Abstract
The observation that T cells can recognize and specifically eliminate cancer cells has spurred interest in the development of efficient methods to generate large numbers of T cells with specificity for tumor antigens that can be harnessed for use in cancer therapy. Recent studies have demonstrated that during encounter with tumor antigen, the signals delivered to T cells by professional antigen presenting cells can affect T cell programming and their subsequent therapeutic efficacy. This has stimulated efforts to develop artificial antigen presenting cells that allow optimal control over the signals provided to T cells. In this review, we will discuss the advantages and disadvantages of cellular and acellular artificial antigen presenting cell systems and their use in T cell adoptive immunotherapy for cancer.
Keywords: immunotherapy, antigen presenting cell, adoptive immunotherapy, artificial antigen presenting cell
Introduction
T cells provide protective immunity to pathogens, and it is increasingly apparent that T cells specific for antigens expressed on malignant cells can be isolated or engineered from cancer patients, and used to treat established malignancy 1-3. Efforts to amplify the T cell response to tumors in patients have focused primarily on two approaches - in vitro isolation, expansion and adoptive transfer of tumor-reactive T cells and immunization of the patient to elicit or expand tumor-specific T cells in vivo 4-6. Recent work has shown that the signals that T cells receive from antigen presenting cells (APC) during and after their initial encounter with tumor antigens can influence their programming and subsequent therapeutic efficacy 7. The inability to regulate exactly the signals and interactions provided by naturally occurring APC has spurred interest in the use of artificial antigen presenting cells (AAPC) to provide greater control over T cell signaling and facilitate the generation of optimally effective T cells for adoptive immunotherapy.
Effective adoptive T cell immunotherapy of cancer requires the isolation or enrichment of T cells specific for tumor-associated antigens (TAA), and their expansion in vitro or in vivo after transfer to numbers sufficient to mediate a therapeutic effect. Expanded T cells must migrate to tumor sites, mediate effector functions that induce tumor cell death, and ideally establish a reservoir of long-lived memory T cells to provide immune surveillance and prevent relapse. AAPC have been shown to be particularly useful for deriving and expanding T cells for immunotherapy, and one can envision that AAPC systems could be employed to induce desired migration, effector function, and programming of cells to establish durable T cell memory. Here, we review advances in AAPC development that could be exploited to facilitate adoptive T cell therapy of cancer.
Current Approaches For Isolation of Tumor-Reactive T Cells
Naturally occurring CD8+ T cells specific for peptide epitopes in TAA are with the notable exception of malignant melanoma and some EBV-associated malignancies, present at low frequency and their isolation and expansion from the blood or from tumor infiltrates is cumbersome and fraught with technical difficulties. The most successful efforts in cancer immunotherapy have isolated melanoma-reactive T cells from infiltrates of surgically resected tumor samples where such T cells are locally enriched 2,8. Culturing tumor explants in high doses of interleukin 2 (IL-2) alone can result in the outgrowth of tumor-reactive T cells that presumably have received a T cell receptor (TCR) signal from antigen expressed by the tumor cells in the culture 8. This approach is constrained by the requirement for surgically excised tumor, which is not always readily available. Other groups have focused on isolating T cells from the peripheral blood of cancer patients using autologous dendritic cells prepared from monocytes and pulsed with tumor antigen(s) as APC 9. This approach is also often unsuccessful, and depending on the tumor antigen and the mode of antigen delivery to the APC, can yield T cells that express a TCR with low avidity for the TAA and are unable to recognize tumor cells. A novel alternative approach involves the use of MHC-streptamers, which are multimerized MHC/peptide complexes that reversibly bind in an antigen-specific manner to TCRs, and allowing magnetic bead for flow sorting isolation and enrichment of even rare T cells specific for a defined antigen 10. This strategy has many potential advantages including the ability to incorporate other phenotypic markers during cell selection that may be indicative of function, capacity for in vivo persistence, and/or migration. However, MHC streptamers are only available for a few TAA, and this methodology has so far been largely used for isolating T cells specific for viral antigens for immunotherapy in immunodeficient stem cell transplant patients.
Recent improvements in gene transduction technologies have allowed the generation of tumor-reactive T cells by engineering polyclonal T cells to express a TAA-specific TCR or chimeric antigen receptor to impart them with tumor specificity, rather than using techniques that require antigen presentation for enrichment of a desired T cell. However, an important issue to consider in the design of TCR gene transfer for clinical applications is that the introduced TCR α or β chains can mispair with endogenous TCR chains and result in autoreactive specificities and toxicity as recently demonstrated in a murine study 11.
All of the current techniques described above to isolate or engineer tumor-reactive T cells for adoptive therapy have limitations, and this has led to the exploration of AAPC, both for the initial activation of antigen-specific T cells and for their subsequent expansion. The development of AAPC has potential advantages in that these systems can theoretically be designed to optimize and precisely control the delivery of signals required for T cell activation and expansion, including interactions between MHC/peptide complexes and the TCR, and adhesion, costimulatory and cytokine signals. Much of this work has focused on in vitro isolation and expansion of T cells for adoptive therapy, although recent work suggests that AAPC may have applications in vivo for priming or boosting antigen-specific T cell responses.
