Abstract
Neutrophil-specific genes are abundant in PBMC microarrays from lupus patients due to presence of low density granulocytes (LDGs) in mononuclear cell fractions. The functionality and pathogenicity of these LDGs have not been characterized. We developed a technique to purify LDGs from lupus PBMCs and assessed their phenotype, function and potential role in disease pathogenesis. LDGs, their autologous lupus neutrophils and healthy control neutrophils were compared in their microbicidal and phagocytic capacities, generation of reactive oxygen species, activation status, inflammatory cytokine profile and type I IFN expression and signatures. The capacity of LDGs to kill endothelial cells and their antiangiogenic potential were also assessed. LDGs display an activated phenotype, secrete increased levels of type I IFNs, TNF-α and IFN-γ, but show impaired phagocytic potential. LDGs induce significant endothelial cell cytotoxicity and synthesize sufficient levels of type I IFNs to disrupt the capacity of endothelial progenitor cells to differentiate into mature endothelial cells. Further, LDG depletion restores the functional capacity of endothelial progenitor cells. We conclude that lupus LDGs are proinflammatory and display pathogenic features, including the capacity to synthesize type I IFNs. They may play an important dual role in premature cardiovascular disease development in SLE by simultaneously mediating enhanced vascular damage while inhibiting vascular repair.
Introduction
The development of systemic lupus erythematosus (SLE) is typically attributed to disruptions in adaptive immunity, leading to the loss of tolerance to self-antigens(1). While the basis for this disruption is unclear, the development and progression of SLE requires T and B cells. Recent evidence suggests that SLE patients also have disruptions in innate immunity (2). Indeed, while the importance of innate immunity in the development and progression of lupus has only begun to been examined, it is likely to have key roles in the regulation of apoptotic load, presentation of potential autoantigens and synthesis of type I IFNs, all of these likely key events in SLE pathogenesis (3, 4). Abnormalities in phenotype and function of monocytes, macrophages, DCs and other components of the innate immune system have clearly been identified in SLE (3) (5) (6). However, while a potential role for neutrophils in lupus pathogenesis and organ damage was described decades ago (7), the exact role that this cell subset plays in SLE has not been well characterized.
Neutrophils provide the key initial innate immune response to infection and destroy bacteria by multiple mechanisms, including the generation of ROS via a respiratory burst, secretion of granules which contain bactericidal proteins and enzymes, and recognition of pattern motifs on the surface of bacteria which induces phagocytosis (8). Neutrophils also produce inflammatory cytokines and eicosanoids, regulate vascular permeability at the site of infection and can induce endothelial damage (9). In this context, the primary role of the neutrophil in the immune response is to inhibit bacterial growth until adaptive immune responses can be initiated. In addition to microbial products, other stimuli such as immune complex tissue deposition can induce a respiratory burst leading to enhanced inflammation and recruitment of additional neutrophils(10). Indeed, disease processes that promote abnormal neutrophil activation can result in tissue damage and potentiation of aberrant immune responses.
Two studies have reported the presence of an abnormal subset of neutrophils in the peripheral circulation of SLE patients (2, 11). Low density neutrophils are present in PBMC preparations derived from adult or pediatric lupus patients. The presence of these cells was established by immunohistochemistry and microscopy of PBMC preparations, as well as through the identification of a “granulocyte gene signature” found in gene expression arrays derived from pediatric lupus PBMCs (2, 11). However, the functional capacity of these cells and their potential to contribute to lupus clinical manifestations had not been explored. In an effort to define more clearly the roles of these cells in SLE, we studied the clinical features of patients with elevated levels of these low-density granulocytes (LDGs), and developed a procedure to rapidly isolate highly-enriched preparations of these cells by negative selection. This allowed us to directly assess the functional capacity of the LDGs relative to normal neutrophils isolated from healthy controls, as well as to lupus normal-density autologous neutrophils. We also assessed their potential pathogenic potential in SLE by measuring their production of proinflammatory cytokines and type I IFNs, and their ability to induce endothelial damage and disrupt endothelial repair.
Materials and Methods
Antibodies
For purification of LDGs, biotinylated Abs recognizing CD3, CD7, CD19, CD79b, CD56, MHCII, CD86, CD235a were obtained from Ancell (Bayport, MN). Characterization of surface molecule expression was performed using FITC-conjugated Abs recognizing CD15, CD16, MHC class II, CD11c, CD66b and CD86; PE-conjugated Abs recognizing CD14 and CD11b; and PE/Cy5-conjugated Abs recognizing CD10 and CD33, all from Ancell. L-selectin-Ab (anti CD62L-PE) was from Southern Biotech (Birmingham, AL).
Patient selection
The University of Michigan institutional review board (IRB) approved this study. Subjects gave informed consent in accordance with the Declaration of Helsinki. Patients fulfilled the revised American College of Rheumatology criteria for SLE(12) and were enrolled from the University of Michigan outpatient Rheumatology clinic and from the Michigan Lupus Cohort. Age and gender- matched healthy controls were recruited by advertisement. Lupus disease activity was assessed by SLE Disease Activity Index (SLEDAI)(13).
Purification of LDGs
Peripheral venous blood from SLE patients was collected in heparinized vacuum containers and processed within 4 h of phlebotomy. PBMCs were isolated by Ficoll/Hypaque gradient, washed twice and contaminating RBCs were lysed by incubation with ice-cold ammonium chloride solution followed by potassium bicarbonate solution or by hypotonic/hypertonic sodium chloride method. Cell pellets were collected by centrifugation, resuspended in PBS/2mM EDTA/0.5% BSA, washed and incubated with 10 µl LDG isolation cocktail (mixture of equal volumes of biotinylated Abs recognizing human CD3, CD7, CD19, CD79b, CD56, MHC class II, CD86 and CD235a) for 30 min on ice, to label T- and B-lymphocytes, NK cells, monocytes and residual RBCs. The labeled cells were washed and cultured with 40 µl of anti-biotin MACS beads for 10 min on ice, followed by addition of a second volume of 40 µl anti-biotin MACS beads mix and 10 min incubation on ice. Cells were washed and applied to a MACS-LS column and non-immobilized cells were recovered by negative selection. The purity of the LDG fraction typically exceeded 95% and was determined by staining with CD15-FITC, CD14-PE and CD10-PE/cy5 by flow cytometry. LDGs were identified as CD15+, CD14lo, CD10+.
Isolation of neutrophils
Normal-density neutrophils were isolated by dextran sedimentation of RBC pellets as described (14). The cells were collected by centrifugation and contained over 95% neutrophils. In additional experiments, and to exclude an effect of the isolation technique on the differences observed between normal-density neutrophils and LDGs, an alternative isolation procedure was performed. After collecting the PBMC fraction, the residual Ficoll/Hypaque suspension was harvested and slowly layered on equal volumes of Histopaque-1119 (Sigma). Neutrophils were recovered by centrifugation at 1440 rpm for 30 min at room temperature. The neutrophil fraction was recovered from the interface, washed twice with PBS and once with ice-cold PBS/2mM EDTA/0.5% BSA. The supernatant was then harvested and the cell pellet was incubated with an Ab cocktail similar to the one used to isolate LDGs by negative selection, as specified above. This was followed by negative selection with magnetic beads, identical to the procedure used to isolate LDGs. The purity of the neutrophil fraction also typically exceeded 95% and was determined by CD15, CD14 and CD10 expression by FACS, as detailed above.
Morphology assessment of LDGs and neutrophils
Cell aliquots (0.1×106) were removed and combined with 1 ml of autologous plasma previously cleared of debris by centrifugation. Cells were collected by brief centrifugation, resuspended in 0.1 ml of clarified autologous plasma, transferred to a cytofuge and spun onto a standard microscope slide. Differential staining was performed on the immobilized cells monolayer using the HEMA3 staining kit from Fisher (Pittsburgh, PA).
Phagocytosis of S.aureus bioparticles
LDGs and neutrophils (1×106 cells) were plated in 96-well plates in 30% autologous serum/HBSS and incubated at 37°C/5% CO2 for 1 h for adherence. PHrodo S. aureus bioparticle conjugates (Invitrogen-A10010) were resuspended in HBSS (final concentration of 1 mg/mL) and sonicated for homogenous dispersion. The pHrodo S. aureus particle suspension was added to the cell cultures and plates were covered and incubated at 37°C for 2 h in the absence of CO2. Fluorescence was read with a plate reader using a 530/25 excitation, 590/35 emission, at a sensitivity of 35–37, following manufacturer’s instructions.
Microbicidal assay
Neutrophil and LDG microbicidal activity was measured using previously published protocols (14). In brief, 2.5×106 LDGs or neutrophils were incubated at 37°C for 20 min in HBSS/10% autologous serum with 2.5×107 S. aureus strain 502A bacteria (ATCC). This was followed by addition of 10 U of lysostaphin (Sigma) to the co-culture to kill any non-engulfed bacteria. Cell aliquots were harvested and lysed at various time-points following addition of lysostaphin, to release internalized viable bacteria. Dilutions of the cell lysates were plated onto Tryptic Soy Agar media (TSA, Acumedia, Lansing, MI) and incubated at 37°C for 16 h. The number of colony forming units/ml (CFU) was then quantified using standard techniques.
Intracellular MPO Concentration
Neutrophils and LDGs were fixed in 4% paraformaldehyde, washed, suspended in PBS/10% DMSO (Sigma), and stored at −80°C prior to MPO staining as described (14). Prior to staining, cells were thawed, permeabilized with 0.2% saponin/PBS (Sigma), washed, blocked with 1% horse serum/1% BSA/0.2% saponin/PBS, and stained at 4°C with either anti-MPO-FITC (BD Biosciences) or isotype control. Cells were washed and MPO expression determined by flow cytometric analysis as mentioned below.
Flow cytometric analysis of cell surface L-selectin
This was performed as previously described(15). In brief, a hydroxamic acid based L-selectin sheddase inhibitor, KD-IX-73-4 (TAPI-0) was purchased from Peptides International (Louisville, KY) and reconstituted in DMSO at 5mg/mL. Lupus LDGs, lupus and control neutrophils were resuspended in PBS/10Mm glucose/0.5% FBS/ 20Mm HEPES and incubated for 10 min at 37 °C in the presence of either vehicle (0.1% (v/v) DMSO) or freshly prepared TAPI-0 (50µg/ml). The cells were then cultured in the presence or absence of 0.1 µg/ml PMA (Sigma) for 10 min at 37°C, followed by addition of RPMI 1640/10% FBS and incubation on ice for 15 min. The samples were centrifuged at 1600 rpm for 5 min at 4° C; cell pellets were resuspended in PBS/1% horse serum/1% BSA and incubated on ice for 30 min with anti-L-Selectin-PE and anti-CD10-PeCy5 (Biolegend) or isotype controls. Samples were washed and fixed with 2% paraformaldehyde and expression of L-selectin on the CD10+ cells was quantified by FACS.
Generation of H2O2
This was quantified as previously described (16). In brief, H2O2 secretion from LDGs or neutrophils (untreated or stimulated with 0.1 µg/mL PMA or 200 µg/mL BSA/anti-BSA immune complexes) was determined by colorimetric analysis using Amplex Red (Molecular Probes, Eugene, OR) reagent, according to the instructions of the manufacturer. A solution containing 50 µM Amplex Red reagent/10 U/mL HRP/PBS was prepared and added to neutrophil or LDG cultures at 37°C for 60 min. Absorbance at 560 nm was then assessed and the H2O2 concentration was determined using an H2O2 standard curve. The detection limit of this method was 0.625 nM H2O2.
Generation of immune complexes
Bovine serum albumin (BSA)/anti-BSA immune complexes were generated as previously described(17). In brief, BSA-1 (Sigma) was added to anti-BSA stock solution (MP Biomedicals, Solon, OH) at a ratio of 1:10, incubated for 30 min at 37°C, then centrifuged at 2500 rpm for 5 min at room temperature and washed twice with PBS. The complexes were resuspended in PBS for a final concentration of 2 mg/ml prior to their use.
Cytokine and eicosanoid quantification
Lupus LDGs, autologous neutrophils and control neutrophils were cultured for 48 h in the presence or absence of 0.1 µg/mL PMA. Supernatants were then harvested and concentration of the cytokines IL-1β, TNF-α, IFN-γ, IL-4, IL-6 and IL-8, as well as the eicosanoids PGE2 and thromboxane (TX)B2 were quantified using a bead multiplex immunoassay kit (Assay Designs, Ann Arbor, MI) as described by the manufacturer. Human IL-17 was quantified in cell supernatants by ELISA (ebioscience, San Diego, CA). Confirmatory experiments were performed for selected cytokines (IL-8 and TNF-α) by assessing their intracellular expression on CD10+ cells as follows. Lupus LDGs and control and lupus neutrophils were incubated with or without 1µg/mL LPS and 1× Brefeldin-A (Biolegend) in IMDM/30%FBS (Invitrogen) at 37° C for 4 h. Cells were harvested, washed twice with PBS/1% horse serum/1% BSA containing 1× Brefeldin-A and incubated with anti-CD10 (Biolegend) or respective isotype for 30 min on ice. Cells were then washed and resuspended in 2% paraformaldehyde overnight at 4°C. This was followed by three washes and incubation in 0.2% saponin/PBS/1% horse serum/1% BSA for 1h on ice, then incubation with anti-IL-8-Alexafluor 488 (Biolegend) or anti-TNF-α-PE (Miltenyi Biotech) or respective isotype control Abs on ice for 1 h. Cells were then washed and fixed in 2% paraformaldehyde, and intracellular expression of IL-8 and TNF-α was assessed on the CD10+ cells by FACS.
