Abstract
Tests of fluid intelligence predict success in a wide range of cognitive activities. Much uncertainty has surrounded brain lesions producing deficits in these tests, with standard group comparisons delivering no clear result. Based on findings from functional imaging, we propose that the uncertainty of lesion data may arise from the specificity and complexity of the relevant neural circuit. Fluid intelligence tests give a characteristic pattern of activity in posterolateral frontal, dorsomedial frontal, and midparietal cortex. To test the causal role of these regions, we examined fluid intelligence in 80 patients with focal cortical lesions. Damage to each of the proposed regions predicted fluid intelligence loss, whereas damage outside these regions was not predictive. The results suggest that coarse group comparisons (e.g., frontal vs. posterior) cannot show the neural underpinnings of fluid intelligence tests. Instead, deficits reflect the extent of damage to a restricted but complex brain circuit comprising specific regions within both frontal and posterior cortex.
Keywords: neuropsychology, frontoparietal cortex, focal brain lesions, cognitive control, IQ
Universal positive correlations between performance on different kinds of task led Spearman (1) to propose that some general or g factor contributes to success in all kinds of cognitive activity. In factor analytic studies, the best single tests of g involve “fluid intelligence” or novel problem-solving (2). Strong performance in such tests is predictive of broad success in many different kinds of cognitive activity, from laboratory tasks to educational and work achievements.
It remains an open question what cognitive or neural processes are measured by fluid intelligence tests. One popular hypothesis (3, 4) links tests of this sort to broad cognitive control functions of frontal and parietal cortex. Examples might include selective activation or bias of cognitive processing (5, 6), detection and use of cognitive conflict (7), assembly and use of sequential mental programs (8, 9), and many more. Although conceptions of cognitive control may vary, such control functions undoubtedly are of importance in many different kinds of behavior, providing a plausible cognitive underpinning for Spearman's proposal of g.
In human functional brain imaging, a strikingly similar pattern of activation is produced by many different cognitive demands, including increased perceptual difficulty, novelty, response conflict, working memory, episodic memory, and semantic memory (10–12). This multiple demand (MD) activity incorporates the lateral prefrontal cortex (LPFC) in and around the inferior frontal sulcus (IFS) and the anterior insula/frontal operculum (AI/FO), the dorsal anterior cingulate/presupplementary motor area (ACC/pre-SMA), a small region of the anterior frontal cortex (AFC), and the intraparietal sulcus (IPS). In putative monkey homologs of MD regions, including posterolateral prefrontal cortex, neural activity is shaped strongly by cognitive context, adapting to code many different kinds of task-relevant information. Broad activity in many different kinds of behavior is a requirement for neural systems linked to g (13, 14), and, indeed, functional imaging studies show strong MD activity during fluid intelligence tests (14, 15).
Important though these functional imaging results may be, they cannot establish whether MD regions have a causal role in supporting fluid intelligence. For this purpose lesion data are critical (16), but classically they have painted a confusing picture of brain systems linked to intelligence. Some authors have highlighted a special role of the frontal cortex (3), whereas others have claimed, conversely, that intelligence is preserved after frontal lobe damage (17). Others have reported similar deficits across frontal and parietal cortex (18). An important recent study showed correlations with g for lesions in several regions of left frontal and parietal cortex as well as for damage to major white matter tracts (19). In this study we examined the specific causal role of MD regions as defined by functional imaging.
Previous lesion work suffers from a number of potential limitations. One limitation concerns comparisons between coarse lesion groups (e.g., frontal vs. posterior). The MD hypothesis predicts deficits associated with specific, quite restricted regions of frontal and parietal damage. Here, we separated damage within and outside MD regions separately for patients with frontal, parietal, and occipitotemporal lesions. A second limitation concerns the link between deficit and specific lesion hotspots. In voxel-based methods, for example, deficits are separately correlated with damage to each separate voxel in the brain (20). When performance depends on a complex circuit, however, no one part of this circuit will be strongly correlated with behavior. Here we examined the separate and joint effects of damage to the different regions of the MD network. A third difficulty arises from the wide variation in fluid intelligence already existing in the normal population. If deficits are not large in comparison with preexisting variability, absolute performance may be linked only weakly to lesion location. To offset this difficulty, we used a prediction equation derived from normal controls to estimate premorbid ability in each patient and linked lesion data not to absolute performance but to estimated ability decrement.
