Abstract
Fibrous aggregates of Tau protein are characteristic features of Alzheimer disease. We applied high resolution atomic force and EM microscopy to study fibrils assembled from different human Tau isoforms and domains. All fibrils reveal structural polymorphism; the “thin twisted” and “thin smooth” fibrils resemble flat ribbons (cross-section ∼10 × 15 nm) with diverse twist periodicities. “Thick fibrils” show periodicities of ∼65–70 nm and thicknesses of ∼9–18 nm such as routinely reported for “paired helical filaments” but structurally resemble heavily twisted ribbons. Therefore, thin and thick fibrils assembled from different human Tau isoforms challenge current structural models of paired helical filaments. Furthermore, all Tau fibrils reveal axial subperiodicities of ∼17–19 nm and, upon exposure to mechanical stress or hydrophobic surfaces, disassemble into uniform fragments that remain connected by thin thread-like structures (∼2 nm). This hydrophobically induced disassembly is inhibited at enhanced electrolyte concentrations, indicating that the fragments resemble structural building blocks and the fibril integrity depends largely on hydrophobic and electrostatic interactions. Because full-length Tau and repeat domain constructs assemble into fibrils of similar thickness, the “fuzzy coat” of Tau protein termini surrounding the fibril axis is nearly invisible for atomic force microscopy and EM, presumably because of its high flexibility.
Keywords: Alzheimers Disease, Heparin, Neurobiology, Neurodegeneration, Neurological Diseases, Protein Assembly, Protein Self-assembly, Protein Stability, Protein-Protein Interactions, Tau
Introduction
Abnormal protein aggregation is a hallmark of a variety of human diseases, especially those appearing at advanced age. Alzheimer disease (AD)4 is the leading cause of dementia in the elderly population and is characterized by two types of protein aggregates. Extracellular amyloid aggregates mainly consist of the Aβ protein, whereas intracellular neurofibrillary tangles consist mainly of fibrils from Tau, a microtubule-binding protein (1, 2). In both cases, the self-assembly of the proteins can be achieved in vitro from recombinant proteins, and mechanisms of these pathological reactions are under intense investigation (3–7). It is hoped that the inhibition of pathological fibrous aggregates or their precursors would reduce the toxic effects on neurons (8). To achieve this goal, knowledge on the building principles of such aggregates would be invaluable. Some progress along this road has been made for the fibrils of the Aβ peptide (40–42 residues). The embedded proteins have a high content of β-strands that fold in a U-shaped manner and stack to form pairs of parallel β-sheets (9). By contrast, Tau is 10 times larger (up to 441 residues, depending on the isoform (Fig. 1)) and largely disordered. Details on the folding and subunit interactions in the aggregated state are mostly unknown; however, β-structure formation is important as well (10–12).
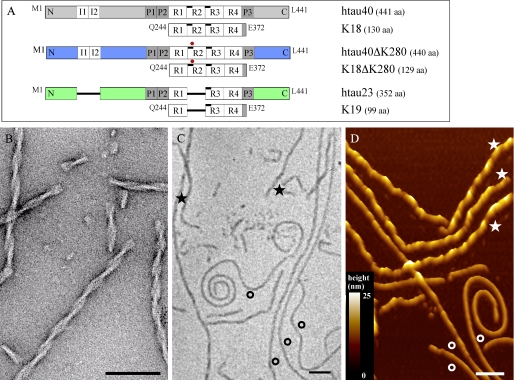
FIGURE 1.
Survey of Tau isoforms, constructs, and fibril assembly. A, sequences of Tau isoforms and constructs assembled into fibrils in the presence of heparin are sketched in a bar diagram. The two hexapeptide motifs (black bars) involved in PHF core formation are located in repeat 2 and 3 (R2 and R3). The position of the Lys280 deletion in hTau40ΔK280 and K18ΔK280 is indicated by red dots. B, EM image of K18ΔK280 fibrils with the typical appearance of PHFs. C, nonselective EM images of hTau40 fibril preparations show a heterogeneous mixture of fibril shapes. D, AFM topographs of the same fibril preparation confirm the heterogeneity of fibril structures but reveal details with superior contrast. Fibril morphologies differ in length, bending, internal twist, periodicity, and thickness (★ for thick fibrils (bright yellow on the color scale) and ○ for thin fibrils (brown on the color scale)). This structural heterogeneity was largely independent of the Tau isoform and construct from which the fibrils self-assembled (supplemental Figs. S1–S3). Notably, although the fibril thickness in EM images is given by the apparent width of the structure, it is reflected by the fibril height in AFM topographs. AFM topographs were recorded in imaging buffer (10 mm Tris-HCl, pH 7.4, 50 mm KCl) and exhibit a full color range that corresponds to vertical scale of 25 nm as indicated by the color scale bar. Length scale bars in B, C, and D correspond to 100 nm.
The fibrils of Tau in AD brains are commonly termed “paired helical filaments” (PHFs) because of their appearance in EM (13). Two protofibrils seem to be wound around one another exposing a crossover repeat of ∼80 nm, a maximal width of ∼22 nm, and a narrow waist of ∼12 nm (see Fig. 3 in Ref. 14). The twisted appearance is variable as follows: ∼10% of Tau fibrils found in AD brains show no twist and are therefore called “straight filaments.” In other diseases with Tau pathology, the crossover repeat is ∼160 nm (15). Likewise, Tau fibrils reassembled in vitro can display a variable twist between filaments assembled from different variants of Tau proteins (e.g. different splicing variants) or even within a given filament. This has caused some concern whether the assembly products observed in vitro reflect those observed in AD brains. A plausible interpretation would be a slight variability in the contacts between subunits, which then can give rise to differences in the overall fibril appearance.
FIGURE 3.
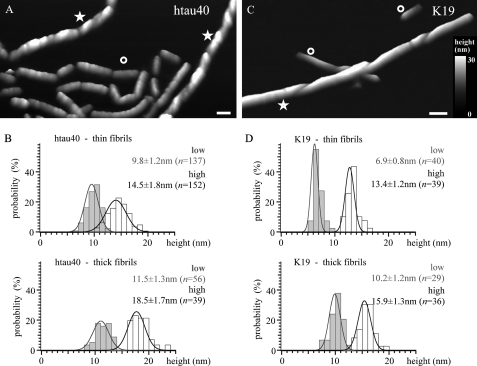
Analysis of Tau fibril thickness. Fibrils with a clear difference in thickness (height) coexist in the same preparation of hTau40 (A) and K19 (C). AFM topographs were taken to perform height measurements of thin (A and C, labeled ○) and thick fibrils (A and C, labeled ★) assembled from hTau40 (B) and K19 (D). The difference in height between thin and thick fibrils ranges from ∼2 nm (lower heights) to ∼4.5 nm (higher heights) for hTau40 fibrils and from ∼2.5 nm (higher heights) to ∼3 nm (lower heights) for K19 fibrils. Full color range corresponds to a vertical scale of 35 nm. Length of scale bars in A and C correspond to 50 nm.
Another fundamental debate concerns the interpretation of the twisted appearance of the Tau fibrils. As an alternative to the “paired helical” structure, it has been proposed that Tau fibrils could be considered as flat ribbons with a width of ∼22 nm and a height of ∼12 nm that twist around their longitudinal axis (16). The apparent groove running down the longitudinal axis could resemble an artifact of staining filling a depression in the fibril, rather than reflecting the division between two joining protofibrillar strands. The interpretation of PHFs as ribbons was mostly based on atomic force microscopy (AFM) and scanning tunneling microscopy (17, 18), but it was viewed with caution because of possible imaging artifacts (19). However, consistent with the “ribbon” interpretation, the ends of filaments or filament fragments usually show clean sharp edges. In contrast, fibrils consisting of two or more protofibrils would be expected to occasionally reveal protruding ends, reminiscent of the protofilament stubs protruding from microtubules (MTs). This is not observed in the case of Tau fibrils. In this study, we therefore use mostly the term “Tau fibrils” rather than PHFs or ribbons to avoid a bias in interpretation.
