Abstract
The aim of this study is to elucidate the effects of interleukin-6 (IL-6) on the expression and activity of the epithelial sodium channel (ENaC), which is one of the key mechanisms underlying tubular sodium reabsorption. M-1 cortical collecting duct cells were treated with IL-6 (100 ng/ml) for 12 h. Real-time polymerase chain reaction and immunoblotting were employed to examine the mRNA and protein abundance. Transepithelial voltage (Vte) and resistance (Rte) were measured with an ohm/voltmeter (EVOM, WPI). The equivalent current was calculated as the ratio of Vte to Rte. Treatment with IL-6 (n = 5) increased the mRNA abundance of α-ENaC by 11 ± 7% (P = not significant), β-ENaC by 78 ± 14% (P = 0.01), γ-ENaC by 185 ± 38% (P = 0.02), and prostasin by 29 ± 5% (P = 0.01), all normalized by β-actin. Treatment with IL-6 increased the protein expression of α-ENaC by 19 ± 3% (P = 0.001), β-ENaC by 89 ± 21% (P = 0.01), γ-ENaC by 36 ± 12% (P = 0.02), and prostasin by 33 ± 6% (P = 0.02). The amiloride-sensitive sodium current increased by 37 ± 5%, from 6.0 ± 0.4 to 8.2 ± 0.3 μA/cm2 (P < 0.01), in the cells treated with IL-6 compared with controls (P = 0.01). Aprotinin (28 μg/ml), a prostasin inhibitor, reduced the amiloride-sensitive sodium current by 61 ± 5%, from 6.1 ± 0.3 to 3.7 ± 0.2 μA/cm2 (P = 0.01). The magnitude of the IL-6-induced amiloride-sensitive sodium current in the presence of aprotinin dropped by 57 ± 2%, from 8.6 ± 0.2 to 4.9 ± 0.2 μA/cm2 (P < 0.01). This study has identified a novel function of IL-6, namely, IL-6 may activate ENaC. Therefore, renal inflammation mediated by IL-6 likely contributes to impaired pressure natriuresis.
Keywords: distal tubule, prostasin, pressure natriuresis
interleukin-6 (IL-6), a pleiotropic cytokine produced by numerous cell types, plays an important role in the inflammation cascade. IL-6 exerts multiple effects on infiltrated inflammatory cells and on structural cells in a variety of tissues and organs, including kidneys (8, 24, 44, 48). Data suggest that IL-6-involved inflammatory processes in the kidney contribute to the pathogenesis of nephropathy (28, 40). IL-6-deficient mice were resistant to HgCl2-induced acute kidney injury compared with wild-type mice (42). Renal function decline in patients with type 1 diabetes was correlated with elevated excretion of IL-6 in urine (67). Renal cortical mRNA expression of IL-6 was 3.4-fold higher in diabetic than in nondiabetic rats, which was significantly associated with urinary albumin excretion (41). In patients with diabetic nephropathy, IL-6 mRNA was expressed by glomerular resident cells and interstitial cells in the renal tissue (56). IL-6 was also shown to be linked to hypertensive glomerular injury (31, 47). Biopsy studies using indirect immunofluorescence microscopy showed that, in patients with kidney disease, IL-6 expression was increased in the area of glomerular and tubular inflammation and tubular atrophy (18, 19).
Furthermore, using mice with knockout of IL-6, Lee et al. (33) found that angiotensin II (ANG II)-induced hypertension was significantly dependent on IL-6. The kidneys of the IL-6 knockout mice were more capable of eliminating a salt load during ANG II infusion than were the kidneys of the wild-type mice. The hypertension caused by a high dose of ANG II and a high-salt diet was attenuated in IL-6 knockout mice (34). These data suggest that there is a powerful effect of IL-6 in mediating the rightward shift in the renal pressure-natriuresis relationship caused by ANG II and high salt intake, providing evidence for an important long-term blood pressure effect of IL-6. In addition, prenatal exposure to IL-6 resulted in hypertension, alterations in the renal and circulatory renin-angiotensin system, and decreased mean urinary sodium excretion in rats (49). However, the mechanisms whereby IL-6 regulates sodium handling remain unknown.
