Abstract
Synaptic vesicle fusion at many synapses has been kinetically separated into two distinct Ca2+-dependent temporal components consisting of a rapid synchronous phase followed by a slower asynchronous component. Mutations in the synaptic vesicle Ca2+ sensor Synaptotagmin 1 (Syt 1) reduce synchronous neurotransmission while enhancing the slower asynchronous phase of release. Syt 1 regulation of vesicle fusion requires interactions mediated by its tandem cytoplasmic C2 domains (C2A and C2B). Although Ca2+ binding by Syt 1 is predicted to drive synchronous release, it is unknown if Ca2+ interactions with either C2 domain is required for suppression of asynchronous release. To determine if Ca2+ binding by Syt 1 regulates these two phases of release independently, we performed electrophysiological analysis of transgenically expressed Syt 1 mutated at Ca2+ binding sites in C2A or C2B in the background of Drosophila Syt 1-null mutants. Transgenic animals expressing mutations that disrupt Ca2+ binding to C2A fully restored the synchronous phase of neurotransmitter release, whereas the asynchronous component was not suppressed. In contrast, rescue with Ca2+-binding mutants in C2B displayed little rescue of the synchronous release component, but reduced asynchronous release. These results suggest that the tandem C2 domains of Syt 1 play independent roles in neurotransmission, as Ca2+ binding to C2A suppresses asynchronous release, whereas Ca2+ binding to C2B mediates synchronous fusion.
Keywords: Drosophila, neuromuscular junction, synaptic transmission, electrophysiology
A hallmark of neuronal communication is the rapid millisecond time scale for synaptic information transfer. The speed of synaptic transmission encompasses a sequence of molecular events, including Ca2+ influx into the presynaptic terminal, Ca2+ triggering of synaptic vesicle fusion, and diffusion and binding of neurotransmitters to postsynaptic receptors. The Ca2+-triggering step for synaptic vesicle fusion is regulated by the synaptic vesicle protein Synaptotagmin 1 (Syt 1). Synaptotagmins are a large family of single-pass transmembrane proteins found on diverse populations of intracellular vesicles, with the Syt 1 subfamily localized to synaptic vesicles (1–3). Synaptotagmins consist of a N-terminal transmembrane segment followed by a cytoplasmic domain with two Ca2+-binding C2 domains. Although C2A and C2B share similar topology, distinct effectors for each domain have been identified (4).
Genetic perturbation studies of Syt 1 have demonstrated an essential role in neurotransmitter release (5–8). Our previous analysis at Drosophila embryonic neuromuscular junctions (NMJs) indicated Syt 1 functions to synchronize rapid fusion of synaptic vesicles to action potentials while reducing a slower asynchronous component of fusion (9). Together with studies at mammalian synapses (10–13), current data support a two Ca2+ sensor model for neurotransmitter release (14), with Syt 1 functioning as the fast synchronous Ca2+ sensor and a second unknown Ca2+ sensor mediating the slower asynchronous phase of release. The synchronizing function of synaptotagmin has also been observed at mammalian systems, with enhanced asynchronous release found at cultured autaptic synapses from Syt 1-KO mice (11, 15) and calyx of Held synapses from Syt 2-KO mice (13). Although the mechanism by which Syt 1 synchronizes release is still being elucidated, it may directly trigger fast vesicle fusion while inhibiting asynchronous release mediated by a second Ca2+ sensor (9). Syt 1 could also suppress asynchronous release by directly competing with the asynchronous Ca2+ sensor for the release machinery (11, 16). Whether Ca2+ binding to Syt 1 is required for these distinct roles in synchronous versus asynchronous release is unclear. To date, Ca2+ binding mutations in the Syt 1 C2A domain have displayed mild phenotypes, whereas C2B Ca2+ binding mutations show profound defects in release (9, 16–18). Here, we compare synchronous and asynchronous release in C2A and C2B Ca2+ binding mutants and find striking contrasts in their effects on neurotransmission. These results indicate that Ca2+ binding to the Syt 1 C2A domain is required for suppression of the asynchronous component of release, whereas Ca2+ binding to C2B triggers synchronous fusion.
