Abstract
We tested the hypothesis that TREK-1, a two-pore domain K channel, is involved with dilations in arteries. Because there are no selective activators or inhibitors of TREK-1, we generated a mouse line deficient in TREK-1. Endothelium-mediated dilations were not different in arteries from wild-type (WT) and TREK-1 knockout (KO) mice. This includes dilations of the middle cerebral artery to ATP, dilations of the basilar artery to ACh, and relaxations of the aorta to carbachol, a cholinergic agonist. The nitric oxide (NO) and endothelium-dependent hyperpolarizing factor components of ATP dilations were identical in the middle cerebral arteries of WT and TREK-1 KO mice. Furthermore, the NO and cyclooxygenase-dependent components were identical in the basilar arteries of the different genotypes. Dilations of the basilar artery to α-linolenic acid, an activator of TREK-1, were not affected by the absence of TREK-1. Whole cell currents recorded using patch-clamp techniques were similar in cerebrovascular smooth muscle cells (CVSMCs) from WT and TREK-1 KO mice. α-linolenic acid or arachidonic acid increased whole cell currents in CVSMCs from both WT and TREK-1 KO mice. The selective blockers of large-conductance Ca-activated K channels, penitrem A and iberiotoxin, blocked the increased currents elicited by either α-linolenic or arachidonic acid. In summary, dilations were similar in arteries from WT and TREK-1 KO mice. There was no sign of TREK-1-like currents in CVSMCs from WT mice, and there were no major differences in currents between the genotypes. We conclude that regulation of arterial diameter is not altered in mice lacking TREK-1.
Keywords: potassium channels, two-pore domain potassium channels (K2P), cerebrovascular circulation, vasodilation, TREK-1 knockout, KCNK2
activation of potassium (K) channels on endothelium and/or vascular smooth muscle constitutes an important step in many mechanisms of vascular dilation (6, 11, 25, 36). Traditionally, K channels from two distinct families were known to serve in this dilator role. One family is characterized by protein subunits having two transmembrane-spanning domains and one pore domain. K channels in this family include inwardly rectifying (Kir) and ATP-sensitive (KATP) K channels. A second family consists of protein subunits having six transmembrane-spanning domains and one pore domain. Members of this family include delayed rectifier or voltage-activated (KV) and calcium-activated (KCa) K channels. Both of these K channel families require the combination of four protein subunits, each contributing one pore domain, to form a fully functional K channel.
More recently, a third family of K channels has been suggested to be involved with dilations. This family consists of protein subunits having four transmembrane-spanning domains and two pore domains for each subunit. Thus, the family has been given the name, two-pore domain K channels (K2P). Unlike the other two families, K2Ps require only two protein subunits, each contributing two pore domains, to form a functional channel. To date, 15 mammalian genes coding for K2P have been identified (16).
At least 10 members of the K2P family are expressed in the vascular system. They include TWIK-1, TWIK-2, TREK-1, TREK-2, TRAAK, TASK-1, TASK-2, TASK-3, TASK-4, and THIK-1 (3, 7, 14, 15, 17–19, 34, 38). Since there are no selective activators or inhibitors for any of the K2P, our understanding of their role is limited. Nevertheless, there are reports to suggest that TREK-1, TASK-1, and TASK-2 are involved with regulation of arterial diameter (3, 14, 15, 17–19). However, more studies are needed to fully demonstrate their function in arteries and arterioles.
The second K2P to be discovered, TREK-1 (gene name KCNK2), is of interest since it is highly expressed in the vascular system (5, 7, 14, 18, 34). In fact, message for TREK-1 is 10-fold more abundant in the middle cerebral artery of the rat than the next most abundant K2P (34). One group of investigators generated TREK-1 knockout (KO) mice (22) and studied vascular responses in basilar and mesenteric arteries (3, 15). These investigators reported that stimulation of endothelial NO production elicited by ACh in basilar arteries and ACh or bradykinin in mesenteric arteries was severely impaired or abolished in arteries from TREK-1 KO mice (3, 15). The authors suggested that the pathway for activating NO synthase is profoundly dysfunctional, likely through altered endothelial Ca2+ regulation, in the absence of TREK-1 (3, 15).
These data suggest that TREK-1 is of fundamental importance in regulating NO production in endothelium. If true, then alterations in TREK-1 expression and/or function could be an important mechanism for impaired NO production in pathological states (39). Therefore, it is imperative to expand these studies involving TREK-1, endothelial NO production, and regulation of vascular tone.
The purpose of this study was to determine whether TREK-1 functions to regulate arterial diameter. First, we generated and validated a TREK-1 KO mouse line to be used in the subsequent studies. The mouse line was of major importance since there are no selective activators or inhibitors for TREK-1. Second, we determined the cardiovascular phenotype in anesthetized mice by comparing wild-type mice to TREK-1 KO mice. Because the heart and blood vessels express TREK-1, it is possible that any dysfunction, if found, would be a result of blood pressure or other cardiac phenotypic differences. Third, we compared endothelium-mediated dilations in aortas, basilar arteries, and middle cerebral arteries from wild-type and TREK-1 KO mice. Fourth, we studied dilations in basilar arteries to polyunsaturated fatty acids, which are nonselective activators of TREK-1. Finally, we studied K+ currents elicited by polyunsaturated fatty acids in single smooth muscle cells isolated from cerebral arteries of wild-type and TREK-1 KO mice.
METHODS
All studies were approved by the Institutional Animal Care and Use Committee at the Baylor College of Medicine.
TREK-1 KO mice.
