Abstract
Objectives
Carotid intima-media thickness (cIMT) is an independent predictor of cardiovascular risk. Furthermore, ethnicity and gender-specific normative data are required to assess cIMT, which are not available for Andean-Hispanics. In addition, data regarding correlates of subclinical atherosclerosis in ethnic population are needed.
Methods
We studied 1448 adults enrolled in a population-based study in Peru. cIMT and carotid plaque were measured with high-resolution ultrasonography. A healthy reference sample (n=472) with no cardiovascular disease, normal weight and normal metabolic parameters was selected to establish normative cIMT values. Correlates of abnormal cIMT and carotid plaque were assessed in the entire population.
Results
In the reference sample, 95th-percentile cIMT values were both age and gender-dependent. In stepwise regression, selected predictors of increasing cIMT were: older age, impaired fasting glucose, diabetes mellitus, higher systolic blood pressure, higher LDL-cholesterol, smoking and male gender. Predictors of carotid plaque included older age, male gender, higher systolic blood pressure, lower diastolic blood pressure and higher LDL-cholesterol. HDL-cholesterol and C-reactive protein were not associated with cIMT or carotid plaque. The lack of association with HDL-cholesterol was confirmed using high performance liquid chromatography.
Conclusions
We present ethnic-specific cutoffs for abnormal cIMT applicable to Andean-Hispanics and correlates of subclinical atherosclerosis in this population. Pending longitudinal studies, our data supports several risk associations seen in other populations and can be used to identify Andean-Hispanics at increased risk for atherosclerotic cardiovascular disease. The lack of association between HDL-C and cIMT or carotid plaque in this population requires further investigation.
Keywords: carotid intima-media thickness, Andean-Hispanics, definitions, cardiovascular disease, Latin America
Introduction
Cardiovascular disease (CVD) has emerged as a leading cause of death in Latin America (1). The early identification of subjects at risk for cardiovascular disease is important because prevention strategies instituted early are likely to have the highest impact in cardiovascular outcomes at the population level and these should be tailored to individual risk. Various cardiovascular risk stratification schemes have been developed using prospective data derived predominantly from Caucasian populations. These are largely based on individual risk factors that independently predict the risk of atherosclerotic cardiovascular disease. Given the ethnic diversity in the profile of CVD, varied risk associations and different levels of genetic–environmental interactions in different populations, such studies performed in Caucasian populations cannot be directly applied to Latin-American populations. Until prospective studies are available, the assessment of markers of subclinical atherosclerosis represents a useful approach to assess the correlates of atherosclerotic risk in Latin-American populations and individuals.
High-resolution carotid ultrasonography can determine the presence or extent of atherosclerosis in situ. Carotid artery intima–media thickness (cIMT) independently predicts the risk of cardiac (myocardial infarction, angina pectoris, coronary intervention) and cerebrovascular events (stroke or transient ischemic attack)(2–13), the involvement of other arterial beds with atherosclerosis (14–16) and is well suited for use in large-scale population studies as a marker of subclinical disease due to the relative simplicity and noninvasive nature of the technique. Appropriate interpretation of individual cIMT should be based on gender, age- and population-specific normative data (17). However, such data are not available for Hispanic populations.
Andean populations represent an important proportion of South American Hispanics. Andean countries have important similarities, including related native Amerindian populations and historical patterns of colonization, which have influenced the patterns of genetic admixture and the cultural characteristics of their inhabitants. Although previous important studies have described cIMT and associated cardiovascular risk factors in Latin-American populations (18–20), studies assessing correlates of cIMT and the presence of carotid plaque in Andean adults are needed. In this study, we aimed to: (1) Establish normative data for cIMT suitable for use among Andean-Hispanics; (2) Assess the correlates of subclinical atherosclerosis assessed by cIMT and the presence of carotid plaque in this population.
Methods
Study population and Sampling Design
The objectives and design of the PREVENCION study have been previously published (21). PREVENCION is a population-based study undertaken in the second largest city in Peru, with a population that is comparable to other urban populations in Peru and resembles urban populations in Andean countries such as Bolivia and Ecuador. This population consists largely of Mestizos (“Mixed”), with the degree of admixture being predominantly Andean-Amerindian (autochthonous Quechua and Aymara), with small contributions from Spanish Whites and minimal contributions from West-African populations. The sampling frame was based on the most recent population and household National Census (21) and the sampling strategy was probabilistic, multistage, clustered and stratified according to geographic location and socioeconomic status. Following initial contact with participants at their household, a comprehensive evaluation was performed at the study headquarters (21–23). We used data from 1448 participants with available carotid ultrasound data [687 (47.4%) men].
For the assessment of normative cIMT values, we selected a healthy reference sample by excluding subjects with any of the following conditions: (1) History of coronary heart disease, heart failure, stroke, peripheral vascular disease, or evidence of previous myocardial infarction in a 12-lead resting electrocardiogram; (2) hypertension (systolic blood pressure [SBP]≥140 mm Hg, diastolic blood pressure [DBP]≥90 mm Hg, or drug treatment for hypertension); (3) diabetes mellitus (fasting blood glucose≥126 mg/dL or pharmacologic treatment for diabetes); (4) low-density lipoprotein cholesterol (LDL-C)>130 mg/dL or pharmacologic therapy for dyslipidemia; (5) current smoking; (6) body mass index≥30 kg/m2. The healthy reference sample consisted of 472 subjects [258 women (54.7%)] aged 20–80 years. The study was approved by the Santa Maria Catholic University Human Research Committee and all participants gave informed consent.
