Abstract
DUOX1 and DUOX2 are members of the NADPH oxidase family that are specifically regulated to produce hydrogen peroxide in epithelia of the thyroid, gastrointestinal tract, and respiratory tract. The determinants of DUOX1 or DUOX2 expression in various tissues have not been established. Using respiratory tract epithelial cells as a model, we investigated changes in DUOX mRNA and protein expression during the first 10 days of differentiation. By comparing a respiratory tract cell line, HBE1, with primary tracheobronchial epithelial (TBE) cells, we determined that DUOX2 was significantly expressed only in cell conditions that included all-trans retinoic acid (ATRA). In HBE1 cells, DUOX2 mRNA increased 6-fold after ATRA treatment. Similarly, ATRA induced a 19-fold increase in DUOX2 mRNA expression in primary TBE cells with parallel increases in DUOX protein and DUOX-mediated H2O2 production as well. In addition, DUOX2 induction by rhinovirus required the presence of ATRA. ATRA had no effect on DUOX1 expression for all the conditions studied. Our data indicate that for respiratory epithelial cells, ATRA is important in the regulation of DUOX2 expression, function, and rhinovirus-mediated DUOX2 inducibility.
Keywords: DUOX1, gene regulation
duox isozymes duox1 and duox2 are dual NADPH oxidase (NOX)-putative heme peroxidases expressed predominantly in epithelial tissues including epithelia of the thyroid, gastrointestinal tract, and respiratory tract (19). They are members of the NOX family that specifically produce hydrogen peroxide (H2O2) in a regulated fashion. The functional significance of DUOX-mediated H2O2 production and the relative contribution of each isoform for functional activity appears to be tissue-specific. For example, DUOX2 is the essential isoform for thyroid hormone synthesis (21), whereas DUOX1 appears to be the major isoform required for wound healing and innate host defense in the respiratory tract (6, 7, 16, 22, 28). In addition, the 5- to 10-fold higher levels of DUOX1 mRNA compared with DUOX2 mRNA in respiratory tract epithelium (17, 27) suggest DUOX1 is the predominant isoform for DUOX-mediated H2O2 production in the airway. However, DUOX2 likely has a higher capacity for H2O2 generation compared with DUOX1 (2, 25) and may have a larger contribution to basal H2O2 production than previously appreciated (15). How the relative expression levels and functional roles of each DUOX isoform are established in tissues remains undetermined.
To begin to address this issue, we investigated some potential factors necessary for DUOX isoform expression in differentiating respiratory tract epithelia. Recent data suggest that DUOX expression in respiratory tract epithelia increases with increasing differentiation (11, 24). Furthermore, it has been proposed that DUOX expression is specifically expressed in ciliated cells. This immediately suggests that DUOX expression is coordinately regulated with cilia cell differentiation. Certainly, costaining of DUOX and cilia in well-differentiated cell cultures supports this model (11, 27). Another possibility is that the transition from submerged conditions to air-liquid interface (ALI) conditions (i.e., in vitro cell culture conditions or during development in vivo) stimulates activation of DUOX transcription and protein expression independent of other features of cellular differentiation. For example, the higher oxygen levels in ALI culture conditions compared with submerged culture conditions may trigger expression of the NOX family members. Alternatively, circulating blood factors/media components may transcriptionally activate DUOX expression. Fischer et al. (12) recently demonstrated that DUOX1 expression was hormonally regulated in fetal alveolar cell cultures, which supports this possibility.
To better understand the relative roles of each DUOX isoform in the respiratory tract, we first sought to understand how DUOX expression is initiated in differentiating respiratory tract epithelium. Because respiratory tract epithelium is known to differentiate in ALI conditions within 7–10 days (33), we chose this period to study changes in DUOX2 expression. We established cell culture conditions to specifically examine whether ALI culture conditions are sufficient for increased DUOX2 expression, whether DUOX2 expression occurs only with conditions that promote cilia formation, or whether media conditions alone are essential for DUOX expression. In addition, we measured transepithelial electrical resistance (Rte) and transepithelial potential (Vte). We utilized these measurements to correlate DUOX expression with physiological indicators of cell differentiation (26).
Based on previous studies of differentiating respiratory tract epithelium (3, 4, 9), we postulated that all-trans-retinoic acid (ATRA) was the key factor responsible for the upregulation of DUOX2. Herein, we explore the changes in DUOX expression, induction, and function that occur in respiratory tract epithelia after long-term treatment with ATRA.
