Abstract
Despite abundant expression of DNA methyltransferases (Dnmt’s) in brain, the regulation and behavioral role of DNA methylation remain poorly understood. We find that Dnmt3a expression is regulated in mouse nucleus accumbens (NAc) by chronic cocaine and chronic social defeat stress. Moreover, NAc specific manipulations that block DNA methylation potentiate cocaine reward and exert antidepressant-like effects, whereas NAc specific Dnmt3a overexpression attenuates cocaine reward and is pro-depressant. On a cellular level, we show that chronic cocaine selectively increases thin dendritic spines on NAc neurons and that DNA methylation is both necessary and sufficient to mediate these effects. These data establish the importance of Dnmt3a in the NAc in regulating cellular and behavioral plasticity to emotional stimuli.
INTRODUCTION
Chronic cocaine and chronic social defeat stress alter gene expression, neuronal plasticity, and ultimately behavior, and we and others have implicated chromatin remodeling in playing a key role in regulating these events in the nucleus accumbens (NAc), a key brain reward region1–9. However, work to date has focused primarily on more labile epigenetic modifications such as histone acetylation and methylation. Given the persistent nature of addiction, an intriguing possibility is whether more stable epigenetic modifications, such as DNA methylation, can more persistently influence gene expression in NAc to maintain this behavior.
Despite abundant neuronal expression of DNA methyltransferases (Dnmt’s)10, little is known about the function of DNA methylation in brain. Behavioral studies suggest that DNA methylation is required for hippocampal-dependent memory formation11, 12. Pharmacological inhibition of DNA methylation blocks long-term potentiation in hippocampus13 and this effect depends on the activity of both Dnmt1 and Dnmt3a11. Moreover, Dnmt inhibition dramatically reduces functional synapses formed by cultured hippocampal neurons as measured by a reduction in mEPSC frequency14. Together, these studies point to DNA methylation playing a crucial role in hippocampus in memory formation and associated effects at the synapse.
In the present study, we focus on the NAc to test if these concepts regarding DNA methylation extend to drug addiction and depression models. By analyzing the role of DNA methylation in the context of both chronic cocaine (a rewarding stimulus with persistent effects) and chronic social defeat stress (an aversive stimulus with persistent effects), these studies complement one another by establishing the influence of this lasting epigenetic modification in NAc across a spectrum of complex behaviors in which this brain region plays an important role. Toward this goal, we identify which specific DNA methyltransferases (Dnmt1, Dnmt3a, or Dnmt3b) are regulated in chronic cocaine and chronic stress paradigms, whether manipulating DNA methylation in NAc affects addictive- and depressive-like behavior, and whether DNA methylation affects synaptic plasticity in NAc.
RESULTS
Transcriptional regulation of Dnmt3a in NAc by cocaine
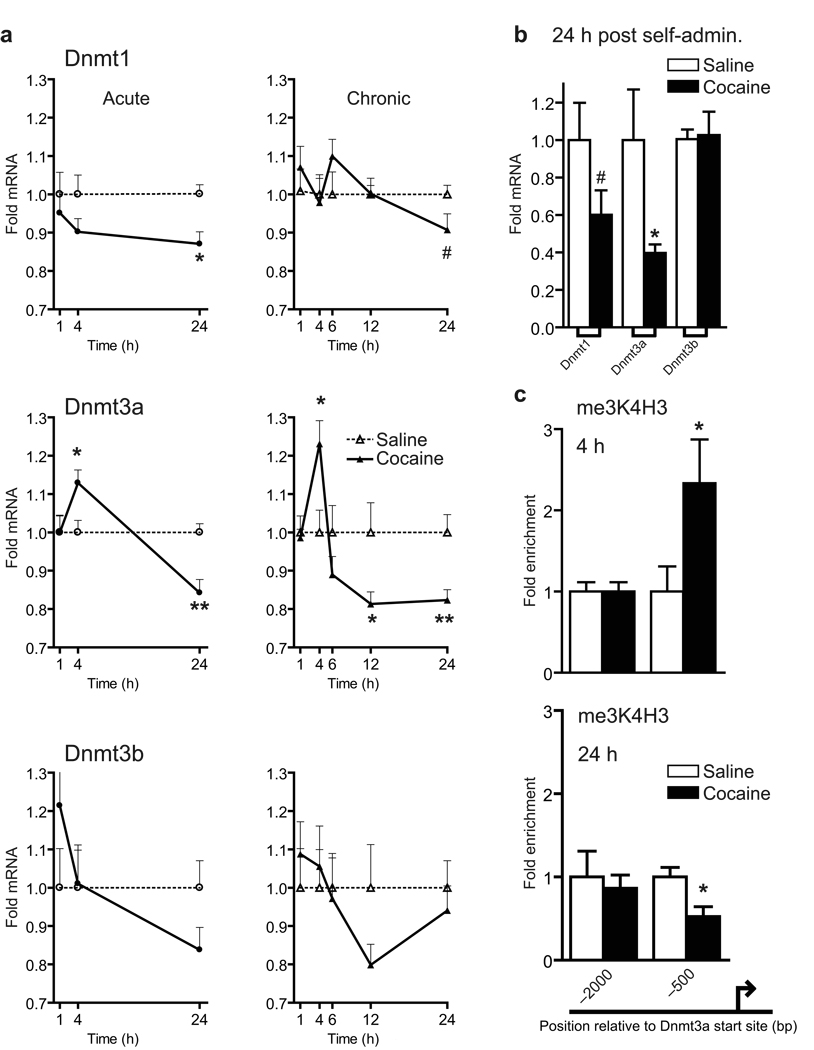
As a first step in determining the role of DNA methylation in cocaine action, we performed quantitative PCR (qPCR) on NAc tissue of mice treated acutely or chronically (7 daily injections) with cocaine for all known Dnmt’s and methyl-binding domain proteins. Among these genes, we found that Dnmt3a is selectively upregulated at 4 hr and downregulated after 24 hr following both acute and chronic cocaine administration, with limited changes observed for the other genes analyzed (Fig. 1a, Supplemental Fig. S1). Dnmt3a’s upregulation during early withdrawal time points is supported by equivalent findings from a recently published microarray study (see supplemental gene lists) performed 4 hr after 15 chronic cocaine