Abstract
A total synthesis of the fostriecin has been achieved in 24 steps from enyne 11. The lactone moiety was installed by a Leighton allylation and Grubbs ring-closing metathesis (RCM) reaction. The highly reactive Z,Z,E-triene moiety was installed via a late stage Suzuki-Miyaura cross coupling of a remarkably stable Z-vinyl boronate. The relative and absolute stereocenters of the C-8,9,11 triol were generated with a regio- and stereoselective asymmetric hydration/oxidation sequence.
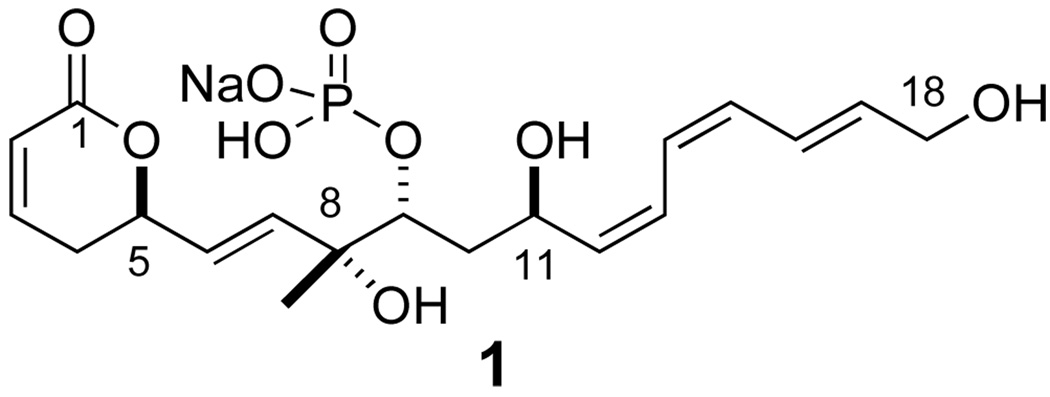
Fostriecin (1, CI-920) is a structurally novel phosphorylated polyene-, polyol- and pyranone-containing natural products, which was isolated from Steptomyces pulveraceus in 1983 (Figure 1).1 In addition to its compelling structural features, fostriecin has been shown to possess significant cytotoxic activity against a broad range of cell lines,2 such as leukemia, lung cancer, breast cancer, and ovarian cancer in vitro, and also antitumor activity against leukemia in vivo.3 This cancer cell activity has been linked to the phosphate portion of the molecule and its ability to inhibit the protein phosphatases 1, 2A and 4 (PP1, PP2A and PP4). Of particular interest is fostriecin’s ability to selectively inhibit phosphatases 2A and 4 over phosphatases 1 (e.g., IC50 = 45 nM for PP1, 1.5 nM for PP2A, and 3.0 nM for PP4).4 Because of its unique structure and compelling biological activity, fostriecin has been extensively studied by both chemists and biologists.
Figure 1.
Fostriecin (1, CI-920)
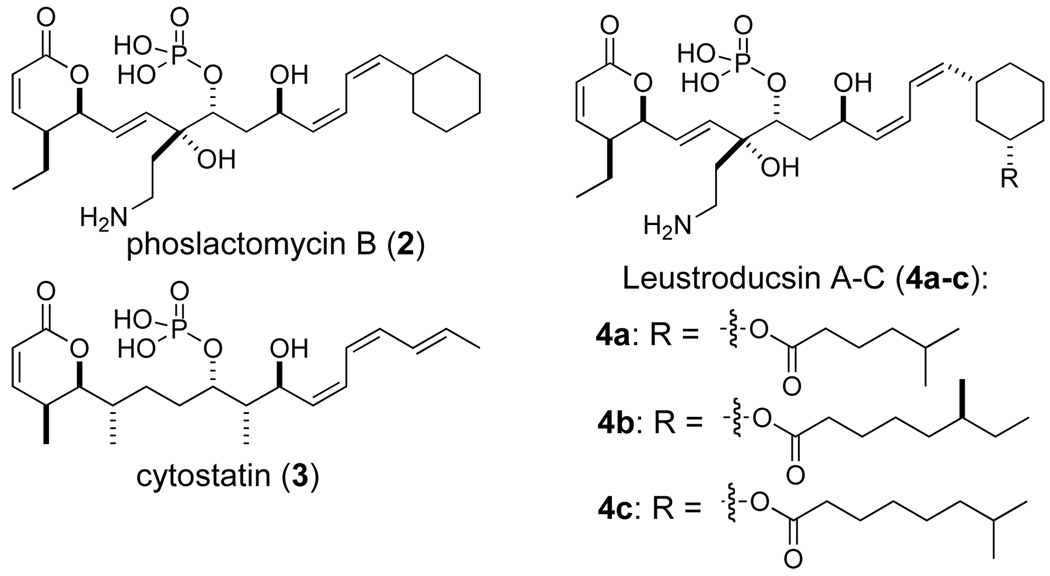
In addition to the mechanism-of-action studies, fostriecin was the subject of several clinical trials at the National Cancer Institute. While its gross structure was known, the complete stereochemical assignment of fostriecin was determined in 1997 by Boger.5 These efforts from Boger also led to its first total synthesis.6a Fostriecin’s potential use as a chemotherapeutic agent has inspired many more total syntheses.6,7 Similarly, the isolation and structural elucidation of related natural products with similar antitumor, antibacterial and antifungal activities have occurred (e.g., phoslactomycins A–F (2);8 cytostatin (3);9 and leustroducsins A–C and H (4a–c) in Figure 2).10
Figure 2.