The epithelial sodium channel (ENaC) consists of at least three subunits (α-ENaC, β-ENaC, and γ-ENaC) and is thought to regulate the final adjustment of renal sodium reabsorption in the distal tubules (51). Therefore, sodium homeostasis, extracellular volume, and, ultimately, blood pressure are maintained by precise regulation of ENaC activity. ENaC activity is controlled by aldosterone, other hormones such as vasopressin, and a network of intracellular factors and multiple accessory regulatory proteins that are not completely understood. Prostasin, a glycosylphosphatidylinositol-anchored serine protease expressed in the distal nephron, has been shown to regulate the proteolytic processing and activation of ENaC subunits in Xenopus oocytes and a mouse cortical collecting duct (CCD) cell line (M-1 cells) (1, 13, 14, 25, 26, 30, 46, 59–61, 63, 64). Prostasin is thought to induce cleavage of an inhibitory peptide from γ-ENaC to activate the channel fully in the cell (9, 10, 25). Because of the impact of ENaC on sodium homeostasis and blood pressure control, the present study was conducted to evaluate the effects of IL-6 on the gene and protein expression of ENaC subunits and the activity of ENaC in CCD cells. In addition, we hypothesize that IL-6 might affect prostasin. We chose M-1 cells, a mammalian cell line derived from microdissected CCD of a mouse transgenic for the early region of SV40, which retains a number of the characteristics of the parental CCDs, including the expression of ENaC, high transepithelial electrical resistance (Rte), and amiloride-sensitive sodium currents (6, 55). Thus, M-1 cells have been valuable tools for studying ENaC and related regulatory proteins (29, 35, 38, 39, 58, 65).
METHODS
Cell culture.
The M-1 cells were obtained and cultured as previously described (38). Briefly, the cultures were initially maintained in a defined medium consisting of equal amounts of Ham's F-12 and low-glucose Dulbecco's modified Eagle's medium (Sigma), supplemented with 2 mmol/l l-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin, 5% fetal bovine serum, growth-promoting factors (6.25 mg/ml transferrin, insulin, and sodium selenite), and 100 nmol/l dexamethasone. After the cells reached confluence, they were passaged to permeable membranes (Transwells, Costar). Cells were synchronized by serum deprivation for 24 h and treated with IL-6 (100 ng/ml) for 12 h. Finally, cells were harvested for mRNA and protein extraction. The Alamar blue viability assay showed that the cell survival rate was ∼85%, after treatment with 100 ng/ml IL-6 for 12 h.
Real-time PCR.
Predesigned TaqMan gene expression assays for mouse α-ENaC (Mm00803386_m1), β-ENaC (Mm00441215_m1), γ-ENaC (Mm00441228_m1), prostasin (Mm00504792_m1), and β-actin (Mm00607939_s1) were purchased from Applied Biosystems (Foster City, CA). Total RNA was extracted from M-1 cells, and 5 μg of total RNA were reverse-transcribed to cDNA with oligo(dT) primers using Superscript III. Real-time PCR was performed with the ABI 7500 fast real-time PCR system (Applied Biosystems). Thermal cycling conditions consisted of 95°C for 20 s, followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. Every sample was done in triplicates. Three negative controls without cDNA template were also included in each plate. The fold changes were determined by the cycle threshold (2−ΔΔCt) method using Sequence Detection version 1.3 software (Applied Biosystems).
Western blot analysis.
Total lysate of the M-1 cells was used to measure the protein levels of the three ENaC subunits. Treated cells were washed three times with PBS, centrifuged, and lysed by PARIS kit (catalog no. AM1921, Ambion).
Membrane fractions of the M-1 cells were used to measure the protein levels of prostasin. As previously described by Chen et al. (13), treated cells were lysed on ice for 1 h with Tris-buffered saline (TBS) containing 1% Triton X-114 (Sigma) and protease inhibitors. After centrifugation at 12,000 rpm for 30 min, the supernatant was placed on a sucrose cushion [6% (wt/vol) sucrose in TBS containing 0.06% Triton X-114]. The solution was then incubated at 37°C for 3 min and centrifuged at 300 g for 3 min at room temperature to separate the detergent phase (pellet) and the aqueous phase. The aqueous phase was removed, and the detergent phase (pellet) was precipitated with acetone and resuspended in ice-cold TBS.
Prepared protein samples were separated with 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membrane was blocked with 5% nonfat milk in PBS + Tween 20 for 1 h and then immunoblotted with commercially available antibodies against α-ENaC (1:500 dilution, rabbit anti-mouse, polyclonal; Affinity BioReagents, Golden, CO), β-ENaC (1:250 dilution, rabbit anti-human, polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA), γ-ENaC (1:500 dilution, rabbit anti-rat, polyclonal; Millipore, Billerica, MA), and prostasin (1:500 dilution, mouse anti-human, monoclonal; Becton, Dickinson, Franklin Lakes, NJ). To check for equal loading, membranes were reprobed with an antibody against β-actin (1:800 dilution; Santa Cruz Biotechnology). Bands were visualized using chemiluminescence substrate followed by exposure to X-ray film. The band densities were measured by densitometry using the Quantity One program (Bio-Rad Laboratories, Hercules, CA). Data are expressed as the ratio of IL-6-treated to control cells after normalization to β-actin expression.