injections15. Dnmt3a’s downregulation at 24 hr is not persistent, since mRNA analysis at 48 hr after the last chronic injection showed no change from control values (data not shown). Together, these data support the notion that Dnmt3a expression in NAc is biphasically regulated in response to each cocaine injection. This biphasic regulation of Dnmt3a appears to occur via transcriptional mechanisms, since chromatin immunoprecipitation (ChIP) analysis of a well known marker of gene activation, histone 3 tri-methyl-Lys4 (me3K4H3), indicated that the Dnmt3a gene, and not the Dnmt1 or Dnmt3b gene, exhibits transiently enhanced (at 4 hr) and suppressed (at 24 hr) me3K4H3 binding at its promoter (Fig. 1c). To provide further relevance to addiction, we analyzed tissue from rats that chronically (13 days) self-administered cocaine and found a significant downregulation in Dnmt3a expression in NAc at 24 hr (P<0.05) after the last cocaine infusion (Fig. 1b). Interestingly, Dnmt1 is significantly downregulated by acute cocaine (P<0.05); however this effect was not significant with chronic cocaine or self-administered cocaine (Fig. 1a,b). Dnmt3b was not regulated under any condition analyzed. Moreover, analysis of relative levels of each Dnmt in mouse and rat NAc revealed that Dnmt3a is by far the most predominant Dnmt expressed in the NAc (Supplemental Fig. S2). Given Dnmt3a’s dynamic regulation by cocaine, and its enrichment in NAc, we focused further on this enzyme subtype.
Fig. 1. Transcriptional regulation of Dnmt3a by chronic cocaine.
(a) qPCR analysis of NAc of acute and chronic (7 days) cocaine treated mice (20 mg/kg/day IP) demonstrated transient increases in Dnmt3a mRNA levels 4 hr after the final injection (*P<0.05, n=14 acute, n=7 chronic) and a decrease after 24 hr (**P<0.005, n=6 acute, n=16 chronic). Dnmt1 (#P=0.08) and Dnmt3b transcripts were not altered significantly by chronic cocaine, however, acute cocaine significantly reduced Dnmt1 expression at 24 hr (*P<0.05, n=6). (b) Dnmt3a mRNA was significantly reduced in the NAc of chronic (13 days) self-administering rats examined 24 hr after the last drug dose (*P<0.05, #P=0.12, n=6 control, n=7 self-administration). (c) ChIP analysis revealed a significant cocaine-induced increase (4 hr) and decrease (24 hr) in me3K4H3 binding −500 bp upstream to the Dnmt3a promoter (*P<0.05, n=4, 5 mice pooled/n), with no regulation seen −2000 bp upstream from the promoter. Data are mean ± sem.
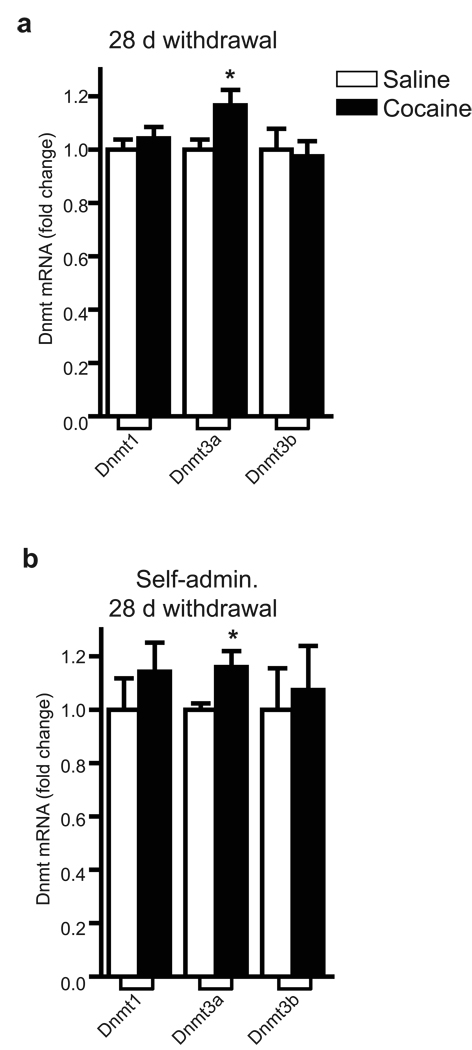
While the molecular events that occur during the transition to the addicted state, such as the aforementioned biphasic regulation of Dnmt3a, are an important component underlying the pathophysiology of addiction, a second major area of research focuses on the mechanisms that maintain the addicted state. As stated in the Introduction, more long-term regulation of Dnmt’s is of particular interest given the theoretical, persisting influence that these enzymes may have on downstream gene targets and behavior. Therefore, we injected mice with cocaine for a more prolonged time period (28 days) and analyzed Dnmt mRNA levels after an additional 28 days of withdrawal. This injection paradigm causes robust and long-lasting molecular and cellular changes, such as increased dendritic spine density on NAc neurons16–18. Surprisingly, under these conditions, we found that Dnmt3a mRNA expression in NAc is selectively increased (Fig. 2a). We also found that Dnmt3a mRNA levels are similarly increased in NAc of rats that underwent 3 weeks of cocaine self-administration followed by 28 days of withdrawal (Fig. 2b). Together, these mRNA data indicate that Dnmt3a is induced in a sustained manner after relatively long periods of cocaine withdrawal, and may thereby play an important role, not only in the transition to addiction, but also in the maintenance of the addicted state.