Related natural products to fostriecin
Unfortunately, due to concerns over the stability of fostriecin, its clinical trials were halted in early Phase I.11 However, interest still exists in new synthetic routes to fostriecin and related molecules. It is hoped that these new routes will provide access to analogues with similar biological activities, yet more desirable physical properties. Furthermore, the unique structural features of fostriecin, which includes an unsaturated lactone, the C-8–C-11 triol monophosphate component, and the conjugated Z,Z,E-trienol, make it an interesting target for synthetic organic chemists.
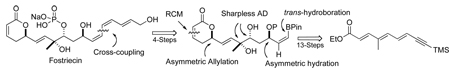
We became interested in the synthesis of fostriecin out of the same desire to develop a new route to the molecule for SAR-type studies, as well as from our ongoing interest in the synthesis of polyol pyranone-containing natural products.12 In particular, we wanted to explore the use of our trienoate asymmetric hydration/oxidation reaction sequence for the C-8,9,11 triol portion of the molecule.13 From a strategic point of view, the pentaene portion of the target molecule made this approach more challenging. Herein we report our successful efforts at regioselectively applying this polyene asymmetric hydration/oxidation reaction sequence in an efficient synthesis of fostriecin. In this regard, our route uniquely uses a late stage trans-hydroboration reaction.14 In addition, it takes advantage of the stereoselective stability of a Z-vinyl boronate intermediate,15,16 which offers a significant alternative for the construction of the Z,Z,E-triene portion of this molecule (vide infra).
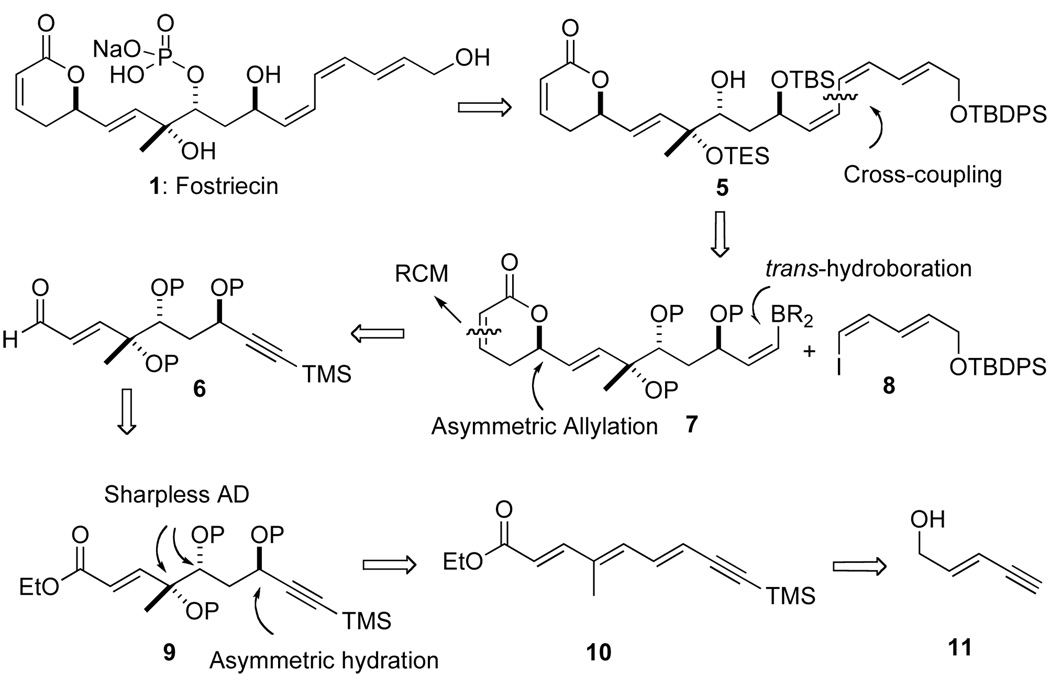
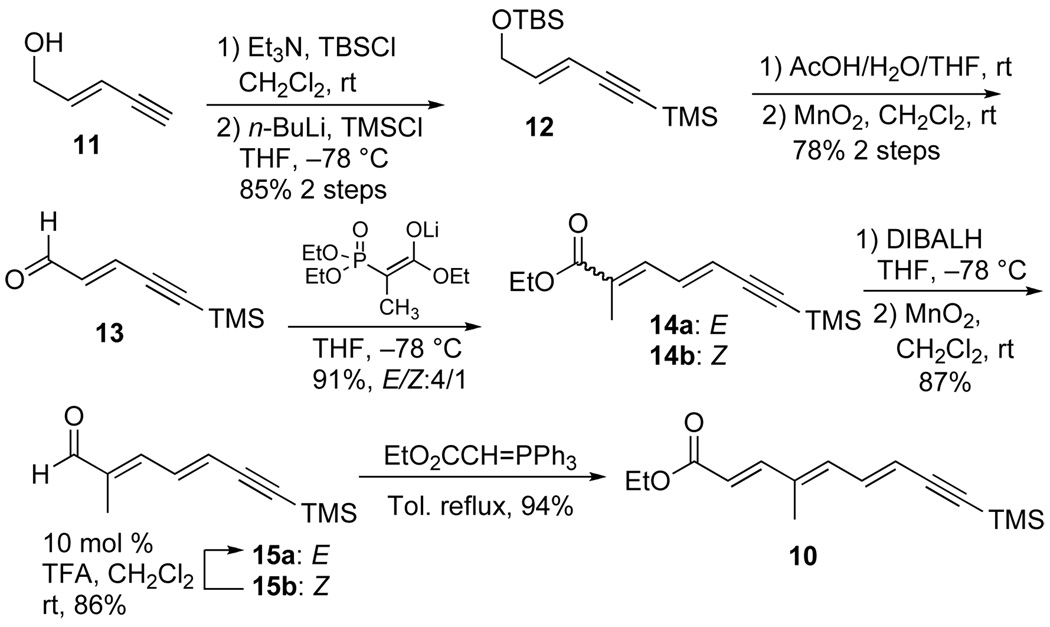
Our synthetic efforts started with an investigation of the chemo- regio-selectivity of the asymmetric oxidation and subsequent reduction of yne-trienoate 10 to install the C-11 propargyl alcohol. While we expected the Sharpless dihydroxylation to occur at the double bond furthest away from the electron-withdrawing group, we have previously found that this reaction can give regioisomers. In practice, our synthesis began with the preparation of the trienoate 10 from commercially available enyne 11 (Scheme 1).