Results
To determine the physiological consequences on synaptic vesicle fusion caused by disrupting Ca2+ binding to the C2A or C2B domain of Syt 1, we generated transgenic Drosophila expressing Syt 1 with mutations in essential Ca2+-binding residues. The C2 domains of Syt 1 form a compact β-sandwich with two Ca2+-binding loops at the apex that penetrate membrane bilayers containing negatively charged lipids. Five highly conserved acidic aspartate residues are ordered within the loops emerging from the C2 domain and directly participate in coordinating Ca2+ binding to Syt 1 (18, 19). For this analysis, Ca2+-binding aspartates were neutralized to asparagines at the key D3 and D4 position of each C2 domain: D282N and D284N in C2A and D416N and D418N in C2B (Fig. 1A). These mutations disrupt Ca2+ binding to the C2A (20) or C2B (17) domains of Syt 1. We expressed the UAS transgenes using GAL 4 under the control of the promoter of the pan-neuronal gene, elav (21), in a Syt 1AD4/Syt 1N13–null background. Immunostaining with anti-Syt 1 antisera (22) demonstrated that the transgenic WT Syt 1 protein, as well as the transgenic C2A and C2B mutant proteins, localized properly at NMJs (Fig. 1B) and expressed at similar levels (Fig. S1).
Fig. 1.
Generation and localization of Syt 1 Ca2+-binding mutations. (A) Schematic diagrams of essential residues that coordinate Ca2+ binding to the Syt 1 C2A and C2B domain, rendered according to Ferandez et al. (18). The key Ca2+ binding D3 and D4 aspartate residues (highlighted in blue) were neutralized to asparagines in the C2A or C2B domain. (B) Immunostaining of hatching stage (21–24 h after fertilization) embryonic NMJs with anti-HRP (green), a neuronal membrane marker to highlight presynaptic terminals, and anti-Syt 1 (magenta). The merged signal is displayed in the bottom image. WT, as well as C2A and C2B mutant (indicated with D > N) proteins from transgenes, show similar levels and distribution of Syt 1 compared with endogenous protein in WT embryos (Left). Null mutants lack anti-Syt 1 immunoreactivity. (Scale bar, 5 μm.)
To examine evoked release properties in Syt 1 rescued animals, we stimulated motor nerves in 4 mM extracellular Ca2+, in which the asynchronous component is obvious, and recorded synaptic currents using whole-cell voltage clamp from body wall muscle number 6 in Drosophila embryos (Fig. 2). As previously observed, Syt 1-null mutants eliminated the synchronous component of release. In contrast, a slower asynchronous release component was uncovered, and it displayed a 100-msec time constant when the latency histogram was fit by an exponential curve. Asynchronous release following an action potential was not confounded from spontaneous miniature synaptic currents, as their frequency is significantly less than 0.1 Hz at these synapses, which have only 20 to 30 active zones at this stage of development. Syt 1-null mutants rescued with a WT Syt 1 transgene showed indistinguishable evoked synaptic currents from WT animals with endogenous Syt 1 (9). Consequently, we used the WT rescued strain as a control for further experiments. Evoked release was fully synchronous, and few asynchronous release events were observed in rescued animals (Fig. 2). To compare the total amount of release observed at Syt 1-null mutant synapses with the control, we measured cumulative charge transfer following stimulation. Total charge, reflecting the number of released vesicles, was reduced by 97.5% in Syt 1-null mutants compared with WT rescues. This finding contrasts with results from mammalian Syt 1 mutant autapses, in which total charge transfer is similar to WT (11, 23). The data more closely matches studies from the calyx of Held synapse in Syt 2-KO mice (13) and from dissociated cultures from Syt 1-KO mice (23), in which total charge is reduced compared with controls. These differences may reflect distinct releasable pool sizes at the various synapses. In summary, we find that Syt 1-null synapses shows asynchronous release with only 2.5% of the total amount of release compared with WT rescues.
Fig. 2.
Altered release properties in Syt 1 C2A and C2B Ca2+ binding mutants. (A) Representative traces of synaptic currents at muscle fiber 6 of hatching stage embryos (21 h after fertilization) evoked by motor nerve stimulation from the genotypes as indicated in Fig. 1. Two representative traces are shown for each genotype. Stimulation artifacts signal the onset of nerve stimulation, indicated by arrows. Arrowheads indicate asynchronous release in null mutants and null animals rescued with C2A mutant Syt 1. (B) Synaptic charge transfer following simulation calculated from the synaptic current. Most synchronous release occurs within 10 ms of stimulation (9). The asynchronous component has an exponential distribution with a time constant of approximately 100 ms, with 95% of asynchronous release occurring within 300 msec after stimulation. We thus quantified the asynchronous component as release events occurring between 10 and 300 ms. (C) Expanded bar graphs quantifying the asynchronous release components for each genotype. (D) Total synaptic charge transfer, including the asynchronous and synchronous components, for each genotype. For C and D, the four groups were analyzed with the Kruskal-Wallis test using a one-way ANOVA by ranks, and significant differences between the groups were found (P < 0.01). **P < 0.01 by Dunn post-hoc multiple comparison test; n = 50 in each genotype. Error bars are SEM. In B–D, WT refers to null mutants rescued with WT Syt 1, and “C2A D > N” and “C2B D > N” refer to null mutants rescued with Ca2+ binding mutants described in Fig. 1. The physiological saline solution contained 4 mM Ca2+ to highlight the asynchronous release component (9).