Electrophysiological transepithelial measurements.
Early passage 11–17 M-1 cells were used for electrophysiological transepithelial measurements. As previously described (35), transepithelial voltage (Vte) and Rte were measured with an ohm/voltmeter (EVOM, WPI, Sarasota, FL). Vte was measured by means of a set of 2 Ag-AgCl electrodes and determined with the apical solution as reference. Rte was measured by passage of current through the cell monolayer and measurement of the resulting voltage gradient across the cells. The equivalent current (Ieq) was calculated as the ratio of Vte to Rte and was normalized by dividing Ieq by the surface area (113 mm2) of active membrane. After evaluations of the dose dependency and time course (see results), a final concentration of IL-6 (100 ng/ml) was used for 12 h to study the effects of IL-6 on ENaC function. To determine the magnitude of ENaC-mediated sodium transport across the apical membrane, an ENaC inhibitor (amiloride, 10 μmol/l) was added to the cell medium for 10 min. Similarly, cells were incubated with aprotinin (28 μg/ml) for 12 h to inhibit the activity of prostasin. Cells were also treated with aldosterone (1 μmol/l) for 12 h from the apical side of the cells as a reference to evaluate the effects of IL-6 on the electrophysiological transepithelial parameters.
Statistical analyses.
Values are means ± SE. Statistical analyses were performed with SPSS 15.0 (SPSS, Chicago, IL). For Western blotting and gene expressions, Student's t-test was used to determine statistical significance between two groups before and after experimental manipulations. For electrophysiological transepithelial measurements, ANOVA was used to determine the statistical significance among groups before and after experimental manipulations. Results were considered significant if P < 0.05.
RESULTS
Effects of IL-6 on the gene expression of ENaC subunits and prostasin.
Treatment with IL-6 (100 ng/ml) for 12 h increased the mRNA abundance of α-ENaC by 11 ± 7% (P = not significant), β-ENaC by 78 ± 14% (P = 0.01), γ-ENaC by 185 ± 38% (P = 0.02), and prostasin by 29 ± 5% (P = 0.01; Fig. 1).
Fig. 1.
Effects of IL-6 on mRNA expression of epithelial sodium channel (ENaC) α-, β-, and γ-subunits and prostasin. M-1 cells, which were serum-deprived for 12 h, were treated with 100 ng/ml IL-6 for 12 h. At 12 h after treatment, total RNA was extracted from M-1 cells, and 5 μg of total RNA were reverse-transcribed to cDNA with oligo(dT) primers. Abundance of each mRNA was normalized for β-actin and compared with control. Values [means ± SE (n = 5)] are expressed as fold increase over control. *P < 0.05 vs. control.
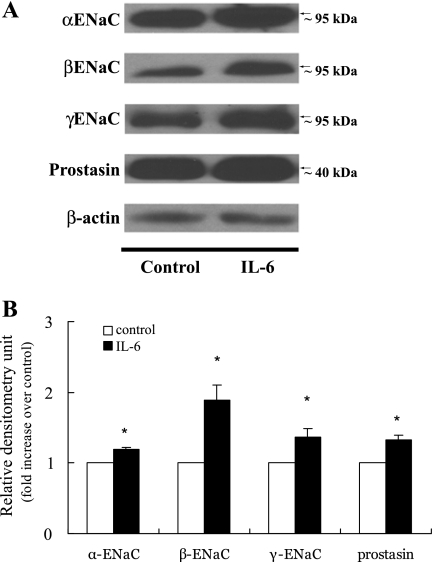
Effects of IL-6 on the protein expression of ENaC subunits and prostasin.
Treatment with IL-6 (100 ng/ml) for 12 h increased the protein expression of α-ENaC by 19 ± 3% (P = 0.001), β-ENaC by 89 ± 21% (P = 0.01), γ-ENaC by 36 ± 12% (P = 0.02), and prostasin by 33 ± 6% (P = 0.02; Fig. 2). In contrast with the previous report, we were not able to detect the presence of prostasin in the cell medium (39), which could be due to the low level of prostasin secreted into the cell medium from the cell membrane.