A further enigmatic aspect of Tau fibrils is the nature of the “fuzzy coat,” which can be digested from the “PHF core” (20). The core protein coincides roughly with the Tau repeat domain of ∼100–120 residues and accounts for only ∼25–30% of the entire Tau protein. Thus, the fuzzy coat should represent the major protein fraction in Tau fibrils, but so far it has escaped detection by microscopic methods. For example, fibrils assembled in vitro from full-length Tau or from the repeat domain alone show only minor differences in diameter by negative stain EM (21). Similar results were obtained for Tau proteins bound to MTs, and the unstructured “projection domain,” which does not bind to MTs. This domain that largely coincides with the Tau termini in the fuzzy coat, is nearly invisible on negatively stained or cryo-preserved unstained MTs (22).
Considering these open questions and the importance of aggregated Tau as a potential target for therapy of AD and related Tauopathies, we initiated a study of Tau isoforms and Tau domains by state-of-the-art AFM. Although AFM cannot provide the spatial resolution of EM, it provides topographs of biological specimens in buffer solution at an exceptional signal-to-noise ratio and a resolution approaching ∼1 nm (23). Thus, AFM allows single biological macromolecules to be observed in molecular detail. In addition, AFM allows manipulating single proteins to characterize their inter- and intramolecular interactions (24, 25). We applied high resolution AFM to reveal detailed structural information of Tau fibrils assembled from six different Tau isoforms and repeat unit constructs. These experiments were conducted in buffer solution at room temperature to guarantee the native integrity of the Tau fibrils. Instead of the commonly reported uniform PHF population, we found that all Tau proteins self-assembled into fibrils that showed populations of variable apparent thickness and twist. Upon mechanical stress as well as exposure to hydrophobic surfaces, the fibrils disassembled into their oligomeric building blocks of ∼15 nm length, which were connected by thin (height ∼2 nm) thread-like stretches. We further demonstrate that hydrophobic and electrostatic interactions play a major role in stabilizing Tau fibrils.
EXPERIMENTAL PROCEDURES
Chemicals and Proteins
Heparin (average molecular mass of 6000 Da), guanidine hydrochloride, and ThS were purchased from Sigma. Full-length human Tau isoforms hTau40, hTau23, hTauΔK280, and Tau constructs K18, K19, and K18ΔK280 (Fig. 1A) were expressed in Escherichia coli and purified by heat treatment and FPLC Mono S chromatography (Amersham Biosciences) as described previously (21). The purity of the proteins was analyzed by SDS-PAGE, and the protein concentrations were determined by absorbance at 214 nm.
PHF Assembly
Aggregation was induced by incubating soluble Tau or Tau constructs typically in the range of 50 μm in volumes of 20 μl at 37 °C in 20 mm BES, pH 7.4, plus 25 mm NaCl buffer with the anionic cofactor heparin 6000 (molar ratio of Tau to heparin = 4:1) for incubation times of ∼3 days for short constructs (K18, K19, and K18ΔK280) or ∼6–7 days for full-length proteins (hTau40, hTau23, and hTauΔK280). The formation of aggregates was monitored by ThS fluorescence and electron microscopy.
ThS Fluorescence
5 μl of 50 μm assembly reactions were diluted to 50 μl with NH4Ac, pH 7, containing 20 μm ThS. Then ThS fluorescence was measured in a Tecan spectrofluorimeter (Crailsheim, Germany) with an excitation wavelength of 440 nm and an emission wavelength of 521 nm (slit width 7.5 nm each) in a 384-well plate (black microtiter 384 plate round well; ThermoLabsystems, Dreieich, Germany). Measurements were carried out at 25 °C, and the background fluorescence was subtracted when needed.
Electron Microscopy
Protein solutions were diluted to 1–10 μm and placed on 600 mesh carbon-coated copper grids for 45 s, washed twice with H2O, and negatively stained with 2% uranyl acetate for 45 s. The samples were examined with Philips CM12 electron microscope at 100 kV.
AFM Sample Immobilization on Mica and HOPG
The Tau sample was diluted in PBS or in adsorption buffer (10 mm Tris-HCl, pH 7.4, 50 mm KCl) to a final concentration of 1–2 μm. A drop (∼20 μl) of the Tau solution was placed onto freshly cleaved mica or highly ordered pyrolytic graphite (HOPG) to allow adsorption of the sample for ∼15 min. Excess protein was removed by rinsing the sample with imaging buffer (10 mm Tris-HCl, pH 7.4, 50 mm KCl). To characterize the influence of ions on the Tau fibril morphology, the imaging buffer (10 mm Tris-HCl, pH 7.4) had varying electrolyte concentrations (0, 10, 50, 166, and 300 mm KCl or 50 mm NaCl). Low pH imaging was done using adsorption and imaging in buffer (10 mm acetate, 10 mm KCl) adjusted to pH 5. For high pH imaging, adsorption and imaging of Tau fibrils were done in 10 mm Tris, 150 mm KCl adjusted to pH 9. When mentioned, HOPG was coated by poly-l-lysine, l-glutamate, or l-threonine (Sigma) using adsorption buffer containing 0.1% of the respective amino acid. To cross-link fibrils adsorbed to mica, they were first adsorbed to mica, then incubated for 30–60 s in adsorption buffer containing 0.5% glutaraldehyde, and subsequently washed several times with imaging buffer. Cross-linking of Tau fibrils in solution prior to surface deposition was achieved by adding 0.5% glutaraldehyde for 15–30 s to the fibril solution containing 1–2 μm Tau protein.
AFM Imaging and Data Analysis
AFM imaging was performed in oscillation mode using a Nanoscope III (Di-Veeco, Santa Barbara, CA) and Si3N4 cantilevers (NPS series, Di-Veeco) exhibiting spring constants of ∼0.32 N/m at resonance frequencies in buffer of 8.5 to 10 kHz. To achieve minimal imaging forces between AFM stylus and sample, the drive amplitude was set between 0.5 and 1.0 V, and the amplitude set point was adjusted manually to compensate for the thermal drift of the AFM. Mean bending angles of Tau fibrils were determined semi-automatically by employing a DNA tracing routine in LabVIEW (National Instruments) (26). The persistence length of each fibril was calculated from the Gaussian variance of bending angles according to Ref. 27.
Fibril heights were measured from cross-sections perpendicular to the long fibril axis. Height profiles were taken along the longitudinal fibril axis. Fibril periodicities were determined from Fourier transformations (IGOR Pro version 5.04B, WaveMetrics Inc.) of topographs along the longitudinal fibril axis (ImageJ version 1.37 software). Gaussian fits to all apparent peaks in the Fourier spectra provided the periodicities further analyzed in histograms to determine the most probable periodicities (Fig. 3). For presentation, all topographs were tilted by 5° (ImageSXM 176-1C, Steve Barrett, Liverpool, UK) to allow perspective view.
RESULTS
Tau Proteins Assemble into Polymorphous Fibrils
In this work we characterize the structure of fibrils assembled in vitro from various Tau isoforms and constructs with different domain compositions (Fig. 1A). Assembly was initiated by the polyanionic cofactor heparin, which helps to overcome the kinetic nucleation barrier and reproduces Tau fibrils with bona fide PHF-like structures (28, 29). The longest isoform in the human central nervous system (CNS), hTau40, contains a repeat domain of four repeats (R1 to R4) and consists of 441 amino acids (aa). The three repeat isoform hTau23 lacks R2 and is 352 aa long. The deletion mutation ΔK280, which has been discovered in Tau-related dementia FTDP-17 (30), significantly enhances the speed of Tau aggregation into fibrils (31). Constructs containing only the repeat domains of hTau40, hTau23, and hTau40ΔK280 are named K18 (130 aa), K19 (99 aa), and K18ΔK280 (129 aa), respectively. Although considerably shorter, the repeat domain-only constructs form fibrils similar to the full-length proteins but are more prone to aggregation, presumably because they are lacking the N- and C-terminal domains that protect the core against unfavorable interactions (7, 32). Randomly picked micrographs of all in vitro preparations showed an unexpected degree of polymorphic fibril appearances in both AFM and EM (Fig. 1, C–D). Compared with EM images, AFM topographs provided a superior contrast. The fibrils had different lengths and their morphology varied in bending, periodicity, twisting, thickness, and substructure. This heterogeneity was independent of the Tau isoform from which the fibrils self-assembled (Fig. 1 and supplemental Figs. S1–S3).