MATERIALS AND METHODS
Cell culture.
Human primary tracheobronchial epithelial (TBE) cells, from tracheas obtained at the University of California at Davis Medical Center (Sacramento, CA) or the National Disease Research Interchange (NDRI; Philadelphia, PA), or HBE1 cells, a papilloma virus-immortalized human TBE cell line kindly provided by Dr. J. Yankaskas from the University of North Carolina (34), were used for all of our experiments. The University of California, Davis Human Subjects Review Committee approved all procedures involved in tissue procurement. Protease-dissociated TBE or HBE1 cells were plated on Transwell (Corning Costar, Corning, NY) chambers (12 mm) at 1–2 × 104 cells/cm2 in LHC Basal Medium/DMEM (1:1) supplemented with insulin (5 μg/ml), transferrin (10 μg/ml), epidermal growth factor (0.5 ng/ml), hydrocortisone (0.1 μM), triiodothyronine (0.01 μM), bovine hypothalamus extract (10 μg/ml), bovine serum albumin (0.5 mg/ml), epinephrine (0.6 μg/ml), phosphorylethanolamine (0.5 μM), ethanolamine (0.5 μM), zinc sulfate (3 μM), ferrous sulfate (1.5 μM), magnesium chloride (0.6 mM), calcium chloride (0.11 mM), and trace elements (selenium, manganese, silicone, molybdenum, vanadium, nickel sulfate, and tin) (14). Once TBE or HBE1 cultures were confluent, they were transferred to ALI culture conditions, and 30 nM ATRA was added to the appropriate cultures for 7–10 days.
Histology.
Cells grown on Transwell membranes with or without ATRA were fixed with 4% paraformaldehyde for 4 h. The membrane-attached fixed cells were then embedded in paraffin and subsequently sectioned at 4-μm thickness for hematoxylin and eosin staining to examine morphological differences.
Electrical measurements.
After cells achieved confluent status, measurements of Rte and Vte were taken on alternate days up to 10 days. PBS, warmed to 37°C, temporarily replaced the media in the apical and basolateral chambers just before each measurement. The tips of the electrode connected to the Millicell-ERS (Millipore, Billerica, MA) electrical resistance system was immersed in the PBS straddling the Transwell membrane, and measurements were taken. The instrument was calibrated before each set of measurements.
Quantitative real-time PCR.
Three micrograms of total RNA was reverse-transcribed with M-MLV Reverse Transcriptase (Promega, Madison, WI) by oligo(dT) primers for 10 min at 70°C, 60 min at 42°C, and 15 min at 70°C in 13-μl reaction volumes. Sample cDNAs were diluted 1:5 in water for use in real-time PCR reactions. Real-time PCR was performed with an ABI 2700 GeneAmp unit (Applied Biosystems, Foster City, CA). Reactions were carried out in 384-well optical reaction plates in a 10-μl final volume containing 5 μl of the SYBR Green (Invitrogen, Carlsbad, CA) PCR master mix, 0.4 μl of 5 μM gene-specific primer mix, 2 μl of diluted sample cDNA, and 2.6 μl of water. Gene-specific primers were designed to selectively amplify DUOX1 or DUOX2, and relative expression values were normalized to β-actin as previously described (17).
Western blotting.
Cells were scraped and lysed in 4°C RIPA buffer (Pierce, Rockford, IL) supplemented with Halt Protease Inhibitor Cocktail and PMSF (Pierce) on ice. The lysate was centrifuged for 10 min at 14,000 rpm at 4°C, and the supernatant was transferred into a fresh tube and stored at −80°C. Total protein concentration was determined using the Bio-Rad DC Protein Assay Kit. Samples were normalized to 30-μg protein, combined with Laemmli buffer (2% SDS, 100 mM dithiothreitol), and heated to 65°C for 15 min and placed on ice. Proteins were run on a 7% NuPAGE Novex Tris-Acetate gel (Invitrogen) and then transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were blocked in 5% fat-free dry milk in PBS and incubated with rabbit anti-DUOX primary antibody (1:10,000) at 4°C for 24 h and secondary goat-anti rabbit horseradish peroxidase (HRP)-coupled antibodies (1:20,000; Pierce) at 25°C for 1 h. Detection was achieved by ECL (Thermo Fisher). The intensity of DUOX protein on the Western blot was normalized to β-actin using MultiGauge v2.3 software (Fujifilm, Cypress, CA).