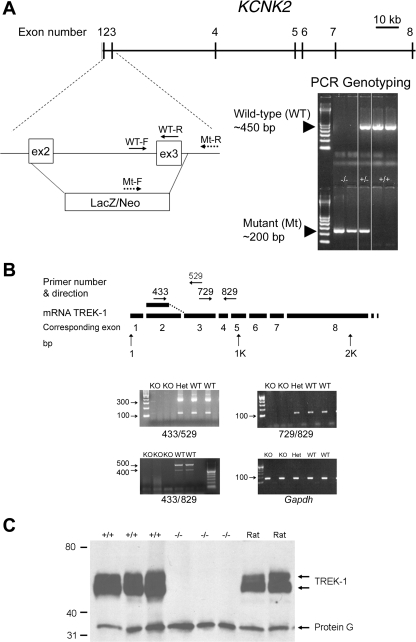
Gene targeting and generation of the TREK-1 mouse line were performed in conjunction with the Texas Institute of Genomic Medicine (Houston, TX). The gene for TREK-1, KCNK2, spans ∼136 kbp of the mouse genome, having eight exons (Fig. 1A). For the mutant gene, a 4 kbp of KCNK2 consisting of the second exon (excluding the first 13 bp), all of the second intron, all of the third exon, and the first 23 bp of the third intron was replaced by a β-galactosidase/neomycin selection cassette (Fig. 1A). The mice were generated on a mixed background of C57BL6J and SV129 strains.
Fig. 1.
A: strategy for gene targeting and generation of KCNK2−/− mice. For the mutant gene, most of exon 2 and all exon 3 were replaced by a β-galactosidase/neomycin (LacZ/Neo) selection cassette. Location of primers for genotyping and PCR products for the wild-type (WT) and mutant (Mt) gene are also shown. WT-F and WT-R represent forward and reverse primers for the WT allele, respectively; Mt-F and Mt-R represent the forward and reverse primers respectively for the mutated allele. B: diagram of TREK-1 mRNA with primer pairs used to detect strands of message. The number for the primers represents the base number of the mRNA with the arrow denoting the direction. The gel shows the presence TREK-1 mRNA in wild-type and heterozygous mice and the absence in TREK-1 knockout (KO) mice. C: Western blots of TREK-1, following immunoprecipitation, in rat, wild-type mice, and TREK-1 KO mice. The lower band marked as Protein G was used in the immunoprecipitation protocol.
PCR genotyping and reverse transcriptase-PCR.
PCR genotyping was conducted using tail DNA (see the online supplemental material and Supplemental Fig. 1A). Reverse transcriptase-PCR was conducted on RNA extracted from brain, aorta, and cerebral arteries (Fig. 1B). All primer pairs spanned at least two exons to avoid amplification of genomic DNA. See the online supplemental material for further details. The PCR products were sequenced by the fluorescent dye-terminator sequencing technique (SeqWright, Houston, TX).
Real-time RT-PCR.
Relative expression of other K2Ps and the large-conductance Ca-activated K channel (BKCa) was measured in cerebral arteries (middle cerebral arteries and basilar artery) and aorta from three adult male TREK-1 KO mice and three adult male WT mice to determine whether there was upregulation of other channels in TREK-1 KO mice. TREK-2 (KCNK10) and TRAAK (KCNK4) were chosen since they are closely related mechanosensitive K2Ps (16). TASK-1 (KCNK3) and TWIK-2 (KCNK6) were chosen because they are highly expressed in cerebral arteries (34). Additionally, relative expression of TWIK-1 (KCNK1) and BKCa (KCNMA1) was measured. After reverse transcription, cDNA was quantified from cerebral arteries and aorta by real-time PCR using SYBR Green PCR Master Mix on an Applied Biosystems 7000 Sequence Detection System. More details of the method and the primers used are presented in the online supplemental material. The efficiency of each primer set was assessed using cDNA from mouse brain and was determined to be >95%. Relative expression of each gene was normalized to GAPDH using the equation: Relative expression = 2(Δct), where Δct = ctK2P − ctGAPDH and ct = the cycle threshold.
Immunoprecipitation.
Immunoprecipitation of TREK-1 was performed as previously described (49). Briefly, TREK-1 from mouse or rat brain homogenates was immunoprecipitated using two C-terminal goat anti-TREK-1 antibodies (100 μl of each, Santa Cruz C-20 and E-19; Santa Cruz Biotechnology, Santa Cruz, CA) and protein G Sepharose beads (GE Healthcare, Piscataway, NJ). TREK-1 was eluted from the beads, and protein was separated by electrophoresis. Membranes were probed overnight with the CT#67 antibody (1:400 dilution, SA Goldstein, University of Chicago), a rabbit polyclonal antibody directed against C-terminal amino acids 371–396 of rat TREK-1 (49). These 26 amino acids of the C-terminal are identical to amino acids 356–381 of mouse TREK-1. After washing, the blots were incubated with horseradish-conjugated goat anti-rabbit antibody (Thermoscientific, Rockford, IL) at a 1:10,000 dilution. Protein expression was detected with enhanced chemiluminescense reaction (SuperSignal, West Femto Maximum Sensitivity Substrate, Thermo Scientific) and measured by ECL Hyperfilm (GE Healthcare).
Cardiovascular phenotyping of KCNK2−/− mice.
Five male KCNK2−/− mice (29.3 ± 1.1 g) and five littermate male KCNK2+/+ mice (28.7 ± 1.0 g) from KCNK2−/+ breeding pairs were anesthetized with pentobarbital sodium (50 mg/kg ip). Body temperature was monitored and maintained at 37°C. ECG was measured from electrodes attached to the paws. Blood flow velocity was measured in the left main coronary artery, the aortic arch, and the abdominal aorta using pulsed Doppler ultrasound (21, 47). Difference in arrivals of the upstroke velocity in the aortic arch and abdominal aorta was used to calculate the aortic pulse wave velocity (47). Velocity of blood flow through the left main coronary artery was recorded at baseline and when mice were ventilated with 1% and 2.5% isoflurane (21). Arterial and intraventricular pressures were measured using a Millar catheter/pressure transducer (SPR1000, 1F ≈ 0.33 mm, Millar Instruments, Houston, TX) inserted into the right carotid artery and advanced into the left ventricle.
Pressurized cerebral arteries.