Laboratory measurements
Samples of venous blood were obtained after at least 8 hours of fast and serum was used for biochemical measurements. Total cholesterol, LDL-C, serum glucose, and triglycerides were measured enzymatically by automated methods (Cobas Mira Assay; Roche, Basel, Switzerland). HDL-C was measured after precipitation of apoB-containing lipoproteins (22). Since we did not find a relationship between HDL-C measured by the precipitation method and cIMT or carotid plaque, sensitivity analyses were performed using a different method. Therefore, serum lipoproteins were additionally analyzed by HPLC. Serum samples were frozen at −80 degrees and shipped to Skylight Biotech laboratories (Tokyo, Japan) where HPLC was performed as previously described (24 25). Briefly, 10 µl of serum was injected into 2 connected columns (300×7.8 mm) of TSKgel LipopropakXL (Tosoh) and eluted by 0.05 mol/l Tris-buffered acetate (TBA, pH 8.0) containing 0.3 mol/l sodium acetate and 0.005% Brij-35. The effluent from the columns was continuously monitored at 550 nm after an online enzymatic reaction with a commercial kit, Determiner L TC (Kyowa Medex). The cholesterol concentration in low-density and high-density lipoproteins was calculated based on the complex chromatograms with the modified Gaussian curve fitting for resolving the overlapping peaks by mathematical treatment as previously described in detail (24 25).
Measurements of cIMT and carotid plaque
High-resolution B-mode carotid ultrasonography was performed with a linear-array, 10-MHz transducer (Sonosite Titan; Sonosite; Bothell, WA). With the subject in the supine position, images were obtained bilaterally from anterior, posterior and lateral views. Both carotid arteries were examined. The transducer was manipulated so that the near and far walls of the common carotid artery (CCA) were parallel to the transducer footprint, and the lumen diameter was maximized in the longitudinal plane. A region 1.0 cm proximal to the carotid bulb was identified, and cIMT of the near and far walls were evaluated as the distance between the lumen-intima interface and the media-adventitia interface. If plaques were present in this area, these were included in the measurements. Measurements of cIMT were performed using the Sonocalc software (Sonosite Titan; Sonosite; Bothell, WA), which performs multiple automated or semi automated measurements along 1 cm and averages them, therefore increasing the accuracy of measurements. The presence of carotid plaque was defined as at least one localized echo structure encroaching into the vessel lumen for which the distance between the media-adventitia interface and the internal side of the lesion was ≥1 mm. Quantification of plaque thickness was made at the site of the maximal encroachment perpendicularly to the vessel wall by measuring the distance between the media-adventitia interface and the lesion surface facing the lumen using digital calipers. All measurements were performed offline in a blinded fashion.
Definitions of abnormal cIMT
We defined age-specific cut points for high cIMT among men and women by generating curves based on 95th-percentile values for each decade of life. These curves were generated by empirical curve estimation procedures in which various regression models were constructed and the model with the highest coefficient of determination (R2) was used. R2 values for models used to determine cut-points for cIMT were 0.96 in women and 0.95 in men.
Statistical Analysis
Data for continuous variables are presented as means. Proportions are presented as frequencies and percentages. We evaluated the effect of traditional risk factors on cIMT (as a continuous variable) using linear regression both with and without adjustment for age and gender. Variables were selected by a stepwise strategy applied to the following variables (with P=0.10 necessary to enter a variable into the model): age, gender, systolic and diastolic pressure, impaired fasting glucose (fasting glucose 100 to 125 mg/dL), diabetes mellitus (fasting glucose ≥126 mg/dL or use of antidiabetic medications), current smoking, triglycerides, C-reactive protein (CRP), LDL-C and HDL-C levels. CRP was log-transformed in all statistical analyses because of a positive skew distribution. A variable that was entered into a model was kept in both models (unadjusted and adjusted for age and gender) in order to permit a direct comparison between models. Interactions between gender, height and weight, and cIMT were tested in all models.
Logistic regression was used to identify independent predictors of a high cIMT or the presence of carotid plaque. All tests were two-sided and α<0.05 was considered to be statistically significant. STATA-10 for Windows (STATA CORP, TX, USA) and SPSS for Windows-v17 (SPSS Inc., Chicago,IL) were used for analyses.
Results
The study population comprised 1448 participants [687 (47.4%) men] aged 20–87 years (mean age 52.4 years), of whom 472 (214 [45.3%] men) were found to be healthy. Important demographic, clinical and laboratory characteristics of the study population are shown in Table 1.
Table 1.
