Abstract
Context: The high incidence of insulin resistance, type 2 diabetes, and metabolic syndrome in Western societies and their impact on quality of life emphasize the importance of identifying underlying susceptibility loci for metabolic diseases. The polycystic ovary syndrome (PCOS) susceptibility locus D19S884 allele 8 (A8) is associated with measures of insulin resistance, β-cell dysfunction, and other metabolic phenotypes in PCOS families. We now investigate the role of D19S884 A8 in pregnancy.
Objective: Using the multiethnic Hyperglycemia and Adverse Pregnancy Outcome cohort, we assessed the associations of D19S884 A8 with measures of maternal glycemia and fetal size.
Design: We tested for association of maternal D19S884 A8 with maternal outcomes (fasting, 1-h, and 2-h plasma glucose, and fasting and 1-h C-peptide from an oral glucose tolerance test) and fetal and maternal D19S884 A8 with fetal outcomes (birth weight, length, head circumference, sum of skin folds, fat mass, cord C-peptide, and 2-h neonatal plasma glucose).
Subjects: We analyzed 4424 Caucasian mothers and 3347 offspring of northern European ancestry, 1957 Thai mothers and 2089 offspring from Bangkok, 1208 Afro-Caribbean mothers and 1209 offspring from Barbados, and 774 Hispanic mothers and 762 offspring from Bellflower, California.
Results: After adjusting for confounding variables and multiple testing, neither maternal nor fetal D19S884 A8 showed significant evidence for association with any of the outcomes tested.
Conclusions: The PCOS susceptibility locus, D19S884 A8, is not a major factor contributing to glycemia during pregnancy or fetal size in a general obstetric population.
The PCOS susceptibility locus D19S884 A8 is not associated with maternal glycemia or fetal size in the Hyperglycemia and Adverse Pregnancy Outcome cohort.
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder affecting approximately 5–10% of women. It occurs among all ethnicities and is the most common hormonal disorder among reproductive-age women (1). PCOS is characterized by hyperandrogenemia and irregular menses and is strongly associated with obesity, increased risk of developing cardiovascular disease, and type 2 diabetes (T2D) (2). Insulin resistance and dysfunction of pancreatic β-cells are central abnormalities of PCOS. Insulin resistance, which is found in 50–70% of PCOS women (3), plays a key role in the predisposition to T2D. In addition, many women with PCOS and their first-degree relatives exhibit inherent defects in β-cell function and abnormal insulin secretion, suggesting a genetic predisposition to β-cell dysfunction possibly initiated during development (4,5,6,7,8).
The intrauterine environment, which is influenced by both maternal and fetal genotypes, is thought to play a critical role in the development of PCOS and other adult-onset disorders. Elevated maternal testosterone levels in healthy women during mid to late gestation are associated with lower birth weight and length (9). In PCOS mothers, offspring have lower birth weight and smaller head circumference than offspring born to control women (10). Furthermore, it has been repeatedly documented that insulin resistance later in life (11), subsequent risk of developing T2D (12), and other metabolic characteristics of PCOS are inversely related to birth weight (13,14). Therefore, it is likely that genetic loci associated with PCOS may negatively influence size at birth.
Insulin resistance is also a feature of the metabolic syndrome, T2D, gestational diabetes mellitus (GDM), and pregnancy. Pregnancy is a physiological state of insulin resistance whereby increased levels of placental hormones and other mediators lead to insulin resistance, causing increased demand on the β-cells (15). This increases as pregnancy progresses, and if the mother is unable to produce enough insulin to overcome insulin resistance, blood glucose levels rise, resulting in GDM. In essence, pregnancy serves as a metabolic stress test and uncovers underlying insulin resistance and β-cell dysfunction. GDM occurs in women who have this underlying metabolic disruption, and like PCOS, GDM identifies women at risk for T2D (16). Furthermore, women with PCOS have a more than 2-fold increased odds of GDM (17) and a 7-fold increased risk of T2D. Because PCOS has significant phenotypic overlap with GDM and T2D, it is conceivable that they also share genetic risk factors.
One approach to ascertain the role of a susceptibility gene in a complex disorder is to test whether that gene contributes to susceptibility of disorders with similar phenotypes. For PCOS, one such susceptibility gene is the gene encoding fibrillin-3 (FBN3). We previously identified a PCOS susceptibility allele that maps to the dinucleotide repeat, D19S884, in intron 55 of FBN3 (18,19,20). D19S884 allele 8 (A8), which has 17 CA repeats, is associated with PCOS, measures of insulin resistance, excess fasting insulin, and other metabolic phenotypes in women with PCOS and their first-degree relatives (19). We therefore hypothesized that D19S884 A8 may also be associated with measures of glycemia, such as increased fasting and 1- and 2-h glucose levels, and measures of β-cell function, such as fasting and 1-h C-peptide levels, in non-PCOS populations, such as pregnant women.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) cohort provides a large and rigorously phenotyped group, ideal for assessing the associations of D19S884 A8 with maternal glycemia and insulinemia during pregnancy. To examine these associations, we genotyped 15,770 mothers and babies of four different ethnicities from the HAPO cohort, and tested for associations between D19S884 A8 and quantitative outcomes representing measures of maternal glycemia and fetal size.