Male WT mice and TREK-1 KO littermates (8–12 wk, 25–33 g) were anesthetized with pentobarbital sodium (50 mg/kg ip) and decapitated. The brain was rapidly removed and placed in chilled buffer. The middle cerebral arteries or the basilar artery were carefully dissected from the brain and mounted on glass pipettes in a vessel chamber (ChuelTech, Houston, TX), as previously described (1, 4, 7, 8). The arteries were pressurized to 75 mmHg, and physiological buffer was perfused through the lumen of each middle cerebral artery at a rate to achieve a shear stress of ∼20 dyn/cm2 on the endothelial surface (20–35 μl/min depending on the artery radius). The basilar arteries were pressurized without luminal flow to closely replicate previous studies in TREK-1 KO mice (3). See the online supplemental material for more information, including the buffer composition. Dilator responses were determined after preconstriction using 3 μM phenylephrine for middle cerebral arteries and 10 nM endothelin-1 for basilar arteries.
Constrictions were expressed as a percent decrease in the diameter of the artery. Dilations were expressed by the following equation: Dilation = 100·(Ddrug − Dconstrictor)/(Dmax − Dconstrictor), where Ddrug is the diameter after administration of the dilator agent, Dconstrictor is the diameter after constriction with either phenylephrine or endothelin-1, and Dmax is the maximum diameter of the artery determined by removing Ca2+ from the buffer.
Electrophysiological studies.
Cerebrovascular smooth muscle cells (CVSMCs) were enzymatically isolated from a basilar artery and two middle cerebral arteries, which had been pooled, using a modification of previously described protocols (see supplemental material online and Refs. 7 and 26). Whole cell currents were measured at room temperature after rupturing the membrane from individual CVSMCs. Currents are expressed as current density (pA/pF) to normalize for differences between cell sizes. The pipette and bath buffers contained 143 and 5.6 mM K+ respectively (see supplemental material online). CVSMCs were held at −50 mV before and between protocols, which was administered every 20 s. For the ramp protocol, the potential was shifted to −60 mV for 30 ms, −120 mV for 30 ms and then ramped to +100 mV over 500 ms.
Data analysis.
The data are reported as means ± SE. Data were analyzed using the two-way repeated-measure ANOVA with a post hoc Tukey test when appropriate. For studies involving real-time reverse-transcriptase PCR, data were analyzed using Students t-test with a Bonferroni correction. P values of less than 0.05 were considered significant.
RESULTS
Characterization of TREK-1 KO mice.
Figure 1A shows the KCNK2 gene, the positions of primers, and the results of PCR genotyping for wild-type and mutant mice. Amplicons of the wild-type (WT) and mutant (Mt) KCNK2 alleles are shown as bands at 450 and 200 bp (Fig. 1A). Expression of KCNK2 mRNA was determined using whole brains from KCNK2+/+ and KCNK2−/− mice (n = 5 each). The schematic location and direction of the primers and the results of the PCR are shown in Fig. 1B. Primer sets spanning any part of exons 2 or 3 failed to amplify a product in KCNK2−/− mice, while the expected amplicons were detected in the KCNK2−/+ and KCNK2+/+ mouse brains (see Fig. 1B and Supplemental Fig. 1 in the online version of this article). Alternative splicing produced two bands as expected (51) that differed by ∼200 bp (Fig. 1B; 433/529 and 433/829). All amplicons were sequenced and confirmed to be valid. Further, analysis of mRNA is provided in the supplemental material online and Supplemental Fig. 1. Fig. 1C shows a Western blot of mouse and rat brains after immunoprecipitation using two antibodies and probing with a third antibody (49). The bands around 34 kDa represent Protein G, which was used in the immunoprecipitation protocol. Rats were used as positive controls, as previously reported (49). The Western blots of brain homogenates from WT mice and rats show two prominent bands at∼50 kDa (Fig. 1C). The upper band of the doublet represents one or both splice variants for TREK-1 (51). The lower band of the doublet represents alternatively translated TREK-1 (49). Of note, the bands near 50 kDa are absent in brain homogenates of TREK-1 KO mice.
Relative expression of K2Ps and BKCa.
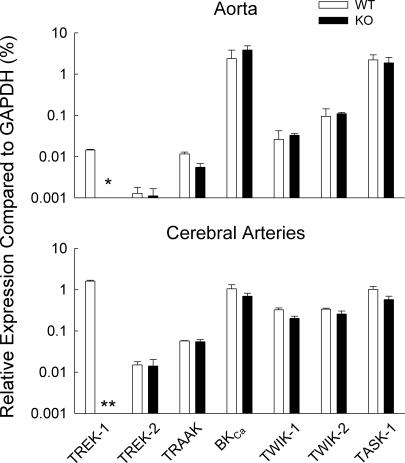
Figure 2 shows the relative expression of BKCa and K2P in aorta and cerebral arteries of WT and TREK-1 KO mice (n = 3 each). Note that mutation of KCNK2 did not alter the expression of TREK-2, TRAAK, TASK-1, TWIK-1, TWIK-2, or BKCa. Figure 2 also shows the relative abundance of mRNA for these K2P and BKCa. TREK-1 is ∼100-fold more abundant in the cerebral arteries than in the aorta of WT mice.
Fig. 2.
Relative expression of mRNA coding for K channels in aorta and cerebral arteries from WT and TREK- KO mice. The mRNA expression is shown as a percentage of the reference mRNA, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). *P = 0.01 after Bonferroni correction; **P < 0.001 after Bonferroni correction.
Cardiovascular phenotype.
Table 1 shows a number of cardiovascular indices obtained from WT and TREK-1 KO mice. Heart rate and blood pressure did not differ between the two genotypes. Peak ventricular pressure and +dP/dtmax, indicators of cardiac systolic function, were not significantly different between genotypes. Similarly, −dP/dtmax and tau (time constant for left ventricle relaxation), which are measures of cardiac diastolic function, were similar. Pulse wave velocity was not significantly changed in the TREK-1 KO mice.
Table 1.