Fig. 2. Prolonged induction of Dnmt3a by chronic cocaine after 28 days of withdrawal.
(a) qPCR analysis of NAc of chronic (28 days) cocaine treated mice (20 mg/kg/day IP) that have undergone 28 days of drug withdrawal demonstrated an increase in Dnmt3a mRNA levels (*P<0.05, n=11). (b) Dnmt3a mRNA expression was significantly increased after 28 days of withdrawal in the NAc of chronic (3 week) self-administering rats (*P<0.05, n=6 control, n=7 self-administration). Data are mean ± sem.
DNA methylation modulates behavioral responses to cocaine
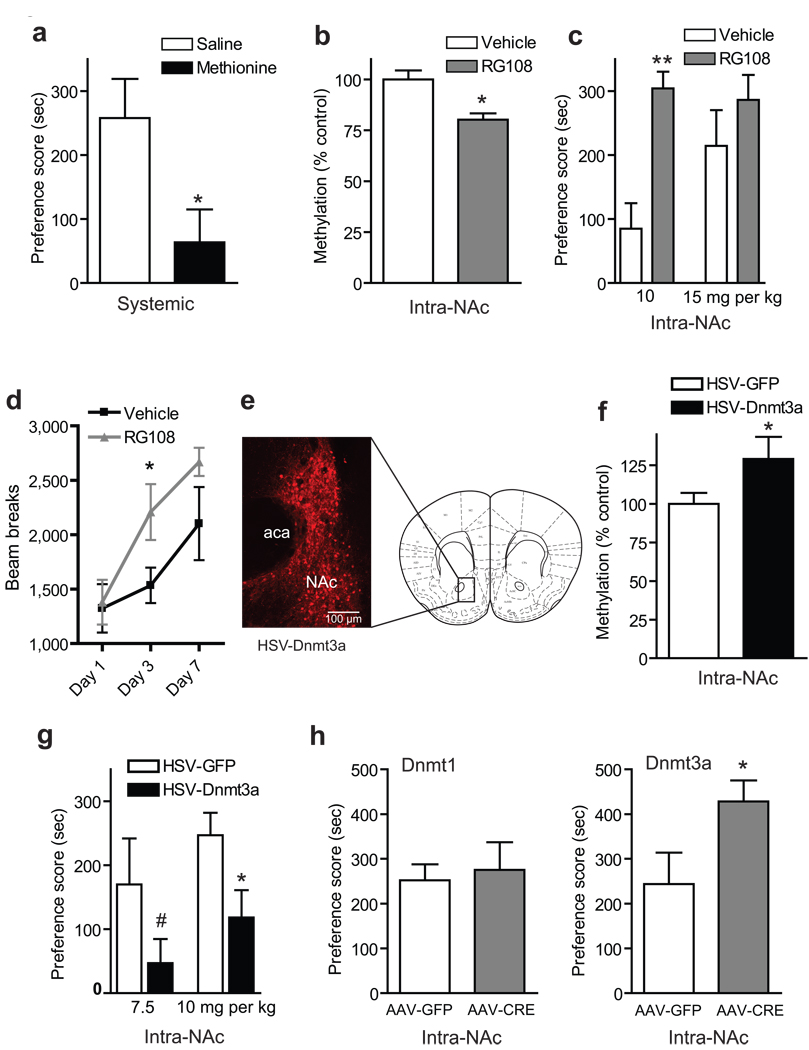
To understand the behavioral significance of the dynamic regulation of Dnmt3a in the NAc by cocaine, we utilized complementary pharmacological and genetic tools to manipulate DNA methylation in this brain region in the context of cocaine conditioned place preference (CPP), which provides an indirect measure of cocaine reward. We first administered methionine (a methyl donor) systemically with an injection regimen reported to hypermethylate specific gene promoters in rodent cortex and striatum19 and found that this manipulation caused a robust decrease in cocaine CPP (Fig. 3a). A key limitation of this experiment is its lack of anatomical and biochemical specificity. Therefore, we next tested whether intra-NAc delivery of RG108, a potent, non-nucleoside inhibitor of DNA methylation20, 21, influences reward behavior. Indeed, at a dose that decreased global DNA methylation, the continuous intra-NAc infusion of RG108 markedly enhanced cocaine CPP as well as the induction of locomotor sensitization to the drug (Fig. 3b–d).