Next, we investigated whether the fibril morphologies depend on the electrolyte and pH of the buffer solution or on the properties of the supporting surface. We adsorbed Tau fibrils to different AFM supports in buffer solutions with electrolyte concentrations ranging between 50 and 500 mm NaCl or KCl and pH of 5.0, 6.0, 7.4, and 9.0. The AFM supports tested were hydrophobic HOPG, HOPG coated with positively charged poly-l-lysine, and hydrophilic mica. In all cases, we could not observe significant variations in the structural properties (thickness, bending, and twisting) of the fibrils (data not shown). Furthermore, we tested whether this polymorphism depends on sample preparation artifacts. Therefore, we imaged all fibrils of the different Tau isoforms in their native state (Fig. 1 and supplemental Figs. S2–S3) and in the fixed state, in which the fibrils were cross-linked before or after adsorption to the microscopy support using 0.5% glutaraldehyde. In all cases, the Tau fibrils imaged in buffer solution showed the same structural diversity. Thus, we conclude that the fibril polymorphism reflects an inherent property of Tau. We characterized the Tau fibril morphologies in more detail below.
Fibrils from Different Tau Proteins Form Equivalent Structural Classes
We first describe AFM topographs of fibrils prepared from the longest human four-repeat Tau isoform, hTau40, and the shortest three-repeat construct, K19 (Fig. 1A and Fig. 2). The fibrils revealed a diversity of appearances in terms of height, twist, coiling, and substructure, whereas the apparent width (measured across the fibrils) remained roughly comparable, about 40–60 nm. This enhanced width is explained by the broadening artifact that results from the nonlinear so-called “stylus convolution” of the fibril with the AFM stylus (see “Experimental Procedures”) (19). In contrast, AFM contours the sample height as well as height variations very accurately with a resolution of ≤0.5 nm (33, 34). We thus decided to judge the diameter of fibrils in AFM topographs from their height (Fig. 3). Fibrils assembled from both hTau40 and K19 could be categorized into three classes as follows: thick corrugated fibrils, thin corrugated fibrils, and thin smooth fibrils (supplemental Table S1).
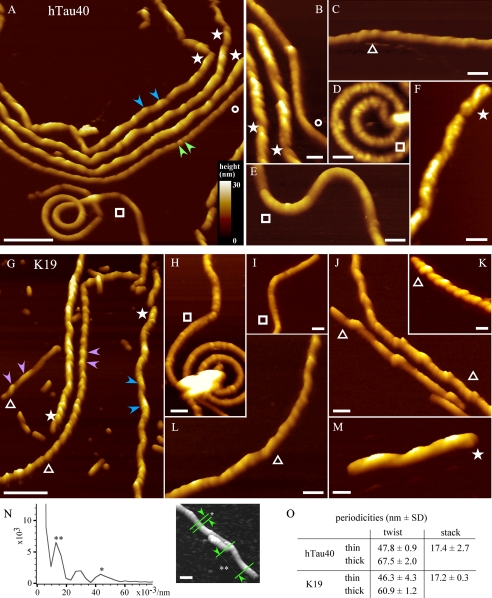
FIGURE 2.
Morphologies of fibrils assembled from full-length Tau and repeat domain. A–F, fibrils from hTau40. G–M, fibrils from the shortened Tau 3-repeat domain K19. With both Tau proteins, a variety of fibril morphologies can be observed by AFM. However, overall the collection of fibrillar structures is remarkably similar, despite the large size difference of hTau40 and K19 (441 versus 99 residues, respectively). There are thin straight fibrils (A and B, labeled ○), thin spiral or wave-like shapes (A, D, E, H, and I, labeled □), and thin fibrils with different degrees of internal twisting (C, G, J, K, and L, labeled ▵). Some fibrils are thicker (indicated by ★, e.g. F and M) than others. To discriminate thick and thin fibrils, their height was quantified from AFM topographs. The ratio of thick to thin fibrils (∼20–75%) depends on the Tau protein and assembly conditions. Different twists can occur in the same fibril (L). Thin fibrils may appear as a stack of smaller subunits (A, green arrowheads, B–E, H–J, and L, with typical spacing of 15–20 nm). On top of this beaded substructure, thin fibrils can have an internal twist (G, purple arrowheads, e.g. C and J) of various periodicities. All thick fibrils show pronounced internal twisting (e.g. A, blue arrowheads, B, F, and M). Periodicities of hTau40 and K19 fibrils were obtained from Fourier transform spectra measured along the fibril axis and correspond to the maxima in the power spectrum (asterisks). N, Fourier transform spectrum obtained for a single thin hTau40 fibril, in this case showing a stack periodicity of 22 nm (*) and a twist periodicity of 77 nm (**). O, most probable periodicities for twisted thin (n = 38) and thick (n = 12) hTau40 fibrils and twisted thin (n = 47) and thick (n = 20) K19 fibrils were attained from Gaussian fits to periodicity distributions. The periodicities of stacked subunits in smooth thin fibrils of hTau40 (n = 16) and K19 (n = 11) were determined in the same way. AFM topographs were recorded in imaging buffer (10 mm Tris-HCl, pH 7.4, 50 mm KCl) and exhibit a full color range that corresponds to a vertical scale of 30 nm. Length scale bars equal 200 nm in A and G and 50 nm in B–F and H–N.
Thick fibrils assembled from hTau40 had thicknesses (AFM heights) between 11.5 ± 1.3 nm (average ±S.D.; n = 39) and 18.5 ± 1.7 nm (n = 56) (Fig. 3, A–B and supplemental Table S1). In the case of the repeat domain K19, the height of thick fibrils varied between 10.2 ± 1.2 nm (n = 36) and 15.9 ± 1.3 nm (n = 29) (Fig. 3, C–D). The pronounced corrugation along the longitudinal axis (★ in Figs. 1 and 2 and supplemental Figs. S2 and S3) indicates a twisted structure with a mean periodicity of 67.5 ± 2.0 nm (n = 16) for hTau40 and 60.4 ± 1.2 nm (n = 20) for K19 (Figs. 2 and 3 and supplemental Table S2). Thick fibrils assembled from hTau40 (∼18.5 nm) and K19 (∼15.9 nm) showed a mean bending angle, q, of 2.2 ± 1.3° (n = 40) and persistence length, lp, of 2.4 ± 0.7 μm (supplemental Table S3). Accordingly, thick fibrils exhibited almost no bending and frequently broke into smaller fragments of variable lengths.
Thin fibrils showed large variations in bending and corrugation (Figs. 2 and 3). Corrugated hTau40 thin fibrils ranged in thickness from 9.8 ± 1.2 nm (n = 137) to 14.5 ± 1.8 nm (n = 152) (Fig. 3B and supplementary Table S1), a mean bending angle of 1.9 ± 1.1° (n = 34), and a persistence length of 3.2 ± 0.6 μm (supplemental Table S3). The thickness of K19 fibrils ranged from 6.9 ± 0.8 nm (n = 40) to 13.4 ± 1.2 nm (n = 39). The mean twist periodicity along the longitudinal fibril axis was 47.8 ± 0.9 nm (hTau40; n = 38) and 46.3 ± 4.3 nm (K19; n = 47) (Fig. 2O and supplemental Table S2). In some cases, variable periodicities were observed in the same fibril (Fig. 2, C and L). The ability of Tau fibrils to bend appeared to be independent of their periodicity. Thin smooth fibrils from hTau40 and K19 that did not reveal obvious corrugations showed an average thickness of 11.0 ± 1.9 nm (n = 140) and 9.4 ± 1.0 nm (n = 33), respectively. Such fibrils were either straight (q = 2.1 ± 0.9°, lp = 4.3 ± 2.5 μm, n = 62) or curved with wavy or spiral-like appearance (q = 2.4 ± 1.4°, lp = 2.0 ± 0.8 mm, n = 10) (Fig. 1, C and D and Fig. 2, A, D and E). If the fibrils were fixed in solution with 0.5% glutaraldehyde prior to adsorption, long straight and large screw-like fibrils were observed (data not shown). We conclude that the wavy and spiral-like fibrils formed upon adsorption of screw-like structured fibrils to the support (Fig. 1, C and D, and Fig. 2, A, D and H). We found that in the case of hTau40, the thick fibrils made up ∼40% (n = 183) of all fibrils, whereas the remaining thin fibrils exhibited thicknesses between ∼10 and 11 nm. Thick and thin fibrils were observed for all Tau isoforms studied, although the abundance of thick fibrils was variable between 20 and 75% (supplemental Table S1). Comparing the periodicity and thickness of thick Tau fibrils characterized here by AFM with the periodicity of ∼80 nm and width of ∼22 nm reported for PHFs by EM (13, 14), it may be concluded that the thick Tau fibrils reflect the commonly reported PHF structure. This interpretation would be in agreement with previous AFM studies (17, 35). However, the coexistence of thin smooth, thin corrugated, and thick fibrils reassembled from the same Tau proteins suggests that these different structural classes may be gathered from Tau proteins showing different assemblies or conformations.