Cell-Based Artificial APC Systems
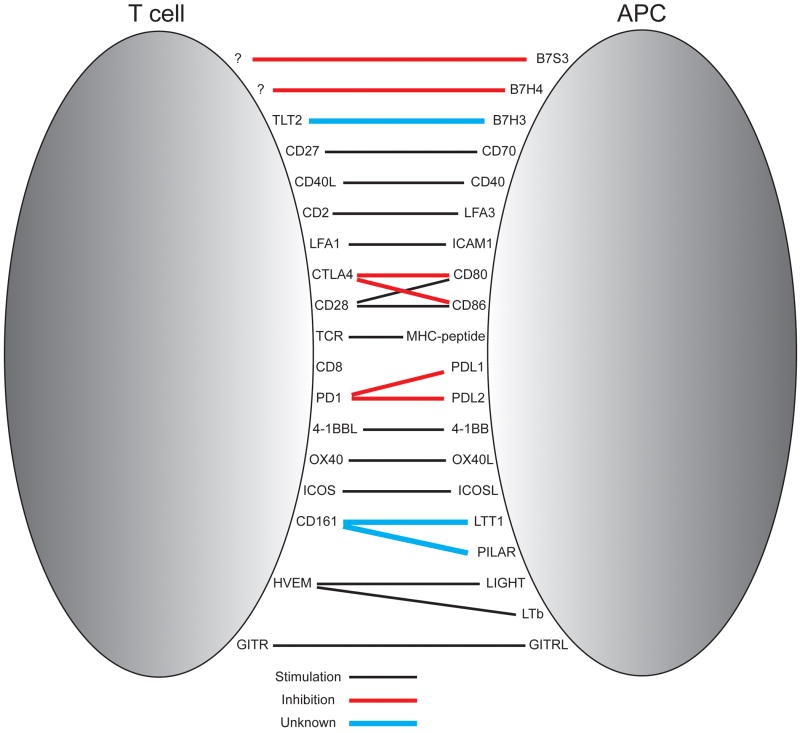
Cellular AAPC have been derived from primary or transformed human or xenogeneic cells that are engineered using retroviral or lentiviral transduction to introduce molecules that provide the necessary TCR, costimulatory, and adhesion events required for immune synapse formation. This strategy can allow stringent control of the delivery of the many positive and negative signals to T cells during their interaction with APC (Figure 1), and cytokines can be introduced into the culture media or produced by transfected AAPC to promote T cell proliferation. One major concern is that cellular AAPC can also present deleterious or negative regulatory signals, although siRNA and zinc finger nucleases could be used to knock down negative regulatory signals and further amplify the T cell stimulatory capacity of AAPC. An advantage of cellular AAPC is that once generated, the cell lines can be qualified and banked and provide a long-term readily accessible source of reagent to use for T cell generation or expansion, without the need to prepare autologous APC or feeder cells that are often required with other T cell culture methods 12.
Figure 1. Ligand-receptor interactions between antigen presenting cells (APC) and T cells.
Interactions that result in costimulation of T cells are depicted in black, and those that result in inhibition of T cells are in red. Interactions depicted in blue indicate situations where the outcome of receptor ligation on T cell function is unknown or controversial.
Allogeneic AAPC
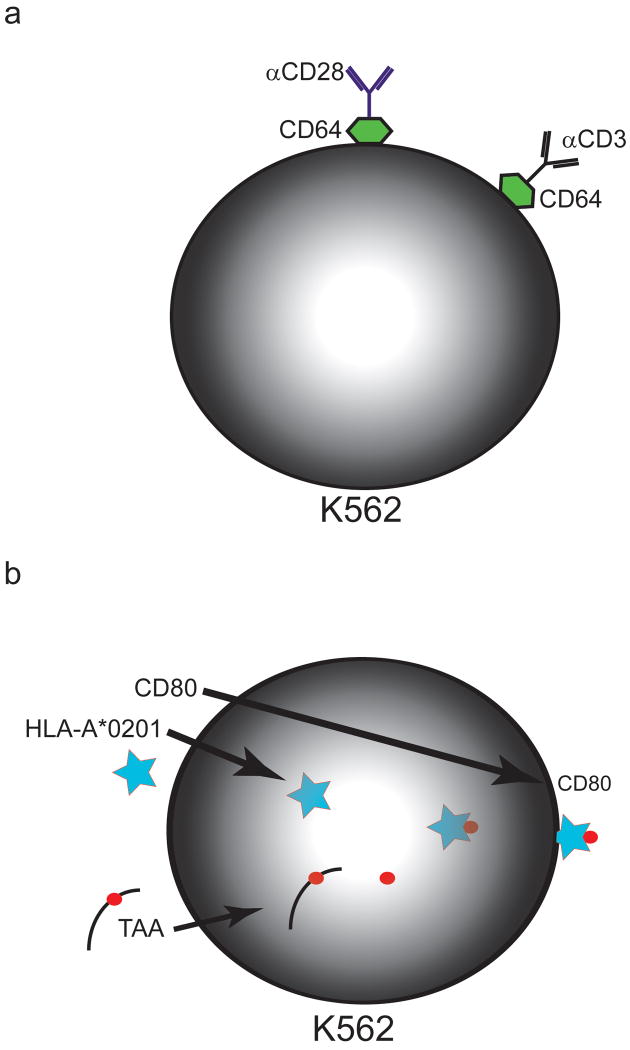
K562 cells are a human erythroleukemia cell line that was derived from a patient with CML in blast crisis, and have been developed as AAPC for both non-specific and specific activation and expansion of human T cells (Figure 2). K562 do not express endogenous HLA A, B or DR molecules, which limits their ability to induce allogeneic T cell proliferation, but do express ICAM-1 and LFA-3 needed to form an effective immune synapse 12-14. When transduced with the human Fc receptors CD32 and CD64, K562 cells can bind and present αCD3 and αCD28 monoclonal antibodies, allowing them to induce non-specific proliferation of T cells 14,15. This approach could be used to expand polyclonal T cells selected by other means for antigen specificity, thus generating an antigen-specific T cell product for immunotherapy 10,16,17. The T cell proliferation induced with K562 AAPC is robust despite their expression of the negative regulatory molecules, PD-L1, PDL2 and B7-H3. K562 cells have been transduced with a variety of costimulatory molecules to further augment signaling, including CD40, CD40L, CD70, CD80, CD83, CD86, ICOSL, GITRL, 4-1BBL and OX40L, which has facilitated dissection of the contributions of each of these molecules to T cell proliferation, and their potential utility in ex vivo CD8+ T cell expansion 13,18. For example, CD8+ T cells stimulated with K562 cells coated with αCD3 mAb and transduced to express 4-1BBL proliferated better and maintained better viability in culture compared to those stimulated with K562 coated with αCD3/αCD28 or αCD3/αCD28 beads 15. K562 cells also express surface IL-15Rα and secrete IL-15, which might contribute to their ability to maintain viability of CD8+ T cells in long term culture, compared to bead-based APCs 18.