Cardiovascular indices in WT and TREK-1 KO mice
| Cardiovascular Index | Units | WT | TREK-1 KO |
|---|---|---|---|
| Heart rate | bpm | 346 ± 33 | 336 ± 23 |
| Systolic blood pressure | mmHg | 97 ± 5 | 94 ± 8 |
| Diastolic blood pressure | mmHg | 70 ± 4 | 59 ± 9 |
| Mean arterial blood pressure | mmHg | 83 ± 4 | 75 ± 9 |
| Pulse pressure | mmHg | 26 ± 2 | 35 ± 4 |
| Peak left ventricular pressure | mmHg | 100 ± 3 | 104 ± 6 |
| +dP/dtmax | mmHg/s | 9879 ± 1439 | 9497 ± 886 |
| −dP/dtmax | mmHg/s | −13755 ± 3257 | −10337 ± 1020 |
| Tau | s | 8.1 ± 1.4 | 7.5 ± 0.8 |
| Pulse wave velocity | cm/s | 568 ± 109 | 654 ± 170 |
Values are expressed as means ± SE; n = 5 for each genotype. WT, wild type; KO, knockout.
Isoflurane, a potent dilator and a nonspecific activator of TREK-1 (41), was used to determine whether the absence of TREK-1 affected the coronary flow reserve. The peak blood flow velocities in the left main coronary arteries were similar in WT and TREK-1 KO mice. The addition of isoflurane (1 and 2.5%) to the ventilatory gas increased peak blood flow velocities to the same degree in each genotype in a concentration-dependent manner (data not shown).
Middle cerebral arteries.
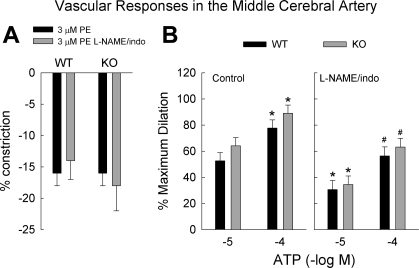
Resting diameters of MCAs after pressurization to 75 mmHg for WT and TREK-1 KO mice were 153 ± 4 μm (n = 25) and 152 ± 4 μm (n = 26), respectively. The constrictor responses to cumulative doses of phenylephrine (0.1–100 μM) were not significantly different between the two genotypes (P = 0.32, n = 9 and 10 for WT and TREK-1 KO mice, respectively; data are not shown). Each artery was preconstricted with 3 μM phenylephrine to study dilator responses (Fig. 3A). Luminal applications of ATP dilated the MCAs in a dose-dependent manner (n = 10 for each genotype; see Fig. 3B). In the presence of 10 μM nitro-l-arginine methyl ester (l-NAME) and 10 μM indomethacin to block NO synthase and cyclooxygenase, respectively, the dilations to luminal ATP were significantly reduced (P < 0.001, n = 8 for WT and 9 for KO mice, Fig. 3B). The dilation remaining after l-NAME and indomethacin is attributed to endothelium-dependent hyperpolarizing factor (EDHF) (1, 6, 52–55). There were no significant differences in the dilation between the genotype in the presence or absence of l-NAME and indomethacin. Dilation to 10−7 and 10−6 M MAHMA NONOate, an NO donor, were similar in MCAs for WT and TREK-1 KO mice (n = 4 for each genotype; data not shown).
Fig. 3.
A: constrictions of middle cerebral arteries to 3 μM phenylephrine (PE) in the absence (n = 9–10 per genotype) or presence of the inhibitors for NO synthase and cyclooxygenase (n = 5 or 6 per genotype), Nitro-l-arginine methyl ester (l-NAME) and indomethacin (indo), respectively. B: dilations of middle cerebral arteries, preconstricted with 3 μM PE, to luminally applied ATP in the absence (n = 10 for each genotype) and the presence of l-NAME and indomethacin (indo) (n = 8 or 9 per genotype). *P < 0.05 compared with corresponding genotype receiving 10−5 M ATP without inhibitors; #P < 0.05 compared with corresponding genotype receiving l-NAME and indomethacin.
Basilar artery.
Because we found no genotypic differences in endothelium-mediated dilations in the MCA, we repeated previous studies using the basilar artery (3). This included pressurization of the artery without luminal flow and administration of dilating agents to the extraluminal bath in an identical concentration. Resting diameters after pressurization to 75 mmHg were not significantly different between genotype [214 ± 7 μm and 223 ± 7 μm for basilar arteries from WT and TREK-1 KO mice, respectively (n = 16 for each group, P = 0.38)]. Constrictions to endothelin-1 (ET-1) were similar in basilar arteries from WT and TREK-1 KO (n = 5; see Supplemental Fig. 2).
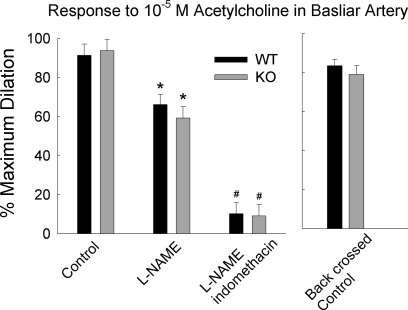
Figure 4 (left) shows dilations of the basilar arteries to 10−5 M ACh when preconstricted with 10 nM ET-1. Blocking NO production with l-NAME decreased the dilation to ACh (P = 0.009) and the combination of l-NAME and indomethacin further reduced the dilation (P < 0.001); however, the genotype of the basilar artery had no significant effect on the responses to ACh (n = 5 or 6 per group).
Fig. 4.
Dilations to ACh in basilar arteries from WT and TREK-1 KO mice in the absence or presence of l-NAME, or l-NAME plus indomethacin (n = 5 or 6 per group). The basilar arteries were preconstricted with 10 nM endothelin-1. The graph to the left shows dilations of basilar artery from WT and TREK-1 KO mice after four generations of back crossing to C57BL6J (n = 5 per genotype). *P < 0.01 compared with corresponding control; #P < 0.001compared to corresponding control and l-NAME-treated groups.