Subjects and Methods
Subjects
The HAPO study is a multiethnic, multicenter, observational, epidemiological study assembled to investigate the associations of varying degrees of glucose intolerance during pregnancy with adverse pregnancy outcomes. HAPO study protocols were approved by the institutional review boards at all centers. Written informed consent was obtained from all participants. Over 31,000 women were enrolled in the HAPO study from 15 different centers in nine countries. Of these, 25,505 women completed oral glucose tolerance tests (OGTTs), and 23,316 babies were delivered (21). In participating HAPO field centers, approximately 90.0% of subjects agreed to participate in genetic studies and had blood drawn for DNA at the time of OGTT. Among these women, approximately 82% of babies had cord blood samples drawn for DNA.
All pregnant women at less than 31 wk gestation were eligible to participate, except women that met one or more of the following criteria (22): age less than 18 yr; delivery planned at another hospital; date of last menstrual period uncertain and no ultrasound estimation from 6–24 wk gestation available; OGTT not completed before 32 wk gestation; a multiple pregnancy; conception using gonadotropin ovulation induction or in vitro fertilization; glucose testing before recruitment; diagnosis of diabetes before or during this pregnancy requiring treatment with medication; participation in another study that may interfere with HAPO; diagnosis of HIV or hepatitis B or C before participation in HAPO; or inability to converse in languages used on field center forms without aid of an interpreter.
To maximize power and reduce the effects of admixture, we selected mothers and babies with the most complete phenotype data from centers that yielded the largest and most homogenous populations. This study was performed using Caucasian subjects from centers in the United Kingdom (Manchester and Belfast), Australia (Brisbane and Newcastle), and North America (Toronto); Thai subjects from Bangkok; Hispanic subjects of Mexican origin from Bellflower, California; and Afro-Caribbean subjects from Barbados. Only women whom gave consent to obtain blood samples for DNA and completed OGTTs were included. To remove the confounding effect of premature delivery on birth weight, we selected babies who were delivered after 37 wk gestation.
Study protocols
Detailed HAPO study methods have been published previously (21,22,23,24). Gestational age was determined from the date of the last menstrual period or from an ultrasound performed between 6 and 24 wk gestation.
OGTT
Mothers provided information on ethnicity, smoking, and family history of diabetes and hypertension and then underwent metabolic evaluation (standard 75-g OGTT and measurement of serum C-peptide) at approximately 28 wk gestation (between 24 and 32 wk) (21,23). Blood samples were obtained for plasma glucose levels at 0, 1, and 2 h; and serum C-peptide levels at 0 and 1 h after glucose challenge. Caregivers and HAPO study staff were “blinded” to the OGTT test results (except when exceeding specific thresholds outlined below).
Glucose analysis and unblinding
Fasting and 2-h OGTT samples were analyzed at each field center laboratory. Values were unblinded if fasting plasma glucose exceeded 5.8 mmol/liter, if 2-h OGTT plasma glucose exceeded 11.1 mmol/liter, or if any plasma glucose value was less than 2.5 mmol/liter. Aliquots of all OGTT samples were analyzed at the HAPO Central Laboratory as previously described (23), and these are the values used in our analyses. Data from unblinded participants were not used in any of the analyses.
Cord-blood plasma glucose and serum C-peptide
Cord blood was collected at delivery to assess fetal β-cell function and was analyzed for plasma glucose and serum C-peptide at the Central Laboratory (23). Neonatal glycemia was determined from blood samples drawn between 1 and 2 h of life.
Neonatal care and anthropometrics
After delivery, infants received customary routine care. Neonatal anthropometrics were obtained within 72 h of delivery and included skin fold thickness at three sites (flank, subscapular, and triceps), weight, length, and head circumference as previously described (24).
Genotyping
DNA was extracted from blood using Autopure LS (Gentra Systems Inc., Minneapolis, MN) and quantified by spectrophotometry at the Northwestern Center for Genetic Medicine Genomics Core. DNA samples were organized randomly on 384-well microtiter plates to eliminate the possibility of spurious associations due to systematic differences in genotyping conditions between plates. For quality control, we randomly placed three Centre d’Etude du Polymorphisme Humain (CEPH) control DNA samples, isolated from lymphoblast cell lines, in each quadrant of the 384-well plates.
The dinucleotide repeat D19S884 was amplified in all subjects using a standard protocol (18,20) with fluorescently labeled primers. For each PCR, 50 ng of genomic DNA was amplified in a total volume of 8 μl in the presence of 200 μm deoxynucleotide triphosphate, 1.5 mm MgCl2, 0.7 U AmpliTaq polymerase, and 0.75 μm of forward primer and 0.75 μm of reverse primer (Applied Biosystems, Foster City, CA). The primer sequences used were as follows: D19S884 forward, 5′-6FAM-TCCCTCAACCCCCCGAGTTC-3′; and reverse 5′-ATGCCCTCAGGACCCCC-3′. PCR products were electrophoresed in the presence of an internal size standard (GeneScan 350 ROX) overnight at room temperature on the Applied Biosystems 3130XL Capillary DNA sequence analysis system, and genotypes were assigned using the GENEMAPPER software version 4.0 (Applied Biosystems). We also visually examined the chromatogram for each sample to confirm the genotype assignment. Identical protocols were used to genotype two other repeat polymorphisms, the TAAAAn repeat in the SHBG gene and the CAGn repeat in the androgen receptor (AR) gene, which we used as covariates in some analyses. The primer sequences used were as follows: SHBG TAAAAn forward, 5′-ATCGCTTGAACTCGAGAGGC-3′; and reverse, 5′-HEX-CAGGGCCTAAACAGTCTAG-3′; and AR CAGn forward, 5′-NED-GTGCGCGAAGTGATCCAGAA-3′; and reverse, 5′-TAGCCTGTGGGGCCTCTACG-3′. For analysis, the multiallelic D19S884 locus was collapsed to a biallelic system for each of the alleles, allowing us to analyze A8 vs. all other alleles.