Fig. 3. DNA methylation regulates cocaine reward.
(a) Chronic (7 days) methionine (0.78 g/kg/2x/day SC) diminished the rewarding effects of cocaine in the CPP paradigm (*P<0.05, n=9). (b) Continuous intra-NAc infusion over 7 days of the DNMT inhibitor RG108 (100 µm) decreased global DNA methylation levels in NAc (*P<0.005, n=6 vehicle, n=7 RG108), (c) increased cocaine CPP at 10 mg/kg IP (**P<0.0005, n=9), (d) and enhanced the induction of locomotor sensitization to chronic cocaine (20 mg/kg IP) (*P<0.05, n=8). (e) Verification of anatomical placement and viral infection in NAc after HSV-Dnmt3a-GFP injection; immunostaining for GFP is shown. Cartoon adapted from MBSC atlas, Figure 18, 1.54 mm Bregma. (f) HSV-Dnmt3a increased global DNA methylation levels (*P<0.05, n=8 HSV-GFP, n=5 HSV-Dnmt3a). (g) Intra-NAc HSV-Dnmt3a significantly attenuated cocaine reward at 10 mg/kg cocaine (*P<0.05, n=10), with a trend seen at a lower dose (#P=0.13, n=10). (h) Intra-NAc AAV-Cre injected into floxed-Dnmt3a mice significantly increased cocaine CPP at 7.5 mg/kg (*P<0.05, n=14 AAV-CRE, n=18 AAV-GFP), with no effect seen in floxed Dnmt1 mice. All raw CPP data are provided in Supplemental Table 1. Data are mean ± sem.
These data suggest that DNA methylation in NAc, possibly via Dnmt3a as the predominant Dnmt in this brain region, negatively regulates cocaine reward. To further test this possibility, we developed a Herpes Simplex Virus (HSV) vector to temporally and specifically overexpress Dnmt3a in the NAc (Fig. 3e). Dnmt3a overexpression increased global DNA methylation in this brain region (Fig. 3f) and, consistent with methionine administration, attenuated cocaine CPP (Fig. 3g). To obtain the converse information, we administered an Adeno-Associated Virus (AAV) vector that expresses Cre recombinase into the NAc of mice homozygous for a floxed Dnmt3a gene. Such NAc specific knock out of Dnmt3a potentiated cocaine CPP, an effect not seen for a similar local knock out of Dnmt1 (Fig. 3h). Importantly, none of these manipulations of DNA methylation in NAc altered baseline locomotor behavior nor did Dnmt3a overexpression impair general tests of learning and memory (Supplemental Fig. S3). These behavioral data, coupled with the dynamic regulation of Dnmt3a expression, suggest that increased Dnmt3a expression in NAc negatively regulates cocaine reward, whereas decreased Dnmt3a enhances cocaine reward.
DNA methylation regulates dendritic spine density in NAc
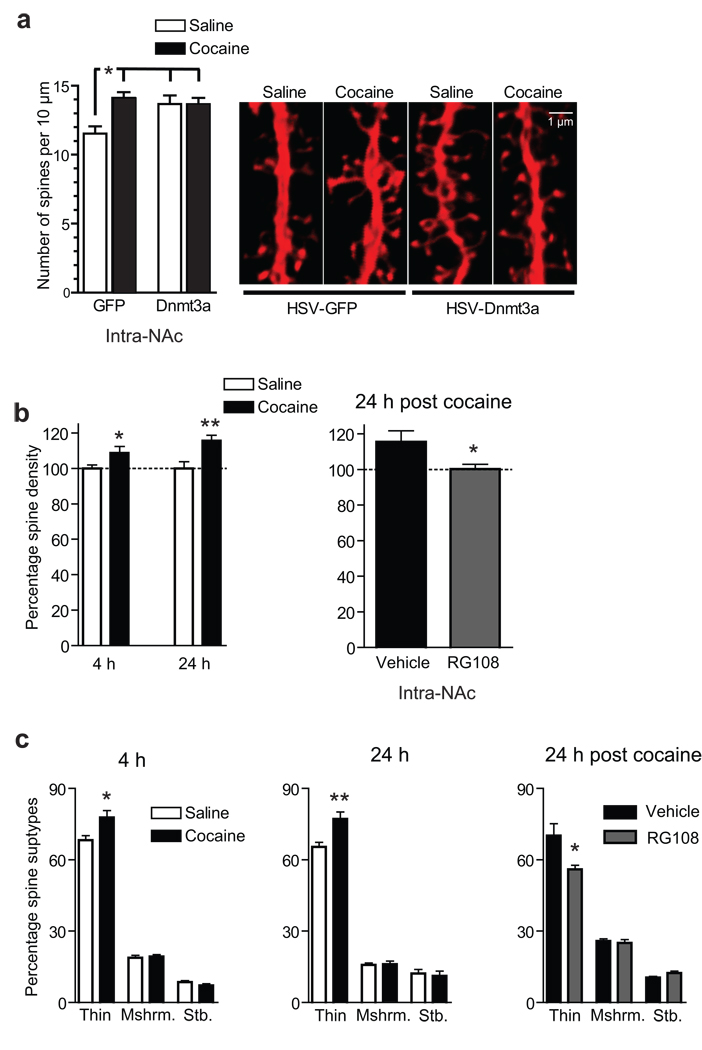
Among the most persistent drug-induced neuroadaptations known is cocaine’s ability to increase dendritic spine density of NAc neurons18. However, the contribution of spinogenesis to the addicted state remains unclear22 (see Discussion). To gain insight into this issue, we analyzed NAc neuron spine density in mice that received intra-NAc HSV-Dnmt3a or a control virus after chronic cocaine or saline treatment. Due to the limited time course of HSV overexpression (which wanes within 6 days of injection), we used a 5-injection chronic cocaine schedule which has been shown to increase spine density 4 hr after the last injection5, 23. As expected, cocaine increased NAc spine density and, interestingly, Dnmt3a overexpression alone was sufficient to increase spine density to cocaine-comparable levels (Fig. 4a).