To analyze the respective contributions of Ca2+ binding to C2A and C2B for Syt 1 function, we compared release properties in transgenic animals expressing a C2A (Syt 1D282N, D284N) or C2B (Syt 1D416N, D418N) mutant in the Syt 1-null background. Striking differences in the effects on the number and kinetics of synaptic vesicle fusion were observed in C2A versus C2B Ca2+ binding mutants. Synapses expressing the C2A domain mutation displayed similar levels of evoked release as WT rescues at 4 mM Ca2+. However, asynchronous release (arrowheads in Fig. 2A) was even more prominent than at Syt 1-null synapses (Fig. 2 B and C). Total charge transfer at C2A mutant synapses was similar to WT rescues (Fig. 2D). In contrast, synapses expressing a Syt 1 transgenic protein with C2B domain mutations largely suppressed the asynchronous component of release (Fig. 2 A–C), similar to synapses expressing WT Syt 1. In contrast, only a small amount of residual synchronous release was observed, and total charge transfer was dramatically reduced, similar to Syt 1-null synapses (Fig. 2 B and D). To precisely analyze the timing of synaptic vesicle fusion in C2A and C2B mutants, we performed a detailed latency analyses (Fig. 3). The latency histogram revealed a large asynchronous release component in C2A mutant rescued animals, which is more enhanced than that observed in null mutants (Fig. 3B). We next assayed spontaneous release rates during the 0.5- to 1-s interval when asynchronous release has subsided. Similar to studies of Syt 1 function at mammalian synapses (24), mutations in C2A or C2B Ca2+-binding sites caused enhanced rates of spontaneous release compared with control or null mutants (Fig. 3C). The presence of robust asynchronous release in the C2A mutant rescue suggests an important role for Ca2+–C2A interactions in suppressing this slower component of fusion. The rescue of the synchronous phase of release by C2A mutants indicates that Ca2+–C2A interactions are not essential for Ca2+-dependent fast neurotransmitter release at Drosophila NMJ synapses. In contrast, the reduction of evoked release by 97% in C2B mutant rescue animals suggests that the Syt 1 C2B domain is the major Ca2+ sensor for synchronous neurotransmitter release. These results separate the requirement for the two C2 domains in the different components of release and indicate that Ca2+–C2B interactions are not essential for suppression of asynchronous fusion.
Fig. 3.
Latency analyses of nerve-evoked synaptic currents. (A) Latencies of synaptic currents within 1 s following nerve stimulation were measured and plotted for each genotype as indicated in Fig. 1. All latencies were counted for each 10-ms bin and presented as the number of events per stimulation in each bin. Examples containing spontaneous bursting activity originating from the CNS were excluded from the analysis. A small number of events triggered by endogenous action potentials, as well as spontaneous miniature release, were observed as random fusion events unrelated to stimulation in the background in each panel. The physiological saline solution contained 4 mM Ca2+ to clearly demonstrate asynchronous release component (9). Bottom: Data shown with enlarged y axis to highlight asynchronous release component. Note that the C2A mutant rescue displays a clear asynchronous component, whereas the C2B mutant rescue does not. In null mutants (Left), the first bin does not show increased height compared with other bins, indicating no contribution of a synchronous component with the exponential distribution of asynchronous release component (9). The numbers analyzed for each genotype were: Syt 1−/− (n = 1,100), WT rescue (n = 471), C2A mutant rescue (n = 429), and C2B mutant rescue (n = 395). (B) Quantification of asynchronous release. We counted release events occurring between 10 and 300 ms per stimulation as the asynchronous component in the same experiments for A. The numbers analyzed for each genotype were the same as those of A. The four groups were analyzed with the Kruskal-Wallis test using a one-way ANOVA by ranks, and significant difference between the groups was found (P < 0.01). **P < 0.01 and *P < 0.05 by Dunn post hoc multiple comparison test. Error bars are SEM. (C) Quantification of spontaneous release. Synaptic currents were recorded between 0.5 s and 1 s after stimulation in the same experiments for A when asynchronous release has subsided. Release frequency per second was calculated per animal. Given occasional sparse action potential firings in motor neurons, these values are an overestimate of miniature release occurring at presynaptic terminals. Results were averaged between animals. The numbers of animal for each genotype were: Syt 1−/− (n = 8), WT rescue (n = 6), C2A mutant rescue (n = 4), and C2B mutant rescue (n = 4). The four groups were analyzed with one-way ANOVA, and significant difference between the groups was found (P < 0.01). **P < 0.01 by Tukey post hoc multiple-comparison test between the WT rescue and each mutant rescue strain. In B and C, WT refers to null mutants rescued with WT Syt 1, and “C2A D > N” and “C2B D > N” refer to null mutants rescued with Ca2+ binding mutants described in Fig. 1.
