Abstract
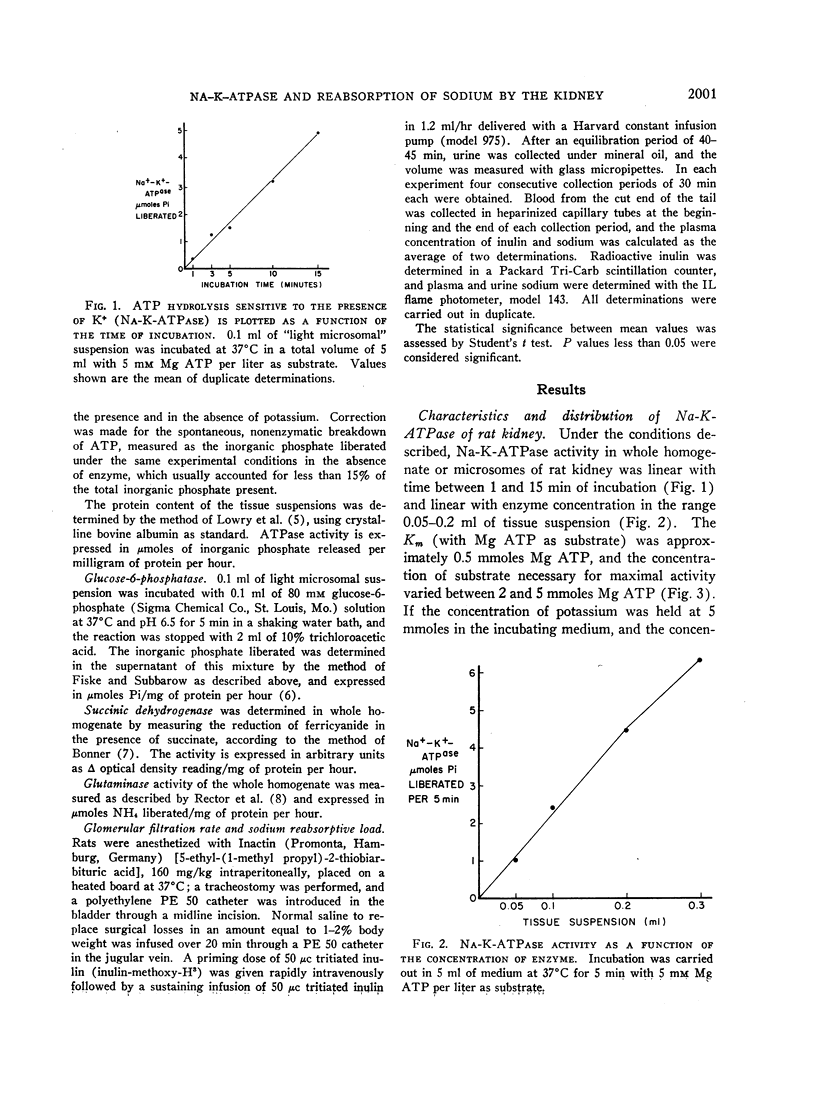
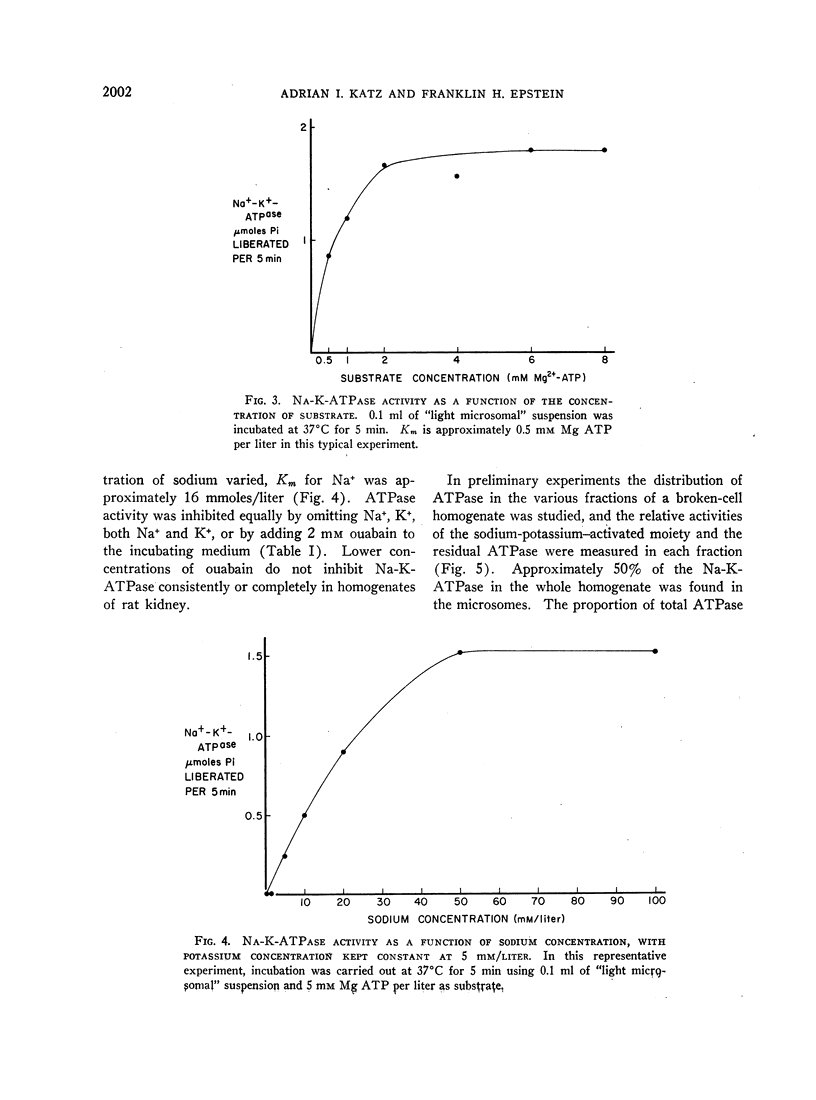
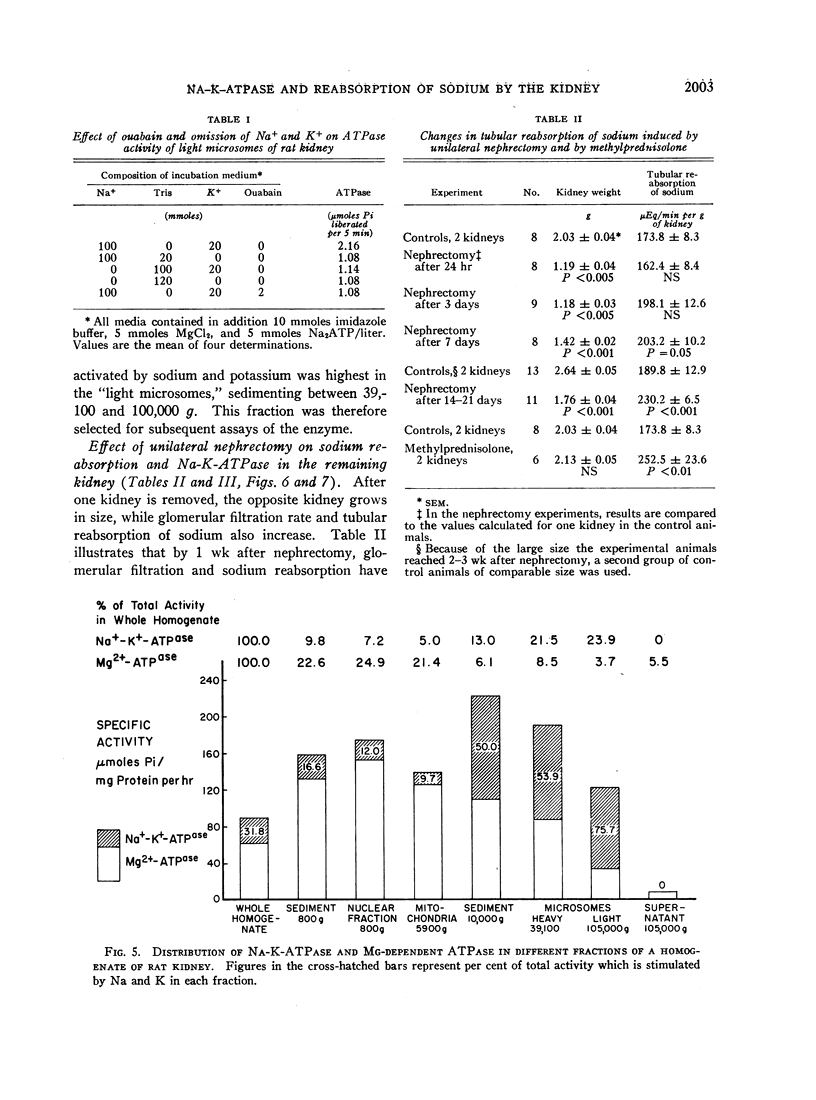
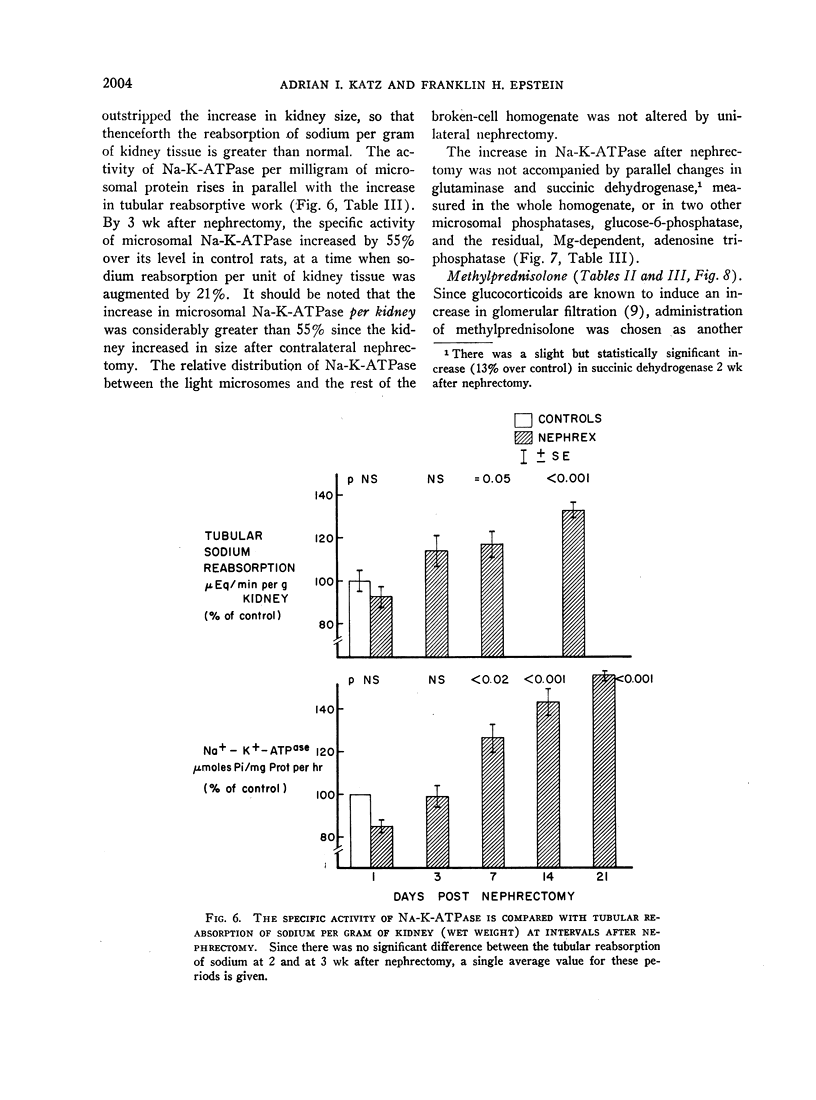
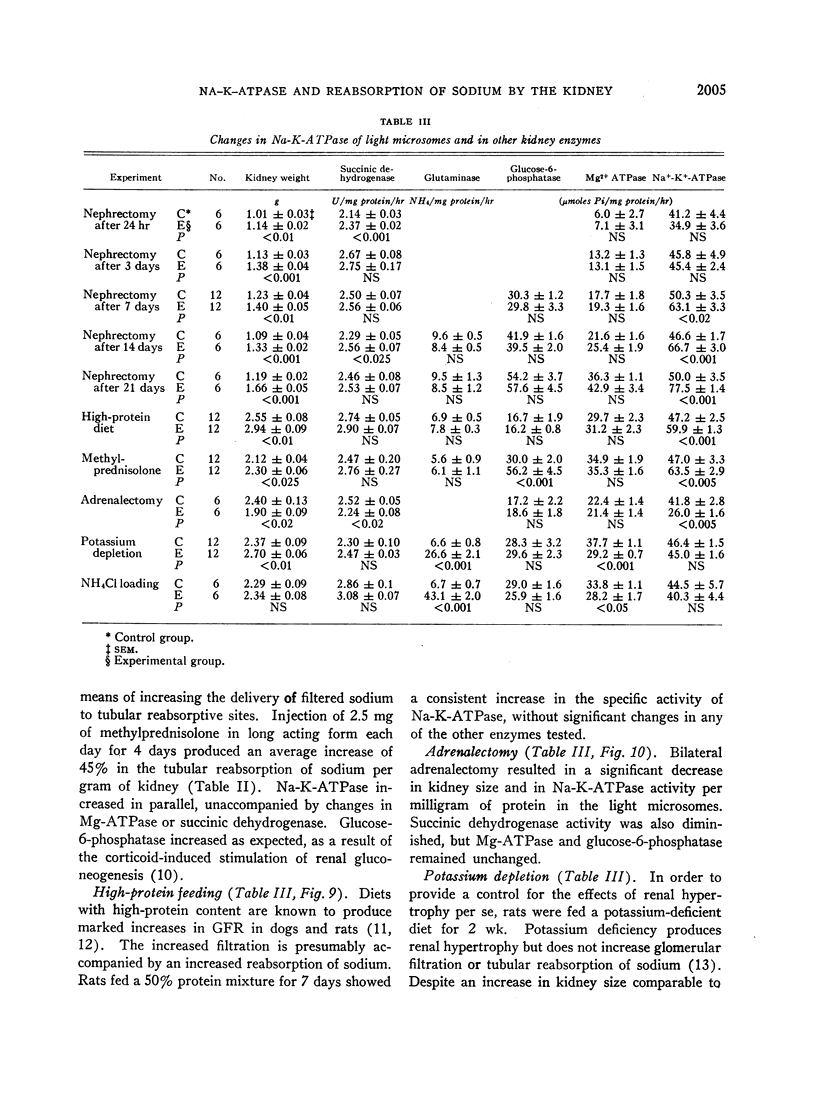
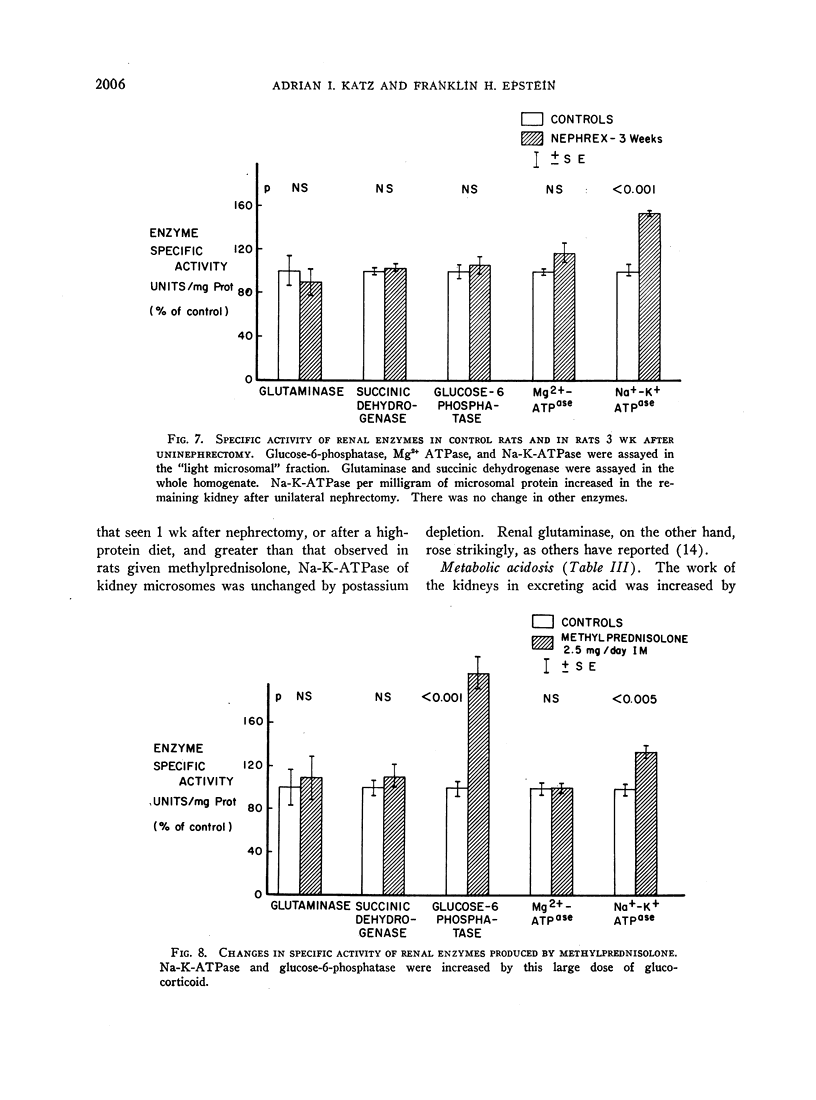
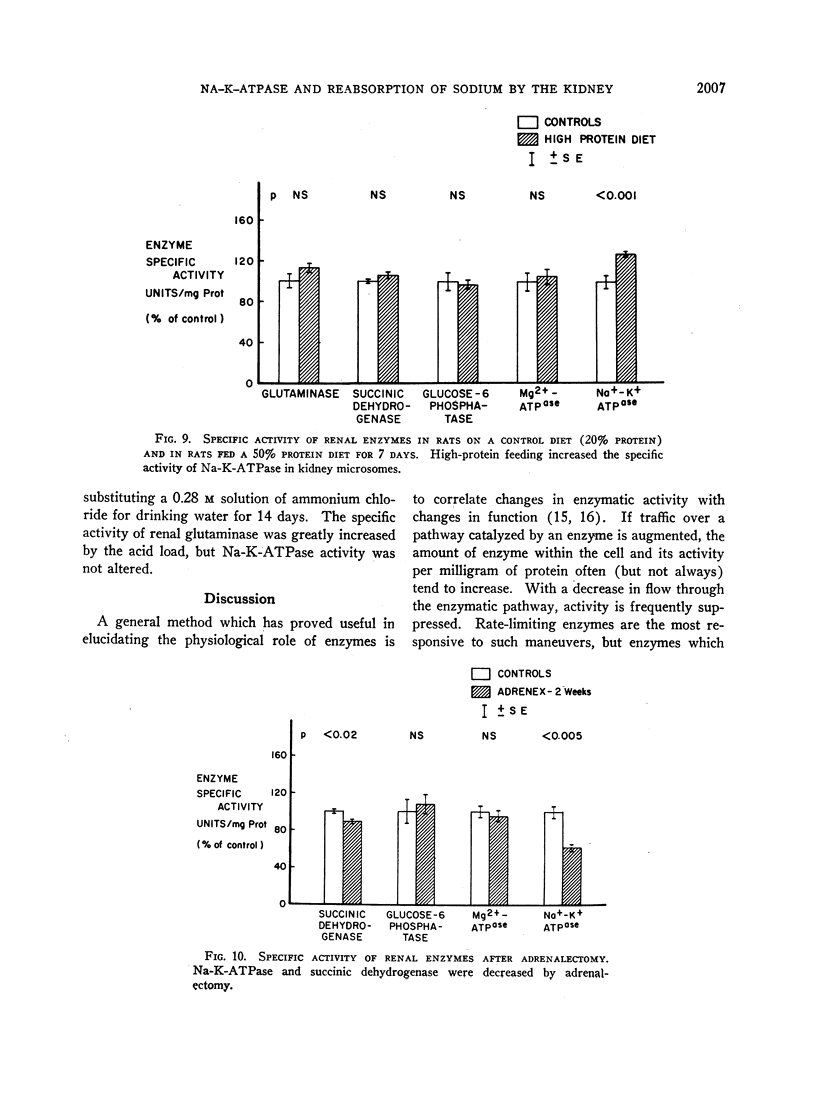
In order to evaluate the possible role of sodium- and potassium-activated adenosine triphosphatase in the active transport of sodium by the renal tubules, we examined the effect of large changes in the tubular reabsorptive load of sodium on the Na-K-ATPase activity of rat kidney homogenates. Glomerular filtration and tubular reabsorption of sodium per gram of kidney tissue increased progressively after contralateral uninephrectomy. This was paralleled by an increase in Na-K-ATPase per milligram of protein in a microsomal fraction of kidney cortex. The importance of this change is underlined by the absence of simultaneous increases in other microsomal enzymes such as glucose-6-phosphatase and Mg++-dependent ATPase, or in succinic dehydrogenase or glutaminase. Similar increases in Na-K-ATPase were observed when the net tubular