Mean age of study participants and age-standardized* population estimates (95% CIs) for important characteristics of the study population
| Men (n=687) |
Women (n=761) |
|
|---|---|---|
| Age, years | 52.7 (51.4–53.9) | 52.1 (51.0–53.3) |
| Current Smoking (%) † | 30.1 (26.1–34.1) | 13.6 (10.7–16.5) |
| Current alcohol consumption (%)† | 56.6 (52.3–60.9) | 22.1 (18.4–25.8) |
| Body Height, cm † | 169.8 (169.1–170.4) | 156.6 (156.1–157.1) |
| Body Weight, Kg † | 76.2 (75.0–77.3) | 63.0 (62.0–63.9) |
| Body Mass Index, kg/m2 † | 26.4 (26.0–26.7) | 25.7 (25.4–26.1) |
| Waist Circumference, cm † | 93.0 (92.2–93.8) | 84.9 (84.0–85.7) |
| Use of hormonal contraceptives (%) | --- | 7.2 (4.8–9.5) |
| Use of hormone-replacement therapy (%) | --- | 2.8 (1.8–3.8) |
| Systolic Blood Pressure, mmHg † | 115.6 (114.6–116.7) | 113.4 (112.3–114.4) |
| Diastolic Blood Pressure, mmHg † | 77.5 (76.8–78.2) | 74.9 (74.2–75.6) |
| Total Cholesterol, mg/dL | 194.6 (191.4–197.9) | 197.2 (194.4–200.1) |
| HDL-Cholesterol, mg/dL † | 45.5 (44.7–46.2) | 48.7 (48.0–49.5) |
| Low HDL-Cholesterol (%)‡ | 28.7 (24.7–32.6) | 56.5 (52.4–60.6) |
| LDL-Cholesterol, mg/dL | 115.2 (112.5–117.9) | 117.2 (115.0–119.3) |
| Triglycerides, mg/dL † | 185.6 (177.9–193.3) | 141.9 (136.8–146.9) |
| C-reactive protein, mg/dL ** | 129.0 (116.7–142.6) | 127.7 (115.6–142.6) |
| Impaired fasting glucose (%) † ‡ | 4.1 (2.4–5.7) | 2.4 (1.6–3.3) |
| Diabetes mellitus (%)‡ | 4.5 (3.0–5.9) | 4.1 (2.7–5.5) |
| Antihypertensive medication use (%) | 8.4 (6.6–10.2) | 11.2 (9.6–12.7) |
| Cholesterol-lowering medication use (%) | 4.5 (2.8–6.3) | 1.8 (1.1–2.5) |
| Hypertension (%) | 18.0 (15.1–21.0) | 17.9 (15.9–18.8) |
| Hypercholesterolemia (%) | 15.9 (12.8–19.0) | 15.2 (12.9–17.6) |
According to World Health Organization estimates on standard world population between 2000–2025
P<0.001 for difference between men and women.
Low HDL-cholesterol was defined as <40 mg/dL in men and <50 mg/dL in women; impaired fasting glucose was defined as fasting blood glucose=100–125 mg/dL; diabetes mellitus was defined as fasting blood glucose ≥126mg/dL or antidiabetic pharmacologic treatment.