Basilar arteries in this study (Fig. 4, left) were from mice on a mixed genetic background of C57BL6J and SV129 strains. Fig. 4 (right) shows that after four backcrosses onto a C57BL6J background, basilar arteries from TREK-1 KO mice dilated to ACh, and these dilations were not significantly different from the dilations of WT littermate mice (n = 5 for each group).
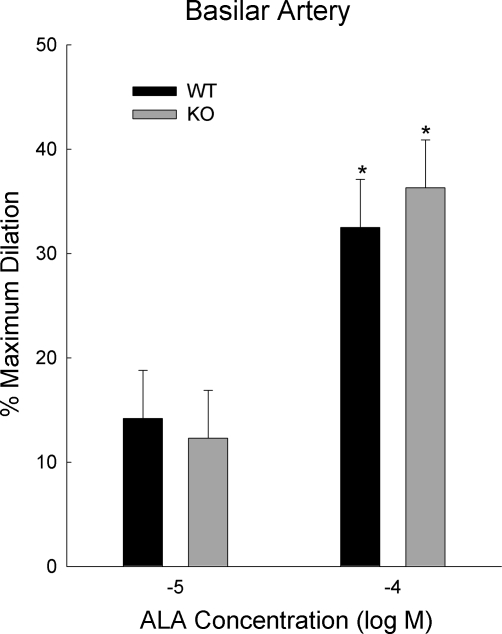
Figure 5 shows dilations to α-linolenic acid (ALA), a nonspecific dilator of TREK-1. The dilations in basilar arteries from WT and TREK-1 KO mice were not significantly different (P = 0.83; n = 5 for each group). The BKCa blocker, penitrem A (200 nM), was present in these studies since α-linolenic acid and other polyunsaturated fatty acids also activate BKCa. At the end of each experiment 10−5 M MAHMA NONOate, an NO donor, was administered. Dilations to MAHMA NONOate were similar in basilar arteries from WT and TREK-1 KO mice (n = 5 for each genotype, data not shown).
Fig. 5.
Dilations of basilar arteries from WT and TREK-1 KO mice to α-linolenic acid (ALA). *P < 0.05 compared with corresponding group given 10−5 M ALA (n = 5 per group).
Aorta.
Isometric constrictions to phenylephrine and endothelium-mediated relaxations to carbachol (a cholinergic agonist) were similar in WT and TREK-1 KO mice (see Supplemental Fig. 3 and supplemental material online).
Electrophysiology studies.
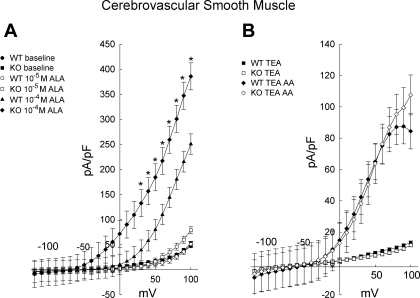
CVSMC sizes, as measured by cell capacitance, were similar between the genotypes. The capacitance, which is directly related to cell surface area, was 12.0 ± 0.4 pF (n = 50) and 12.4 ± 0.3 pF (n = 56) for CVSMCs from WT and TREK-1 KO, respectively (P = 0.41). Whole cell currents recorded in CVSMCs from WT and TREK-1 KO mice were almost identical (Fig. 6A and Supplemental Fig. 4). Figure 6A shows the effects of 10−5 and 10−4 M α-linolenic acid on whole cell currents in CVSMCs from WT and TREK-1 KO mice. The vehicle for α-linolenic acid, ETOH, had no effect on current when given alone (data not shown). Interestingly, CVSMCs from TREK-1 KO showed an enhanced response to 10−4 M α-linolenic acid compared with WT. Repeated-measures ANOVA revealed a significant interaction between genotype and membrane potential (P = 0.002; n = 7 and 4 for WT and TREK-1 KO, respectively).
Fig. 6.
A: effects of ALA (10 and 100 μM) on current density in cerebrovascular smooth muscle cells from WT (n = 7) and TREK-1 KO (n = 4) mice. *P < 0.05 compared with WT exposed to 100 μM α-linolenic acid. B: effects of 10 μM arachidonic acid (AA) on current density of cerebrovascular smooth muscle cells from WT (n = 9) and TREK-1 KO (n = 7) mice. Tetraethylammonium (TEA; 10 mM) was present in all studies shown in 6B.
Arachidonic acid is one of the most widely used tools used to activate TREK-1 (10, 20, 33, 51). Figure 6B shows whole cell currents before and after the addition of 10−5 M arachidonic acid. Tetraethylammonium (TEA; 10 mM), a blocker of BKCa that does not inhibit TREK-1 (42), was present in all experimental conditions. Arachidonic acid increased the whole cell currents in both CVSMCs for both genotypes. However, unlike α-linolenic acid, there was no significant difference between currents from WT and TREK-1 KO mice (P = 0.88; n = 9 and 7, respectively).
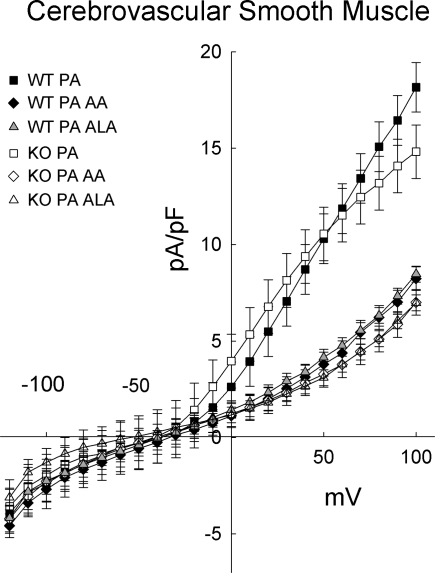
Figure 7 shows the whole cell currents after the addition of α-linolenic acid (n = 12 each for group) or arachidonic acid (n = 7 WT and n = 6 KO) when 200 nM penitrem A (PA), a BKca blocker, was present in the bath. Penitrem A not only prevented the increase in currents elicited by α-linolenic or arachidonic acid, but the whole cell currents actually decreased upon application of either α-linolenic or arachidonic acid. Inhibition of voltage-sensitive K channels by polyunsaturated fatty acid may account for the decreased current (9). In the absence of penitrem A, the decrease in whole cell current due to the inhibition of voltage-sensitive K channels is masked by the much larger increase in BKCa currents. The vehicle for penitrem A, DMSO, had no effect on whole cell currents when administered alone (data not shown). Similar to penitrem A, 200 nM iberiotoxin, another selective BKCa blocker, prevented the increase in currents upon administration of 10−4 M α-linolenic acid or 10−5 M arachidonic acid (data not shown).