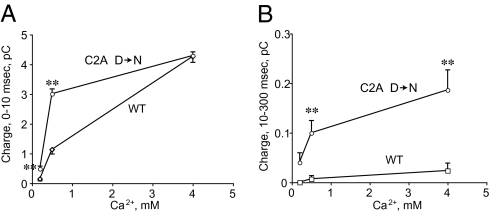
To further characterize the role of the Syt 1 C2A domain in release, we extended the analysis of the synchronous and asynchronous release components at C2A mutant synapses. In addition, we examined the Ca2+ dependence of synaptic transmission in the C2A mutant rescues compared with WT rescues (Fig. 4). Surprisingly, synapses expressing the C2A domain mutation displayed larger synaptic currents at lower Ca2+ concentrations, not only in the asynchronous component (Fig. 4B), but also in synchronous release (Fig. 4A). In high extracellular Ca2+ (4 mM), synchronous release in WT rescued animals was comparable to that observed in C2A mutant rescued strains. These findings are consistent with the hypothesis that reduced suppression of synaptic vesicle fusion via the C2A domain enhances vesicle release.
Fig. 4.
Comparison of the Ca2+ dependency of release between WT and C2A mutant rescues. (A) The synchronous release component measured as charge transfer within 10 ms following nerve stimulation is plotted. Note that release at 0.2 mM and 0.5 mM Ca2+ is significantly larger in the C2A mutant rescue that in WT rescue. (B) The asynchronous release component measured as charge transfer from 10 ms to 300 ms following stimulation is plotted. Note that the C2A mutant rescue shows a dramatic enhancement of asynchronous release at all Ca2+ concentrations tested. **P < 0.01 by Mann-Whitney U test. Asynchronous release at 0.2 mM in WT rescue was too rarely observed to allow statistical analysis; n = 50 for each Ca2+ concentration in each genotype. Error bars are SEM. WT refers to null mutants rescued with WT Syt 1 and “C2A D > N” refers to null mutants rescued with the C2A Ca2+ binding mutant described in Fig. 1.
Previous studies in Drosophila demonstrated that Syt 1-null mutants have a smaller releasable vesicle pool as revealed by hypertonic stimulation (9, 25). This reduction in the releasable vesicle pool is hypothesized to result from a lack of Syt 1 binding to the soluble N-ethylmaleimide–sensitive fusion protein attachment protein receptor (SNARE) complex, the fusion machinery required for synaptic vesicle release (26). The loss of SNARE binding by Syt 1 may disrupt intimate docking of synaptic vesicles with the plasma membrane, resulting in loss of synchronous fusion and reduction in hypertonic-induced release. To determine whether the phenotypes observed in C2A and C2B mutations are caused by abnormalities in synaptic vesicle pool size, we performed hypertonic stimulation to measure fusion-competent vesicles. As shown in Fig. 5, synapses expressing either transgenic Syt 1 protein with C2A or C2B mutations rescued hypertonic-induced release to the same level as that observed in WT Syt 1 rescued animals. These results indicate that phenotypes observed at C2A and C2B mutant synapses are not secondary to abnormal vesicle pool sizes. In the case of the C2B mutant transgene, which shows only residual levels of synchronous release, the findings imply that synaptic vesicles are normally docked and fusion-competent, but cannot be triggered to fuse by Ca2+ influx. In summary, the defects in evoked transmission at synapses expressing C2A and C2B mutations reflect changes in release properties, as opposed to alterations in synaptic vesicle pool size.
Fig. 5.
Hypertonic-induced release reveals restoration of the readily releasable pool in the C2A and C2B mutant rescues. (A) Sample traces of hypertonic-induced release for each genotype. Hypertonic stimulated release was achieved by puffing 500 mM sucrose solution onto the NMJ for 3 s (bars) in the absence of Ca2+. (B) Quantification of hypertonic stimulated release for null mutants and WT, C2A, and C2B mutant rescues. Miniature synaptic currents occurring within 5 s after the onset of stimulation were counted. The four groups were analyzed with the Kruskal-Wallis test using a one-way ANOVA by ranks, and significant difference between the groups was found (P < 0.01). **P < 0.01 by Dunn post-hoc multiple comparison test between the null and each rescue strain. The numbers of samples analyzed for each genotype were: Syt 1−/− (n = 9), WT rescue (n = 18), C2A mutant rescue (n = 25), and C2B mutant rescue (n = 20). Error bars are SEM.