geometric mean
HDL= High-density lipoprotein; LDL=Low-density lipoprotein;
Distribution of cIMT values in the healthy reference sample
In the reference sample, men demonstrated higher cIMT than women (0.63 vs. 0.57; P<0.001). A significant correlation was observed between increasing age and mean cIMT for both men (R=0.53; P<0.001) and women (R=0.64; P<0.001). The gender difference in cIMT in the reference sample persisted after adjustment for age (0.61 vs. 0.59 mm; P=0.01). Therefore, the distribution of cIMT according to age was analyzed separately in men and women.
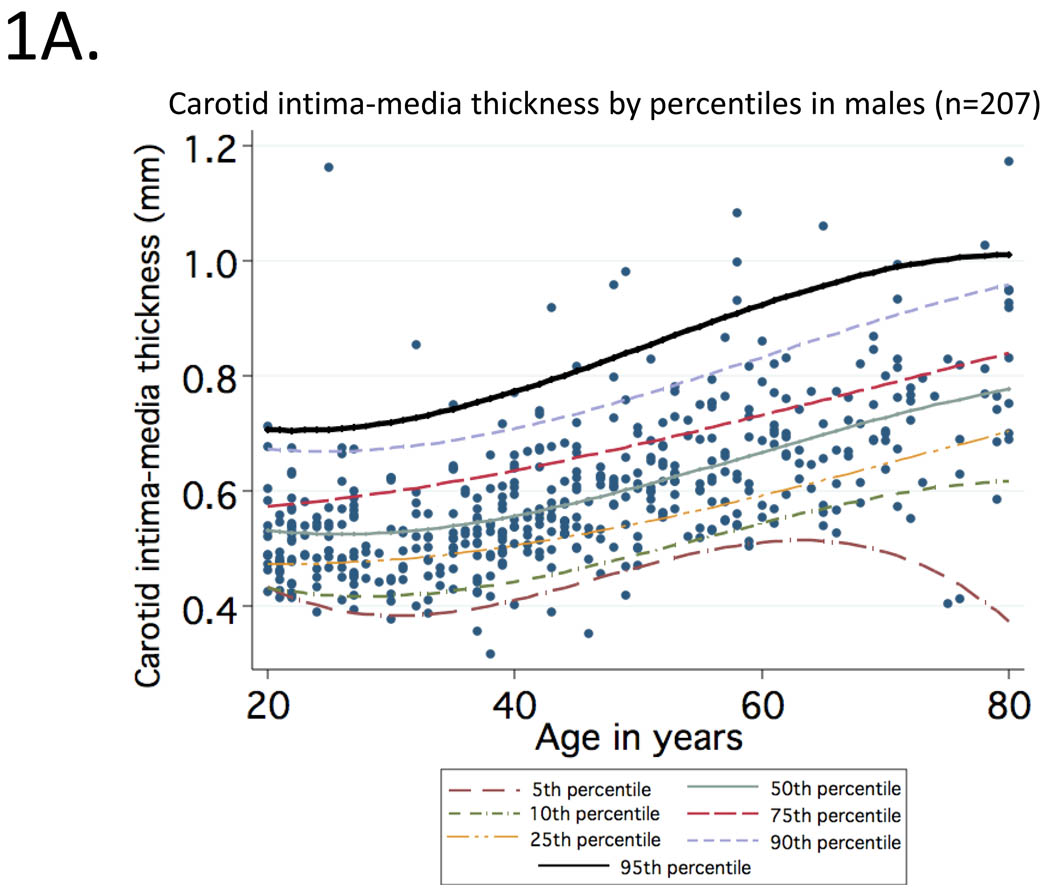
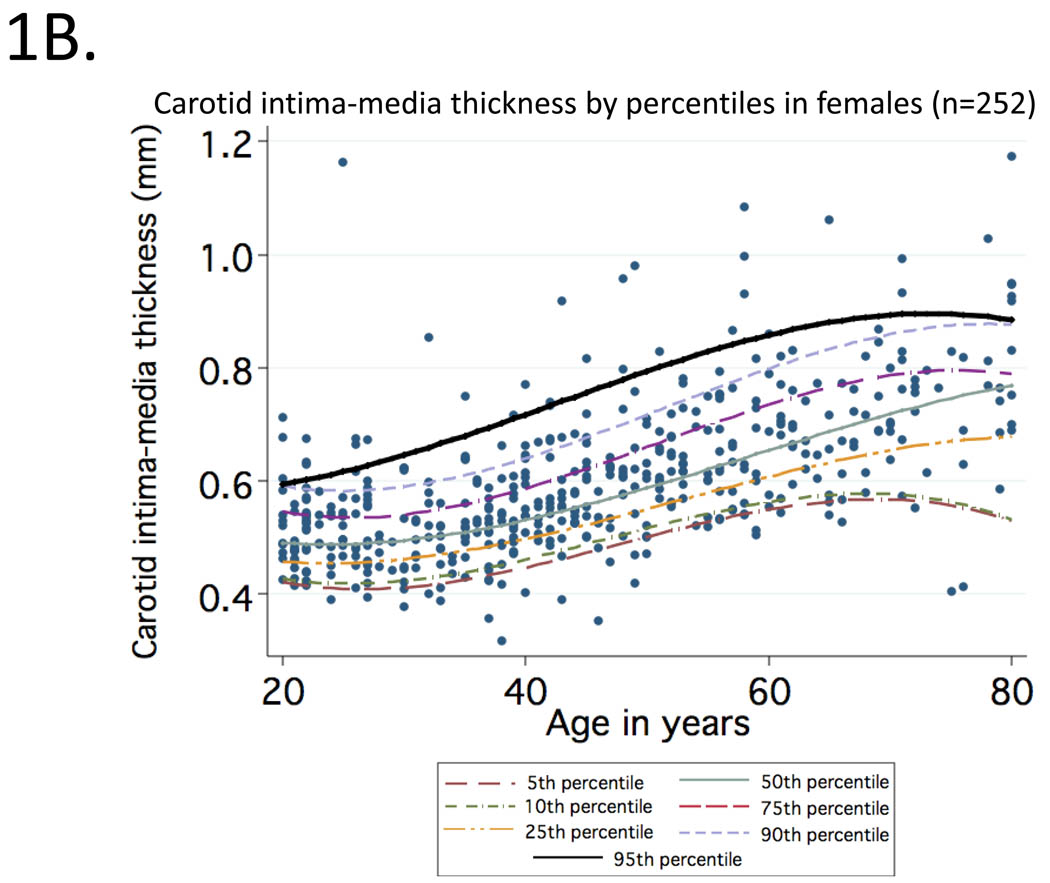
Mean (95% CI) values of cIMT (in mm) observed at CCA for healthy participants aged 20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69 and 70 to 80 were: 0.50 (0.49–0.53), 0.52 (0.50–0.53), 0.58 (0.55–0.61), 0.63 (0.61–0.65), 0.68 (0.66–0.70), 0.76 (0.70–0.80). Curves representing percentile distributions according to age in men and women are shown in Figure 1. The 95th percentiles (95% CI) among men (Figure 1A) in these age groups were 0.67 (0.59–1.09), 0.67 (0.59–0.85), 0.75 (0.68–0.92), 0.82 (0.75–1.07), 0.81 (0.74–0.86) and 0.98 (0.88–1.17), respectively (range=0.32 to 1.17). Corresponding values among women (Figure 1B) were 0.58 (0.56–0.62), 0.61 (0.58–0.64), 0.69 (0.64–0.91), 0.76 (0.72–0.91), 0.86 (0.77–1.06) and 0.91 (0.78–0.92), respectively (range=0.35 to 1.06).
Figure 1.
Percentile distributions for carotid intima-media thickness among men (1A) and women (1B) included in the reference sample according to age.
Predictors of cIMT and carotid plaque
In the entire sample, linear regression showed an association between increasing cIMT and age, male gender, SBP, DBP, LDL-C, triglycerides, C-reactive protein, current smoking, body mass index, impaired fasting glucose and diabetes mellitus. All of the associations persisted after adjustment for age and gender except for triglycerides and C-reactive protein (Table 2).
Table 2.