Fibrils from Different Tau Proteins Reveal Similar Subperiodicities
In addition to the coarse height variations of Tau fibrils, high resolution AFM topographs revealed corrugations of smaller magnitude with periodicities of 17.4 ± 2.7 nm (n = 16) and 17.2 ± 0.3 nm (n = 11) in thin smooth and thin twisted fibrils of hTau40 and K19 (Fig. 2, B, D, E, H, I, and J and supplemental Table S2), respectively. Similar observations were made for fibrils assembled from other Tau isoforms and constructs (supplemental Figs. S2 and S3 and supplemental Table S2). Occasionally, we observed similar periodic substructures in thick Tau fibrils. However, due to the strong corrugation of thick fibrils, their finer substructures could hardly be contoured by the scanning AFM stylus. The described variations in thickness, twist, and bending of Tau fibrils reflect their structural heterogeneity. In summary, all fibrils showed similar small height corrugations that indicated a periodic substructure of ∼17–19 nm. Based on this common structural feature, it may be concluded that there is a uniform building block from which Tau fibrils assemble. In the next experiments, we mechanically disassembled individual Tau fibrils to reveal further insight into their stability and architecture.
Mechanical Dissection of Tau Fibrils
To obtain topographs of unperturbed Tau fibrils, the forces applied by the scanning AFM stylus had to be kept below 100 pN (Figs. 1–3). In the following experiments, we first imaged hTau40 fibrils at such minimal forces (Fig. 4). Then, we slightly increased the imaging forces to ∼150–200 pN to apply mechanical stress to selected regions of Tau fibrils (force from left-to-right in Fig. 4, A and B). After this, the fibrils were re-imaged at minimal forces. The applied mechanical stress was sufficient to induce the stepwise disassembly of the fibrils into smaller fragments (Fig. 4, arrowheads). Other fibrils were simply displaced. High resolution topographs (Fig. 4, D and E) showed that the fragments of mechanically disassembled hTau40 fibrils were 9.0 ± 2.0 nm high (n = 102) (supplemental Fig. S4C) and remained connected by thread-like structures exhibiting heights of 2.6 ± 1.0 nm (n = 74). The lengths of the disassembled fragments were broadly distributed between 9 and 55 nm showing five preferred peaks around 14.9 ± 0.1, 21.3 ± 0.3, 26.2 ± 0.6, 36.0 ± 0.1, and 48.4 ± 1.5 nm (n = 224) (Fig. 4F). The shortest fragment length (∼15 nm) resembled the short periodicity of ∼17 nm along the axis of the fibrils. The increments to longer fragments varied from 5 to 12 nm, for both hTau40 and K19, indicating similar mechanical constraints for both types of fibrils. Interestingly, sonication for ∼10 min (120 Watt) prior to adsorption to mica induced structurally similar disassembly of Tau fibrils (data not shown). Such sonication-induced fragments could function as nucleation seeds for fibril re-assembly (32). However, chemical cross-linking with 0.5% glutaraldehyde prevented the mechanically induced disassembly of Tau fibrils even when applying much higher stressing forces (data not shown). Mechanically induced disassembly of Tau fibrils was observed for all Tau isoforms and occurred independently of ionic strength (up to 300 mm KCl or NaCl) and pH (from 5 to 9).
FIGURE 4.
Mechanically and chemically induced disassembly of Tau fibrils. A and B, mechanical stress can induce the disassembly of hTau40 fibrils into smaller fragments. This phenomenon was observed for fibrils of every Tau protein when increasing the force applied to the AFM stylus to ∼150–200 pN. This disassembly emerges in the AFM fast scanning direction (dashed horizontal lines in topographs) where the lateral interaction forces between AFM stylus and fibril are highest. Repeatedly imaging the same surface area (A, panels I–III, and B, panels I–III) shows the consecutive fragmentation of hTau40 fibrils. Changes in fibril structure are indicated by arrowheads before and after fragmentation. Green arrowheads in A indicate the dislocation of a fibril accompanied by fragmentation and extension. C, spontaneous disassembly of hTau40 fibrils upon exposure to a hydrophobic surface (HOPG). As in the case of mechanical fragmentation, fibrils disassemble into smaller fragments typically 15–25 nm in length. D and E, high resolution AFM topographs of mechanically disassembled fibrils on mica highlight thread-like connections between the fragments. The cross-section plotted along a disassembled fibril (E) indicates a pronounced height difference between fragments (9.0 ± 2.0 nm; mean ± S.D.) and thread-like connections (2.6 ± 1.0 nm). F, histogram of fragment lengths (n = 224). The multimodal distribution shows three major peaks at 14.9, 21.3, and 26.2 nm (all maxima indicated on right). AFM topographs were recorded in imaging buffer and exhibit full color ranges that correspond to a vertical scale of 20 nm (A and B) or 10 nm (C and E). The length of all scale bars corresponds to 100 nm.
To mechanically disassemble thick Tau fibrils, we had to apply higher scanning forces (∼300 pN). In most cases, these thick fibrils broke into ∼200–500-nm-long fragments of thick Tau fibrils that showed sharp edges (supplemental Figs. S2H and S3K). Occasionally, mechanically stressed thick twisted Tau fibrils disassembled, similar to thin fibrils, into much smaller fragments. Our experiments showed that mechanical stress could induce the disassembly of Tau fibrils into shorter fragments. However, despite breakage, the fragments remained somehow connected by thread-like structures. But what were the interactions that hold the fragments in Tau fibrils together? We address this question and investigate interactions that contribute to Tau fibril stability below.
Hydrophobic and Electrostatic Interactions Stabilize Tau Fibrils
It has been proposed that the core of PHFs consists of stacked β-strands formed by the repeat domains of Tau (36) that are stabilized by hydrophobic interactions around the hexapeptide motifs (Fig. 1A), although the outer surface of Tau fibrils is largely charged or polar (37). The interaction of proteins with solid surfaces can induce conformational changes that destabilize the protein, often accompanied by a loss in activity (reviewed in Ref. 38). This is especially true for hydrophilic proteins adsorbed to hydrophobic surfaces, where the hydrophobic core of the native protein folds toward the hydrophobic support (39, 40) leading to malfunction and denaturation. We decided to investigate whether a similar effect would denature Tau fibrils when adsorbed to a hydrophobic surface. When adsorbing Tau fibrils in low salt (≤50 mm NaCl or KCl) to the hydrophobic surface of HOPG, the majority of fibrils spontaneously disassembled (Fig. 4C and supplemental Fig. S4B). This disassembly was similar to that observed upon applying mechanical stress. The disassembled fibrils showed fragments that remained connected by thin thread-like chains having heights of 9.3 ± 2.3 nm (n = 27) and 1.5 ± 1.3 nm (n = 21), respectively (supplemental Fig. S4D). When the fibrils were exposed to HOPG for long times (>75 h), all fibrils disassembled, and the support was covered with a thin layer showing a height of 1.3 ± 0.2 nm (n = 23) (data not shown). Such fragmentation of Tau fibrils on HOPG was observed for full-length proteins and for repeat domain constructs at pH 7.4 at low ionic strength (≤50 mm NaCl or KCl) (Fig. 4C and supplemental Fig. S4, B–F). However, when increasing the electrolyte concentration to ≥200 mm KCl or NaCl, the fibrils stopped disassembling. By contrast, when adsorbed to hydrophilic mica, Tau fibrils were structurally stable for many hours (>72 h) in a broad pH range of 5–9, independently of the electrolyte composition and concentration. The disassembly of Tau fibrils was also inhibited when cross-linked by 0.5% glutaraldehyde before exposure to the hydrophobic support. Furthermore, coating the hydrophobic HOPG prior to fibril adsorption with either positively charged (poly-l-lysine), negatively charged (l-glutamate), or neutral polar (l-threonine) amino acids prevented Tau fibrils from disassembly (supplemental Fig. S4G). In the latter case, the fibril morphologies were similar to that of intact fibrils on mica and of fibrils that had been fixed with glutaraldehyde before adsorption. These observations suggest that the exposure of Tau fibrils to a hydrophobic environment competes with hydrophobic interactions that stabilize the fibril. If this competition of hydrophobic interactions is lost, the fibrils disassemble into smaller fragments.