Fig. 4. DNA methylation regulates NAc dendritic spine density.
(a) Dnmt3a overexpression mimicked cocaine’s ability to increase dendritic spine density of NAc medium spiny neurons compared to saline GFP controls (*P<0.05, n=5 both HSV-GFP conditions, n=6 both HSV-Dnmt3a conditions). Representative confocal scans of GFP immunostained NAc neurons infected with either GFP or Dnmt3a-GFP viruses. To correspond with the shortened timescale of HSV expression, these analyses were performed 4 hr after 5 cocaine injections (20mg/kg IP). (b) Using an alternate method involving direct injection of Lucifer yellow into identified neurons, we found that chronic (7 days) cocaine treatment also significantly increased spine density at 4 hr (*P<0.05, n=7 saline, n=8 cocaine) and 24 hr (**P<0.01, n=8 saline, n=7 cocaine) after the last cocaine injection. Continuous (7 days) intra-NAc RG108 infusion significantly reduced spine density 24 hr after the last chronic cocaine injection (*P<0.05, n=4 Vehicle infused, n=5 RG108 infused). Data are expressed as % change in spine density relative to saline controls. (c) NeuronStudio spine type analysis of the same images from (b) revealed that chronic cocaine selectively increased thin spines both 4 and 24 hr after the last cocaine injection (*P<0.05, **P<0.01). Likewise, intra-NAc RG108 selectively reduced thin spines of chronic cocaine injected animals (*P<0.05). None of these conditions significantly affected the number of mushroom (mshrm) or stubby (stb) spines. Data are mean ± sem.
We next performed a more detailed spine analysis under identical conditions where we found Dnmt3a expression to be cocaine regulated: at 4 and 24 hr following the last of 7 daily cocaine injections (Fig. 1a). Here, we imaged Lucifer yellow filled NAc medium spiny neurons and analyzed dendrites using NeuronStudio software; these techniques are amenable to spine density as well as spine type analysis24. We found an increased spine density at both 4 hr (P<0.05) and 24 hr (P<0.01) of withdrawal from chronic cocaine (Fig. 4b). Moreover, spine type analysis revealed that these effects at both time points are due to a selective increase in thin spines, with no effect seen in mushroom or stubby spines (Fig. 4c). The shared increase in thin spine density suggests a mechanistic link for these two time points, which might reflect a lasting consequence of the transient induction of Dnmt3a seen at 4 hr. To test this hypothesis, we followed the same chronic cocaine dosing regimen while continuously delivering the Dnmt inhibitor, RG108, into the NAc and analyzed spine density and spine type 24 hr after the last cocaine dose. We found that RG108 completely blocks cocaine-induced spinogenesis (Fig. 4c). Moreover, spine type analysis revealed that this effect is due to a specific effect on thin spines (Fig. 4c).
DNA methylation in NAc regulates depression-like behavior
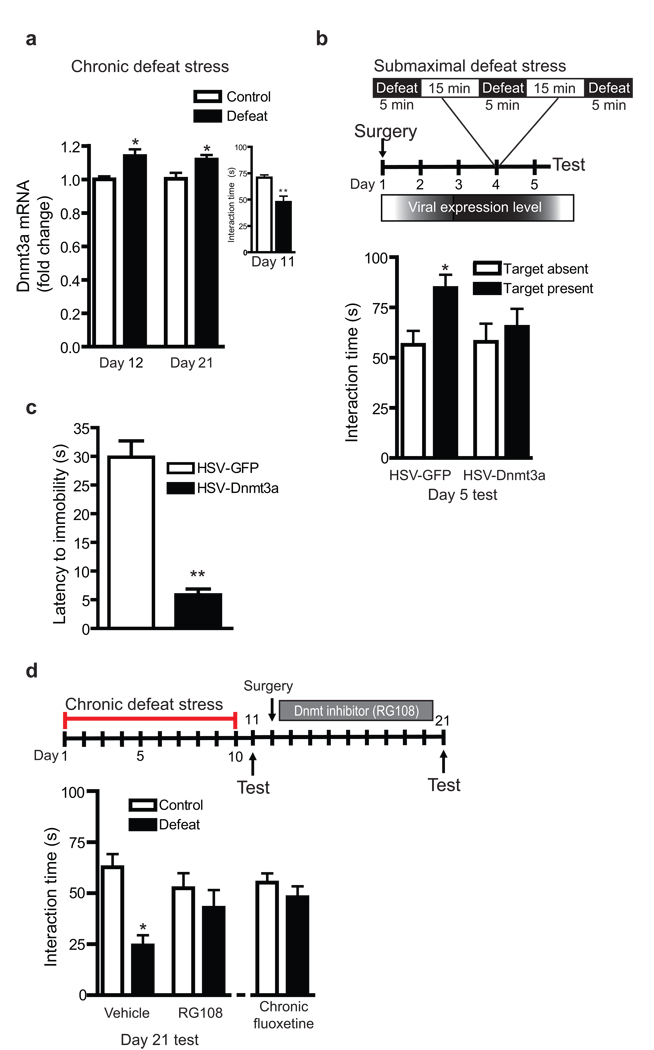
In addition to playing an important role in addictive behavior, the NAc is known to be critically involved in depression and, as with cocaine models, labile histone modifications such as acetylation and methylation in NAc have been implicated in rodent models of depression1, 7, 25. However, the role of DNA methylation in depressive behavior remains unexplored. We therefore analyzed mRNA levels for Dnmt’s and methyl-binding domain proteins in NAc at 1 and 10 days after chronic (10 days) social defeat stress—an ethologically-relevant model of depression that induces several depressive-like behaviors including prolonged social avoidance26. We found increased Dnmt3a levels in the NAc at both time points after chronic defeat stress (Fig. 5a). In contrast, no regulation was seen for any of the other proteins studied (Supplemental Fig. S4). As well, no significant regulation of Dnmt3a mRNA was observed 90 min after the last defeat nor 2 or 24 hrs after an acute defeat (data not shown).
