Abstract
In a series of four experiments, we explored whether pigeons complete partially occluded moving shapes. Four pigeons were trained to discriminate between a complete moving shape and an incomplete moving shape in a two-alternative forced-choice task. In testing, the birds were presented with a partially occluded moving shape. In Experiment 1, none of the pigeons appeared to complete the testing stimulus; instead, they appeared to perceive the testing stimulus as incomplete fragments. However, in Experiments 2, 3, and 4, three of the birds appeared to complete the partially occluded moving shapes. These rare positive results suggest that motion may facilitate amodal completion by pigeons, perhaps by enhancing the figure-ground segregation process.
The broad spatial pattern of our visual world projects onto a small two-dimensional retina in each eye. Our visual system extracts useful information from these retinal patterns and reconstructs them into a meaningful visual scene representing the three-dimensional world. During this complex reconstructive process, the visual system appears to interpolate the features (contour, color, and texture) of an object that are partially hidden from view. This interpolation process is called amodal or visual completion (Michotte, Thinès, & Crabbé, 1964/1991; Kanizsa, 1979; Palmer, 1999; for a review, see Sekuler & Murray, 2001).
Several behavioral studies (Nakayama, Shimojo, & Silverman, 1989; Rensink & Enns, 1998), physiological investigations (Bakin, Nakayama, & Gilbert, 2000; Corballis, Fendrich, Shapley, & Gazzaniga 1999; Giersch, Humphreys, Boucart, & Kovács, 2000; Murray, Kersten, Olshausen, Schrater, & Woods, 2002; Schiller, 1995; Sugita, 1999), and an analytical model (Grossberg, 2003) all suggest that the process of amodal completion begins in the early stages of visual processing. It is, therefore, plausible to expect that the system responsible for amodal completion in human adults may be shared with human infants and even nonhuman animals.
Developmental research has discovered that infants do not exhibit amodal completion behavior immediately after birth. The groundbreaking study of amodal completion in infants was conducted by Bower (1967), who tested 1-month-old infants with operant conditioning methods. The results showed that infants trained with a partially occluded triangle exhibited generalization to a complete triangle. However, Kellman and Spelke (1983) later failed to replicate this result with 4-month-old infants using visual habituation and response recovery methods. Nevertheless, Kellman and Spelke (1983; Kellman, Spelke, & Short, 1986) did find common motion between the visible parts of an occluded rod to be a strong cue for the perception of object unity by 4-month-old infants; these infants appeared to perceive two commonly-moving rods as a single object, implying that they amodally completed the rod when it was placed behind a box.
Subsequent to the Kellman and Spelke (1983) project, most studies of amodal completion behavior in infants have been conducted in the context of “object unity.” Jusczyk, Johnson, Spelke, and Kennedy (1999) confirmed that common motion is a better cue for object unity than edge alignment, synchronous color change, or synchronous brightness change of an occluded rod. The results of these and other studies of infants’ perception of amodal completion—or object unity (for summaries, see Johnson, 2003)—are consistent with one another in that completion occurred in infants 4 months old and older, and that common motion was required to support amodal completion behavior (see also Quinn, Brown, & Streppa, 1997).
In several nonhuman primate studies, empirical evidence suggests that squirrel monkeys (Nagasaka & Osada, 2000), Japanese macaques (Sugita, 1999), rhesus macaques (Bakin, et al., 2000; Fujita, 2001; Osada & Schiller, 1994; Schiller, 1995), baboons (Deruelle, Barbet, Depsy, & Fagot, 2000; Fagot, Barbet, Parron, & Deruelle, 2006), and chimpanzees (Sato, Kanazawa, & Fujita, 1997) may amodally complete a partially occluded object. Moreover, other studies have explored amodal completion behavior in non-primate species. Bengalese finches (Okanoya & Takahashi, 2000), chickens (Forkman, 1998; Forkman & Vallortigara, 1999; Lea, Slater, & Ryan, 1996; Regolin & Vallortigara, 1995; Regolin, Marconato, & Vallortigara, 2004), and mice (Kanizsa, Renzi, Conte, Compostela, & Guerani, 1993) may also exhibit amodal completion behavior.
The subjects in these nonhuman animal experiments cover a very wide range of species; peculiarly, all but one species in those studies have shown evidence of amodal completion behavior. Despite at least ten research efforts in over 20 years, no positive evidence has ever been obtained for amodal completion behavior in pigeons (Aust & Huber, 2006; Cerella, 1980; DiPietro, Wasserman, & Young, 2002, Experiment 1; Fujita, 2001; Fujita & Ushitani, 2005; Sekuler, Lee, & Shettleworth, 1996; Shimizu, 1998; Ushitani & Fujita, 2005; Ushitani, Fujita, & Yamanaka, 2001; Watanabe & Furuya, 1997).
Although pigeons have thus far failed to show evidence of amodal completion behavior, it may be premature to conclude that they cannot do so. Most prior pigeon studies were conducted with static stimuli. As noted above, common motion facilitates 4-month-old human infants’ amodal completion behavior. Perhaps common motion also plays a similarly important role in amodal completion for pigeons. The present study explored pigeons’ capacity to exhibit amodal completion behavior by training and testing the birds with moving stimuli.
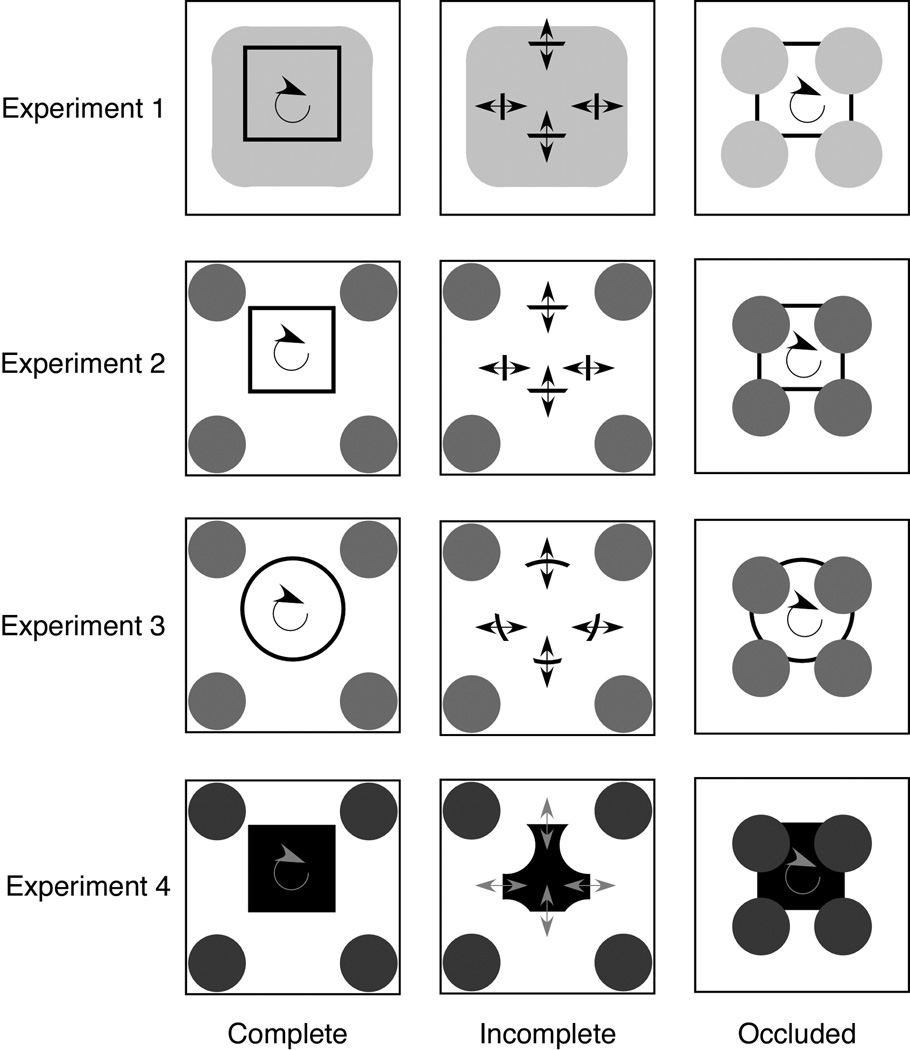
We examined pigeons’ amodal completion behavior by using the moving stimuli that are depicted in Figure 1. We created these stimuli according to the methods of Lorenceau and Shiffrar (1992; Shiffrar & Lorenceau, 1996). In general, human observers report that a square is moving in a circular trajectory behind four occluders when they view the rightmost display in the first row of Figure 1 (Occluded stimulus in Experiment 1). However, if the hue and luminance of the occluders are equal to those of the background and only the visible portions of the occluded shape are presented (Incomplete stimulus in Experiment 1), then the perception of the stimulus is dramatically changed; when human observers view the center display in the first row of Figure 1, they do not report seeing a square, but they instead report seeing two pairs of parallel lines with the opposite pairs moving in unison. This experimental task has been productively deployed in studying “motion integration” or “motion interpretation.”