Scheme 1.
Fostriecin (1) retrosynthesis
In order to protect the terminal acetylene, the primary alcohol of 11 was first protected as a silyl ether (TBSCl/Et3N) and then a TMS group was added to the terminal acetylenic position (n-BuLi/trimethylchlorosilane) to give fully protected enynol 12 in 85% yield for two steps (Scheme 2). Selective deprotection of the silyl-ether with aqueous acetic acid in THF afforded an allylic alcohol, which was directly oxidized with MnO2 to provide aldehyde 13 (78%). Horner-Emmons reaction of triethyl-2-phosphonopropionate with aldehyde 13 provided dienynes 14a/b in 91 % yield as a 4:1 mixture of E/Z-isomers. While the E/Z-double bond isomers could be separated by SiO2 chromatography on a preparative scale it was easier to carry the mixture forward. Exposure of a CH2Cl2 solution of the two isomers 14a/b with 2.5 equiv of DIBAL-H at −78 °C provided a mixture of allylic alcohols, which without purification were oxidized with MnO2 to afford aldehydes 15a/b (87% yield/2 steps). At this stage, the undesired double bond isomer 15b was readily converted into the desired isomer 15a by treating the mixture with 10 mol % TFA in CH2Cl2 (86% of > 20:1 ratio). Finally, a stabilized Wittig olefination between aldehyde 15a with EtO2CCH=PPh3 in toluene at reflux gave trienoate 10 in 94% yield (E/Z ratio > 20/1).
Scheme 2.
Synthesis of trieneoate 10
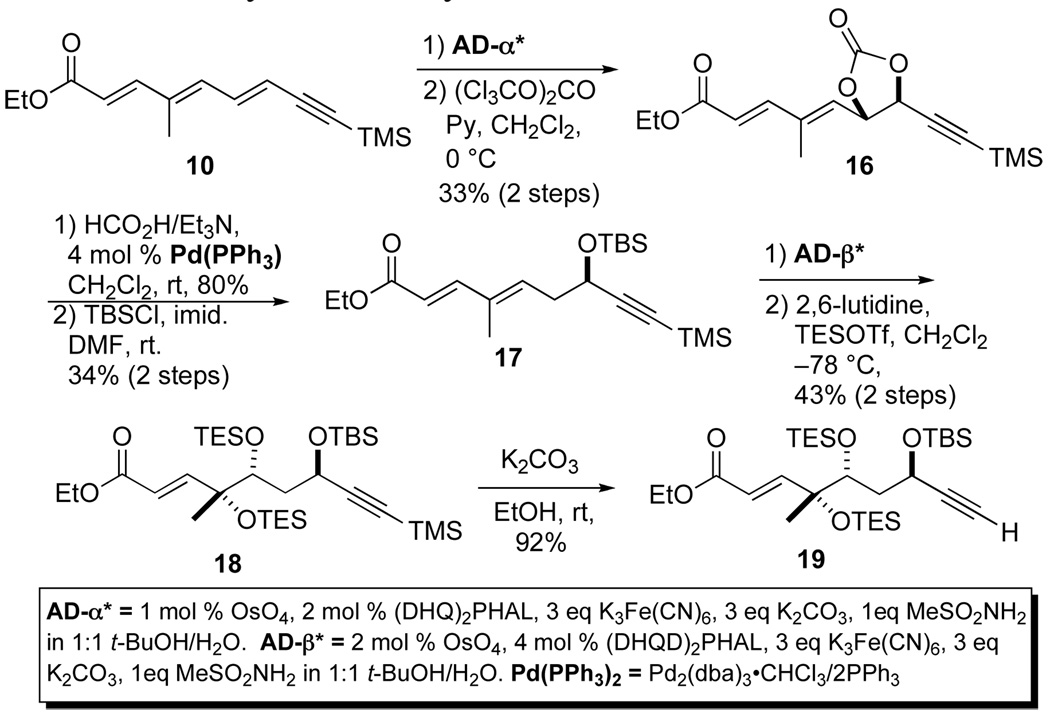
With a practical approach to the trienoate 10 in hand, we turned our attention to the asymmetric hydration/oxidation sequence of 10 to provide a suitably protected triol 19 (Scheme 3).17 This began with an asymmetric dihydroxylation and subsequent palladium catalyzed reduction of the most electron deficient double bond of 10. Exposure of 10 to Sharpless AD-mix-α reagent system regioselectively provided the corresponding diol,18 which was subsequently converted (triphosgene/Py) to a carbonate 16 (80% for 2 steps). Treatment of 16 with 2.0 mol % Pd2(dba)3•CHCl3, 4.0 mol % PPh3 and a mild hydride source (3 equiv, Et3N/HCO2H) provided a propargylic alcohol which was then protected to a silyl ether 17 (64% yield for 2 steps). A second Sharpless dihydroxylation with the AD mix-β reagent system of dienoate 17 cleanly gave a diol product, which was then protected as a bis-triethylsilyl ether 18 (TESOTf/2,6-lutidine) in good overall yield (62% for 2 steps) and diastereomeric and enantiomeric purity (>96% ee and dr). The terminal trimethyl silyl group was then selectively removed (K2CO3 in EtOH) to give 19 (92%).