Fig. 5. Regulation of depression-like behavior by Dnmt3a.
(a) qPCR of NAc taken 1 or 10 days after chronic (10 days) social defeat shows a significant increase in Dnmt3a levels at both time points (*P<0.05). Inset displays social avoidance, a depressive-like behavior, observed in these mice (**P<0.005, n=16 control, 26 defeated). (b) Naïve mice infused intra-NAc with HSV-GFP or HSV-Dnmt3a were subjected to a submaximal protocol of social defeat. HSV-GFP mice showed a significant increase in interaction with a social target (F1,42 = 5.219, *P<0.05) as would be expected from control mice25, whereas HSV-Dnmt3a mice lacked this phenotype (P>0.05), a pro-depressive-like response. (c) Intra-NAc injection of HSV-Dnmt3a significantly decreased latency to immobility on Day 2 of the rat forced-swim test, also a pro-depressive-like response (**P<0.001 n=7). (d) Conversely, intra-NAc infusion (10 days) of RG108, initiated one day after the last defeat episode, completely reversed the chronic social defeat-induced social avoidance exhibited by vehicle-infused mice (F1,44 = 12.876 **P<0.001), an effect equivalent to that seen for chronic (20 mg/kg/day IP, 14 days) fluoxetine administration (P>0.05). Data are mean ± sem.
We next tested the influence of Dnmt3a on susceptibility to social defeat by subjecting mice, which received HSV-GFP or HSV-Dnmt3a injections into the NAc, to submaximal defeat stress. In this paradigm, animals undergo just one day of defeat, which is not sufficient to induce social avoidance; in fact, normal mice display increased interaction with a social target under these conditions26, 27. As expected, HSV-GFP mice exhibit this significantly increased interaction time (P<0.05). In contrast, Dnmt3a overexpression in NAc attenuates social interaction, consistent with a pro-depressive-like phenotype (Fig. 5b). To complement these data, we assessed a second model of depressive-like behavior28 and found that Dnmt3a overexpression significantly reduces the latency to immobility (P<0.001) in the rat forced swim test (Fig. 5c), also a pro-depression-like effect. These data suggest that the prolonged induction of Dnmt3a in NAc by chronic social defeat stress promotes depressive behavior. To further test this hypothesis, we continuously infused the Dnmt inhibitor RG108 into the NAc between 1 and 10 days after defeat stress, when we observed increased Dnmt3a mRNA expression in this brain region. We found that such local RG108 infusion reversed social avoidance in defeated mice, an effect similar to that observed by the standard antidepressant fluoxetine (Fig. 5d), indicating that Dnmt inhibition in this brain region exerts antidepressant-like effects.
DISCUSSION
Results of the present study demonstrate that Dnmt3a expression is subject to dynamic regulation in NAc by two types of chronic emotional stimuli. Chronic social defeat stress induces a persistent upregulation of Dnmt3a in this brain region. In contrast, cocaine biphasically regulates Dnmt3a expression on a short timescale, whereas with longer-term withdrawal persisting upregulation of Dnmt3a is observed as well. Functional experiments establish that the cocaine-induced downregulation of Dnmt3a enhances cocaine reward, whereas upregulation of Dnmt3a exerts the opposite effect. Likewise, functional experiments in the chronic defeat stress and forced swim paradigms suggest that prolonged upregulation of Dnmt3a in NAc drives depressive-like behavior. Taken together, these data suggest that an appropriate balance of DNA methylation in NAc crucially gates behavioral responses to emotional stimuli—a hypermethylated state dampens responses to rewarding stimuli and heightens responses to aversive stimuli, conversely, a hypomethylated state heightens responses to rewarding stimuli and dampens responses to aversive stimuli.