Figure 1.
The four sets of stimuli used in the study. Each set comprised three movies (Complete, Incomplete, and Occluded). The Complete and Incomplete stimuli were presented in training and testing. The Occluded stimuli were presented only in testing. The arrows depict the movement of the shapes and were not shown in the actual stimuli. The full set of stimuli in color can be seen at: http://www.psychology.uiowa.edu/Faculty/Wasserman/ACMovies/Figure1.html
Here, we used these stimuli to see if pigeons spontaneously exhibit “motion integration” or “motion interpretation.” That is, we aimed to see whether or not pigeons perceive a complete shape when they are presented with a partial shape moving behind occluders, thereby suggesting the involvement of amodal completion. We report four experiments in which we asked whether object motion supports amodal completion behavior in pigeons. We initially trained pigeons to discriminate two movie stimuli: one of a Complete object (left column of Figure 1) and the other of an Incomplete object (center column of Figure 1). We then showed them the Occluded object movie (right column of Figure 1). If the pigeons were to classify the Occluded object movie as “complete,” then this result would constitute evidence that pigeons can amodally complete partially occluded moving objects.
Experiment 1
Method
Subjects
The subjects were four feral pigeons (Columba livia) kept at 85% of their free-feeding weights by controlled daily rations. The pigeons had earlier participated in amodal completion studies in which static 2-D stimuli were used; they had failed to show evidence of amodal completion in much the same way as had pigeons in all of the previously published research reports that we reviewed earlier. The birds had never before seen moving stimuli in the experimental apparatus.
Apparatus
The pigeons were trained in four operant conditioning boxes detailed by Gibson, Wasserman, Frei, and Miller (2004). The boxes were located in a dark room with continuous white noise. The stimuli were presented on a 15-in LCD monitor located behind an AccuTouch® resistive touchscreen (Elo TouchSystems, Fremont, CA). A food cup was centered on the rear wall level with the floor; a rotary food dispenser delivered 45-mg Noyes food pellets through a vinyl tube into the cup. A houselight on the rear wall provided illumination during the session. Each chamber was controlled by an Apple® eMac® computer. The experimental procedure was programmed in HyperCard (Version 2.4, Apple Computer, Inc., Cupertino, CA).
Stimuli
The stimuli consisted of three movies: Complete square, Incomplete square, and Occluded square (top row of Figure 1). The movies were made with Flash MX (Version 7.2, Macromedia, Inc., San Jose, CA) and were composed of 35 frames. Each stimulus (4.0 cm high and 4.0 cm wide) was placed in the center of the screen. The choice keys were two 2.7 × 2.4 cm white rectangles, each containing distinctive Macintosh icons, which were located above and below or left and right of the movie stimulus; those placements were counterbalanced across the birds. The rest of the screen was black.
The Occluded stimulus consisted of two parts: an outline of a square (19 mm height and width, 0.6 mm thickness) and four occluders. The Occluded stimulus was drawn in black on a white background; the inside of the square was also white. The square moved in a circular trajectory behind the four occluders during each presentation; the occluders (13 mm diameter) were gray circles placed over the four corners of the square. The Complete stimulus showed the full contour of the square, whereas the Incomplete stimulus showed the same portions of the square that were visible in the Occluded stimulus. Both Complete and Incomplete stimuli were drawn on a round-cornered gray square on a white background. The luminance of the square was the same as that of the occluders in the Occluded stimulus. All of the movies that were used in our study can be seen at http://www.psychology.uiowa.edu/Faculty/Wasserman/ACMovies/Figure1.html.
Procedure
Pretraining
During pretraining, the pigeons were taught to peck a square that was placed in the center of the display or at one of four smaller squares that were placed around the central square. After the birds reached an “observing response” criterion (Fixed Ratio 20 for each square), the birds proceeded to training.
Training
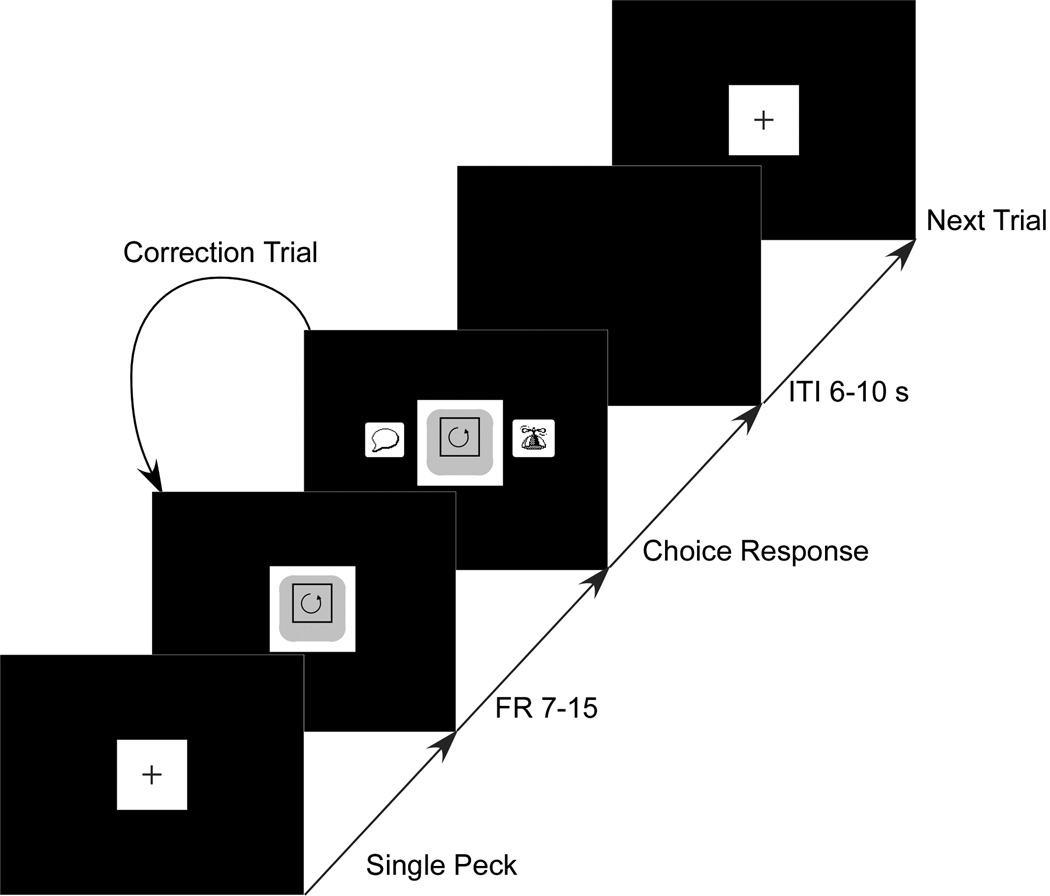
The pigeons were trained on a standard two-alternative forced-choice task. Figure 2 shows the sequence of events in the course of a training trial. The trial began with the presentation of a black cross centered on a white square (7 × 7 cm) in the middle of the display. After a peck at the square, either the Complete or Incomplete moving stimulus appeared. The birds were required to peck at the stimulus a fixed number of times (“observing responses”), which varied for different birds from 7 to 15 pecks per trial. After the final peck, two choice report keys (“complete” and “incomplete”) appeared. After a choice response to either of the keys, the moving stimulus and the keys were removed from the screen. A correct choice was followed by a food-pellet reinforcer and an intertrial interval (ITI) which ranged from 6 to 10 s (mean of 8 s); then, a new trial followed. If the bird chose the incorrect response, then the house light darkened from 6 to 10 s (mean of 8 s) and the bird had to complete one or more correction trials until it made the correct choice. The correction trials were identical to the choice trials. The correction trials and occasional incomplete sessions were not used in data analysis.