Discussion
Neurotransmitter release at synapses is regulated by two kinetically distinct Ca2+ sensors. A low-affinity Ca2+ sensor mediates the rapid synchronous component of transmitter release, whereas a second Ca2+ sensor supports a slower asynchronous phase of fusion. Syt 1 has emerged as the primary candidate for the low-affinity Ca2+ sensor promoting synchronous release (reviewed in refs. 27–29). How Syt 1 and the asynchronous Ca2+ sensor regulate the two distinct phases of release, and whether there are competitive interactions between the two fusion pathways, are unclear. To examine the requirements of Ca2+ binding to the C2A or C2B domain of Syt 1 in regulating the kinetics of vesicle fusion, we rescued Syt 1-null mutants with transgenes disrupting Ca2+ binding to either domain. Both C2A and C2B Ca2+ binding mutants fully restored the releasable synaptic vesicle pool triggered by hypertonic stimulation, suggesting Ca2+ binding to Syt 1 is not required for its role in supplying the readily releasable pool through vesicle docking. Alternatively, Ca2+ binding to either C2 domain may be sufficient for supplying the readily releasable pool. Further studies with mutations disrupting Ca2+ binding to both C2 domains will be required to test these alternative models. Recordings of nerve-evoked synaptic currents revealed contrasting roles for the two C2 domains in triggering fusion. Transgenic rescue animals expressing Syt 1 with C2A mutations displayed enhanced asynchronous release and retained robust synchronous fusion, whereas Syt 1 with C2B mutations largely suppressed the asynchronous component, but limited ability to rescue synchronous fusion. These observations suggest distinct roles for Syt 1 during the fusion process. First, the Syt 1 C2B domain is the major Ca2+ sensor for fast synchronous neurotransmitter release, whereas C2A Ca2+ binding is dispensable for triggering synaptic vesicle fusion. Second, Ca2+ binding to the C2A domain is critical for suppressing asynchronous release, whereas C2B Ca2+ binding is not essential in this role. Third, the enhanced asynchronous release observed in the absence of Syt 1 does not require reduction of the synchronous component, as observed in the C2A mutant rescue experiments. These data indicate that Syt 1 and the asynchronous Ca2+ sensor independently regulate synaptic vesicle fusion without mutually competing as previously proposed (16).
The role of the C2B domain of Syt 1 as a Ca2+ sensing module was suggested by its structure (18) and biochemical properties (30). Genetic manipulations of Syt 1 in Drosophila confirmed that Ca2+–C2B domain interactions were essential for synaptic neurotransmitter release (17), whereas Ca2+–C2A interactions were not (31). Critical roles for the C2B domain of Syt 1 were also demonstrated using transfection of C2B Ca2+ binding mutants into Syt 1-deficient hippocampal cultured neurons. This approach indicated that Ca2+ binding into the slot formed by the D2 and D3 aspartates of C2B were required for triggering vesicle fusion (16). Our C2B domain mutation analysis, which used disruptions of both the D3 and D4 aspartates, is consistent with these observations and support a critical role for the C2B domain of Syt 1 in Ca2+-triggered synaptic vesicle fusion.