Fig. 2.
Effects of IL-6 on protein expression of α-ENaC, β-ENaC, γ-ENaC, and prostasin. A: M-1 cells, which were serum-deprived for 12 h, were treated with 100 ng/ml IL-6, and protein expression was determined by immunoblotting (10% SDS-PAGE). B: bands in A were quantified by densitometry and expressed as fold increase over control. Values are means ± SE (n = 5). *P < 0.05 vs. control.
Amiloride-sensitive Ieq in response to IL-6.
IL-6 concentrations of 10, 100, 150, and 200 ng/ml were used to test the potential dose dependency of Ieq (n = 3 for each dose). The amiloride-sensitive Ieq was increased only ∼5% by 10 ng/ml IL-6 (P > 0.05) and ∼37% by 100 ng/ml IL-6 (P < 0.01). The higher doses, 150 and 200 ng/ml, however, appeared to decrease the amiloride-sensitive Ieq ∼30% (P = 0.04) and ∼40% (P = 0.01), respectively. Thus, 100 ng/ml was used to test the effects of IL-6 on amiloride-sensitive Ieq in the M1-cells. Next, the effects of 100 ng/ml IL-6 were evaluated at 30 min, 1 h, 6 h, 12 h, 24 h, and up to 48 h (n = 3 for each time point). The amiloride-sensitive Ieq remained unchanged at 30 min, 1 h, and 6 h (P > 0.05). At 24 and 48 h, amiloride-sensitive Ieq was decreased up to 42% (P = 0.001) and 56% (P = 0.04), respectively. However, at 12 h, the amiloride-sensitive Ieq significantly increased (Fig. 3). As such, in the following experiments, M-1 cells were treated with IL-6 at 100 ng/ml for 12 h.
Fig. 3.
Stimulation of equivalent short-circuit current (Ieq) by IL-6. IL-6 (100 ng/ml) significantly increased Ieq by 37 ± 5% compared with nontreated cells. Amiloride almost completely inhibited IL-6-induced Ieq, and preincubation of IL-6 for 12 h did not counterbalance the inhibitory effect of amiloride on Ieq. Values are means ± SE (n = 3). *P < 0.05 vs. control.
Amiloride, an ENaC inhibitor at a concentration of 10 μmol/l, almost completely abolished Ieq. Conversely, aldosterone (1 μmol/l) increased the amiloride-sensitive Ieq by 66 ± 5%. The aldosterone-induced Ieq was inhibited by 31 ± 4% by aprotinin (28 μg/ml), a prostasin inhibitor. The amiloride-sensitive Ieq increased by 37 ± 5%, from 6.0 ± 0.4 to 8.2 ± 0.3 μA/cm2 (P < 0.01), in the cells treated with IL-6 (100 ng/ml) compared with controls. Amiloride almost completely inhibited IL-6-induced Ieq, and IL-6 did not counterbalance the inhibitory effect of amiloride on Ieq (Fig. 3). After 12 h of incubation, aprotinin (28 μg/ml), a prostasin inhibitor, reduced the amiloride-sensitive Ieq by 61 ± 5%, from 6.4 ± 0.2 to 3.4 ± 0.3 μA/cm2 (P < 0.01), by reducing Rte and Vte (Table 1). In contrast, IL-6 did not alter Rte but increased Vte from 8.4 ± 0.2 to 12.3 ± 0.8 mV and, in turn, increased Ieq. The magnitude of the IL-6-induced amiloride-sensitive Ieq in the presence of aprotinin dropped by 57 ± 2%, from 8.4 ± 0.2 to 4.9 ± 0.2 μA/cm2 (P < 0.01). Furthermore, IL-6 did not alter the amiloride-sensitive Ieq in the cells pretreated with aprotinin (Fig. 4).
Table 1.
Effects of IL-6 on Rte and Vte
| Group | Rte, Ω·cm2 | Vte, mV | Ieq, μA/cm2 |
|---|---|---|---|
| Control | 292.3 ± 7.5 | 8.4 ± 0.2 | 6.4 ± 0.2 |
| Aprotinin | 262.0 ± 20.4* | 4.0 ± 0.3* | 3.4 ± 0.3* |
| IL-6 | 307.3 ± 6.7 | 12.3 ± 0.8* | 8.9 ± 0.4* |
| IL-6 + aprotinin | 250.7 ± 6.8* | 5.6 ± 0.4* | 4.9 ± 0.2* |
| Aprotinin + IL-6 | 254.0 ± 15.9* | 4.2 ± 0.8* | 3.6 ± 0.5* |
Values are means ± SE. Treatment with aprotinin (28 μg/ml) for 12 h decreased transepithelial resistance (Rte) and voltage (Vte); treatment with IL-6 (100 ng/ml) for 12 h did not alter Rte but markedly increased Vte. In cells pretreated with IL-6 for 12 h (IL-6 + aprotinin), aprotinin decreased Rte and Vte; in cells pretreated with aprotinin for 12 h (aprotinin + IL-6), IL-6 did not alter Rte or Vte. Ieq, transepithelial equivalent current.