reabsorption of sodium was increased by feeding the animals a high-protein diet or after injection of methylprednisolone. On the other hand, Na-K-ATPase was lowered when tubular transport of sodium was reduced by bilateral adrenalectomy. The results of these experiments show that renal Na-K-ATPase changes in an adaptive way when renal reabsorption of sodium is chronically increased or diminished and support the hypothesis that this enzyme system is involved in the process by which sodium is actively transported across the renal tubule.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONTING S. L., CANADY M. R. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE AND SODIUM TRANSPORT IN TOAD BLADDER. Am J Physiol. 1964 Nov;207:1005–1009. doi: 10.1152/ajplegacy.1964.207.5.1005. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L., HAWKINS N. M. Studies on sodium-potassium-activated adenosinetriphosphatase. IV. Correlation with cation transport sensitive to cardiac glycosides. Arch Biochem Biophys. 1962 Sep;98:413–419. doi: 10.1016/0003-9861(62)90206-0. [DOI] [PubMed] [Google Scholar]
- BOWER B. F. SITE OF CARDIAC GLYCOSIDE INHIBITION OF CATION TRANSPORT. Nature. 1964 Nov 21;204:786–787. doi: 10.1038/204786a0. [DOI] [PubMed] [Google Scholar]
- CADE J. R., SHALHOUB R. J., CANESSA-FISCHER M., PITTS R. F. Effect of strophanthidin on the renal tubules of dogs. Am J Physiol. 1961 Feb;200:373–379. doi: 10.1152/ajplegacy.1961.200.2.373. [DOI] [PubMed] [Google Scholar]
- CHARNOCK J. S., POST R. L. STUDIES OF THE MECHANISM OF CATION TRANSPORT. I. THE PREPARATION AND PROPERTIES OF A CATION-STIMULATED ADENOSINE-TRIPHOSPHATASE FROM GUINEA PIG KIDNEY CORTEX. Aust J Exp Biol Med Sci. 1963 Oct;41:547–560. [PubMed] [Google Scholar]
- Chignell C. F., Titus E. Effect of adrenal steroids on a Na+- and K+-requiring adenosine triphosphatase from rat kidney. J Biol Chem. 1966 Nov 10;241(21):5083–5089. [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F. H., Katz A. I., Pickford G. E. Sodium- and potassium-activated adenosine triphosphatase of gills: role in adaptation of teleosts to salt water. Science. 1967 Jun 2;156(3779):1245–1247. doi: 10.1126/science.156.3779.1245. [DOI] [PubMed] [Google Scholar]