The anti-DUOX primary antibody was generated by our laboratory using a pET-24d plasmid containing a portion of DUOX1 (8), which was generously provided by Xavier De Deken and Greg Conner. Four liters of Escherichia coli containing the pET-24d plasmid were grown up shaking at 37°C and induced with isopropyl β-d-thiogalactopyranoside (IPTG), final concentration 1 mM, for expression of the DUOX1 protein fragment. DUOX1 protein fragments were harvested using the native conditions for soluble protein and denaturing conditions for the inclusion bodies from the Macherey-Nagel (Bethlehem, PA) NucleoSpin protocol. Purification of the DUOX1 protein fragments were achieved using Millipore Ultracel 30- and 10-kDa spin columns. Evaluation of protein purity was performed with Western blot analysis using previously generated anti-DUOX primary antibody from the pET-24d plasmid that has been shown to recognize both DUOX1 and DUOX2 protein (8). Purified protein fractions were sent to New England Peptide (NEP; Gardner, MA) to inject into two New Zealand White rabbits to generate polyclonal antibodies. Two production bleeds per rabbit were harvested by NEP, and we tested the serum for specificity and efficiency for recognizing DUOX protein.
H2O2 measurements.
Cells were plated on Transwell (Corning Costar) chambers (12 mm) and transferred to ALI conditions after they reached confluent status. After 10 days under ALI conditions, H2O2 measurements were performed. The cells were pretreated with or without 20 μM (or 2 μM; see the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site) diphenyleneiodonium (DPI) in media in the basolateral chamber for 30 min to inhibit DUOX activity (15), and thapsigargin (final concentration 1 μM) was added to the appropriate wells to stimulate DUOX activity (13) before a 10-min incubation of 150-μl 1:1 ratio of PBS to 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red; 100 μM) combined with 0.1 U/ml HRP in the apical chamber. Samples (50 μl) were removed from the apical chamber and loaded in a 96-well microplate. For each experiment, the amount of apical H2O2 was determined using an H2O2 standard curve from 0 to 32 μM. Fluorescence was read on a Packard FluoroCount Microplate Fluorometer (excitation 530 nm, emission 590 nm; GMI, Ramsey, MN).
Viral infection.
Rhinovirus 1B (RV1B) stocks were obtained from Dr. Wai-Ming Lee (University of Wisconsin), and viral titers were determined by plaque assay as described previously (10). HBE1 cells were treated with ATRA for 7 days followed by infection with RV1B for 24 h at 33°C. PBS with or without RV1B were added apically to HBE1 cells. For RV1B infection, a multiplicity of infection (MOI) of 1 was used by adding a volume of RV1B that provided 1 × 106 plaque-forming units (PFU) to 1 × 106 respiratory tract epithelial cells. This protocol was based on previously published techniques (20).
Statistical analysis.
Data are presented as original values or as means with SE; n refers to the number of replicates. Comparison between two treatment groups was done using paired t-tests. Effect of multiple treatments was tested using ANOVAs followed by Tukey-Kramer multiple comparisons test. Statistical testing was done using GraphPad Prism 5. Resulting P values were given, and P < 0.05 was considered significant.
RESULTS
Morphological differences between lung epithelial cells grown with or without ATRA.
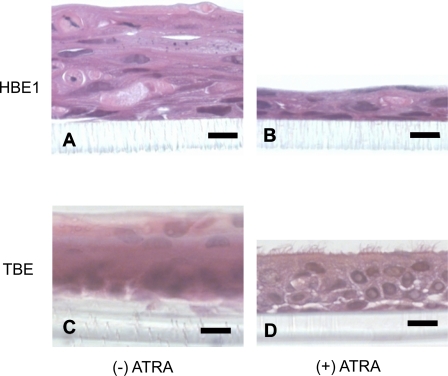
To verify that our ALI cell culture conditions produced the morphological changes we anticipated, we performed cross-sectional staining of HBE1 cells and primary TBE cells grown in culture for 7 days with or without ATRA (Fig. 1). As expected, HBE1 cells and primary cells grown without ATRA displayed a stratified squamous epithelial phenotype (Fig. 1, A and C). In addition, primary TBE cells developed multiple keratinized layers that were not observed with HBE1 cells (data not shown). HBE1 cells treated with ATRA displayed an epithelial phenotype that ranged from simple squamous or cuboidal to stratified squamous layers without evidence for cilia formation (Fig. 1B). In contrast, primary TBE cells treated with ATRA displayed a pseudostratified columnar ciliated epithelial phenotype, similar to in vivo respiratory tract epithelia (Fig. 1D).