P < 0.05 vs. control.
Fig. 4.
Inhibition of IL-6-induced Ieq stimulation. Aprotinin (28 μg/ml), a prostasin inhibitor, reduced the amiloride-sensitive Ieq by 61 ± 5%. Magnitude of the IL-6-induced Ieq dropped by 57 ± 2% after 12 h of incubation in aprotinin. IL-6 did not alter Ieq in cells pretreated with aprotinin for 12 h. Values are means ± SE (n = 3). *P < 0.05 vs. control.
DISCUSSION
The present study is one of the first to examine the distal tubular effects of IL-6, and the major finding is that exogenous IL-6 appears to augment ENaC expression and activity in M-1 CCD cells in vitro. M-1 cells treated with IL-6 exhibit an increase in the mRNA and protein levels of ENaC subunits and prostasin. The significant increase in Ieq in response to IL-6 is abolished by amiloride, indicating that the electrophysiological induction likely is of ENaC origin. Furthermore, the IL-6 induced Ieq is aprotinin-sensitive, suggesting that prostasin could contribute to the IL-6-related ENaC pathway. Our data imply that IL-6 might play a key role in sodium handling in the kidney through coordinated regulation of ENaC and serine proteases such as prostasin.
Physiological stimuli for the synthesis of IL-6 include norepinepherine, ANG II, other cytokines, and even IL-6 itself (62). Whether IL-6 originates locally or systemically, IL-6 is the main circulating cytokine in linking systemic inflammation with local pathology (43). High salt intake, aldosterone, and ANG II have been shown to increase circulatory IL-6 level in Dahl-salt sensitive rats, hypertensive rats, diabetic rats, and normotensive humans (7, 36, 53, 57). High circulating levels of IL-6 have been correlated with increased blood pressure in spontaneously hypertensive rats and apparently healthy men and might be an independent risk factor for future myocardial infarction in humans (5, 11, 45, 50). It was suggested that IL-6 could be a determinant of diastolic blood pressure in children with obesity-related hypertension (21). Our results suggest that IL-6 might impair natriuresis via ENaC activation, which could partly contribute to salt sensitivity in subclinical inflammatory conditions such as diabetes, obesity, hypertension, and kidney diseases.
Inflammation and proinflammatory cytokines have been demonstrated to affect the function of ENaC. Transforming growth factor-β1 (TGF-β1) was shown to antagonize the action of aldosterone on sodium transport by the rat inner medullary collecting ducts in primary cell culture (27, 54). Specifically, TGF-β1 decreased the endogenous prostasin mRNA and protein expression by 50 ± 12% and 44 ± 12%, respectively, and the amiloride-sensitive 22Na uptake by 35.9 ± 4.8% in cultured M-1 cells, in which the IκBα and NF-κB/Rel signaling system might be involved (58). In another independent study, the TGF-β-induced reduction of amiloride-sensitive Ieq was alleviated in the NH2-terminal-truncated Smad4-overexpressed M1 cells, suggesting that TGF-β1 may decrease the ENaC functionality via a Smad4-dependent pathway (12). Wakida et al. (65) demonstrated that treatment of M-1 cells with TGF-β1 significantly increased the protein expression of protease nexin-1, an endogenous serine protease inhibitor of prostasin. Data on tumor necrosis factor (TNF)-α, however, have been inconsistent. In rats with streptozotocin-induced diabetes, DiPetrillo et al. (15, 17) showed that TNF-α stimulated sodium uptake in the distal tubule cells, providing a possible mechanism for TNF-induced sodium retention and early diabetic nephropathy. Furthermore, while TNF altered distal tubule sodium transport chronically and acutely, chronic TNF exposure led to distal tubule sensitization that permitted acute TNF-induced