanisms underlying PI(3,5)P2-dependent activation of TRPMLs, we tested the effect of PI(3,5)P2 on several gain-of-function mutations in TRPML1 channels 20. Recently, we and others found that a spontaneous mutation (Varitint Waddler; Va) in the 5th transmembrane domain of TRPML3 dramatically increased TRPML3 channel activity (Refs. 5–6). Further analysis suggested that Va is a gating mutation that locks TRPML1-3 channels in an open non-gating state 5–6. In contrast with ITRPML1, whole-endolysosome ITRPML1-Va exhibited large basal currents (1,190 ± 254 pA/pF at −140 mV, N = 5), that failed to increase with PI(3,5)P2 application (0.99 ± 0.01 of basal, N = 3; Fig. 1i, 1k, 1l). TRPML1-R427P is a partial gain-of-function mutation 20. Whole-endolysosome ITRPML1-R427P exhibited a substantial basal current (349 ± 80 pA/pF at −140 mV, N = 3), but was only modestly activated by PI(3,5)P2, at 3.7 ± 1.3 fold of basal activity (N = 7) (Fig. 1j–l). Collectively, these results suggested that PI(3,5)P2 activated TRPML1 by increasing the open probability.
Activation of endogenous TRPML-like currents by PI(3,5)P2
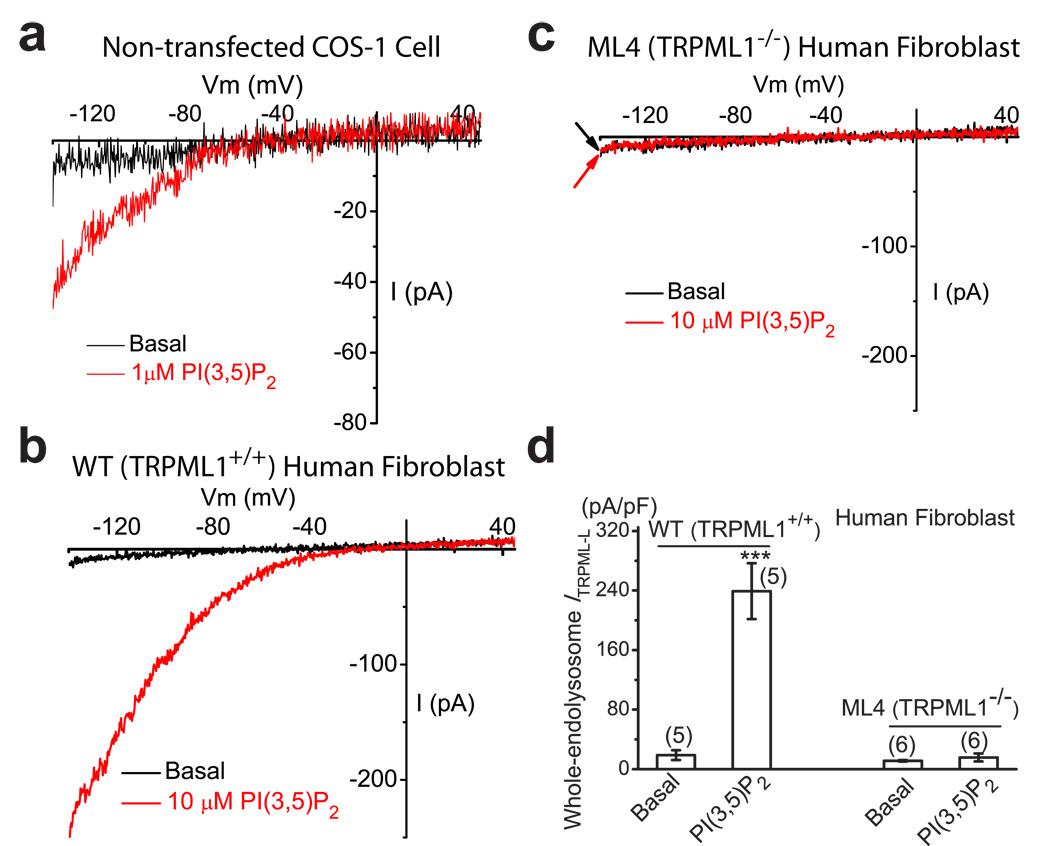
Next, we investigated whether PI(3,5)P2 activated endogenous TRPMLs. Using enlarged LELs isolated from non-transfected Cos-1 cells, we were able to record PI(3,5)P2–activated, inwardly rectifying TRPML-like currents (ITRPML-L) in 4 out of 20 vacuoles (Fig. 2a). ITRPML-L was 68 ± 4 pA/pF at −140 mV (N = 4). In enlarged LELs isolated from wild-type (TRPML1+/+) human fibroblast cells, however, PI(3,5)P2–activated ITRPML-L was detected in 5 of 5 tested vacuoles (Fig. 2b), with an average current amplitude larger than Cos-1 cells (239 ± 37 pA/pF at −140 mV, N = 5; Fig. 2d). In contrast, enlarged LELs from human ML4 (TRPML1−/− or abbreviated as ML1−/−) fibroblasts showed no significant PI(3,5)P2–activated ITRPML-L (15.7 ± 5.2 pA/pF at −140 mV, N = 6; Fig. 2c, 2d). These results indicated that PI(3,5)P2 activated endogenous TRPMLs, and that TRPML1 was the primary or sole functional TRPML channel in the endolysosome of human fibroblast, although a previous study reported the expression of TRPML2 and TRPML3 in human fibroblasts 25.
Figure 2. PI(3,5)P2 activates endogenous TRPML-like currents in the endolysosomal membranes.
a) An endogenous inwardly rectifying TRPML-like current (ITRPML1-L) activated by PI(3,5)P2 (10 µM) in a vacuole isolated from a non-transfected Cos-1 cell. b) Large PI(3,5)P2-activated ITRPML1-L in a vacuole isolated from wild type (WT) human skin fibroblast cell. c) Lack of significant PI(3,5)P2-activated ITRPML1-L in a vacuole isolated from a human ML4 (TRPML1−/−) skin fibroblast cell. d) Endogenous PI(3,5)P2-activated ITRPML-L in WT and TRPML1−/− human fibroblasts. For statistical analysis: ***, P < 0.001; NS (non-significant), P > 0.05).