We previously demonstrated that vesicle fusion mediated by application of external salines containing high K+ or Ca2+ ionophores is larger in Syt 1-null mutants than WT animals (9), indicating an inhibitory function of Syt 1 in addition to its positive role in driving synchronous release. Our current studies indicate that Ca2+ binding to the C2B domain is dispensable for the suppression of asynchronous release, suggesting Ca2+-C2A–dependent interactions can negatively regulate the slow component of fusion. These observations are consistent with studies at mammalian synapses, as Syt 1 C2B Ca2+-binding mutants also suppress asynchronous release at hippocampal synapses (16). A prior model for the synchronization role of Syt 1 postulated that it directly competes with the asynchronous Ca2+ sensor for binding sites on the SNARE complex (16). Based on this model, asynchronous release is eliminated by binding of Syt 1 to SNARE complexes, preventing access of the asynchronous sensor to the fusion machinery. The data from our analysis of the C2A Ca2+ binding mutant suggest a simple competition model cannot account for the effects observed. As the enhancement of asynchronous release observed in the C2A mutant rescue occurs without a corresponding reduction in synchronous fusion, it does not require a competitive reduction of the synchronous component. Support for the competition model was based on observations that total release between Syt 1 and WT neurons was unchanged at autapses (11), but this is not observed at the calyx of Held (13), in dissociated hippocampal cultures (23), and our present study. Rather, the data suggest an inhibitory role in preventing asynchronous release mediated by Ca2+ binding to the C2A domain. Previous studies in hippocampal neurons support an inhibitory role for the C2A domain of Syt 1 in exocytosis. Stevens and Sullivan (32) found that neuronal Syt 1 cultures rescued with C2A mutated at the D3 and D4 Ca2+ binding residues showed larger EPSCs at lower Ca2+ concentrations when EPSC size is normalized to maximal response, generating a similar Ca2+ dependence curve to that observed in Drosophila (Fig. 4). Although distinct from the enhanced asynchronous release we describe, an inhibitory function for Ca2+–C2A interactions (and to a lesser extent, Ca2+–C2B) in spontaneous release has also been reported at mammalian cortical synapses (24). Similarly, we observed that transgenic rescue with mutations in C2A or C2B Ca2+ binding sites results in an elevation of spontaneous release, suggesting a conserved role for Syt 1 in this process. The lack of an increase in spontaneous release in Syt 1-null mutant embryonic synapses is likely a result of the known role of the protein in docking (33), and the corresponding reduction in the readily releasable pool revealed by hypertonic sucrose application (9). Our data indicate that C2A and C2B Ca2+ binding sites are not required for the “docking” function of Syt 1 (or that either can suffice), resulting in more docked vesicles than in the null case, and thus a greater chance to reveal a role in suppressing spontaneous release. This larger readily releasable pool of vesicles may also contribute to the enhanced asynchronous release observed in the C2A Ca2+ binding mutant rescues compared with that in Syt 1-null mutant. Consistent with the in vivo observations, in vitro reconstituted membrane assays also suggest a potential inhibitory role for Syt 1, which can be released by addition of Ca2+ (34).
The underlying molecular interactions that mediate Syt 1’s activity in suppressing asynchronous fusion and triggering synchronous release are still unclear. Although Syt 1 can bind to SNARE complexes in a Ca2+-independent manner (35), Ca2+ enhances Syt 1-SNARE interactions (36, 37). Ca2+–C2A binding is important for such high-affinity SNARE binding (36), suggesting a reduction in tight coupling between Syt 1 and SNAREs may be a physical substrate for the loss of asynchronous release suppression in the C2A Ca2+-binding mutant. We hypothesize that Ca2+-independent SNARE binding would contribute to synaptic vesicle docking and intimate association between the two merging membranes. Ca2+-independent SNARE binding would enhance hypertonic-induced release by positioning synaptic vesicles adjacent to the plasma membrane. Consistent with this model, a reduction in synaptic vesicle docking has been observed by EM (23, 33) and functionally demonstrated by loss of hypertonic-induced release (9, 23, 25) in Syt 1-null mutants. A Ca2+-independent docking function is also supported by the observation that Ca2+ binding to C2A or C2B is dispensable for hypertonic-induced release (Fig. 5). Similarly, work at the calyx of Held synapse demonstrates that the Syt 2 R399, 400Q mutant, which retains normal Ca2+ sensing, reduces the tight coupling between Ca2+ influx and release probably as a result of incorrect positioning of synaptic vesicles, suggesting Ca2+-independent docking of synaptic vesicles is promoted by Syt 1 (38).
Ca2+–C2B interactions have recently been demonstrated to drive bending of target membranes via lipid tubulation, a property not associated with C2A activity (39). The efficiency of Ca2+–C2A binding as a release trigger would likely be weaker than C2B, as a result of its inability to bend the plasma membrane into a fusion-promoting conformation. When Ca2+ binding to the C2A domain is disrupted, an enhanced synchronous release triggered by Ca2+ binding to the C2B domain might be expected if C2A–Ca2+ functions as a clamp, as shown in Fig. 4. How Ca2+ binding to C2A normally suppresses asynchronous release is unclear, but we speculate that high-affinity C2A Ca2+ binding would allow this domain to hold Ca2+ until its concentration subsides to resting levels, driving a conformation of Syt 1 that would suppress the slow phase of release until C2A–Ca2+ disengages. Further studies will be required to define precise molecular interactions required for the function of each C2 domain during vesicle fusion.