Power analysis
We used CaTSQT2 version 0.0.1 (25) to calculate the power to detect associations with A8 in each ethnic population, assuming an additive model, at significance levels of α = 0.001, 0.01, and 0.05. We calculated power to detect effect sizes that account for 0.1 to 1.0% of the variance (Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Statistical analysis
Because D19S884 A8 is associated with PCOS, we tested for associations between A8 and quantitative outcomes representative of those seen in PCOS women and their offspring. These analyses tested for associations between maternal D19S884 A8 and maternal outcomes, maternal D19S884 A8 and fetal outcomes, and fetal D19S884 A8 and fetal outcomes. Associations were initially examined in all mothers or babies together, while adjusting for field center, and then examined in each ethnicity separately.
Outcomes for mothers were glucose levels (fasting, 1-h, and 2-h), C-peptide levels (fasting and 1-h), and mean arterial pressure [MAP; diastolic pressure + (systolic pressure − diastolic pressure)/3], all measured at the OGTT. Outcomes for neonates were birth weight, length, head circumference, sum of skin folds, fat mass, cord C-peptide, and 2-h plasma glucose. Fat mass was calculated using the equation 1000 × (0.39055 × birth weight/1000 + 0.0453 × flank skin fold − 0.03237 × length + 0.54657) (24), adapted from Catalano et al. (26).
Associations between D19S884 A8 and quantitative outcomes were assessed through linear regressions, with single outcomes under an additive model using PLINK version 1.05 (27), integrated into the BC/SNPmax database version 2.5.9 (Biocomputing Platforms Ltd., Espoo, Finland). Natural log (ln) transformation was used to achieve normal distribution for maternal and neonatal C-peptide levels and neonatal sum of skin folds. Mendelian error checking of genotypes was performed with PedCheck, version 1.00 (28) through BC/SNPmax.
Three models (I, II, and III) were analyzed for each outcome. Model I was an unadjusted model, with the exception of the Caucasian group, in which we adjusted for field center. Model II was a minimally adjusted model that included adjustment for the three variables thought to be the major confounders for maternal outcomes (maternal age, BMI, and parity), and for fetal outcomes (gender, gestational age at delivery, and parity). Model III was the fully adjusted model, adjusting for all covariates included in models I and II as well as MAP, maternal height, and gestational age of the baby at OGTT for maternal outcomes; and maternal age, BMI, height, gestational age and MAP at OGTT, maternal fasting plasma glucose and fasting C-peptide, ethnicity, and smoking status for fetal outcomes.
The following additional analyses were performed using model III: 1) including or excluding the SHBG and AR genotypes as covariates; 2) assuming an additive model or a dominant model; and 3) performing association testing of all additional D19S884 alleles with an allele frequency of more than 5% in each ethnicity. For the A8 analyses, Bonferroni corrections were made to adjust for the testing of multiple traits (maternal traits, n = 6; fetal traits, n = 7). Analyses of additional D19S884 alleles were corrected for the testing of multiple traits and multiple alleles (n = 6–8, depending on the ethnicity). Pcorrected values <0.05 were considered significant.
Results
Characteristics of the HAPO Study participants and measurements of outcomes have been reported (21,24). The number of samples genotyped for each center and ethnicity are given in Table 1. The largest group is Caucasians (3174 mother/baby pairs, n = 7771), whereas the smallest is Hispanics (Mexican-American) (740 mother/baby pairs, n = 1536). The racial distribution is 50.1% Caucasian, 25.2% Asian, 15.1% Afro-Caribbean, and 9.6% Hispanic. The CEPH control samples genotyped with a 90.3% success rate and had a plate to plate concordance of 99.8%. Two percent of the participant samples were selected randomly and regenotyped without knowledge of previously assigned genotypes. Among these, 99.9% concordance was observed. We obtained D19S884 genotypes for 94.7% of the samples. Mother-baby pairs (n = 226 pairs) with one or more Mendelian discrepancies, as well as individuals with other non-Mendelian errors (n = 176), were excluded from the analyses.
Table 1.