Our analysis of dendritic spine density on NAc neurons demonstrates that DNA methylation is an essential mediator of cocaine-induced spinogenesis: Dnmt3a overexpression in NAc mimics the cocaine-induced increase in spines, while RG108 infusion into this region blocks cocaine’s action. A key question is how such a role for Dnmt3a in NAc spine regulation relates to the highly dynamic regulation seen for Dnmt3a expression. Since Dnmt3a overexpression alone is sufficient to regulate spine density, and because DNA demethylation is an enzymatically unfavorable chemical reaction and debate still exists regarding the enzymatic basis of active DNA demethylation, we speculate that the transient reduction in Dnmt3a expression, such as what we observe 24 hr after chronic cocaine, is likely not of a sufficient timescale to downregulate spines29. Rather, we speculate that the transient increase in Dnmt3a expression seen 4 hr after each cocaine exposure leads to the progressive accumulation of DNA methylation and is thereby responsible for the overall induction of NAc dendritic spines. The fact that a highly persistent increase in Dnmt3a expression is seen at 4 weeks of withdrawal, a time point when NAc spine density is robustly induced as revealed by multiple laboratories and methods16–18, is consistent with our hypothesis. The mechanisms responsible for the complex time course of Dnmt3a regulation in NAc–with early induction, quickly followed by suppression, and then followed by a slowly developing but very sustained induction–is unknown and requires future exploration (see below). Likewise, the persistent induction of Dnmt3a in NAc after chronic social defeat stress raises the novel possibility that NAc spine density may be regulated under these conditions as well, something in fact observed in preliminary investigations30.
Our findings that Dnmt3a induction increases NAc spine density, while it attenuates cocaine reward, highlights an important question in the addiction field: What is the behavioral relevance of cocaine’s induction of dendritic spines on NAc neurons? Several conflicting reports exist on this subject22. Robinson and colleagues positively correlated spine induction with increased locomotor sensitization31. Three studies that directly manipulated genes (ΔFosB, NFκB, or G9a) in NAc, known to regulate spines, found that manipulations that block spine induction also block cocaine’s behavioral responses5, 23. However, two other studies yielded conflicting results: blocking CDK5 or activating MEF2 blocks cocaine-induced spinogenesis but enhances cocaine reward17, 32. The pattern seen for Dnmt3a matches these latter findings. The basis for these paradoxical results is unknown. One possibility is that regulation of different types of spines might exert very different functional effects on NAc neurons and consequently on behavior. A recent study reported highly complex regulation of various spine types over a course of chronic cocaine exposure and withdrawal33, and we show a selective effect of DNA methylation on the regulation of thin spins. The observations that Dnmt3a induces thin spines, but blunts cocaine’s behavioral effects, raise the possibility that the induction of thin spines actually represents a homeostatic adaptation that serves to oppose the behavioral effects of cocaine. Clearly, this question requires much further investigation.
The electrophysiological function of cocaine-induced, DNA methylation-dependant thin spines remains speculative at this point, however, interesting electrophysiological correlates have been reported under similar cocaine injection regimens. In association with the increase in thin spines, chronic cocaine causes: 1) a reduction in firing rate in the NAc shell34, 2) synaptic depression (decreased AMPA/NMDA ratio) during early (24 hrs) withdrawal35, and 3) synaptic potentiation (increased AMPA/NMDA ratio) during late (10–14 days) withdrawal35. First, some evidence exists for spines driving neuronal firing rate, as it was found recently that the spine density reductions that are associated with dopamine depletion induce a homeostatic response of increased firing of medium spiny neurons36. Second, synaptic depression, indicated by decreased AMPA/NMDA ratio, may represent an increased pool of AMPA receptor-lacking synapses, which are also known to be regulated by cocaine37. Since thin spines are thought to represent highly plastic, newly formed spines which lack AMPA receptors, we speculate that cocaine-induced DNA methylation generates such less responsive thin spines38. Furthermore, It is also possible that, as these spines mature, they may incorporate AMPA receptor and thereby contribute to the increased AMPA/NMDA ratio as well as behavioral sensitization found during later withdrawal. Finally, the influence of DNA methylation on synaptic plasticity is heavily supported in the hippocampal literature. In cultured hippocampal neurons, consistent with our discovery that RG108 reduces cocaine-induced spine density of NAc neurons, Dnmt inhibition causes a marked reduction in functional synapses in an activity-dependant manner as measured by a reduction in mEPSC frequency14. Moreover, both Dnmt inhibition and conditional knock out of Dnmt1 and Dnmt3a block long-term potentiation, a process thought to involve the formation and/or consolidation of dendritic spines11, 12. These studies point to the importance of understanding the electrophysiological changes brought about by DNA methylation in the NAc. Such studies will provide key insight into whether cocaine-induced, DNA methylation-dependent changes in thin spines are a cause or consequence of changes in firing rate and/or synaptic depression.
