Abstract
Obese humans and animals exhibit reduced functioning of the dopamine (DA) system in the nucleus accumbens (NAc). The question addressed here is whether this change in NAc DA can be detected in Sprague-Dawley rats that are prone to obesity on a fat-rich diet but still at normal body weight. Rats were subgrouped as “obesity-prone” (OP) or “obesity-resistant” (OR), based on their weight gain during 5 days of access to a high-fat diet, and were then shifted to a lower-fat chow diet before microdialysis testing was performed. The OP rats compared to OR rats exhibited markedly reduced basal levels of DA in the NAc. After a high-fat challenge meal, both OP and OR rats showed a significant increase in extracellular DA and its metabolites; however, the NAc DA of the OP rats still remained at reduced levels. Also, the increase in DA and metabolite levels observed in OR rats after systemic administration of a fat emulsion was not evident in the OP rats, which instead showed no change in DA and a decrease in its metabolites. These results demonstrate, first, that fat can stimulate accumbal DA release and, second, that outbred rats prone to overeating and becoming obese on a palatable, fat-rich diet exhibit reduced signaling in the mesolimbic DA system while still at normal weight, suggesting that it may be causally related to their excess consummatory behavior.
Keywords: obesity-prone, obesity-resistant, fat, triglycerides, Intralipid, rat
Introduction
Overeating of palatable food may be due to any number of factors, including endocrine, metabolic, and neurobiological processes [1–4]. Diets in industrialized countries contain increasingly more fat, and elevated intake of fat-rich, energy-dense foods invariably promotes weight gain [5]. Importantly, not all humans or animals given access to dietary fat over-consume it [6–7]. When placed on a diet relatively high in fat and energy content, Sprague-Dawley rats exhibit a wide range of eating and body weight patterns, with one sub-set prone to overeating and becoming obese and another eating normally and remaining at normal weight on the same diet [6, 8]. The reasons for these differential profiles remain unclear, but one strong possibility is a difference in reward or satiety signaling in response to consumption of the fat-rich diet.
Dopamine (DA) in the nucleus accumbens (NAc) plays a major role in food reward [9], as well as in the motivation to seek and eat food [9–11]. Injection of DA in the NAc can facilitate food intake [12–13], and acute ingestion of fat or injection of a lipid emulsion, both of which increase levels of circulating triglycerides (TG) [14–16], can stimulate extracellular DA release, particularly in the shell region [17–19]. Animals made obese from chronic consumption of a high-fat diet show major differences from non-obese animals in accumbal DA function. These obese animals have lower levels of electrically-evoked DA in NAc slices compared to chow-fed rats [18], suggesting that they have lower basal levels of DA. Also, animals that have chronically consumed fat have decreased DA turnover in the NAc [20]. In humans, obese adults exhibit a deficiency in DA signaling in the striatum, as they have diminished availability of the D2 receptor in this region compared to lean individuals [21]. Obese adolescent girls show less activation of the striatum in response to consumption of a fat-rich milkshake than do lean girls [22]. Further, body mass index correlates negatively with activity in dopaminergic brain regions during gastric distention that normally occurs during food consumption [23]. These results together suggest that obese individuals have lower DA functioning, not just basally but also in response to food and fat consumption, and that this disturbance may contribute to an increased drive to consume rewarding foods high in fat.
It is not clear, however, whether this lower DA functioning observed in already obese subjects actually precedes and is causally related to the obesity or whether it is simply a consequence of it. To determine this, it is important to investigate subjects prior to or very early in the development of obesity. As with obese outbred rats, inbred rats that are prone to developing obesity have lower levels of NAc DA compared to those bred to be obesity-resistant, as assessed by both microdialysis and electrophysiology [24]. These studies in selectively-bred rats illustrate how one can detect significant differences in pre-obese animals to elucidate mechanisms that may contribute to the development of obesity. Similar experiments in outbred animals, however, are lacking, as it can be difficult to identify rats early on that are prone to overeating and obesity. In outbred, Sprague-Dawley rats given ad libitum access to a high-fat, high-energy diet, there is evidence that animals which overeat a fat-rich diet and eventually become obese can be readily identified by their weight gain during the first few days of access to the diet [8, 25–26]. These “obesity-prone” (OP) rats compared to their “obesity-resistant” (OR) counterparts, after just five days on the high-fat diet, start to show some disturbances in brain function, including elevated expression of the orexigenic peptide, galanin, in the hypothalamus [8] that is known to preferentially stimulate intake and weight gain on a high-fat diet [27]. There is further evidence that circulating lipids, in particular TG, which are also increased in OP rats [8, 28], may be responsible for stimulating galanin expression and may contribute to their increase in food intake on a fat-rich diet [16, 28].
In the present study, we first tested whether these outbred OP rats, while still at normal body weight and maintained on a standard lab chow diet, already show differences from OR rats in their accumbal DA levels, both basally and in response to dietary fat. We also examined the possibility that the differences between these groups in their accumbal DA may be related to differences in their circulating levels of TG, which are found to impact on neurochemical processes in the brain that control consummatory behavior. We hypothesized that OP rats would have lower basal and fat-induced NAc DA release but higher fat-induced TG levels than OR rats. Our results highlight a novel mechanism, involving circulating lipids and accumbal DA levels, which may contribute to the propensity of OP rats to over-consume a fat-rich diet.