Quantification of IFN-α mRNA
A total of 2 × 106 control or lupus neutrophils or autologous lupus LDGs were plated and left untreated or stimulated with either PMA (100ng/mL) for 1 h or G-CSF (50ng/mL) for 16 h. Total RNA was prepared with Tri-pure (Roche, Indianapolis, IN). RNA template was digested with DNase I (Invitrogen), then reverse-transcribed to cDNA using Superscript II reverse transcriptase (Invitrogen). Oligonucleotide primers (Integrated DNA Technologies, Coralville, IA) used in the reactions were as follows: Universal IFNα : 5'-TCC ATG AGA TGA TCC AGC AG-3' (forward), 5'-ATT TCT GCT CTG ACA ACC TCC C-3' (reverse); HPRT1: 5’-TTG GTC AGG CAG TAT AAT CC-3’ (forward), 5’-GGG CAT ATC CTA CAA CAA AC-3’(reverse). Real-time PCR reactions were run on an ABI PRISM 7900HT in duplicate using 2 × SYBR GREEN PCR master mix (Applied Biosystems, Foster City, CA). These IFN-α primers essentially amplify all described forms of IFN-α (18). Since IFN-α genes are monoexonic, it is critical to remove all genomic DNA from RNA preparation, and this was verified prior to RNA use.
Type I IFN bioassay
LDGs, autologous neutrophils or control neutrophils were isolated and cultured alone or in the presence of 0.1 µg/mL PMA for 1 h or 50 ng/mL recombinant G-CSF for 16 h. In additional experiments neutrophils and LDGs were transfected with Poly (I:C) (Calbiochem,La Jolla, CA) using the Amaxa Biosystems nucleoporator (Lonza, Walkersville, MD) with program Y01 as previously reported (19). In brief, 2 × 106 neutrophils or LDGs were resuspended in 100 µl complete nucleofector solution (Lonza) containing 10µg Poly(I:C) (Sigma), transferred to a nucleoporation cuvette, electroporated and then immediately transferred into 24-well tissue culture plates. Supernatants were harvested 16 h after transfection.
Induction of IFN-inducible genes by LDG or neutrophil supernatants was determined using a described bioassay (20, 21) that quantifies specific IFN-inducible genes by cell supernatants on cultured target epithelial cells, with some modifications. To this end, HeLa cells (ATCC; Manassas, VA) were cultured in DIFCO/10% FBS/ nonessential amino acids/ 10 mM Hepes at 37°C in 5% CO2; plated at 2×105 cells/ well in a 24-well plate; and exposed to 100% LDG–supernatant or recombinant IFN-α2b (1 kU/well, Schering, Kenilworth, NJ, used as positive control) for 6 h. Tri-Pure was added and cells were stored at −70°C until RNA extraction. cDNA was prepared and real-time PCR reactions were run as mentioned above. The type I IFN-inducible genes quantified by this assay were IFN-induced protein-44 (IFI44), IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), and double-stranded RNA-activated protein kinase (PRKR). Primers for these genes have been previously described (20). Samples were normalized to media alone after normalization to housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT-1), and results were reported as fold induction/media.
Endothelial cell cytotoxicity assay
The capacity of lupus LDGs and lupus and control, neutrophils to induce HUVEC)\ cytotoxicity was assessed by flow cytometry. In brief, HUVECs were cultured in MCDB131 (Gibco-Carlsbad, CA) basal media supplemented with EGM-2MV (without hydrocortisone) bullet kit (Lonza), in 0.2. % gelatin coated 24- well plates (Fisher, Pittsburgh, PA) and activated with TNF-α and IFN-γ for 18 h before exposure to neutrophils, as previously described(21). PMA-activated LDGs, autologous neutrophils or control neutrophils were co-cultured with HUVECs at a 1:2 effector:target ratio for 16–20 h. LDGs and neutrophils were harvested and HUVECs were exposed to 0.05% trypsin-EDTA (Gibco,Carlsbad, CA) and centrifuged at 1600 rpm for 5 min. Cells were resuspended in 2% horse serum/PBS and 104–105 cells incubated with PE-conjugated-anti-human CD146 (BD Pharmingen, San Diego, CA, an endothelial cell marker), PE/Cy5 anti-human CD10 (Biolegend, San Diego, CA, an LDG/neutrophil marker), and APC- or FITC-Annexin-V (BD Pharmingen, an apoptosis marker). Cells were then washed and fixed in 4% paraformaldehyde. The percentage of apoptotic endothelial cells was identified on cells that co-stained for CD146 and Annexin-V, in the PE/Cy5 anti-human CD10-negative gate. Experiments were performed in the presence or absence of a transwell (Corning Inc.; Lowell, MA) that separated HUVECS from LDGs/ neutrophils. Findings were confirmed using a bioluminescence cytotoxicity assay (aCellatox assay, Cell Technology Inc, Mountain View, CA) that quantitatively measures GAPDH release, according to the manufacturer’s instructions.
Assessment of the capacity of EPCs and CACs to become mature endothelial cells
Control or SLE PBMCs (4 × 106/mL) were cultured in endothelial cell-specific enrichment medium (EBM2; Cambrex, East Rutherford, NJ) supplemented with 20% FBS, bovine brain extract, and epithelial growth factor (EGF) as described by our group(21). Media was changed 120 h after plating, then every 3 days. On day 15, cells were incubated with markers of mature endothelial cells, including diI–acetylated low density lipoprotein (LDL) (Biomedical Technologies, Stoughton, MA), and FITC–UEA-1 (ICN, Irvine, CA). Cells were analyzed by fluorescent microscopy using a Leica DMIRB fluorescent inverted microscope (Bannockburn, IL). All images were acquired at room temperature using live cells in PBS without mounting media. Images were acquired with an objective magnification of × 5, × 10, or × 20 (for × 50, × 100, or × 200 total magnification, respectively). The numeric apertures for the objective lenses of the fluorescent microscope were as follows: × 5 = 0.15; × 10 = 0.3; × 20 = 0.4. Images were acquired with an Olympus DP30BW camera (Olympus Corporation, Tokyo, Japan) using the acquisition software Olympus-BSW (Olympus). Final processing was done with Adobe Photoshop CS2 (San Jose, CA). In some of the experiments PBMCs were depleted of plasmacytoid DCs (pDCs) using the anti-CD304 BDCA-4 microbead Kit or of LDGs using anti-CD10 beads (both from Miltenyi), prior to plating the rest of PBMCs under proangiogenic conditions. The capacity of these culture supernatants to induce type I IFN-responsive genes on epithelial cell lines was measured as stated above and as previously described (21).
Statistical analysis
Difference between means was analyzed using Student t test or analysis of variance (ANOVA) with pos-hoc analysis with SPSS v.14 (SPSS, Chicago, IL). To determine whether treatment with immunosuppressants was associated with phenotypic/functional abnormalities, univariate linear regression was performed. Vascular repair markers were modeled separately as dependent variables, with medications modeled as dichotomous independent predictors. For the IFN-inducible gene experiments, 2-group comparisons of continuous data that had a normal distribution were assessed using t tests. The Kruskal-Wallis nonparametric test was used to compare the study groups for the values of the IFN-inducible genes because the data were not normally distributed.
Results
Demographics and patient population
Demographic and clinical information of patients and controls enrolled for all the experiments performed in this study is included in Table I. There were no significant differences between SLE patients and controls with regard to age or gender. Overall, 60% of SLE patients studied had serological and/or clinical evidence of active disease (measured as a SLE disease activity index (SLEDAI) >2). Approximately a quarter of the lupus patients included in the study had current or past history of lupus nephritis.
Table 1.
Demographic and clinical characteristics of patients
| VARIABLE | CONTROL n=110 | SLE n=190 |
|---|---|---|
| Females, % | 82 | 90 |
| Age, mean (SEM) | 38 (7) | 41 (5) |
|
Disease activity, mean SLEDAI (SEM) |
_____ | 6.5 (4.8) |
| SLEDAI < 2, % | _____ | 40 |
| SLEDAI ≥2, % | _____ | 60 |
| Medications, % | _____ | |
| Antimalarials | _____ | 65 |
| Azathioprine | _____ | 5 |
| Mycophenolate mofetil | _____ | 30 |
| Cyclophosphamide | _____ | 2.1 |
| Methotrexate | _____ | 3.6 |
| Prednisone < 0.5 mg/kg/d | _____ | 52 |
| Prednisone 0.5–1 mg/kd/d | _____ | 6 |
| Prednisone > 1 mg/kg/d | _____ | 1 |
| No immunosuppression | _____ | 18 |
| Past or current lupus nephritis, % |
_____ | 30 |
| Positive antiphospholipid Ab, β2-glycoprotein I Ab, and/or lupus anticoagulant, % |
_____ | 22 |
---— indicates not applicable; Ab: antibody; SLEDAI: Systemic Lupus Erythematosus disease activity index; SEM: standard error of the mean
Characterization of lupus LDGs
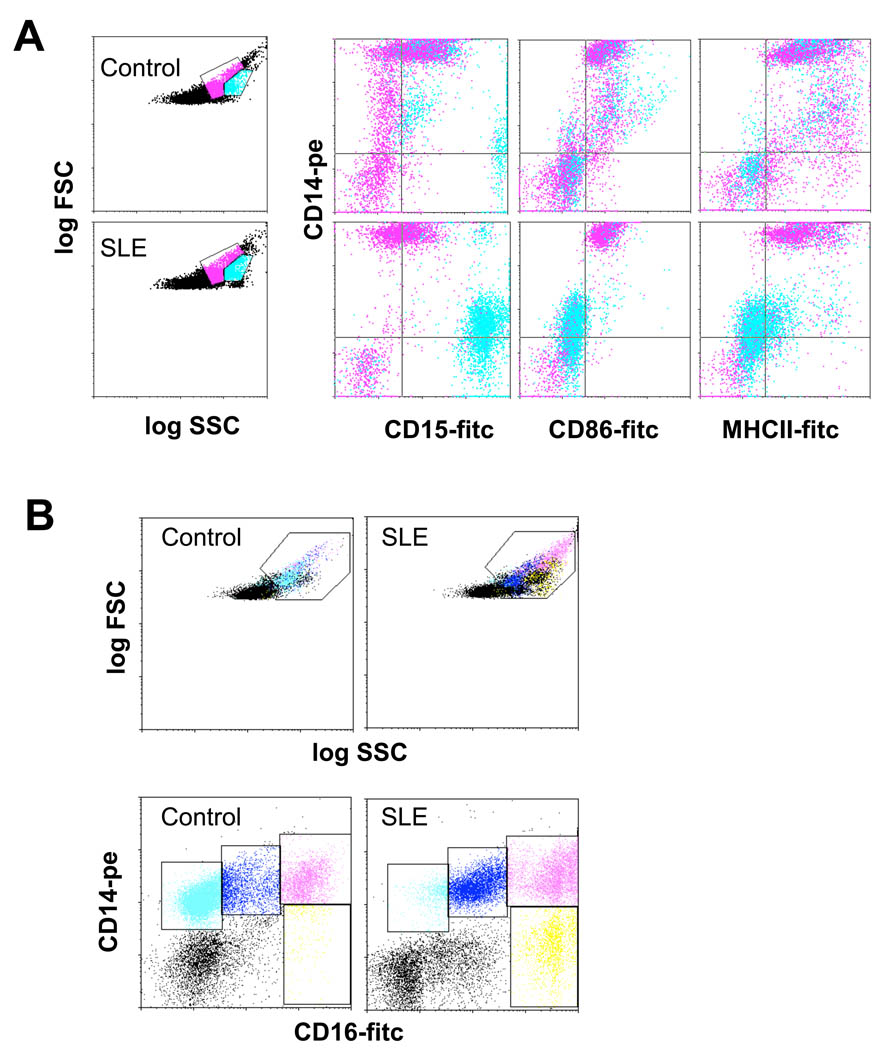
The presence of LDGs was initially confirmed in adult SLE patients (Figure 1). The LDG population segregated directly adjacent to the monocyte pool by flow cytometric analysis using a dual log scale of forward and side scatter intensity (Figure1A). The monocytes and LDGs could be clearly distinguished in lupus samples based upon expression of the neutrophil marker CD15 (Lewisx) and the monocyte marker CD14 (Figure 1A). Monocytes were CD14+/CD15lo while LDGs had a CD14lo/CD15+ profile. Indeed, greater than 95% of the cells in the monocyte gate were CD14+/CD15lo. Monocytes could be further distinguished from LDGs by their expression of MHC class II and the costimulatory molecule CD86 (B7.2), as well as by their lack of expression of the membrane peptidase CD10 (Fig.1B and not shown). In contrast, lupus LDGs expressed CD10, but lacked MCH class II and CD86 (Figure 1A). Analysis of CD86 and CD16 revealed several subpopulations. Most healthy control monocytes displayed the resting phenotype of CD86+CD16−, while SLE monocytes had the more activated phenotype of CD86+CD16+. In contrast, LDGs were CD16hi/CD86− (Figure 1B).