Maternal and baby DNA samples by field center and ethnicity
| Center | Ethnicity | Total mothers | Total babies | Mother-offspring pairs | Total |
|---|---|---|---|---|---|
| Belfast | Caucasian (UK) | 1,292 | 997 | 955 | 2,289 |
| Manchester | Caucasian (UK) | 1,092 | 624 | 598 | 1,716 |
| Brisbane | Caucasian (Australia) | 911 | 677 | 632 | 1,588 |
| Newcastle | Caucasian (Australia) | 478 | 408 | 362 | 886 |
| Toronto | Caucasian (NA) | 651 | 641 | 627 | 1,292 |
| Total Caucasian | 4,424 | 3,347 | 3,174 | 7,771 | |
| Bangkok | Thai | 1,957 | 2,089 | 1,795 | 4,046 |
| Barbados | Afro-Caribbean | 1,208 | 1,209 | 1,174 | 2,417 |
| Bellflower | Hispanic | 774 | 762 | 740 | 1,536 |
| Total overall | 8,363 | 7,407 | 6,883 | 15,770 |
Genotyping
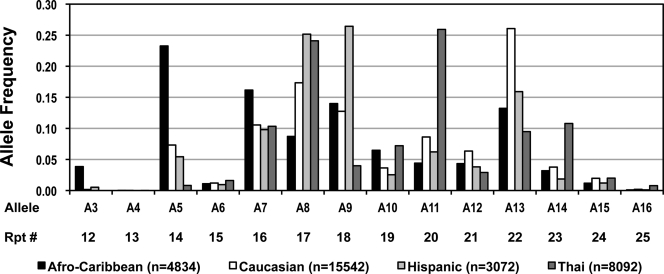
D19S884 allele designations and their respective number of repeats are defined in Fig. 1. We identified 23 alleles at the D19S884 locus, A0–A22 (range, 9 to 31 CA repeats). To our knowledge, this is the greatest number of alleles at this locus identified in any study to date and is due to our large sample size and ethnic diversity. The frequency of these alleles varied widely across populations, and for each ethnic group the distribution of alleles with a frequency greater than 0.005 is presented in Fig. 1. Alleles A0–A2 and A17–22 were very rare and were not seen in all populations (data not shown). Among Caucasians, the repeat allele frequencies were similar to those previously reported by Urbanek et al. (20) for CEPH Caucasian families and by others (29,30). A7 through A13 were common to all ethnicities (frequencies, ≥0.03). The most common alleles were A13 in Caucasians (0.26), A9 in Hispanics (0.26), A5 in Afro-Caribbeans (0.23), and A11 in Thais (0.26). The Caucasians have seven alleles with greater than 0.05% frequency; whereas the Hispanics, Afro-Caribbeans, and Thais only have six alleles (Fig. 1). A8, the PCOS susceptibility allele, contains 17 CA repeats and was the second most common allele in three of the four ethnicities [Caucasian (0.17), Hispanic (0.25), and Thai (0.24)], but was considerably lower in the Afro-Caribbean population (0.09).
Figure 1.
Frequencies of D19S884 (CA)n alleles and repeat number by ethnic group in the HAPO study. The repeat number (Rpt #) represents the number of CA repeats present at the corresponding allele. Only alleles with frequencies greater than 0.005 are shown. Black bars, Afro-Caribbeans; white, Caucasians; light gray, Hispanics; dark gray, Thais. n, Number of chromosomes.
Power analysis
We calculated the power to detect associations with A8 in each of the ethnicities in our cohort assuming an additive model, detecting effect sizes that account for 0.1, 0.5, and 1.0% of the variance. The Hispanic babies were our least powered group, with the smallest sample number (n = 706). However, we had over 80% power to detect associations with neonatal 2-h plasma glucose, our least powered trait, with an uncorrected α-level of 0.05, assuming that A8 explains 0.1% of the phenotypic variance. Neonatal 2-h plasma glucose is the trait with the fewest number of measurements recorded in our cohort, with only 92.7% of babies having values for this trait. We also had 100% power to detect associations with this trait with an uncorrected α-level of 0.001, assuming that A8 explains 0.5% of the variance (Supplemental Table 1).
Statistical analyses
Our results for A8 are presented in Table 2 for both unadjusted (model I) and fully adjusted (model III) analyses. The P values for the minimally adjusted results (model II) were very similar to those of the fully adjusted model for all outcomes analyzed and therefore are not shown. For model III, adjusting for the SHBG and AR genotypes as covariates using the shortest allele, longest allele, or the average of the two alleles, did not alter the P values (data not shown). After adjusting for confounding variables, the strongest evidence for association with maternal A8 was observed with ln 1-h C-peptide levels in Thai mothers, where there is a 2.62% increase in C-peptide levels per risk allele (Puncorrected = 0.024). The strongest evidence for association with fetal A8 was observed with the ln sum of skin folds, where there is a 0.86% decrease in the sum of skin folds per risk allele (Puncorrected = 0.041) in all babies analyzed together. However, none of these findings remain statistically significant after Bonferroni correction for multiple testing. Further A8 analyses performed by analyzing the male and female babies separately (data not shown) also did not yield any significant results after correction for multiple testing.
Table 2.