Suppression of ITRPML1 by a decrease in PI(3,5)P2
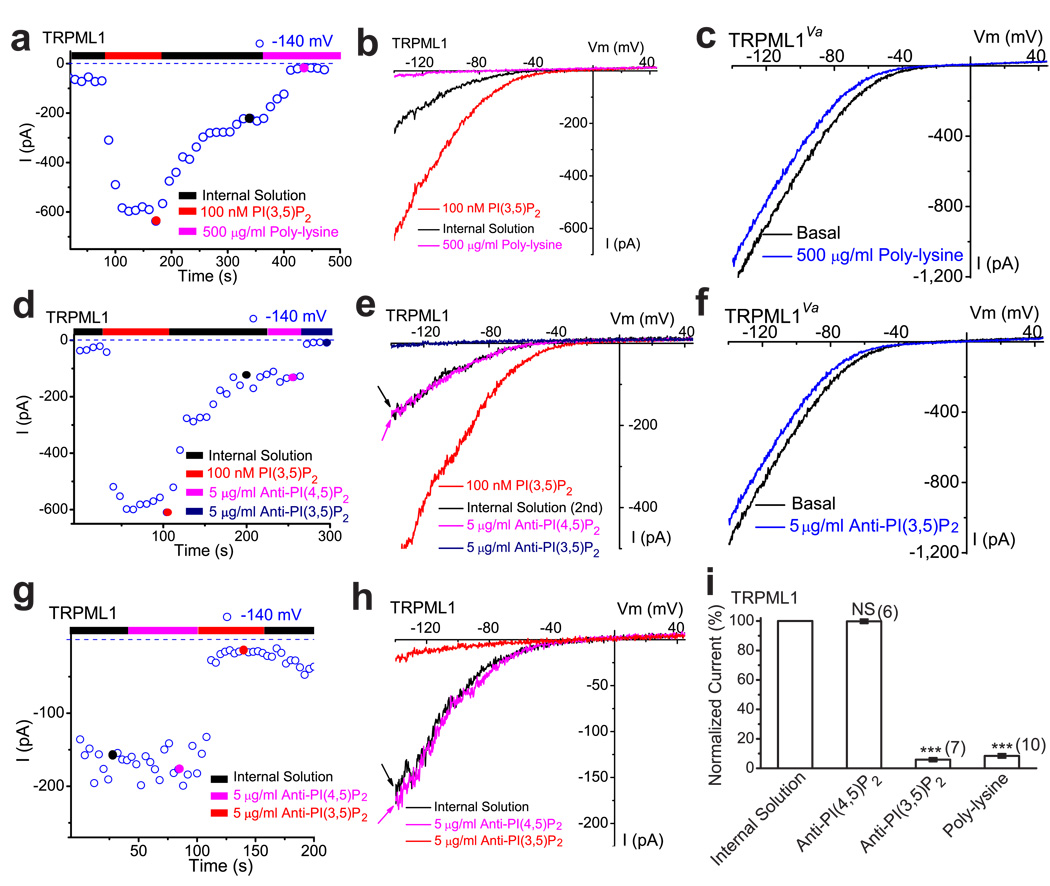
Whole-endolysosome ITRPML1 exhibited a decay or slow return to steady-state levels upon washout of diC8 PI(3,5)P2 (Fig. 3a). If PI(3,5)P2 levels underlie the basal pre-PI(3,5)P2, or the post-PI(3,5)P2 activity of TRPML1, diminishing the PI(3,5)P2 level would be expected to decrease the ITRPML1. One method of “chelating” PIPs is adding polycations such as poly-lysine to the cytoplasmic side of the membrane, where they bind the negatively-charged phosphate head groups of PIPs in an electrostatic manner 26–27. Poly-lysine at 500 µg /ml rapidly inhibited post-PI(3,5)P2 ITRPML1, but not ITRPML1-Va (Fig. 3a–c), indicating that poly-lysine primarily affected TRPML1 (activation) gating. In support, a neutralizing anti- PI(3,5)P2 antibody at 5 µg /ml significantly inhibited post-PI(3,5)P2 ITRPML1, but not ITRPML1-Va (Fig. 3d–f). In contrast, no significant inhibition was seen with an anti-PI(4,5)P2 antibody (Fig. 3d, 3e). To further investigate whether the basal ITRPML1 prior to PI(3,5)P2 treatment was caused by basal levels of PI(3,5)P2 in the endolysosomal membrane, we investigated a subset of vacuoles with large basal currents of > 100 pA at −140 mV. Strong inhibitions were seen with poly-lysine (92 ± 1%, N = 7) or anti-PI(3,5)P2 antibody (94 ± 1%, N = 10), but not with anti-PI(4,5)P2 antibody (3 ± 1%, N = 4; Fig. 3g–i).
Figure 3. A decrease in PI(3,5)P2 by chelating agents suppresses TRPML1 channel activity in the endolysosomal membrane.
a) Post- PI(3,5)P2 quasi-steady state ITRPML1 was inhibited by bath (internal/cytoplasmic) application of poly-lysine (500 µg/ml) to an enlarged endolysosome from a TRPML1-EGFP-expressing Cos-1 cell. ITRPML1 increased with addition of 100 nM PI(3,5)P2, but gradually reduced to a quasi-steady state level upon washout. b) Representative traces of ITRPML1 at three time points, as shown in a: upon PI(3,5)P2 application (red), washout (black), and poly-lysine application (magenta). c) Lack of poly-lysine (500 µg/ml) effect on whole-endolysosome ITRPML1-Va. d) Post- PI(3,5)P2 quasi-steady state ITRPML1 was inhibited by bath application of neutralizing anti-PI(3,5)P2(5 µg/ml), but not anti-PI(4,5)P2 (5 µg/ml). e) Representative traces of ITRPML1 at three time points, as shown in d. f) Lack of anti- PI(3,5)P2 (5 µg/ml) effect on whole-endolysosome ITRPML1-Va. g, h) Large basal pre- PI(3,5)P2 ITRPML1 was inhibited by bath application of neutralizing anti-PI(3,5)P2 (5 µg/ml), but not anti-PI(4,5)P2. i) ITRPML1 was inhibited by more than 90% by bath (internal/cytoplasmic) application of poly-lysine (500 µg/ml) or PI(3,5)P2 antibody.
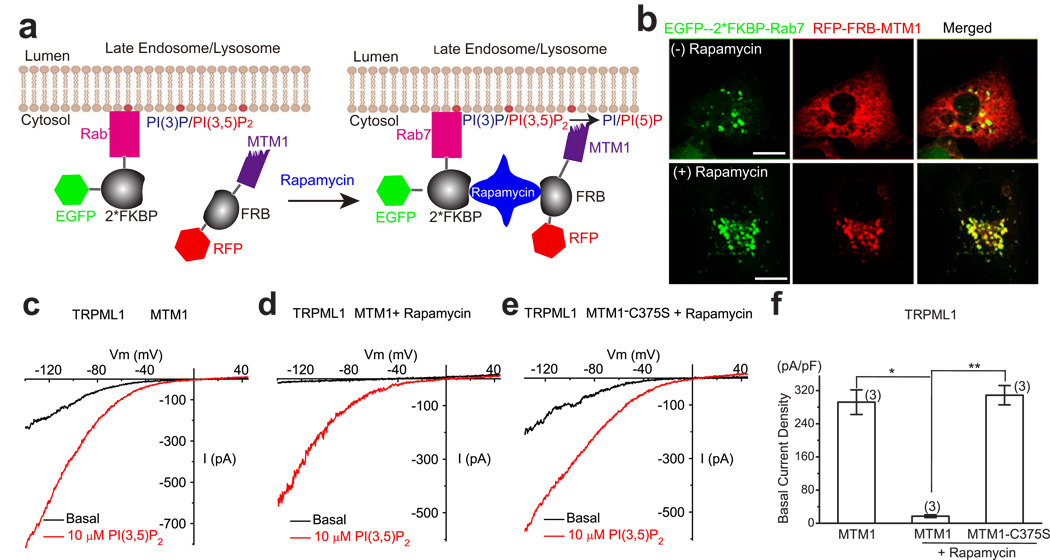
To further confirm that PI(3,5)P2 is an endogenous activator of TRPML1, we also recorded basal ITRPML1 after depleting the PI(3,5)P2 level by overexpressing MTM1, a PI-3 phosphatase that can convert PI(3,5)P2 and PI(3)P into PI(5)P and PI, respectively 11,15. A rapamycin-dependent heterodimerization system was used to recruit the otherwise cytosolic MTM1 28(see Fig. 4a, 4b). In cells expressing both RFP-FRB-MTM1 and EGFP-2*FKBP-Rab7, rapamycin induced a rapid recruitment of MTM1 to Rab7-positive LEL membranes (Fig. 4b). We noticed that the basal ITRPML1was consistently larger for vacuoles isolated from Cos-1 cells with longer (> 5h) pretreatment of vacuolin-1 (data not shown). In vacuoles isolated from MTM1-transfected cell, large basal ITRPML1 was seen before rapamycin treatment (Fig. 4c). Following recruitment of MTM1 (Fig. 4d), but not the inactive mutant (C375S) MTM1 (Fig. 4e), however, a large suppression of basal whole-endolysosome ITRPML1 was seen (Fig. 4d–f). Collectively, these results suggested that PI(3,5)P2 levels were the primary determinant of TRPML1 channel activity in the endolysosome.