activation of ENaC in diabetic rats (16). On the contrary, in cultured A6 distal nephron cells, inhibition of ENaC by TNF-α was mediated by ceramide through protein kinase C (2). The mRNA expressions of α-ENaC, β-ENaC, and γ-ENaC were depressed after treatment of mice and cultured M-1 cells with TNF-α and IL-1β, respectively (52). Interferon-γ also altered ENaC function in vitro and in vivo (52). Furthermore, lipopolysaccharide-induced downregulation of ENaC expression was inhibited in knockout mice with a deficiency for TNF-α, IL-1 receptor-1, or interferon-γ. In addition, TNF-α and IL-1β inhibited electrogenic sodium absorption in rat distal colon by mRNA expression regulation of β-ENaC and γ-ENaC (3). The effects of IL-2, IL-4, and IL-13 on sodium absorption, possibly via ENaC, were also shown in colon or bronchial epithelium (4, 20), although their impact on renal ENaC has not been studied. Masilamani et al. (37) demonstrated that elevated circulating aldosterone (due to dietary salt restriction or aldosterone infusion) markedly increased the abundance of α-ENaC protein without increasing the abundance of β-ENaC and γ-ENaC. In the present study, the responses of the gene and protein expression of ENaC subunits to IL-6 also seem to be heterogeneous, although they could be attributable to antibodies from different commercial sources in terms of the Western blot data. IL-6 consistently and markedly enhanced the mRNA and protein expression of β-ENaC, but the increase in the protein expression of γ-ENaC and α-ENaC was relatively small. IL-6 did not alter the gene expression of α-ENaC. The increase in the gene and protein expression of prostasin was also marginal, such that the biological impact of these subtle changes is in question. The aldosterone-induced Ieq (66 ± 5%) was more than twice as high as the IL-6-induced Ieq (37 ± 5%), suggesting that aldosterone is a more powerful ENaC regulator than is IL-6. Unlike aprotinin, IL-6 did not seem to inhibit the Rte. Instead, treatment with IL-6 resulted in the increment of voltage and, subsequently, the elevation of Ieq, indicating that IL-6 might interact with ENaC to increase amiloride-sensitive sodium current. More than half (57 ± 2%) of the IL-6-induced Ieq was reduced by the inhibition of aprotinin, indicating that the stimulatory effect of IL-6 on ENaC activity involves the proteolytic processing. However, cautions are needed in the data interpretation, because aprotinin, also known as bovine pancreatic trypsin inhibitor, is a nonspecific inhibitor of prostasin (69). Several other serine proteases, such as kallikrein and trypsin, are also inhibited by aprotinin, so that other serine proteases may be induced by IL-6. The involvement of prostasin in the IL-6-ENaC conduit needs to be further studied in vitro and in vivo. It is likely that IL-6 could affect ENaC via signal transduction pathways, such as the NF-κB cascade (66, 32), which may be also involved in prostasin activity (58, 65). Furthermore, our data with a single dose (100 ng/ml) at a single time point (12 h) need to be interpreted with caution. In addition, we did not separate the immature from the mature form of the ENaC subunits, which is another limitation, since channel processing and trafficking may play an important role in the response of ENaC to the stimuli in the kidney (22, 23).
We speculate that, apart from ENaC, IL-6 may regulate other renal sodium channels, such as the sodium/hydrogen exchanger (NHE3) and sodium-chloride cotransporter in the proximal tubules, which collectively contribute to the tubular sodium reabsorption. In particular, prostasin is believed to be expressed in the proximal tubules (68) and may affect these sodium transporters.