Figure 2.
The sequence of events in the course of a trial. FR stands for Fixed Ratio and ITI stands for Intertrial Interval.
Each daily training session contained 80 blocks of 2 trials (total of 160 trials) and each trial presented either a Complete or an Incomplete moving stimulus. Training continued until the pigeons reached a criterion of at least 80% correct for each stimulus and 85% correct overall for 2 consecutive sessions.
Testing
During each testing session, the pigeons received 184 trials. The first 40 trials involved 20 presentations of each training stimulus and were treated as warm-up trials, which were not used in data analysis. The following 144 trials contained 16 blocks; each block (9 trials) included 4 presentations of the 2 training stimuli plus 1 Occluded testing stimulus. The trials in each block were randomly presented. The birds received reinforcement for any responses (nondifferential reinforcement) on the testing trials. Testing sessions continued for 3 days. If a pigeon failed to complete a testing session, then it was returned to training until it again reached criterion.
Behavioral Measures
The percentage of choices of the “complete” key (number of responses to “complete” key divided by total number of responses to both “complete” and “incomplete” keys times 100) during testing was our dependent measure. For all statistical tests, alpha was set at 0.05.
Results and Discussion
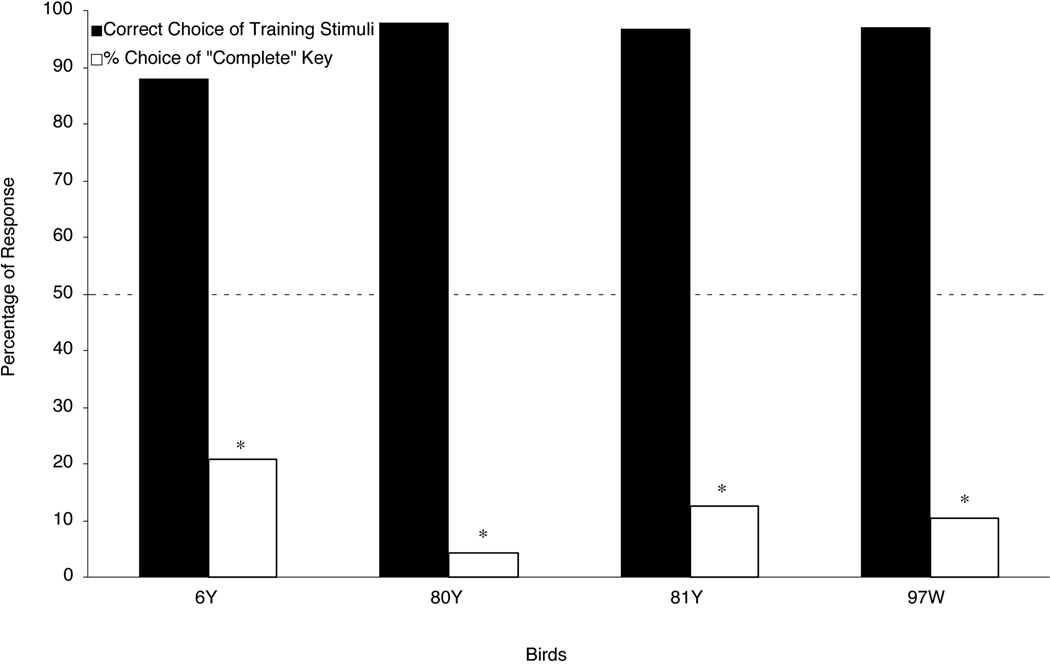
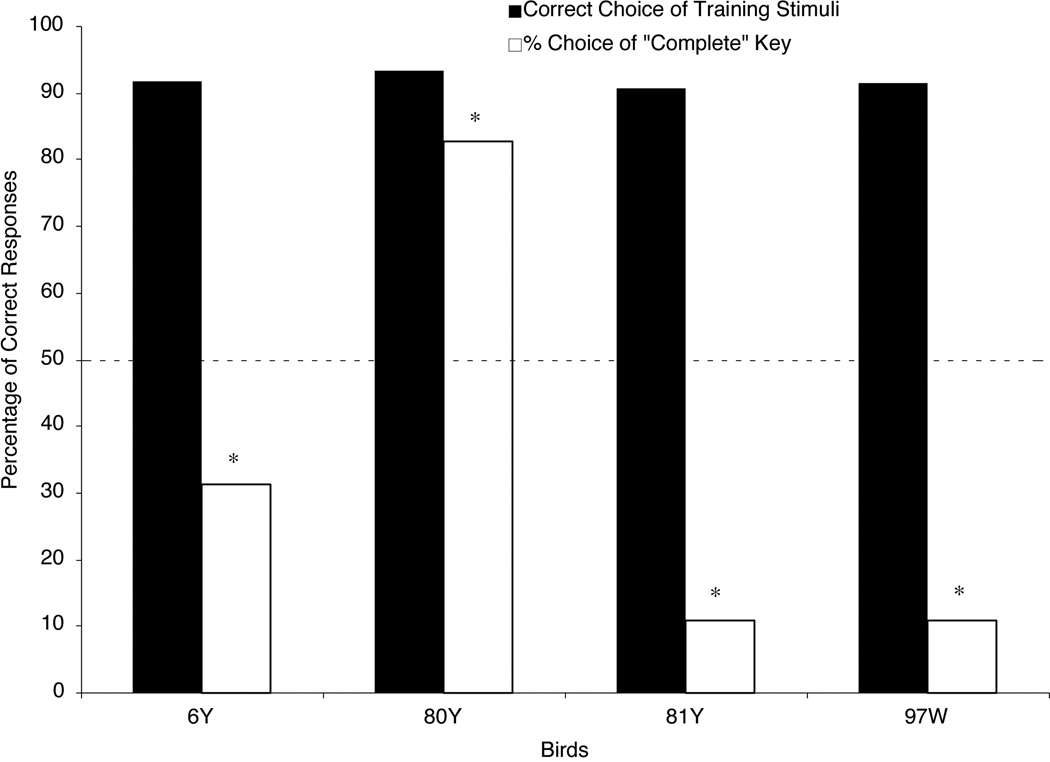
The pigeons took a mean of 13.3 sessions (range: 8 to 16 sessions) to finish discrimination training. Figure 3 shows the percentage of correct responses to the training stimuli during the testing sessions; these scores were near or above 90% correct, indicating that the birds’ responding to the training stimuli was substantially free of interference from the interpolated testing trials. Figure 3 also shows the percentage of choice responses to the “complete” report key when the pigeons were presented with an Occluded testing stimulus. If the birds perceived a square moving behind occluders in the Occluded testing stimulus, then these scores should have been reliably higher than chance (50%). But, the pigeons responded in just the opposite fashion; they predominately chose the “incomplete” key rather than the “complete” key. This trend was statistically significant for all four birds. Two-tailed t-tests revealed that the percentages of “complete” responses made to the Occluded testing stimulus were lower than the 50% chance level (6Y: t(47) = −4.92; 80Y: t(47) = −15.73; 81Y: t(47) = −7.77; 97W: t(47) = −8.88). These results suggest that the pigeons did not amodally complete the square in the Occluded testing stimulus, despite the fact that motion has been reported to be a robust cue for object unity in human infants.