2. Methods and procedures
2.1. Subjects
Male Sprague-Dawley rats (N = 55 total), weighing approximately 250 g at the onset of experiments, were obtained from Taconic Farms (Germantown, NY). They were housed individually in the Princeton University animal vivarium on a reversed 12-h light/dark cycle, with lights off at 6:00 am. All rats were maintained ad libitum on lab chow (LabDiet #5001, PMI Nutrition International, Richmond, IN) and water, except when indicated in the experimental design. All procedures were conducted in accordance with the Princeton University Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines on the ethical use of animals.
2.2. Classification as obesity-prone or obesity-resistant
Rats were characterized as OP or OR based on their 5-day weight gain on a high-fat diet, as previously described [8]. The high-fat diet used in this experiment was presented in round glass jars in the home cage, and it contained 50% fat (82% lard and 18% vegetable oil), 25% carbohydrate (30% dextrin, 30% cornstarch and 40% sucrose) and 25% protein (100% casein), supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals, Costa Mesa, CA) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals, Costa Mesa, CA). The diet contained 5.15 kcal/g gross energy. At the start of the study while maintained on lab chow and water, rats (N=37) were adapted acutely to the high-fat diet with a single, 15-kcal meal given early in the dark cycle for each of 3 days. Following this, chow was removed, and animals were given ad libitum access to the high-fat diet for 5 consecutive days, during which time daily measurements were taken of body weight and caloric intake. Based on their 5-day weight gain which is strongly, positively correlated with their daily high-fat diet intake, animals were rank ordered and classified as OP (highest tertile) or OR (lowest tertile). Elevated intake of the high-fat diet in the OP animals has been previously shown to cause a 50% greater accumulation of body fat after 3 weeks of ad libitum access to this high-fat diet [8]. After classification of the subgroups, the high-fat diet was removed and replaced with ad libitum lab chow for at least two weeks, to normalize body weight and caloric intake in the OP and OR rats before the start of the tests.
2.3. Surgery
Surgery to implant guide cannulas for microdialysis was performed at least one week after the animals were returned to chow and returned to normal body weight. Animals (N = 16, randomly selected from the OP and OR groups) were anesthetized with 20 mg/kg xylazine and 100 mg/kg ketamine (i.p.), supplemented with ketamine as needed. Using a stereotaxic instrument, bilateral 21-gauge stainless-steel guide cannulas were implanted and aimed at the posterior medial accumbens shell, with coordinates +1.2 mm anterior to bregma, 0.8 mm lateral to midsagittal sinus, and 4.0 mm ventral to the surface of the level skull.
2.4. Microdialysis and DA assays
After one week of recovery, a microdialysis probe was inserted and cemented in place, protruding 5 mm from the guide cannula to reach the intended site in the accumbens shell (9 mm ventral to skull surface). Probes were fixed in place and perfusion begun approximately 16 h before the microdialysis session, to allow neurotransmitter recovery to stabilize. Microdialysis probes were constructed of silica glass tubing (37 μm inner diameter, Polymicro Technologies Inc., Phoenix, AZ) inside of a 26-gauge stainless steel tube, with a cellulose tubing tip sealed at the end with epoxy cement (6000 MW, 0.2 mm outer diameter × 2.0 mm long, Spectrum Medical Co., Los Angeles, CA) [29]. Probes were perfused with buffered Ringer’s solution (142 mM NaCl, 3.9 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 1.35 mM Na2HPO4, 0.3 mM NaH2PO4, pH 7.3) at a flow rate of 0.5 μL/min. This was increased to 1.0 μL/min 2 h before and throughout the microdialysis session. Samples were collected every 20 min during the session.
Dopamine and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), were analyzed by reverse phase, high-performance liquid chromatography with electrochemical detection. Samples (20 μL each) were injected directly into a 20-μL sample loop leading to a 10-cm column with 3.2 mm bore and 3 μm, C-18 packing (Brownlee Co. Model 6213, San Jose, CA). The mobile phase contained 60 mM sodium phosphate, 100 μM EDTA, 1.24 mM heptanesulfonic acid, and 5% vol/vol methanol, with a pH of 3.6. Dopamine, DOPAC and HVA were measured with a coulometric detector (ESA Co. model 5100A, Chelmsford, MA), with the conditioning potential set at +500 mV, and the working cell potential at −400 mV.
2.5. Specific Experiments
Three experiments were performed to measure extracellular accumbens DA levels in the OP and OR rats (N = 16). In Experiment 1, basal levels of accumbens DA were measured in each subgroup under conditions where no food was available. In Experiment 2, the OP and OR rats were given a small, 15-kcal high-fat challenge meal, and its effects on extracellular DA and metabolites, as well as circulating TG, were measured. In Experiment 3, rats were injected with 10 kcal of the fat emulsion, Intralipid (5 mL i.p.; 20% fat, Baxter Healthcare Corporation, Deerfield, IL), and its effects on extracellular DA and the metabolites as well as circulating TG, were measured.
For microdialysis, each rat was brought from its home cage to the microdialysis cage the night before experimentation, at which time the microdialysis probe was implanted. Food was removed from the cage 12 h before the microdialysis session began. On the first day, microdialysis samples were collected starting 4 h into the dark phase, at which time water was also removed from the cage. After a stable baseline was reached (variation no more than 10% in 3 continuous samples), rats were given a 15-kcal, challenge high-fat meal for 20 min, and samples were collected both during this meal and for the following 60 min. Food and water were then returned to the cage. On the second day, food was again removed from the cage 12 h before the microdialysis session, and the samples were collected starting 4 h into the dark phase, with water removed. After 3 stable baseline samples were collected, rats were injected with Intralipid (10 kcal, 5 mL, i.p.) and microdialysis samples were collected for the following 4 h. After testing, the rats were then returned to their home cages where they remained on ad libitum rodent chow and were later tested for circulating levels of TG.