Scheme 3.
Asymmetric hydration and oxidation of triene 10
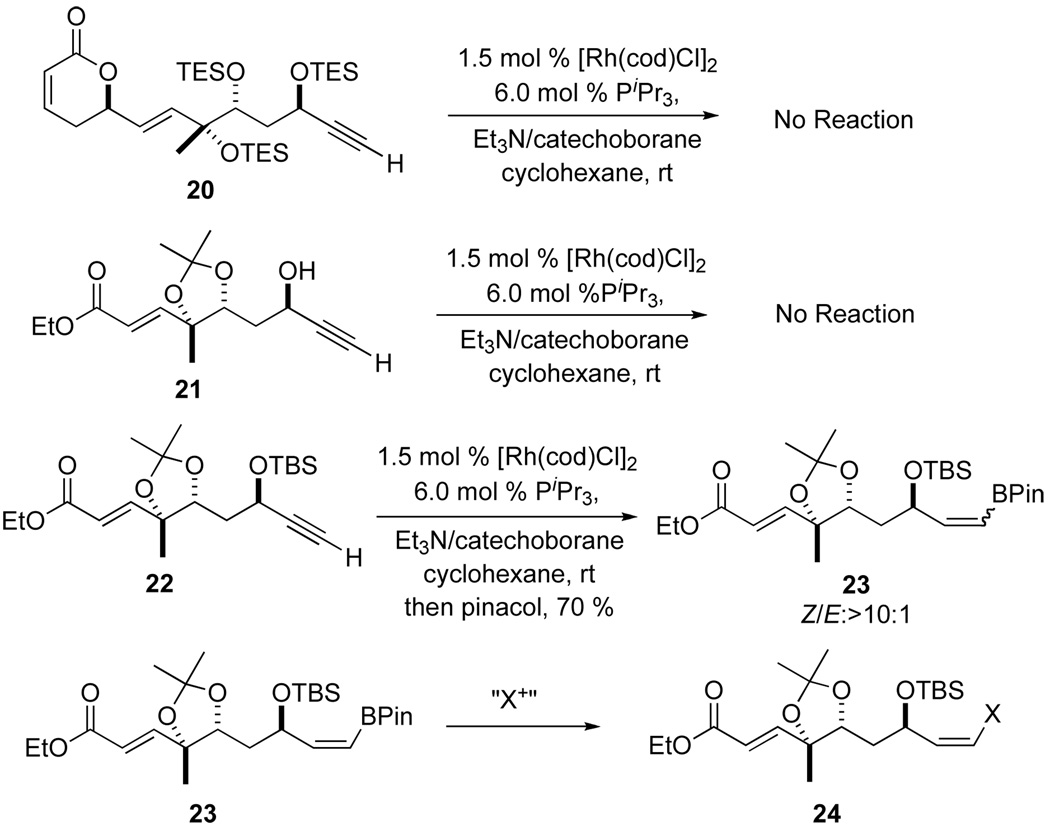
With the desired triol-stereochemistry of fostriecin installed we next turned to the key rhodium catalyzed trans-hydroboration reaction (Scheme 4).14 Because of the perceived instability of the organoborane intermediates, we first investigated the trans-hydroboration of late stage intermediates like 20 with the pyranone ring installed. Unfortunately, when terminal alkyne 20 was exposed to Miyaura’s typical procedure no desired products were detected. A similar lack of reactivity was observed for the propargyl alcohol 21. Success was finally achieved when both the hydroxyl group and pyranone ring were removed. Thus when the TBS-protected propargyl alcohol 22 was exposed to the Miyaura’s conditions (1.5 mol % Rh[(cod)Cl]2, 6 mol % of Pi-Pr3, triethylamine, catechol borane then pinacol)14 the desired trans-hydroboration proceeded smoothly to give Z-vinylborane 23 (78%, >10:1). To our surprise both the vinyl borane 23 was stable to silica gel chromatography and all attempts to stereoselectively convert the Z-vinyl boronate into a vinyl halide like 24 (e.g., NBS and I2).
Scheme 4.