Perspectives and Significance
Taken together, we have demonstrated that exposure of M-1 cells to IL-6 might upregulate ENaC expression. Exogenous IL-6 increases amiloride-sensitive Ieq in CCD cells in vitro. The present study also envisages the possibility that IL-6 might induce ENaC activation through proteolytic processes, although more mechanistic approaches are required to prove its causal effects. IL-6, a multifunctional cytokine, has been shown to be increased in hypertension, diabetes, and obesity, all of which demonstrate impaired sodium handling. Although further studies are required to fully elucidate the detailed regulatory mechanisms and biological implications of IL-6-induced ENaC activation in cells, animal models, and human subjects, our data could provide a potential mechanism linking sodium homeostasis derangement with chronic subclinical inflammatory conditions, including diabetes and diabetic nephropathy, obesity, hypertension, and kidney disease.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-077230 and HL-085817.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol 12: 1114–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-α through protein kinase C. Am J Physiol Renal Physiol 293: F1178–F1186, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Barmeyer C, Amasheh S, Tavalali S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. IL-1β and TNFα regulate sodium absorption in rat distal colon. Biochem Biophys Res Commun 317: 500–507, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Barmeyer C, Horak I, Zeitz M, Fromm M, Schulzke JD. The interleukin-2-deficient mouse model. Pathobiology 70: 139–142, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens 19: 149–154, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bens M, Chassin C, Vandewalle A. Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells. Pflügers Arch 453: 133–146, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 63: 1791–1800, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Boswell RN, Yard BA, Schrama E, van Es LA, Daha MR, van der Woude FJ. Interleukin 6 production by human proximal tubular epithelial cells in vitro: analysis of the effects of interleukin-1α (IL-1α) and other cytokines. Nephrol Dial Transplant 9: 599–606, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its α-subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension 38: 399–403, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Chang CT, Hung CC, Chen YC, Yen TH, Wu MS, Yang CW, Phillips A, Tian YC. Transforming growth factor-β1 decreases epithelial sodium channel functionality in renal collecting duct cells via a Smad4-dependent pathway. Nephrol Dial Transplant 23: 1126–1134, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Chen LM, Skinner ML, Kauffman SW, Chao J, Chao L, Thaler CD, Chai KX. Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J Biol Chem 276: 21434–21442, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol 111: 127–138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol 284: F113–F121, 2003 [DOI] [PubMed] [Google Scholar]
- 16.DiPetrillo K, Coutermarsh B, Soucy N, Hwa J, Gesek F. Tumor necrosis factor induces sodium retention in diabetic rats through sequential effects on distal tubule cells. Kidney Int 65: 1676–1683, 2004 [DOI] [PubMed] [Google Scholar]
- 17.DiPetrillo K, Gesek FA. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am J Nephrol 24: 352–359, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Fukatsu A, Matsuo S, Tamai H, Sakamoto N, Matsuda T, Hirano T. Distribution of interleukin-6 in normal and diseased human kidney. Lab Invest 65: 61–66, 1991 [PubMed] [Google Scholar]
- 19.Fukatsu A, Matsuo S, Yuzawa Y, Miyai H, Futenma A, Kato K. Expression of interleukin 6 and major histocompatibility complex molecules in tubular epithelial cells of diseased human kidneys. Lab Invest 69: 58–67, 1993 [PubMed] [Google Scholar]
- 20.Galietta LJ, Pagesy P, Folli C, Caci E, Romio L, Costes B, Nicolis E, Cabrini G, Goossens M, Ravazzolo R, Zegarra-Moran O. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 168: 839–845, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Garanty-Bogacka B, Syrenicz M, Syrenicz A, Gebala A, Lulka D, Walczak M. Serum markers of inflammation and endothelial activation in children with obesity-related hypertension. Neuroendocrinol Lett 26: 242–246, 2005 [PubMed] [Google Scholar]
- 22.Hanwell D, Ishikawa T, Saleki R, Rotin D. Trafficking and cell surface stability of the epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells. J Biol Chem 277: 9772–9779, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hill WG, An B, Johnson JP. Endogenously expressed epithelial sodium channel is present in lipid rafts in A6 cells. J Biol Chem 277: 33541–33544, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Dohi K, Ishikawa H, Ohmoto Y. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol 143: 3949–3955, 1989 [PubMed] [Google Scholar]
- 25.Hughey RP, Carattino MD, Kleyman TR. Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens 16: 444–450, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Husted RF, Matsushita K, Stokes JB. Induction of resistance to mineralocorticoid hormone in cultured inner medullary collecting duct cells by TGF-β1. Am J Physiol Renal Fluid Electrolyte Physiol 267: F767–F775, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Ihm CG, Jeong KW, Lee SH, Lee TW, Park JK. Effects of therapeutic agents on the inflammatory and fibrogenic factors in IgA nephropathy. Nephrology (Carlton) 12Suppl 3: S25–S26, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Iwashita K, Kitamura K, Narikiyo T, Adachi M, Shiraishi N, Miyoshi T, Nagano J, Tuyen DG, Nonoguchi H, Tomita K. Inhibition of prostasin secretion by serine protease inhibitors in the kidney. J Am Soc Nephrol 14: 11–16, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kleyman TR, Myerburg MM, Hughey RP. Regulation of ENaCs by proteases: an increasingly complex story. Kidney Int 70: 1391–1392, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Kusaka M, Mackenzie HS, Ziai F, Hancock WW, Tilney NL. Recipient hypertension potentiates chronic functional and structural injury of rat renal allografts. Transplantation 74: 307–314, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lebowitz J, Edinger RS, An B, Perry CJ, Onate S, Kleyman TR, Johnson JP. IκB kinase-β (IκKβ) modulation of epithelial sodium channel activity. J Biol Chem 279: 41985–41990, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Lee DL, Leite R, Fleming C, Pollock JS, Webb RC, Brands MW. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension 44: 259–263, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Hering-Smith KS, Schiro FR, Hamm LL. Serine protease activity in M-1 cortical collecting duct cells. Hypertension 39: 860–864, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension 48: 1050–1057, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC-α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakhoul NL, Hering-Smith KS, Gambala CT, Hamm LL. Regulation of sodium transport in M-1 cells. Am J Physiol Renal Physiol 275: F998–F1007, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, Nonoguchi H, Chen LM, Chai KX, Chao J, Tomita K. Regulation of prostasin by aldosterone in the kidney. J Clin Invest 109: 401–408, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Navarro JF, Milena FJ, Mora C, Leon C, Garcia J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 26: 562–570, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papanicolaou DA, Vgontzas AN. Interleukin-6: the endocrine cytokine. J Clin Endocrinol Metab 85: 1331–1333, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Raasveld MH, Weening JJ, Kerst JM, Surachno S, ten Berge RJ. Local production of interleukin-6 during acute rejection in human renal allografts. Nephrol Dial Transplant 8: 75–78, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767–1772, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc 1: 4–9, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl: S12–S22, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Sakai A, Kawano M, Kuramoto A. Interleukin-6 produced by renal-cell carcinoma cells and progression of multiple myeloma. N Engl J Med 324: 1893–1894, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Samuelsson AM, Alexanderson C, Molne J, Haraldsson B, Hansell P, Holmang A. Prenatal exposure to interleukin-6 results in hypertension and alterations in the renin-angiotensin system of the rat. J Physiol 575: 855–867, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanz-Rosa D, Oubina MP, Cediel E, de Las Heras N, Vegazo O, Jimenez J, Lahera V, Cachofeiro V. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-κB/IκB system. Am J Physiol Heart Circ Physiol 288: H111–H115, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Schafer JA. Abnormal regulation of ENaC: syndromes of salt retention and salt wasting by the collecting duct. Am J Physiol Renal Physiol 283: F221–F235, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Schmidt C, Hocherl K, Bucher M. Regulation of renal glucose transporters during severe inflammation. Am J Physiol Renal Physiol 292: F804–F811, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol 93: 817–824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes JB. Physiologic resistance to the action of aldosterone. Kidney Int 57: 1319–1323, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Stoos BA, Naray-Fejes-Toth A, Carretero OA, Ito S, Fejes-Toth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int 39: 1168–1175, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Suzuki D, Miyazaki M, Naka R, Koji T, Yagame M, Jinde K, Endoh M, Nomoto Y, Sakai H. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes 44: 1233–1238, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Tuyen DG, Kitamura K, Adachi M, Miyoshi T, Wakida N, Nagano J, Nonoguchi H, Tomita K. Inhibition of prostasin expression by TGF-β1 in renal epithelial cells. Kidney Int 67: 193–200, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Vallet V, Horisberger JD, Rossier BC. Epithelial sodium channel regulatory proteins identified by functional expression cloning. Kidney Int Suppl 67: S109–S114, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Vallet V, Pfister C, Loffing J, Rossier BC. Cell-surface expression of the channel activating protease xCAP-1 is required for activation of ENaC in the Xenopus oocyte. J Am Soc Nephrol 13: 588–594, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol 8: 253–278, 1990 [DOI] [PubMed] [Google Scholar]
- 63.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol 120: 191–201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol 11: 828–834, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Wakida N, Kitamura K, Tuyen DG, Maekawa A, Miyoshi T, Adachi M, Shiraishi N, Ko T, Ha V, Nonoguchi H, Tomita K. Inhibition of prostasin-induced ENaC activities by PN-1 and regulation of PN-1 expression by TGF-β1 and aldosterone. Kidney Int 70: 1432–1438, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-κB activation in the intestinal epithelia. J Immunol 171: 3194–3201, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Wolkow PP, Niewczas MA, Perkins B, Ficociello LH, Lipinski B, Warram JH, Krolewski AS. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 19: 789–797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu JX, Chao L, Chao J. Molecular cloning, tissue-specific expression, and cellular localization of human prostasin mRNA. J Biol Chem 270: 13483–13489, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem 269: 18843–18848, 1994 [PubMed] [Google Scholar]