Association of individual atherosclerotic risk factors with carotid intima-media thickness in linear regression models with and without adjustment for age and gender (n=1407).
| Without adjustment for age and gender | After adjustment for age and gender | |||||
|---|---|---|---|---|---|---|
| β (95%CI) | Standardized β | P value | β (95%CI) | Standardized β | P value | |
| Age (per 10 years) | 0.066 (0.062 to 0.070) | 0.632 | < 0.001 | -- | ||
| Sex (men vs women) | 0.021 (0.003 to 0.039) | 0.061 | 0.02 | -- | ||
| Systolic blood pressure (per 10mmHg) | 0.037 (0.033 to 0.042) | 0.412 | < 0.001 | 0.012 (0.008 to 0.016) | 0.133 | < 0.001 |
| Diastolic blood pressure (10mmHg) | 0.037 (0.033 to 0.042) | 0.412 | < 0.001 | 0.012 (0.008 to 0.016) | 0.133 | < 0.001 |
| HDL-cholesterol (per 10 mg/dL) | −0.0008 (−0.0098 to −0.0082) | −0.0045 | 0.866 | −0.005 (−0.012 to 0.002) | −0.031 | 0.143 |
| LDL-cholesterol (per 10 mg/dL) | 0.012 (0.009 to 0.014) | 0.219 | < 0.001 | 0004 (0.002 to 0.006) | 0.081 | < 0.001 |
| Triglycerides (per 10 mg/dL) | 0.003 (0.002 to 0.004) | 0.158 | < 0.001 | 0.0005 (−0.0003 to 0.0013) | 0.028 | 0.192 |
| C-reactive protein (natural log) | 0.021 (0.013 to 0.030) | 0.135 | < 0.001 | −0.0001 (−0.0072 to 0.0069) | −0.0009 | 0.97 |
| Smoking (current yes or no) | −0.027 (−0.051- to −0.003) | −0.059 | 0.026 | 0.021 (0.002 to 0.040) | 0.046 | 0.029 |
| Body mass index (per 5 units) | 0.041 (0.031 to 0.051) | 0.215 | < 0.001 | 0.011 (0.003 to 0.018) | 0.055 | 0.009 |
| Impaired fasting glucose * | 0.144 (0.105 to 0.183) | 0.186 | < 0.001 | 0.063 (0.032 to 0.095) | 0.082 | < 0.001 |
| Diabetes mellitus † | 0.146 (0.111to 0.181) | 0.207 | < 0.001 | 0.070 (0.041 to 0.098) | 0.098 | < 0.001 |
All models are age-standardized according to World Health Organization estimates on standard world population between 2000–2025. ;
Defined as fasting glucose 100–125 mg/dL;
Fasting blood glucose ≥126 mg/dL or use of antidiabetic medications. CI= Confidence Interval; HDL=high-density lipoprotein; LDL=low-density lipoprotein
In stepwise regression, selected predictors of increasing cIMT were: age, impaired fasting glucose, diabetes mellitus, SBP, LDL-C (all P<0.001), current smoking (P=0.015) and gender (P=0.016) (Table 3).
Table 3.
Risk factors associated with increasing carotid intima-media thickness selected on stepwise linear regression (n=1407)*
| β Coefficient (95% CI) |
Standardized β | P value | |
|---|---|---|---|
| R2=0.44 | |||
| Age (per 10 years) | 0.056 (0.050–0.060) | 0.531 | < 0.001 |
| Sex (men vs. women) | 0.017 (0.003–0.031) | 0.050 | 0.016 |
| Systolic blood pressure (per 10 mmHg) | 0.012 (0.007–0.016) | 0.127 | < 0.001 |
| LDL-cholesterol (per 10 mg/dL) | 0.004 (0.002–0.006) | 0.077 | < 0.001 |
| Smoking (current yes or no) | 0.023 (0.004–0.042) | 0.050 | 0.015 |
| Impaired fasting glucose † | 0.062 (0.031–0.093) | 0.079 | < 0.001 |
| Diabetes mellitus ‡ | 0.063 (0.034–0.091) | 0.089 | < 0.001 |
Analyses are standardized according to World Health Organization estimates on standard world population between 2000–2025.
Fasting blood glucose 100–125 mg/dL;
Fasting blood glucose ≥126 mg/dL or use of antidiabetic medications.
CI= Confidence Interval; LDL = low-density lipoprotein.
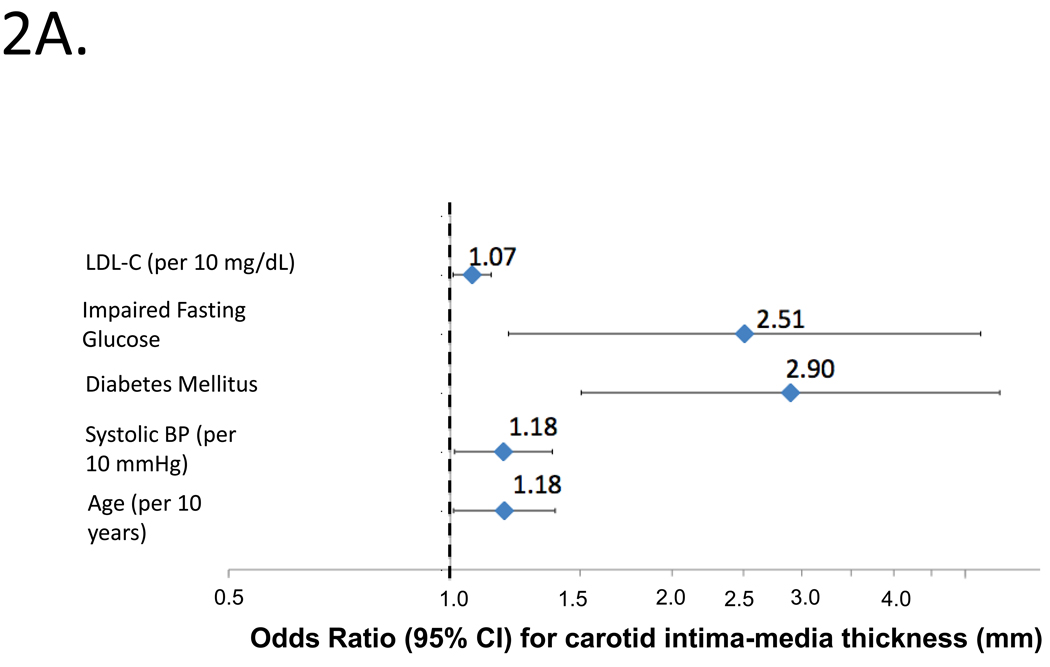
Figure 2A shows independent predictors of high cIMT by logistic regression. Independent predictors of high cIMT were age, SBP, diabetes mellitus, impaired fasting glucose and LDL-C (top panel; model c-statistic=0.702).