Our results provide clear support for the MD hypothesis. Among 80 patients with stable, focal cerebral lesions, we find loss of fluid intelligence to be associated specifically with damage to MD regions.
Results
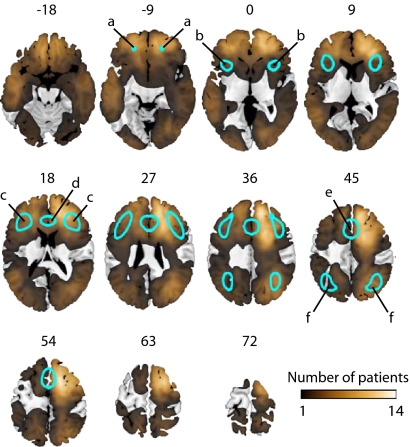
For each patient, current fluid intelligence was measured using two well-established tests (21, 22). The premorbid score on each test was estimated from a multiple regression equation, derived from healthy controls, predicting fluid intelligence score from patient age and reading vocabulary (23, 24). Each patient's lesion was traced onto an anatomical MRI and normalized to Montreal Neurological Institute (MNI) space. Only patients with lesions confined entirely to either frontal or posterior (occipital, temporal, and parietal) cerebral hemispheres were included. In MNI space, MD regions were derived from a prior review of functional activation in a diverse set of tasks (11), and comprised restricted areas of frontal and parietal cortex (Fig. 1). The distribution of lesions in our sample (Fig. 1) provided wide brain coverage both within and outside MD regions. Each patient's lesion was analyzed for volume of damage within the a priori-defined MD circuit as well as for total (whole-brain) lesion volume. Behavioral deficits (discrepancy between measured postmorbid and estimated premorbid scores) were correlated against these lesion characteristics and are reported with Pearson's r and accompanying one-tailed P value.
Fig. 1.
MD regions (blue outlines) shown on standard slices from the MNI template brain. Slices are numbered by z level in MNI space. MD regions encompass restricted areas of frontal and parietal cortex, including (a) anterior frontal cortex; (b) anterior insula/frontal operculum; (c) inferior frontal sulcus; (d) anterior cingulate; (e) presupplementary motor area; (f) intraparietal sulcus. Color scale shows lesion overlap for our patient sample; note wide brain coverage both within and outside the restricted MD regions.
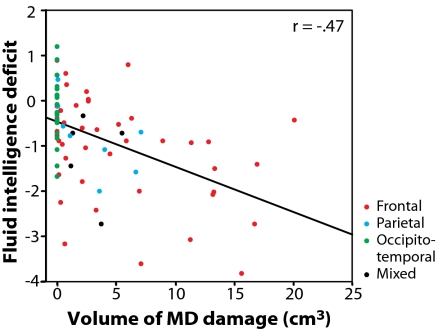
Our first question concerned the overall relation between fluid intelligence deficit and total volume of damage within the MD circuit. In the patient group as a whole (n = 80), fluid intelligence deficit was significantly correlated with total volume of MD lesion(r = −0.47; P < 0.001) (Fig. 2). A specific role for MD cortex would imply that this correlation should remain significant even when total lesion volume is partialled out. This result would show that, for fixed total lesion volume, deficit increases with increasing MD and decreasing non-MD tissue damage. Indeed, the correlation with MD lesion volume remained significant after partialling out whole-brain lesion volume (r = −0.32; P = 0.002).
Fig. 2.
Correlation between volume of damage to MD regions and fluid intelligence deficit in the whole patient group (n = 80). Fluid intelligence deficit indicates the average discrepancy between measured postmorbid and estimated premorbid score on two tests of novel problem solving (postmorbid minus premorbid).
A series of further analyses was conducted to clarify the basis for this result. First, we classified patients into three groups according to whether their lesion affected the frontal (n = 44), parietal (n = 9), or occipitotemporal (n = 22) lobe. Patients with lesions affecting more than one lobe were excluded from this analysis. We then carried out an analysis of covariance on behavioral deficit scores in the three groups, covarying lesion volume. This analysis revealed a significant difference between groups (F2,72 = 3.36; P = 0.040). Post hoc pairwise analyses revealed that the group difference was driven by preserved performance in the group with occipitotemporal lesions (no MD damage) relative to the group with frontal lesions (P = 0.012). Performance in the group with parietal lesions was intermediate.