DISCUSSION
Tau Fibrils Are Polymorphic but Show Common Properties
The observed polymorphism of Tau fibrils may resemble different ways of how Tau assembles into fibrils. The flexibility of the different fibril classes, as judged by their persistence length (supplemental Table S3), was found to be very similar to that observed for β-lactoglobulin amyloid fibrils (∼1.6 μm) (41) and in the range of actin fibers (∼15 μm) (42). We found that the persistence length of each fibril class, i.e. thin smooth, thin corrugated, and thick fibrils, remained unchanged for various buffer conditions and supporting surfaces. In addition the persistence length for each fibril class was the same among different Tau isoforms. These results, in combination with the rather high persistence length in the micromolar range, support the view that the different Tau fibrils resemble distinct, structurally well defined assemblies of low flexibility. Despite their rather large structural heterogeneity, Tau fibrils share common features. First, thick and thin fibrils exposed an ∼17–19-nm periodicity. The protein mass per fibril length obtained from STEM measurements on purified unstained fibrils (63) suggested an average density of ∼3.5 mol·nm−1 full-length hTau40 and ∼4.5 mol·nm−1 in the fibrils. Accordingly, an ∼17-nm subunit would consist of 17 nm·3.5 mol/nm ≈60 hTau40 molecules and 17 nm·4.5 mol/nm ≈77 K19 molecules. Such dimensions of a subunit seem reasonable when compared with oligomers of other amyloid-forming proteins like Aβ (∼40 molecules Aβ (43)) or the prion protein (14–28 molecules PrPsc (44)). Second, all thin and thick fibrils showed the same minimal height (thickness) of ∼9–11 nm. Third, all fibrils could disassemble into smaller fragments that remained connected by a thread-like structure. Fourth, hydrophobic interactions seem to play a major role for the integrity of the fibrils, regardless of the given Tau isoform and the presence of the N and C termini. Fifth, electrostatic interactions could be tuned to compensate destabilizing hydrophobic interactions. All fibrils assembled from Tau isoforms and constructs investigated in this work showed these features, suggesting that the assembly of Tau into differently shaped fibrils may follow common mechanisms.
To generate different fibril structures, different assembly mechanisms may coexist for the same Tau protein. Because different Tau isoforms and constructs form a similar polymorphic set of fibril structures that all contain β-stacked Tau repeat domains (45–47), it may be speculated that all Tau protein assemblies share common structural similarities. For the coexisting polymorphic fibril shapes one could think of different explanations. On the one hand, a variation in the β-stacking of Tau molecules could cause a set of different subunits, each of which assemble into a different fibril structure. Otherwise, Tau may assemble into structurally similar subunits that organize differently to form polymorphic fibril shapes. For MTs, it is known that minute differences in the tubulin binding angles result in different curvature of their protofilaments (48–50). Applying this principle to Tau fibrils, small variations in the Tau subunit conformations or interactions could result in fibrils that have different curvatures in their solution state, which, in turn, appear as different twisting degrees when adsorbed to a flat surface. For example, a noncurved flat ribbon would appear as a thin smooth fibril on the surface, although a slightly curved flat ribbon would adsorb as a thin corrugated fibril. Thick corrugated fibrils could be described as fibrils that are highly and regularly curved in solution and twist even after adsorption to the surface. Although all Tau isoforms and constructs showed a similar structural polymorphism, the frequency at which certain fibril morphologies were populated was characteristic for each Tau variant (supplemental Table S1). One may speculate that the structural polymorphism of Tau fibrils is related to the properties of the Tau protein (10). Several examples for different fibril morphologies were reported for Aβ and other amyloid fibrils (51–55). Similarly to Tau fibrils, these amyloid fibrils differ in twist and bending. Tau fibrils can also show differences in their crossover repeats, namely straight fibrils and PHFs, that can coexist in in vitro fibril preparations of different Tau proteins (56, 57). It has been discussed that this structural variability could reflect different protein assemblies. When AFM imaging fibrils of the FTDP-17 (frontotemporal dementia and parkinsonism linked to chromosome 17)-related deletion mutants (supplemental Fig. S3), hTau40ΔK280 and K18ΔK280, fibril morphologies and dimensions were similar to wild-type hTau40. However, the greater β-sheet propensity of the ΔK280 mutation increases the Tau aggregation rate (58) and changes the ratio of smooth and twisted thin fibrils (supplemental Fig. S3 and Table S1). For example, K18 assembles predominantly into thin smooth fibrils, but K18ΔK280 assembles into both thin smooth and thin twisted fibrils. Additionally, the formation of thick twisted fibrils is largely enhanced for K18ΔK280 (50% thick fibrils, see supplemental Table S1) compared with wild-type K18 (20% thick fibrils). These alterations in the fibril morphology distributions are less pronounced when comparing hTau40 with hTau40ΔK280 (supplemental Table S1). From these results, it may be assumed that certain fibril morphologies contain higher β-sheet percentage than others. To test this hypothesis would require selecting Tau fibrils by their morphology and analyzing their specific β-sheet content by other methods, i.e. circular dichroism.
Thin and Thick Tau Fibrils, Flat and Twisted Ribbons?
One critical parameter to distinguish fibrils of different morphological classes is to measure their height in AFM topographs. It may be concluded that thick fibrils represent PHFs. Two alternative PHF models have been discussed in the literature. One model assumes a single flat ribbon structure with an internal twist around the fibril axis (59–61). The other model suggests two protofibrils twisted around each other, hence the name paired helical (13, 16, 17). The coexistence of thin and thick Tau fibrils is suggestive of the interpretation that PHFs consist of two strands. However, in our high resolution AFM topographs, the majority of thick fibrils (∼95%) do not expose characteristic substructures that could be assigned to two smaller fibrils. In addition, a two-stranded fibril should show a much lower flexibility than single protofibrils. In contrast, the persistence length of thick fibrils was found to be slightly lower than that of thin smooth or thin twisted fibrils (supplemental Table S3), which indicates a higher flexibility of the thick fibrils. When breaking upon adsorption to a surface or mechanical stress, Tau fibrils never showed double ends as it would be expected when breaking up a two-stranded fibril. Moreover, when mechanically stressed, thin and thick fibrils disassembled into fragments that were connected by one thread-like chain. We therefore conclude that these thick fibrils are not composed of two individual fibrils.
Thick fibrils showed height corrugations ranging from ∼11 to 19 nm (hTau40) and from ∼10 to 16 nm (K19). The mean thin fibril height was ∼11 nm for hTau40 and ∼9 nm for K19. Thus, thick and thin fibrils show the same minimum height of ∼9–11 nm. The thin fibrils clearly suggest that Tau assembles into flat ribbons. Apparently, these Tau ribbons can be corrugated, and in some cases we have observed the transition of a smooth thin ribbon into a corrugated thin ribbon. However, such a thin Tau ribbon, if twisted around its longitudinal axis, could easily form a thick twisted fibril. Thus, the polymorphic Tau fibrils would expose the same basic architecture of a ribbon providing a consistent explanation of the observed structures. Small differences in the interactions between the Tau subunits may induce changes in their packaging and lead either to flat, slightly corrugated, or twisted Tau ribbons or fibrils. In summary, our data suggest that the appearance of thin and thick Tau fibrils by AFM is based on similar ribbon-like structures. The periodicity and corrugation observed in the majority of thick Tau fibrils is likely to be explained by a twisted ribbon-like fibril (59, 62). If this twisted fibril would be stretched out it would show a similar height and width as a thin Tau fibril (reminiscent of a coiled telephone cord after stretching). The height and width of such a flat “thin” Tau ribbon would be ∼9–11 and ∼15–18 nm, respectively. Surprisingly, the dimensions of the ribbons are nearly independent of the Tau protein from which it was assembled, even though the Tau proteins differed more than 4-fold in mass. Possible explanations are discussed below.
