Abstract
Bacterial nitric oxide synthases (bNOS) are present in many Gram-positive species and have been demonstrated to synthesize NO from arginine in vitro and in vivo. However, the physiological role of bNOS remains largely unknown. We show that NO generated by bNOS increases the resistance of bacteria to a broad spectrum of antibiotics, enabling the bacteria to survive and share habitats with antibiotic-producing microorganisms. NO-mediated resistance is achieved through both the chemical modification of toxic compounds and the alleviation of the oxidative stress imposed by many antibiotics. Our results suggest that the inhibition of NOS activity may increase the effectiveness of antimicrobial therapy.
Bacterial and eukaryotic nitric oxide synthases (NOS), which produce NO by catalyzing the oxidation of l-arginine to l-citrulline, are structurally and mechanistically related (1, 2). Although bacterial NOS (bNOS) lacks the essential reductase domain, it uses available cellular reductases to generate NO in vivo (3). Previously, we demonstrated that bNOS protects bacteria against oxidative stress (4, 5). This function of bNOS is essential for the virulence of Bacillus anthracis (5), and bNOS also helps Streptomyces turgidiscabies to infect plants (6). However, genes that encode bNOS are also present in the genomes of numerous nonpathogenic soil bacteria (table S1) (3).
To determine the function of bNOS, we used Phenotype MicroArray (fig. S1 and table S2). Wild-type (wt) and nos mutant strains of B. subtilis showed no growth differences in various media and nutrient supplements, but many bactericidal chemicals preferentially suppressed growth of the nos mutant (Table 1 and fig. S1). Although they are very diverse, these chemicals have to manifest their antibacterial activity via a common mechanism that is targeted by endogenous NO. Bactericidal antibiotics—such as lactams, aminoglycosides, and quinolones—exert their toxicity, at least in part, by promoting the formation of reactive oxygen species (ROS) (7). NO protects Gram-positive bacteria against oxidative stress by a mechanism that, in principle, could also explain NO-mediated cell resistance to antibiotics (4, 5) (fig. S2). To test this hypothesis and to determine the mechanisms of NO-mediated antibiotic resistance, we investigated the effects of NO on bacterial killing by three different antimicrobials: acriflavine, pyocyanin, and cefuroxime.
Table 1.
bNOS protection against a wide spectrum of antimicrobials. A representative list of chemicals from the Phenotype MicroArray screen that preferentially inhibit the growth of nos-deficient B. subtilis. Negative numbers indicate the relative growth inhibition (as provided by Biolog, fig. S1) of the Δnos strain compared to that of the wt strain.