Results of association studies
| Trait | Ethnicity | A8 frequency (%) | Model I (unadjusted)
|
Model III (fully adjusted)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | β | Puncorrected | Pcorrected | n | β | Puncorrected | Pcorrected | |||
| Maternal genotype vs. maternal phenotype | ||||||||||
| Fasting plasma glucose (mg/dl) | All | 19 | 8016 | −0.13310 | 0.36 | 1.00 | 7845 | −0.0407 | 0.75 | 1.00 |
| Afro-Caribbean | 9 | 1131 | −0.03482 | 0.95 | 1.00 | 1131 | −0.0417 | 0.93 | 1.00 | |
| Caucasian | 17 | 4183 | −0.00832 | 0.97 | 1.00 | 4178 | −0.0476 | 0.78 | 1.00 | |
| Hispanic | 25 | 730 | 0.05603 | 0.90 | 1.00 | 730 | −0.0926 | 0.82 | 1.00 | |
| Thai | 24 | 1805 | −0.15130 | 0.53 | 1.00 | 1805 | −0.0703 | 0.76 | 1.00 | |
| 1-h plasma glucose (mg/dl) | All | 19 | 8016 | −0.96330 | 0.13 | 0.89 | 7845 | −0.6666 | 0.26 | 1.00 |
| Afro-Caribbean | 9 | 1131 | −1.32700 | 0.52 | 1.00 | 1131 | −1.6930 | 0.37 | 1.00 | |
| Caucasian | 17 | 4183 | −0.13320 | 0.88 | 1.00 | 4178 | −0.0669 | 0.94 | 1.00 | |
| Hispanic | 25 | 730 | −3.06900 | 0.13 | 0.89 | 730 | −3.6470 | 0.06 | 0.39 | |
| Thai | 24 | 1805 | −0.78650 | 0.50 | 1.00 | 1805 | −0.4784 | 0.67 | 1.00 | |
| 2-h plasma glucose (mg/dl) | All | 19 | 8016 | −1.03300 | 0.03 | 0.23 | 7845 | −0.6415 | 0.15 | 1.00 |
| Afro-Caribbean | 9 | 1131 | −1.24700 | 0.45 | 1.00 | 1131 | −1.7550 | 0.25 | 1.00 | |
| Caucasian | 17 | 4183 | −0.13920 | 0.83 | 1.00 | 4178 | −0.0387 | 0.95 | 1.00 | |
| Hispanic | 25 | 730 | −1.11000 | 0.44 | 1.00 | 730 | −1.4570 | 0.29 | 1.00 | |
| Thai | 24 | 1805 | −1.42700 | 0.11 | 0.79 | 1805 | −1.2600 | 0.14 | 0.99 | |
| ln fasting C-peptide (μg/liter) | All | 19 | 7984 | −0.00002 | 1.00 | 1.00 | 7814 | 0.0018 | 0.68 | 1.00 |
| Afro-Caribbean | 9 | 1131 | −0.00512 | 0.82 | 1.00 | 1131 | 0.0018 | 0.93 | 1.00 | |
| Caucasian | 17 | 4152 | 0.00705 | 0.35 | 1.00 | 4147 | 0.0013 | 0.82 | 1.00 | |
| Hispanic | 25 | 730 | −0.00084 | 0.96 | 1.00 | 730 | −0.0053 | 0.69 | 1.00 | |
| Thai | 24 | 1805 | −0.00003 | 1.00 | 1.00 | 1805 | 0.0052 | 0.52 | 1.00 | |
| ln 1-h C-peptide (μg/liter) | All | 19 | 7962 | 0.00476 | 0.46 | 1.00 | 7796 | 0.0057 | 0.36 | 1.00 |
| Afro-Caribbean | 9 | 1123 | −0.00923 | 0.74 | 1.00 | 1123 | 0.0003 | 0.99 | 1.00 | |
| Caucasian | 17 | 4144 | 0.00627 | 0.51 | 1.00 | 4139 | 0.0013 | 0.88 | 1.00 | |
| Hispanic | 25 | 730 | −0.02185 | 0.24 | 1.00 | 730 | −0.0236 | 0.18 | 1.00 | |
| Thai | 24 | 1803 | 0.02363 | 0.04 | 0.30 | 1803 | 0.0259 | 0.02 | 0.17 | |
| MAP (mm Hg) | All | 19 | 8016 | −0.03420 | 0.82 | 1.00 | 7845 | −0.1018 | 0.53 | 1.00 |
| Afro-Caribbean | 9 | 1131 | −0.28990 | 0.63 | 1.00 | 1131 | −0.1639 | 0.76 | 1.00 | |
| Caucasian | 17 | 4183 | 0.04577 | 0.84 | 1.00 | 4178 | 0.0248 | 0.90 | 1.00 | |
| Hispanic | 25 | 730 | −0.25970 | 0.59 | 1.00 | 730 | −0.3175 | 0.47 | 1.00 | |
| Thai | 24 | 1805 | −0.08503 | 0.77 | 1.00 | 1805 | 0.0531 | 0.85 | 1.00 | |
| (Continued) | ||||||||||
Table 2A.