Figure 2. Examples of cellular artificial APC systems.
A) K562 erythroleukemia cells can be transduced with the human Fcγ receptor, CD64, to allow Fc binding of antibodies to CD3 and CD28. Other costimulatory signals could be delivered alone or in concert with CD28 by adding specific antibodies.
B) K562 erythroleukemia cells can be transduced with HLA-A*0201 and a TAA. Proteosomal processing of the expressed TAA results in presentation of TAA-derived peptide antigens in conjunction with the transduced HLA-A*0201. Costimulation can be provided by transduction with CD80 (or other costimulatory molecules as desired).
In more recent work, K562 have been transduced with HLA-A*0201 and HLA-DR*0401 to allow presentation of exogenously loaded peptide antigens, or endogenously processed antigens introduced by transfection 13,18,19. Preliminary studies have shown that CD8+ T cells specific for HLA-A*0201-restricted epitopes from Mart-1 and influenza matrix protein (FMP) could be generated after stimulation of CD8+ T cells with K562 cells transduced with HLA-A*0201, CD80, CD83 and Mart-1- or FMP-expressing minigenes 20. K562 cells transduced to express a truncated CD19 molecule have also been used to expand CD8+ T cells that were modified to express a CD19 chimeric antigen receptor that consists of a single chain Fv (scFv) specific for CD19 linked to T cell receptor signaling molecules. The K562 AAPC were more effective in inducing T cell growth than an OKT3-based rapid expansion protocol that used large numbers of CD19+ LCL and PBMC as feeder cells, which may indicate the need to have control over the signal strength delivered to T cells 21.
Other allogeneic tumor cell lines have also been transduced with HLA and costimulatory molecules, or with cytokines such as GM-CSF or IL-12, and investigated as AAPC to present uncharacterized TAA 22,23. This approach has been investigated in detail as a strategy for vaccination in murine models to correct deficiencies in TAA presentation by tumor cells or to amplify cross presentation of TAA by DC, and has been reviewed in detail elsewhere 22-26.
Xenogeneic AAPC
One of the earliest cell based AAPC systems used Drosophila melanogaster cells that were transduced to stably express murine or human MHC and costimulatory molecules, exogenously loaded with peptide TAA, and used to induce proliferation of murine and human cells 27-29. Drosophila based AAPC do not process and present endogenous antigens on the introduced MHC molecules, which limits their use for studies in which processing of antigen is required such as the expansion of uncharacterized TAA-reactive T cells for adoptive immunotherapy 27,28. However, the inability of Drosophila cells to process endogenous antigens protects against the induction of xenoreactivity. The Drosophila AAPC system was used to derive and expand melanoma-reactive T cells for a clinical study of adoptive T cell therapy in melanoma patients 29. Drosophila-derived artificial APC transduced with HLA-A*0201 and pulsed with a tyrosinase peptide were used to expand tyrosinase specific CD8+ T cells to up to 5×108 cells for infusion into melanoma patients. Modest clinical responses and no evidence of clinically significant xenoreactivity were noted 29. However, there are significant drawbacks with Drosophila APCs, particularly their inability to survive in culture at 37°C and to stimulate CD8+ T cell proliferation in the absence of PBMC feeder support 28,29.
Murine NIH/3T3 fibroblasts transduced to express MHC and costimulatory molecules have been used to study T cell-APC interactions in mice and humans, and appear to be more promising candidates than Drosophila AAPC for expanding human CD8+ T cells for adoptive immunotherapy 14,30. Unlike Drosophila AAPC, NIH/3T3 cells present endogenously processed antigens, and can therefore stimulate CD8+ T cell responses to both exogenously loaded and transfected antigen 14,30-32. NIH/3T3 have been transduced with a gene encoding a viral or tumor antigen and with human HLA-A2, CD80, ICAM-1, and LFA-3, and these AAPC successfully stimulated T cells specific for influenza, CMV, and for the hTERT and WT1 TAA. Moreover, TAA-reactive CD8+ T cells expanded ∼1 × 105 fold after five in vitro stimulations with NIH/3T3 AAPC 14,31,32. Similarly, NIH/3T3 AAPC that were transduced to express CD80, 4-1BBL and cell surface PSMA were effective in expanding human T cells transduced to express a PSMA-specific chimeric antigen receptor, and the resulting T cells were effective in treating PSMA-positive tumors in a humanized mouse model 33. In comparative studies NIH/3T3 were more efficient than Drosophila AAPC, and as efficient as autologous adherent PBMC and EBV-transformed B cells for CD8+ T cell expansion, without the requirement for adding feeder cells to the culture system 31. To broaden applicability, NIH/3T3 cells have been transduced to express a number of human HLA molecules to provide a panel of AAPC that can be used to derive T cells for immunotherapy depending on the HLA type of the patient 31,34.
Synthetic or Exosomal Artificial APC Systems
The concept of utilizing AAPC for in vitro T cell expansion stemmed from the initial observation that T cells could be induced to proliferate independently of cognate antigen by stimulation with mitogenic αCD3 to provide a TCR signal, and αCD28 to provide costimulation. CD8+ T cell proliferation induced by αCD3 and αCD28 required stabilization of the Fc portion of each mAb, either by Fc receptors or by immobilization onto solid phase surfaces, such as tissue culture plates. Although robust proliferation could be achieved after culture of T cells with αCD3 and αCD28 coated plates, the interaction between T cell and the synthetic surface did not closely mimic the events occurring in a natural immune synapse, which was a perceived disadvantage when compared with cell based AAPC systems. However, efforts to improve the dynamics of acellular systems have included the development of bead-based AAPC, and the incorporation of ligands into liposomes and supported planar membrane structures 35-38.