Figure 4. A decrease in PI(3,5)P2 level by a translocatable lipid phosphatase suppresses TRPML1 channel activity in the endolysosomal membrane.
a) Recruitment of MTM1 to endolysosomal membranes by rapamycin-dependent heterodimerization of RFP-FRB-MTM1 and EGFP-2*FKBP-Rab7. Rab7 is a LEL-specific Rab protein. MTM1 is a PI-3 phosphatase that can convert PI(3,5)P2 and PI(3)P into PI(5)P and PI, respectively. b) Rapamycin-dependent heterodimerization of RFP-FRB-MTM1 and EGFP-2*FKBP-Rab7 alters subcellular localization of MTM1. Cos-1 cells were transfected with both RFP-FRB-MTM1 and EGFP-2*FKBP-Rab7. Rapamycin (500 nM; 20 min) treatment promotes co-localization of MTM1-RFP with Rab7-EGFP. Scale Bar = 10 µm. c-f) The effects of MTM1 on ITRPML1. Cos-1 cells were co-transfected with human TRPML1-myc, RFP-FRB-MTM1 or RFP-FRB-MTM1-C375S, and EGFP-2*FKBP-Rab7. MTM1 was recruited to LEL membranes by rapamycin (500 nM) -dependent heterodimerization of RFP-FRB-MTM1 and EGFP-2*FKBP-Rab7. c) ITRPML1 in MTM1-transfected cells before rapamycin treatment. d) ITRPML1 in MTM1-transfected cells after rapamycin treatment. e) ITRPML1 in MTM1-C357S-transfected cells after rapamycin treatment. f) Differential effects of WT and inactive mutant (C375S) MTM1 on basal whole-endolysosome ITRPML1 For statistical analysis: *, 0.01 < P < 0.05; **, P < 0.01).
Binding of PI(3,5)P2 to the N-terminus of TRPML1 in vitro
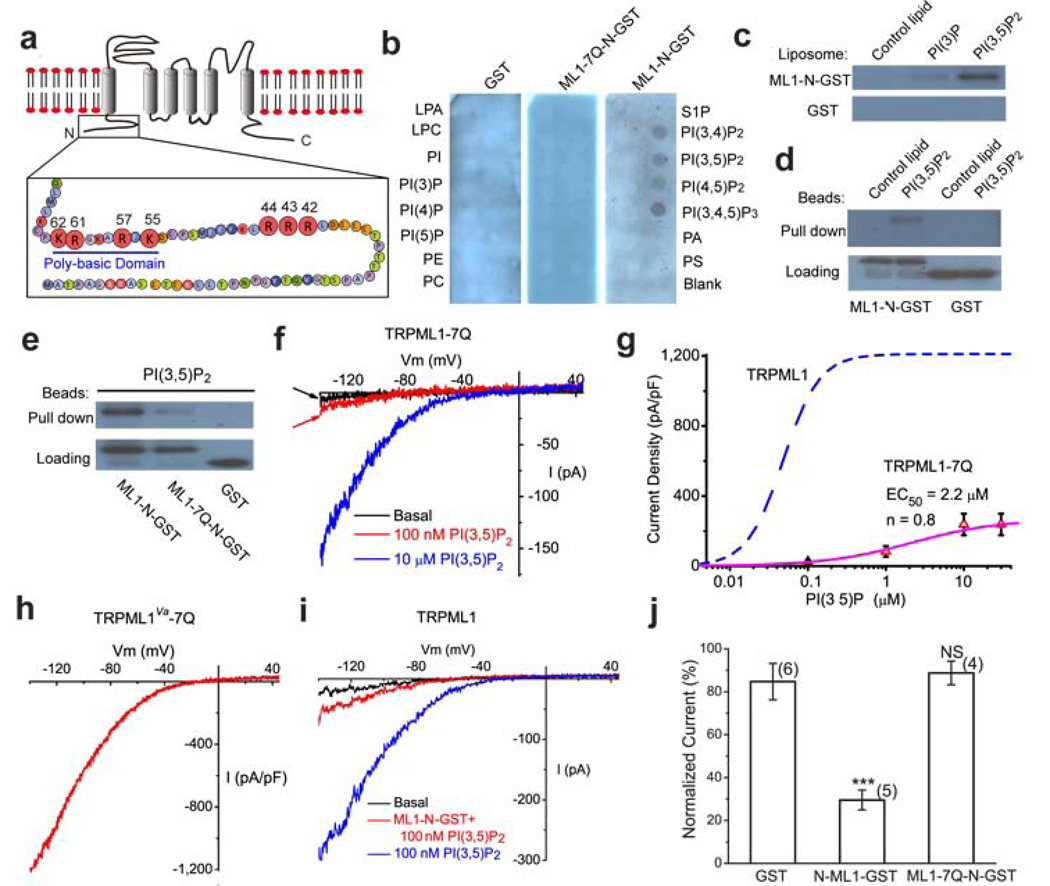
Phosphoinoitides are known to bind with high affinity to PI-binding modules such as PH or FYVE domain, or to poly-basic region with unstructured clusters of positively-charged amino acid residues, such as Arg and Lys, in an electrostatic manner 27. PI(4,5)P2 can bind directly to the cytoplasmic N- and C- termini of several plasma membrane TRPs 26,29. Notably, the intracellular N terminus of TRPML1 has a poly-basic region 26 (see Fig. 5a). To test whether PI(3,5)P2 binds directly to TRPML1, we fused GST to the entire N-terminus of mouse TRPML1, up to residue 69 (see Fig. 5a for putative membrane topology). The protein, GST-TRPML1-N (abbreviated as GST-ML1-N), was used to probe phosphoinositides immobilized on a nitrocellulose membranes 26–27,29. GST-ML1-N, but not GST alone, bound to PIP3 and PIP2s, but not to other PIPs or phospholipids (Fig. 5b). A liposome assay showed that GST-ML1-N strongly associated with PI(3,5)P2, but not PI(3)P or control liposomes (Fig. 5c). In a pull-down assay, agarose beads conjugated with PI(3,5)P2, but not control lipids, pulled down GST-ML1-N (Fig. 5d). Taken together, these results suggested that PI(3,5)P2 bound directly in vitro to the cytoplasmic N-terminus of TRPML1.
Figure 5. Direct binding of PI(3,5)P2 to the TRPML1 N-terminus requires multiple positively-charged amino acid residues.
a) The cytoplasmic N-terminus of TRPML1 contains a poly-basic region and clusters of positively charged amino acid residues as potential PI(3,5)P2 binding sites. The positively charged amino acid residues (Arg and Lys) that were mutated into neutral amino acids Gln (Q) in this study are shown with enlarged circles and their amino acid residue numbers. b) Protein-lipid overlays. The strip contained 15 different types of lipids: PA, phosphatidic acid; S1P, sphingosine-1-phosphate. Three purified proteins were used to probe the strip: GST alone (left panel), GST-fused to the N-terminal fragment of TRPML1 (ML1-N-GST; right panel), and Gln-substituted mutant of ML1-N-GST (ML1-7Q-N-GST; middle). Proteins were detected with anti-GST antibodies. c) Liposome pull-down assay. Liposomes were incubated with purified GST-fusion proteins, centrifuged, and associated proteins visualized by Western blot with GST antibodies. d) Binding of GST-ML1-N to agarose beads conjugated to PI(3,5)P2, but not control lipids; GST alone failed to pull down PI(3,5)P2-conjugated beads. e) Compared to GST-ML1-N, GST-ML1-7Q-N exhibited significantly weaker binding to PI(3,5)P2-conjugated agarose beads. f) Whole-endolysosome ITRPML1- 7Q was weakly activated by high concentrations of PI(3,5)P2. g) PI(3,5)P2 dose dependence of ITRPML1- 7Q. Dotted line indicates the dose dependence of ITRPML1 (repotted from Fig. 1d). h) Large basal whole-endolysosome ITRPML1-Va-7Q. Charge-removing Gln substitutions (7Q) were introduced into the gain-of-function Va background. i) GST-ML1-N peptide (5 µg/ml) reduced PI(3,5)P2-dependent activation of whole-endolysosome ITRPML1·. j) Charge-removing Gln-substituted substitutions (7Q) abolished the inhibitory effect of GST-ML1-N peptide.