Fig. 1.
Full differentiation of primary tracheobronchial epithelial (TBE) cells in air-liquid interface (ALI) culture conditions requires all-trans retinoic acid (ATRA). Human bronchial epithelial HBE1 and primary TBE cells were grown in ALI culture conditions with or without ATRA for 7 days followed by fixation in 4% paraformaldehyde and embedded in paraffin. Cross sections of membrane-adherent cells were then stained with hematoxylin and eosin for morphological analysis. HBE1 cells grown without ATRA (A) displayed a stratified squamous epithelial phenotype compared with HBE1 cells treated with ATRA (B), which displayed a simple squamous or thin stratified squamous epithelial phenotype. C: primary TBE cells grown without ATRA displayed a keratinized stratified squamous epithelial phenotype, whereas those treated with ATRA (D) displayed a pseudostratified columnar ciliated epithelial phenotype similar to respiratory epithelium in vivo. Black bar represents 10 μm.
Assessment of the electrical properties of HBE1 and TBE cells.
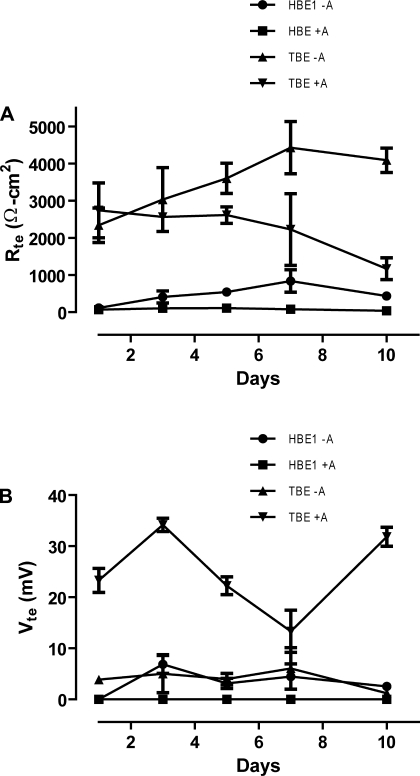
To further assess differentiation of HBE1 and TBE cells grown with or without ATRA, we measured for the presence of two main physiological indicators of mature respiratory tract epithelia, barrier function (Rte) and Vte (26). HBE1 cells grown without ATRA develop significant Rte. However, HBE1 cells treated with ATRA were unable to develop tight junctions as evidenced by the low Rte values (Fig. 2A) despite the apparent continuous cell layer seen on histology. Primary TBE cells treated with or without ATRA were capable of developing an adequate epithelial barrier consistent with the morphological changes we observed (Fig. 2A). In contrast, only ATRA-treated primary cells demonstrated evidence for effective Vte. HBE1 cells grown with or without ATRA and primary TBE cells grown without ATRA demonstrated negligible Vte (Fig. 2B).
Fig. 2.
ATRA-treated primary TBE cells alone demonstrate electrical properties of a well-differentiated respiratory tract epithelium. HBE1 cells and primary TBE cells were grown in Transwell chambers until confluent, followed by serial measurements of transepithelial electrical resistance (Rte) and transepithelial potential (Vte) over a 10-day period starting with the 1st day of ALI conditions. A: changes in Rte values for HBE1 cells or primary TBE cells treated with or without ATRA (A). B: changes in Vte values for HBE1 cells or primary TBE cells treated with or without ATRA. Displayed Rte values (Ω·cm2) were corrected for background resistance of the membrane with PBS alone (background Rte was ∼125 Ω/well). Vte values (mV) represent absolute Vte after the probe was zeroed in PBS alone. A Vte >10 mV and an Rte >300 Ω·cm2 was considered significant. Means and SE of at least 3 separate experiments are displayed.
Expression of DUOX1/2 in HBE1 and primary TBE cells.