Polystyrene beads as artificial APC for non-specific T cell expansion
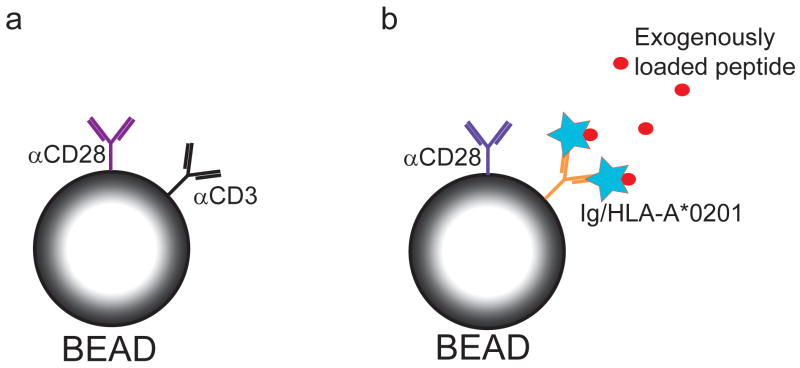
The development of mono-disperse spherical polymer beads to which protein could be non-specifically bound heralded a new era in AAPC technology 37. Polystyrene beads between 5-6 μm in diameter, manufactured under stringent conditions that resulted in uniformity of size and composition, were found to be the optimal size for stimulation of T cells compared to those of smaller dimensions; and once coated with αCD3 allowed the delivery of an antigen-independent signal to polyclonal T cells in a format considered to more closely approximate an immune synapse than solid phase planar systems. In addition to stimulation of the TCR signaling pathway through CD3, costimulatory or inhibitory signals can be provided by covalently binding agonistic or antagonistic ligands and/or antibodies to the beads (Figure 3A). Latex beads can be manufactured to allow delivery of defined signals, enabling excellent control of delivered signals compared to cell-based AAPC 39,40. This approach has successfully been exploited in multiple studies to investigate the signals involved in the T cell response to TCR ligation and to expand T cells for in vitro and in vivo use in patients 41,42.
Figure 3. Examples of bead-based artificial APC systems.
A) Antibodies to CD3 and CD28 are covalently bound to polystyrene beads to provide antigen independent T cell stimulation. B) Antigen specificity can be imparted to polystyrene beads by binding HLA-Ig fusion proteins, which can then be exogenously loaded with peptide antigen.
Clinical studies of adoptive immunotherapy have used αCD3/αCD28 paramagnetic bead-based AAPC to expand autologous CD3+ T cells, which were then infused into individuals with non-Hodgkin's lymphoma or CML after autologous CD34+ selected hematopoietic stem cell transplantation (HSCT) 43,44. The beads were efficiently removed by magnetic depletion before infusion. Robust in vitro T cell expansion was observed and although antitumor responses were noted in both studies, it was difficult to determine the contribution of the infused T cells because the patients also received cytotoxic conditioning. CD3+ T cells from an allogeneic stem cell transplant donor have also been expanded with αCD3/αCD28 beads and infused in doses of 1 × 106 – 1 × 108 CD3+ cells/kg to post transplant patients that had relapsed with hematologic malignancies after allogeneic transplant 45. The CD4:CD8 T cell ratio remained constant during the expansion and a mean of 113 +/- 26.3 fold expansion was achieved during a 12 day culture. Clinical responses were encouraging but could not be directly ascribed to the activated donor leukocyte infusions (DLI) as all patients also received conventional DLI and many received chemotherapy prior to DLI.
An interesting clinical application utilized αCD3/αCD28 beads for expanding T cells from leukapheresis products prepared from multiple myeloma patients shortly after immunization against Streptococcus pneumoniae, followed by infusion of the expanded cells early after autologous HSCT 46,47. The procedure resulted in rapid CD4+ and CD8+ T cell recovery after HSCT and augmented specific immunity to pneumococcal antigen. However, significant toxicity was noted as cell infusion early after HSCT resulted in a syndrome similar to autologous graft versus host disease, with more extensive tissue injury appearing with earlier T cell infusion 46,47. It is possible that the autoimmunity was due to T cell infusion in a lymphopenic setting, although a contribution of the bead expansion to altering the threshold for T cell signaling to self-antigen could not be excluded.
Polystyrene beads as artificial APC for antigen-specific T cell expansion
Bead-based AAPC have been endowed with antigen specificity by coating the bead with MHC-peptide single chain construct dimers or tetramers (Figure 3B). Such antigen-specific beads were used successfully to stimulate murine ova-specific CD8+ T cells and tumor-reactive T cells specific for the melanoma antigen TRP-2 48,49. A similar approach was used to expand human HA-1-specific CD8+ T cells and to generate IL-13Rα2-specific CD8+ T cells to target glioma cells 40,50,51. An improvement that circumvents the need to coat the bead with preformed MHC-peptide single chain dimers is to bind dimeric HLA-A2-immunoglobulin fusion molecules to the bead, which can then be loaded exogenously with peptide antigen. This strategy was combined with bound αCD28 to provide costimulation and effectively expanded antigen-specific T cells ex vivo 52. Human Mart-1-specific CD8+ T cells expanded using this approach were adoptively transferred to NOD/SCID mice with melanoma xenografts and exhibited equivalent antitumor activity as T cells expanded using peptide-pulsed MoDC 53. HLA-Ig AAPC also generated a higher percentage of polyfunctional influenza virus-specific T cells compared with T cells derived by stimulation with autologous MoDC 54. This data is encouraging in view of the observation that the induction of polyfunctional T cells correlates with effective immunization 55,56, but the mechanisms responsible for programming of polyfunctional responses require additional study, and it is uncertain whether such T cells derived by AAPC stimulation will be more effective for adoptive immunotherapy of cancer.