Critical amino-acid residues in PI(3,5)P2 binding
To further map the PI(3,5)P2 binding sites, we systematically replaced positively-charged amino acid residues (Arg and Lys) within and adjacent to the poly-basic region with non-charged Gln residues and assayed Gln-substituted, purified GST-fusion proteins for PI(3,5)P2 binding. We also tested whether PI(3,5)P2 activated Gln-substituted TRPML1 channels using whole-endolysosome recordings. A most dramatic decrease in PI(3,5)P2 binding and activation (Fig. 5e–g) was observed in a 7Q mutant, with seven substitutions (R42Q/R43Q/R44Q/K55Q/R57Q/R61Q/K62Q; Fig. 5a). In contrast to GST-ML1-N, GST-ML1-7Q-N failed to bind significantly to PI(3,5)P2 or to other PIPs in the PIP strip and pull down assays (Fig. 5b, 5e). Considering the specificity of PI(3,5)P2 for TRPML1 activation, one plausible explanation for the apparent discrepancy between our biochemical assays (see Fig. 5b) and electrophysiological measurements (see Fig. 1f) is that the purified GST-ML1-N protein fragment did not recapitulate the specificity of PIP-binding of full length TRPML1 in the endolysosomal membrane. Nevertheless, the binding affinity of ML1-N to PI(3,5)P2 was dramatically reduced by removing the charges with the 7Q mutations (Fig. 5e), suggesting that multiple positively charged amino acid residues are critical for PI(3,5)P2 binding.
Compared to wild type (WT) TRPML1, TRPML1-7Q was only weakly activated by high concentrations of PI(3,5)P2, with a maximal response (efficacy) that was approximately 20% of ITRPML1 (EC50= 2.2 ± 2 µM, Hill slope (n) = 0.8, N = 6 vacuoles) (Fig. 5f, 5g). Like TRPML1Va, TRPML1Va−7Q also exhibited large basal currents (Fig. 5h), suggesting a relatively specific effect of 7Q mutations on PI(3,5)P2-dependent activation. Consistent with the PI(3,5)P2 binding-assay results, we found that GST-ML1-N, but not GST-ML1-7Q-N, competitively reduced the activation effect of PI(3,5)P2 (Fig. 5i, 5j). Collectively, our results suggested that PI(3,5)P2 bound directly to the cytoplasmic N-terminus of TRPML1, resulting in conformational changes that favor opening of TRPML1.
PI(3,5)P2 in vacuolar Ca2+ release in yeast
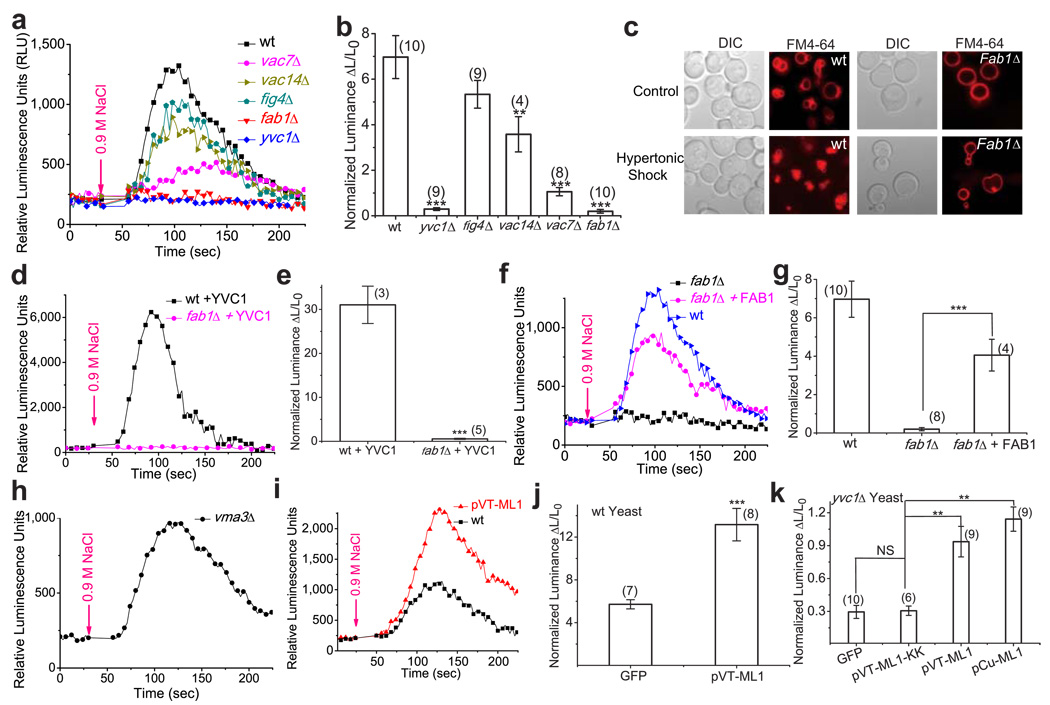
TRPML1 is a Ca2+-permeable channel 30–31, so activation by PI(3,5)P2 may lead to Ca2+ release from endolysosomes. Although PIKfyve/Fab1 is a PI 5-kinase responsible for PI(3,5)P2 generation in both yeast and mammalian cells 11,13–15, the extracellular signals that lead to activation of PIKfyve in mammalian cells have not been identified. Furthermore, endolysosomal Ca2+ release has been difficult to detect in mammalian cells because of the small size of the endolysosome 5,32. In contrast, Fab1-mediated PI(3,5)P2 production has been well documented in yeast 11,13. For example, hyperosmotic shock increases PI(3,5)P2 levels more than 20-fold within a few minutes 11,13. Interestingly, hyperosmotic shock is also known to induce Ca2+ release from yeast vacuoles, which share many similar features with mammalian lysosomes. Induction of Ca2+ release is through Yvc1/TRPY1, a TRP-channel homolog in yeast, and occurs with a slightly faster, but otherwise comparable time-course of PI(3,5)P2 elevation 33. Based on our data, we hypothesized that hyperosmolarity-induced vacuolar Ca2+ release would be dependent on elevated PI(3,5)P2 levels. To test this, we used an a luminescence assay based on aequorin, a genetic Ca2+ sensor, 33 to measure changes in cytosolic Ca2+ ([Ca2+]cyt) levels. Consistent with previous reports 33, hyperosmotic shock by addition of 0.9 M NaCl to the culture medium induced a rapid and dramatic increase of [Ca2+]cyt. The time required for peak [Ca2+]cyt increase was 48 ± 4 sec, (N = 10 yeast colonies/experiments); the increase of luminescence was 700 ± 94% over basal (N = 4; Fig. 6a, 6b). In the yvc1Δ mutant, however, no significant increase in [Ca2+]cyt was seen (29 ± 6 % over basal, N = 4), consistent with the hypothesis that the [Ca2+]cyt increase resulted primarily from vacuolar release through Yvc133. Interestingly, in the fab1Δ mutant, hyperosmotic shock also failed to induce any significant increase in [Ca2+]cyt (20 ± 8 %, N = 4; Fig. 6a, 6b), in comparison with 700 % for the wild-type cells. Both yvc1Δ and fab1Δ cells responded to 14% ethanol or 0.03 % SDS (data not shown), which is known to induce Ca2+ influx 33. Consistent with previous studies 11,13, fab1Δ mutant yeasts failed to exhibit vacuolar fragmentation in response to hyperosmotic shock (Fig. 6c).