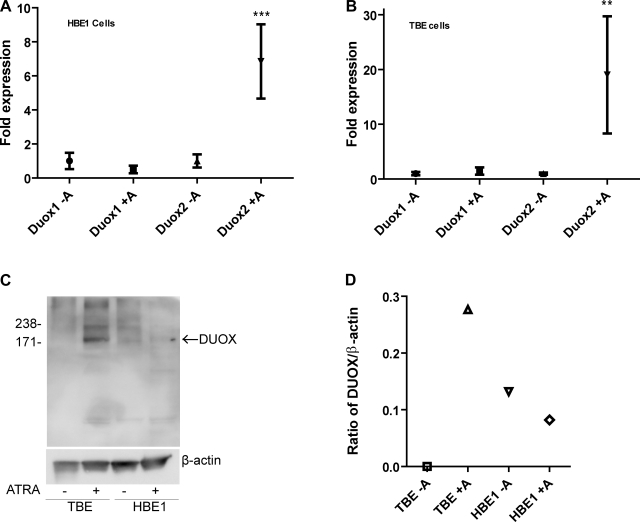
To correlate features of airway cell differentiation to DUOX expression, we measured both DUOX mRNA and protein expression after 7 days in ALI conditions. ATRA significantly upregulated DUOX2 mRNA expression 6-fold in HBE1 (Fig. 3A) and 19-fold in primary TBE cells (Fig. 3B). Similarly, DUOX protein expression in primary TBE cells was undetectable in Western blots from cells not treated with ATRA but readily identified in cells treated with ATRA (Fig. 3C). In contrast, DUOX protein expression was minimally detected in HBE1 cells treated with or without ATRA (Fig. 3C). Even after normalizing DUOX band intensity to β-actin, we were unable to detect a difference in DUOX protein expression between treatment groups for HBE1 cells (Fig. 3D).
Fig. 3.
Changes in dual NADPH oxidase-putative heme peroxidase (DUOX) mRNA and protein expression after treatment with ATRA. HBE1 cells and primary TBE cells were grown in ALI culture conditions with or without 30 nM ATRA for 7 days followed by harvest for either RNA or protein. A: quantitative real-time PCR (qRT-PCR) analysis of DUOX1 and DUOX2 mRNA expression from HBE1 cells with or without ATRA. ***P = 0.0004 (by ANOVA). B: qRT-PCR analysis of DUOX1 and DUOX2 mRNA expression from primary TBE cells with or without ATRA. **P = 0.0073 (by ANOVA). Data are expressed as fold induction of the gene of interest normalized to β-actin. C: a total of 30 μg of protein from each sample was loaded onto a 7% SDS-PAGE gel, and Western blotting for DUOX was performed using anti-DUOX antibody as described in materials and methods. The membrane was stripped, and Western blotting for β-actin was performed to ensure equal protein loading. Arrow identifies predicted molecular mass of DUOX protein (kDa). D: band intensity was measured using MultiGauge v2.3 software (Fujifilm), and the ratio of DUOX band intensity to β-actin band intensity was calculated. Results are representative of at least 3 separate experiments.
Regulation of DPI-inhibitable H2O2 generation by ATRA.
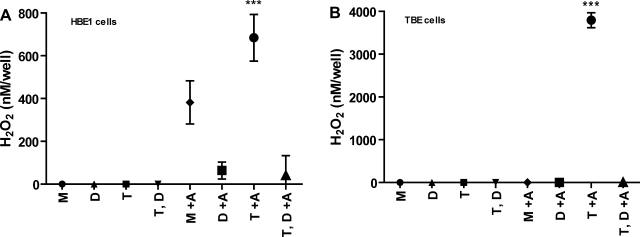
Apical H2O2 production was measured in HBE1 cells and primary TBE cells treated with or without ATRA. For each condition, cells were also treated short-term with either media alone, with 1 μM thapsigargin to stimulate DUOX-mediated H2O2 production (13), or with 20 μM DPI to inhibit DUOX-mediated H2O2 production (15). HBE1 cells grown without ATRA produced nondetectable levels of apical H2O2, whereas HBE1 cells treated with ATRA produced moderate levels of DPI-inhibitable H2O2. Both baseline and thapsigargin-inducible H2O2 were substantially inhibited by DPI. For primary TBE cells, only ATRA-treated, thapsigargin-stimulated cells produced measurable levels of apical H2O2 after 10 min. This high level of H2O2 generation was inhibited in the presence of DPI, strongly suggesting that DUOX was the peroxide source (Fig. 4, Supplemental Fig. S1). Lower doses of DPI (2 μM) produced similar degrees of DPI-inhibitable H2O2 production (Supplemental Figs. S2 and S3).