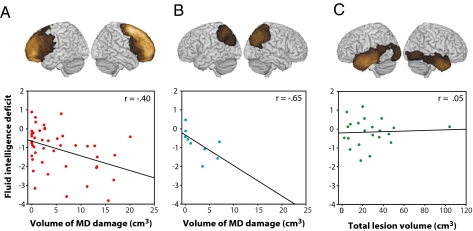
Next, we used multiple regressions to assess the prediction of fluid intelligence deficit from volumes of damage within and outside MD regions in each group separately. In the group with frontal lesions (n = 44), only MD lesion volume was retained as a significant predictor (r = −0.40; P = 0.004) (Fig. 3A). The correlation between behavioral deficit and MD lesion volume also remained significant if non-MD lesion volume was first partialled out (r = −0.27; P = 0.037). The same was true of the smaller group with parietal lesions (n = 9). Only the extent of MD damage was retained as a significant predictor (r = −0.63; P = 0.035) (Fig. 3B), and the correlation remained significant when non-MD lesion volume was partialled out (r = −0.65; P = 0.042). In the group with occipitotemporal lesions (n = 22), in which there was no MD damage, lesion volume was not correlated with behavioral deficit (r = 0.05; P = 0.41) (Fig. 3C).
Fig. 3.
Lesion characteristics predicting fluid intelligence deficit in patient subgroups. Individual regression lines show correlation between fluid intelligence deficit and (A) volume of MD damage in patients with frontal damage (n = 44), (B) volume of MD damage in patients with parietal damage (n = 9), and (C) whole-brain lesion volume in patients with occipitotemporal damage (n = 22). Icons above the graphs show lesion density overlap maps for each patient subgroup.
A further analysis estimated the magnitude of IQ deficit after MD damage compared with non-MD damage within the frontal lobe. For one of our tests—the Cattell Culture Fair—norms allow test scores to be transformed into conventional IQ scores (21). Using this test alone, we carried out a multiple regression predicting IQ deficit (measured postmorbid IQ minus estimated premorbid IQ) from volumes of MD and non-MD damage in the group of patients with frontal lesions. The multiple regression was significant (F2,42 = 4.44; P = 0.018), and, as before, the extent of MD damage was a significant predictor of IQ deficit after the extent of non-MD damage was partialled out (t43 = −1.77; P = 0.042), whereas the converse partial correlation was not significant (t43 = −1.35; P = 0.092). Regression slopes show that, after partialling out the contribution of non-MD damage, 10 cm3 of frontal MD damage causes a deficit of 6.4 IQ points, compared with 0.8 IQ points for each 10 cm3 of frontal cortex outside the MD network in the converse comparison. A similar result was obtained when data from all patients with frontal and posterior lesions were included in the regression (6.5 IQ points for MD damage compared with 1.0 IQ points for non-MD damage).
Finally, we tested the contribution of each MD region individually. For each region, correlation with the fluid intelligence deficit was tested after first partialling out the correlation with each of the other three MD regions and non-MD lesion volume. This analysis tests whether damage to each MD region contributes individually to the prediction of fluid intelligence loss after the effect of all of the other regions and non-MD volume has been taken into account. The partial correlations were significant for the LPFC (r = −0.31; P = 0.004), ACC/pre-SMA (r = −0.33; P = 0.002), AFC (r = −0.32; P = 0.003), and IPS (r = −0.29; P = 0.005) regions, suggesting that each of the MD regions made a unique contribution to fluid intelligence loss.
MD regions were defined by applying an arbitrary threshold to a previous set of functional imaging data derived from a wide range of tasks (Materials and Methods). The threshold was chosen to match typical functional activations on tasks designed to test fluid intelligence. Repeating our analyses using a range of different thresholds (Materials and Methods) did not change the pattern of results. Although the boundaries we have defined for MD regions cannot be exact, they perform well in defining regions of damage most closely linked to fluid intelligence deficit.
Discussion
In contrast to prior lesion studies of fluid intelligence (e.g., 3, 25, 26), we tested the role of a specific, distributed brain circuit. Based on findings from functional imaging, we predicted deficits from specific regions of damage in lateral frontal, dorsomedial frontal, and midparietal cortex. The results showed good convergence of functional imaging and lesion results. Within each of the predicted regions, volume of damage was predictive of fluid intelligence deficit, whereas outside these regions damage was not predictive. Because human brain lesions are variable and uncontrolled, anatomical conclusions from neuropsychological studies often are fairly coarse. Guided by functional imaging, however, our analysis defines surprisingly specific regions within frontal and parietal cortex with a causal role in fluid intelligence.