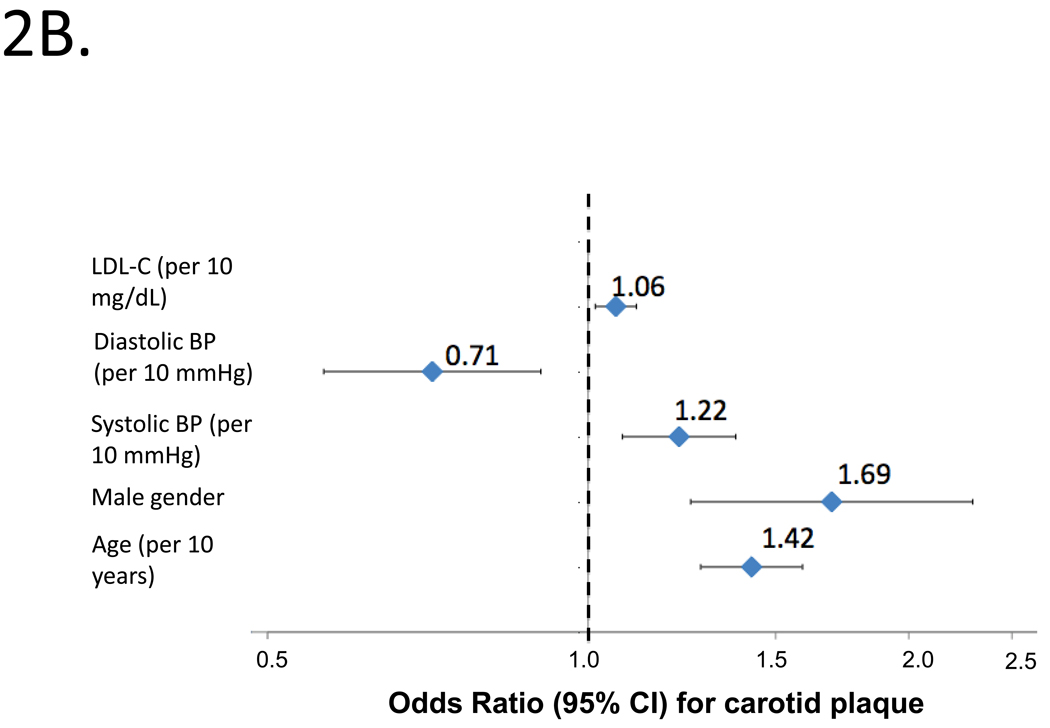
Figure 2.
Independent predictors of abnormal cIMT (2A) or carotid plaque (2B) by logistic regression in the entire study population. Odds ratios and 95%CIs are shown.
Figure 2B shows independent predictors of carotid plaque by logistic regression, which included age, male gender, SBP, DBP and LDL-C (bottom panel; model c-statistic=0.697). Higher SBP was independently associated with an increased risk of carotid plaque (OR=1.22; 95%CI=1.08–1.38; P=0.002), whereas higher DBP was independently associated with a lower risk of carotid plaque (OR=0.71; 95%CI=0.57 to 0.90; P=0.005).
Additional analyses regarding the association between HDL-C and cIMT/carotid plaque
Given the lack of association between HDL-C and either cIMT or the presence of carotid plaque, we performed additional analyses using data derived from HPLC, which were available from 782 subjects. HDL-C measured by HPLC was not associated with cIMT or after adjustment for age, gender, SBP, DBP, diabetes mellitus, current smoking, LDL-C, CRP (β per 10 mg/dL-increase= 0.005; 95%CI=−0.006 to 0.015; Standardized β=0.025; P=0.37). In contrast, LDL-C measured by HPLC was an independent predictor of cIMT (β per 10 mg/dL-increase=0.009; 95%CI=0.004 to 0.014; Standardized β=0.11; P<0.001). HDL-C measured by HPLC was not predictive of cIMT even when only subjects not taking lipid-lowering therapy (n=750) were analyzed (β per 10 mg/dL-increase=0.005; 95%CI= −0.005 to 0.016; Standardized β=0.03; P=0.31). HDL-C did not predict carotid plaque (OR=0.91; 95%CI=0.78–1.06; P=0.23), even when subjects taking lipid-lowering therapy were excluded from the analysis (OR=0.92; 95%CI=0.79–1.08; P=0.31). Results were consistent in gender-stratified analyses.
Discussion
We report on the cross-sectional associations between carotid atherosclerosis and classic cardiovascular risk factors in a large population-based sample of Andean-Hispanics. We also provide, for the first time, normative data for this population, which can be used to define abnormally high cIMT values in Andean-Hispanic adults aged 20–80 years. We show that cIMT in this population is associated with age, gender, SBP, LDL-C, current smoking, impaired fasting glucose and diabetes mellitus, but not with C-reactive protein or HDL-C levels. Carotid plaque is associated with age, male gender, SBP, DBP and LDL-C but not HDL-C.