Figure 1. Identification of LDGs in lupus PBMC fractions.
Healthy control or SLE PBMCs were stained for markers of the monocyte or granulocyte lineages and analyzed by FACS. A. Gates which contained predominantly lymphocytes, monocytes and granulocytes were established in dual-log scattergrams. Granulocytes (blue) and monocytes (pink) are distinguished based on CD14, CD15, CD86 and MHC class II expression. Monocytes express high levels of CD14 and are positive for CD86 and MHC class II, while CD15 is weak or absent. Granulocytes present in the PBMC fraction are CD15hi, CD14lo and negative for CD86 and MHC class II. Similar results were seen in 2 additional controls and 5 additional SLE patients. B. Analysis of CD86 and CD16 revealed several subpopulations. Most healthy control monocytes display the resting phenotype of CD86+CD16− (light blue), while SLE monocytes have the more activated phenotype of CD86+CD16+ (blue). The CD16hi cells can be further divided based on CD86 expression. The CD16hi/CD86− pool (yellow) likely represents LDGs, while the CDhi/CD86+ population (pink) possibly reflects conjugates of CD16hi granulocytes and CD86+ monocytes.
Using surface expression of CD14 and CD15 as a guide, it was possible to construct scatter gates which discriminated LDGs from monocytes present in lupus PBMCs.
The forward and side scatter profile was used to determine the relative levels of LDGs in PBMCs from healthy individuals and SLE patients, and confirmed by CD14/CD15 coexpression. PBMCs from 22 healthy individuals showed an average of 5% LDGs. This likely represents contaminating mature degranulated neutrophils in the PBMC preparations since most control samples were completely devoid of contaminating granulocytes. By comparison, all SLE preparations contained LDGs, which represented an average of 17% of total PBMCs, with a range of 1.2% to 54% (n=65, p<0.05). When total numbers of neutrophils present in the PBMC subset were compared, there was a highly significant difference between lupus patients and healthy controls (mean 0.26×106+0.08×106 cells/ml of blood versus 0.0046×106±0.008×106cells/ml of blood, respectively; P=0.007) indicating an absolute significant increase which was not related to the lymphopenia commonly observed in SLE. LDGs accounted for more than 25% of the total PBMCs in 12 of 65 SLE samples (19% of SLE patients). The clinical characteristics of this subset of SLE patients with more than 25% LDGs of the total PBMCs were examined in greater detail and revealed that 83% of patients with elevated levels of LDGs had skin involvement (including vasculitis) and/or synovitis. In contrast, these clinical complications were not observed in patients with PBMC profiles comparable to those of healthy controls. There was no significant correlation between age, disease duration and/or use of immunosuppressive drugs or corticosteroid dose and the presence of LDGs, consistent with results from the two previous publications that reported this cell subset (2, 11). Indeed, these cells were present in SLE patients who were not currently using medications to treat their disease (either because of new-onset or due to remission of disease), as well as in individuals with various doses of immunosuppressives, corticosteroids or antimalarials (not shown).
The LDG population was then examined in greater detail. To this end, a negative-selection approach to label and remove T and B lymphocytes, NK cells, monocytes and erythrocytes from PBMCs by magnetic-bead assisted cell sorting was developed (Figure 2). Negative selection was necessary because commercially available kits used to purify neutrophils using positive selection rely on positive selection based on expression of CD15 or CD16 which could potentially interfere with subsequent cell-based assays of cell activation and cytotoxicity.
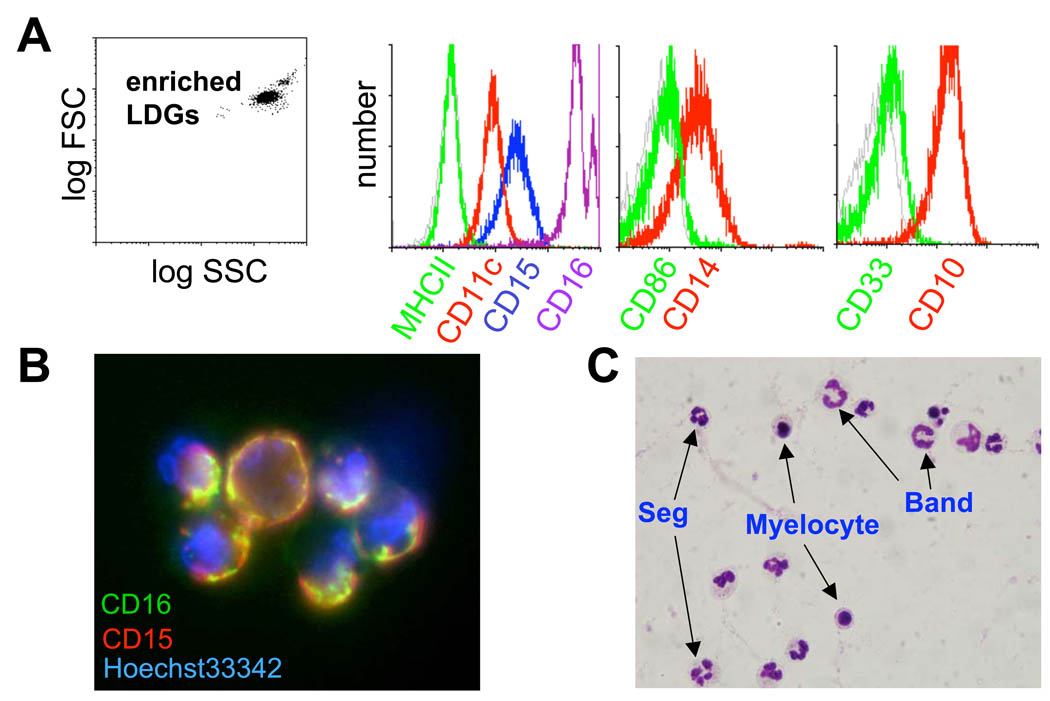
Figure 2. Enrichment of lupus LDGs.
LDGs were isolated from lupus PBMC fractions by negative selection with magnetic beads. A. With this approach highly enriched granulocytes were obtained. The cells express granulocyte markers (high levels of CD15, CD16, CD11c, CD14 and CD10). The position of the isotype control Ab is shown in the gray histogram. Similar results were seen in 2 additional SLE patients. B. Enriched granulocytes were stained with CD16-FITC and CD15-biotin followed by Cy3-streptavidin; nuclei were counterstained with Hoechst 33342. Surface expression and co-distribution of CD15 and CD16 is apparent (original total magnification 1000x). Similar result was seen in one additional SLE patient. C. Differential staining of the enriched granulocyte fraction obtained from lupus PBMCs reveals that the cells are neutrophils and have a range of nuclear morphologies. Segmented (Seg) and band neutrophils are apparent, as well as myelocyte-like cells (total magnification 1000×). Similar results were seen in 3 additional SLE patients.
PBMCs from SLE patients were incubated with a cocktail of biotin-conjugated monoclonal antibodies (Abs) recognizing CD3, CD7, CD19, CD79b, CD56, CD86, MHC class II and glycophorin A, then tagged with anti-biotin Ab coupled superparamagnetic beads, and depleted in a magnetic field. The resulting cell suspension was highly enriched for the LDGs (~95%) as demonstrated by FACS analysis for granulocyte markers FcγRIII (CD16), CD15 and CD10. CD33, a marker expressed on developing or immature granulocytes, was only very weakly expressed (Figure 2A), and expression of other early progenitor markers CD34, and Flt-3 (CD135) was not detected. Taken together, the profile of surface molecular expression was consistent with a mature neutrophil phenotype. In addition, LDGs expressed platelet/endothelial cell adhesion molecule (PECAM/CD31), CD11c (Figure 2A and table II), as well as the receptors for granulocyte colony stimulating factor (G-CSFR, CD114) and granulocyte-macrophage colony stimulating factor (GM-CSFR, CD116) (Table II). IL-3 receptor α chain (CD123) and macrophage colony stimulating factor receptor (CD115) were not detectable. There was a trend for CD10 and CD11c mean fluorescent intensity to be lower in the lupus LDGs when compared to autologous lupus neutrophils and control neutrophils but the difference was not statistically significant (Table 2). Overall, when compared to autologous normal-density lupus neutrophils or control neutrophils, LDGs expressed comparable levels of the markers mentioned above (Table II). The phenotype of lupus LDGs was also confirmed by fluorescence microscopy (Figure 2B).
Table 2.
Expression of cell surface markers in LDGs and neutrophils.
| MARKER(%(MFI)) | LDGs | Lupus neutrophils | Control neutrophils |
|---|---|---|---|
| CD10 | 93.7±11 (121±20) | 95.8±1.5(265±50) | 97±0.5(255.7±80) |
| CD11c | 97.3± 0.1(37.8±1) | 98.6±0.1 (91.3±30) | 98.2±0.3 (73±20) |
| CD14 | 68.08±10(63±20) | 83.6±3(71.4±20) | 76.7±8(56.7±11) |
| CD15 | 97.3±0.7(359.9±200) | 98±0.2 (200±90) | 97.8±0.2 (198±70) |
| CD16 | 98±0.01 (373±80) | 99±0.03 (482±80) | 97±0.6(384±100) |
| CD31 | 98±0.4(138±10) | 99±0.08(162±30) | 99±0.08(240±40) |
| CD114 | 72±16 (30±10) | 65±13 (26±6) | 63±18 (25±4) |
| CD116 | 5.6±0.5 (2.5±1.2) | 7±2.2 (2.9±1.1) | 4.2±2.5 (3.6±1.5) |
LDGs: low density granulocytes; MFI: mean fluorescence intensity.
To further assess activation status, SLE neutrophils, autologous LDGs and neutrophils isolated from healthy volunteers, were analyzed for surface expression of CD66b and CD11b. Elevated expression of these molecules on the cell surface is associated with cell activation due to vesicular membrane fusion during exocytosis of neutrophil granules (22). Both CD66b and CD11b were elevated on the cell surface of lupus neutrophils and LDGs, compared to normal neutrophils (CD66b: 59.6±5.9 (lupus LDGs); 49.1±4.9 (lupus neutrophils); 24.8±0.5(control neutrophils); CD11b: 27.7±3.6 (lupus LDGs); 24.7± 3.5 (lupus neutrophils); 13.2±1.1 (control neutrophils); results represent mean fluorescence intensity + SEM of 5 controls and 13 lupus patients; p<0.05 when comparing control neutrophils to lupus LDGs and neutrophils; p=not significant when comparing LDGs and autologous neutrophils).
L-selectin is constitutively expressed at high levels on the surface of resting neutrophils, and these molecules are shed upon neutrophil stimulation in a distinctly robust manner and play a role in regulating leukocyte rolling velocity in vivo(23). The shedding of L-selectin is distinct, because it is unusually rapid and resistant to common protease inhibitors(24). However, certain hydroxamic acid-base metalloproteinase inhibitors prevent L-selectin shedding from the surface of neutrophils(15). This has been previously demonstrated by showing that exposure of neutrophils to various agents induces rapid loss of L-selectin from the cell surface and that this is prevented by the metalloproteinase inhibitor. To additionally assess LDG activation status, we compared L-selectin shedding after PMA stimulation among the various cell subsets. Prior to stimulation, there were no significant differences in L-selectin expression between LDGs, lupus neutrophils and control neutrophils when assessing both % expression and MFI (not shown).