Certainly, fluid intelligence tests are complex, with alternative solutions to be developed and assessed, novel strategies to be considered, and multiple sources of information to be combined. A variety of cognitive control functions have been linked to frontal and parietal cortex, and certainly the parts of the MD system are heterogeneous in terms of cellular architecture, connectivity, and other factors. Possibly, different MD regions underlie different cognitive control functions (27, 28), all contributing to fluid intelligence; alternatively, individual cognitive control functions typically may depend on multiple MD regions. Further work is needed to clarify how the broad concept of fluid intelligence may be divided usefully into finer cognitive components.
Although g is well measured by tests of fluid intelligence, a second conventional method—used in tests like the Wechsler Adult Intelligence Scale (29)—is to average performance on a wide variety of different subtests. Although the average across subtests may be well correlated with fluid intelligence and with g, individual subtests have specific verbal, spatial, or other content. Correspondingly, factor analyses of such tests show separate as well as shared variance between subtests, and different subtests are affected by different patterns of brain damage (30). To the extent that the common element reflects fluid intelligence, however, our data suggest specific association with MD cortex (19).
Our data go well beyond previous demonstrations of fluid intelligence impairment after different cortical lesions (3). They show that impairment is associated most closely with damage to a surprisingly restricted frontoparietal system, incorporating the IFS, AI/FO, AFC, ACC/pre-SMA, and IPS. In line with the broad cognitive control functions of this circuit, our data show a causal role in fluid intelligence.
Materials and Methods
Participants.
Patients were recruited from the Cambridge Cognitive Neuroscience Research Panel (Cambridge, UK) (n = 70) and from the Institute of Cognitive Neurology Research Database (Buenos Aires, Argentina) (n = 10). Patients were selected for chronic, focal, adult-onset lesions restricted either to frontal or posterior cortex, of varied etiology (Table S1). Exclusion criteria were visual field cut, overt aphasia, preinsult history of epilepsy, or unsuitability for MRI. Mean age was 51.3 y (SD = 12.9 y). Following common neuropsychological practice, premorbid IQ was assessed using either the revised National Adult Reading Test (23) or the equivalent Word Accentuation Test (24), as appropriate for first language. Mean premorbid IQ was 109.1 (SD = 13.1). Control subjects (n = 33), recruited from the Medical Research Council Cognition and Brain Sciences Unit Volunteer Panel, were matched carefully to the patient group for age (mean age = 48.4 y; SD = 12.9 y) and premorbid IQ (mean = 109.5; SD = 12.3). All participants gave written informed consent to take part. The study was approved by the Cambridge Local Research Ethics Committee, Cambridge, UK.
Neuropsychological Assessment.
Patients and control subjects were assessed on two problem-solving tests with previously established high g loading: the Cattell Culture Fair (Scale 2 Form A) (21) and Letter Sets from the Educational Testing Service Kit of Factor-Referenced Tests (22). The Culture Fair consists of four timed sets of problems (series completions, odd-one-out, matrices, topological relations) involving geometrical figures. In Letter Sets problems, subjects must determine which of five sets of four letters differs in some way from the remainder.
Estimation of Deficit.
Data from controls were used to derive two multiple-regression equations, one predicting Culture Fair score from age and premorbid IQ, and the other similarly predicting Letter Sets score. These equations then were used to estimate patient premorbid scores. For each test, the estimated premorbid score was subtracted from the observed score and transformed to a z-score by dividing by the SD of residuals in the relevant control-group regression. Premorbid/current discrepancies from the two tests were averaged together to give a single measure of fluid intelligence deficit.
MD Regions.
MD regions were defined using data from a prior review of activity associated with a diverse set of cognitive demands (11), following the kernel method (31). To ensure symmetrical regions of interest, all peaks from the original review first were projected onto a single hemisphere. A point was placed at the location of each peak, and the resulting image was smoothed (15-mm FWHM) and thresholded at 3.5 times the height of a single peak. The resulting regions were mirrored onto the opposite hemisphere, producing bilateral regions in posterior LPFC extending from the posterior part of the IFS dorsally to the AI/FO ventrally (center of mass in MNI space ±38 25 21), AFC (±21 44–9), IPS (±35–58 41), and ACC/pre-SMA (±6 23 39). To examine the importance of the specific threshold chosen, analyses were repeated at thresholds ranging from 1.75 to 4.375 times peak height.