| Δnos inhibition | Chemical | Proposed biological effect/chemical type |
|---|---|---|
| −76 | 5-Chloro-7-iodo-8-hydroxyquinoline | DNA intercalator/quinolone |
| −62 | 8-Hydroxyquinoline | DNA intercalator/quinolone |
| −67 | 8-Hydroxy-5-nitroquinoline | DNA intercalator/quinolone |
| −80 | Novobiocin | DNA intercalator/quinolone |
| −209 | Acriflavine | DNA intercalator/acridine |
| −44 | 9-Aminoacridine | DNA intercalator/acridine |
| −109 | Prochlorperazine | DNA intercalator/phenothiazine |
| −77 | Chlorpromazine | DNA intercalator/phenothiazine |
| −45 | Prochlorperazine | DNA intercalator/phenothiazine |
| −68 | Penimepicycline | Protein synthesis/tetracycline |
| −64 | Sisomicin | Protein synthesis/aminoglycoside |
| −40 | Gentamicin | Protein synthesis/aminoglycoside |
| −252 | Cephaloridine | Cell wall metabolism/cephalosporin |
| −124 | 7-Aminocephalosporanic acid | Cell wall metabolism/cephalosporin |
| −110 | Cefotaxime | Cell wall metabolism/cephalosporin |
| −173 | Cefuroxime | Cell wall metabolism/cephalosporin |
| −114 | Ampicillin | Cell wall metabolism/lactam |
| −79 | Moxalactam | Cell wall metabolism/lactam |
| −78 | 6-Aminopenicillanic acid | Cell wall metabolism/lactam |
| −73 | Amoxicillin | Cell wall metabolism/lactam |
| −39 | Azlocillin | Cell wall metabolism/lactam |
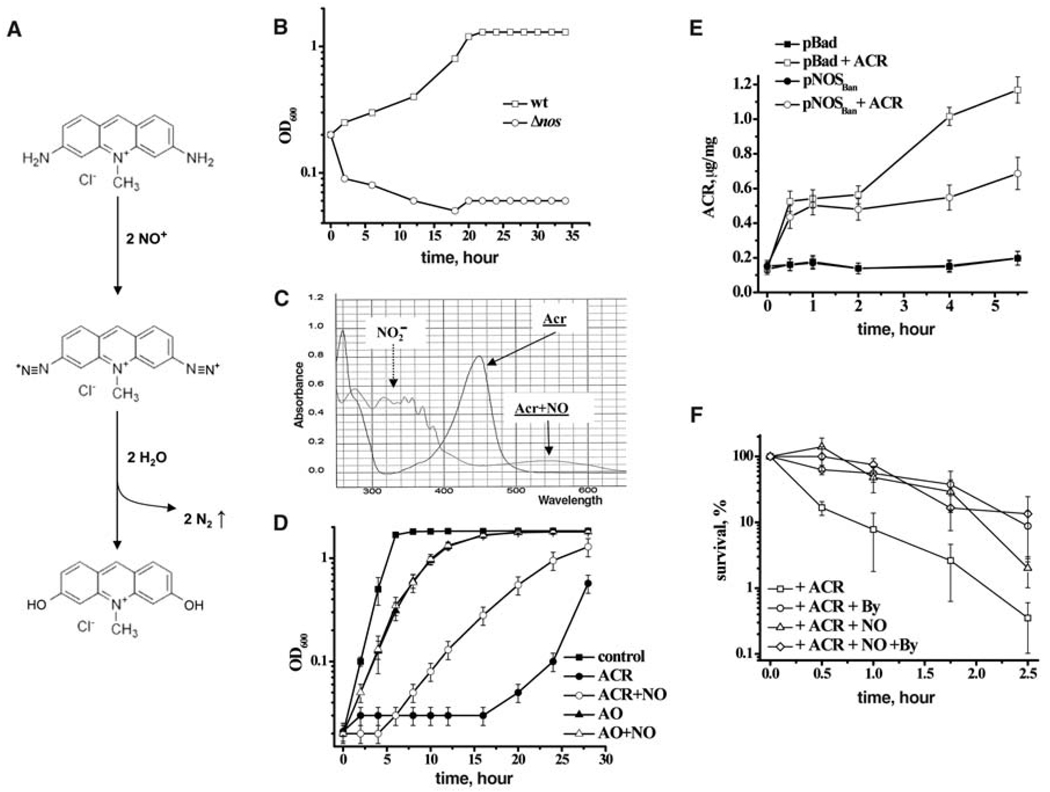
Acriflavine (ACR; Fig. 1A) was the most potent inhibitor of the Δnos strain among the DNA intercalators group of antibiotics (Table 1, Fig. 1B, and fig. S3). ACR carries two aromatic amino groups that are essential for its toxicity (8). Products of NO oxidation (NO+) readily nitrosate arylamino moieties (Fig. 1A) (9, 10). The resulting aryldiazonium cations are quickly hydrolyzed with the release of N2 gas and the formation of less-toxic dihydroxyacridine derivatives (Fig. 1A). Indeed, mixing ACR with NO resulted in gas formation and a color change from orange to faint blue. Spectral data support the proposed reaction (Fig. 1C): The 450-nm peak of ACR is converted into a wider ~550-nm peak resulting from the by-products of intermolecular diazonium crosslinks (10). We premixed ACR with NO in growth media before inoculating it with bacteria. This resulted in reduced killing of either B. subtilis or Staphylococcus aureus by the ACR (Fig. 1D and fig. S4). NO by itself did not affect the growth of bacteria at this concentration. Moreover, acridine orange (AO), in which the arylamino groups are methylated and unable to interact with NO+, was unaffected by NO treatment (Fig. 1D). The antibacterial effect of AO was significantly less than that of ACR, apparently because of the methylated NH2 groups. Thus, bNOS-generated NO modifies ACR directly, thereby decreasing its potency in vivo.