As noted earlier, the mechanisms responsible for cocaine’s short-term biphasic regulation of Dnmt3a mRNA expression are unknown. In hippocampus, Dnmt3a was also found to be rapidly upregulated by fear conditioning, but the expression pattern following this upregulation over longer time points has not been assessed. Since Dnmt inhibition in hippocampus blocks memory formation, it was presumed in this study that Dnmt3a’s transient increase initiates downstream methylation of target genes that ultimately influence fear memory12. This is analogous to our proposal that the transient increases in Dnmt3a that occur with each cocaine injection cause more lasting regulation of cellular and behavioral plasticity. In our study, the fact that me3K4H3 binding to the Dnmt3a promoter is commensurately associated with Dnmt3a mRNA regulation at both the 4 and 24 hr time points suggests that a histone 3, Lys4 methyltransferase, such as KMT2A (also known as MLL1), may regulate Dnmt3a’s expression. Additionally, me3K4H3 is well known to inversely correlate with DNA methylation at particular gene promoters, raising the intriguing possibility that Dnmt3a may feed back and regulate its own mRNA expression39. On a general level, one hypothesis that may explain Dnmt3a’s complex regulation could be a transcriptional response to the rapid fluctuations in levels of dopamine or BDNF that are seen with cocaine exposures40. This is an intriguing possibility since: 1) BDNF protein, like Dnmt3a mRNA, also accumulates with prolonged cocaine withdrawal41, and 2) chronic social defeat stress induces lasting enhancement of BDNF signaling in NAc26 and, as we show here, also causes lasting increases in Dnmt3a expression in this brain region.
Finally, these data, in conjunction with studies of histone acetylation and methylation1, 5–7, 25, support an emerging model that epigenetic alterations in NAc have profound effects on the regulation of emotional behavior in models of both drug addiction and depression. The regulation is complex, with different epigenetic mechanisms apparently exerting distinct effects on behavioral endpoints. A promising line of future research would be to assess the behavioral function of additional enzymes that mediate still other forms of epigenetic modifications. In parallel, the gene targets (and their degree of overlap) for these various enzymes remain largely unknown. The elaboration of these targets should help us identify the many ways in which epigenetic mechanisms, including Dnmt3a-mediated DNA methylation, regulate NAc neuronal function to mediate the complex behavioral phenotypes of addiction and depression. It is also important to explore in future studies how Dnmt3a regulation and other epigenetic modifications in NAc contribute to the complex interactions known to exist between cocaine and stress vulnerabilities.
In this study, we found that both chronic cocaine and stress differentially regulate the de-novo DNA methyltransferase, Dnmt3a, in NAc to control dendritic spine plasticity and behavioral responses to these stimuli. We find that Dnmt3a activity in NAc in vivo is necessary for cocaine-induced increases in dendritic spine density selectively for the thin type of spines. Our findings also suggest that the rewarding responses to cocaine negatively correlate with thin spines and also raise the possibility that depressive responses to chronic stress may positively correlate with increased spine density. Taken together, these observations implicate a new epigenetic modification–DNA methylation–in the molecular mechanisms controlling cocaine- and stress-induced structural and behavioral plasticity and could ultimately lead to the development of improved treatments for drug addiction and depression.
METHODS
Methods and associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse and National Institute of Mental Health. We thank Dr. R. Jaenisch for the generous gift of the Dnmt3a plasmid, and W.G. Janssen for animal perfusions. We also thank Dr. A.J. Robison for helpful discussion and comments on manuscript.
Footnotes
Additional Supplementary Information is linked to the online version of the paper at www.nature.com/natureneuroscience/.
Author Contributions: Q.L. and E.J.N. were responsible for overall study design. Q.L., J.F., I.M., and J.W.K. designed, conduced, and analyzed RNA and ChIP experiments. Q.L., V.V., H.E.C., I.M., B.W., and R.S.O. designed and performed CPP, locomotor sensitization, and learning and memory behavioral experiments. Q.L., V.V., R.S.O., B.W., S.D.I., and C.A.B. designed and conducted submaximal defeat, forced swim, and social defeat experiments. Q.L., D.D., D.M.D., J.H.M., B.W., and E.L.W. designed and conducted dendritic spine analysis. Q.L. carried out confocal imaging of dendritic spines, D.D. performed single-cell filling, V.V., H.E.C., QL, and B.W. performed stereotaxic surgeries, W.R. and Q.L. performed HSV cloning of Dnmt3a, F.H., H.W., M.A.N., Y.R., A.J.E., M.K., and Y.L.H. designed and performed self-administration experiments, R.L.N. prepared new HSV vectors and performed quality control experiments on all HSV vectors, E.M. prepared new AAV vectors and performed quality control experiments on all AAV vectors, A.X. performed ChIP-chip statistical analyses, G.F. provided floxed Dnmt mice, and Q.L. and E.J.N. wrote the paper with the help of the other authors.
Author Information Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Covington HE, 3rd, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Levine AA, et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renthal W, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder FA, et al. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33:2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Levenson JM, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 14.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heiman M, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulipparacharuvil S, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 19.Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci U S A. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brueckner B, et al. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005;65:6305–6311. doi: 10.1158/0008-5472.CAN-04-2957. [DOI] [PubMed] [Google Scholar]
- 21.Metivier R, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 22.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010 doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson MB, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 29.Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Christoffell D, et al. Nuclear Factor kappa B regulates synaptic and behavioral plasticity induced by chronic stress. Soc. Neurosci. Abs. 2010 [Google Scholar]
- 31.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 32.Norrholm SD, et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen HW, et al. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azdad K, et al. Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion. PLoS One. 2009;4:e6908. doi: 10.1371/journal.pone.0006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Weber M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 40.Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 41.Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrot M, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2'-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci U S A. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 46.Clark MS, et al. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linhart HG, et al. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–3122. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vialou V, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 50.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.