- Fisher C. J., Stetten M. R. Parallel changes in vivo in microsomal inorganic pyrophosphatase, pyrophosphate-glucose phosphotransferase and glucose 6-phosphatase activities. Biochim Biophys Acta. 1966 May 26;121(1):102–109. doi: 10.1016/0304-4165(66)90352-7. [DOI] [PubMed] [Google Scholar]
- GOLDSMITH C., RECTOR F. C., Jr, SELDIN D. W. Evidence for a direct effect of serum sodium concentration on sodium reabsorption. J Clin Invest. 1962 Apr;41:850–859. doi: 10.1172/JCI104542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., Klose R. M., Windhager E. E. Effect of ionic substitutions on distal potential differences in rat kidney. Am J Physiol. 1966 Sep;211(3):560–568. doi: 10.1152/ajplegacy.1966.211.3.560. [DOI] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. The problem of clinical vasopressin resistance: in vitro studies. Ann Intern Med. 1961 Apr;54:700–709. doi: 10.7326/0003-4819-54-4-700. [DOI] [PubMed] [Google Scholar]
- HOLLIDAY M. A., EGAN T. J. Changes in GFR and C-H2O before and after repair of K deficiency in rats. Am J Physiol. 1962 Apr;202:773–776. doi: 10.1152/ajplegacy.1962.202.4.773. [DOI] [PubMed] [Google Scholar]