Fuzzy Coat
It is widely accepted that Tau assembly into fibrils is mediated by interactions of β-strands in their repeat domains (45, 58). However, the structure of the Tau domains outside the repeats in aggregated fibrils remains an open question. Limited digestion of PHFs removes these domains, leaving behind a PHF core built essentially from the repeat domains (60, 63). Thus, it has been suggested that the Tau termini form a fuzzy coat surrounding the PHF (20). However, the nature of this coat has remained elusive. We hoped to shed light on this issue by comparing Tau fibrils with different domain compositions. For example, in the case of full-length Tau, the repeat domain includes ∼27% of the mass (∼120/441 aa, see Fig. 1A), so that the cross-section dimensions of fibrils from the full-length protein should be almost four times larger than fibers from the repeat domain only. However, our topographs show that both full-length and short Tau constructs assemble into structurally similar thick and thin fibrils. The height difference between thick fibrils from hTau40 and K18 was only ∼2 nm (supplemental Table S1) and even less when comparing thick fibrils of hTau23 versus K19 and hTau40ΔK280 versus K18ΔK280. Such small differences are in agreement with electron microscopy data showing the width difference between fibrils assembled from hTau40 and K18 is minute (1–2 nm) and poorly detectable (31). These differences in AFM height and corresponding EM width are too small to account for the “missing mass” of the Tau terminal domains. From structural studies, the Tau terminal domains are described as protruding from the fibril surface like a polymer brush (21, 64). This model is supported by AFM force spectroscopy measurements on a brush of Tau molecules that were projecting away from their anchoring surface (65). In this case, repulsive electrostatic forces between the protruding termini and the approaching AFM tip could be detected, which decreased with increasing salt concentrations in the surrounding medium. This is reminiscent with the behavior of a brush consisting of densely packed, unstructured poly-electrolyte chains.
The conformation and thus extension of surface-immobilized unstructured polypeptides in solution largely depends on their surface density (reviewed in Ref. 66). A dense packing enhances the polypeptide extension to form a polymer brush, although separation of neighboring polypeptides, large enough to prevent chain interactions, results in chain collapse onto the surface (67). According to this model of a polymer brush, there are different scenarios to explain how the unstructured Tau termini may establish the fuzzy coat of Tau fibrils.(i) A high packing density of Tau on the fibril surface would force their termini to fully protrude. In this case, the contribution of the termini to the fibril thickness would be that of extended polypeptide chains and much different for full-length Tau and terminally truncated Tau constructs. Assuming that every peptide of the N (∼240 aa) and C termini (∼70 aa) contributes ∼0.3 nm to the polypeptide length, fibrils assembled from hTau40 would show a reasonably thick coat of ∼20–70 nm. (ii) A medium or lower packing density of Tau molecules would allow their termini to collapse onto the fibril surface. The thickness of such a collapsed layer would be in the range of the radius of gyration of the polypeptide in solution, Rg, and can be estimated using the freely jointed chain model for flexible polymers (68). For proteins, each peptide bond can be modeled as one subunit with a length of ∼0.4 nm, and a Kuhn length b of 0.4–0.8 nm describes the elastic behavior of the polymer (69, 70). The height of an isolated unstructured collapsed polypeptide is then given by Hcol ≈ b·N3/5 (67), where N is the number of peptides in the peptide chain. A layer of collapsed Tau N-terminal domain (∼240 aa) and C-terminal domain (∼70 aa) on the fibril surface would show minimum heights of Hcol, Nterm ≈0.4 nm·2433/5 ≈10.8 nm and Hcol, Cterm ≈0.4 nm·693/5 ≈5.1 nm, respectively. These values are much closer to the ∼1–2 nm coat thickness detected in AFM and EM than the contribution expected for an extended polymer brush. However, these estimates are valid for isolated polymer chains on a surface and thus still underestimate the thickness of the polymer brush that would be generated by many Tau proteins assembled into a fibril (assuming ∼4 Tau molecules per nm fibril length (63)). (iii) The third scenario assumes that the Tau termini protrude from the fibril surface at a similar medium to low density but show a stretched out conformation. In this case, the termini would form a loose but extended hydrophilic polymer brush. Such a soft brush may be too flexible to be imaged by AFM and stained for EM imaging. It has been assumed that during AFM imaging, loose polymer brushes may escape the AFM stylus (reviewed in Ref. 66). For the flexible termini of full-length Tau bound to MTs, a loose polymer brush model with a comparable low apparent polymer chain extension has been proposed (22). Tau stabilizes MTs by binding to their outer surface via several regions in the repeat domain, although the termini protrude brush-like (71), similar to the fuzzy coat that is thought to surround PHFs. AFM imaging of MTs that were coated with Tau molecules revealed a continuous ∼1 nm thick layer on their outer surface (72), which indicates that the bound repeat domain of Tau but not the loose terminal polymer brush was detected. Accordingly, this phenomenon may explain the surprisingly small contribution of the fuzzy coat to the fibril diameter of ∼2 nm. In conclusion, our data suggest that unstructured Tau termini protrude from the fibril surface into the surrounding medium to form a loose fur-like brush, which explains the nature of the so-called fuzzy coat.
Stability of Tau Fibrils
Mechanical as well as chemical stress induces Tau fibril disassembly into fragments that remain somehow connected by thread-like chains. Whereas thin Tau fibrils already disassembled at scanning forces of ∼150–200 pN, thicker Tau fibrils required higher scanning forces of ∼300 pN to disassemble. Thereby, thick fibrils tended to break into shorter fibrils, even though their flexibility was slightly higher compared with thin twisted or smooth fibrils. However, these fragments disassembled from thick fibrils could further fragment into small fragments that were connected by thread-like chains, such as observed for the disassembly of thin fibrils. Exposure to a hydrophobic surface induced spontaneous disassembly of Tau fibrils into “pearl chains.” Thick fibrils first broke into smaller pieces that then further fragmented such as observed for thin fibrils. For adsorption times of ∼30 min, the number of Tau fibrils remaining intact was higher for thick ones (supplemental Fig. S4F, arrowheads). Upon exposure to the hydrophobic HOPG surface over longer times (>75 h), all thin and thick fibrils completely disassembled into thread-like chains. These results indicate that thicker Tau fibrils are more stable than the thinner ones but that the disassembling structures are similar. Furthermore, it can be concluded that chemically and mechanically induced disassembly of Tau fibrils follows similar ways and produces distinct structural entities.
Currently, the modes of toxicity of different assembly forms, namely monomers, oligomers, and fibrils, as well as their impact on in vivo aggregation are extensively discussed for Tau (73) and for other amyloidic proteins like the prion protein and amyloid-β (reviewed in Ref. 74). Recently, it was shown that a human mutant Tau domain, which is highly prone to aggregate, can induce the coaggregation of endogenous wild-type Tau in mice (75). Oligomeric Tau aggregates, when added to the culture medium, become endocytosed and then function as nucleation seeds for intracellular aggregation of endogenous Tau in cultured neural C17 cells (76). So far, no such propagation of aggregation on cells was observed for intact Tau fibrils. However, the mechanically or chemically induced fragmentation of fibrils into oligomers as we show here in vitro could also reflect a way to generate nucleation seeds for in vivo aggregation of Tau proteins.
Fragments and Thread-like Chains of Thick and Thin Fibrils Look Similar
The fragments have a size of ∼15 nm (length) × ∼9 nm (height) × 15–18 nm (width) and therefore are too large to reflect single Tau molecules. Therefore, they must reflect larger assemblies of Tau molecules. It may be speculated whether the thread-like connectors could be Tau termini. At this stage, we cannot state how many Tau molecules make up one oligomeric Tau fragment and how many termini may be required to form the thread-like connections between the fragments.