Fig. 7.
In the presence of 200 nM penitrem A (PA), a selective blocker of large-conductance Ca-activated K channels, currents decreased with the addition of either 10 μM AA or 100 μM ALA (n = 6–12 per group) in cerebrovascular smooth muscle cells from WT and TREK-1 KO mice.
DISCUSSION
The purpose of this study was to determine whether TREK-1 functions to regulate arterial diameter. Because there are no selective activators or inhibitors, we generated mice that did not express TREK-1. From our results, we conclude that regulation of arterial diameter is not altered in mice lacking TREK-1. This conclusion is based on the following observations: 1) Dilator responses were similar in arteries from WT and TREK-1 KO mice (Figs. 3B, 4, and 5, and Supplemental Fig. 3B). In addition, the increase in blood velocity in the left main coronary artery was similar in WT and TREK-1 KO mice after the addition of isoflurane, a nonselective activator of TREK-1 (41), to the ventilatory gas. 2) Freshly isolated CVSMCs did not show TREK-1-like currents (Figs. 6 and 7, and Supplemental Fig. 4). Our conclusion is strengthened and amplified by the confirming results from arteries of multiple origins or vascular beds, the use of multiple vasoactive agents, and the electrophysiological studies.
We report that dilator responses were not altered in arteries from TREK-1 KO mice. These studies include velocity measurement in the left main coronary artery to isoflurane (data not shown), dilations of the middle cerebral artery to ATP (Fig. 3B), dilations of the basilar to ACh (Fig. 4), dilations of the basilar artery to α-linolenic acid (Fig. 5), and relaxations of the aorta to carbachol (Supplemental Fig. 3B). Not only were the responses similar in these arteries from WT and TREK-1 KO mice, but the NO and EDHF components were identical in the middle cerebral arteries (Fig. 3B), and the NO and cyclooxygenase-dependent components were identical in the basilar arteries of WT and TREK-1 KO mice (Fig. 4). Given that we used only one or two concentrations of the dilating agents, it is possible that we missed subtle differences in the concentration response curves of the dilators.
We observed increased currents in CVSMCs obtained from both WT and TREK-1 KO mice after administering either of the polyunsaturated fatty acids, α-linolenic, or arachidonic acid (Fig. 6). While polyunsaturated fatty acids activate TREK-1 (10, 20, 33, 51), they also can activate BKCa and the K2Ps, TREK-2, TRAAK, TWIK-2, and THIK-1 (12, 27, 35, 40, 44, 46). Interestingly, the currents elicited by α-linolenic acid, but not arachidonic acid, were greater in CVSMCs from TREK-1 KO mice than those from WT mice. We have no explanation for this enhanced current for one polyunsaturated fatty acid, α-linolenic, but not another, arachidonic acid. Regardless of the magnitude of the current, selective BKCa inhibitors, penitrem A, or iberiotoxin, completely abolished the α-linolenic and arachidonic acid currents in both genotypes (Fig. 7). We conclude that the polyunsaturated fatty acids that stimulated currents in CVSMCs from either WT or TREK-1 KO mice were a result of BKCa activation. Thus, TREK-1-like currents were not observed in CVSMCs from WT mice after blockade of BKCa and there were no differences in currents between the genotype. The electrophysiological studies reinforce the idea that TREK-1 is not involved with regulation of arterial diameter.
Our results in isolated basilar arteries differ from those of a previous study that used a different strain of TREK-1 KO mice (3). Although we found no differences in dilator responses in the basilar arteries from WT and TREK-1 KO mice, Blondeau et al. (3) reported that dilations elicited by ACh and α-linolenic acid were abolished or severely attenuated in basilar arteries from their strain of TREK-1 KO mice. Given this discrepancy, we felt that several pertinent points should be discussed.
One possibility for the discrepancy is that our mouse model is not a true TREK-1 KO line. Because our model was integral to these studies, it was thoroughly characterized in terms of gene structure, message transcribed, and protein translated. PCR genotyping and reverse transcriptase PCR confirmed the insertion of the β-galactosidase/neomycin selection cassette and the absence of exon 3 and most of exon 2 (Fig. 1, A and B). Finally, Western blot analysis, following immunoprecipitation, conclusively demonstrated that TREK-1 protein was absent in the KO mice (Fig. 1C). This is the first Western blot that has been published to date using TREK-1 KO mice. The antibodies used for the immunoprecipitation and Western blot should detect any TREK-1 being translated, regardless of which one of the multiple start codons was used to initiate protein translation. Thus, our studies conclusively demonstrate that our mouse line does not express TREK-1.
Different knockout strategies for Kv channels have produced different phenotypes in mice (37). It is possible that the different strategies used to generate the two TREK-1 knockout strains account for the differences in the results in isolated basilar arteries. In our strategy, both the second and third exons of KCNK2 were deleted, removing all start codons. The earliest in-frame start codon of the mutated KCNK2 mRNA corresponds to a portion of the last transmembrane domain. Additionally, our knockout should express β-galactosidase and a neomycin selection protein (see Fig. 1A). It is possible that these two alien proteins could alter function in the cell; however, it is difficult to envision that their expression would restore TREK-1 function in the knockout arteries. The previous strategy for the TREK-1 knockout strain, which was used by Blondeau et al. (3), removed only the third exon (22). A start codon in exon 2 would be retained in the mutated TREK-1 with the possibility that a protein, 35 amino acids in length, could be expressed. This protein would consist of 15 amino acids from the N-terminal of TREK-1 and a 20-amino acid nonsense sequence coded by a frame-shift before an early stop codon. If translated, this peptide could bind and interfere with the posttranslational modification of other K channels, particularly other K2Ps. Similar scenarios have occurred where a truncation of one Kv channel isoform disrupted the expression of other Kv isoforms by producing a dominant-negative effect (13, 37, 45). While this scenario is possible, we believe it to be unlikely. Even if this short peptide is expressed, a dominant-negative effect of this short peptide seems impractical given that it should not bind to critical segments of other K2P channels.