Figure 6. Ca2+ release from yeast vacuoles after hyperosmostic shock is dependent on PI(3,5)P2 production.
a) Representative Ca2+ response measured with aequorin-mediated luminescence in wild-type (wt), yvc1Δ, and PI(3, 5P)2-deficient strains (vac7Δ, vac14Δ, fig4Δ, fab1Δ) upon hyperosmotic shock induced by addition of 0.9 M NaCl. b) Quantitative data to show luminescence responses upon hyperosmotic shock in different yeast strains. Fold-response was normalized to basal luminescence prior to shock. c) Hyperosmotic shock increases the number of vacuoles and decreases the vacuolar volume in wild type, but not fab1 mutant yeast strains. Both WT and fab1Δ cells were labeled with FM4-64 dye to visualize vacuole volume and the number of vacuole lobes. Cells were treated with 0.45M NaCl and viewed by fluorescence microscopy. d, e) Overexpression of YVC1 in fab1Δ cells failed to restore hyperosmolarity-induced Ca2+ response. f, g) Overexpression of FAB1 in fab1Δ cells restored hyperosmolarity-induced Ca2+ response. h, i) Overexpression of TRPML1 in WT yeast cells increased hyperosmolarity-induced Ca2+ response. j) Hyperosmotic shock induces vacuolar Ca2+ release in vma3 mutant yeast strains. k) Overexpression of TRPML1, but not pore mutant TRPML1-KK (in pCu and pVT expression vectors), in yvc1Δ yeast cells resulted in small but significant hyperosmolarity-induced Ca2+ response.
Fab1 kinase activity is regulated by Vac7, Vac14 and Fig4, mutations in which cause a partial or nearly complete loss of Fab1-mediated PI(3,5)P2 production 11–13,15,34. All these mutants exhibited reduced vacuolar Ca2+ release: 533 ± 60 % (N = 4) for fig4Δ, 357 ± 77 % (N = 4) for vac14Δ, and 106 ± 18 % (N = 4) for vac7Δ (Fig. 6a, 6b). The degree of Ca2+-release reduction (fab1Δ > vac7Δ>vac14Δ> fig4Δ) correlated well with the severity of the defect of PI(3,5)P2 production in these strains 11. The loss of Ca2+ response in fab1Δ cells was not due to reduced expression of Yvc1 (Fig. 6d, 6e), as transformation of Yvc1-GFP failed to restore the response. In contrast, when wild-type yeast FAB1 was transformed into fab1Δ mutant yeast, hyperosmolarity-induced [Ca2+]cyt increase was largely restored (Fig. 6f, 6g). Mutant fab1Δ yeast reportedly hypo-acidify the vacuole 11,13, which could result in reduced Ca2+ release because of a decrease in H+-dependent refilling of vacuolar Ca2+ stores mediated by Vcx1 (vacuolar Ca2+-H+ exchanger) 35. However, vcx1Δ cells exhibit normal Ca2+ release in response to hyperosmotic shock 33, indicating that the alternate Pmc1 (vacuolar Ca2+ ATPase)-mediated refilling system is sufficient to maintain vacuolar Ca2+ stores. In support, we found that hypo-acidified cells lacking vma3, an essential component of the vacuolar ATPase 35, exhibited normal [Ca2+]cyt release (Fig. 6h). These results suggested that the Fab1-mediated PI(3,5)P2 generation system was required for hyperosmolarity-induced, Yvc1-dependent vacuolar Ca2+ release. Although TRPML1 shares a degree of sequence homology to Yvc1, they differ significantly in their channel properties 5,36. To test whether TRPML1 functions in yeast, we overexpressed TRPML1 in both a wild-type and yvc1Δ background. Overexpression of TRPML1 in wild-type yeast resulted in a significant increase in Ca2+ release in response to hyperosmotic shock (Fig. 6i, 6j). In yvc1Δcells, overexpression of TRPML1, but not a non-functional pore mutant of TRPML1 (TRPML1-KK), resulted in a partial rescue of the [Ca2+]cyt response, with a 114 ± 14% increase over basal luminescence (N = 9) (Fig. 6k). In contrast, overexpression of TRPML1 in fab1Δ failed to result in significant [Ca2+]cyt response (Supplementary Fig. S5), while overexpression of YVC1 in yvc1Δcells completely restored the [Ca2+]cyt response (Supplementary Fig. S6). The rescue might be incomplete because Yvc1, but not TRPML1, is activated by cytoplasmic Ca2+ and mechanical force 36, both of which might have synergistic effects with PI(3,5)P2. Nevertheless, these results suggested that regulation of lysosomal Ca2+ channels by PI(3,5)P2 might be a conserved mechanism in eukaryotic cells.
TRPML1 and PI(3,5)P2 in endolysosomal trafficking
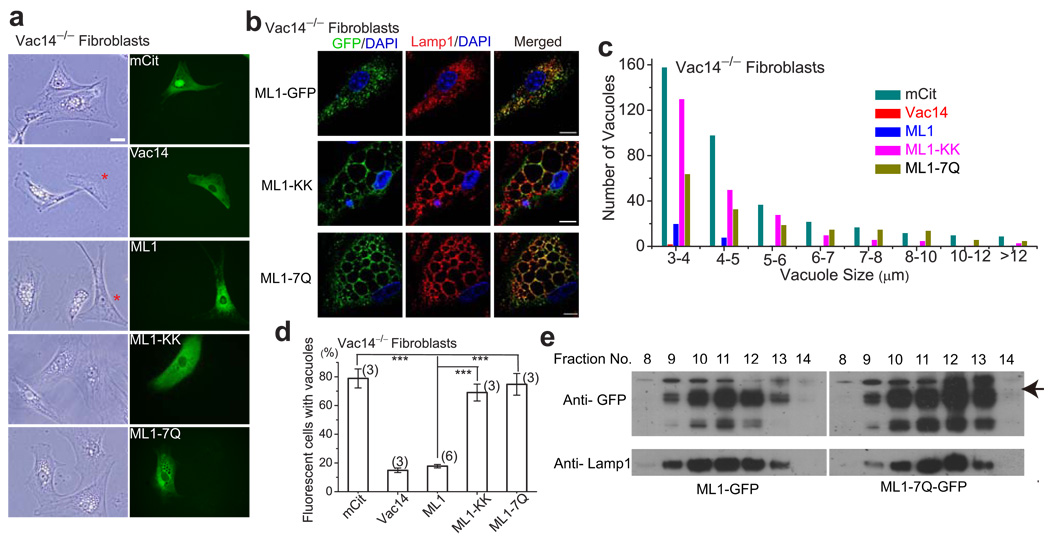
The data presented here suggest that TRPMLs might be activated downstream of the PI(3,5)P2 increase, to trigger membrane fusion and fission (see Supplementary Fig. S1). If this hypothesis is correct, expression of TRPML1, which often exhibits substantial basal activity in heterologous systems 5, might alleviate trafficking defects in PI(3,5)P2-deficient cells. In cultured Vac14−/− fibroblast cells, the trafficking defects from PI(3,5)P2 deficiency were reflected by enlarged vacuoles/LELs (> 3 up to 12 µm in diameter) in 79 ± 7% of the cells (N = 3 experiments with >100 cells) (Fig. 7a; Ref. 12). Only approximately 5–10 % of WT cells were vacuolated (< 4 µm; data not shown). Transfection of a wild-type Vac14 construct was sufficient to restrict vacuoles to 15 ± 2% of cells (N = 3 experiments; Fig. 7a). Interestingly, we were able to rescue the vacuolar phenotype by transfection of TRPML1, which showed vacuolation in 18 ± 1 % cells (N = 6), but not pore-mutant TRPML1 (ML1-KK) with 69 ± 6% vacuolation (N = 3), or the PI(3,5)P2-insensitive mutant TRPML1 (ML1-7Q) with 75 ± 7% vacuolation (N = 3) (Fig. 7a–d). Although ML1-7Q still localized to Lamp1-positive compartments (Fig. 7b, 7e), large vacuoles were seen in the majority of ML1-7Q-transfected Vac14−/− fibroblasts (Fig. 7a–d). Collectively, these results suggested that TRPML1 channel activity and PI(3,5)P2 sensitivity played important roles in controlling vacuole size.