Limitations of polystyrene beads as artificial APC
Bead-based AAPC can provide good control of signal delivery, but the approach has several limitations. The interactions between T cells and beads are not identical to those between T cells and a natural APC or cell based AAPC, and signals provided by surface-bound antibodies are not identical to those provided by natural ligands presented in a lipid bilayer. Spherical beads are also incapable of the dynamic remodeling that is established at the immune synapse between professional APC and T cells. The expansion and viability of T cells can also be a problem with bead-based culture systems. Early studies found that CD8+ T cells expanded with αCD3/αCD28 beads less well than their CD4+ counterparts, and the viability of CD8+ T cells was poor after long periods of in vitro culture 18,57. Substitution of 4-1BBL costimulation for αCD28 resulted in a marked improvement in the expansion and survival of CD8+ T cells, which illustrates the need for a better understanding of the signaling requirements needed to program T cells for survival after adoptive therapy 18. In the case of antigen-specific beads, HLA-Ig molecules are not currently available for all HLA alleles, although a panel of beads coated with HLA molecules present at high frequency in a given population could be constructed and enable coverage of a large percentage of the population with an ‘off the shelf’ antigen-specific artificial APC system 58. Another limitation is that antigens presented by latex beads are not processed and presented to a responding T cell, mandating that the target TAA must be known and provided in a processed form. The incorporation of additional signaling molecules requires purification of recombinant protein and linking the protein to the bead, which is often more difficult than transfecting cell-based AAPC. Control of local release of cytokines is also difficult in bead-based AAPC systems, although efforts have been made to overcome this limitation by manufacturing poly(lactide-co-glycolide) (PLGA) beads incorporating encapsulated cytokine delivery systems 59,60. Despite these limitations, bead based AAPC are being evaluated for expanding T cells for adoptive therapy and analysis of the persistence and function of such T cells in vivo will assist in improving this methodology for future clinical applications.
Lipid Vesicles and Exosomes
The importance of mobility of molecules that participate in an immune synapse between a natural APC and a T cell has been recognized as a critical component in efficient TCR signaling, and antigen presentation by exosomes, liposomes and other lipid preparations might represent a means to recapitulate this in an artificial setting.
Lipid vesicles comprised of cholesterol and phosphatidylcholine have been used primarily in vitro as tools to study molecular interactions at cell membrane surfaces and have not yet been aggressively explored as AAPC for deriving T cells for adoptive immunotherapy 61. However, the concept that an engineered lipid surface could improve immune synapse formation is attractive, and recent studies have begun to evaluate such reagents as AAPC.
Immunosomes are comprised of virus-like particles (VLPs) that have budded from lipid rafts of HEK293 cells infected with Moloney murine leukemia virus 62. By modifying the scFv of OKT3 and T cell costimulatory ligands with GPI anchors to allow localization to lipid raft-based VLPs, and transducing them into HEK293, immunosomes were obtained that nonspecifically stimulated T cells in vitro 62. Antigen-specific T cells could be induced by substituting the OKT3 scFv with H2Kb and a minigene encoding a peptide antigen. Despite the cumbersome nature of the engineering to produce these AAPC, it highlights a new approach to generating better immune synapse formation. Other studies have approached this problem by using GM1 liposomes bound with cholera toxin B and neutravidin to anchor αCD3, αCD28, and LFA-1 to stimulate T cell responses in vitro 63. In comparisons with αCD3/αCD28 beads, they were found to be similarly effective; however, the use of cholera toxin B may limit the clinical application of this approach 63.
Exosomes are vesicles secreted from cellular endosomes that present antigen with HLA class I and II molecules, and provide costimulatory, and adhesion signals to T cells 64. Exosomes derived from dendritic cells were used as an artificial acellular vaccine for murine mastocytoma and mammary carcinoma, and induced anti-tumor immune responses in vivo, despite exhibiting relatively weak T cell stimulation in vitro 64. Tumor-derived exosomes also appear to induce more efficient T cell stimulation in vivo than in vitro, suggesting that cross-priming by endogenous professional APCs might be required for optimal T cell stimulation 64,65. The requirement for cross-priming to induce CD8+ T cell proliferation by tumor derived exosomes suggests this approach may not be the optimal AAPC preparation for in vitro expansion of T cells for adoptive immunotherapy 14. Whether this limitation also applies to DC-derived exosomes remains to be determined, although the generation of sufficient autologous DC to prepare exosomes remains a barrier to broad utility of DC based products.
AAPC for Inducing or Expanding Tumor-reactive T Cells In Vivo
Eliciting effective tumor-reactive T cell responses in humans by vaccination has proven challenging, despite using highly specialized professional APC preparations, viral vectors, and gene modified tumor cells to present or deliver tumor antigens 66. This at least in part reflects the difficulty circumventing self-tolerance mechanisms that prevent the activation of T cells to TAA, which are often normal self-proteins. Conceptually, AAPC systems could be useful to overcome this problem, but critical insights into how to apply such an approach are presently lacking. A more immediate application of AAPC for which a strong rationale and some data currently exists, is to use AAPC to boost T cell responses that are established by adoptive T cell transfer 67.
Autologous AAPC to elicit and expand antigen-specific T cells in vivo
T cells that were transduced to express foreign marker genes such as hygromycin or neomycin phosphotransferase, or the HSV viral thymidine kinase (HSV-TK) suicide gene, and then adoptively transferred to humans induced potent CD8+ T cell responses to the introduced transgene products, demonstrating that transferred T cells have potent APC capacity in humans 68-70. This clinical observation was followed up by murine and clinical studies in which T cells were modified to express melanoma antigens and used as a vaccine, which demonstrated the induction of T cell immunity to a self-antigen and antitumor efficacy 71,72.
The potential use of T cells as APC (T-APC) to boost the level of adoptively transferred T cells in vivo was recently shown in nonhuman primates. In this work, a CD8+ T cell response to a cytomegalovirus antigen was established by adoptive transfer of a virus-specific T cell clone, and the response was then boosted to a higher level in vivo by a single intravenous infusion of autologous T-APC pulsed with the CMV peptide 73. These findings suggest that T cells, which express MHC and costimulatory molecules and traffic widely in vivo when administered intravenously, and are easy to obtain and transduce, are themselves a promising AAPC 68. Current studies in nonhuman primates are focused on optimizing the use of T-APC for boosting transferred T cell immunity, and could enable the use of much lower antigen-specific T cell doses for immunotherapy.
Acellular antigen presenting systems for augmenting T cell immunity in vivo
The use of bead based AAPC systems for in vivo activation of T cells faces considerable challenges. However, in murine studies MHC-Ig AAPC have been administered in vivo and shown to effectively expand adoptively transferred tumor-reactive CD8+ T cells 74. Other murine studies used beads coated with Mart-1 tetramer and αCD28 as a tumor vaccine and found that in vivo administration could induce proliferation of naïve Mart-1-specific CD8+ T cells 75. While no bead-related toxicity was reported after in vivo administration in mice, the possibility of microembolic or foreign body reactions remains present, and is likely to limit clinical applications.