Figure 3.
The percentage of each bird’s choice responses in Experiment 1 testing. Filled bars show the percentage of correct responses to the training stimuli. Open bars show the percentage of choices on Occluded testing trials made to the “complete” report key. The dashed line shows chance responding (50%). The asterisks represent choice scores that were significantly below chance (p < .05).
In most prior studies, pigeons categorized a partially occluded object as neither complete nor incomplete (Cerella, 1980; Ushitani & Fujita, 2005, Experiment 2; Watanabe & Furuya, 1997): that is, they showed no systematic perception of the stimulus. But, the current experiment found that the birds classified the Occluded square as “incomplete.” The difference between the Incomplete stimulus and the Occluded stimulus was the presence of the occluders; perhaps, the birds ignored the occluders altogether when they viewed the testing display. Indeed, in the current experiment, the occluders were painted grey on a white background (Figure 1, Experiment 1). Therefore, the Occluded testing stimuli used here might not have provided a distinct enough contrast for the pigeons to perceive the circles as occluders. As well, the interior of the square in the Occluded stimulus was white, whereas it was grey in the Complete stimulus. If the birds encoded and remembered the moving grey square in the Complete stimulus, then they might have discriminated the Occluded stimulus from the Complete stimulus and failed to report the presence of the Occluded square. We addressed these concerns in Experiment 2.
Experiment 2
In Experiment 1, all of the birds reported the Occluded stimulus as “incomplete.” This result might have been due to particular features of the Occluded stimulus, such as the brightness of the background, the brightness of the square, and the shading of the occluders. Thus, the birds might not have seen the four circles in the Occluded stimulus as occluders obstructing the moving square; amodal completion requires recognition of the occluded object and the occluding object. In Experiment 2, we used stimuli in which the occluders were painted red. This modification of adding color contrast to luminance contrast between the occluders and the background should enhance the salience of the occluders, which in turn might facilitate amodal completion behavior. In addition, we painted the interior of the square objects with same white color in all three versions of the movies, in order to make the stimuli more similar to each other. This change too might facilitate the pigeons’ discriminative transfer of “complete” responses to the Occluded testing stimulus.
Method
Subjects
The same four pigeons from Experiment 1 continued to serve.
Apparatus
The apparatus was the same as that used in Experiment 1.
Stimuli
The second row in Figure 1 shows the three movie stimuli that were used in the current experiment. The Occluded stimulus consisted of two parts: an outline of a square (13 mm height and width, 0.6 mm thickness) and four occluders. The square was drawn in black on a white background (33 mm height and width); the inside of the square was also white. The square moved in a circular trajectory behind the occluders during each stimulus presentation. The occluders (9-mm diameter) were red circles placed over the four corners of the square. The Complete stimulus showed the full contour of the square, whereas the Incomplete stimulus showed the same portions of the square that were visible in the Occluded stimulus. Both Complete and Incomplete stimuli were drawn on a white background (33 mm height and width); the same occluders in the Occluded stimulus were placed in the corners of the background.
Procedure
The training and testing procedures were the same as in Experiment 1.
Behavioral Measures
The behavioral measures were the same as in Experiment 1.
Results and Discussion
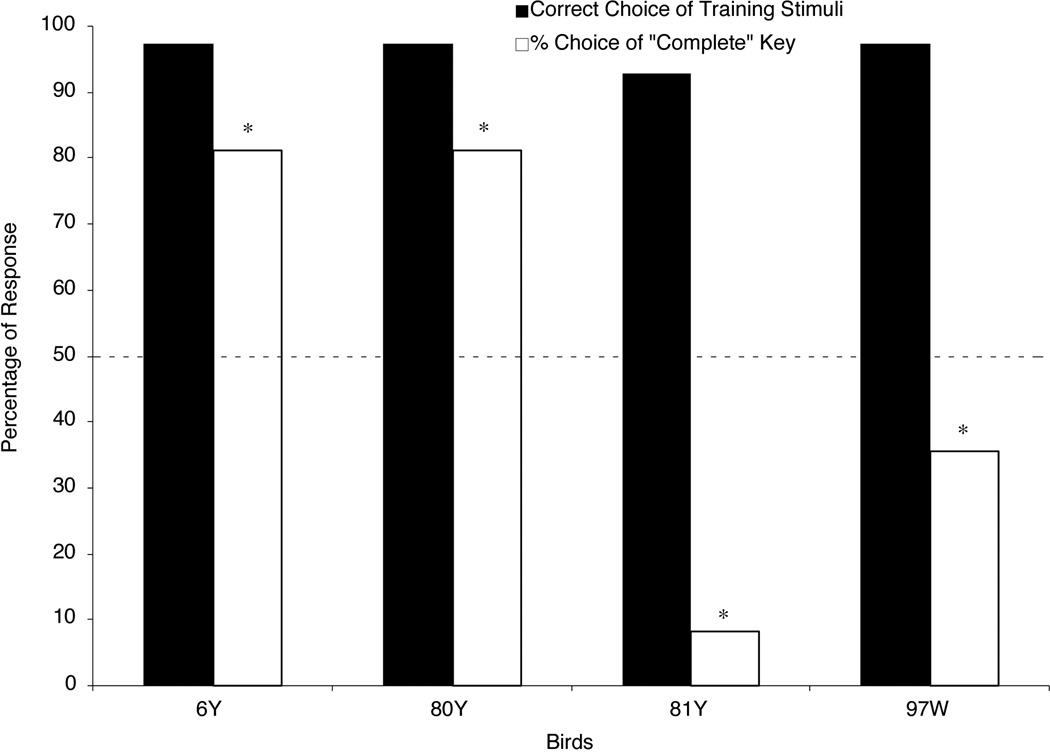
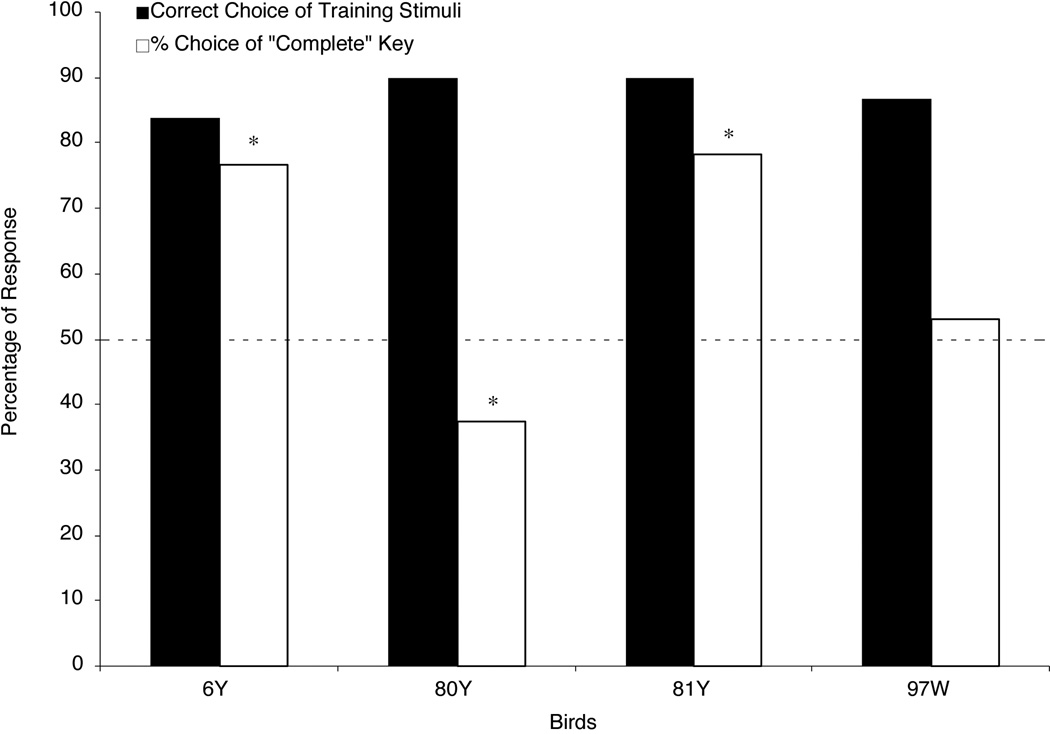
The pigeons took a mean of 2.8 sessions (range: 2 to 4 sessions) to finish discrimination training. Figure 4 shows the percentages of correct responses to the training stimuli during the testing sessions; these scores all exceeded 90% correct. Figure 4 also shows the percentages of choice responses to the “complete” key when the pigeons were presented with Occluded testing stimuli. Two birds (6Y and 80Y) robustly responded to the “complete” key and the other two birds (81Y and 97W) reliably responded to the “incomplete” key to different degrees when they viewed the Occluded testing stimulus.
Figure 4.
The percentage of each bird’s responses in Experiment 2. Filled bars show the percentage of correct responses to the training stimuli. Open bars show the percentage of choices on Occluded testing trials made to the “complete” report key. The dashed line shows chance responding (50%). The asterisks represent choice scores that were either significantly above or below chance (p < .05).