Unlike the previous study (3), dilations to ACh were similar in basilar arteries from WT and TREK-1 KO mice. The TREK-1 KO mice used by Blondeau et al. (3) were on a C57BL6J background. Our mice were a mixed C57BL6J/SV129 background. Even after backcrossing our mixed strain to C57BL6J mice for four generations, we still observed robust dilations to ACh in basilar arteries from TREK-1 KO mice (Fig. 4). Four generations of backcrossing should produce mice that were on average 94% of the C57BL6J strain. Our backcrossing studies leave little room for strain differences to account for the differences in results of our study and the previous study (3).
Unlike the previous studies (3), dilations to the nonspecific activator of TREK-1, α-linolenic acid, were similar in basilar arteries from WT and TREK-1 KO mice (Fig. 5). Because polyunsaturated fatty acids, including α-linolenic acid, not only activate TREK-1 but also BKCa, we included penitrem A to block the confounding effects of BKCa activation. Contrary to our study, Blondeau et al. (3) reported that dilations to α-linolenic acid and other polyunsaturated fatty acids were either completely abolished or severely attenuated in arteries from TREK-1 KO mice, even without any BKCa blockers present in the preparation. Since BKCa are highly expressed in cerebral arteries (see Fig. 2 and Ref. 31) and are activated by polyunsaturated fatty acids, it would appear that BKCas are somehow silenced in basilar arteries of TREK-1 KO mice used in the previous study (3).
In our study, we measured K+ currents in CVSMCs from WT and KO mice. We did not find TREK-1 or TREK-1-like currents in the WT mice. In fact, currents in WT and knockout were very similar. This observation is consistent with our conclusion that neither α-linolenic acid nor arachidonic acid dilates cerebral arteries by activating TREK-1 on smooth muscle. However, these electrophysiological studies in CVSMCs cannot address any altered function in endothelium that would involve NO production. While Blondeau et al. (3) reported differences in dilator responses in the basilar artery from WT and TREK-1 knockout mice to α-linolenic acid, no electrophysiological studies were included that demonstrated the presence and absence of TREK-1 currents in WT and TREK-1 KO mice, respectively.
Both our TREK-1 KO strain of mice and the previous strain (3, 15, 22) are normotensive. Given the severity of the endothelial dysfunction previously reported (3, 15), it is surprising that the TREK-1 KO mice used in the other studies were normotensive. However, it is possible that not all arteries, express TREK-1. Endothelial dysfunction to a selected number of arteries, which otherwise express TREK-1, may not be sufficient to affect blood pressure as a whole in the TREK-1 KO mice.
Perspectives and Significance
K channels, in general, are known to be involved with various cellular processes, including control of vessel diameter, regulatory and apoptotic volume regulation, cell migration, and proliferation (23, 24, 32). With the discovery that K2Ps, including TREK-1, are expressed in arteries (3, 7, 14, 15, 17–19, 34, 38), the question arises regarding their function. In this study, we find no evidence that TREK-1 is involved with the regulation of arterial diameter. However, it must be pointed out that our results in cerebral arteries are not consistent with another study using a different TREK-1 KO strain (3). While we cannot explain this discrepancy, we do wish to point out that a role for TREK-1 in the regulation of vascular diameter is controversial. TREK-1 could be involved with other cellular processes. For example, members of the TASK subgroup of the K2P family are involved with regulatory and apoptotic volume regulation (2, 28, 30, 48). In the mouse embryo, apoptosis recruits a K channel whose characteristics closely resemble that of TREK-1 (50). One role that is particularly appealing is volume regulation since TREK-1 is a mechanosensitive ion channel (42, 43). Even without a change in the extracellular osmolarity, regulation of cell volume is required for normal cellular homeostasis. In addition, hormones often utilize volume regulation as a mechanism to exert their effects (29). There is much to be learned regarding the functional role of K2P, in general, and TREK-1, specifically, in vascular tissues.