After stimulation and in the absence of a metalloproteinase inhibitor, there was comparable downregulation of cell surface L-selectin expression among the 3 groups (−12.1±2.5 for lupus LDGs; −12.4±3 for lupus neutrophils; −13.9±4.2 for control neutrophils; results represent mean change in % cell surface L-selectin expression from baseline ± SEM of 4 independent experiments, p=not significant). Similar results were found when MFI was analyzed (−9.8±3.5 for lupus LDGs; −10.4±3.2 for lupus neutrophils; −14±4.2 for control neutrophils; results represent mean change in MFI cell surface L-selectin expression from baseline±SEM; p=not significant). When a metalloproteinase inhibitor was added, shedding was abrogated in the 3 groups and again there were no significant differences among groups (17.3±3 lupus LDGs; 15.5±2 for lupus neutrophils; 13±1.5 for control neutrophils; results represent mean change in % cell surface L-selectin expression from baseline ±SEM of 4 independent experiments; p=not significant). Similar results were found when MFI was analyzed (5.4±3 lupus LDGs; 5.3±2.5 for lupus neutrophils;5.5±1.8 for control neutrophils; results represent mean change in MFI cell surface L-selectin expression±SEM; p=not significant). Thus, relative to healthy control neutrophils, both LDGs and autologous lupus neutrophils have an activated phenotype on the basis of surface molecule expression of CD66b and CD11b ex vivo but do not differ in L-selectin expression and shedding before and after stimulation.
However, examination of LDGs by differential staining and microscopy suggested an immature phenotype consistent with a previous report in pediatric lupus patients(2). Using microscopy, the stages of neutrophil development are distinguished by changes in nuclear morphology. Early or immature neutrophils possess round or ovoid nuclei while more developed cells have indented, band or segmented nuclei. Despite the apparent mature phenotype demonstrated by surface molecule expression (Figures 1 and 2), differential staining of the enriched LDGs revealed a mixed population with cells with band, lobular or myelocyte-like nuclei (Figure 2C). The distribution of cells displaying the different nuclear morphologies varied between samples, but the LDGs were always less segmented and more lobular than the corresponding lupus autologous normal-density neutrophils or the neutrophils isolated from healthy individuals (not shown). Approximately 60 % of the LDGs were polymorphonuclear, with the rest of the cells having a band, lobular or myelocyte- like nuclei. This is in contrast with the autologous normal density neutrophils and the control neutrophils in which >90% were polymorphonuclear. Overall then, while both LDGs and their autologous lupus neutrophils displayed surface markers of activation, their nuclear morphology differed significantly.
LDGs display neutrophil function but have decreased phagocytic potential
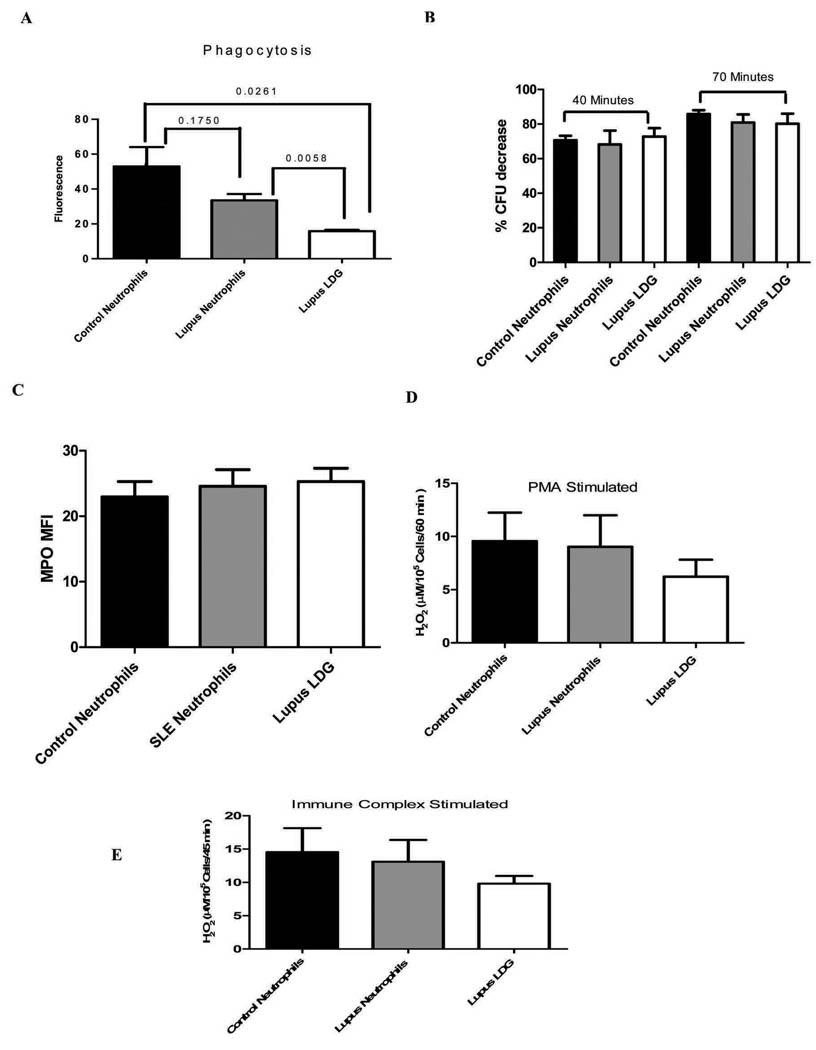
Next, the functional capacity of LDGs was evaluated. Measurements included the capacity to engulf and kill bacteria, determining the intracellular levels of MPO granules, and ability to mount a respiratory burst and to generate ROS. The phagocytic function of neutrophils is crucial for host defense and is mediated by FcγR and complement receptors (25). As shown in Figure 3A, LDGs displayed decreased phagocytosis of S. aureus when compared to healthy control or lupus autologous neutrophils. However, bactericidal activity of the engulfed bacteria was comparable between LDGs, autologous neutrophils or healthy control neutrophils (Figure 3B). Further, LDGs displayed comparable levels of intracellular MPO expression (Figure 3C), as well as comparable degrees of respiratory burst, as assessed by hydrogen peroxide synthesis after PMA or immune complex stimulation (Figures 3D and E and not shown, either using normal-density neutrophil isolation by dextran or by negative selection). Overall, these results indicate that LDGs have phenotypic and functional features of normal neutrophils but, despite an overall activated phenotype based on expression of surface receptors, their capacity to phagocytose bacteria appears to be significantly impaired.
Figure 3. Neutrophil function of LDGs.
A. Phagocytic capacity of bacteria is impaired in lupus LDGs. Results represent mean± SEM fluorescence of 5 control and 5 lupus patients each performed in triplicate, as assessment of phagocytosis of S. aureus particles. P values are included. B. Bactericidal activity is preserved in LDGs. Bar graph displays the percentage S.aureus CFU decrease observed between 10 to 40 min and 10 to 70 min after the addition of lysostaphin in lupus LDGs, autologous lupus neutrophils or control neutrophils. Results represent the mean ± SEM from 5 lupus and 5 control patients, each performed in triplicate; p=not significant between groups. C. Intracellular MPO levels in LDGs are similar to normal density neutrophils. Results represent the mean±SEM mean fluorescent intensity of intracellular MPO in LDGs and neutrophils (5 control and 5 lupus patients; p=not significant). D. LDGs do not differ from neutrophils in hydrogen peroxide synthesis capacity after PMA stimulation. Results represent mean±SEM H2O2 concentration (µM/105 cells/ 60 min) of 5 control and 5 lupus samples, each performed in triplicate; p=not significant. E. LDGs do not differ from neutrophils in hydrogen peroxide synthesis capacity after immune complex stimulation. Results represent mean±SEM H2O2 concentration (µM/105 cells/ 45 min) of 5 control and 5 lupus samples, each performed in triplicate; p=not significant.
LDGs synthesize increased levels of proinflammatory cytokines
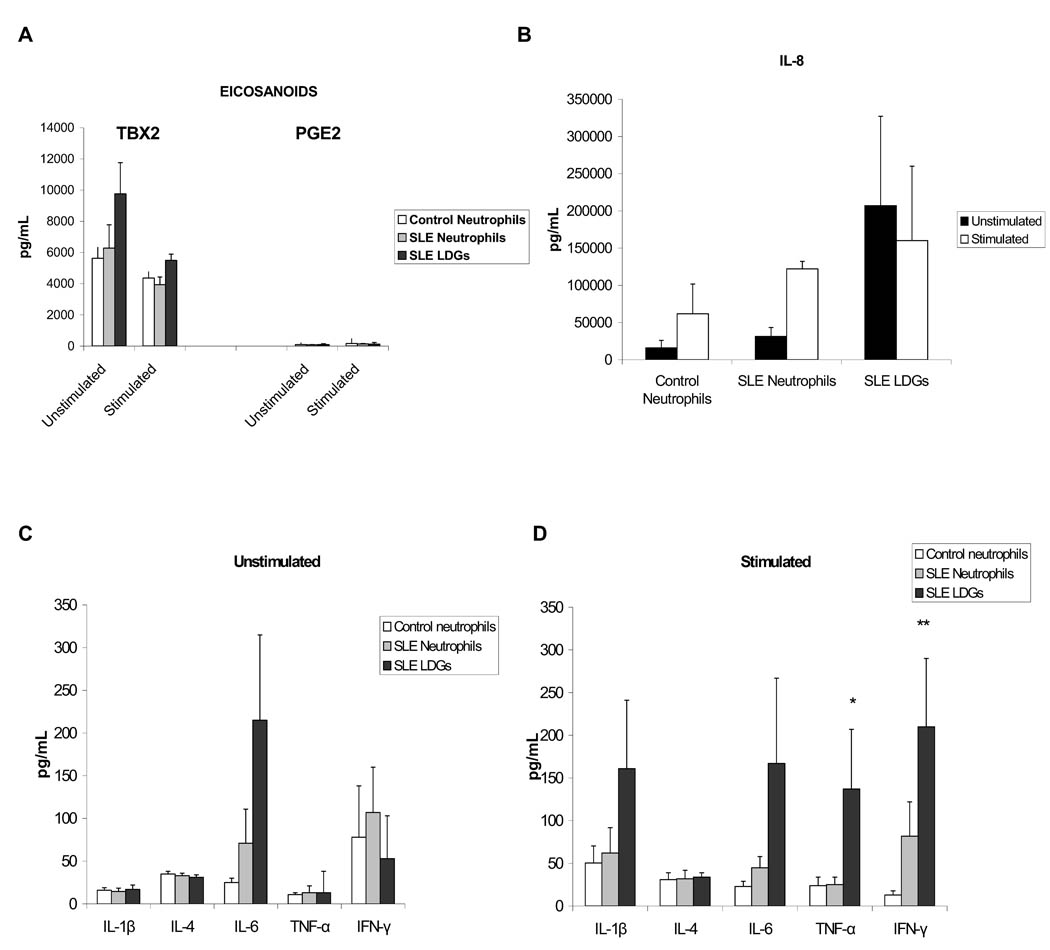
During migration, neutrophils are activated resulting in the generation of arachidonic acid metabolites, ROS, proteases (26) and various cytokines that may play an additional role in the progression of inflammation to either resolution or to a chronic inflammatory response (27). Secretion of nine cytokines and two eicosanoids was assessed in the supernatants of resting or PMA-activated LDGs, autologous neutrophils or control neutrophils, using cytometric bead arrays. Under resting conditions, there were no statistically significant differences in eicosanoid (PGE2 and TBX2) or cytokine secretion among the three groups of cells (Figures 4 A,B,C), although there was a trend for higher IL-8 and IL-6 concentrations in the LDG supernatants, when compared to autologous or control neutrophils (Figure 4 B–C). Upon stimulation with PMA, LDGs secreted significantly increased levels of TNF-α when compared to healthy control or autologous lupus neutrophils, and of IFN-γ when compared to control neutrophils (Figure 4D). There was also a trend for LDGs to secrete higher levels of IL-6, IL-1β and IL-8 than autologous lupus or control neutrophils upon stimulation, but the differences did not reach statistical significance (Figure 4B and D). There were no significant differences in eicosanoid secretion among the three groups of cells after stimulation (Figure 4A). Neither LDGs nor neutrophils secreted detectable levels of IL-17, as measured by ELISA (not shown). Additional experiments assessing intracellular expression on CD10+ cells of IL-8 (the cytokine most abundantly secreted) and TNF-α (the cytokine that showed the most significant difference between LDGs and other neutrophil groups) confirmed significant enhanced expression of TNF-α and a trend for higher expression of IL-8 in the LDG group upon stimulation (Table 3). These experiments also indicate that the differences in cytokine secretion observed by ELISA were not due to a small subset of contaminating non-neutrophil cells. Overall, these results indicate that LDGs secrete increased levels of proinflammatory cytokines upon stimulation.
Figure 4. LDGs secrete increased levels of proinflammatory cytokines.
Results represent A- eicosanoid and B–D- cytokine concentration in cell supernatants in pg/mL. Results are shown using unstimulated or PMA-stimulated cells and measurements were done with supernatants harvested after 48 h in culture.; * P<0.05 when comparing LDGs to autologous neutrophils and control neutrophils and ** P<0.05 when comparing LDGs to control neutrophils. Results represent mean±SEM of 10 controls and 10 SLE patients.
Table 3.