The dual roles of the Syt 1 C2 domains is consistent with observations that synaptotagmins have universally evolved with both a C2A and C2B domain throughout the animal kingdom (40). Systematic comparison between C2A and C2B Ca2+ binding mutations in inhibitory transmission in mammalian cortical cultures also supports unique roles for both C2 domains in regulating fusion (41), suggesting C2A and C2B have important functions in neurotransmission from invertebrates to mammals. In summary, we conclude that the C2A domain of Syt 1 suppresses asynchronous release, whereas the C2B domain acts as a synchronous fusion trigger. This dual role for Syt 1 allows neurotransmitter release to be tightly regulated, with vesicle fusion occurring only at high Ca2+ concentrations, thus synchronizing release to the action potential.
Materials and Methods
Drosophila Strains and Mutagenesis.
Drosophila melanogaster were cultured on standard medium at 22 °C. DNA constructs for UAS-Syt 1C2B-D3,4N encoding Syt 1D416N, D418N were obtained from N. E. Reist (Colorado State University, Fort Collins, CO). DNA for UAS-Syt 1 C2A-D3,4N encoding Syt 1D282N, D284N was generated using the QuikChange multi site-directed mutagenesis kit (Stratagene) with the primer ctcgtgtttgccattttcAacttcAatcgc. All transgenic strains were generated using standard microinjection into white- embryos. UAS transgenes were expressed using a GAL4 driver under control of the pan-neuronal elav promoter (21) on the third chromosome in the Syt 1-null background. Null mutants lacking endogenous Syt 1 were generated by crossing Syt 1N13, an intragenic Syt 1 deficiency (7), with Syt 1AD4, which truncates Syt 1 before the transmembrane domain (8). These null alleles were recombined with a chromosome containing the muscle-specific myosin heavy chain (Mhc) null mutant, Mhc1, to inhibit muscle contraction and facilitate stable recordings as previously described (42). The Mhc1 mutant had no observed effect on synapse formation, neurotransmitter release, or postsynaptic glutamate receptor clustering (9, 42). Mutant chromosomes were placed over a CyO balancer containing actin-driven GFP to allow unambiguous identification of embryos with Syt 1 null and Mhc1 backgrounds.
Electrophysiological Analysis.
Synaptic currents were recorded with the patch-clamp technique in whole-cell configuration from embryonic muscle fiber 6 in segments A2 to A5 that were maintained at a holding potential of −60 mV. Embryos were aged 21 to 24 h after fertilization and recorded in HL3.1 saline solution (43) (in mM: NaCl, 70; KCl, 5; MgCl2, 5.5; NaHCO3, 10; trehalose, 5; sucrose, 115; Hepes-NaOH, 5; pH 7.2) as described (42), using an Axopatch 200B amplifier (Axon Instrument) at 23 °C to 24 °C. External saline solutions with various concentrations of Ca2+ were prepared by replacing MgCl2 with CaCl2. The internal solution in patch pipettes contained: (in mM) CsCl, 158; ATP, 2; EGTA, 5; Hepes-NaOH, 10, pH 7.1. Motor nerves were positioned in a suction electrode at their site of emergence from the CNS for nerve stimulation. Before recording, embryos were treated for 1 min with 0.4 mg/mL collagenase (type IV; Sigma) in 0.1 mM Ca2+ saline solution. For nerve stimulation, 2 to 5 μA of positive current was passed for 1 ms through a suction electrode, which contained HL3.1 saline solution and was tightly attached to the nerve. For hypertonic-stimulated release, 500 mM sucrose dissolved in Ca2+-free HL-3.1 saline solution was included in a puff pipette with a 1-μm tip, and placed in close vicinity of the boundary between muscles 6 and 7, where the nerve terminal is ending. Hypertonic solution was puffed using positive pressure for 3 s. Slow responses originating from electrically coupled muscle fibers were excluded in subsequent analysis in all genotypes. We performed these experiments in Ca2+-free saline solution to avoid enhancements of presynaptic release mediated by retrograde signaling downstream of postsynaptic Ca2+ influx (44, 45). Statistical analysis was performed using Prism 5 software (GraphPad). The variation of data between animals (average of five animals) was small compared with the larger variation in data from one animal. Thus, results were combined and the number of events recorded shown as N.
Immunostaining.
Immunostaining on filleted embryos was performed as previously described (46). FITC-conjugated IgG against HRP, which labels neuronal cell membranes, was purchased from Cappel and used at 1:1,000. DSYT2 against Syt 1 (22) was used at 1:1,000. Immunoreactive proteins were visualized on a Zeiss Pascal confocal microscope using fluorescent secondary antibodies (Molecular Probes/Chemicon).
Supplementary Material
Acknowledgments
We thank Chip Quinn and members of the Littleton and Yoshihara laboratories for helpful scientific discussions and Drs. J. R. Lemos and M. Francis for critical reading. This work was supported by National Institutes of Health Grants NS40296 (to J.T.L.) and MH85958 (to M.Y.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000606107/-/DCSupplemental.