Figure 7. Overexpression of TRPML1 rescues the enlarged endolysosome phenotype of PI(3,5)P2-deficient mouse fibroblasts.
a, b) The effects of overexpression of WT TRPML1 and pore (ML1-KK) or PI(3,5)P2-insensitive (ML1-7Q) mutant TRPML1 on the number and size of the vacuoles in Vac14−/− fibroblasts. Cultured Vac14−/− mouse fibroblast cells exhibited variable numbers (1–20) of large (> 3 µm) vacuoles/endolysosomes. Non-vacuolated cells are indicated with asterisk. Scale Bar = 20 µm. b) TRPML1, ML1-KK, and ML1-7Q proteins were co-localized in Lamp1-positive compartments of Vac14−/− fibroblast cells. c) Large vacuoles in 75% of vector (mCit) -transfected Vac14−/− fibroblast cells. Overexpression of Vac14-mCit or EGFP-ML1 reduced the percentage (of enlarged vacuoles) to approximately 15%, while the 75% of EGFP-ML1-KK or EGFP-ML1-7Q-transfected cells contained enlarged vacuoles. d) Histogram analysis of the vacuole size/number in Vac14−/− fibroblasts transfected with indicated constructs. e) Fractionation analysis reveals co-localization of TRPML1 and TRPML1-7Q with Lamp-1. Gradient cellular fractionations were obtained using ultracentrifugation. Both TRPML1 and TRPML1-7Q proteins were concentrated in Lamp1-rich fractionations.
Discussion
We used biochemical binding assays to demonstrate that PI(3,5)P2 binds directly to the N-terminus of TRPML1. Using whole-endolysosomal patch-clamp recordings, we showed that PI(3,5)P2 robustly activates TRPML1 in the endolysosome. The effect of PI(3,5)P2 was strikingly specific; none of the other PIPs activated TRPML1. Using a spectrum of yeast mutant strains with variable degrees of PI(3,5)P2 deficiency, we established a high-degree of correlation between the amount of vacuolar Ca2+ release and the levels of PI(3,5)P2. Finally, we showed that overexpression of WT, but not the PIP2-insensitive variant of TRPML1, was sufficient to rescue the trafficking defects in PI(3,5)P2-deficient mammalian cells, as demonstrated by observation of enlarged vacuoles. Our identification of an endolysosome-localizing Ca2+ channel that is activated by the endolysosome-specific PI(3,5)P2 provides a previously unknown link between these two important regulators of intracellular membrane trafficking.
Similar trafficking defects are seen in both TRPML1−/−, and PI(3,5)P2-deficient cells. For example, LEL-to-Golgi retrograde trafficking, a process requiring membrane fission, is defective in both TRPML1−/− cells 5,19,37–39, and in PI(3,5)P2-deficient cells 11–15,34. Furthermore, both TRPML1 and PI(3,5)P2-metabolizing enzymes are implicated in membrane-fusion processes like exocytosis 11,15,20, and lysosomal fusion with autophagosomes 6,40. Thus, both TRPML1 and PI(3,5)P2 play active roles in membrane fission and fusion. However, the cellular defects of PI(3,5)P2-deficient cells are generally more severe than TRPML1−/− cells. PI(3,5)P2 is proposed to have at least five independent functions, including: recruitment of cytosolic proteins to define organelle specificity; functional regulation of endolysosomal membrane proteins; determination of physical properties and fusogenic potential of endolysosomal membranes; serving as precursor for PI(3)P or PI(5)P, and modulation of endolysomal pH 9,11,15. Hence, activation of TRPML1 might define a subset of the multiple functions of PI(3,5)P2. Such activation, however, may provide an essential spatial and temporal regulation of endolysosomal dynamics. Membrane fusion and fission are highly coordinated processes requiring an array of cytosolic and membrane-bound proteins and factors. A local increase in PI(3,5)P2 likely recruits protein complexes required to generate the membrane curvature necessary for membrane fusion and fission 15. A local increase in PI(3,5)P2 could also activate TRPML1 to elevate juxtaorganellar Ca2+, which binds to a putative Ca2+ sensor protein such as Synaptotagmin/CaM 3 or ALG-2 41, to exert effects on SNARE proteins or lipid bilayer fusion 15,42. A similar mechanism might be employed by NAADP and TPC channels to regulate membrane trafficking in the endolysosome32,43.
The remarkable specificity of PI(3,5)P2 in activating TRPML1 is consistent with the role of Ca2+ in controlling the direction and specificity of membrane traffic 1,3. Although we identified several positively charged amino acid residues as potential PI(3,5)P2 binding sites, electrostatic interaction alone is unlikely to provide a high affinity PI(3,5)P2 binding pocket 26–27. Thus, additional structural determinants such as hydrophobic amino acid residues must also contribute to specificity, by interacting with the lipid portion of PI(3,5)P2. Because of the low abundance of PI(3,5)P2 11, such specificity might be a pre-requisite for PI(3,5)P2 and TRPML1 to control the trafficking direction in the late-endocytic pathway. In other organelles, however, other PIPs and intracellular Ca2+ channels are likely to provide machinery necessary for Ca2+-dependent membrane fission and fusion. Within LELs, membrane fusion and fission are likely to occur in sub-organellar compartments that are enriched for both TRPMLs and PIKfyve. While TRPML-mediated juxtaorganellar Ca2+ transients might be captured using real-time live-imaging methods, these seemingly “spontaneous” events may correlate with membrane fusion and fission events that can be simultaneously monitored with fluorescence-imaging approaches.
Methods
Molecular biology and biochemistry
Full-length mouse TRPML1, 2, and 3 were cloned into the EGFP-C2 (Clontech) or mCherry vector 20–21,31. TRPML1 non-conducting pore mutant (D471K/D472K; abbreviated TRPML1-KK) and PIP2-insensitive mutant (R42Q/R43Q/R44Q/K55Q/R57Q/R61Q/K62Q; abbreviated TRPML1-7Q) were constructed using a site-directed mutagenesis kit (Qiagen). For glutathione S-transferase (GST) fusion constructs, DNA fragments corresponding to the N- (amino acid residues 1–69) terminal regions of mouse TRPML1 were generated by PCR amplification and cloned into the EcoRI and XhoI site of pGEX4T1, in frame to generate GST-fusion protein plasmids (pGEX-ML1-N). For overexpression of TRPML1 in yeast, full-length mouse TRPML1 was cloned into the XhoI and EcoRI site of the pCuGFP416 vector, and the XhoI and Hind III site of the pVT102U-GFP vector. FKBP*2 fragment was PCR-amplified from EGFP-FKBP12-Rab5 and inserted into the HindIII and XhoI site of EGFP-Rab7 vector. RFP-FRB-MTM1 and EGFP-FKBP*2-Rab5 were kind gift from Dr.Banasfe Larijani. All constructs were confirmed by sequencing, and protein expression was verified by Western blot. HEK293T, Cos-1, human fibroblasts, or mouse primary fibroblast cells were transiently transfected with TRPML1-3 and the TRPML1 mutants for electrophysiology, biochemistry, live-cell imaging, and confocal imaging. Confocal images were taken using a Leica (TCS SP5) microscope. TRPML1 Western blot analyses were performed with an anti-GFP monoclonal antibody (Covance).