Fig. 1.
Mechanisms of bNOS protection against acriflavine. (A) Proposed chemistry of NO-mediated detoxification of acriflavine (ACR). (B) Optical density (OD) growth curves of B. anthracis wt (Sterne) and Δnos in the presence of ACR (8 µg/ml). (C) Changes in absorbance spectra of ACR upon interaction with NO. (D) OD growth curves of B. subtilis in the presence of MAHMA (NO) and ACR, or acridine orange (AO). Data are shown as the mean ± SE from three experiments. (E) NO-dependent degradation of ACR in vivo. The plot shows intracellular ACR concentration normalized per milligram of total protein of E. coli harboring either empty vector (pBad) or pNOSBan grown in the presence or absence of ACR. Data are shown as the mean ± SE from three experiments. (F) NO protects against ACR-induced ROSs. B. subtilis were pretreated with 0.5 mM bipyridyl (By) or 100 µM NO donor (NO) followed by ACR. The percentage of surviving cells was determined by counting colony-forming units (CFU), and is shown as the mean ± SD from three experiments.
To demonstrate that endogenous NO caused ACR modification, we used a strain of Escherichia coli that expressed B. anthracis NOS. This strain produces NO upon induction with arabinose (3). ACR accumulation can be monitored directly in vivo by detecting changes in its characteristic yellow color. The rate of intracellular ACR accumulation and its overall concentration diminished greatly in NO-producing cells compared with the empty vector control (Fig. 1E). Thus, endogenous NO must be responsible for ACR nitrosation, which would be catalyzed in vivo by transition metals (11, 12) and micellar chemistry (10).
The direct reaction of ACR with NO+ reduced its toxicity only partially (Fig. 1D), suggesting that the efficient protection against ACR by endogenous NO involves additional mechanism(s). Because quinolones kill E. coli by promoting oxidative stress (7), and because NO protects cells against ROS (3–5), we examined whether ACR also kills bacteria via ROS formation. Pretreatment of cells with bipyridyl, an iron chelator that efficiently suppresses the damaging Fenton reaction (13), substantially decreased the toxicity of ACR (Fig. 1F). Furthermore, NO pretreatment (3 min before antibiotic addition) was as effective as bipyridyl in protecting against ACR (Fig. 1F) but failed to further protect cells that were pretreated with bipyridyl (Fig. 1F), indicating that both chemicals acted through the same pathway, i.e., by suppressing the Fenton reaction. A direct interaction between NO and antibiotics was excluded, because NO has a very short life in biological solutions and must have been eliminated as nitrite within the first seconds of pretreatment (14, 15). Together, the ACR results lead to two conclusions: (i) ACR kills bacteria, at least in part, by a ROS-dependent mechanism; (ii) the mechanism of NO-mediated protection against ACR is twofold: NO directly modifies ACR, making it less toxic, and, at the same time, it also protects against ACR-induced oxidative stress.
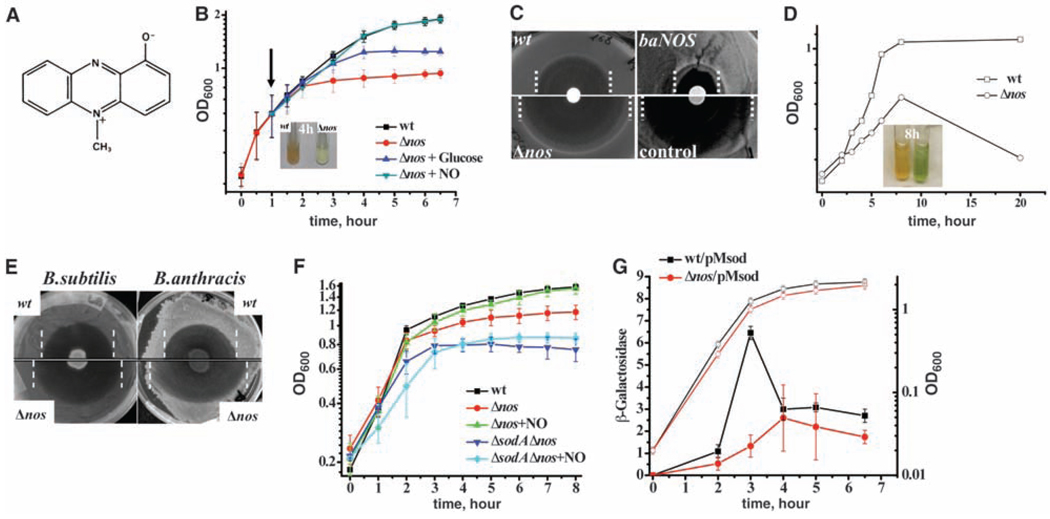