2.6. Blood collection and metabolite assessment
To understand their relationship to accumbens DA, circulating levels of TG were measured in response to a high-fat meal challenge or the injection of Intralipid. For Experiment 2, the effect of the 1-h high-fat meal (15 kcal) was measured in a separate set of OP or OR rats (n = 9/group), starting 5 h into the dark cycle, one hour after lab chow was removed from the home cage. For Experiment 3, the effect of Intralipid was measured in the same rats used for microdialysis (OP: n = 8; OR: n = 8), several days following the completion of microdialysis. Starting 5 h into the dark cycle, food was removed from the home cage, and the animals were injected with Intralipid (10 kcal, 5 mL, i.p.). One hour after the start of the high-fat meal or injection of Intralipid, tail vein or trunk blood, respectively, was drawn for measurement of TG levels. Serum was then assayed using a Triglyceride Assay kit (Sigma-Aldrich Co., St. Louis, MO).
2.7. Histology
At the end of the experiment, histology was performed to verify microdialysis probe placements. Rats were sacrificed by rapid decapitation, and the brains were kept in formalin for a minimum of 1 week. They were then sliced in 40 μm sections on a freezing microtome and slide-mounted for microscopic verification. Once visualized, probe tracks were plotted using the atlas of Paxinos and Watson [30]. Three animals had probes more than 0.5 mm from the target region and were therefore discarded from the analysis. In addition, the microdialysis probes of two other rats failed overnight between Experiments 2 and 3, thus data was not collected for these two rats in Experiment 3.
2.8. Data analysis
Experiment 1 data were analyzed by independent samples two-tailed t-tests, in addition to Pearson’s correlation. Microdialysis data from Experiments 2 and 3 were converted to percent of the mean of the three baseline samples. They were analyzed by two-way repeated measures ANOVA (with group as the between-subject factor and time as the within-subject factor) and then by one-way repeated measures ANOVA and Neuman-Keuls post-hoc tests, for time within each group when justified. Microdialysis data from Experiment 2, without conversion, were also analyzed by two-way repeated measures ANOVA (with group as the between-subject factor and time as the within-subject factor) and then by independent samples two-tailed t-tests. All TG data were analyzed by independent samples two-tailed t-tests. All values are expressed as mean ± SEM.
3. Results
3.1. Basal levels of accumbens DA in obesity-prone and obesity-resistant rats
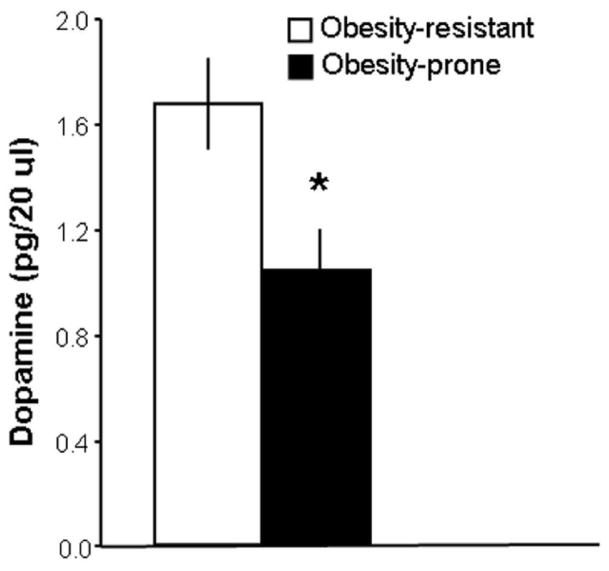
Rats (N = 37) were given 5 days of access to the high-fat diet for group classification. The rats were designated as OP or OR, based on their weight gain on this diet (7.9 ± 0.4 vs. 4.1 ± 0.2 g/day) that, in turn, was strongly, positively correlated with their daily intake of the high-fat diet (r = 0.81, p<0.001). While the OP rats at the end of the 5-day classification period had significantly higher body weight (335 ± 6 vs. 314 ± 3 g, p<0.05) and caloric intake (115 ± 3 vs. 94 ± 4 kcal/day, p<0.001) compared to the OR rats, their shift to a lab chow diet for two weeks allowed the OP animals to return to a similar body weight as the OR rats (369 ± 4 vs. 355 ± 6 g, ns), at which time the microdialysis tests were performed. In the OP (n = 5) compared to OR (n = 8) animals, the results revealed significantly lower, basal levels of extracellular DA in the NAc shell (−38%, p<0.05) (Fig. 1). These results demonstrate that, in the absence of high-fat food, the OP rats at normal body weight already have a different neurochemical milieu in the NAc, reduced DA signaling, compared to the OR rats.
Fig. 1.
Obesity-prone rats (n = 5) that over-consume a fat-rich diet have lower basal extracellular accumbens DA levels than obesity-resistant rats (n = 8). Data are mean ± SEM, *p < 0.05 vs. obesity-resistant.