Fig. 4.
Apical H2O2 production in HBE1 and TBE cells treated with or without ATRA. HBE1 cells (A) and primary TBE cells (B) were grown in ALI culture conditions with or without 30 nM ATRA for 7 days followed by measurements of H2O2 using Amplex Red assays: M, media alone; D, diphenyleneiodonium (20 μM); T, thapsigargin (1 μM); and T,D, thapsigargin/diphenyleneiodonium cotreatment. Values are expressed as nM H2O2/well/10 min by comparing with an H2O2 standard curve. Data represent the means and SE of 3 independent experiments with 9 wells per treatment group. ***P < 0.0001 (by ANOVA).
Rhinovirus-mediated DUOX2 expression requires ATRA.
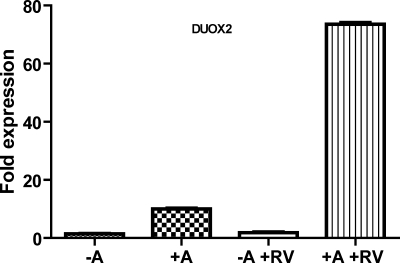
We (17) previously have shown that rhinovirus infection significantly induces DUOX2 expression after 24 h in primary TBE cells. Further investigation demonstrated that DUOX2 mRNA inducibility by virus differed between primary TBE cells, grown with ATRA supplementation, and HBE1 cells, typically grown without ATRA (unpublished data). Based on these results, we postulated that this differential induction was due to the presence of ATRA. To evaluate this notion, we infected ATRA-untreated and ATRA-treated HBE1 cells with minor serotype RV1B at an MOI of 1. As shown in Fig. 5, RV1B infection did not increase DUOX2 mRNA expression in HBE1 cells. However, in the presence of ATRA, RV1B significantly upregulated DUOX2 mRNA expression levels in HBE1 cells 10-fold higher than the induction of DUOX2 by ATRA alone. This 10-fold induction was similar in magnitude to the fold induction we observed in primary TBE cells.
Fig. 5.
DUOX2 mRNA expression levels in HBE1 cells after rhinovirus (RV) infection with or without ATRA. HBE1 cells were grown in ALI culture conditions with or without 30 nM ATRA for 7 days and then infected with RV for 24 h. Quantitative PCR analysis of DUOX2 mRNA expression in RV-infected cells was compared with RV-uninfected cells. Data are expressed as fold induction of DUOX2 normalized to β-actin by defining 2−ΔCt cycle threshold value from the ATRA-untreated, uninfected sample as 1.
DISCUSSION
Current literature suggests that DUOX expression in the respiratory tract correlates with cell differentiation (1, 11, 24). In this study, we have shown that ATRA is a key component for DUOX2 mRNA and protein expression as well as inducibility by rhinovirus. By comparing primary TBE cells with HBE1 cells, however, we have been able to demonstrate that ATRA is not sufficient for DUOX protein expression in HBE1 cells. Together, these data suggest that ATRA is required for normal DUOX2 promoter activation and mRNA expression but that additional components are required for functional protein expression.
A well-differentiated respiratory tract epithelium is characterized morphologically by a pseudostratified columnar epithelium containing ciliated cells, mucus-secreting cells, and basal cells. Consistent with previous reports (3, 31, 32, 36), we demonstrated that ATRA is essential for the proper maturation of respiratory tract epithelium. However, only primary TBE cells developed pseudostratified columnar morphology and cilia formation. HBE1 cells did not form cilia or develop other features consistent with a mature epithelium.
Similarly, only the ATRA-treated primary cells generated Rte and Vte values that were consistent with a mature epithelium. Electrical properties for well-differentiated respiratory tract epithelium are highly dependent on the media and matrix used. However, Rte values of 300–600 Ω·cm2 and Vte values of 10–30 mV are suggested to be representative of an in vivo airway epithelium (26, 29). Values below this range reflect cell cultures that are unable to achieve the polarization required for a fully functional epithelium. ATRA-untreated HBE1 and primary TBE cells were able to generate substantial tight junctions but were unable to produce Vte values expected from a functional respiratory tract epithelium. Although Rte for primary TBE cells initially were outside the range expected, we observed values closer to the normal range with increasing days on culture. Vte measures for the ATRA-treated primary TBE cells were well within the expected values throughout the observation period.