Pyocyanin (1-hydroxy-5-methyl-phenazine, PYO) is one of many antimicrobials that resemble ACR structurally (Fig. 2A). It is a natural toxin synthesized by Pseudomonas aeruginosa and has broad clinical and antibiotic effects (16, 17). Because both P. aeruginosa and B. subtilis inhabit the same soil niche, we hypothesized that endogenous NO could defend B. subtilis against PYO. Indeed, PYO inhibited the growth of the nos deletion strain to a much greater extent than it did the growth of wt B. subtilis (Fig. 2B). In contrast to the Δnos cells, which ceased growth in liquid culture within 3 hours of incubation with PYO, the wt cells continued to grow and metabolized all the PYO, as shown by the disappearance of its characteristic blue color (Fig. 2B). Consistently, the PYO killing zone was larger for Δnos cells on agar plates (Fig. 2C). The addition of exogenous NO completely restored the growth of the Δnos cells in the presence of PYO (Fig. 2B). Such NO-mediated growth recovery occurs even though NO was added hours before the onset of growth inhibition by PYO (Fig. 2B), providing evidence that NO signaling initiates a mechanism of persistent defense against PYO. Finally, to demonstrate the protective effect of endogenous NO against PYO, we integrated the B. anthracis nos gene into the chromosome of B. subtilis Δnos cells under control of the arabinose-inducible promoter (3). The expression of B. anthracis NOS (baNOS) increased resistance to PYO both in liquid culture and on agar plates (Fig. 2C and fig. S5). The deletion of the nos gene in B. anthracis greatly increased its sensitivity to PYO (Fig. 2D), which correlates with the greater intrinsic activity of B. anthracis NOS (3, 5). Because deletion of bNOS in S. aureus also sensitized this pathogen to PYO (fig. S6), these results show that NOS-mediated protection against PYO is a general phenomenon of all NOS-containing bacteria.
Fig. 2.
NO-mediated defense against P. aeruginosa and its mechanism. (A) Chemical structure of the PYO toxin. (B) B. subtilis-generated NO allows growth in the presence of PYO. Growth curves of B. subtilis 6051 (wt) and Δnos strains after the addition of 25 µM PYO (time 0) are shown. After 1 hour, an NO donor (green triangles) or glucose (blue triangles) was added to Δnos cells. Data are shown as the mean ± SE from three experiments. The inset shows tubes with wt (left) and Δnos (right) cultures after a 4-hour incubation with PYO. (C) bNOS expression renders cells resistant to PYO. A paper disk saturated with 10 mM PYO was placed on the B. subtilis bacterial lawn. baNOS indicates B. subtilis expressing nos from B. anthracis. Zone borders are marked with dashed lines. (D) Deletion of the nos gene sensitizes B. anthracis to PYO. Growth curves of B. anthracis Sterne (squares) and Δnos (circles) strains grown in 100 µM PYO are shown. The inset shows tubes with the Sterne (left) and Δnos (right) strains after an 8-hour incubation with PYO. (E) Endogenous NO protects B. subtilis and B. anthracis from P. aeruginosa. Five microliters of a P. aeruginosa PA14 overnight culture was placed on the Bacilli lawns on P agar plates. Lysis zone borders are marked with dashed lines. (F) SodA is critical for bacterial defense against PYO. Experimental conditions were as in (B), except that wt B. subtilis 168 was used as a background strain for all the mutants. Values are the means ± SD from three independent experiments. (G) bNOS controls SodA expression. The lacZ reporter was placed under a chromosomal copy of the sodA promoter (pMsod) in B. subtilis 168 (wt) and Δnos strains. Cultures were sampled to measure the growth [optical density at 600 nm (OD600), open symbols] and β-galactosidase activity (solid symbols). Means ± SD from three experiments are given.