- Hierholzer K., Wiederholt M., Stolte H. Hemmung der Natriumresorption im proximalen und distalen Konvolut adrenalektomierter Ratten. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;291(1):43–62. [PubMed] [Google Scholar]
- Hook J. B., Williamson H. E. Lack of correlation between natriuretic activity and inhibition of renal Nak-activated ATPase. Proc Soc Exp Biol Med. 1965 Nov;120(2):358–360. doi: 10.3181/00379727-120-30536. [DOI] [PubMed] [Google Scholar]
- IACOBELLIS M., MUNTWYLER E., GRIFFIN G. E. Enzyme concentration changes in the kidneys of protein- and/or potassium-deficient rats. Am J Physiol. 1954 Sep;178(3):477–482. doi: 10.1152/ajplegacy.1954.178.3.477. [DOI] [PubMed] [Google Scholar]
- JONES V. D., LOCKETT G., LANDON E. J. A CELLULAR ACTION OF MERCURIAL DIURETICS. J Pharmacol Exp Ther. 1965 Jan;147:23–31. [PubMed] [Google Scholar]
- KAMAT V. B., WALLACH D. F. SEPARATION AND PARTIAL PURIFICATION OF PLASMA-MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA MICROSOMES. Science. 1965 Jun 4;148(3675):1343–1345. doi: 10.1126/science.148.3675.1343. [DOI] [PubMed] [Google Scholar]