Two-tailed t-tests confirmed that the percentage of “complete” key choices made to the Occluded testing stimuli was significantly higher than the 50% chance level for Birds 6Y and 80Y [6Y: t(47) = 5.49; 80Y: t(47) = 5.49]. These results suggest that these two pigeons amodally completed the moving square when it was occluded by the red circles. These data represent rare signs of amodal completion behavior in pigeons. But, statistical analysis also disclosed that Birds 81Y and 97W chose the “complete” key significantly less often than the 50% chance level when they viewed the Occluded testing stimulus [81Y: t(47) = −10.34; 97W: t(47) = −2.09]. These results suggest that these two birds classified the Occluded testing stimulus as “incomplete,” just as they had in Experiment 1.
Why might the pigeons have exhibited such inconsistent trends? Perhaps the pigeons simply expressed a spatial report key preference when they were shown the Occluded testing stimulus; thus, these results might not truly reflect the transfer of discriminative responding to the Occluded testing stimuli. We addressed this matter in Experiment 3.
Experiment 3
In Experiment 2, two birds classified the Occluded testing stimulus as “complete,” whereas the other two birds classified it as “incomplete.” The method used in Experiment 2 could not rule out the possibility that the birds responded on the Occluded testing trials with strong spatial report key preferences. In Experiment 3, we randomized the placements of the “complete” and “incomplete” report keys in an effort to discourage the birds from displaying a spatial report key preference. In addition, we also explored the effect of two different types of reinforcement procedures on Occluded testing trials.
Method
Subjects
The same four pigeons from Experiments 1 and 2 continued to serve.
Apparatus
The apparatus was the same as in Experiments 1 and 2.
Stimuli
The third row in Figure 1 shows the stimuli that were used in the current experiment. The Occluded stimulus consisted of two parts: an outline of a circle (16 mm diameter, 0.6 mm thickness) and four occluders. The circle was drawn in black on a white background (33 mm diameter); the interior of the circle was also white. The circle moved in a circular trajectory behind the occluders during each stimulus presentation. The occluders (9 mm diameter) were red circles placed over the circle. The Complete stimulus showed the full contour of the circle, whereas the Incomplete stimulus showed the same portions of the circle that were visible in the Occluded stimulus. Both Complete and Incomplete stimuli were drawn on a white background (33 mm height and width); the same occluders in the Occluded stimulus were placed in the corners of the background.
The choice keys were two 2.7 × 2.4 cm rectangles painted blue or yellow, which were located above and below or left and right of the movie stimulus; those placements were counterbalanced across birds. The blue key was assigned to “complete” reports and the yellow key was assigned to “incomplete” reports for two pigeons (6Y and 80Y); the assignments were reversed for the other two pigeons (81Y and 97W). The placements of the blue and yellow keys were randomized on each trial.
Procedure
Each bird took part in 4 testing sessions after training to criterion. The birds did not receive reinforcement for any report responses (extinction) on Occluded testing trials for the first 2 testing days; for the following 2 testing days, the birds received reinforcement for any report responses (nondifferential reinforcement) on Occluded testing trials. The rest of the procedure was conducted in the same way as in Experiment 2.
Behavioral Measures
The behavioral measures were the same as in Experiments 1 and 2.
Results and Discussion
The pigeons took a mean of 8.25 sessions (range: 5 to 12 sessions) to complete discrimination training. As the overall trends in the birds’ responding were similar for both types of testing conditions, we consolidated the data for the following data analysis. Figure 5 shows the percentages of correct responses to the training stimuli during the testing sessions. These scores were near or above 90% correct. Figure 5 also shows the percentages of choice responses to the “complete” key when the pigeons were presented with an Occluded testing stimulus. One bird (80Y) robustly responded to the “complete” key when it viewed an Occluded testing stimulus; but, the other three birds (6Y, 81Y, and 97W) robustly responded to the “incomplete” key when they viewed an Occluded testing stimulus. Two-tailed t-tests confirmed that the percentage of choices made to the “complete” key was reliably higher than the 50% chance level for Bird 80Y [t(63) = 6.9], whereas the other three birds chose the “complete” key reliably less often than the 50% chance level [6Y: t(63) = −3.2; 81Y: t(63) = −9.9; 97W: t(63) = −9.9].
Figure 5.
The percentage of each bird’s responses in Experiment 3. Filled bars show the percentage of correct responses to the training stimuli. Open bars show the percentage of choices on Occluded testing trials made to the “complete” report key. The dashed line shows chance responding (50%). The asterisks represent choice scores that were either significantly above or below chance (p < .05).