The current structural model of PHFs suggests a partial hydrophobic core of stacked β-strands and a mostly charged hydrophilic outer surface (21). From an entropic point of view, a protein exposed to a sufficiently hydrophobic surface unfolds and attaches its formerly hydrophobic interior to the surface (40). This unfolding minimizes the hydrophobic surface exposed to water. Such denaturation of proteins interacting with a hydrophobic surface is well known (77). Our experiments show that exposure to hydrophobic surfaces can be easily used to destabilize and disassemble Tau fibrils. However, coating the hydrophobic surface with polar or charged molecules inhibited the spontaneous fragmentation of Tau fibrils. We also observed that increasing the electrolyte concentration prevented Tau fibrils from destabilizing and disassembling when exposed to hydrophobic surfaces. With increasing electrolyte concentrations, both attractive and repulsive electrostatic interactions between surface charges become compensated by counter ions (78). Hydrophobic interactions remain mainly unaffected by the electrolyte. The fact that compensating of electrostatic interactions prevents Tau fibrils from hydrophobically induced denaturation suggests that the electrostatic interactions rather tend to destabilize Tau fibrils (79, 80). Such electrostatic contributions would not be restrained to the fuzzy coat of the fibril surface because we observe the same phenomenon for full-length hTau40 and the terminally truncated Tau constructs. On the other hand, polyelectrolyte brushes extend in low salt conditions and collapse in environments with increased salt concentration (81). This reduction in chain extension was also observed for surface-immobilized soluble Tau by AFM (65). A collapse of the Tau termini in the fuzzy coat would increase the charge density on the fibril surface, which may be the mechanism behind the protection of the Tau fibrils against hydrophobic induced disassembly at higher and physiological relevant salt concentrations. The protective effect of the surface charge density that is generated by coating the hydrophobic HOPG surface with charged amino acids would strengthen this hypothesis. To examine the critical density of such a protective “charge coat” and its actual relevance for protecting the fibrils from disassembling is an interesting issue for prospective studies. However, all these results emphasize the key role of hydrophobic interactions to maintain the integrity of Tau fibrils. Stretching this idea further, chemically induced fibril destruction could also play a role in vivo. Following the suggested nucleation-elongation pathway of Tau aggregation (82), the production of smaller oligomeric aggregates, which can function as nucleation seeds (32) and can be transferred to other cells by unknown intermediate steps (83), may be a trigger of Tau aggregation in AD or other Tauopathy brains. In this context it could be of greatest interest to examine the physiological conditions that destabilize Tau fibrils and result in their fragmentation.
Supplementary Material
Acknowledgments
We thank Ch. Bippes and J. Helenius (Basel, Switzerland) for helpful discussions, I. Lindner (Hamburg, Germany) for excellent technical assistance, and J. Biernat (Hamburg) for expert advice.
This work was supported by the Deutsche Forschungsgemeinschaft (to D. M.), Max-Planck Society (to D. J. M. and E. M. M.), and VW Foundation Grant 1/82544 (to E. M. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3, Figs. S1–S4, and additional text.
- AD
- Alzheimer disease
- aa
- amino acid
- AFM
- atomic force microscopy
- HOPG
- highly ordered pyrolytic graphite
- PHF
- paired helical filament
- MT
- microtubule
- ThS
- thioflavine S
- BES
- 2-[bis(2-hydroxyethyl)amino]ethanesulfonic acid
- N
- newton.
REFERENCES
- 1.Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Nat. Rev. Neurosci. 8, 663–672 [DOI] [PubMed] [Google Scholar]
- 2.Finder V. H., Glockshuber R. (2007) Neurodegener Dis. 4, 13–27 [DOI] [PubMed] [Google Scholar]
- 3.Iqbal K., Liu F., Gong C. X., Alonso Adel C., Grundke-Iqbal I. (2009) Acta Neuropathol. 118, 53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebs M. R., Domike K. R., Donald A. M. (2009) Biochem. Soc. Trans. 37, 682–686 [DOI] [PubMed] [Google Scholar]
- 5.Mandelkow E., von Bergen M., Biernat J., Mandelkow E. M. (2007) Brain Pathol. 17, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minati L., Edginton T., Bruzzone M. G., Giaccone G. (2009) Am. J. Alzheimers Dis. Other Demen. 24, 95–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder L. I., Guillozet-Bongaarts A. L., Garcia-Sierra F., Berry R. W. (2005) Biochim. Biophys. Acta 1739, 216–223 [DOI] [PubMed] [Google Scholar]
- 8.Bulic B., Pickhardt M., Schmidt B., Mandelkow E. M., Waldmann H., Mandelkow E. (2009) Angew. Chem. Int. Ed. Engl. 48, 1740–1752 [DOI] [PubMed] [Google Scholar]
- 9.Nelson R., Sawaya M. R., Balbirnie M., Madsen A. Ø., Riekel C., Grothe R., Eisenberg D. (2005) Nature 435, 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeganathan S., von Bergen M., Mandelkow E. M., Mandelkow E. (2008) Biochemistry 47, 10526–10539 [DOI] [PubMed] [Google Scholar]
- 11.Mukrasch M. D., Biernat J., von Bergen M., Griesinger C., Mandelkow E., Zweckstetter M. (2005) J. Biol. Chem. 280, 24978–24986 [DOI] [PubMed] [Google Scholar]
- 12.von Bergen M., Barghorn S., Biernat J., Mandelkow E. M., Mandelkow E. (2005) Biochim. Biophys. Acta 1739, 158–166 [DOI] [PubMed] [Google Scholar]
- 13.Kidd M. (1963) Nature 197, 192–193 [DOI] [PubMed] [Google Scholar]
- 14.Crowther R. A. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 2288–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ksiezak-Reding H., Morgan K., Mattiace L. A., Davies P., Liu W. K., Yen S. H., Weidenheim K., Dickson D. W. (1994) Am. J. Pathol. 145, 1496–1508 [PMC free article] [PubMed] [Google Scholar]
- 16.Pollanen M. S., Markiewicz P., Goh M. C. (1997) J. Neuropathol. Exp. Neurol. 56, 79–85 [DOI] [PubMed] [Google Scholar]
- 17.Pollanen M. S., Markiewicz P., Bergeron C., Goh M. C. (1994) Am. J. Pathol. 144, 869–873 [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno-Herrero F., Pérez M., Baró A. M., Avila J. (2004) Biophys. J. 86, 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen M. J., Hud N. V., Balooch M., Tench R. J., Siekhaus W. J., Balhorn R. (1992) Ultramicroscopy 42, 1095–1100 [DOI] [PubMed] [Google Scholar]
- 20.Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4506–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barghorn S., Davies P., Mandelkow E. (2004) Biochemistry 43, 1694–1703 [DOI] [PubMed] [Google Scholar]
- 22.Santarella R. A., Skiniotis G., Goldie K. N., Tittmann P., Gross H., Mandelkow E. M., Mandelkow E., Hoenger A. (2004) J. Mol. Biol. 339, 539–553 [DOI] [PubMed] [Google Scholar]
- 23.Engel A., Müller D. J. (2000) Nat. Struct. Biol. 7, 715–718 [DOI] [PubMed] [Google Scholar]
- 24.Allison D. P., Hinterdorfer P., Han W. (2002) Curr. Opin. Biotechnol. 13, 47–51 [DOI] [PubMed] [Google Scholar]
- 25.Müller D. J., Sapra K. T., Scheuring S., Kedrov A., Frederix P. L., Fotiadis D., Engel A. (2006) Curr. Opin. Struct. Biol. 16, 489–495 [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Herrero F., Seidel R., Johnson S. M., Fire A., Dekker N. H. (2006) Nucleic Acids Res. 34, 3057–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivetti C., Codeluppi S. (2001) Ultramicroscopy 87, 55–66 [DOI] [PubMed] [Google Scholar]