3.2. High-fat meal-induced rise in DA in obesity-prone and obesity-resistant rats
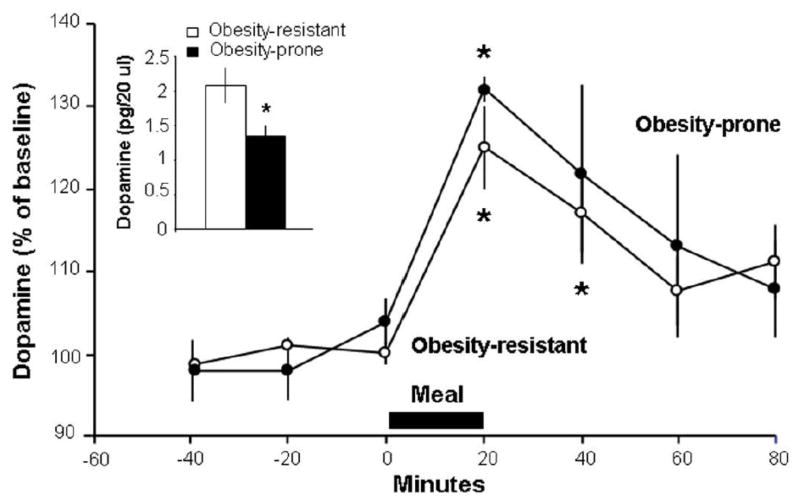
This experiment, with measurements of accumbens DA and also of circulating TG that may affect DA signaling, tested the OP and OR rats in terms of their responsiveness to a high-fat meal challenge, to determine whether their differences in consumption may be related to different reward signals from the diet. One hour after a high-fat meal (15 kcal) that was fully consumed, measurements of circulating TG revealed significantly higher levels (+58%) in the OP compared to OR rats (195 ± 39 vs. 123 ± 14 mg/dl, p<0.05). Across both subgroups (OR: n = 8; OP: n = 5), the measurements of DA release in the NAc shell after the fat-rich meal revealed elevated levels over time (F(6,66) = 13.67, p<0.001) (Fig. 2). After the start of the meal, DA levels increased in the OR rats (F(6,42) = 8.70, p<0.001), significantly at both 20 min (125 ± 5%, p<0.001) and 40 min (117 ± 5%, p<0.05), and in the OP rats (F(6,24) = 5.29, p<0.01), although only at 20 min (132 ± 1%, p<0.01). Despite their similar relative increase in DA (F(1,11) = 0.35, ns), the OP and OR rats exhibited differences in their absolute levels, with DA during the first 40 min after the high-fat meal still significantly lower in the OP rats compared to OR rats (1.3 ± 0.1 vs. 2.1 ± 0.2 pg/20 μl, p<0.05) (Fig. 2), consistent with results in Experiment 1.
Fig. 2.
In response to a 20-min high-fat meal, obesity-prone rats (n = 5; solid circles) and obesity-resistant rats (n = 8; open circles) have the same relative increase in extracellular DA in the nucleus accumbens. Data are mean ± SEM, *p < 0.05 vs. baseline. Inset: Absolute levels of DA after the high-fat meal remain lower in obesity-prone compared to obesity-resistant rats. Data are mean ± SEM, *p < 0.05 vs. obesity-resistant.
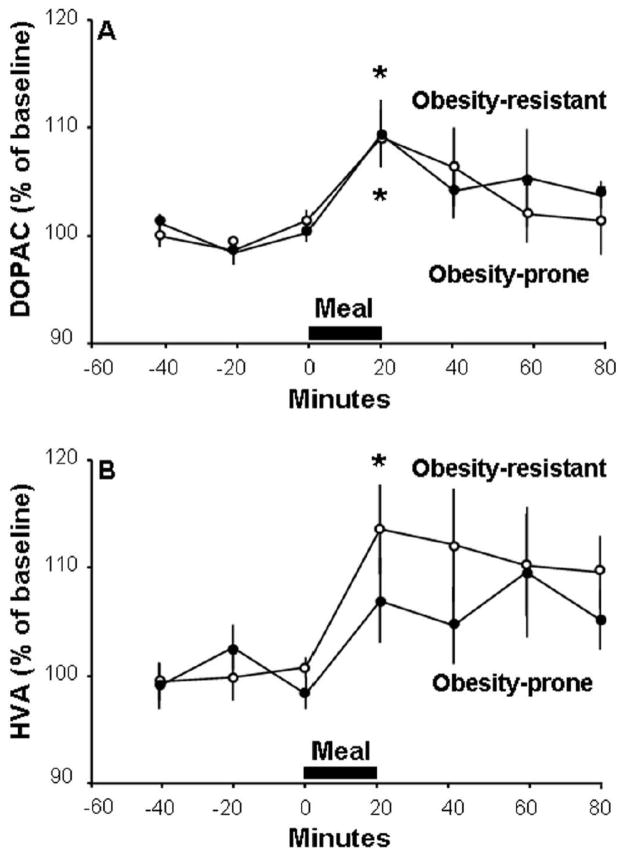
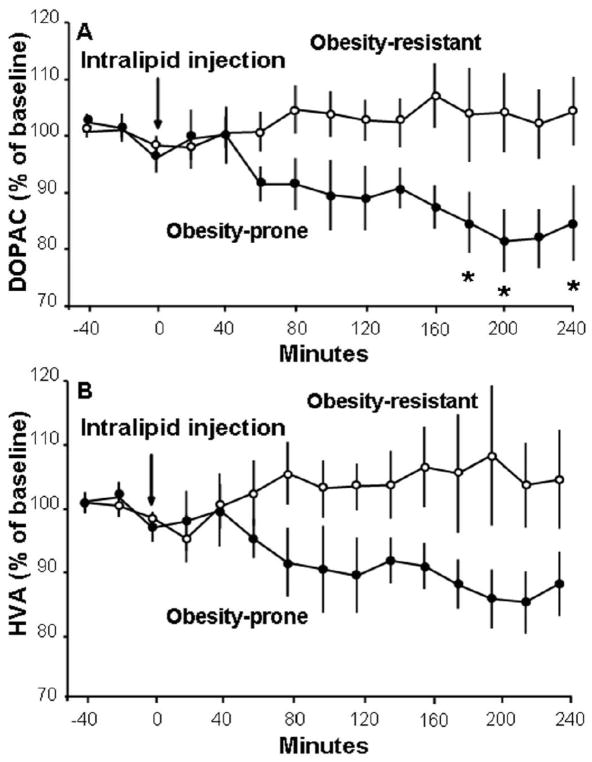
A similar pattern was also observed with the DA metabolites in response to the high-fat meal (Fig. 3). The OP and OR rats were similar in their DOPAC levels (F(1,11) = 0.08, ns) (Fig. 3A) that changed over time in both the OR rats (F(6,42) = 2.72, p<0.05) and OP rats (F (6,24) = 3.20, p<0.05) with a small increase at 20 min (109 ± 3%, p<0.05 for both groups), but these subgroups exhibited differences in their meal-induced changes in HVA levels. Although similar in their overall levels (F(1,11) = 0.80, ns) (Fig 3B), the OR rats showed a significant increase over time (F(6,42) = 3.90, p<0.01), with a peak of 113 ± 4% at 20 min (p<0.05), but the OP rats showed no change in HVA levels after the meal (F(6,24) = 1.87, ns). Together, these results demonstrate that the OP rats, while responding to a fat-rich meal with an increase in accumbal DA release, still have significantly lower absolute levels of DA compared to the OR rats and exhibit less change in the DA metabolites, while showing higher circulating levels of TG.