Neuroradiological Assessment.
T1-weighted spoiled gradient-recalled MRI scans (resolution 1 × 1 × 2 mm) were acquired for all patients. Lesions were traced by a neurologist (F.M.) who was blind to experimental results, and scans subsequently were normalized using cost-function lesion masking (32). The derived normalization parameters then were used to normalize lesion tracings, which were used to calculate whole-brain, MD, and non-MD lesion volumes. Lesion tracing was carried out using MRIcro (www.mricro.com; ref. 33); normalization and volume calculations were performed using SPM5 (www.fil.ion.ucl.ac.uk/spm).
Supplementary Material
Acknowledgments
This work was funded by the Medical Research Council (UK) Intramural Program U.1055.01.001.00001.01. A.W. was supported by a Domestic Research Studentship/Millennium Scholarship funded by the University of Cambridge and the Newton Trust. F.M. was funded by grants from the Institute of Cognitive Neurology and the Libertad y Desarrollo Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007928107/-/DCSupplemental.
References
- 1.Spearman C. The Abilities of Man. New York: Macmillan; 1927. [DOI] [PubMed] [Google Scholar]
- 2.Cattell RB. Abilities: Their Structure, Growth and Action. Boston: Houghton-Mifflin; 1971. [Google Scholar]
- 3.Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- 4.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. discussion 154–187. [DOI] [PubMed] [Google Scholar]
- 5.Norman DA, Shallice T. Chip Report 99. La Jolla, CA: Univ of California, San Diego; 1980. Attention to Action: Willed and Automatic Control of Behaviour. [Google Scholar]
- 6.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 7.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 8.Duncan J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Luria AR. Higher Cortical Functions in Man. London: Tavistock; 1966. [Google Scholar]
- 10.Duncan J. EPS Mid-Career Award 2004: Brain mechanisms of attention. Q J Exp Psychol (Colchester) 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- 11.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 12.Nyberg L, et al. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- 13.Duncan J, et al. Goal neglect and Spearman's g: Competing parts of a complex task. J Exp Psychol Gen. 2008;137:131–148. doi: 10.1037/0096-3445.137.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Duncan J, et al. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 15.Lee KH, et al. Neural correlates of superior intelligence: Stronger recruitment of posterior parietal cortex. Neuroimage. 2006;29:578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Nachev P, Mah YH, Husain M. Functional neuroanatomy: The locus of human intelligence. Curr Biol. 2009;19:R418–R420. doi: 10.1016/j.cub.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 18.Warrington EK, James M, Maciejewski C. The WAIS as a lateralizing and localizing diagnostic instrument: A study of 656 patients with unilateral cerebral lesions. Neuropsychologia. 1986;24:223–239. doi: 10.1016/0028-3932(86)90055-2. [DOI] [PubMed] [Google Scholar]
- 19.Gläscher J, et al. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci USA. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates E, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 21.Institute for Personality and Ability Testing . Measuring Intelligence with the Culture Fair Tests. Champaign, IL: Institute for Personality and Ability Testing; 1973. [Google Scholar]
- 22.Ekstrom RB, French JW, Harmon HH, Derman D. ETS Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- 23.Nelson HE. National Adult Reading Test. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- 24.Burin DI, Jorge RE, Arizaga RA, Paulsen JS. Estimation of premorbid intelligence: The word accentuation test—Buenos Aires version. J Clin Exp Neuropsychol. 2000;22:677–685. doi: 10.1076/1380-3395(200010)22:5;1-9;FT677. [DOI] [PubMed] [Google Scholar]
- 25.Tranel D, Manzel K, Anderson SW. Is the prefrontal cortex important for fluid intelligence? A neuropsychological study using Matrix Reasoning. Clin Neuropsychol. 2008;22:242–261. doi: 10.1080/13854040701218410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso A, De Renzi E, Faglioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligence tasks. Brain. 1973;96:715–728. doi: 10.1093/brain/96.4.715. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 28.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- 30.Gläscher J, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusack R, Mitchell DJ, Duncan J. Discrete object representation, attention switching, and task difficulty in the parietal lobe. J Cogn Neurosci. 2010;22:32–47. doi: 10.1162/jocn.2009.21194. [DOI] [PubMed] [Google Scholar]
- 32.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- 33.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.