GRANTS
This research was supported by an American Heart Association predoctoral fellowship (0815342F) from the South Central Affiliate (to K. Namiranian) and National Institutes of Health Grants RO1 NS46666 (to R. M. Bryan), P01 NS038660 (R. M. Bryan), RO1 HL088435 (to S. P. Marrelli), R01 HL22512 (to C. J. Hartley), and K25 HL73041 (to A. K. Reddy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1.Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol Heart Circ Physiol 291: H223–H230, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C, Guy N, Barhanin J, Poujeol P. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol 122: 177–190, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res 101: 176–184, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bryan RM, Jr, Eichler MY, Swafford MWG, Johnson TD, Suresh MS, Childres WF. Stimulation of α2 adrenoceptors dilates the rat middle cerebral artery. Anesthesiology 85: 82–90, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bryan RM, Jr., Joseph BK, Lloyd E, Rusch NJ. Starring TREK-1: the next generation of vascular K+ channels. Circ Res 101: 119–121, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bryan RM, Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology 102: 1261–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bryan RM, Jr, You J, Phillips SC, Andresen JJ, Lloyd EE, Rogers PA, Dryer SE, Marrelli SP. Evidence for two-pore domain potassium channels in rat cerebral arteries. Am J Physiol Heart Circ Physiol 291: H770–H780, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bryan RM, Jr, Marrelli SP, Steenberg ML, Schildmeyer LA, Johnson TD. Effects of luminal shear stress on cerebral arteries and arterioles. Am J Physiol Heart Circ Physiol 280: H2011–H2022, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Danthi S, Enyeart JA, Enyeart JJ. Modulation of native TREK-1 and Kv1.4 K+ channels by polyunsaturated fatty acids and lysophospholipids. J Membr Biol 195: 147–164, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Enyeart JA, Danthi SJ, Enyeart JJ. TREK-1 K+ channels couple angiotensin II receptors to membrane depolarization and aldosterone secretion in bovine adrenal glomerulosa cells. Am J Physiol Endocrinol Metab 287: E1154–E1165, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev 78: 53–97, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J 17: 3297–3308, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folco E, Mathur R, Mori Y, Buckett P, Koren G. A cellular model for long QT syndrome. Trapping of heteromultimeric complexes consisting of truncated Kv11 potassium channel polypeptides and native Kv14 and Kv15 channels in the endoplasmic reticulum. J Biol Chem 272: 26505–26510, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol 142: 192–202, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garry A, Fromy B, Blondeau N, Henrion D, Brau F, Gounon P, Guy N, Heurteaux C, Lazdunski M, Saumet JL. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: the endothelial link. EMBO Rep 8: 354–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein SAN, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV Nomenclature and Molecular Relationships of Two-P Potassium Channels. Pharmacol Rev 57: 527–540, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gonczi M, Szentandrassy N, Johnson IT, Heagerty AM, Weston AH. Investigation of the role of TASK-2 channels in rat pulmonary arteries: pharmacological and functional studies following RNA interference procedures. Br J Pharmacol 147: 496–505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J 38: 305–318, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FEJ. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res 93: 957–964, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol 546: 625–639, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Effects of isoflurane on coronary blood flow velocity in young, old and ApoE(−/−) mice measured by Doppler ultrasound. Ultrasound Med Biol 33: 512–521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J 23: 2684–2695, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Phys Rev 89: 193–277, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Jackson WF. Potassium channels and proliferation of vascular smooth muscle cells. Circ Res 97: 1211–1212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation 4: 35–50, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kim D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci 24: 648–654, 2003 [DOI] [PubMed] [Google Scholar]
- 28.L'hoste S, Poet M, Duranton C, Belfodil R, Barriere HE, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem 282: 36692–36703, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247–306, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Lauritzen I, Zanzouri M, Honore E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels. J Biol Chem 278: 32068–32076, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology 21: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Liu LY, Hoffman GE, Fei XW, Li Z, Zhang ZH, Mei YA. Delayed rectifier outward K+ current mediates the migration of rat cerebellar granule cells stimulated by melatonin. J Neurochem 102: 333–344, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Huang H, Wang W, Wang J, Sachs F, Niu W. Stretch-activated potassium channels in hypotonically induced blebs of atrial myocytes. J Membr Biol 226: 17–25, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lloyd EE, Marrelli SP, Bryan RM., Jr cGMP does not activate two-pore domain K+ channels in cerebrovascular smooth muscle. Am J Physiol Heart Circ Physiol 296: H1774–H1780, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd EE, Marrelli SP, Namiranian K, Bryan RM., Jr Characterization of TWIK-2, a two-pore domain K+ channel, cloned from the rat middle cerebral artery. Exp Biol Med (Maywood) 234: 1493–1502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K+ channel function in mice: what have we learned, and where do we go from here? Circ Res 89: 944–956, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, Wilhelm J, Morty RE, Brau ME, Weir EK, Kwapiszewska G, Klepetko W, Seeger W, Olschewski H. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res 98: 1072–1080, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Phys Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci 24: 339–346, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2: 422–426, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17: 4283–4290, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel AJ, Lazdunski M, Honoré E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol 13: 422–428, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Patel AJ, Maingret F, Magnone V, Fosset M, Lazdunski M, Honoré P. ETWIK2, an inactivating domain K+ channel 2. J Biol Chem 275: 28722–28730, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Persson AS, Klement G, Almgren M, Sahlholm K, Nilsson J, Petersson S, Arhem P, Schalling M, Lavebratt C. A truncated Kv1.1 protein in the brain of the megencephaly mouse: expression and interaction. BMC Neurosci 6: 65, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem 276: 7302–7311, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Reddy AK, Taffet GE, Li YH, Lim SW, Pham TT, Pocius JS, Entman ML, Michael LH, Hartley CJ. Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng 52: 1771–1783, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Skatchkov SN, Eaton MJ, Shuba YM, Kucheryavykh YV, Derst C, Veh RW, Wurm A, Iandiev I, Pannicke T, Bringmann A, Reichenbach A. Tandem-pore domain potassium channels are functionally expressed in retinal (Muller) glial cells. Glia 53: 266–276, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA. Alternative translation initiation in rat brain yields K(2P)2.1 potassium channels permeable to sodium. Neuron 58: 859–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trimarchi JR, Liu L, Smith PJ, Keefe DL. Apoptosis recruits two-pore domain potassium channels used for homeostatic volume regulation. Am J Physiol Cell Physiol 282: C588–C594, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Xian TL, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res 69: 86–97, 2006 [DOI] [PubMed] [Google Scholar]
- 52.You J, Johnson TD, Marrelli SP, Bryan RM., Jr Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol Heart Circ Physiol 277: H893–H900, 1999 [DOI] [PubMed] [Google Scholar]
- 53.You J, Marrelli SP, Bryan RM., Jr Role of cytoplasmic phospholipase A2 in endothelium-derived hyperpolarizing factor dilations of rat middle cerebral arteries. J Cereb Blood Flow Metab 22: 1239–1247, 2002 [DOI] [PubMed] [Google Scholar]
- 54.You JP, Johnson TD, Marrelli SP, Mombouli JV, Bryan RM., Jr P2u-receptor mediated release of endothelium-derived relaxing factor/nitric oxide and endothelium-derived hyperpolarizing factor from cerebrovascular endothelium in rats. Stroke 30: 1125–1133, 1999 [DOI] [PubMed] [Google Scholar]
- 55.You J, Golding EM, Bryan RM., Jr Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. Am J Physiol Heart Circ Physiol 289: H1077–H1083, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.