Fig. 3.
In response to a 20-min high-fat meal, obesity-prone rats (n = 5; solid circles) and obesity-resistant rats (n = 8; open circles) have similar relative changes in DA metabolites in the nucleus accumbens. A. Both groups of rats have significant elevations in levels of 3,4-dihydroxyphenylacetic acid (DOPAC). B. Only obesity-resistant rats have significantly elevated levels of homovanillic acid (HVA). Data are mean ± SEM, *p < 0.05 vs. baseline.
3.3. Intralipid-induced rise in DA in obesity-prone and obesity-resistant rats
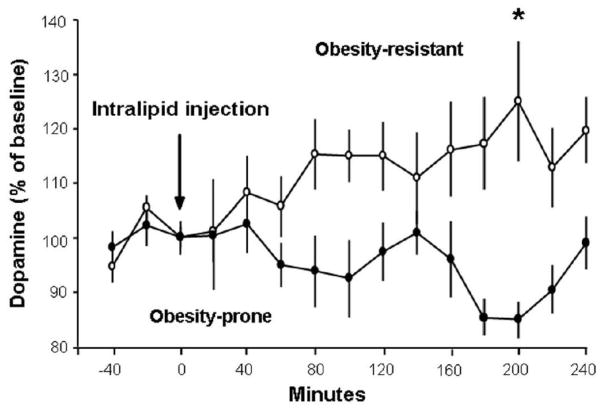
The findings in Experiment 2, suggesting that OP compared to OR rats after a fat-rich meal have lower DA in association with elevated TG, led us to test the possibility that these OP rats might also exhibit reduced accumbal DA release in response to peripheral administration of a fat emulsion, which bypasses the mouth and raises circulating TG. The animals in this experiment (OR: n = 6; OP: n = 5) were given an i.p. injection of Intralipid (10 kcal). At 1 h after injection, the OP rats once again had significantly higher TG levels compared to OR rats (296 ± 44 vs. 104 ± 9 mg/dl, p<0.05). In association with this difference in TG, the OP rats also exhibited a strikingly different pattern of accumbal DA release and metabolism (F(1,9) = 9.46, p<0.05) (Fig. 4). In the OR rats, DA was significantly elevated across time (F(14,70) = 2.51, p<0.001), with levels peaking at 125 ± 10% compared to baseline at 200 min after injection (p<0.01). This is in contrast to the OP rats, which exhibited no significant change in DA levels after Intralipid injection (F(14,56) = 1.59, ns).
Fig. 4.
In response to injection of a lipid emulsion, obesity-prone rats (n = 5; solid circles) release less DA in the nucleus accumbens compared to obesity-resistant rats (n = 6; open circles). Data are mean ± SEM, *p < 0.05 vs. baseline.
The same general pattern also occurred with the DA metabolites, with OR rats showing higher levels than the OP rats (Fig. 5). There was a significant difference between the OP and OR rats in their DOPAC levels (F(1,9) = 5.51, p<0.05) but not HVA levels (F(1,9) = 3.54, ns). With no change in DOPAC levels across time in the OR rats (F(14,70) = 0.77, ns), the group difference actually reflected a decrease in levels in OP animals (F(14,56) = 3.93, p<0.001), which remained below baseline at 180 min (85 ± 5%, p<0.05), 200 min (81 ± 6%, p<0.01), and 240 min (85 ± 7%, p<0.05) after injection. Together, these results demonstrate that OP rats, which appear to metabolize the injected fat emulsion differently, have a significantly diminished DA response to the circulating lipids, which in the OR rats markedly increase DA release.
Fig. 5.