Intracellular cytokine expression in LDGs and neutrophils.
| Cytokine | LDG unstimulated |
LDG stimulated |
Lupus neutrophils unstimulated |
Lupus neutrophils stimulated |
Control neutrophils unstimulated |
Control neutrophils stimulated |
|---|---|---|---|---|---|---|
| IL-8 (%±SEM) |
9.3±1 | 11.6±3 | 6.5±2 | 8.3±1.5 | 5±1.5 | 6.9±2.4 |
| IL-8 (MFI±SEM) |
33±13.2 | 42±10.9 | 29±9.2. | 40±3.46 | 27±11.7 | 34±7 |
| TNF-α (%±SEM) |
49.6±10 | 57±8 | 48±2.5 | 52±1.5 | 45±10 | 54.3±8 |
| TNF-α (MFI±SEM) |
70±25 | 2688±960** | 39.3±80 | 62.6±18 | 38±0.1 | 50±0.2 |
MFI=mean fluorescence intensity; SEM= standard error of the mean
= P<0.05 when comparing LDG versus control or lupus neutrophils.
LDGs synthesize increased levels of type I IFNs
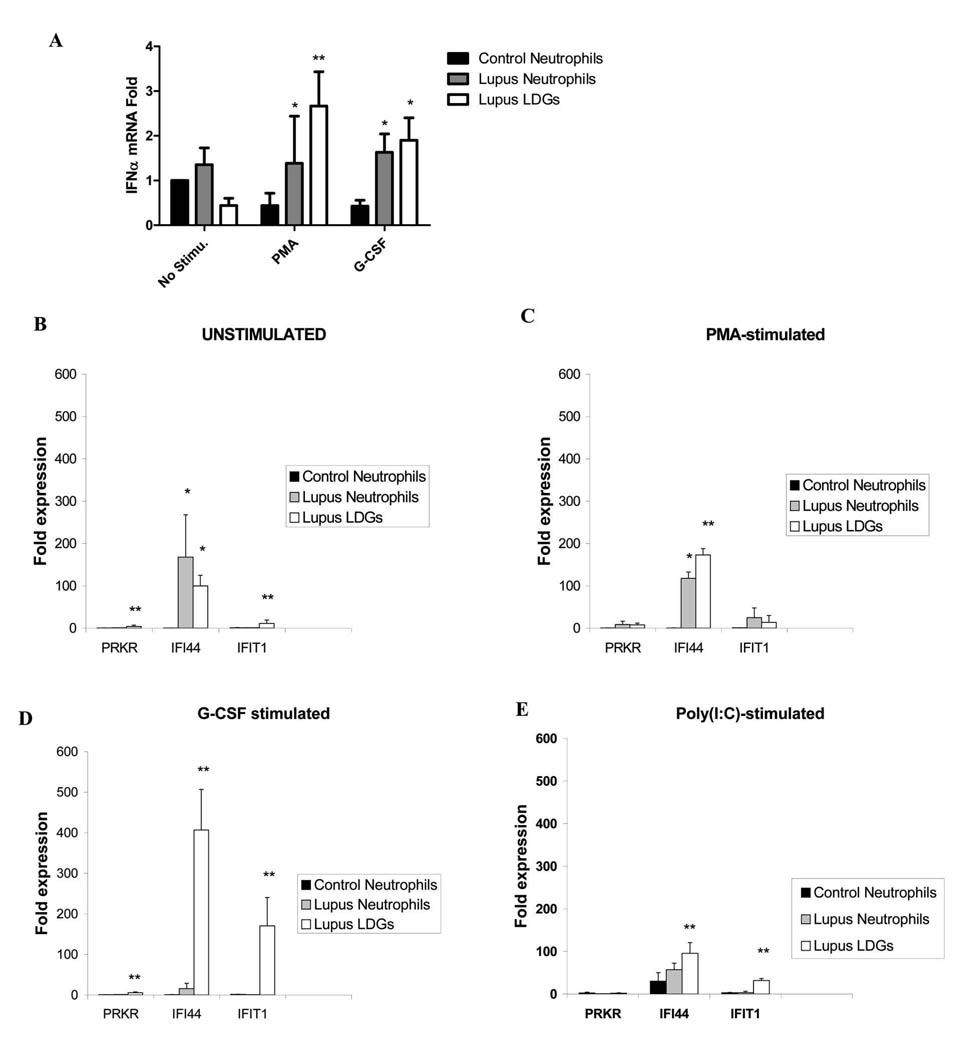
Several studies have implicated a key role for IFN-α, and potentially other type I IFNs, in SLE pathogenesis (2, 4, 6, 28). While the exact sources of the increased levels of IFN-α in SLE are not known, depletion experiments have demonstrated that pDCs contribute only part to the increased expression of this molecule in this disease (6). Since mature neutrophils can produce this cytokine in response to specific stimuli(18, 29), we quantified IFN-α mRNA levels in SLE LDGs, their autologous neutrophils and healthy control neutrophils in response to PMA and G-CSF. As shown in Figure 5A, upon activation with PMA, LDGs expressed significantly higher levels of IFN-α mRNA than control or autologous lupus neutrophils. Further, both LDGs and autologous lupus neutrophils expressed higher levels of IFN-α mRNA upon stimulation with G-CSF. Since these cells could synthesize other type I IFNs besides IFN-α and, to confirm that increased levels of type I IFNs were being synthesized by LDGs, we assessed the capacity of LDGs and neutrophil supernatants to induce type I IFN signatures on an epithelial cell line, using a bioassay reported by Hua et al(20) and previously used by our group with some modifications(21). In this bioassay, an epithelial cell line is exposed to the supernatants of lupus or control cells to assess the induction of type I IFN-inducible genes on the cell line. Supernatants from unstimulated, PMA-activated and Poly(I:C)-transfected LDGs caused a significant induction of type I IFN signatures on epithelial cell lines, when compared to control and autologous lupus neutrophils (Figure 5B, C and E). This was significantly enhanced upon simulation with G-CSF (Figure 5D). Overall, these data indicated that lupus neutrophils synthesize higher amounts of type I IFNs than control neutrophils and this is significantly enhanced in the LDG group. These results were confirmed when normal-density neutrophils were isolated by negative selection after gradient centrifugation (Figure 1 supplementary materials) indicating that the differences observed among groups were not due to the isolation techniques.
Figure 5. Lupus LDGs express increased levels of IFN-α mRNA and their supernatants induce enhanced levels of type I IFN-inducible genes.
A. Results represent mean±SEM of IFN-α mRNA expression, after adjusting for housekeeping gene (HPRT1). Results are from 4 controls and 5 SLE patients. B–D. Results represent fold induction of IFN-inducible genes (mRNA) on epithelial cell lines by neutrophil supernatants and are presented as mean ± SEM fold-induction by supernatants from control neutrophils (n=5), SLE neutrophils or autologous lupus LDGs (n=8 each). Data are normalized to HPRT-1, *p<0.05 when comparing to control neutrophils and **p<0.05 when comparing LDGs to both control and lupus neutrophils.
LDGs alter the balance of endothelial cell damage and repair
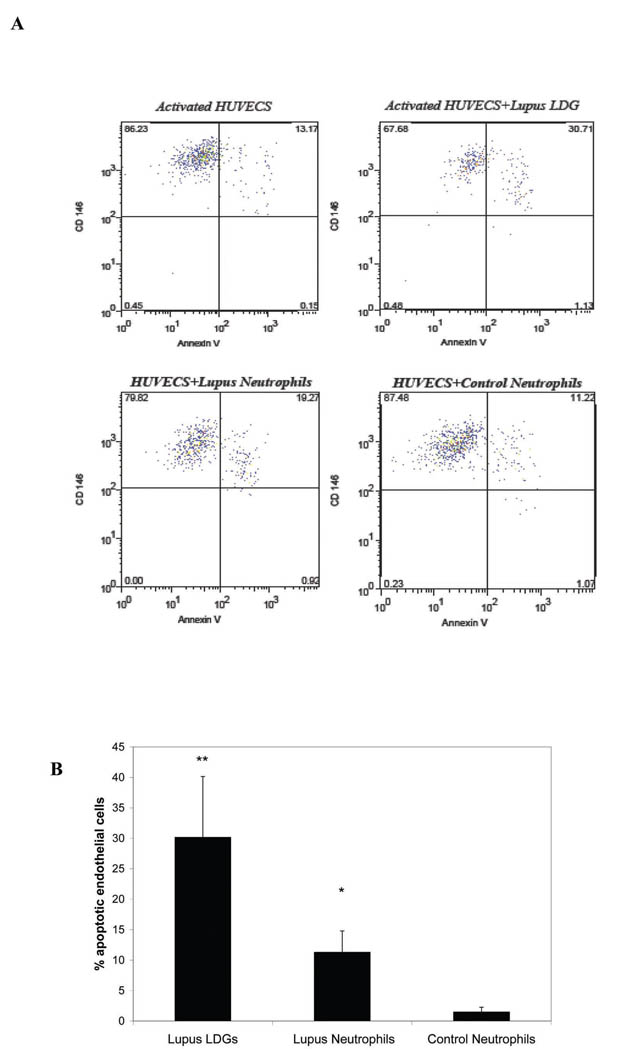
SLE patients develop a striking increase in the risk of premature CV complications due to accelerated atherosclerosis (30). As a potential mechanism leading to enhanced CV damage, our group has shown that SLE patients develop an imbalance between vascular damage and repair, characterized by accelerated endothelial cell apoptosis and abnormal phenotype and function of the cells involved in vascular repair (21, 31). These abnormalities correlate with the development of endothelial dysfunction (31), a predictor of atherosclerosis development in the general population. The mechanisms that induce accelerated endothelial cell apoptosis in SLE remain to be determined. Given the potential role of neutrophils in the induction of damage to the endothelium in other pathologic conditions (32), we examined if LDGs can harm endothelial cells. Lupus neutrophils induced significantly higher levels of cytotoxicity of endothelial cells than control neutrophils (Figure 6). However, the induction of endothelial cytotoxicity was much more marked by LDGs when compared to lupus or control neutrophils. Endothelial cytotoxicity was completely abolished with the use of a transwell, indicating that direct contact between the neutrophil effectors and the endothelial cell target is required to induce cell death. Similar results were obtained when normal-density neutrophils were isolated using negative selection after gradient centrifugation (lupus LDGs 28.2± 5.8; lupus neutrophils 9.1±4.1; control neutrophils 2.5±2.5; results represent % of apoptotic HUVECs after overnight exposure to LDGs, autologous lupus neutrophils or control neutrophils. Results represent mean ±SEM of 6 SLE and 4 controls samples, p<0.05 when comparing lupus neutrophils to control neutrophils and lupus LDGs to lupus neutrophils and p<0.01 when comparing LDGs to control neutrophils. These findings were confirmed using a bioluminescence cytotoxicity assay (not shown).
Figure 6. Lupus LDGs are cytotoxic to the endothelium.
A. Results show representative dot plots of endothelial cell apoptosis induced by LDGs, autologous lupus neutrophils or control neutrophils. Neutrophils representing CD10+ cells are excluded from analysis. CD146+ cells represent endothelial cells and Annexin V + cells are considered apoptotic or early necrotic. Numbers represent % expression in the various quadrants. Apoptotic endothelial cells are CD146+Annexin V+. B. Bar graphs represent % of apoptotic HUVECs after overnight exposure to LDGs, autologous lupus neutrophils or control neutrophils. Results represent mean +SEM of 7 SLE and 9 controls samples, *p<0.05 when comparing lupus neutrophils to control neutrophils and ** p<0.01 when comparing LDGs to autologous lupus neutrophils and to control neutrophils.