Issues To Consider For AAPC Systems
Recent studies have highlighted the potential of adoptive CD8+ T cell immunotherapy in cancer patients. However, despite encouraging initial clinical responses, lack of T cell persistence and disease recurrence remain common problems after T cell therapy. A major focus for the field is now on strategies to enable the transfer and establishment of durable immunity to cancer. The human CD8+ T cell compartment contains distinct T cell subsets that appear to differ strikingly in their capacity to persist after in vitro expansion and adoptive transfer. Experiments in our lab in non-human primates have shown that effector T cell clones derived from TCM cells can persist long term, revert to quiescent TCM and TEM in vivo after adoptive transfer, self-renew and migrate to memory cell niches, whereas those derived from TEM cells rapidly die in vivo 73. A study in a murine model demonstrated that naïve TCR transgenic T cells activated short term in vitro also have superior survival properties and antitumor efficacy compared with TEM 76. In this model, long term culture of any T cell subset abrogated persistence and antitumor activity 76. Alternative less frequent subsets of T cells in both mice and humans have been suggested to have stem cell like properties, and may provide a unique source of T cells for immunotherapy 77,78.
AAPC systems have compelling advantages for deriving and expanding T cells of desired specificity, including more ready development under Good Manufacturing Practice conditions, and providing off-the-shelf reagents for clinical use. There are also disadvantages and an optimal system has not yet been defined (Table 1). A theoretical advantage of AAPC is that culture conditions including the choice of APC, cytokines, and/or pharmacologic modifiers can be carefully evaluated for T cell expansion to potentially impart or retain instructional programs that allow T cells to persist, function, and migrate in desired fashion after adoptive transfer. Achieving this goal will require detailed analysis that not only examines the in vivo fate of T cell products derived under different culture conditions but correlates this with gene expression and/or epigenetic signatures. The recent success of adoptive T cell therapy in both solid tumors and hematologic malignancies suggests that this will be an important pursuit to improve outcome and broaden the application of this modality of cancer therapy.
Table 1.
| SYSTEM | SOURCE | ANTIGEN DEPENDENT OR INDEPENDENT | AMENABLE TO TRANSDUCTION | ANTIGEN PROCESSING | CLINICAL TRIALS | COMMENTS |
|---|---|---|---|---|---|---|
| Cellular | ||||||
| K562 | Human erythroleukemia | Both | Yes | Yes | No | Transduced Fc receptors can bind αCD3 or HLA-Ig to allow antigen-specific or non-specific CD8+ T cell expansion Better CD8+ T cell proliferation when expressing 4-1BBL than αCD28 Has been prepared as GMP-compliant stock Might require HLA transduction for antigen presentation |
| 3T3 | Murine fibroblast | Both | Yes | Yes | No | Minimal evidence of xenoreactivity Requires HLA transduction for antigen presentation |
| Insect | Drosophila Schneider S2 cell line | Both | Yes | No | Yes | Lysed at 37°C Exogenous antigen only Minimal evidence of xenoreactivity |
| Acellular | ||||||
| αCD3/αCD28 beads | Synthetic | Independent | No | No | Yes | Standardized manufacture Excellent control of delivered signals Better CD8+ T cell proliferation when 4-1BBL is substituted for αCD28 Can substitute or add costimulatory or inhibitory molecules |
| HLA-Ig beads | Synthetic | Dependent | No | No | No | Excellent control of delivered signals Can substitute other HLA-Ig Exogenous antigen loading only |
Acknowledgments
Funding and support: The authors acknowledge funding from the Thomsen Family, Fred Hutchinson Cancer Research Center Breast Cancer Research Program, and NIH grants CA18029, CA114536, AI53193 and AI086683-01.
References
- 1.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilla L, Rivoltini L, Patuzzo R, et al. Multipeptide vaccination in cancer patients. Expert Opinion on Biological Therapy. 2009;9:1043–1055. doi: 10.1517/14712590903085109. [DOI] [PubMed] [Google Scholar]
- 5.Bendandi M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer. 2009;9:675–681. doi: 10.1038/nrc2717. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Chang CH, Goldenberg DM. Novel strategies for improved cancer vaccines. Expert Review of Vaccines. 2009;8:567–576. doi: 10.1586/erv.09.11. [DOI] [PubMed] [Google Scholar]
- 7.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Shelton TE, et al. Generation of Tumor-Infiltrating Lymphocyte Cultures for Use in Adoptive Transfer Therapy for Melanoma Patients. Journal of Immunotherapy. 2003 July/August;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurokawa T, Oelke M, Mackensen A. Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. International Journal of Cancer. 2001;91:749–756. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1141>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Neudorfer J, Schmidt B, Huster KM, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320:119–31. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 16:565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 12.Paulos C, Suhoski M, Plesa G, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunologic Research. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering Artificial Antigen-presenting Cells to Express a Diverse Array of Co-stimulatory Molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JV, Latouche JB, Riviere I, et al. The ABCs of artificial antigen presentation. Nat Biotech. 2004;22:403–410. doi: 10.1038/nbt955. [DOI] [PubMed] [Google Scholar]
- 15.Maus MV, Thomas AK, Leonard DGB, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotech. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 16.Becker C, Pohla H, Frankenberger B, et al. Adoptive tumor therapy with T lymphocytes enriched through an IFN-[gamma] capture assay. Nat Med. 2001;7:1159–1162. doi: 10.1038/nm1001-1159. [DOI] [PubMed] [Google Scholar]