Continued
| Trait | Ethnicity | A8 frequency (%) | Model I (unadjusted)
|
Model III (fully adjusted)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | β | Puncorrected | Pcorrected | n | β | Puncorrected | Pcorrected | |||
| Fetal genotype vs. fetal phenotype | ||||||||||
| Length (cm) | All | 19 | 6706 | 0.02040 | 0.67 | 1.00 | 6534 | 0.0069 | 0.87 | 1.00 |
| Afro-Caribbean | 9 | 1127 | 0.14400 | 0.45 | 1.00 | 1127 | 0.0831 | 0.64 | 1.00 | |
| Caucasian | 17 | 2870 | −0.02054 | 0.79 | 1.00 | 2847 | −0.0386 | 0.57 | 1.00 | |
| Hispanic | 25 | 718 | 0.10100 | 0.34 | 1.00 | 718 | 0.1087 | 0.25 | 1.00 | |
| Thai | 24 | 1841 | −0.03994 | 0.54 | 1.00 | 1841 | −0.0041 | 0.94 | 1.00 | |
| Birth weight (g) | All | 19 | 6706 | −2.33100 | 0.82 | 1.00 | 6534 | −7.0260 | 0.43 | 1.00 |
| Afro-Caribbean | 9 | 1127 | 29.96000 | 0.38 | 1.00 | 1127 | 17.3400 | 0.58 | 1.00 | |
| Caucasian | 17 | 2870 | 2.60800 | 0.88 | 1.00 | 2847 | −2.8850 | 0.84 | 1.00 | |
| Hispanic | 25 | 718 | −0.69630 | 0.98 | 1.00 | 718 | 1.1080 | 0.96 | 1.00 | |
| Thai | 24 | 1841 | −30.9600 | 0.05 | 0.36 | 1841 | −19.2400 | 0.16 | 1.00 | |
| Head circumference (cm) | All | 19 | 6707 | −0.03450 | 0.26 | 1.00 | 6535 | −0.0408 | 0.15 | 1.00 |
| Afro-Caribbean | 9 | 1127 | 0.09222 | 0.40 | 1.00 | 1127 | 0.0368 | 0.73 | 1.00 | |
| Caucasian | 17 | 2871 | −0.02436 | 0.63 | 1.00 | 2848 | −0.0307 | 0.49 | 1.00 | |
| Hispanic | 25 | 718 | −0.06203 | 0.38 | 1.00 | 718 | −0.0552 | 0.39 | 1.00 | |
| Thai | 24 | 1841 | −0.09274 | 0.06 | 0.41 | 1841 | −0.0632 | 0.15 | 1.00 | |
| ln sum skin folds (mm) | All | 19 | 6700 | −0.00746 | 0.09 | 0.66 | 6528 | −0.0086 | 0.04 | 0.29 |
| Afro-Caribbean | 9 | 1127 | 0.00748 | 0.53 | 1.00 | 1127 | 0.0032 | 0.78 | 1.00 | |
| Caucasian | 17 | 2869 | −0.00643 | 0.37 | 1.00 | 2846 | −0.0065 | 0.34 | 1.00 | |
| Hispanic | 25 | 714 | −0.02341 | 0.07 | 0.49 | 714 | −0.0232 | 0.06 | 0.39 | |
| Thai | 24 | 1840 | −0.01068 | 0.17 | 1.00 | 1840 | −0.0064 | 0.37 | 1.00 | |
| Fat mass (g) | All | 19 | 6668 | −3.35500 | 0.35 | 1.00 | 6496 | −4.8190 | 0.14 | 0.99 |
| Afro-Caribbean | 9 | 1119 | 8.56000 | 0.44 | 1.00 | 1119 | 5.2400 | 0.62 | 1.00 | |
| Caucasian | 17 | 2856 | −0.94060 | 0.88 | 1.00 | 2833 | −2.0420 | 0.70 | 1.00 | |
| Hispanic | 25 | 715 | −7.54300 | 0.46 | 1.00 | 715 | −7.5090 | 0.41 | 1.00 | |
| Thai | 24 | 1828 | −12.3100 | 0.02 | 0.16 | 1828 | −8.3070 | 0.08 | 0.59 | |
| ln cord C-peptide (μg/liter) | All | 19 | 6994 | −0.00190 | 0.73 | 1.00 | 6808 | 0.0005 | 0.92 | 1.00 |
| Afro-Caribbean | 9 | 1126 | 0.00923 | 0.60 | 1.00 | 1126 | 0.0144 | 0.38 | 1.00 | |
| Caucasian | 17 | 3082 | −0.00828 | 0.33 | 1.00 | 3053 | −0.0037 | 0.65 | 1.00 | |
| Hispanic | 25 | 717 | 0.00644 | 0.66 | 1.00 | 717 | 0.0064 | 0.64 | 1.00 | |
| Thai | 24 | 1911 | 0.00213 | 0.82 | 1.00 | 1911 | 0.0012 | 0.89 | 1.00 | |
| Neonatal 2-h glucose (mg/dl) | All | 19 | 5845 | −0.68970 | 0.07 | 0.46 | 5689 | −0.7036 | 0.06 | 0.43 |
| Afro-Caribbean | 9 | 1035 | −0.91910 | 0.46 | 1.00 | 1035 | −0.9854 | 0.42 | 1.00 | |
| Caucasian | 17 | 2073 | −1.02200 | 0.10 | 0.70 | 2052 | −1.0640 | 0.08 | 0.58 | |
| Hispanic | 25 | 706 | −0.66450 | 0.50 | 1.00 | 706 | −0.6172 | 0.52 | 1.00 | |
| Thai | 24 | 1895 | −0.36710 | 0.55 | 1.00 | 1895 | −0.4731 | 0.43 | 1.00 | |
Analyses on additional D19S884 alleles with 0.05% frequency in each ethnicity were also performed. After correcting for multiple alleles and multiple traits, significant evidence for a positive association was observed between maternal A9 (19 repeats) and neonatal 2-h plasma glucose [2.30 mg/dl per allele (95% confidence interval, 1.04, 3.5); Pcorrected = 0.019]. This effect was specific to female babies [2.91 mg/dl per allele (95% confidence interval, 1.22, 4.6); Pcorrected = 0.041] and was not observed in males. After correction for multiple testing, we observed no statistically significant evidence for an inverse association between any alleles and any traits in any population studied.