These results suggest that one pigeon amodally completed the moving circle, but that the other three birds did not. These results further suggest that one of the pigeons (6Y) that predominantly chose the “complete” key in Experiment 2, may have been expressing a spatial report key preference when the bird was shown the Occluded testing stimuli.
Experiment 4
In all three of the preceding experiments, the pigeons were presented with an “outline” of a shape in the movie stimuli. However, the objects in our visual world also have salient surfaces; only rarely do we perceive the bare outlines of objects. Therefore, objects with a discriminable surface should be more natural in pigeons’ visual experience than line-drawn objects. Furthermore, Kellman (2003) has proposed two processes for visual interpolation in studies of human perception (Kellman & Shipley, 1991; Yin, Kellman, & Shipley, 1997, 2000): contour interpolation and surface interpolation. He argued that the contour interpolation process connects oriented edges across a gap and that surface interpolation integrates visible regions. Moreover, his studies showed that these two interpolation systems are separately operative for visual interpolation. So, we surmised that adding surface properties might prove to be a practical cue for pigeons’ amodal completion behavior. We thus devised this final investigation (Experiment 4), in which filled objects were used, in order to explore the effect of surface cues on pigeons’ amodal completion behavior.
Method
Subjects
The same four pigeons from Experiments 1, 2, and 3 continued to serve.
Apparatus
The apparatus was the same as in Experiments 1, 2, and 3.
Stimuli
The fourth row of Figure 1 depicts the stimuli that were used in this experiment. The Occluded stimulus consisted of two parts: a square (13 mm height and width, 0.6 mm thickness) and four occluders. The square was painted black on a white background (33 mm height and width). The square moved in a circular trajectory behind the occluders during each presentation. The occluders (9 mm diameter) were green circles placed over the four corners of the square. The Complete stimulus showed the whole shape of the square, whereas the Incomplete stimulus showed the same portion of the square that was visible in the Occluded stimulus. Both Complete and Incomplete stimuli were drawn on a white background (33 mm height and width); the same occluders in the Occluded stimulus were placed in the corners of the background.
The choice keys were two 2.7 × 2.4 cm white rectangles, each containing a triangular picture (an upright triangle with a texture of vertical lines or an inverted triangle with a texture of horizontal lines), were located to the top-left and bottom-right or to the top-right and bottom-left of the movie stimulus; those placements were counterbalanced across birds. The key containing the upright triangle was made the “complete” report response and the key containing the inverted triangle was made the “incomplete” report response for two birds (81Y and 97W); the assignments were reversed for the other two birds (6Y and 80Y). The placements of the keys were randomized on each trial.
Procedure
The procedure was the same as in Experiment 3.
Behavioral Measures
The behavioral measures were the same as in the three prior experiments.
Results and Discussion
The pigeons required many more sessions (6Y: 38; 80Y: 30; 81Y: 156; 97W: 31) to complete discrimination training than in the earlier experiments. Figure 6 shows the percentages of correct responses to the training stimuli during the testing sessions. These scores were near or above 90% correct. Figure 6 also shows the percentages of choice responses to the “complete” key when the pigeons were presented with an Occluded testing stimulus. Two birds (6Y and 81Y) responded predominately to the “complete” key, one bird (80Y) responded predominately to the “incomplete” key, and the final bird (97W) responded indiscriminately to both keys when presented with the Occluded testing stimulus.
Figure 6.
The percentage of each bird’s responses in Experiment 4. Filled bars show the percentage of correct responses to the training stimuli. Open bars show the percentage of choices on Occluded testing trials made to the “complete” report key. The dashed line shows chance responding (50%). The asterisks represent choice scores that were either significantly above or below chance (p < .05).
Two-tailed t-tests confirmed that the percentages of report responses made to the “complete” key were significantly higher than the 50% chance level for Birds 6Y and 81Y [6Y: t(63) = 5.0; 81Y: t(63) = 5.4]. These results suggest that these two pigeons amodally completed the moving square when it was presented along with surface cues. But, statistical analysis also disclosed that Bird 97W equivalently chose the “complete” and “incomplete” keys [t(63) = 0.5, p = 0.6] and that Bird 80Y chose the “complete” key significantly less often than the 50% chance level [t(63) = −2.0]. Curiously, Bird 80Y had been the only pigeon in Experiment 3 to exhibit reliable choice of the “complete” key to the Occluded testing stimulus.
General Discussion
In the present series of four experiments, we explored pigeons’ amodal completion behavior by using moving visual stimuli (Figure 1). In Experiment 1, we found that the pigeons perceived broken lines instead of a complete square in the Occluded testing stimulus. We suspected that the birds might have ignored the grey occluders because of the weak contrast disparity between the light gray occluders and the white background. In Experiment 2, in which we painted the occluders red, the results indicated that two of the birds that had not done so in Experiment 1 now perceived a complete square in the Occluded testing stimulus; the other two birds still appeared to have seen broken lines in the Occluded testing stimulus. Because of the experimental procedures that we used in Experiment 2, this pattern of results could have arisen from the pigeons’ exhibiting a spatial report key preference when they were shown the Occluded testing stimuli. For this reason, in Experiments 3 and 4, we randomized the locations of the choice report keys. We also used a filled shape with distinctive surface properties as the focal visual object in Experiment 4. Now in these two experiments, three of the four pigeons evidenced completion of partially occluded moving objects; these three birds reliably reported “complete” when they were presented with an Occluded moving stimulus. These data represent rare instances of amodal completion behavior in pigeons and suggest that motion may be an important factor in the process.
In an earlier study, Ushitani et al. (2001, Experiment 1) used a white rod moving behind a grey belt and found that pigeons did not exhibit amodal completion behavior. This finding is consistent with the results of our own Experiment 1. But, when Ushitani et al. (Experiments 2, 3, and 4) later used a red occluding belt, their pigeons still perceived the occluded moving rod to be multiple broken rods. What is responsible for the disparate results between their study and ours?
One possibility is the experimental apparatus that they used. Ushitani et al. (2001) used a CRT monitor and we used an LCD display for stimulus presentation. Pigeons’ visual temporal resolution is much higher than humans’ and it reaches 145 Hz (Emmerton, 1983). Hence, the pigeons may have detected the flickering of the CRT monitor, thereby changing their perception of the moving stimuli. Furthermore, Yamaguchi, Kitamura, and Ito (2003) found that pigeons’ discrimination of paintings on an LCD display worsened when the same paintings were presented on a CRT monitor. Finally, Ikebuchi and Okanoya (1999) reported that male zebra finches and Bengalese finches showed robust courtship displays to video images of conspecific females when the videos were presented on an LCD display, but not when they were presented on a CRT monitor. Therefore, the failure to find evidence of amodal completion behavior by pigeons in Ushitani et al. (2001) may have been due to suboptimal experimental methods.
These considerations notwithstanding, if we focus on the behavior of each of our individual pigeons, then evidence of consistency is in short supply. Consider only the data from Experiments 3 and 4, in which our experimental methods ought to have been the most sensitive and reliable. In Experiment 3, only one bird (80Y) chose the “complete” key when it viewed the Occluded testing stimulus, suggesting that the pigeon perceived a complete moving square behind the occluders. But, the same bird chose the “incomplete” key in Experiment 4; this trend was statistically significant, suggesting that the pigeon did not see a complete square in this later experiment. Recall that, when we conducted Experiment 4, we had hoped that providing the target shape with surface cues would help the birds to complete it; this was certainly not the case for this particular pigeon.