To recapitulate soil conditions, we co-cultured P. aeruginosa with B. subtilis and B. anthracis on P agar, which stimulates PYO production. A drop of P. aeruginosa PA14 was placed on a bacilli lawn for overnight incubation (Fig. 2E). PA14 is a clinical isolate that produces a large quantity of PYO (18). PA14 kills both B. subtilis and B. anthracis; however, the lysis zones were substantially larger for the nos mutant cells than for the wt cells of both species (Fig. 2E). To verify that NOS-dependent cell viability was indeed caused by PYO detoxification by NO in vivo, we tested P. aeruginosa that was deficient in PYO production, PYO(–), and found that it made smaller lysis zones of equal size for both Δnos and wt B. subtilis (fig. S7). Thus, PYO is one of the key factors that P. aeruginosa uses to combat bacilli. Bacilli use endogenous NO to reduce the oxidative stress associated with PYO toxicity (see below), thereby defending themselves against killing by P. aeruginosa.
In contrast to ACR, PYO does not have arylamino groups to react with NO+. Consistently, premixing NO with PYO did not result in a color change or attenuation of PYO toxicity (fig. S8) (19), indicating that NO-mediated protection was not due to direct chemical interaction between NO+ and PYO, but rather required a cellular response mechanism. We noticed that PYO did not significantly affect the exponential phase of growth of either the wt or the Δnos cells, but PYO did arrest the growth of the Δnos cells at stationary phase (Fig. 2B). During the stationary phase, a redox cycling agent, such as PYO, promotes superoxide anion formation. Indeed, PYO toxicity has been associated with ROSs (17, 20). Consistently, the presence of glucose, which prolonged fermentative growth, delayed the onset of PYO growth inhibition (Fig. 2B) (21).
B. subtilis carries only one superoxide dismutase (SOD), SodA, which confers resistance to endogenous superoxide and superoxide-generating agents (22, 23). The deletion of sodA rendered B. subtilis more sensitive to PYO (Fig. 2F and fig. S9), even in the presence of exogenous NO (Fig. 2F). We found that sodA expression increased sharply in wt cells during the late exponential phase of growth (22) but was abolished in Δnos cells (Fig. 2G), indicating that bNOS is required for SodA activation, which, in turn, provides resistance to PYO.
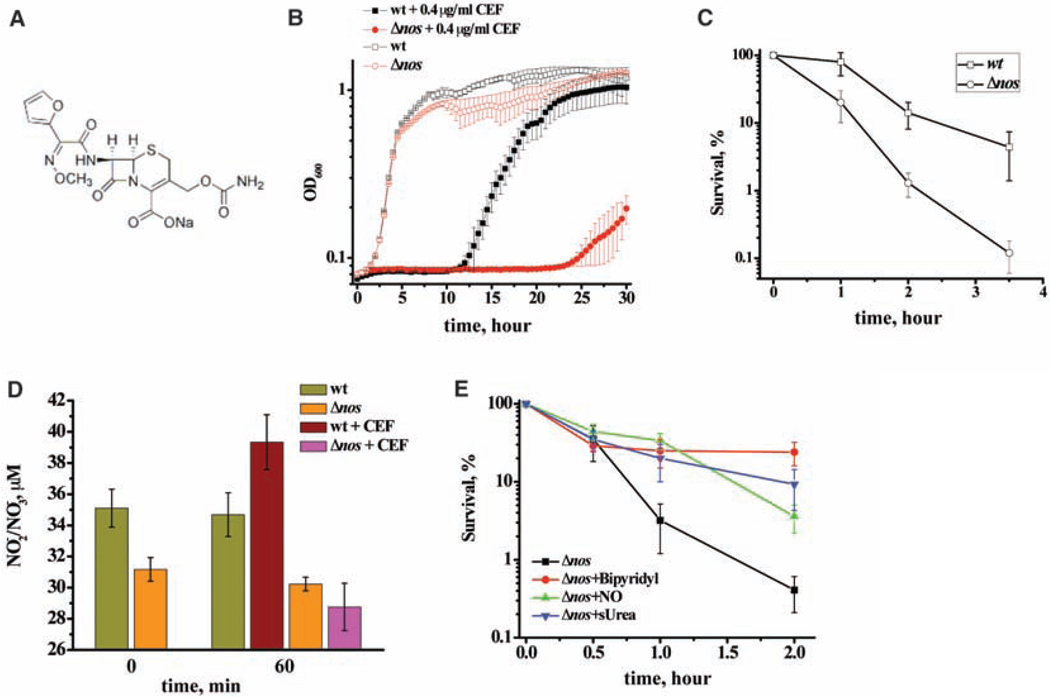