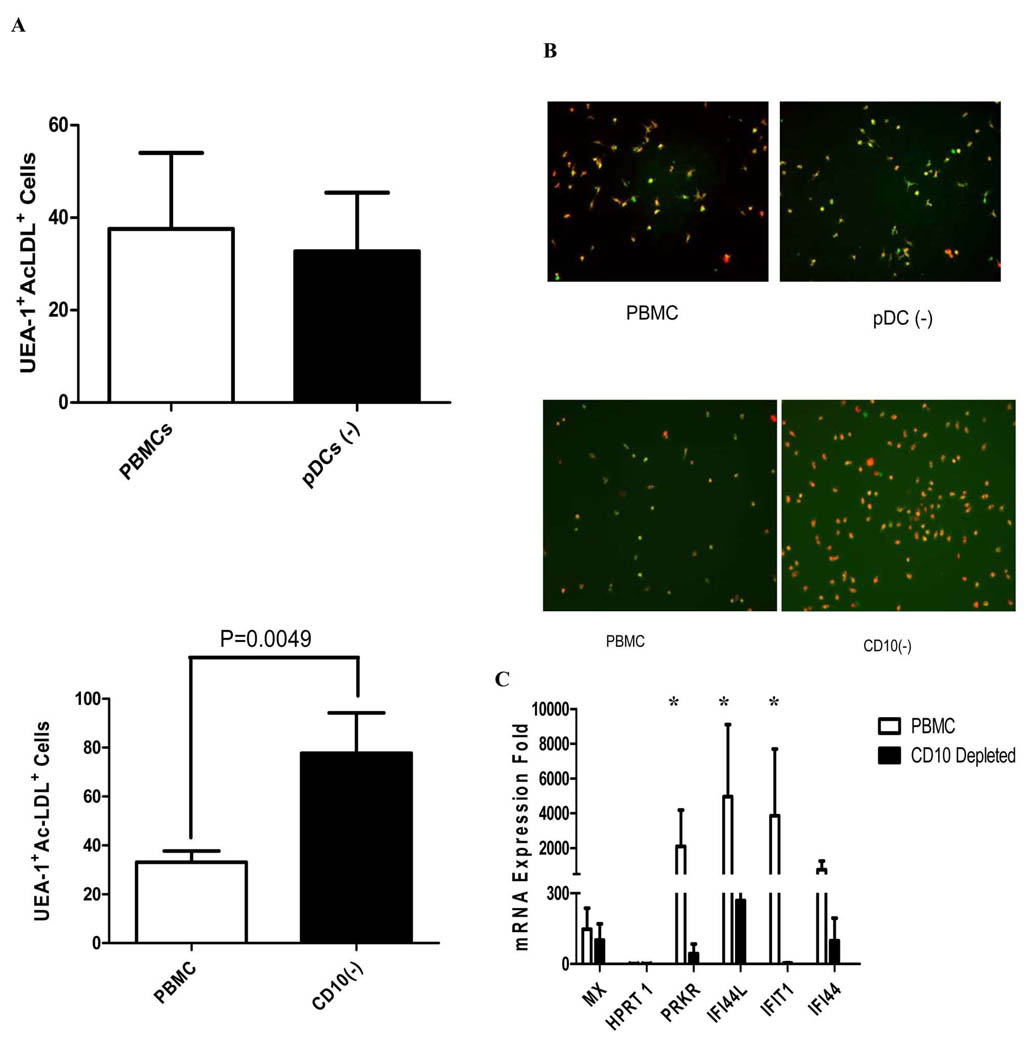
Previously, our group and others showed that SLE patients display abnormal phenotype and function of cells involved in blood vessel repair (EPCs and myeloid CACs) due to the antiangiogenic effects of IFN-α and, potentially, other type I IFNs (21, 33). Among various abnormalities, we previously reported that lupus PBMCs containing EPCs/CACs that are plated under proangiogenic stimulation, strikingly fail to form a mature endothelial cell monolayer after 7–14 days in culture (21). This is in contrast with healthy control PBMCs which typically differentiate into mature endothelial cells during proangiogenic stimulation. Given that LDGs contaminate lupus PBMCs and synthesize increased levels of IFN-α, we assessed if LDG depletion from EPC/CAC containing-PBMCs prior to plating of proangiogenic cultures would promote the normal phenotype and capacity to form an endothelial cell monolayer. In contrast to pDC depletion from lupus PBMC preparations, which did not restore the functional capacity of PBMCs to differentiate into mature endothelial cells and did not decrease type I IFN synthesis, LDG depletion led to a striking restoration of the normal capacity of the PBMCs to differentiate into mature endothelial cells and a pronounced decrease in type I IFN synthesis (Figure 7 A–C). These results indicate that LDGs contribute to type I IFN production in the proangiogenic cultures and appear to be an important factor that leads to the aberrant phenotype and function of EPCs/CACs in this disease.
Figure 7. LDG depletion improves the capacity of EPCs/CACs to become mature endothelial cells by abrogating type I IFN activity.
Lupus PBMCs were cultured under proangiogenic conditions and incubated at different time-points during culture with diI–acetylated LDL and FITC–UEA-1. Mature endothelial cells were identified by co-expression of these markers. A. Bar graphs represent number of mature endothelial cells per power field at day 7, when comparing initial plating of unfractionated lupus PBMCs versus PBMCs depleted of pDCs (pDC(−)) (top) or LDGs (CD10 depletion, CD10(−)) (bottom). Results are mean± SEM of 4 independent experiments for pDC depletion and 6 independent experiments for LDG depletion; p=NS for pDC depletions and p=0.0049 for LDG depletion. B. Results are representative images of mature endothelial cells obtained after 7 days in culture, when comparing initial plating of lupus unfractionated PBMCs versus PBMCs depleted of either pDCs (top) or LDGs (bottom) exposed to proangiogenic stimulation. Lupus CD10 depletion, but not pDC depletion, resulted in enhanced numbers of mature endothelial cells. C. Bar graphs represent the capacity of supernatants obtained at day 7 from lupus unfractionated PBMCs, PBMCs depleted of either pDCs or LDGs (all cultured under proangiogenic stimulation) to induce type I IFN-inducible genes in epithelial cell lines. Results are mean± SEM of 4 independent experiments for pDC depletion and 6 independent experiments for LDG depletion; *p<0.05 when comparing type I IFN induction between total PBMCs and CD10-depleted PBMC cultures.
DISCUSSION
Despite considerable advances identifying the importance of adaptive immunity in the pathogenesis of lupus, the study of innate immune responses in SLE was neglected for many years. In recent years, however, experimental evidence has indicated that lupus patients also have significant disruptions in innate immunity (2, 6). The role of neutrophils in the pathogenesis of SLE was proposed decades ago, particularly their potential role in nephritis (7); however, the exact role that these cells play in the pathogenesis of autoimmune responses and organ damage in this disease has been incompletely characterized.
Over the last couple of decades, there have been two studies that reported the presence of an abnormal subset of neutrophils in the peripheral circulation of SLE patients. The results of these studies suggest that these cells are aberrant in SLE. In the first study, Hacbarth et al reported that Ficoll-Hypaque density gradient preparations of PBMCs from adult patients with SLE were highly contaminated with low buoyant density neutrophils. These neutrophils were proposed to be activated and activation was considered secondary to a plasma effect of an uncharacterized molecule(11). In the second study by Bennett et al, gene array analysis of PBMCs from pediatric lupus patients revealed increased expression of genes related to the development and function of granulocytes, which was not associated to medication use(2). This "granulocyte signature" present in lupus PBMCs was confirmed by the presence of highly granular cells in the mononuclear cell subset which covered all stages of granulocyte development, including pro-, myelo- and meta-myelocytes, bands and segmented neutrophils, similar to what we are now reporting in adult SLE. Interestingly, the “granulocyte signature” in the pediatric lupus PBMCs was coincident with an “IFN-signature”. While not usually considered IFN-α-producing cells, mature neutrophils are capable of producing IFN-α in response to certain stimuli (29). Thus, the co-association of the granulocyte and IFN signatures could have indicated neutrophil-derived IFN-α expression and/or IFN-α-mediated inhibition of neutrophil maturation (34–36). From our study in adult SLE it appears that LDGs, and to a lesser extent normal-density lupus neutrophils, are indeed capable of synthesizing and secreting higher amounts of IFN-α (and potentially other type I IFNs) and that LDGs in fact account for significant type I IFN activity in lupus PBMCs.
Several studies have implicated a key role for IFN-α in SLE pathogenesis, both in disease initiation and the development of flares and organ severity in human and murine systems (6, 37–41). Although the exact source of increased IFN-α in SLE had not been completely characterized, depletion experiments have demonstrated that pDCs contribute only part of the IFN-α (6, 42, 43). The expression of Fcγreceptors on the surface of LDGs suggests that anti-DNA immune complexes, present in the blood of patients with SLE, could be delivered to intracellular TLR9 via Fcγ-mediated activation and internalization, as described for DCs (44–46). Although a previous study suggests that neutrophils are not responsive to TLR9 agonists alone (47), this may reflect the inability of the ligands to be properly internalized (48–50). Thus, TLR9 ligands delivered to the receptor as part of immune complexes present in lupus serum (51) could be an additional source that would stimulate LDGs to synthesize IFN-α. A recent report indicates that neutrophils are able to activate type I IFN responses via helicase recognition, as Poly(I:C)-transfected human neutrophils express elevated mRNA levels of various type I IFNs as well as IFN-responsive genes (18). Alternatively, mature neutrophils are known to produce IFN-α in response to engagement of the GCSF receptor (29). LDGs express GCSF-R and type I IFN production by neutrophils was indeed enhanced by GCSF and Poly (I:C), particularly in the lupus LDGs. It is possible that IFN-α synthesis by LDGs and neutrophils may promote its own expression. Both GCSF and IFN-α utilize the Jak/Stat signaling pathways to induce gene expression(52–54). Thus, IFN-α production by GCSF-stimulated neutrophils may promote additional IFN-α expression through a positive feedback signaling mechanism.
Neutrophils are central effector cells in the mediation of the host’s nonspecific response to inflammation, which usually results in the successful sequestration of inflammatory lesions. However, during accelerated or unchecked inflammation, the recruitment and activation of neutrophils can lead to the development of disease states with significant tissue damage. Damage is mediated by ROS as well as by the release of proinflammatory cytokines and eicoisanoids. While neutrophils are generally not thought to be an important source of de novo synthesis of polypeptide mediators, recent progress has shown that they are able to synthesize cytokines in response to a variety of inflammatory stimuli and during certain pathological conditions (55). The expression profiles of neutrophil-derived cytokines are similar with those of monocytes/macrophages, including the proinflammatory cytokines TNF-α and IL-1β (56, 57), IFN-γ(58–60) and CC and CXC chemokines (including IL-8 and MIP-1α) (57, 61). As such, inhibition of neutrophil-derived cytokines is viewed as a potentially useful strategy for therapeutic immunointervention. In our study, while ROS and eicosanoid production was not enhanced in LDGs when compared to control or autologous lupus neutrophils, these cells present a proinflammatory phenotype characterized by augmented TNF-α, IFN-α and IFN-γ secretion which may promote and enhance tissue damage. While the beneficial versus deleterious role of TNF-α in SLE remains a matter of discussion, animal models show that this molecule can be harmful in murine lupus (62, 63) and TNF-α blockade in MRL/lpr mice and other lupus murine models has been proven to decrease disease severity(64, 65). TNF-α is overexpressed in human lupus nephritis(66–68) and refractory cutaneous disease (69) and this increase is associated with worsening kidney histological activity (70). Whether LDGs represent an important source of this cytokine in blood or specific tissues in which TNF-α may have deleterious effects remains to be determined. With regards to IFN-γ, levels of this cytokine in serum and in tissue have been found to be elevated in SLE and correlate with the development of nephritis (71, 72) and with overproduction of autoAbs (73). Taken together, our results indicate that LDGs secrete enhanced levels of proinflammatory cytokines that have been reported to potentially play an important role in tissue damage in SLE.
Aberrant apoptotic cell death, phagocytic uptake, and their interplay may induce autoantibody production and autoimmunity (74). Neutrophil phagocytosis and chemotaxis have been previously reported to be impaired in SLE patients (75) with different mechanisms proposed including autoAbs and serum cytokines (76–78). We confirmed a trend for decreased phagocytosis of bacteria in normal-density lupus neutrophils, although there was no significant difference when compared to control neutrophils. In contrast, LDGs display a profound decrease in their capacity to phagocytose bacteria. These results may indicate a potential deficient ability for the clearance of infectious agents and increased predisposition for infections in SLE. Indeed, patients with this disease have a higher infection rate than the general population and episodes of bacteremia are associated with an unfavorable long-term outcome in this patient population(79). Whether this increased predisposition and poor outcome to infections is mainly secondary to immunosuppressive drugs or to abnormalities in bacterial phagocytosis/clearance secondary to the disease remains to be determined.
Patients with SLE have a strikingly higher risk of developing CV complications when compared to age- and gender-matched controls (30, 80). Our group and others have proposed that this is due to a strong imbalance between vascular damage (endothelial cell apoptosis) and repair (by EPCs, CACs and other cells crucial for vasculogenesis)(21, 31, 81). We and others have proposed that type I IFNs play a crucial role in the induction of aberrant vascular repair as neutralization of IFN-α or type I IFN receptor leads to abrogation of the abnormal capacity of lupus EPCs/CACs to become mature endothelial cells(21, 33). Further, IFN-α is clearly cytotoxic to EPCs(21). Other groups have previously shown that pDCs are not the primary source of enhanced IFN-α synthesis in SLE but the precise subset(s) involved in humans with this disease remain unclear (6, 82). We have now shown that it is the LDGs, and not pDCs, which appear to induce the enhanced IFN-α production that leads to abnormal EPC/CAC function in vitro and, potentially, in vivo in SLE. This and the observation that LDGs are cytotoxic to the endothelium suggest that this neutrophil subset may play an important role in the induction of premature vascular damage in SLE. Indeed, in ischemic heart disease, apoptosis of endothelial cells and aberrant vascular repair have been shown to contribute to disease progression and events (83–85). Therefore, future strategies aimed at characterizing the origin of these cells and therapeutic mechanisms to deplete them are warranted.