On the other hand, when the other three birds (6Y, 81Y, and 97W) were presented with the Occluded testing stimulus in Experiment 3, they chose the “incomplete” key, suggesting that they perceived broken lines. Yet, in Experiment 4, two out of these three birds (6Y and 81Y) appeared to complete the Occluded testing stimulus; thus, surface cues might have affected these pigeons’ perception of the Occluded testing stimulus. Finally, the results from the fourth bird (97W) never exceeded chance in Experiments 3 or 4, suggesting that this bird never perceived a complete square in the Occluded testing stimulus—with or without surface cues available.
At this time, we cannot explain these inconsistent results among our pigeons. The choice behaviors of Birds 6Y, 81Y, and 97W in Experiment 3 and Bird 80Y in Experiment 4 replicated previous results in pigeons (e.g., Sekuler et al., 1996); that is, the pigeons appear to have discriminated the stimuli by using local features. Indeed, our Incomplete stimuli showed the same portions of the shapes that were visible in Occluded stimuli. If our birds had used these common features for discrimination of the task stimuli, then they should have treated the Occluded stimuli as Incomplete. On the other hand, the same birds (80Y in Experiment 3 and 6Y and 81Y in Experiment 4) showed the opposite choice tendency when the Occluded and Incomplete stimuli shared common features. Therefore, our pigeons seem to have solved the discrimination task by using other cues, quite possibly global interpolated shape formed by amodal completion. So, overall, our albeit inconsistent positive results do generally suggest that an object’s motion may play an important role in the amodal completion behavior of pigeons, especially in light of the numerous failures to obtain amodal completion behavior with static stimuli and in light of the fact that these same pigeons had failed to evidence amodal completion behavior with static visual stimuli in experiments that preceded the ones reported here.
It might be of relevance to note that the perception of the moving stimulus that we used in the current study is actually bistable in humans; when we pay particular attention to local features of the stimulus, we can see broken rods or an ambiguous area moving behind the occluders although we see complete object most of the time. Some prior studies (Sekuler et al., 1996; Experiments 1 and 3, Ushitani & Fujita, 2005) as well as the current study showed that the pigeons classify occluded objects as incomplete. Thus, the pigeons might have a tendency to pay attention to the partial features even when those features are moving in unison. Perhaps the current inconsistent results might be due to the unique perceptual behavior of pigeons.
Kellman, Guttman, and Wickens (2001) suggested that object segregation is required in the early stages of amodal completion. These authors argued that motion information is more robust for object segregation than are luminance or chromatic discontinuities, because motion is more highly correlated with object boundaries. Furthermore, research has found that 4-month-old infants and still older human children require common motion to support amodal completion behavior (for summaries, see Johnson, 2003). As well, research in our own laboratory has disclosed that specific training experiences can enhance pigeons’ recognition of partially occluded objects (DiPietro et al., 2002; Lazareva, Wasserman, & Biederman, 2007). These studies suggest that the improvements obtained in pigeons’ recognizing partially occluded objects is due to training experiences which encouraged the birds to segregate the objects from the occluders. Perhaps the moving objects used in the current study also encouraged the pigeons to segregate the target shapes from the occluders, thereby helping the birds to complete the partially occluded shapes.
This interesting possibility merits further experimental scrutiny. So too does the interaction of stimulus properties and past experience. Segregating object from background is surely foundational to navigating in the visual world. Such successful navigation may need to be cultivated by active experience with relevant stimulus information.
Acknowledgements
This research was supported by National Institute of Mental Health Grant, MH47313. We thank Michelle Miner for her assistance in editing this manuscript and in collecting these data. We also thank Olga Lazareva, Leyre Castro, and Andrea Frank for their help in collecting these data. Moreover, we strongly appreciate the technical assistance of Joshua James Reynolds in conducting this research.
References
- Aust U, Huber L. Does the use of natural stimuli facilitate amodal completion in pigeons? Perception. 2006;35:333–349. doi: 10.1068/p5233. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Nakayama K, Gilbert CD. Visual responses in monkey areas V1 and V2 to three-dimensional surface configuration. Journal of Neuroscience. 2000;20:8188–8198. doi: 10.1523/JNEUROSCI.20-21-08188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower TGR. Phenomenal identity and form perception in an infant. Perception & Psychophysics. 1967;2:74–76. [Google Scholar]
- Cerella J. The pigeon’s analysis of pictures. Pattern Recognition. 1980;12:1–6. [Google Scholar]
- Corballis PM, Fendrich R, Shapley R, Gazzaniga MS. Illusory contours and amodal completion: Evidence for a functional dissociation in callosotomy patients. Cognitive Neuroscience. 1999;11:459–466. doi: 10.1162/089892999563535. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Barbet I, Depsy D, Fagot J. Perception of partially occluded figures by baboons (Papio papio) Perception. 2000;29:1483–1497. doi: 10.1068/p3071. [DOI] [PubMed] [Google Scholar]
- DiPietro NT, Wasserman EA, Young ME. Effects of occlusion on pigeons' visual object recognition. Perception. 2002;31:1299–1312. doi: 10.1068/p3441. [DOI] [PubMed] [Google Scholar]
- Emmerton J. Vision. In: Abs M, editor. Physiology and behavior of the pigeon. New York, NY: Academic Press; 1983. pp. 245–266. [Google Scholar]
- Fagot J, Barbet I, Parron C, Deruelle C. Amodal completion by baboons (Papio papio): contribution of background depth cues. Primates. 2006;47:145–150. doi: 10.1007/s10329-005-0165-5. [DOI] [PubMed] [Google Scholar]
- Fujita K. Perceptual completion in rhesus monkeys (Macaca mulatta) and pigeons (Columba livia) Perception and Psychophysics. 2001;63:115–125. doi: 10.3758/bf03200507. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ushitani T. Better living by not completing: A wonderful peculiarity of pigeon vision? Behavioural Processes. 2005;69:59–66. doi: 10.1016/j.beproc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Forkman B. Hens use occlusion to judge depth in a two-dimensional picture. Perception. 1998;27:861–867. doi: 10.1068/p270861. [DOI] [PubMed] [Google Scholar]
- Forkman B, Vallortigara G. Minimization of modal contours: An essential cross-species strategy in disambiguating relative depth. Animal Cognition. 1999;2:181–185. [Google Scholar]
- Gibson BM, Wasserman EA, Frei L, Miller K. Recent advances in operant conditioning technology: A versatile and affordable computerized touchscreen system. Behavior Research Methods, Instruments, & Computers. 2004;36:355–362. doi: 10.3758/bf03195582. [DOI] [PubMed] [Google Scholar]
- Giersch A, Humphreys GW, Boucart M, Kovács I. The computation of occluded contours in visual agnosia: Evidence for early computation prior to shape binding and figure-ground coding. Cognitive Neuropsychology. 2000;17:731–759. doi: 10.1080/026432900750038317. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Filling-in the forms: Surface and boundary interactions in visual cortex. In: Pessoa L, DeWeerd P, editors. Filling-in: From perceptual completion to cortical reorganization. New York, NY: Oxford University Press; 2003. pp. 13–37. [Google Scholar]