Endogenous NO effectively diminishes lactam toxicity against B. subtilis (Table 1). Although a major target for lactams is cell wall biosynthesis, other toxicity mechanisms are involved; for example, ampicillin kills E. coli by inducing ROS. This bactericidal effect is abolished by the addition of the iron chelator bipyridyl or the ROS scavenger thiourea (7). Because NO/NO+ protect Bacilli against oxidative stress (fig. S2) (4, 5), and they do not react with lactams directly (fig. S10), we propose that NOS activity renders bacteria resistant to lactams by suppressing oxidative stress in a similar, general manner, as demonstrated for ACR and PYO (fig. S2). Indeed, cefuroxime (CEF, Fig. 3A) kills nos-deficient S. aureus and B. subtilis much more efficiently than it kills wt cells (Fig. 3B and 3C). Moreover, pretreatment with exogenous NO temporarily protects cells against CEF toxicity (Fig. 3E). Similar protection can be achieved by addition of the iron chelator bipyridyl or the radical scavenger thiourea (Fig. 3E).
Fig. 3.
The mechanism of bNOS protection against cefuroxime. (A) Chemical structure of cefuroxime (CEF). (B) bNOS-dependent growth of S. aureus in the presence of CEF. Overnight cultures of S. aureus 4220 and its Δnos derivative were diluted into fresh LB media containing 0.4 µg/ml CEF. Cells were grown in triplicate at 37°C with aeration using a Bioscreen C automated growth analysis system. (C) nos deletion renders B. subtilis more sensitive to cefuroxime. B. subtilis 6051 and Δnos strains were grown to OD600 ~1.0, followed by the addition of cefuroxime (25 µg/ml) (time 0). The survival was determined by counting CFU and is shown as the mean ± SD from three independent experiments. (D) Stimulation of bNOS activity by antibiotic treatment. Conditions were the same as in (C). The graph demonstrates the changes in the total nitrate/nitrite concentration in wt and Δnos cultures before and after the addition of CEF (50 µg/ml). (E) NO protects B. subtilis against ROS-mediated CEF toxicity. Conditions were the same as in (C). Cells were pretreated with 0.5 mM bipyridyl (an iron chelator) or 100 µM NO donor or 150 mM thiourea (a ROS scavenger) for 3 min, followed by CEF (50 µg/ml). The percentage of surviving cells was determined by colony formation and is shown as the mean ± SD from four experiments.
bNOS gene expression was not affected by the antibiotic. However, when CEF was added, an increase of the end products of NO oxidation (nitrite/nitrate) occurred in the growing culture of the wt B. subtilis, but not in the Δnos cells (Fig. 3C), indicating that the antibiotic stimulated bNOS activity (24). Using a cell-permeable fluorescent NO sensor (CuFL) (3, 5, 25), we also detected the increase of NO production after treatment with the antibiotic in vivo (fig. S11).
Our results show that bacteria use NOS as a part of their defense system against other microorganisms. Because the pathogens, including B. anthracis and S. aureus, have NOS, which protects them against antibiotics and immune attack (5), the inhibition of this enzyme could serve as an effective antibacterial intervention.