The mechanisms by which LDGs and lupus neutrophils in general induce enhanced damage of endothelial cells remains to be fully characterized and is likely to be multifactorial, given the proinflammatory profile of these cells. Neutrophils can directly cause damage to vascular endothelium through a variety of mechanisms (9, 86–89). This typically does not occur when neutrophils are suspended in the bloodstream but rather when they are adherent to endothelium or are in contact with extracellular matrix proteins in the interstitium. Thus, adhesion of neutrophils is crucial in inflammatory injury and it is possible that enhanced proinflammatory cytokine synthesis as well as other yet unidentified molecules could play a role in enhancing LDG adherence to endothelium and promoting enhanced cytotoxicity (89). Indeed, endothelial cell death induced by LDGs was blunted when their contact with endothelial cells was eliminated with a transwell. Further, high LDG levels correlated with vascular inflammation in SLE patients, which further indicates that these cells may contribute to aspects of lupus related to vascular damage or inflammation.
The origin of the LDGs remains unclear. While the cells display some phenotypic properties of activated neutrophils, they do not differ in their activation status from autologous lupus neutrophils and they express comparable levels of MPO and generate equivalent levels of ROS when compared to control and lupus neutrophils. These observations indicate that these cells do not represent a population of in vivo-activated and degranulated lupus neutrophils. Further, the nuclear morphology indicates potential disruptions in their development and the presence of more immature forms. GM-CSF secreting PBMCs have been identified in SLE patients and GMCSF levels have been reported to be elevated in SLE (90); it is therefore possible that this cytokine could play a role in accelerated mobilization of neutrophil precursors from the bone marrow (91). This seeming contradiction in assessment of development state of LDGs depending upon surface molecule expression and nuclear morphology may also be indicative of disruptions in neutrophil development in SLE, possibly due to the effects of type I IFNs and/or other yet unidentified mechanisms. Overall, it appears that LDGs differ from their autologous normal-density neutrophils in that they have a different nuclear morphology, show strikingly diminished phagocytic potential, enhanced capacity to kill endothelial cells and significant increases in TNF-α and IFN-α synthesis upon stimulation. However, more studies are needed to assess whether LDGs represent a distinct population of lupus neutrophils with specific disruptions in neutrophil development, or merely a more aberrant/activated subset within the spectrum of polymorphonuclear cells present in patients with this disease. Similarly, it is unclear what the significance is of the trend observed for decreased CD10 expression in LDGs, as assessed by mean fluorescent intensity by FACS, when compared to control or autologous lupus neutrophils. CD10 is a cell surface enzyme with neutral metalloendopeptidase activity that cleaves and inactivates multiple proinflammatory and vasoactive molecules(92) and whose expression is directly related to neutrophil mean age(93). There is evidence that neutrophil CD10 expression decreases significantly in response to in vivo inflammatory challenges (94). Importantly, CD10 is only expressed by segmented neutrophils and not by earlier myeloid progenitors. While a CD10−/CD16low phenotype has been proposed to identify greater numbers of phenotypically immature neutrophils than does cellular morphology alone(93), the vast majority of LDGs expressed CD10, even if maybe at lower levels than the other neutrophils subsets. Nevertheless, this observation further suggests that potential abnormalities in neutrophil development may be involved in the generation of LDGs.
Our results also indicate that the differences observed between LDGs and normal-density neutrophils are not due to the isolation technique, as similar results were observed when two different isolation methods were used to obtain the normal-density cells. This is in consensus with previous studied that indicate that magnetic bead isolation techniques do not lead to changes in neutrophil activation when compared to other isolation methods(95).
Future studies should also investigate if these aberrant cells are also present in individuals with other systemic autoimmune diseases (without representing mere activated degranulated cells). Over the last few years, evidence has accumulated indicating that increased type I IFN signatures are present in patients with other systemic autoimmune diseases including primary Sjögren syndrome (96, 97), progressive systemic sclerosis (98, 99), psoriasis (100, 101) and inflammatory myopathies (102). Interestingly, all these conditions are associated to an increased CV risk (103–106). It will therefore be important to assess the role of neutrophils in enhanced type I IFN activity and the role of these cells in vascular damage in these diseases.
Overall, we have characterized in detail the phenotype of a low-density neutrophil subset which appears to be present in higher numbers in SLE patients with distinct clinical manifestations. LDGs have preserved neutrophil function overall but display impairments in phagocytic potential, have a proinflammatory phenotype and induce vascular damage, suggesting that they may contribute to the accelerated atherosclerosis observed in SLE patients. The potential role of these cells in lupus pathogenesis (in part mediated by enhanced type I IFN synthesis) and on organ damage warrants further investigation.
Supplementary Material
Acknowledgments
We thank Caroline Mohai and Jennifer Johnson for help with patient recruitment. We also thank Dr. David Markovitz and Dr. Philip Cohen for critical review of the manuscript and Dr. Nirit Mor-Vaknin for helpful discussions.
Non-standard abbreviations
- CV
Cardiovascular
- CACs
circulating angiogenic cells
- DCs
dendritic cells
- diI
1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate
- EPCs
endothelial progenitor cells
- H2O2
hydrogen peroxide
- LDGs
low density granulocytes
- MPO
myeloperoxidase
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- UEA-1
Ulex europaeus agglutinin-1
Footnotes
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
GRANT SUPPORT
This work was supported by an Innovative Research Grant (IRG) from the Arthritis Foundation, a grant from the Lupus Foundation of America, a Collaborative Grant from the Michigan Institute from Clinical and Health Research (MICHR)/CTSA (PHS grant UL1RR024986); PHS grant HL088419 and by the Anthony S. Gramer Fund in Inflammation Research (all to MJK). This work was also supported (in part) by the Carol and Herb Amster Lupus Research Fund and the Michael and Marcia Klein Lupus Research Fund (to WJM) and by NIH through the University of Michigan's Cancer Center Support Grant (P30 CA46592) and the Rheumatic Disease Core Center Grant (P30 AR48310).
The authors have no conflicting financial interests.
REFERENCES
- 1.Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2005;17:518–522. doi: 10.1097/01.bor.0000170479.01451.ab. [DOI] [PubMed] [Google Scholar]
- 2.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, Lee KD, Gavalchin J, Kaplan MJ. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J Immunol. 2006;176:2095–2104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan MJ, Lewis EE, Shelden EA, Somers E, Pavlic R, McCune WJ, Richardson BC. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–6029. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 6.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 7.Camussi G, Cappio FC, Messina M, Coppo R, Stratta P, Vercellone A. The polymorphonuclear neutrophil (PMN) immunohistological technique: detection of immune complexes bound to the PMN membrane in acute poststreptococcal and lupus nephritis. Clin Nephrol. 1980;14:280–287. [PubMed] [Google Scholar]
- 8.Malech HL. The role of neutrophils in the immune system: an overview. Methods in molecular biology (Clifton, N.J. 2007;412:3–11. doi: 10.1007/978-1-59745-467-4_1. [DOI] [PubMed] [Google Scholar]
- 9.Weiss SJ, Young J, LoBuglio AF, Slivka A, Nimeh NF. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981;68:714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzocchi-Machado CM, Alves CM, Azzolini AE, Polizello AC, Carvalho IF, Lucisano-Valim YM. Fcgamma and complement receptors: expression, role and co-operation in mediating the oxidative burst and degranulation of neutrophils of Brazilian systemic lupus erythematosus patients. Lupus. 2002;11:240–248. doi: 10.1191/0961203302lu172oa. [DOI] [PubMed] [Google Scholar]
- 11.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, Nauseef WM. Isolation and Functional Analysis of neutrophils. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0723s19. 7.23. [DOI] [PubMed] [Google Scholar]
- 15.Allport JR, Ding HT, Ager A, Steeber DA, Tedder TF, Luscinskas FW. L-selectin shedding does not regulate human neutrophil attachment, rolling, or transmigration across human vascular endothelium in vitro. J Immunol. 1997;158:4365–4372. [PubMed] [Google Scholar]
- 16.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker BA, Hagenlocker BE, Stubbs EB, Jr, Sandborg RR, Agranoff BW, Ward PA. Signal transduction events and Fc gamma R engagement in human neutrophils stimulated with immune complexes. J Immunol. 1991;146:735–741. [PubMed] [Google Scholar]
- 18.Tamassia N, Le Moigne V, Rossato M, Donini M, McCartney S, Calzetti F, Colonna M, Bazzoni F, Cassatella MA. Activation of an immunoregulatory and antiviral gene expression program in poly(I:C)-transfected human neutrophils. J Immunol. 2008;181:6563–6573. doi: 10.4049/jimmunol.181.9.6563. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JL, Ellis BA, Munafo DB, Brzezinska AA, Catz SD. Gene transfer and expression in human neutrophils. The phox homology domain of p47phox translocates to the plasma membrane but not to the membrane of mature phagosomes. BMC immunology. 2006;7:28. doi: 10.1186/1471-2172-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 21.Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, McCune WJ, Kaplan MJ. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto TK, Kahn J, Migaki G, Mainolfi E, Shirley F, Ingraham R, Rothlein R. Regulation of L-selectin expression by membrane proximal proteolysis. Agents Actions Suppl. 1995;47:121–134. doi: 10.1007/978-3-0348-7343-7_11. [DOI] [PubMed] [Google Scholar]
- 25.Brown EJ. Complement receptors, adhesion, and phagocytosis. Infect Agents Dis. 1992;1:63–70. [PubMed] [Google Scholar]
- 26.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. The American review of respiratory disease. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 27.Wertheim WA, Kunkel SL, Standiford TJ, Burdick MD, Becker FS, Wilke CA, Gilbert AR, Strieter RM. Regulation of neutrophil-derived IL-8: the role of prostaglandin E2, dexamethasone, and IL-4. J Immunol. 1993;151:2166–2175. [PubMed] [Google Scholar]
- 28.Crow MK, Wohlgemuth J. Microarray analysis of gene expression in lupus. Arthritis Res Ther. 2003;5:279–287. doi: 10.1186/ar1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirafuji N, Matsuda S, Ogura H, Tani K, Kodo H, Ozawa K, Nagata S, Asano S, Takaku F. Granulocyte colony-stimulating factor stimulates human mature neutrophilic granulocytes to produce interferon-alpha. Blood. 1990;75:17–19. [PubMed] [Google Scholar]
- 30.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:338–346. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, Chakrabarti A, Richardson BC, Shelden E, McCune WJ, Kaplan MJ. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 32.Ward PA, Varani J. Mechanisms of neutrophil-mediated killing of endothelial cells. J Leukoc Biol. 1990;48:97–102. doi: 10.1002/jlb.48.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S, Butfiloski EJ, Sobel ES, Reeves WH, Segal MS. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3759–3769. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 34.Verma DS, Spitzer G, Gutterman JU, Zander AR, McCredie KB, Dicke KA. Human leukocyte interferon preparation blocks granulopoietic differentiation. Blood. 1979;54:1423–1427. [PubMed] [Google Scholar]
- 35.Verma DS, Spitzer G, Gutterman JU, Beran M, Zander AR, McCredie KB. Human leukocyte interferon-mediated granulopoietic differentiation arrest and its abrogation by lithium carbonate. Am J Hematol. 1982;12:39–46. doi: 10.1002/ajh.2830120106. [DOI] [PubMed] [Google Scholar]
- 36.Broxmeyer HE, Lu L, Platzer E, Feit C, Juliano L, Rubin BY. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983;131:1300–1305. [PubMed] [Google Scholar]
- 37.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 38.Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003;15:548–556. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Dall'era MC, Cardarelli PM, Preston BT, Witte A, Davis JC., Jr Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis. 2005;64:1692–1697. doi: 10.1136/ard.2004.033753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 41.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 42.Blomberg S, Eloranta ML, Magnusson M, Alm GV, Ronnblom L. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2524–2532. doi: 10.1002/art.11225. [DOI] [PubMed] [Google Scholar]