Endolysosomal electrophysiology
Endolysosomal electrophysiology was performed in isolated endolysosomes using a modified patch-clamp method 20–21. HEK293 or Cos-1 cells were used for all the heterologous expression experiments and were transfected using Lipofectamine 2000 (Invitrogen) with TRPML1 or mutants fused to GFP or mCherry. Human skin fibroblast cells from a ML4 patient (TRPML1−/−, clone GM02048) and age-matched control (TRPML1+/+, clone GM00969) were obtained from the Coriell Institute for Medical Research (NJ, U.S.A). LEL size is usually < 0.5 µm, which is suboptimal for patch clamping. We therefore treated cells with 1 µM vacuolin-1, a lipid-soluble polycyclic triazine that can selectively increase the size of endosomes and lysosomes, for ~1h 44. Large vacuoles (up to 5 µm; capacitance = 0.68 ± 0.05 pF, N = 44 vacuoles) were observed in most vacuolin-treated cells. Occasionally, enlarged LELs were obtained from TRPML1-transfected cells without vacuolin-1 treatment. No significant difference in TRPML channel properties were seen for enlarged LELs obtained with or without vacuolin-1 treatment. Vacuoles positive for both mCherry-TRPML1 and EGFP-Lamp1 were considered enlarged LELs. Whole-endolysosome recordings were performed on isolated enlarged LELs. In brief, a patch pipette (electrode) was pressed against a cell and quickly pulled away to slice the cell membrane. Enlarged LELs were released into a dish and identified by monitoring EGFP-TRPML1, the mCherry-TRPML1 or EGFP-Lamp1 fluorescence. Unless otherwise stated, bath (internal/cytoplasmic) solution contained 140 mM K-Gluconate, 4 mM NaCl, 1 mM EGTA, 2 mM Na2-ATP, 2 mM MgCl2, 0.39 mM CaCl2, 0.1 mM GTP, 10 mM HEPES (pH adjusted with KOH to 7.2; free [Ca2+]i approximately 100 nM). Pipette ( luminal) solution was pH 4.6 standard extracellular solution (modified Tyrode’s) with 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, 10 mM glucose (pH adjusted with NaOH). All bath solutions were applied via a fast perfusion system to achieve a complete solution exchange within a few seconds. Data were collected using an Axopatch 2A patch clamp amplifier, Digidata 1440, and pClamp 10.0 software (Axon Instruments). Whole-endolysosome currents were digitized at 10 kHz and filtered at 2 kHz. All experiments were conducted at room temperature (21–23°C), and all recordings were analyzed with pCLAMP10 (Axon Instruments, Union City, CA), and Origin 8.0 (OriginLab, Northampton, MA). All PIP antibodies are from Echelon Biosciences Inc. All PIPs are from A.G. Scientific, Inc.
GST fusion proteins
To purify GST-tagged proteins, Escherchia coli BL21DE3 was transformed with empty pGEX vectors or pGEX-TRPML1-N (abbreviated as pGEX-ML1-N). After growth to approximately OD600 = 0.6 in a SuperBroth medium supplemented with ampicillin, expression was induced with IPTG (1 mM) for 7 h at 37°C. Cells were collected and resuspended in 30 ml of ice-cold PBS supplemented with protease inhibitor cocktail, 0.5 mM EDTA, and deoxytibonuclease, and lysed with a French press. Cell lysates in 1% Triton-X100 were incubated with 2 mL mixed glutathione Sepharose (GE Healthcare) for 1 h at 4°C. After three washes with 30 mL PBS, proteins were eluted with 7 mL elution buffer (10 mM Glutathione, 50 mM Tris, pH 8).
Lipid Strip Binding Assay
Lipid binding analysis of GST-ML1-N and GST-ML1-7Q-N fusion proteins was conducted using PIP Strips (Echelon Biosciences Inc.), with each spot containing 100 pmol active lipids. Membranes were blocked with Phosphate Buffered Saline Tween (PBST) solution (supplemented with 3% fatty acid-free bovine serum albumin) for 1 h at room temperature, and incubated with 0.5–3 µg GST-fusion protein in blocking buffer overnight. After six washes, the membranes were incubated with a mouse anti-GST antibody (1:5000, Sigma) for 1 h at room temperature, and secondary antibody HRP-labeled goat anti-mouse (1:5000) was added before detection by enhanced chemiluminescence.
PIP bead binding assay
Purified GST fusion proteins (10 µg) were diluted in 1 mL binding buffer (50 mM Tris, 150 mM NaCl, 0.25% NP-40, pH 7.5) and incubated with 50 µl of 50% lipid-conjugated Sepharose beads (Echelon Biosciences Inc.) for 2 h. After three washes, proteins were eluted from the PIP beads by heating at 50°C for 10 min in 2X SDS-PAGE sample buffer and visualization by Western blot.
Liposome binding assay
Purified GST fusion proteins (3 µg) were incubated with 20 µl liposome (Echelon Biosciences Inc.) in 1 mL binding buffer (50 mM Tris, 150 mM NaCl, 0.05% Nonidet P-40, pH 7.5) for 10 min. Liposomes were pelleted at 16,000 × g for 10 min and washed multiple times with binding buffer. Bound fractions were analyzed by Western blot.
Yeast Strains
Wild-type and mutant yeast strains are listed in Table 1. Strains were grown at 24°C or 30°C in either YPD (yeast extract/peptone/glucose) or synthetic complete (SC) minimal medium.
Table 1.
Yeast strains.
| Genotype | Strains | Genetic background | References |
|---|---|---|---|
| WT | LWY7217 | MATa leu2,3–112 ura3-52 his3-Δ200 trp1- Δ901 lys2-Δ801 suc2-Δ9 |
Ref. 34 |
| vac7Δ | LWY2054 | LWY 7217; vac7Δ∷HIS3 | Ref. 34 |
| WT | LWY 7235 | MATa leu2,3–112 ura3-52 his3-Δ200 trp1- Δ901 lys2-Δ801 suc2-Δ9 |
Ref. 34 |
| vac14Δ | LWY 5177 | LWY 7235; vac14Δ∷TRP1 | Ref. 34 |
| fig4Δ | LWY 6474 | LWY 7235; fig4Δ∷TRP1 | Ref. 34 |
| yvc1Δ | LWY 6848 | LWY 7235; yvc1Δ∷Kan | This study |
| WT | SEY6210 | MATa leu2-3,112 ura3-52 his3-Δ200 trp1- Δ901 lys2-Δ801 suc2-Δ9 |
Ref. 14 |
| fab1Δ | fab1Δ2 | SEY6210; fab1Δ∷HIS3 | Ref. 14 |
| vma3Δ | Vma3Δ | MATa his3-Δ1 leu2Δ0, Met15Δ0 ura3Δ0 vmaΔ∷ Kan MX |
Open Biosystems |
Yeast Ca2+ imaging
Yeast Ca2+ imaging experiments were performed using an aequorin-based genetic Ca2+ sensor method 33. PEVP11/AEQ plasmid was kindly provided by Dr. Patrick H. Masson (University of Wisconsin-Madison, Madison, WI). Transformed yeasts were inoculated from a saturated overnight culture to OD600 ~ 0.3 in a synthetic defined (SD) medium with 3 µM coelenterazine, and grown overnight (OD600 = 2–3) at 24°C or 30°C to convert apo-aequorin to aequorin. For each experiment, an aliquot of 250 µl was harvested, re-suspended in 100 µl SD medium, and transferred to 94-well plates for luminescence measurement. Baseline luminescence was recorded every 3 s for 30 s using a PHERAstar plate reader (BMG Labs). Hyperosmotic shock was performed by adding an equal volume of SD medium (100 µl) containing 1.8 M NaCl to a final concentration of 0.9 M. All experiments were concluded with 14% ethanol or 0.03 % SDS, which resulted in maximal luminescence and served as a positive control for aequorin transformation.