- Ikebuchi M, Okanoya K. Male zebra finches and Bengalese finches emit directed songs to the video images of conspecific females projected onto a TFT display. Zoological Science. 1999;16:63–70. [Google Scholar]
- Johnson SP. Development of fragmented versus holistic object perception. In: Schwarzer G, Leder H, editors. The development of face processing. Ashland, OH: Hogrefe & Huber Publishers; 2003. pp. 3–17. [Google Scholar]
- Jusczyk PW, Johnson SP, Spelke ES, Kennedy LJ. Synchronous change and perception of object unity: Evidence from adults and infants. Cognition. 1999;71:257–288. doi: 10.1016/s0010-0277(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Kanizsa G. Organization in Vision: Essays on Gestalt Perception. New York, NY: Praeger; 1979. [Google Scholar]
- Kanizsa G, Renzi P, Conte S, Compostela C, Guerani L. Amodal completion in mouse vision. Perception. 1993;22:713–721. doi: 10.1068/p220713. [DOI] [PubMed] [Google Scholar]
- Kellman PJ. Visual perception of objects and boundaries: A four-dimensional approach. In: Kimchi R, Behrmann M, Olson CR, editors. Perceptual organization in vision: behavioral and neural perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 155–201. [Google Scholar]
- Kellman PJ, Guttman S, Wickens T. Geometric and neural models of contour and surface interpolation in visual object perception. In: Shipley TF, Kellman PJ, editors. From fragments to objects: Segmentation and grouping in vision. New York, NY: Elsevier Science; 2001. pp. 183–245. [Google Scholar]
- Kellman PJ, Shipley TF. A theory of visual interpolation in object perception. Cognitive Psychology. 1991;23:141–221. doi: 10.1016/0010-0285(91)90009-d. [DOI] [PubMed] [Google Scholar]
- Kellman PJ, Spelke ES. Perception of partially occluded objects in infancy. Cognitive Psychology. 1983;15:483–524. doi: 10.1016/0010-0285(83)90017-8. [DOI] [PubMed] [Google Scholar]
- Kellman PJ, Spelke ES, Short KR. Infant perception of object unity from translatory motion in depth and vertical translation. Child Development. 1986;57:72–76. [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA, Biederman I. Pigeons’ recognition of partially occluded objects depends on specific training experience. Perception. 2007;36:33–48. doi: 10.1068/p5583. [DOI] [PubMed] [Google Scholar]
- Lea SEG, Slater AM, Ryan CME. Perception of object unity in chicks: A comparison with the human infant. Infant Behavior and Development. 1996;19:501–504. [Google Scholar]
- Lorenceau J, Shiffrar M. The role of terminators in motion integration across contours. Vision Research. 1992;32:263–273. doi: 10.1016/0042-6989(92)90137-8. [DOI] [PubMed] [Google Scholar]
- Michotte A, Thinès G, Crabbé G. Amodal completion of perceptual structures. In: Thinès G, Costall A, Butterworth G, editors. Michotte&’s experimental phenomenology of perception. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 140–167. (Original work published 1964) [Google Scholar]
- Murray SO, Kersten D, Olshausen BA, Schrater P, Woods DL. Shape perception reduces activity in human primary visual cortex. Proceedings of the National Academy of Sciences of the United State of America. 2002;99:15164–15169. doi: 10.1073/pnas.192579399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka Y, Osada Y. Subjective contours, amodal completion, and transparency in animals. Japanese Journal of Animal Psychology. 2000;50:63–73. [Google Scholar]
- Nakayama K, Shimojo S, Silverman GH. Stereoscopic depth: Its relation to image segmentation, grouping, and recognition of partially occluded objects. Perception. 1989;18:55–68. doi: 10.1068/p180055. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Takahashi M. Shikaku-teki hokan e no seitaigaku-teki apuroochi [Ecological approach to visual completion] Kokoro no hattatsu: ninchi-teki seicho no kikoo 1999. 2000:34–41. Reports of the Grant-in-aid for Scientific Research for Priority Areas. [Google Scholar]
- Osada Y, Schiller PH. Can monkeys see objects under conditions of transparency and occlusion? Investigative Ophthalmology & Visual Science. 1994;35:1664. [Google Scholar]
- Palmer SE. Organizing objects and scenes. In: Palmer SE, editor. Vision science – photons to phenomenology. Cambridge, MA: MIT Press; 1999. pp. 254–310. [Google Scholar]
- Quinn PC, Brown CR, Streppa ML. Perceptual organization of complex visual configurations by young infants. Infant Behavior & Development. 1997;20:35–46. [Google Scholar]
- Regolin L, Marconato F, Vallortigara G. Hemispheric differences in the recognition of partly occluded objects by newly hatched domestic chicks (Gallus gallus) Animal Cognition. 2004;7:162–170. doi: 10.1007/s10071-004-0208-0. [DOI] [PubMed] [Google Scholar]
- Regolin L, Vallortigara G. Perception of partially occluded objects by young chicks. Perception & Psychophysics. 1995;57:971–976. doi: 10.3758/bf03205456. [DOI] [PubMed] [Google Scholar]
- Rensink RA, Enns JT. Early completion of occluded objects. Vision Research. 1998;38:2489–2505. doi: 10.1016/s0042-6989(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Sato A, Kanazawa S, Fujita K. Perception of object unity in a chimpanzee (Pan troglodytes) Japanese Psychological Research. 1997;39:191–199. [Google Scholar]
- Schiller PH. Effect of lesions in visual cortical area V4 on the recognition of transformed objects. Nature. 1995;376:342–344. doi: 10.1038/376342a0. [DOI] [PubMed] [Google Scholar]
- Sekuler AB, Lee JA, Shettleworth SJ. Pigeons do not complete partially occluded figures. Perception. 1996;25:1109–1120. doi: 10.1068/p251109. [DOI] [PubMed] [Google Scholar]
- Sekuler AB, Murray RF. Amodal completion: A case study in grouping. In: Shipley TF, Kellman PJ, editors. From fragments to objects–Segmentation and Grouping in vision. New York, NY: Elsevier Science; 2001. pp. 265–293. [Google Scholar]
- Shiffrar M, Lorenceau J. Increased motion linking across edges with decreased luminance contrast, edge width and duration. Vision Research. 1996;36:2061–2067. doi: 10.1016/0042-6989(95)00283-9. [DOI] [PubMed] [Google Scholar]
- Shimizu T. Conspecific recognition in pigeons (Columba livia) using dynamic video images. Behaviour. 1998;135:13–53. [Google Scholar]
- Sugita Y. Grouping of image fragments in primary visual cortex. Nature. 1999;401:269–272. doi: 10.1038/45785. [DOI] [PubMed] [Google Scholar]
- Ushitani T, Fujita K. Pigeons do not perceptually complete partially occluded photos of food: An ecological approach to the “pigeon problem. ”. Behavioural Processes. 2005;69:67–78. doi: 10.1016/j.beproc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Ushitani T, Fujita K, Yamanaka R. Do pigeons (Columba livia) perceive object unity? Animal Cognition. 2001;4:153–161. doi: 10.1007/s100710100088. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Furuya I. Video display for study of avian visual cognition: From psychophysics to sign language. International Journal of Comparative Psychology. 1997;10:111–127. [Google Scholar]
- Yamaguchi T, Kitamura K, Ito M. The effect of visual displays for stimulus presentation on pigeons’ discrimination of paintings. Japanese Journal of Animal Psychology. 2003;53:11–15. [Google Scholar]
- Yin C, Kellman PJ, Shipley TF. Surface completion complements boundary interpolation in the visual integration of partly occluded objects. Perception. 1997;26:1459–1479. doi: 10.1068/p261459. [DOI] [PubMed] [Google Scholar]
- Yin C, Kellman PJ, Shipley TF. Surface integration influences depth discrimination. Vision Research. 2000;40:1969–1978. doi: 10.1016/s0042-6989(00)00047-x. [DOI] [PubMed] [Google Scholar]