Supplementary Material
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/325/5946/1380/DC1
Materials and Methods
SOM Text
Figs. S1 to S11
Tables S1 and S2
References and Notes
References and Notes
- 1.Pant K, Bilwes AM, Adak S, Stuehr DJ, Crane BR. Biochemistry. 2002;41:11071. doi: 10.1021/bi0263715. [DOI] [PubMed] [Google Scholar]
- 2.Stuehr DJ. Biochim. Biophys. Acta. 1999;1411:217. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 3.Gusarov I, et al. J. Biol. Chem. 2008;283:13140. doi: 10.1074/jbc.M710178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gusarov I, Nudler E. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13855. doi: 10.1073/pnas.0504307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shatalin K, et al. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1009. doi: 10.1073/pnas.0710950105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson EG, et al. Chem. Biol. 2008;15:43. doi: 10.1016/j.chembiol.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. Cell. 2007;130:797. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Wainwright M. J. Antimicrob. Chemother. 2001;47:1. doi: 10.1093/jac/47.1.1. [DOI] [PubMed] [Google Scholar]
- 9.NO does not react with nucleophiles directly. However, products of NO oxidation (NO+) readily nitrosate arylamino moieties (Fig. 1A). Such products appear intracellularly via reaction with transition metals (such as Fe3+ or Cu2+) (11, 12). Also, NO autoxidation is accelerated markedly due to the process of micellar catalysis, which is mediated by proteins’ hydrophobic pockets and membranes in vivo (10).
- 10.Nedospasov A, Rafikov R, Beda N, Nudler E. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13543. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Proc. Natl. Acad. Sci. U.S.A. 2009;106:4671. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster MW, Liu L, Zeng M, Hess DT, Stamler JS. Biochemistry. 2009;48:792. doi: 10.1021/bi801813n. [DOI] [PubMed] [Google Scholar]
- 13.Asad NR, Leitao AC. J. Bacteriol. 1991;173:2562. doi: 10.1128/jb.173.8.2562-2568.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moncada S, Palmer RM, Higgs EA. Pharmacol. Rev. 1991;43:109. [PubMed] [Google Scholar]
- 15.Stamler JS, Singel DJ, Loscalzo J. Science. 1992;258:1898. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 16.Lau GW, Hassett DJ, Ran H, Kong F. Trends Mol. Med. 2004;10:599. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Baron SS, Rowe JJ. Antimicrob. Agents Chemother. 1981;20:814. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahme LG, et al. Science. 1995;268:1899. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 19.Vukomanovic DV, et al. Biochem. J. 1997;322:25. doi: 10.1042/bj3220025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan HM, Fridovich I. J. Bacteriol. 1980;141:156. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.B. subtilis catabolizes glucose and other sugars to pyruvate during exponential growth (26). Instead of oxidizing pyruvate further, they excrete it as acetoin, thereby limiting the respiratory chain activity (26). In contrast, during the stationary phase, acetoin is reused from the media leading to the increase of oxidative phosphorylation.
- 22.Inaoka T, Matsumura Y, Tsuchido T. J. Bacteriol. 1998;180:3697. doi: 10.1128/jb.180.14.3697-3703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaoka T, Matsumura Y, Tsuchido T. J. Bacteriol. 1999;181:1939. doi: 10.1128/jb.181.6.1939-1943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitrate/nitrite in the media is not a quantitative reflection on actual NO production. NO is synthesized intracellularly and freely diffuses out of the cell where it is oxidized to nitrate/nitrite. Much of this nitrate/nitrite is taken up and used by bacteria. Thus, the net increase in nitrate/nitrite in the media is appreciably less than the amount of NO that is actually synthesized. Five to seven micromolar of NO2−/NO3− per hour (~100 nM/min) is a large increase. Previously we demonstrated that only a single treatment with 30 µM NO renders cells resistant to oxidative stress (4). Sustained NO production at higher levels is toxic [e.g., (27)]. Cells need to produce the optimal level of NO in response to toxins, enough to suppress the Fenton reaction, but not enough to affect the respiratory chain and glycolysis.
- 25.Lim MH, Xu D, Lippard SJ. Nat. Chem. Biol. 2006;2:375. doi: 10.1038/nchembio794. [DOI] [PubMed] [Google Scholar]
- 26.Fisher SH, Sonenshein AL. Annu. Rev. Microbiol. 1991;45:107. doi: 10.1146/annurev.mi.45.100191.000543. [DOI] [PubMed] [Google Scholar]
- 27.Moore CM, Nakano MM, Wang T, Ye RW, Helmann JD. J. Bacteriol. 2004;186:4655. doi: 10.1128/JB.186.14.4655-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.This research was supported by the NIH Director’s Pioneer Award to E.N.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.