- 43.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 44.Vallin H, Perers A, Alm GV, Ronnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol. 1999;163:6306–6313. [PubMed] [Google Scholar]
- 45.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 48.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda K, Yu P, Kirschning CJ, Schlatter B, Schmitz F, Heit A, Bauer S, Hochrein H, Wagner H. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol. 2005;174:6129–6136. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 50.Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, Schettini J, Raiden S, Geffner J. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33:3164–3174. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 51.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 52.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur AG, Barbieri G, Witthuhn BA, Schindler C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 53.Numata A, Shimoda K, Kamezaki K, Haro T, Kakumitsu H, Shide K, Kato K, Miyamoto T, Yamashita Y, Oshima Y, Nakajima H, Iwama A, Aoki K, Takase K, Gondo H, Mano H, Harada M. Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway. J Biol Chem. 2005;280:12621–12629. doi: 10.1074/jbc.M408442200. [DOI] [PubMed] [Google Scholar]
- 54.Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, Dummer R, Simon HU, Yousefi S. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279:44123–44132. doi: 10.1074/jbc.M405883200. [DOI] [PubMed] [Google Scholar]
- 55.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 56.Kermarrec N, Selloum S, Plantefeve G, Chosidow D, Paoletti X, Lopez A, Mantz J, Desmonts JM, Gougerot-Pocidalo MA, Chollet-Martin S. Regulation of peritoneal and systemic neutrophil-derived tumor necrosis factor-alpha release in patients with severe peritonitis: role of tumor necrosis factor-alpha converting enzyme cleavage. Critical care medicine. 2005;33:1359–1364. doi: 10.1097/01.ccm.0000166359.47577.57. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura A, Hara Y, Kaneko T, Kato I. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. Journal of periodontal research. 1997;32:279–286. doi: 10.1111/j.1600-0765.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 58.Sohn EJ, Paape MJ, Connor EE, Bannerman DD, Fetterer RH, Peters RR. Bacterial lipopolysaccharide stimulates bovine neutrophil production of TNF-alpha, IL-1beta, IL-12 and IFN-gamma. Veterinary research. 2007;38:809–818. doi: 10.1051/vetres:2007033. [DOI] [PubMed] [Google Scholar]
- 59.Bogdan C, Schleicher U. Production of interferon-gamma by myeloid cells--fact or fancy? Trends Immunol. 2006;27:282–290. doi: 10.1016/j.it.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]
- 61.Ko HJ, Lim SS. Production of macrophage inflammatory protein (MIP)-1alpha and MIP-1beta by human polymorphonuclear neutrophils stimulated with Porphyromonas endodontalis lipopolysaccharide. Journal of endodontics. 2002;28:754–757. doi: 10.1097/00004770-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–3054. [PubMed] [Google Scholar]
- 63.Yokoyama H, Kreft B, Kelley VR. Biphasic increase in circulating and renal TNF-alpha in MRL-lpr mice with differing regulatory mechanisms. Kidney Int. 1995;47:122–130. doi: 10.1038/ki.1995.14. [DOI] [PubMed] [Google Scholar]
- 64.Su X, Zhou T, Yang P, Edwards CK, 3rd, Mountz JD. Reduction of arthritis and pneumonitis in motheaten mice by soluble tumor necrosis factor receptor. Arthritis Rheum. 1998;41:139–149. doi: 10.1002/1529-0131(199801)41:1<139::AID-ART17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 65.Edwards CK, 3rd, Zhou T, Zhang J, Baker TJ, De M, Long RE, Borcherding DR, Bowlin TL, Bluethmann H, Mountz JD. Inhibition of superantigen-induced proinflammatory cytokine production and inflammatory arthritis in MRL-lpr/lpr mice by a transcriptional inhibitor of TNF-alpha. J Immunol. 1996;157:1758–1772. [PubMed] [Google Scholar]
- 66.Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067–1074. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- 67.Gabay C, Cakir N, Moral F, Roux-Lombard P, Meyer O, Dayer JM, Vischer T, Yazici H, Guerne PA. Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol. 1997;24:303–308. [PubMed] [Google Scholar]
- 68.Malide D, Russo P, Bendayan M. Presence of tumor necrosis factor alpha and interleukin-6 in renal mesangial cells of lupus nephritis patients. Hum Pathol. 1995;26:558–564. doi: 10.1016/0046-8177(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 69.Zampieri S, Alaibac M, Iaccarino L, Rondinone R, Ghirardello A, Sarzi-Puttini P, Peserico A, Doria A. Tumour necrosis factor alpha is expressed in refractory skin lesions from patients with subacute cutaneous lupus erythematosus. Ann Rheum Dis. 2006;65:545–548. doi: 10.1136/ard.2005.039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aderka D, Wysenbeek A, Engelmann H, Cope AP, Brennan F, Molad Y, Hornik V, Levo Y, Maini RN, Feldmann M, et al. Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthritis Rheum. 1993;36:1111–1120. doi: 10.1002/art.1780360812. [DOI] [PubMed] [Google Scholar]
- 71.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, Fukuda K, Kanai H, Nakashima H, Otsuka T, Hirakata H. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–2160. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 72.Chan RW, Lai FM, Li EK, Tam LS, Chow KM, Lai KB, Li PK, Szeto CC. Intrarenal cytokine gene expression in lupus nephritis. Ann Rheum Dis. 2007;66:886–892. doi: 10.1136/ard.2006.063123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan MJ. Apoptosis in systemic lupus erythematosus. Clin Immunol. 2004;112:210–218. doi: 10.1016/j.clim.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 75.de la Fuente H, Richaud-Patin Y, Jakez-Ocampo J, Gonzalez-Amaro R, Llorente L. Innate immune mechanisms in the pathogenesis of systemic lupus erythematosus (SLE) Immunol Lett. 2001;77:175–180. doi: 10.1016/s0165-2478(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 76.Yu CL, Sun KH, Tsai CY, Wang SR. Inhibitory effects of anticardiolipin antibodies on lymphocyte proliferation and neutrophil phagocytosis. Ann Rheum Dis. 1991;50:903–908. doi: 10.1136/ard.50.12.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu CL, Chang KL, Chiu CC, Chiang BN, Han SH, Wang SR. Defective phagocytosis, decreased tumour necrosis factor-alpha production, and lymphocyte hyporesponsiveness predispose patients with systemic lupus erythematosus to infections. Scand J Rheumatol. 1989;18:97–105. doi: 10.3109/03009748909099924. [DOI] [PubMed] [Google Scholar]
- 78.Biswas D, Mathias A, Dayal R, Aggarwal A, Misra R, Naik S. Presence of antibodies to SSB/La is associated with decreased phagocytic efficiency of neutrophils in patients with systemic lupus erythematosus. Clinical rheumatology. 2008;27:717–722. doi: 10.1007/s10067-007-0776-x. [DOI] [PubMed] [Google Scholar]
- 79.Chen MJ, Tseng HM, Huang YL, Hsu WN, Yeh KW, Wu TL, See LC, Huang JL. Long-term outcome and short-term survival of patients with systemic lupus erythematosus after bacteraemia episodes: 6-yr follow-up. Rheumatology (Oxford) 2008;47:1352–1357. doi: 10.1093/rheumatology/ken196. [DOI] [PubMed] [Google Scholar]
- 80.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 81.Westerweel PE, Luijten RK, Hoefer IE, Koomans HA, Derksen RH, Verhaar MC. Haematopoietic and endothelial progenitor cells are deficient in quiescent Systemic Lupus Erythematosus. Ann Rheum Dis. 2007;66:865–870. doi: 10.1136/ard.2006.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee PY, Weinstein JS, Nacionales DC, Scumpia PO, Li Y, Butfiloski E, van Rooijen N, Moldawer L, Satoh M, Reeves WH. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 84.Valgimigli M, Agnoletti L, Curello S, Comini L, Francolini G, Mastrorilli F, Merli E, Pirani R, Guardigli G, Grigolato PG, Ferrari R. Serum from patients with acute coronary syndromes displays a proapoptotic effect on human endothelial cells: a possible link to pan-coronary syndromes. Circulation. 2003;107:264–270. doi: 10.1161/01.cir.0000045665.57256.86. [DOI] [PubMed] [Google Scholar]
- 85.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26:257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
- 86.Zhao ZQ, Sato H, Williams MW, Fernandez AZ, Vinten-Johansen J. Adenosine A2-receptor activation inhibits neutrophil-mediated injury to coronary endothelium. The American journal of physiology. 1996;271:H1456–H1464. doi: 10.1152/ajpheart.1996.271.4.H1456. [DOI] [PubMed] [Google Scholar]
- 87.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol. 1996;149:1617–1626. [PMC free article] [PubMed] [Google Scholar]
- 88.Wang JH, Redmond HP, Watson RW, Condron C, Bouchier-Hayes D. Induction of heat shock protein 72 prevents neutrophil-mediated human endothelial cell necrosis. Arch Surg. 1995;130:1260–1265. doi: 10.1001/archsurg.1995.01430120014002. [DOI] [PubMed] [Google Scholar]
- 89.Varani J, Ginsburg I, Schuger L, Gibbs DF, Bromberg J, Johnson KJ, Ryan US, Ward PA. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989;135:435–438. [PMC free article] [PubMed] [Google Scholar]
- 90.Fiehn C, Wermann M, Pezzutto A, Hufner M, Heilig B. [Plasma GM-CSF concentrations in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropathy] Zeitschrift fur Rheumatologie. 1992;51:121–126. [PubMed] [Google Scholar]
- 91.Willeke P, Schluter B, Schotte H, Erren M, Mickholz E, Domschke W, Gaubitz M. Increased frequency of GM-CSF secreting PBMC in patients with active systemic lupus erythematosus can be reduced by immunoadsorption. Lupus. 2004;13:257–262. doi: 10.1191/0961203304lu1009oa. [DOI] [PubMed] [Google Scholar]
- 92.Connelly JC, Skidgel RA, Schulz WW, Johnson AR, Erdos EG. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985;82:8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orr Y, Wilson DP, Taylor JM, Bannon PG, Geczy C, Davenport MP, Kritharides L. A kinetic model of bone marrow neutrophil production that characterizes late phenotypic maturation. American journal of physiology. 2007;292:R1707–R1716. doi: 10.1152/ajpregu.00627.2006. [DOI] [PubMed] [Google Scholar]
- 94.Kaneko T, Stearns-Kurosawa DJ, Taylor F, Jr, Twigg M, Osaki K, Kinasewitz GT, Peer G, Kurosawa S. Reduced neutrophil CD10 expression in nonhuman primates and humans after in vivo challenge with E. coli or lipopolysaccharide. Shock. 2003;20:130–137. doi: 10.1097/01.shk.0000068326.68761.34. [DOI] [PubMed] [Google Scholar]
- 95.Zahler S, Kowalski C, Brosig A, Kupatt C, Becker BF, Gerlach E. The function of neutrophils isolated by a magnetic antibody cell separation technique is not altered in comparison to a density gradient centrifugation method. J Immunol Methods. 1997;200:173–179. doi: 10.1016/s0022-1759(96)00206-2. [DOI] [PubMed] [Google Scholar]
- 96.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren's syndrome treated with etanercept. Arthritis Rheum. 2007;56:3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes and immunity. 2009;10:285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim D, Peck A, Santer D, Patole P, Schwartz SM, Molitor JA, Arnett FC, Elkon KB. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- 99.Airo P, Ghidini C, Zanotti C, Scarsi M, Cattaneo R, Caimi L, Imberti L. Upregulation of myxovirus-resistance protein A: a possible marker of type I interferon induction in systemic sclerosis. J Rheumatol. 2008;35:2192–2200. doi: 10.3899/jrheum.080418. [DOI] [PubMed] [Google Scholar]
- 100.Eriksen KW, Lovato P, Skov L, Krejsgaard T, Kaltoft K, Geisler C, Odum N. Increased sensitivity to interferon-alpha in psoriatic T cells. J Invest Dermatol. 2005;125:936–944. doi: 10.1111/j.0022-202X.2005.23864.x. [DOI] [PubMed] [Google Scholar]
- 101.Nestle FO, Gilliet M. Defining upstream elements of psoriasis pathogenesis: an emerging role for interferon alpha. J Invest Dermatol. 2005;125:xiv–xv. doi: 10.1111/j.0022-202X.2005.23923.x. [DOI] [PubMed] [Google Scholar]
- 102.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, Beggs AH, Amato AA, Greenberg SA. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56:3784–3792. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, Kaufmann R, Vogl TJ, Boehncke WH. Psoriasis: a possible risk factor for development of coronary artery calcification. The British journal of dermatology. 2007;156:271–276. doi: 10.1111/j.1365-2133.2006.07562.x. [DOI] [PubMed] [Google Scholar]
- 104.Vaudo G, Bocci EB, Shoenfeld Y, Schillaci G, Wu R, Del Papa N, Vitali C, Delle Monache F, Marchesi S, Mannarino E, Gerli R. Precocious intima-media thickening in patients with primary Sjogren's syndrome. Arthritis and rheumatism. 2005;52:3890–3897. doi: 10.1002/art.21475. [DOI] [PubMed] [Google Scholar]
- 105.Hettema ME, Zhang D, de Leeuw K, Stienstra Y, Smit AJ, Kallenberg CG, Bootsma H. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther. 2008;10:R49. doi: 10.1186/ar2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soltesz P, Der H, Kerekes G, Szodoray P, Szucs G, Danko K, Shoenfeld Y, Szegedi G, Szekanecz Z. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clinical rheumatology. 2009;28:655–662. doi: 10.1007/s10067-009-1118-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.