Substrate optimization for the trans-hydroboration
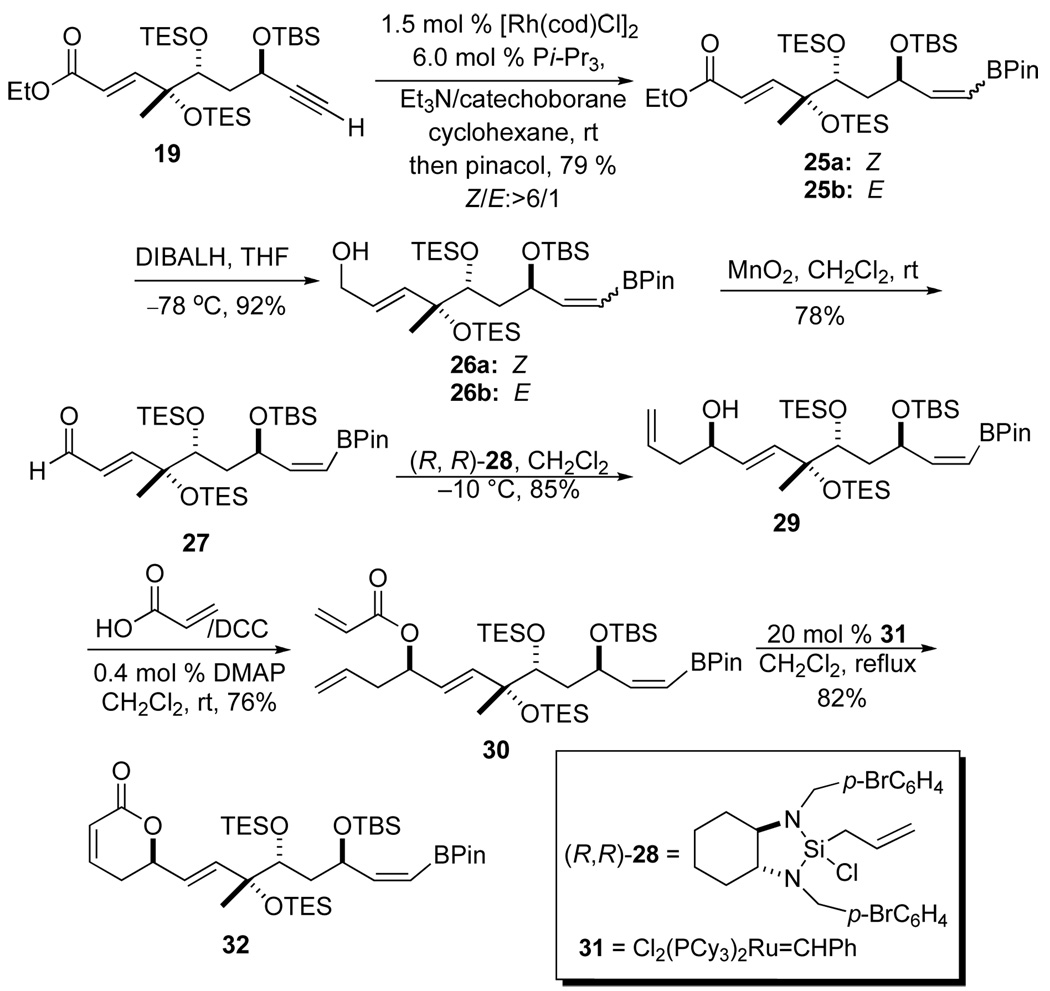
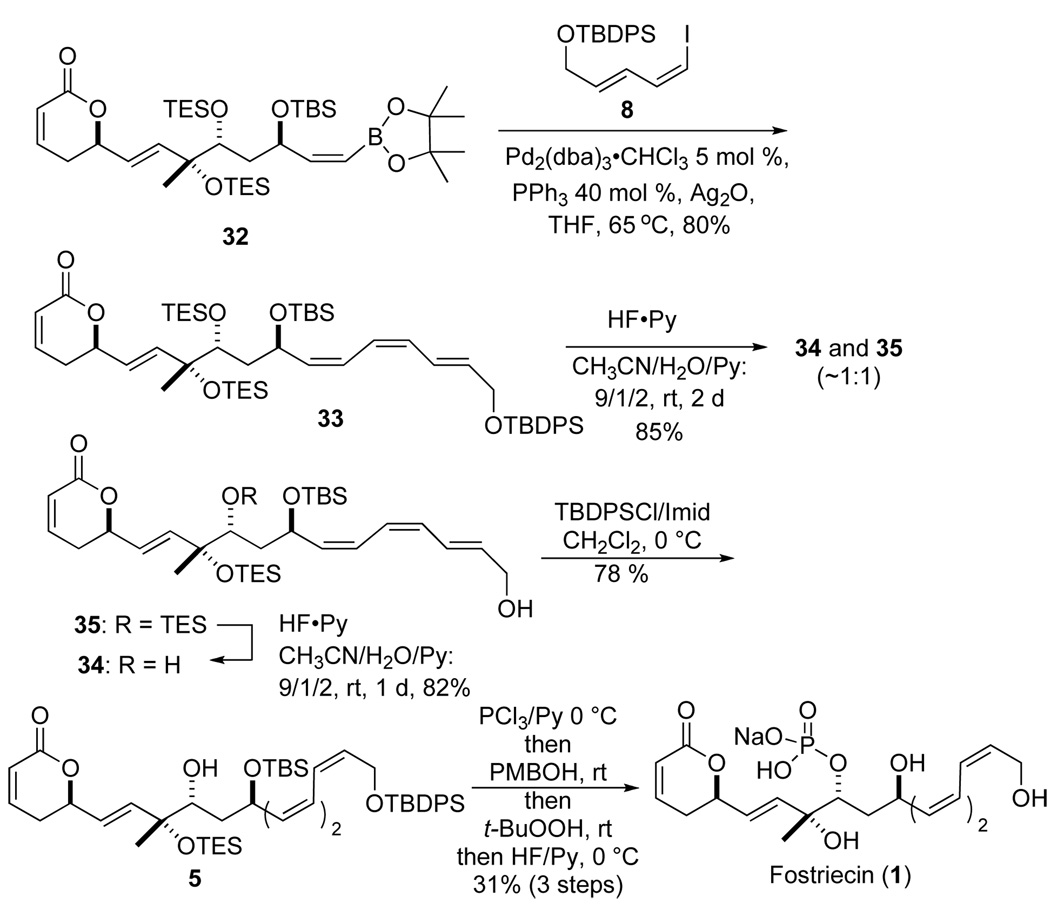
For reasons associated with the end-game protecting group strategy, we had to move forward with the bis-TES ether protected vinyl boranes 25a/b for installation of the pyranone ring and the E,E,Z-triene. When the more suitably protected alkyne 19, with a bis-TES-ether protecting groups, was exposed to the same conditions a slightly less selective trans-hydroboration reaction occurred to give 25a, along with the undesired cis-hydroboration product 25b in a 6:1 ratio of inseparable isomers. Once again vinyl boronate 25a/b was remarkably stable to silica gel chromatography and several subsequent synthetic transformations to install the pyranone ring (Scheme 5). To our delight, the Z-vinyl boronate displayed remarkable stability to the reaction conditions. For instance, when the mixture of esters 25a/b was exposed to excess DIBALH a good yield of allyl alcohols 26a/b were isolated (92% yield, >6/1: Z/E ratio). Moreover, when the mixture of allylic alcohols was oxidized with MnO2 only aldehyde 27 was isolated as a single vinylborane isomer in 78% yield. Diastereoselective allylation of aldehyde 27 was achieved by simple exposure to a solution of the Leighton allylsilane reagent (R,R)-28 (0.2 M in CH2Cl2)19 at −10 °C providing allylic alcohol 29 in 85% yield with near perfect stereocontrol (>99% ee and dr). A DCC-promoted esterification with acrylic acid (3 equiv) and allylic alcohol 29 afforded a tetraene 30 in 76% yield. Exposure of a refluxing CH2Cl2 solution of the tetraene 30 to the Grubbs catalyst 31 (10 mol %) resulted in a clean cyclization to pyranone 32 in 82% yield.
Scheme 5.
Trans-hydroboration and pyranone installation
With both the vinyl boronate 32 and vinyl iodide 8 in hand, we then investigated the Suzuki–Miyaura cross coupling.20 Our initial efforts using a Pd(PPh3)4/Ag2O system led to no reaction. Further investigations led to an optimal condition, which used a 20% Pd/PPh3 system (20% Pd2(dba)3•CHCl3/80%PPh3). These conditions smoothly coupled vinyl boronate 32 and vinyl iodide 8 affording the Z,Z,E-triene 33 in excellent alkene stereoselectivity (>20:1) and 80 % yield.