After injection of a lipid emulsion, obesity-prone rats (n = 5; solid circles) have lower levels of DA metabolites in the nucleus accumbens than obesity-resistant rats (n = 6; open circles). A. Obesity-prone rats have decreased levels of dihydroxyphenylacetic acid (DOPAC). B. There are no significant differences between groups in levels of homovanillic acid (HVA). Data are mean ± SEM, *p < 0.05 vs. baseline.
3.4. Histology
Histology revealed that microdialysis probes were located primarily in the medial shell region of the NAc (Fig. 6).
Fig. 6.
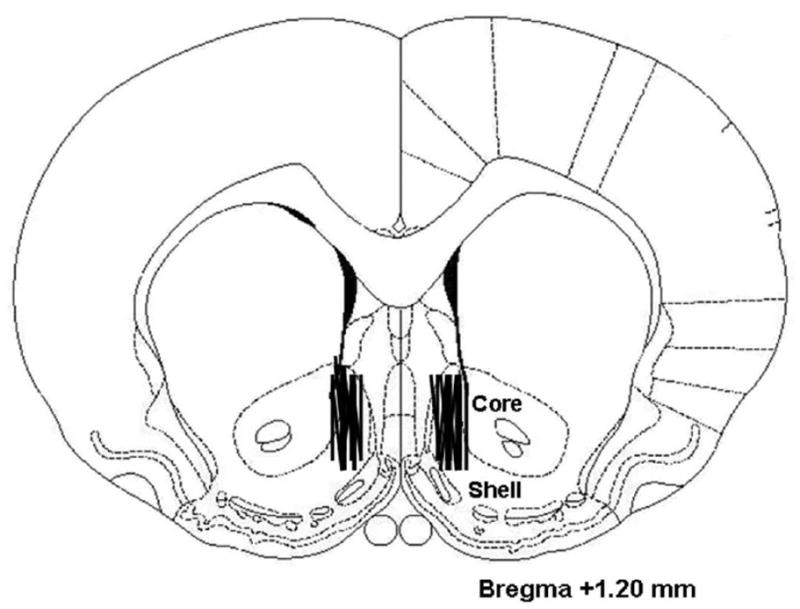
Histology showed that microdialysis samples were drawn primarily from the medial nucleus accumbens shell. Adapted from The Rat Brain, compact 3rd edition, G. Paxinos and C. Watson, Copyright 1997, with permission from Elsevier.
4. Discussion
4.1. Basal DA is reduced in obesity-prone rats
The question addressed in the present study was whether outbred OP rats, prior to their development of obesity, show differences from OR rats in their basal accumbal DA levels, as well as their DA levels in response to fat intake or administration. The results demonstrated that OP rats, which consume excessive calories when offered a high-fat diet, have markedly lower extracellular DA in the NAc shell compared to OR rats. These results are consistent with a recent study of inbred OP rats [24], which compared to inbred OR rats also show decreased extracellular DA using microdialysis. Recent work by Davis and colleagues, showing chronic intake of a high-fat diet to decrease accumbal DA turnover in Long-Evans rats [20], might suggest that the greater intake by the OP compared to OR animals in the present study during their 5-day classification period contributed to the decrease in DA. This possibility seems unlikely, however, since the effects in the Davis study were comparable regardless of whether the fat was consumed ad libitum or was restricted to match a chow control group. The difference in DA signaling between the OP and OR rats could be due to a number of different factors, with one likely candidate involving the adiposity hormone, leptin. After 5 days on the high-fat diet, outbred rats designated as OP, while only slightly heavier than the OR rats, already have significantly higher levels of leptin [8], which after a high-fat meal accurately predicts long-term over-consumption on a high-fat diet [31]. Infusion of leptin is found to inhibit accumbal DA release [32], while in vitro bath application of leptin suppresses DA cell firing in the ventral tegmental area [33], which contains the DA cells that project to the NAc. Leptin levels were not assessed in the present animals as the fat meal or Intralipid injection given prior to blood sampling would have obscured the results. If, however, the elevated leptin levels seen in the OP rats on a high-fat diet [8] do remain high even after they are switched to a standard lab chow diet, as in the present study, this hormone may act via DA cell bodies in the ventral tegmental area to maintain accumbal DA at generally low levels.
4.2. Low DA can facilitate food intake
The precise role of DA in driving food intake is still a matter of debate, but there is some evidence that low levels of DA may lead to an increase in consumption. On the one hand, a rise in accumbal DA can enhance feeding and food-seeking behavior [9], and intra-accumbal injection of DA agonists can increase food intake [12, 34], particularly at a lower dose [35]. On the other hand, levels of DA do not rise during food deprivation [36–37], and NAc injection of a DA antagonist does not decrease overall free-feeding [38–39]. One correlate of the DA hypothesis holds that animals enjoy the effects of a rise in DA and work to enhance DA levels when they are low [40]. This appears to be the case in humans and animals given antipsychotic medications that act in part as DA antagonists at the D2 receptor. Human patients administered these medications exhibit enhanced food intake and binge eating, which leads to increased weight gain [41–43]. A similar effect with these medications is seen in female rats and mice [44–45]. Since low DA functioning can lead to increased food intake [40], it may be the lower basal levels of accumbal DA in the OP rats that cause their increased consumption of a high-fat diet.