Discussion
This is the first study to examine D19S884 A8 in a non-PCOS cohort. D19S884 A8 has been shown to be associated with PCOS in multiple studies of populations of European ancestry (20,31,32). D19S884 maps to chromosome 19p13.2 within the FBN3 gene. FBN3 encodes fibrillin-3, one of the three members of the fibrillin family of extracellular matrix proteins. Fibrillins provide structural integrity to connective tissues and regulate the activity of members of the TGFβ superfamily (33). Members of the TGFβ family have been implicated in PCOS, T2D, insulin resistance, and glucose homeostasis (34,35,36). Thus, it was plausible that if members of the TGFβ pathway are implicated in various states of insulin resistance, then FBN3, a potential extracellular regulator of this pathway, may also play a role in regulating maternal glycemia because pregnancy is a natural state of insulin resistance.
D19S884 A8 is associated with insulin resistance and other characteristics of the metabolic syndrome in women with PCOS and their first-degree relatives. A8 is associated with higher fasting insulin levels and elevated homeostasis model of assessment for insulin resistance in women with PCOS independent of obesity (19,20), as well as increased fasting proinsulin levels and proinsulin:insulin ratios in brothers of women with PCOS, suggestive of β -cell dysfunction (19). It is unknown whether the association between A8 and measures of insulin resistance and/or glycemia is specific to PCOS or whether A8 is associated with these metabolic features in the absence of PCOS. Therefore, we hypothesized that in a nonpathogenic insulin-resistant state, such as pregnancy, A8 might be associated with elevated glucose levels and/or elevated C-peptide levels.
We tested for associations between A8 and six metabolic quantitative traits in 8363 women of four different ethnicities from the HAPO cohort (Table 1). The HAPO cohort is an exceptional cohort due to its multiethnic nature and large number of subjects with glucose concentrations that span the full range of normal OGTT values (22,24). For the HAPO study, phenotypes were measured in an identical manner across all centers, and all metabolic assays were carried out in a central laboratory in an effort to avoid center-to-center variation. Due to the large sample size, this study was powered to detect even relatively subtle changes in phenotypic measurements. However, we did not find any evidence for association between D19S884 A8 and maternal glycemia or C-peptide levels.
In addition to its association with adult phenotypes, D19S884 A8 may also be associated with fetal size. A prenatal origin of PCOS has been postulated (37), and women with PCOS are reported to be born small for gestational age (38) and may subsequently give birth to smaller babies (10). Furthermore, polymorphisms in numerous genes impacting maternal glycemia or fetal insulin production affect fetal size (39,40). We anticipated that the presence of A8 in the mother, causing elevated maternal insulin resistance and glucose levels, might be associated with an increase in fetal size. Conversely, the presence of A8 in the baby, causing increased exposure to fetal testosterone or β-cell dysfunction, may be associated with a decrease in birth weight and length. We therefore tested for association between measures of fetal size and D19S884 A8 status in mothers and newborns. We did not find significant evidence of association between either maternal or fetal D19S884 A8 and any of the measures of fetal size tested.
Although other D19S884 alleles have been tested for association with PCOS in cohorts of European ancestry, none have shown significant associations (26). However, these studies have been relatively small, and it is therefore plausible that: 1) there could be an allele with a small effect that can only be detected in a larger cohort such as HAPO; or 2) other alleles may be associated with PCOS, glycemia, or fetal size in other ethnicities. We did find statistically significant association between A9 and neonatal 2-h glucose levels in female babies. These findings need to be investigated further to determine their relevance in fetal glycemia.
In this study, we tested for association between D19S884 A8, a known PCOS susceptibility locus, and maternal glycemia and C-peptide levels, as well as fetal size in babies of the HAPO cohort. We did not detect significant evidence for association between D19S884 A8 and any phenotype tested. Therefore, we conclude that D19S884 A8 is not a general mediator of glycemia, insulin resistance, or fetal size in this cohort and that the metabolic effects of A8 observed in women with PCOS and their family members may be specific to PCOS.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant P50 HD44405 and by Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarships Program award PGS-D. This work was also supported by Grants R01 DK067459 and U01 HG004415 from the National Institute of Diabetes, Digestive, and Kidney Diseases and the National Human Genome Research Institute. The HAPO study was funded by Grants R01-HD34242 and R01-HD34243 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Diabetes and Digestive and Kidney Diseases, and by the American Diabetes Association. Members of the HAPO Study Cooperative Research Group are listed in the appendix of Ref. 21.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 5, 2010
Abbreviations: A8, Allele 8; AR, androgen receptor; GDM, gestational diabetes mellitus; ln, natural log; MAP, mean arterial pressure; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; T2D, type 2 diabetes.