Despite the fact that HBE1 cells did not differentiate into a mature respiratory tract epithelium, DUOX2 mRNA expression increased significantly in these cells with the addition of ATRA. In fact, when we compared absolute cycle threshold (CT) values for DUOX2 mRNA between ATRA-treated primary TBE and HBE1 cells, the values were nearly identical (data not shown). The discordance between morphological and physiological measures of differentiation and DUOX2 expression in ATRA-treated HBE1 cells supports the notion that ATRA is the dominant factor in determining DUOX2 transcriptional activity.
However, our data suggest that ATRA is not the sole factor determining DUOX2 protein expression. The amount of H2O2 generation in ATRA-treated primary TBE cells was consistent with previously published literature (11), suggesting 7–10 days was an adequate period for DUOX maturation. Importantly, despite highly similar absolute ΔCT values for DUOX2 mRNA in ATRA-treated primary TBE and HBE1 cells, Western blots and H2O2 measurements indicated significantly lower protein levels in HBE1 cells. Using similar total protein loading, we were able to clearly see a DUOX band in ATRA-treated primary cells but not in HBE1 cells. Based on the H2O2 measurements, it appears that ATRA did affect DUOX2 protein levels in HBE1 cells but that these differences were below the detection limit of the Western blots. In addition, repeat H2O2 measurements using different frozen stocks of HBE1 cells demonstrated variable basal and DPI-inhibitable H2O2 values (see supplementary data) raising doubts that DUOX2 was the predominant source of H2O2 in this cell line. These data suggest that ATRA-independent components are required for effective DUOX2 translation and functional activity, which are not efficiently expressed in HBE1 cells.
DUOX1 mRNA levels did not change with or without ATRA in both primary TBE and HBE1 cells, supporting the notion that the changes we observed were DUOX2-specific. The lack of any DUOX staining for ATRA-untreated primary cells was unexpected. Because the DUOX antibody we used will detect both DUOX1 and DUOX2, we expected to see some DUOX1 protein even in the absence of ATRA. Factors leading to squamous metaplasia may specifically suppress DUOX1 expression, or the period of observation may have been too short for robust DUOX1 expression. A recent report by Rada et al. (24) supports the latter possibility.
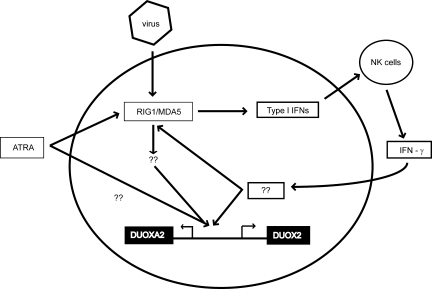
Based on our (17) previous findings that DUOX2 expression is increased by Th1 cytokines and rhinovirus infection, we hypothesized that DUOX2 is an important part of the antiviral response. In this report, we demonstrated that rhinovirus-mediated DUOX2 transcription requires ATRA. In parallel, retinoic acid is important for RIG-I induction (35). Given the important roles for RIG-I/MDA5 in sensing rhinovirus double-stranded RNA and inducing the type I IFN responses in human airway epithelial cells (5, 18, 30), these data suggest that DUOX2 is an essential component of an ATRA-mediated antiviral signaling network in respiratory tract epithelium. We speculate that ATRA works directly, or indirectly through RIG-I/MDA5 and other transcription factors, to activate the DUOX2 promoter and induce transcription when the cell is infected with virus. The RIG-I/MDA5-mediated induction of IFN-β and IFN-β-mediated effector molecules recruits IFN-γ-producing immune cells, which further amplifies DUOX2 expression (17) (Fig. 6). The precise roles of and regulatory mechanisms responsible for DUOX2 in antiviral host defense are currently being investigated in our laboratory.
Fig. 6.
Proposed schematic of the signaling network for DUOX2 induction during viral infection. ATRA is required for DUOX2 promoter activation after viral infection. This potentially is mediated through an ATRA-dependent factor such as RIG-I/MDA5. The RIG-I/MDA5-mediated induction of IFN-β and IFN-β-mediated effector molecules recruit IFN-γ-producing immune cells that further amplify DUOX2 expression. NK, natural killer; ??, potential pathways that have not been experimentally verified.