- 28.Goedert M., Jakes R., Spillantini M. G., Hasegawa M., Smith M. J., Crowther R. A. (1996) Nature 383, 550–553 [DOI] [PubMed] [Google Scholar]
- 29.Pérez M., Valpuesta J. M., Medina M., Montejo de Garcini E., Avila J. (1996) J. Neurochem. 67, 1183–1190 [DOI] [PubMed] [Google Scholar]
- 30.van Swieten J. C., Bronner I. F., Azmani A., Severijnen L. A., Kamphorst W., Ravid R., Rizzu P., Willemsen R., Heutink P. (2007) J. Neuropathol. Exp. Neurol. 66, 17–25 [DOI] [PubMed] [Google Scholar]
- 31.Barghorn S., Zheng-Fischhöfer Q., Ackmann M., Biernat J., von Bergen M., Mandelkow E. M., Mandelkow E. (2000) Biochemistry 39, 11714–11721 [DOI] [PubMed] [Google Scholar]
- 32.Friedhoff P., von Bergen M., Mandelkow E. M., Davies P., Mandelkow E. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engel A., Schoenenberger C. A., Müller D. J. (1997) Curr. Opin. Struct. Biol. 7, 279–284 [DOI] [PubMed] [Google Scholar]
- 34.Hansma H. G., Hoh J. H. (1994) Annu. Rev. Biophys. Biomol. Struct. 23, 115–139 [DOI] [PubMed] [Google Scholar]
- 35.Moreno-Herrero F., Valpuesta J. M., Pérez M., Colchero J., Barö A. M., Avila J., Montejo De Garcini E. (2001) J. Alzheimers Dis. 3, 443–451 [DOI] [PubMed] [Google Scholar]
- 36.von Bergen M., Friedhoff P., Biernat J., Heberle J., Mandelkow E. M., Mandelkow E. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5129–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andronesi O. C., von Bergen M., Biernat J., Seidel K., Griesinger C., Mandelkow E., Baldus M. (2008) J. Am. Chem. Soc. 130, 5922–5928 [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi K., Sakiyama T., Imamura K. (2001) J. Biosci. Bioeng. 91, 233–244 [DOI] [PubMed] [Google Scholar]
- 39.Kauzmann W. (1959) Adv. Protein Chem. 14, 1–63 [DOI] [PubMed] [Google Scholar]
- 40.Meyer E. E., Rosenberg K. J., Israelachvili J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15739–15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagis L. M., Veerman C., van der Linden E. (2004) Langmuir 20, 924–927 [DOI] [PubMed] [Google Scholar]
- 42.Yanagida T., Nakase M., Nishiyama K., Oosawa F. (1984) Nature 307, 58–60 [DOI] [PubMed] [Google Scholar]
- 43.Orte A., Birkett N. R., Clarke R. W., Devlin G. L., Dobson C. M., Klenerman D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14424–14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silveira J. R., Raymond G. J., Hughson A. G., Race R. E., Sim V. L., Hayes S. F., Caughey B. (2005) Nature 437, 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berriman J., Serpell L. C., Oberg K. A., Fink A. L., Goedert M., Crowther R. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9034–9038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goux W. J., Kopplin L., Nguyen A. D., Leak K., Rutkofsky M., Shanmuganandam V. D., Sharma D., Inouye H., Kirschner D. A. (2004) J. Biol. Chem. 279, 26868–26875 [DOI] [PubMed] [Google Scholar]
- 47.Skrabana R., Sevcik J., Novak M. (2006) Cell. Mol. Neurobiol. 26, 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gigant B., Curmi P. A., Martin-Barbey C., Charbaut E., Lachkar S., Lebeau L., Siavoshian S., Sobel A., Knossow M. (2000) Cell 102, 809–816 [DOI] [PubMed] [Google Scholar]
- 49.Mandelkow E. M., Mandelkow E., Milligan R. A. (1991) J. Cell Biol. 114, 977–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogales E., Wolf S. G., Downing K. H. (1998) Nature 391, 199–203 [DOI] [PubMed] [Google Scholar]
- 51.Fändrich M., Meinhardt J., Grigorieff N. (2009) Prion 3, 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meinhardt J., Sachse C., Hortschansky P., Grigorieff N., Fändrich M. (2009) J. Mol. Biol. 386, 869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buée L., Delacourte A. (1999) Brain Pathol. 9, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paravastu A. K., Leapman R. D., Yau W. M., Tycko R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18349–18354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stromer T., Serpell L. C. (2005) Microsc. Res. Tech. 67, 210–217 [DOI] [PubMed] [Google Scholar]
- 56.Frost B., Ollesch J., Wille H., Diamond M. I. (2009) J. Biol. Chem. 284, 3546–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato S., Nakamura H. (1990) Acta Neuropathol. 81, 125–129 [DOI] [PubMed] [Google Scholar]
- 58.von Bergen M., Barghorn S., Li L., Marx A., Biernat J., Mandelkow E. M., Mandelkow E. (2001) J. Biol. Chem. 276, 48165–48174 [DOI] [PubMed] [Google Scholar]
- 59.Ruben G. C., Wang J. Z., Iqbal K., Grundke-Iqbal I. (2005) Microsc. Res. Tech. 67, 175–195 [DOI] [PubMed] [Google Scholar]
- 60.Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4884–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crowther R. A. (1990) Biochim. Biophys. Acta 1096, 1–9 [DOI] [PubMed] [Google Scholar]
- 62.Wischik C. M., Crowther R. A., Stewart M., Roth M. (1985) J. Cell Biol. 100, 1905–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Bergen M., Barghorn S., Müller S. A., Pickhardt M., Biernat J., Mandelkow E. M., Davies P., Aebi U., Mandelkow E. (2006) Biochemistry 45, 6446–6457 [DOI] [PubMed] [Google Scholar]
- 64.Dickson D. W., Ksiezak-Reding H., Liu W. K., Davies P., Crowe A., Yen S. H. (1992) Acta Neuropathol. 84, 596–605 [DOI] [PubMed] [Google Scholar]
- 65.Mukhopadhyay R., Hoh J. H. (2001) FEBS Lett. 505, 374–378 [DOI] [PubMed] [Google Scholar]
- 66.Bright J. N., Woolf T. B., Hoh J. H. (2001) Prog. Biophys. Mol. Biol. 76, 131–173 [DOI] [PubMed] [Google Scholar]
- 67.de Gennes P. G. (1980) Macromolecules 13, 1069 [Google Scholar]
- 68.Flory P. J. (1969) Statistical Mechanics of Chain Molecules, pp. 432, Interscience Publisher, New York [Google Scholar]
- 69.Oesterhelt F., Rief M., Gaub H. E. (1999) New J. Phys. 1, 6.1–6.11 [Google Scholar]
- 70.Su T., Purohit P. K. (2009) Acta Biomater. 5, 1855–1863 [DOI] [PubMed] [Google Scholar]
- 71.Mukrasch M. D., von Bergen M., Biernat J., Fischer D., Griesinger C., Mandelkow E., Zweckstetter M. (2007) J. Biol. Chem. 282, 12230–12239 [DOI] [PubMed] [Google Scholar]
- 72.Schaap I. A., Hoffmann B., Carrasco C., Merkel R., Schmidt C. F. (2007) J. Struct. Biol. 158, 282–292 [DOI] [PubMed] [Google Scholar]
- 73.Congdon E. E., Duff K. E. (2008) J. Alzheimers Dis. 14, 453–457 [DOI] [PubMed] [Google Scholar]
- 74.Krammer C., Schätzl H. M., Vorberg I. (2009) Prion 3, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sydow A., Mandelkow E. M. (2010) Neurodegener. Dis. 7, 28–31 [DOI] [PubMed] [Google Scholar]
- 76.Frost B., Jacks R. L., Diamond M. I. (2009) J. Biol. Chem. 284, 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gray J. J. (2004) Curr. Opin. Struct. Biol. 14, 110–115 [DOI] [PubMed] [Google Scholar]
- 78.Leckband D., Israelachvili J. (2001) Q. Rev. Biophys. 34, 105–267 [DOI] [PubMed] [Google Scholar]
- 79.Pace C. N., Shirley B. A., McNutt M., Gajiwala K. (1996) FASEB J. 10, 75–83 [DOI] [PubMed] [Google Scholar]
- 80.Lins L., Brasseur R. (1995) FASEB J. 9, 535–540 [DOI] [PubMed] [Google Scholar]
- 81.Moya S., Azzaroni O., Farhan T., Osborne V. L., Huck W. T. (2005) Angew. Chem. Int. Ed. Engl. 44, 4578–4581 [DOI] [PubMed] [Google Scholar]
- 82.Congdon E. E., Kim S., Bonchak J., Songrug T., Matzavinos A., Kuret J. (2008) J. Biol. Chem. 283, 13806–13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brundin P., Melki R., Kopito R. (2010) Nat. Rev. Mol. Cell Biol. 11, 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.