- KLEINMAN L. I., RADFORD E. P., Jr, TORELLI G. UREA AND INULIN CLEARANCES IN UNDISTURBED, UNANESTHETIZED RATS. Am J Physiol. 1965 Mar;208:578–584. doi: 10.1152/ajplegacy.1965.208.3.578. [DOI] [PubMed] [Google Scholar]
- LANDON E. J., NORRIS J. L. Sodium- and potassium-dependent adenosine triphosphatase activity in a rat-kidney endoplasmic reticulum fraction. Biochim Biophys Acta. 1963 May 14;71:266–276. doi: 10.1016/0006-3002(63)91081-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landon E. J., Jazab N., Forte L. Aldosterone and sodium-potassium-dependent ATPase activity of rat kidney membranes. Am J Physiol. 1966 Oct;211(4):1050–1056. doi: 10.1152/ajplegacy.1966.211.4.1050. [DOI] [PubMed] [Google Scholar]
- MALNIC G., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF RENAL POTASSIUM EXCRETION IN THE RAT. Am J Physiol. 1964 Apr;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., BURG M. Effect of strophanthidin on electrolyte excretion in the chicken. Am J Physiol. 1960 Jul;199:49–54. doi: 10.1152/ajplegacy.1960.199.1.49. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZMANN H. J., WINDHAGER E. E., SOLOMON A. K. Single proximal tubules of the Necturus kidney. II. Effect of 2, 4-dinitro-phenol and ouabain on water reabsorption. Am J Physiol. 1958 Dec;195(3):570–574. doi: 10.1152/ajplegacy.1958.195.3.570. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- TAYLOR C. B. The effect of mercurial diuretics on adenosinetriphosphatase of rabbit kidney in vitro. Biochem Pharmacol. 1963 Jun;12:539–550. doi: 10.1016/0006-2952(63)90129-1. [DOI] [PubMed] [Google Scholar]
- VOGEL G., TERVOOREN U. DIE BEDEUTUNG VON KALIUM FUER DIE RENAL TUBULAEREN TRANSPORTE VON NATRIUM UND CALCIUM UND FUER DIE WIRKUNG KARDIOTONER STEROIDE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Jun 2;284:103–107. [PubMed] [Google Scholar]
- WALLACH D. F., KAMAT V. B. PLASMA AND CYTOPLASMIC MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA. Proc Natl Acad Sci U S A. 1964 Sep;52:721–728. doi: 10.1073/pnas.52.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Singhal R. L., Stamm N. B., Fisher E. A., Mentendiek M. A. Regulation of enzymes involved in gluconeogenesis. Adv Enzyme Regul. 1964;2:1–38. doi: 10.1016/s0065-2571(64)80003-0. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]