Various studies have explored the relationship between cardiovascular disease and cIMT in different ethnic populations (2–13), but few in Latin America (18–20). We showed that cIMT is directly correlated with increasing age even among healthy individuals. This is in line with previous data, indicating that cIMT is a useful marker of arterial aging. Our normative data can be used to estimate the “arterial age” of individual subjects by comparing individual measurements with the percentile distributions described herein. This is an intuitive and promising approach for risk stratification, which requires further validation.
Pending prospective data from Hispanic populations, our findings demonstrate that various classic cardiovascular risk factors are associated with carotid atherosclerosis (and presumably, cardiovascular risk) in Andean-Hispanic adults and support risk stratification schemes based on these classic cardiovascular risk factors in this population. Risk factors associated with cIMT in our study included age, gender, SBP, LDL-C, current smoking, impaired fasting glucose and diabetes mellitus. Because cIMT may be affected both by atherosclerosis and wall hypertrophy, we also assessed the predictors of focal plaque, which may be more representative of atherosclerosis than cIMT and more informative for predicting cardiovascular risk (26). Predictors that included older age, male gender, a higher SBP and a higher LDL-C. In contrast, a higher DBP was associated with a decreased prevalence of carotid plaque (i.e., lower DBP was independently associated with plaque). This reflects a relationship between higher pulse pressure (difference between systolic and diastolic blood pressure) and carotid plaque. Pulse pressure is a marker of large artery stiffness; therefore, our findings provide further support for an association between atherosclerosis and large artery stiffness. This is likely to be related to shared pathogenic mechanisms and risk factors, since large artery stiffness is more closely related to arteriosclerosis (a medial wall process) than atherosclerosis per se (27).
It should be noted that an important proportion of the variability in cIMT and presence of carotid plaque remains unexplained by conventional risk factors in this population, highlighting the importance of identifying novel risk factors for atherosclerosis in this population. The c-statistics of our logistic regression models to detect plaque and high-cIMT (treated as a dichotomous variable) were similar, but the determinants of plaque and cIMT were not identical. Further studies are required to assess the value of plaque versus cIMT for cardiovascular risk prediction in this population and potential ethnic differences regarding the role of classic versus novel risk factors as determinants of carotid atherosclerosis and cardiovascular risk.
Interestingly, HDL-C levels were not associated with cIMT or carotid plaque in this population, in contrast with what has been consistently reported in other populations (28). This was true for HDL-C measured by the precipitation method and for HDL-C measured by HPLC. We, as well as others, have consistently reported a surprisingly high prevalence of low HDL-C in Andeans (22 29), particularly among younger women. In our population, a strikingly high prevalence of low HDL-C was observed despite a relatively low prevalence of diabetes mellitus. The metabolism of HDL-C is complex and it is possible that genetic or environmental factors in Amerindian populations uniquely influence measured serum HDL-C and its association with atherosclerosis. For instance, genetic polymorphisms in apolipoprotein E, hepatic lipase, and cholesteryl-ester transfer protein, apoprotein A-IV and several other candidate genes have been shown or proposed to impact HDL-C levels in other populations. Various environmental factors (such as diet) may be responsible for the high prevalence of low HDL-C in this population, but do not explain the apparent lack of association with cIMT observed in our study despite our large sample size. It is possible that specific sub-fractions of HDL lipoprotein particles have an association with cIMT in this population and this will be addressed in future studies. Clearly, more studies regarding the role of HDL-C on cardiovascular risk are needed in Andean populations are also needed before it can be confidently assumed that the risk associations observed in other studies apply to this population.
In addition to the clinical and epidemiologic implications of our reported associations, the normative data provided for cIMT in our study will allow the application of this simple, non-invasive technique in individual subjects from this ethnic group. Importantly, the American Society of Echocardiography recommends population-specific normative data according to age, gender, and ethnicity (17), and our study provides this important normative data for Andean-Hispanic adults since data from Caucasian populations cannot be directly applied to Andean-Hispanics.
Strengths of our study include our large sample size, our population-based sampling strategy and the fact that a single physician conducted the carotid ultrasonic examination since it is generally accepted that the intersonographer variability for B-mode measurements is greater than the intrasonographer variability (17). Our study is limited by its cross-sectional nature. In addition, our examination was limited to the CCA, which may not have detected the presence of atherosclerosis in other vascular beds or more distal segments of the carotid artery. Finally, we did not have referent participants from other ethnic groups (such as a comparable Caucasian population evaluated with identical methods to specifically assess the effect of ethnicity). However, pending prospective data, our findings provide important insights into the determinants of subclinical vascular disease in this population. A longitudinal study is needed for a better evaluation of the relationships between cardiovascular risk factors, subclinical atherosclerosis and the risk of cardiovascular events in this population.
Acknowledgments
Sources of funding: The PREVENCION study was supported by the Santa Maria Research Institute, AQP, Peru. JAC is supported by National Institutes of Health grant RO1-HL080076 and American Heart Association National Research Award #0885031N. CAP is supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and NIH Office of Women’s Health and Research through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Junichiro Takahashi and Gen Toshima are employees of Skylight Biotech (Tokyo, Japan) which provide commercially available services for HPLC measurements of serum lipoproteins. The other authors have no conflicts of interest.