4.3. Fat-induced DA release fails to compensate for low levels in obesity-prone rats
After a 15-kcal high-fat meal, which stimulates DA, OP rats in the present study showed an increase in DA release and levels of DA metabolites that was similar in magnitude to the effect exhibited by OR rats, peaking at the end of the meal and then declining thereafter. Despite this meal-induced change, however, the absolute levels of DA still remained significantly lower in the OP compared to OR rats after the meal, suggesting that OP rats would have to eat more of the high-fat diet to attain the same levels of DA as the OR rats. This higher intake, in fact, occurred during the 5-day ad libitum access, as the OP rats consumed significantly more high-fat diet. While previous work has shown that accumbal DA is increased by ingestion of a cafeteria diet high in both fat and carbohydrate [18] or by sham feeding of fat [17], this is the first paper to demonstrate that voluntary ingestion of fat alone is sufficient to elevate DA levels. Given that palatable meals are generally known to enhance DA levels in the NAc [46], the high-fat-induced rise in DA in the present study may be due specifically to the orosensory reward produced by the fat itself. This is suggested by the findings, for example, that the rewarding tastes of sucrose [47–48] or fat-rich corn oil [17] in sham feeding rats are sufficient to enhance the release of accumbens DA. Notably, the OP and fat-preferring, Osborne-Mendel strain of rat is found to have taste receptor cells that are less responsive to polyunsaturated fatty acids than those of the fat-avoiding, S5B/PL rat strain [49]. This suggests that the Sprague-Dawley OP compared to OR rats, at normal body weight, may be less able to detect the fat content of the high-fat meal and thus need to consume substantially more of the high-fat diet to achieve the same DA levels as the OR rats with access to the same diet. On the other hand, there is evidence that rats and mice can detect the taste of fat even when they are anosmic or the diet texture is held constant [50–51], suggesting that the DA response to fat may also be due to other factors in addition to orosensory stimulation.
4.4. Accumbal DA in obesity-prone rats is unresponsive to circulating lipids
When the orosensory reward of fat is bypassed with direct injection of a lipid emulsion that increases TG levels, Sprague-Dawley rats are found to exhibit an increase in levels of accumbens DA and its metabolites, similar to that seen after the consumption of fat [19]. In the present experiment, this effect is clearly evident in the OR rats after injection of Intralipid. With the rise in TG, this increase in DA levels may be due, in part, to the actions of fatty acids, the breakdown products of TG, which are found to reduce DA uptake in cell lines that express the DA transporter [52–53], leading to an increase in extracellular levels of DA. The possibility that fatty acids increase DA is further supported by evidence that hypothalamic levels of both DA and enzymes associated with DA metabolism are increased in situations where levels of fatty acids are increased, such as after chronic intake of fat [54] or fasting [55]. The present study showed the fat-induced rise in accumbal DA to be absent in OP rats, which after Intralipid exhibited no change in DA and a decrease in its metabolites. These results suggest that OP rats are less sensitive than OR rats to physiological signals such as circulating lipids, causing them to have less lipid-induced stimulation of DA release and thus increase their consummatory behavior to achieve desired DA levels. This finding is similar to that observed in the OP, fat-preferring Osborne-Mendel rats, which are less responsive than fat-avoiding S5B/PL rats to the satiating effect of duodenal Intralipid infusion [56]. It is also consistent with the generally reduced sensitivity of OP rats to other satiety factors, such as leptin and insulin [57–60]. Recent evidence suggests that this decreased responsiveness to circulating lipids in OP animals may be due, in part, to the reduced transport of fatty acids across the blood brain barrier. Inbred rats prone to the development of obesity have reduced liver gene expression of CD36, a fatty acid transporter, compared to those resistant to the development of obesity [61]. Thus, the OP rats may consume more of a high-fat diet, in part, due to their decreased responsiveness to the physiological signaling of meal consumption.
4.5. Obesity-prone rats have an increased TG response after a fat meal or lipid injection
This decreased responsiveness to physiological signaling in OP rats may be related to the results obtained with measurements of circulating TG. In response to a high-fat meal or injection of Intralipid, levels of TG were consistently higher in the OP compared to OR animals. This finding with a high-fat meal is consistent with previous studies showing OP animals to have higher TG levels after a single high-fat meal [31] or 5 days of ad libitum high-fat diet intake [8]. The present finding with Intralipid is novel and confirms this increased TG response using a different mode of fat presentation. Elevated TG levels in OP rats in response to fat are also consistent with findings of elevated levels of leptin and insulin after fat consumption in these animals [8, 25, 31]. In response to fatty acid administration, OP rats generally exhibit reduced fat oxidation compared to OR rats [61–62]. This suggests that their metabolism of the fat-induced TG would also be decreased, reducing the availability of fatty acids for stimulating DA. Thus, as with elevated levels of leptin and insulin reflecting reduced sensitivity to these hormones [57–58], the OP rats of the present study at normal weight with increased TG levels may also be less sensitive to TG and their breakdown products in terms of their effects on accumbal DA signaling after consumption of a high-fat meal or injection of a fat emulsion. Thus, in addition to reduced orosensory detection, this reduced DA responsiveness to circulating lipids may contribute to the failure of OP rats to properly regulate their intake on a high-fat diet.
4.6. Summary and conclusions
The present results support the idea that OP rats over-consume a high-fat diet to raise their levels of accumbal DA, which are basally lower than in OR rats. Consumption of a high-fat diet, while increasing NAc DA, does not bring DA to the same absolute levels in OP as in OR rats, indicating that OP rats are likely to consume substantially more of the diet in order to achieve the same DA concentrations as OR rats. Our findings suggest that the accumbal DA system of OP rats is less sensitive to specific physiological signals of ingestion related to dietary fat, such as elevated circulating TG levels, which ultimately impact on the DA system. Consistent with that seen in already-obese individuals, these neurochemical disturbances are found here to be evident in normal-weight animals that are prone to obesity, suggesting that they may be underlying causes of their overeating and eventual obesity on a high-fat diet. Together, these results point to a novel mechanism, involving circulating lipids and accumbal DA levels, that contributes to fat over-consumption in certain individuals.