References
- 1.Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 3.Adolfsen B, Saraswati S, Yoshihara M, Littleton JT. Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment. J Cell Biol. 2004;166:249–260. doi: 10.1083/jcb.200312054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai J, Chapman ER. The C2 domains of synaptotagmin—partners in exocytosis. Trends Biochem Sci. 2004;29:143–151. doi: 10.1016/j.tibs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca2+-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 6.Geppert M, et al. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 7.Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiAntonio A, Schwarz TL. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 9.Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- 10.Goda Y, Stevens CF. Readily releasable pool size changes associated with long term depression. Proc Natl Acad Sci USA. 1998;95:1283–1288. doi: 10.1073/pnas.95.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiki T, Augustine GJ. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J Neurosci. 2004;24:6127–6132. doi: 10.1523/JNEUROSCI.1563-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang ZP, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, et al. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada WM, Zucker RS. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992;61:671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin OH, et al. Sr2+ binding to the Ca2+ binding site of the synaptotagmin 1 C2B domain triggers fast exocytosis without stimulating SNARE interactions. Neuron. 2003;37:99–108. doi: 10.1016/s0896-6273(02)01145-5. [DOI] [PubMed] [Google Scholar]
- 16.Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C(2)B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez I, et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 19.Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: A novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 20.Ubach J, Zhang X, Shao X, Südhof TC, Rizo J. Ca2+ binding to synaptotagmin: How many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campos AR, Rosen DR, Robinow SN, White K. Molecular analysis of the locus elav in Drosophila melanogaster: A gene whose embryonic expression is neural specific. EMBO J. 1987;6:425–431. doi: 10.1002/j.1460-2075.1987.tb04772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Dean C, Arthur CP, Dong M, Chapman ER. Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I. J Neurosci. 2009;29:7395–7403. doi: 10.1523/JNEUROSCI.1341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Pang ZP, Shin OH, Südhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fergestad T, et al. Targeted mutations in the syntaxin H3 domain specifically disrupt SNARE complex function in synaptic transmission. J Neurosci. 2001;21:9142–9150. doi: 10.1523/JNEUROSCI.21-23-09142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Söllner T, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 27.Yoshihara M, Adolfsen B, Littleton JT. Is synaptotagmin the calcium sensor? Curr Opin Neurobiol. 2003;13:315–323. doi: 10.1016/s0959-4388(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 28.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 29.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai RC, et al. The C2B domain of synaptotagmin is a Ca2+-sensing module essential for exocytosis. J Cell Biol. 2000;150:1125–1136. doi: 10.1083/jcb.150.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C(2)A domain. Nature. 2002;418:336–340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- 32.Stevens CF, Sullivan JM. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron. 2003;39:299–308. doi: 10.1016/s0896-6273(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 33.Reist NE, et al. Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J Neurosci. 1998;18:7662–7673. doi: 10.1523/JNEUROSCI.18-19-07662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chicka MC, Hui E, Liu H, Chapman ER. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+ Nat Struct Mol Biol. 2008;15:827–835. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rickman C, Davletov B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J Biol Chem. 2003;278:5501–5504. doi: 10.1074/jbc.C200692200. [DOI] [PubMed] [Google Scholar]
- 36.Earles CA, Bai J, Wang P, Chapman ER. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J Cell Biol. 2001;154:1117–1123. doi: 10.1083/jcb.200105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch KL, et al. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol Biol Cell. 2007;18:4957–4968. doi: 10.1091/mbc.E07-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young SM, Jr, Neher E. Synaptotagmin has an essential function in synaptic vesicle positioning for synchronous release in addition to its role as a calcium sensor. Neuron. 2009;63:482–496. doi: 10.1016/j.neuron.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craxton M. Synaptotagmin gene content of the sequenced genomes. BMC Genomics. 2004;5:43. doi: 10.1186/1471-2164-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin OH, Xu J, Rizo J, Südhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci USA. 2009;106:16469–16474. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshihara M, Suzuki K, Kidokoro Y. Two independent pathways mediated by cAMP and protein kinase A enhance spontaneous transmitter release at Drosophila neuromuscular junctions. J Neurosci. 2000;20:8315–8322. doi: 10.1523/JNEUROSCI.20-22-08315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- 44.Malgaroli A, Tsien RW. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature. 1992;357:134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- 45.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 46.Yoshihara M, Rheuben MB, Kidokoro Y. Transition from growth cone to functional motor nerve terminal in Drosophila embryos. J Neurosci. 1997;17:8408–8426. doi: 10.1523/JNEUROSCI.17-21-08408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.