With all the carbon atoms and stereocenters in place all that was needed to complete the synthesis was the selective deprotection and phosphorylation of the C-9 TES-group followed by global deprotection. Unfortunately, the selective deprotection of silyl ether 33 required a three-step sequence. First exposure of 33 to HF•Py gave products of C-9 TES and C-18 TBDPS deprotection 34 along with mono-deprotected products 35. A subsequent re-exposure of 35 to HF•Py gave improved yields of the bis-deprotected diol 34. Finally, the primary hydroxyl group of 34 was selectively protected with TBDPSCl/imidazole to give silyl ether 5. Product 5 was the intermediate reported by Imanishi and others.6 Using the Imanishi protocol 2 mg of protected dephospho-fostriecin 5 was converted into fostriecin 1 (~ 0.5 mg), which 1HNMR (600 MHz in D2O) and HRMS matched those of the natural material.6a,g,21
In summary, an enantioselective synthesis of fostriecin has been accomplished in 24 steps from commercially available starting material (11). This highly enantio- and diastereocontrolled route illustrates the utility of an iterative Sharpless AD reaction, Noyori asymmetric reduction, asymmetric Leighton allylation, Rh-catalyzed trans-hydroboration and Suzuki–Miyaura cross-coupling reaction sequence. It is also worth noting that these new routes start from achiral sources. Importantly, these key reactions are performed under catalyst control. This strategy should facilitate the syntheses of fostriecin analogues and similar structural natural products. Efforts along these lines will be reported in due course.
Supplementary Material
Scheme 6.
Fostriecin End-Game
Acknowledgment
We are grateful to NIH (GM088839) and NSF (CHE- 0749451) for the support of our research program.
Footnotes
Supporting Information Available: Complete experimental procedures and spectral data for all new compounds can be found in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Tunac JB, Graham BD, Dobson WE. J. Antibiot. 1983;36:1595. doi: 10.7164/antibiotics.36.1595. [DOI] [PubMed] [Google Scholar]; (b) Stampwala SS, Bunge RH, Hurley TR, Wilmer NE, Brankiewicz AJ, Steinman CE, Smitka TA, French JC. J. Antibiot. 1983;36:1601. doi: 10.7164/antibiotics.36.1601. [DOI] [PubMed] [Google Scholar]; (c) Hokanson GC, French JC. J. Org. Chem. 1985;50:462. [Google Scholar]
- 2.(a) Lewy DS, Gauss C-M, Soenen DR, Boger DL. Curr. Med. Chem. 2002;9:2005. doi: 10.2174/0929867023368809. [DOI] [PubMed] [Google Scholar]; (b) De Jong RS, De Vries EGE, Mulder NH. Anti- Cancer Drugs. 1997;8:413. [PubMed] [Google Scholar]
- 3.Reviews: Jackson RC, Fry DW, Boritzki TJ, Roberts BJ, Hook KE, Leopold WR. Adv. Enzyme Regul. 1985;23:193. doi: 10.1016/0065-2571(85)90048-2. Scheithauer W, Hoff DDV, Clark GM, Shillis JL, Elslager EF. Eur. J. Clin. Oncol. 1986;22:921. doi: 10.1016/0277-5379(86)90057-x.