References
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Sam S, Dunaif A 2003 Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab 14:365–370 [DOI] [PubMed] [Google Scholar]
- Legro RS, Castracane VD, Kauffman RP 2004 Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 59:141–154 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Finegood DT 1996 β-Cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 81:942–947 [DOI] [PubMed] [Google Scholar]
- Sam S, Legro RS, Bentley-Lewis R, Dunaif A 2005 Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4797–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A 2006 Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Nat Acad Sci USA 103:7030–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Sung YA, Legro RS, Dunaif A 2008 Evidence for pancreatic β-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism 57:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colilla S, Cox NJ, Ehrmann DA 2001 Heritability of insulin secretion and insulin action in women with polycystic ovary syndrome and their first degree relatives. J Clin Endocrinol Metab 86:2027–2031 [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Jacobsen G, Romundstad P 2006 Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol 155:365–370 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F 2005 Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 20:2122–2126 [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsén T, Tuomilehto J, Jaddoe VW, Osmond C, Barker DJ 2002 Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 45:342–348 [DOI] [PubMed] [Google Scholar]
- Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D 2000 The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 133:176–182 [DOI] [PubMed] [Google Scholar]
- Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM 2003 Is birth weight related to later glucose and insulin metabolism? A systematic review. Diabet Med 20:339–348 [DOI] [PubMed] [Google Scholar]
- Mi J, Law C, Zhang KL, Osmond C, Stein C, Barker D 2000 Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann Intern Med 132:253–260 [DOI] [PubMed] [Google Scholar]
- Homko C, Sivan E, Chen X, Reece EA, Boden G 2001 Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab 86:568–573 [DOI] [PubMed] [Google Scholar]
- Buchanan TA 2001 Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab 86:989–993 [DOI] [PubMed] [Google Scholar]
- Lo JC, Feigenbaum SL, Escobar GJ, Yang J, Crites YM, Ferrara A 2006 Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care 29:1915–1917 [DOI] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss 3rd JF, Spielman RS, Dunaif A 1999 Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci USA 96:8573–8578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek M, Sam S, Legro RS, Dunaif A 2007 Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab 92:4191–4198 [DOI] [PubMed] [Google Scholar]
- Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2005 Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab 90:6623–6629 [DOI] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA 2008 Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002 [Google Scholar]
- 2002 The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet 78:69–77 [DOI] [PubMed] [Google Scholar]
- Nesbitt GS, Smye M, Sheridan B, Lappin TR, Trimble ER 2006 Integration of local and central laboratory functions in a worldwide multicentre study: Experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials 3:397–407 [DOI] [PubMed] [Google Scholar]
- 2009 Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skol AD, Scott LJ, Abecasis GR, Boehnke M 2006 Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet 38:209–213 [DOI] [PubMed] [Google Scholar]
- Catalano PM, Thomas AJ, Avallone DA, Amini SB 1995 Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol 173:1176–1181 [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE 1998 PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl MJ, Hatzirodos N, Irving-Rodgers HF, Zhao ZZ, Painter JN, Hickey TE, Gibson MA, Rainey WE, Carr BR, Mason HD, Norman RJ, Montgomery GW, Rodgers RJ 2009 Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod 15:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villuendas G, Escobar-Morreale HF, Tosi F, Sancho J, Moghetti P, San Millán JL 2003 Association between the D19S884 marker at the insulin receptor gene locus and polycystic ovary syndrome. Fertil Steril 79:219–220 [DOI] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2006 Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab 91:4112–4117 [DOI] [PubMed] [Google Scholar]
- Tucci S, Futterweit W, Concepcion ES, Greenberg DA, Villanueva R, Davies TF, Tomer Y 2001 Evidence for association of polycystic ovary syndrome in Caucasian women with a marker at the insulin receptor locus. J Clin Endocrinol Metab 86:446–449 [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC 2003 Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33:407–411 [DOI] [PubMed] [Google Scholar]
- Herder C, Zierer A, Koenig W, Roden M, Meisinger C, Thorand B 2009 Transforming growth factor-β1 and incident type 2 diabetes: results from the MONICA/KORA case-cohort study, 1984–2002. Diabetes Care 32:1921–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL 2007 FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA 104:1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Middelberg-Bisping K, Drewes C, Schatz H 1996 Elevated plasma levels of transforming growth factor-β 1 in NIDDM. Diabetes Care 19:1113–1117 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Franks S 2002 Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol 174:1–5 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Potau N, Francois I, de Zegher F 1998 Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 83:3558–3562 [DOI] [PubMed] [Google Scholar]
- Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, Ellard S, Ferrer J, Hattersley AT 2007 Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 4:e118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio R, Bellanné-Chantelot C, Moreno JC, Morel V, Calle H, Alonso M, Mustieles C 2002 Nine novel mutations in maturity-onset diabetes of the young (MODY) candidate genes in 22 Spanish families. J Clin Endocrinol Metab 87:2532–2539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.