- 17.Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Snyder KM, Suhoski MM, et al. 4-1BB Is Superior to CD28 Costimulation for Generating CD8+ Cytotoxic Lymphocytes for Adoptive Immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler MO, Lee JS, Ansén S, et al. Long-Lived Antitumor CD8+ Lymphocytes for Adoptive Therapy Generated Using an Artificial Antigen-Presenting Cell. Clinical Cancer Research. 2007;13:1857–1867. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 20.Hirano N, Butler MO, Xia Z, et al. Efficient Presentation of Naturally Processed HLA Class I Peptides by Artificial Antigen-Presenting Cells for the Generation of Effective Antitumor Responses. Clinical Cancer Research. 2006;12:2967–2975. doi: 10.1158/1078-0432.CCR-05-2791. [DOI] [PubMed] [Google Scholar]
- 21.Numbenjapon T, Serrano LM, Chang WC, et al. Antigen-independent and antigen-dependent methods to numerically expand CD19-specific CD8+ T cells. Experimental Hematology. 2007;35:1083–1090. doi: 10.1016/j.exphem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Obermann S, Petrykowska S, Manns MP, et al. Peptide-β2-microglobulin-major histocompatibility complex expressing cells are potent antigen-presenting cells that can generate specific T cells. Immunology. 2007;122:90–97. doi: 10.1111/j.1365-2567.2007.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasawatari S, Tadaki T, Isogai M, et al. Efficient priming and expansion of antigen-specific CD8+ T cells by a novel cell-based artificial APC. Immunol Cell Biol. 2006;84:512–521. doi: 10.1111/j.1440-1711.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- 24.de Gruijl T, van den Eertwegh A, Pinedo H, et al. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunology, Immunotherapy. 2008;57:1569–1577. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. The Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardoll DM. Cancer Cancer vaccines: a road map for the next decade. Current Opinion in Immunology. 1996;8:619–621. doi: 10.1016/s0952-7915(96)80076-8. [DOI] [PubMed] [Google Scholar]
- 27.Cai Z, Brunmark A, Jackson MR, et al. Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14736–14741. doi: 10.1073/pnas.93.25.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun S, Cai Z, Langlade-Demoyen P, et al. Dual Function of Drosophila Cells as APCs for Naive CD8+ T Cells: Implications for Tumor Immunotherapy. Immunity. 1996;4:555–564. doi: 10.1016/s1074-7613(00)80482-3. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell MS, Darrah D, Yeung D, et al. Phase I Trial of Adoptive Immunotherapy With Cytolytic T Lymphocytes Immunized Against a Tyrosinase Epitope. J Clin Oncol. 2002;20:1075–1086. doi: 10.1200/JCO.2002.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 30.Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotech. 2000;18:405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- 31.Papanicolaou GA, Latouche JB, Tan C, et al. Rapid expansion of cytomegalovirus-specific cytotoxic T lymphocytes by artificial antigen-presenting cells expressing a single HLA allele. Blood. 2003;102:2498–2505. doi: 10.1182/blood-2003-02-0345. [DOI] [PubMed] [Google Scholar]
- 32.Dupont J, Latouche JB, Ma C, et al. Artificial Antigen-Presenting Cells Transduced with Telomerase Efficiently Expand Epitope-Specific, Human Leukocyte Antigen-Restricted Cytotoxic T Cells. Cancer Res. 2005;65:5417–5427. doi: 10.1158/0008-5472.CAN-04-2991. [DOI] [PubMed] [Google Scholar]
- 33.Zhong XS, Matsushita M, Plotkin J, et al. Chimeric Antigen Receptors Combining 4-1BB and CD28 Signaling Domains Augment PI3kinase/AKT/Bcl-XL Activation and CD8+ T Cell-mediated Tumor Eradication. Mol Ther. 2009;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasan AN, Kollen WJ, Trivedi D, et al. A Panel of Artificial APCs Expressing Prevalent HLA Alleles Permits Generation of Cytotoxic T Cells Specific for Both Dominant and Subdominant Viral Epitopes for Adoptive Therapy. J Immunol. 2009;183:2837–2850. doi: 10.4049/jimmunol.0804178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelhard VH, Strominger JL, Mescher M, et al. Induction of secondary cytotoxic T lymphocytes by purified HLA-A and HLA-B antigens reconstituted into phospholipid vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:5688–5691. doi: 10.1073/pnas.75.11.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi M, Brian AA, McConnell HM. Binding of cytotoxic T-lymphocytes to supported lipid monolayers containing trypsinized H-2Kk. Molecular Immunology. 1983;20:1227–1231. doi: 10.1016/0161-5890(83)90147-5. [DOI] [PubMed] [Google Scholar]
- 37.Curtsinger J, Deeths MJ, Pease P, et al. Artificial cell surface constructs for studying receptor-ligand contributions to lymphocyte activation. Journal of Immunological Methods. 1997;209:47–57. doi: 10.1016/s0022-1759(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann SH, Mescher MF. Secondary cytolytic T lymphocyte stimulation by purified H-2Kk in liposomes. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:2488–2492. doi: 10.1073/pnas.78.4.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter S, Herrgen L, Schoor O, et al. Cutting Edge: Predetermined Avidity of Human CD8 T Cells Expanded on Calibrated MHC/Anti-CD28-Coated Microspheres. J Immunol. 2003;171:4974–4978. doi: 10.4049/jimmunol.171.10.4974. [DOI] [PubMed] [Google Scholar]
- 40.Schilbach K, Kerst G, Walter S, et al. Cytotoxic minor histocompatibility antigen HA-1-specific CD8+ effector memory T cells: artificial APCs pave the way for clinical application by potent primary in vitro induction. Blood. 2005;106:144–149. doi: 10.1182/blood-2004-07-2940. [DOI] [PubMed] [Google Scholar]
- 41.Anel A, O'Rourke AM, Kleinfeld AM, et al. T cell receptor and CD8-dependent tyrosine phosphorylation events in cytotoxic T lymphocytes: activation of p56 lck by CD8 binding to class I protein. European Journal of Immunology. 1996;26:2310–2319. doi: 10.1002/eji.1830261007. [DOI] [PubMed] [Google Scholar]
- 42.Nunès JA, Collette Y, Truneh A, et al. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7-1, activates p21ras. The Journal of Experimental Medicine. 1994;180:1067–1076. doi: 10.1084/jem.180.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 44.Rapoport AP, Levine BL, Badros A, et al. Molecular remission of CML after autotransplantation followed by adoptive transfer of costimulated autologous T cells. Bone Marrow Transplant. 2003;33:53–60. doi: 10.1038/sj.bmt.1704317. [DOI] [PubMed] [Google Scholar]