Yeast vacuole labeling
Log-phase yeast cells were labeled with FM4-64 dye (Molecular Probes) 34. Phase contrast and fluorescence images were taken using a Zeiss microscope with a 100X objective.
Mouse fibroblast vacuole assay
Vac14−/− mouse fibroblast cells were isolated and cultured as described previously 12. Briefly, fibroblasts were transiently transfected by electroporation (260 V, 950 µF) with 100 µg of the following expression constructs: mCit, mCit-Vac14, GFP-ML1, GFP-ML1-KK, or GFP-ML1-7Q. Cells were grown on 100-mm plates to 90% confluence and distributed to six 35-mm plates after electroporation. Cells were fixed with 4% paraformaldehyde 24 h after electroporation. Fibroblasts were considered to be vacuolated if they had at least one enlarged (> 3 µm) cytoplasmic vacuole.
Cellular fractionation
To perform lysosomal fractionation studies 45, cell lysates were obtained by Dounce homogenization in a homogenizing buffer (0.25 M sucrose, 1 mM Na2EDTA, 10 mM HEPES, pH 7.0). Lysates were then centrifuged at 1900 × g at 4°C for 10 min to remove the nuclei and intact cells. Post-nuclear supernatants underwent ultracentrifugation through a Percoll density gradient using a Beckman L8-70 ultracentrifuge. An ultracentrifuge tube was layered with 2.5 M sucrose, 18% Percoll in homogenizing buffer, and the post-nuclear supernatant on top. Centrifugation was 67,200 × g at 4°C for 1.5 h in a Beckman Coulter 70.1 Ti Rotor. Samples were fractionated into 16 samples of unequal volume. Top fractions contained minimal cellular components, bottom fractions contained concentrated bands of cellular organelles and were separated into smaller fraction volumes.
Confocal imaging
All images were taken using a Leica (TCS SP5) confocal microscope. Lamp1 antibody was from the Iowa Hybridoma Bank.
Data analysis
Data are presented as the mean ± standard error of the mean (SEM). Statistical comparisons were made using analysis of variance (ANOVA). A P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
This work was supported by start-up funds to H.X. from the Department of MCDB and Biological Science Scholar Program, the University of Michigan, an NIH RO1 grant (NS062792 to H.X), pilot grants (to H.X.) from the UM Initiative on Rare Disease Research, Michigan Alzheimer’s Disease Research Center, National Multiple Sclerosis Society, and NIH R01 GM50403 to L.S.W. We thank Amy Chang for her constant assistance/support and providing yeast strains, Patrick Masson for the pEVP11/AEQ plasmid, Dan Kilonsky for pCuGFP416 vector, Rob Botelho for Fab1 strains, Dr. Pietro De Camili for CFP-FRB-MTM1 and CFP-FKBP*2-Rab5 constructs, Dr.Banasfe Larijani for RFP-FRB-MTM1 and EGFP-FKBP*2-Rab5 constructs, Dr. Martha Cyert for Yvc1-GFP construct, and David Ginsburg’s lab for the electroporator. We are grateful to S. Park, S. Punthambaker, S. Liu, and C. Li for assistance, and L. Yue, D. Ren, and M. Meisler for comments on an earlier version of the manuscript. We appreciate the encouragement and helpful comments from other members of the Xu laboratory.
Footnotes
Author Contributions
X.P.D., D.S., and X.W. contributed equally to this work. H.X., M.D., and X.P.D. designed research; X.P.D., D.S., X.W., T.D., and Z.Q. performed research. X.L., X.C., Y.Z., L.S.W., M.D. contributed new regents; X.P.D., D.S., X.W., T.D., Z.Q., and H.X. analyzed the data; and H.X., X.P.D., and D.S. wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO reports. 2007;8:236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 3.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nature reviews. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 4.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. Journal of neurochemistry. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1-3 channels. FEBS letters. 2010 doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puertollano R, Kiselyov K. TRPMLs: in sickness and in health. American Journal of physiology. 2009;296:F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun M, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Human molecular genetics. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 8.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiological reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 9.Bonangelino CJ, et al. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. Journal of cell biology. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow CY, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. The Biochemical journal. 2009;419:1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell. 2006;5:723–731. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botelho RJ, Efe JA, Teis D, Emr SD. Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Molecular biology of the cell. 2008;19:4273–4286. doi: 10.1091/mbc.E08-04-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poccia D, Larijani B. Phosphatidylinositol metabolism and membrane fusion. The Biochemical journal. 2009;418:233–246. doi: 10.1042/BJ20082105. [DOI] [PubMed] [Google Scholar]
- 16.Jin N, et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P2 in yeast and mouse. EMBO. 2008;27:3221–3234. doi: 10.1038/emboj.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen J, et al. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nature cell biology. 2009;11:769–776. doi: 10.1038/ncb1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow CY, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. The American jounral of human genetics. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic (Copenhagen, Denmark) 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong XP, et al. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. The Journal of biological chemistry. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke FT, et al. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Current biology. 1998;8:1219–1222. doi: 10.1016/s0960-9822(07)00513-1. [DOI] [PubMed] [Google Scholar]
- 23.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. Journal of cell biology. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudge SA, Anderson DM, Emr SD. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Molecular biology of cell. 2004;15:24–36. doi: 10.1091/mbc.E03-05-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeevi DA, Frumkin A, Offen-Glasner V, Kogot-Levin A, Bach G. A potentially dynamic lysosomal role for the endogenous TRPML proteins. The Journal of pathology. 2009;219:153–162. doi: 10.1002/path.2587. [DOI] [PubMed] [Google Scholar]
- 26.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO. 2008;27:2809–2816. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoncu R, et al. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Molecular cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm C, et al. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Delling M, Li L, Dong X, Clapham DE. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18321–18326. doi: 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denis V, Cyert MS. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. The Journal of cell biology. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duex JE, Tang F, Weisman LS. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI(3,5P)2 synthesis and turnover. The Journal of cell biology. 2006;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baars TL, Petri S, Peters C, Mayer A. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Molecular biology of the cell. 2007;18:3873–3882. doi: 10.1091/mbc.E07-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer CP, et al. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson EG, Schaheen L, Dang H, Fares H. Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC cell biology. 2007;8:54. doi: 10.1186/1471-2121-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treusch S, et al. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson CJ, Lenk GM, Meisler MH. Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Human molecular genetics. 2009;18:4868–4878. doi: 10.1093/hmg/ddp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. The Journal of biological chemistry. 2009;284:36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiological reviews. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 43.Zhu MX, Ma J, Parrington J, Galione A, Mark Evans A. TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS letters. 2010 doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huynh C, Andrews NW. The small chemical vacuolin-1 alters the morphology of lysosomes without inhibiting Ca2+-regulated exocytosis. EMBO reports. 2005;6:843–847. doi: 10.1038/sj.embor.7400495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca2+ channel TRPML3 regulates membrane trafficking and autophagy. Traffic (Copenhagen, Denmark) 2009;10:1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.