A recent study by Mühlbauer et al. (23) demonstrated that H2O2 levels were significantly increased in the thyroid of rats when treated with pharmacological doses of 13-cis-retinoic acid. Because DUOX2 is the predominant source of H2O2 in the thyroid, it is likely that this increase in H2O2 production is DUOX2-mediated. Of interest, pharmacological doses of ATRA had no effect on H2O2 production in the thyroid. These differences may be due to tissue specificity, species differences, or a dose-dependent effect. However, these findings highlight the notion that retinoic acid is a critical regulatory factor for DUOX2 in multiple tissues.
Based on absolute changes in mRNA levels, HBE1 cells, and potentially other respiratory tract cell lines, serve as a reasonable model to study DUOX2 gene regulation as long as media are supplemented with ATRA. Consistent with previous suggestions (15), however, DUOX2 protein expression and functional activity may be inadequately modeled by the use of cell lines; whether this remains true for other DUOX-expressing tissues remains to be determined.
GRANTS
The work was supported in part by the Ruth L. Kirschstein National Research Service Awards (T32-HL-07103) and the National Heart, Lung, and Blood Institute (R01-HL-085311 and R01-HL-085311-02S1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Reen Wu (University of California at Davis) for the HBE1 cells. We greatly appreciate the gift of the DUOX pET-24d plasmid from Xavier De Deken (Université Libre de Bruxelles) and Greg Conner (University of Miami).
REFERENCES
- 1.Allaoui A, Botteaux A, Dumont JE, Hoste C, De Deken X. Dual oxidases and hydrogen peroxide in a complex dialogue between host mucosae and bacteria. Trends Mol Med 15: 571–579, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280: 30046–30054, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cardoso WV, Williams MC, Mitsialis SA, Joyce-Brady M, Rishi AK, Brody JS. Retinoic acid induces changes in the pattern of airway branching and alters epithelial cell differentiation in the developing lung in vitro. Am J Respir Cell Mol Biol 12: 464–476, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Chang WH, Reddy SP, Di YP, Yoneda K, Harper R, Wu R. Regulation of thioredoxin gene expression by vitamin A in human airway epithelial cells. Am J Respir Cell Mol Biol 26: 627–635, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, Yagi S, Dolganov G, Boushey H, Avila P, Wu R. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol 34: 192–203, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conner GE, Salathe M, Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med 166: S57–S61, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett 581: 271–278, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Di YP, Harper R, Zhao Y, Pahlavan N, Finkbeiner W, Wu R. Molecular cloning and characterization of spurt, a human novel gene that is retinoic acid-inducible and encodes a secretory protein specific in upper respiratory tracts. J Biol Chem 278: 1165–1173, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol 38: 59–64, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1506–L1514, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Forteza R, Salathe M, Miot F, Forteza R, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 462–469, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183–206, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, Conner GE. Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med 47: 1450–1458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 17: 1502–1504, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579: 4911–4917, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Souza N, Dolganov G, Dubin R, Sachs LA, Sassina L, Sporer H, Yagi S, Schnurr D, Boushey HA, Widdicombe JH. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol 286: L373–L381, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med 347: 95–102, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 175: 174–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mühlbauer M, da Silva AC, Marassi MP, Lourenço AL, Ferreira AC, de Carvalho DP. Retinoic acid modulation of thyroid dual oxidase activity in rats and its impact on thyroid iodine organification. J Endocrinol 205: 271–277, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol 181: 4883–4893, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigutto S, Hoste C, Dumont JE, Corvilain B, Miot F, De Deken X. Duox1 is the main source of hydrogen peroxide in the rat thyroid cell line PCCl3. Exp Cell Res 313: 3892–3901, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell Dev Biol 39: 56–62, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem 279: 36454–36461, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282: 3213–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Widdicombe JH. Use of cultured airway epithelial cells in studies of ion transport. Am J Physiol Lung Cell Mol Physiol 258: L13–L18, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol 22: 41–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu R, Yankaskas J, Cheng E, Knowles MR, Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis 132: 311–320, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J 10: 2398–2403, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol Lung Cell Mol Physiol 262: L713–L724, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA, Jr, Sarkadi B, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol Cell Physiol 264: C1219–C1230, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev 227: 54–65, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Yoon JH, Gray T, Guzman K, Koo JS, Nettesheim P. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am J Respir Cell Mol Biol 16: 724–731, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.