- 4.Reviews: Walsh AH, Cheng A, Honkanen RE. FEBS Lett. 1997;416:230. doi: 10.1016/s0014-5793(97)01210-6. Hastie CJ, Cohen PTW. FEBS Lett. 1998;431:357. doi: 10.1016/s0014-5793(98)00775-3.
- 5.Boger DL, Hikota M, Lewis BM. J. Org. Chem. 1997;62:1748. [Google Scholar]
- 6.(a) Boger DL, Ichikawa S, Zhong W. J. Am. Chem. Soc. 2001;123:4161. doi: 10.1021/ja010195q. [DOI] [PubMed] [Google Scholar]; (b) Chavez DE, Jacobsen EN. Angew. Chem., Int. Ed. 2001;40:3667. doi: 10.1002/1521-3773(20011001)40:19<3667::aid-anie3667>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]; (c) Reddy YK, Falck JR. Org. Lett. 2002;4:969. doi: 10.1021/ol025537r. [DOI] [PubMed] [Google Scholar]; (d) Wang YG, Kobayashi Y. Org. Lett. 2002;4:4615. doi: 10.1021/ol020193q. [DOI] [PubMed] [Google Scholar]; (e) Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T. Chem. Commun. 2002;7:742. doi: 10.1039/b201302a. [DOI] [PubMed] [Google Scholar]; (f) Esumi T, Okamoto N, Hatakeyama S. Chem. Commun. 2002;24:3042. doi: 10.1039/b209742g. [DOI] [PubMed] [Google Scholar]; (g) Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T. J. Am. Chem. Soc. 2003;125:8238. doi: 10.1021/ja030133v. [DOI] [PubMed] [Google Scholar]; (h) Fujii K, Maki K, Kanai M, Shibasaki M. Org. Lett. 2003;5:733. doi: 10.1021/ol027528o. [DOI] [PubMed] [Google Scholar]; (i) Trost BM, Frederiksen MU, Papillon JPN, Harrington PE, Shin S, Shireman BT. J. Am. Chem. Soc. 2005;127:3666. doi: 10.1021/ja042435i. [DOI] [PubMed] [Google Scholar]; (j) Maki K, Motoki R, Fujii K, Kanai M, Kobayashi T, Tamura S, Shibasaki M. J. Am. Chem. Soc. 2005;127:17111. doi: 10.1021/ja0562043. [DOI] [PubMed] [Google Scholar]; (k) Hayashi Y, Yamaguchi H, Toyoshima M, Okado K, Toyo T, Shoji M. Org. Lett. 2008;10:1405. doi: 10.1021/ol800195g. [DOI] [PubMed] [Google Scholar]; (l) Robles O, McDonald F. Org. Lett. 2009;11:5498. doi: 10.1021/ol902365n. [DOI] [PubMed] [Google Scholar]; (m) Shibahara S, Fujino M, Tashiro Y, Okamoto N, Esumi T, Takahashi K, Ishihara J, Hatakeyama S. Synthesis. 2009;17:2953. [Google Scholar]; (n) Sarkar S, Wanzala E, Shibahara S, Takahashi K, Ishihara J, Hatakeyama S. Chem. Commun. 2009;39:5907. doi: 10.1039/b912267b. [DOI] [PubMed] [Google Scholar]
- 7.For the synthesis of related molecules, see: Cossy J, Pradaux F, BouzBouz S. Org. Lett. 2001;3:2233. doi: 10.1021/ol016116x. Kiyotsuka Y, Igarashi J, Kobayashi Y. Tetrahedron Lett. 2002;43:2725. Ramachandran V, Liu H, Reddy V-R, Brown HC. Org. Lett. 2003;5:3755. doi: 10.1021/ol035516c. Shimada K, Kaburagi Y, Fukuyama T. J. Am. Chem. Soc. 2003;125:4048. doi: 10.1021/ja0340679. Bialy L, Waldmann H. Chem.-Eur. J. 2004;10:2759. doi: 10.1002/chem.200305543. Wang Y-G, Takeyama R, Kobayashi Y. Angew. Chem., Int. Ed. 2006;45:3320. doi: 10.1002/anie.200600458. Lawhorn BG, Boga SB, Wolkenberg SE, Colby DA, Gauss C-M, Swingle MR, Amable L, Honkanen RE, Boger DL. J. Am. Chem. Soc. 2006;128:16720. doi: 10.1021/ja066477d. Shibahara S, Fujino M, Tashiro Y, Takahashi K, Ishihara J, Hatakeyama S. Org. Lett. 2008;10:2139. doi: 10.1021/ol8004672. Miyashita K, Tsunemi T, Hosokawa T, Ikejiri M, Imanishi T. J. Org. Chem. 2008;73:5360. doi: 10.1021/jo8005599. Druais V, Hall MJ, Corsi C, Wendeborn SV, Meyer C, Cossy J. Org. Lett. 2009;11:935. doi: 10.1021/ol8029142. Knig CM, Gebhardt B, Schleth C, Dauber M, Koert U. Org. Lett. 2009;11:2728. doi: 10.1021/ol900757k. For a synthesis/structural correction of sultriecin, see: Burke CP, Haq N, Boger DL. J. Am. Chem. Soc. 2010;132:2157. doi: 10.1021/ja9097252.