References
- 1.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb Vasc Biol. 1991 doi: 10.1161/01.atv.11.5.1245. [DOI] [PubMed] [Google Scholar]
- 3.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common Carotid Intima-Media Thickness and Risk of Stroke and Myocardial Infarction : The Rotterdam Study. Circulation. 1997;96(5):1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, et al. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 5.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of Coronary Heart Disease Incidence with Carotid Arterial Wall Thickness and Major Risk Factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 1997;146(6):483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 6.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. American Journal of Epidemiology. 2000;151(5):478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 7.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, et al. Comparison Between Measures of Atherosclerosis and Risk of Stroke: The Rotterdam Study. Stroke. 2003;34(10):2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura A, Iso H, Imano H, Ohira T, Okada T, Sato S, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke; a Journal of Cerebral Circulation. 2004;35(12):2788–2794. doi: 10.1161/01.STR.0000147723.52033.9e. [DOI] [PubMed] [Google Scholar]
- 9.Rosvall M, Janzon L, Berglund G, Engström G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. Journal of Internal Medicine. 2005;257(5):430–437. doi: 10.1111/j.1365-2796.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 10.Murakami S, Otsuka K, Hotta N, Yamanaka G, Kubo Y, Matsuoka O, et al. Common carotid intima-media thickness is predictive of all-cause and cardiovascular mortality in elderly community-dwelling people: Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomedicine & Pharmacotherapy = Biomédecine & Pharmacothérapie. 2005;59 Suppl 1 doi: 10.1016/s0753-3322(05)80010-1. S49-53-S49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke; a Journal of Cerebral Circulation. 2006;37(1):87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 13.Chien K-L, Su T-C, Jeng J-S, Hsu H-C, Chang W-T, Chen M-F, et al. Carotid artery intima-media thickness, carotid plaque and coronary heart disease and stroke in Chinese. PloS One. 2008;3(10) doi: 10.1371/journal.pone.0003435. e3435-e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allan PL, Mowbray PI, Lee AJ, Fowkes FGR. Relationship Between Carotid Intima-Media Thickness and Symptomatic and Asymptomatic Peripheral Arterial Disease: The Edinburgh Artery Study. Stroke. 1997;28(2):348–353. doi: 10.1161/01.str.28.2.348. [DOI] [PubMed] [Google Scholar]
- 15.Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, et al. Arterial Wall Thickness Is Associated With Prevalent Cardiovascular Disease in Middle-Aged Adults : The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26(3):386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 16.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 17.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89-90-93-11; quiz 89-90. [DOI] [PubMed] [Google Scholar]
- 18.Casella IB, Sotelo FJB, Yamazaki Y, Presti C, Vassoler A, Melo HAH. Comparison of common carotid artery intima-media thickness between Brazilian Euro-descendants and Afro-descendants with atherosclerosis risk factors. Clinics (São Paulo, Brazil) 2009;64(7):657–664. doi: 10.1590/S1807-59322009000700009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos RD, Nasir K. Insights into atherosclerosis from invasive and non-invasive imaging studies: Should we treat subclinical atherosclerosis? Atherosclerosis. 2009;205(2):349–356. doi: 10.1016/j.atherosclerosis.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Verçoza A, Baldisserotto M, de Los Santos C, Poli-de-Figueiredo C, d'Avila D. Cardiovascular Risk Factors and Carotid Intima-Media Thickness in Asymptomatic Children. Pediatric Cardiology. 2009 doi: 10.1007/s00246-009-9493-3. [DOI] [PubMed] [Google Scholar]
- 21.Medina-Lezama J, Chirinos JA, Zea Diaz H, Morey O, Bolanos JF, Munoz-Atahualpa E, et al. Design of PREVENCION: a population-based study of cardiovascular disease in Peru. Int J Cardiol. 2005;105(2):198–202. doi: 10.1016/j.ijcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Medina-Lezama J, Zea-Diaz H, Morey-Vargas OL, Bolanos-Salazar JF, Munoz-Atahualpa E, Postigo-MacDowall M, et al. Prevalence of the metabolic syndrome in Peruvian Andean hispanics: the PREVENCION study. Diabetes Res Clin Pract. 2007;78(2):270–281. doi: 10.1016/j.diabres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Medina-Lezama J, Morey-Vargas OL, Zea-Diaz H, Bolanos-Salazar JF, Corrales-Medina F, Cuba-Bustinza C, et al. Prevalence of lifestyle-related cardiovascular risk factors in Peru: the PREVENCION study. Rev Panam Salud Publica. 2008;24(3):169–179. doi: 10.1590/s1020-49892008000900003. [DOI] [PubMed] [Google Scholar]
- 24.Hara I, Okazaki M. High-performance liquid chromatography of serum lipoproteins. Methods Enzymol. 1986;129:57–78. doi: 10.1016/0076-6879(86)29062-x. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki M, Usui S, Ishigami M, Sakai N, Nakamura T, Matsuzawa Y, et al. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol. 2005;25(3):578–584. doi: 10.1161/01.ATV.0000155017.60171.88. [DOI] [PubMed] [Google Scholar]
- 26.Simon A, Megnien JL, Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 30(2):182–185. doi: 10.1161/ATVBAHA.109.196980. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson IB, McEniery CM, Cockcroft JR. Arteriosclerosis and atherosclerosis: guilty by association. Hypertension. 2009;54(6):1213–1215. doi: 10.1161/HYPERTENSIONAHA.109.142612. [DOI] [PubMed] [Google Scholar]
- 28.Amarenco P, Labreuche J, Touboul P-J. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 2008;196(2):489–496. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Florez H, Silva E, Fernandez V, Ryder E, Sulbaran T, Campos G, et al. Prevalence and risk factors associated with the metabolic syndrome and dyslipidemia in White, Black, Amerindian and Mixed Hispanics in Zulia State, Venezuela. Diabetes Res Clin Pract. 2005;69(1):63–77. doi: 10.1016/j.diabres.2004.11.018. [DOI] [PubMed] [Google Scholar]