- 45.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 46.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 47.Rapoport AP, Stadtmauer EA, Aqui N, et al. Rapid Immune Recovery and Graft-versus-Host Disease–like Engraftment Syndrome following Adoptive Transfer of Costimulated Autologous T Cells. Clinical Cancer Research. 2009;15:4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tham EL, Jensen PL, Mescher MF. Activation of antigen-specific T cells by artificial cell constructs having immobilized multimeric peptide-class I complexes and recombinant B7-Fc proteins. Journal of Immunological Methods. 2001;249:111–119. doi: 10.1016/s0022-1759(00)00335-5. [DOI] [PubMed] [Google Scholar]
- 49.Lu X, Jiang X, Liu R, et al. Adoptive transfer of pTRP2-specific CTLs expanding by bead-based artificial antigen-presenting cells mediates anti-melanoma response. Cancer Letters. 2008;271:129–139. doi: 10.1016/j.canlet.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 50.Oosten LEM, Blokland E, van Halteren AGS, et al. Artificial antigen-presenting constructs efficiently stimulate minor histocompatibility antigen-specific cytotoxic T lymphocytes. Blood. 2004;104:224–226. doi: 10.1182/blood-2003-07-2461. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X, Lu X, Liu R, et al. HLA Tetramer–Based Artificial Antigen-Presenting Cells Efficiently Stimulate CTLs Specific for Malignant Glioma. Clinical Cancer Research. 2007;13:7329–7334. doi: 10.1158/1078-0432.CCR-07-1025. [DOI] [PubMed] [Google Scholar]
- 52.Oelke M, Maus MV, Didiano D, et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–625. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 53.Durai M, Krueger C, Ye Z, et al. In vivo functional efficacy of tumor-specific T cells expanded using HLA-Ig based artificial antigen presenting cells (aAPC) Cancer Immunology, Immunotherapy. 2009;58:209–220. doi: 10.1007/s00262-008-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ndhlovu ZM, Oelke M, Schneck JP, et al. Dynamic regulation of functionally distinct virus-specific T cells. Proceedings of the National Academy of Sciences. 107:3669–3674. doi: 10.1073/pnas.0915168107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 56.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. The Journal of Experimental Medicine. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laux I, Khoshnan A, Tindell C, et al. Response Differences between Human CD4+ and CD8+ T-Cells during CD28 Costimulation: Implications for Immune Cell-Based Therapies and Studies Related to the Expansion of Double-Positive T-Cells during Aging. Clinical Immunology. 2000;96:187–197. doi: 10.1006/clim.2000.4902. [DOI] [PubMed] [Google Scholar]
- 58.Oelke M, Schneck J. Overview of a HLA-Ig based “Lego-like system” for T cell monitoring, modulation and expansion. Immunologic Research. doi: 10.1007/s12026-009-8156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steenblock ER, Fahmy TM. A Comprehensive Platform for Ex Vivo T-cell Expansion Based on Biodegradable Polymeric Artificial Antigen-presenting Cells. Mol Ther. 2008;16:765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- 60.Steenblock ER, Wrzesinski SH, Flavell RA, et al. Antigen presentation on artificial acellular substrates: modular systems for flexible, adaptable immunotherapy. Expert Opinion on Biological Therapy. 2009;9:451–464. doi: 10.1517/14712590902849216. [DOI] [PubMed] [Google Scholar]
- 61.Prakken B, Wauben M, Genini D, et al. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat Med. 2000;6:1406–1410. doi: 10.1038/82231. [DOI] [PubMed] [Google Scholar]
- 62.Derdak SV, Kueng HJ, Leb VM, et al. Direct stimulation of T lymphocytes by immunosomes: Virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules. Proceedings of the National Academy of Sciences. 2006;103:13144–13149. doi: 10.1073/pnas.0602283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zappasodi R, Di Nicola M, Carlo-Stella C, et al. The effect of artificial antigen-presenting cells with preclustered anti-CD28/-CD3/-LFA-1 monoclonal antibodies on the induction of ex vivo expansion of functional human antitumor T cells. Haematologica. 2008;93:1523–1534. doi: 10.3324/haematol.12521. [DOI] [PubMed] [Google Scholar]
- 64.Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 65.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 66.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor Regression and Autoimmunity after Reversal of a Functionally Tolerant State of Self-reactive CD8+ T Cells. The Journal of Experimental Medicine. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger C, Flowers ME, Warren EH, et al. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riddell SR, Elliott M, Lewinsohn DA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–23. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 70.Jensen MC, Popplewell L, Cooper LJ, et al. Anti-Transgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Re-directed T Cells in Humans. Biology of Blood and Marrow Transplantation. doi: 10.1016/j.bbmt.2010.03.014. In Press, Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fontana R, Bregni M, Cipponi A, et al. Peripheral blood lymphocytes genetically modified to express the self/tumor antigen MAGE-A3 induce antitumor immune responses in cancer patients. Blood. 2009;113:1651–1660. doi: 10.1182/blood-2008-07-168666. [DOI] [PubMed] [Google Scholar]
- 72.Russo V, Cipponi A, Raccosta L, et al. Lymphocytes genetically modified to express tumor antigens target DCs in vivo and induce antitumor immunity. The Journal of Clinical Investigation. 2007;117:3087–3096. doi: 10.1172/JCI30605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ugel S, Zoso A, De Santo C, et al. In vivo Administration of Artificial Antigen-Presenting Cells Activates Low-Avidity T Cells for Treatment of Cancer. Cancer Res. 2009;69:9376–9384. doi: 10.1158/0008-5472.CAN-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen C, Zhang J, Xia L, et al. Induction of tumor antigen-specific cytotoxic T cell responses in naïve mice by latex microspheres-based artificial antigen-presenting cell constructs. Cellular Immunology. 2007;247:28–35. doi: 10.1016/j.cellimm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naïve rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8(+) memory stem cells. Nat Med. 2009 doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turtle CJ, Swanson HM, Fujii N, et al. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–44. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]