- 8.(a) Fushimi S, Shimazu A, Seto H. J. Antibiot. 1989;42:1019. doi: 10.7164/antibiotics.42.1019. [DOI] [PubMed] [Google Scholar]; (b) Fushimi S, Furihata K, Seto H. J. Antibiot. 1989;42:1026. doi: 10.7164/antibiotics.42.1026. [DOI] [PubMed] [Google Scholar]; (c) Ozasa T, Suzuki K, Sasamata M, Tanaka K, Kobori M, Kadota S, Nagai K, Saito T, Watanabe S, Iwanami M. J. Antibiot. 1989;42:1331–1338. doi: 10.7164/antibiotics.42.1331. [DOI] [PubMed] [Google Scholar]; (d) Ozasa T, Tanaka K, Sasamata M, Kaniwai H, Shimizu M, Matsumoto H, Iwanami M. J. Antibiot. 1989;42:1339. doi: 10.7164/antibiotics.42.1339. [DOI] [PubMed] [Google Scholar]; (e) Shibata T, Kurihara S, Yoda K, Haruyama H. Tetrahedron. 1995;51:1999. [Google Scholar]
- 9.(a) Amemiya M, Someno R, Sawa R, Naganawa H, Ishizuka M, Takeuchi T. J. Antibiot. 1994;47:541. doi: 10.7164/antibiotics.47.541. [DOI] [PubMed] [Google Scholar]; (b) Amemiya M, Ueno M, Masuda T, Nishida C, Hamada M, Ishizuka M, Takeuchi T. J. Antibiot. 1994;47:536. doi: 10.7164/antibiotics.47.536. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kohama T, Enokita R, Okazaki T, Miyaoka H, Torikata A, Inukai M, Kaneko I, Kagasaki T, Sakaida Y, Satoh A, Shiraishi A. J. Antibiot. 1993;46:1503. doi: 10.7164/antibiotics.46.1503. [DOI] [PubMed] [Google Scholar]; (b) Kohama T, Nakamura T, Kinoshita T, Kaneto I, Shiraishi A. J. Antibiot. 1993;46:1512. doi: 10.7164/antibiotics.46.1512. [DOI] [PubMed] [Google Scholar]
- 11.De Jong RS, Mulder NH, Uges DRA, Sleijfer DT, Hoppener FJP, Groen HJM, Willemse PHB, van der Graaf WT, de Vries EGE. Br. J. Cancer. 1999;79:882. doi: 10.1038/sj.bjc.6690141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Gao D, O'Doherty GA. Org. Lett. 2005;7:1069. doi: 10.1021/ol047322i. [DOI] [PubMed] [Google Scholar]; (b) Gao D, O’Doherty GA. J. Org. Chem. 2005;70:9932. doi: 10.1021/jo051681p. [DOI] [PubMed] [Google Scholar]
- 13.(a) Guo G, Mortensen MS, O’Doherty GA. Org. Lett. 2008;10:3149. doi: 10.1021/ol801055b. [DOI] [PubMed] [Google Scholar]; b) Li M, O’Doherty GA. Org. Lett. 2006;8:6087. doi: 10.1021/ol062595u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li M, O’Doherty GA. Org. Lett. 2006;8:3987. doi: 10.1021/ol061439k. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ahmed MdM, Mortensen MS, O’Doherty G. J. Org. Chem. 2006;71:7741. doi: 10.1021/jo061200h. [DOI] [PMC free article] [PubMed] [Google Scholar]; For other oxidation/reduction asymmetric hydration approaches, see: Gansauer A, Fan C-A, Keller F, Keil J. J. Am. Chem. Soc. 2007;129:3484. doi: 10.1021/ja0686211.
- 14. Ohmura T, Yamamoto Y, Miyaura N. J. Am. Chem. Soc. 2000;122:4990. For its use in synthesis, see: ref 6c.
- 15.For an example of a stable vinyl boronate in natural product synthesis, see: Penner M, Rauniyar V, Kaspar LT, Hall DG. J. Am. Chem. Soc. 2009;131:14216. doi: 10.1021/ja906429c.
- 16.We found these vinyl-boronates rival the vinyl-trifluoroborates in terms of reaction compatibility, see: Molander GA, Cooper DJ. J. Org. Chem. 2008;73:3885. doi: 10.1021/jo800383e.
- 17.Hunter TJ, O’Doherty GA. Org. Lett. 2002;4:4447. doi: 10.1021/ol0269502. [DOI] [PubMed] [Google Scholar]
- 18.(a) Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483. [Google Scholar]; (b) Zhang Y, O'Doherty GA. Tetrahedron. 2005;61:6337. [Google Scholar]
- 19.Kubota K, Leighton J. Angew. Chem., Int. Ed. 2003;42:946. doi: 10.1002/anie.200390252. [DOI] [PubMed] [Google Scholar]
- 20.For a review: Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457.
- 21.The optical rotation data for our synthetic fostriecin did not match that of the natural material. This could be a result of both the different concentration and solvent. However, our optical rotation data for 5 did match that reported by Imanishi, see supporting information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.