Research Highlights.
Rats prone to obesity show reduced levels of dopamine in the nucleus accumbens
Rats show increased levels of accumbal dopamine after ingestion of dietary fat
Rats prone to obesity have a reduced accumbal dopamine response to intake of fat
Obesity-prone rats have a reduced dopamine response to injection of a fat emulsion
Reduced dopamine in obesity-prone rats may lead them to over-consume dietary fat
Acknowledgments
This research was supported by NIH grant DA 21518. We extend thanks to Olga Karatayev for her expert advice on the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 4.Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Joint WHO/FAO Expert Consultation; World Health Organization. WHO Technical Report Series 916. Geneva: 2002. Diet, nutrition and the prevention of chronic diseases: report of a joing WHO/FAO expert consultation. [Google Scholar]
- 6.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–9. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- 7.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32 (Suppl 7):S109–19. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–78. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 12.Pal GK, Thombre DP. Modulation of feeding and drinking by dopamine in caudate and accumbens nuclei in rats. Indian J Exp Biol. 1993;31:750–4. [PubMed] [Google Scholar]
- 13.Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–45. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 14.Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007;292:E561–70. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- 15.Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–12. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- 16.Gaysinskaya VA, Karatayev O, Chang GQ, Leibowitz SF. Increased caloric intake after a high-fat preload: relation to circulating triglycerides and orexigenic peptides. Physiol Behav. 2007;91:142–53. doi: 10.1016/j.physbeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 18.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–9. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rada P, Avena NM, Karatayev O, Chang GQ, Hoebel BG, Leibowitz SF. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2008. Accumbens dopamine is stimulated by dietary fat and also by injection of a lipid emulsion that increases circulating triglycerides: possible role in fat-induced overeating, Program No. 713.3. [Google Scholar]
- 20.Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–63. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 22.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomasi D, Wang GJ, Wang R, Backus W, Geliebter A, Telang F, et al. Association of body mass and brain activation during gastric distention: implications for obesity. PLoS One. 2009;4:e6847. doi: 10.1371/journal.pone.0006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–6. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagliassotti MJ, Knobel SM, Shahrokhi KA, Manzo AM, Hill JO. Time course of adaptation to a high-fat diet in obesity-resistant and obesity-prone rats. Am J Physiol. 1994;267:R659–64. doi: 10.1152/ajpregu.1994.267.3.R659. [DOI] [PubMed] [Google Scholar]
- 26.Gayles EC, Pagliassotti MJ, Prach PA, Koppenhafer TA, Hill JO. Contribution of energy intake and tissue enzymatic profile to body weight gain in high-fat-fed rats. Am J Physiol. 1997;272:R188–94. doi: 10.1152/ajpregu.1997.272.1.R188. [DOI] [PubMed] [Google Scholar]
- 27.Yun R, Dourmashkin JT, Hill J, Gayles EC, Fried SK, Leibowitz SF. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides. 2005;26:2265–73. doi: 10.1016/j.peptides.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Circulating triglycerides after a high-fat meal: predictor of increased caloric intake, orexigenic peptide expression, and dietary obesity. Brain Res. 2009;1298:111–22. doi: 10.1016/j.brainres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez L, Stanley BG, Hoebel BG. A small, removable microdialysis probe. Life Sci. 1986;39:2629–37. doi: 10.1016/0024-3205(86)90119-0. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. Stereotaxic Coordinates. 3. San Diego: Academic Press; 1997. The Rat Brain. [Google Scholar]
- 31.Leibowitz SF, Chang GQ, Dourmashkin JT, Yun R, Julien C, Pamy PP. Leptin secretion after a high-fat meal in normal-weight rats: strong predictor of long-term body fat accrual on a high-fat diet. Am J Physiol Endocrinol Metab. 2006;290:E258–67. doi: 10.1152/ajpendo.00609.2004. [DOI] [PubMed] [Google Scholar]
- 32.Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–7. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 33.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA, Fotuhi M, Colle LM. Facilitation of feeding by nucleus accumbens amphetamine injections: latency and speed measures. Pharmacol Biochem Behav. 1989;32:769–72. doi: 10.1016/0091-3057(89)90031-2. [DOI] [PubMed] [Google Scholar]
- 35.Evans KR, Vaccarino FJ. Intra-nucleus accumbens amphetamine: dose-dependent effects on food intake. Pharmacol Biochem Behav. 1986;25:1149–51. doi: 10.1016/0091-3057(86)90102-4. [DOI] [PubMed] [Google Scholar]
- 36.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–50. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–15. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–21. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- 39.Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–82. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 40.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry. 2001;62:231–8. doi: 10.4088/jcp.v62n0404. [DOI] [PubMed] [Google Scholar]
- 42.Blouin M, Tremblay A, Jalbert ME, Venables H, Bouchard RH, Roy MA, et al. Adiposity and eating behaviors in patients under second generation antipsychotics. Obesity (Silver Spring) 2008;16:1780–7. doi: 10.1038/oby.2008.277. [DOI] [PubMed] [Google Scholar]
- 43.Kluge M, Schuld A, Himmerich H, Dalal M, Schacht A, Wehmeier PM, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol. 2007;27:662–6. doi: 10.1097/jcp.0b013e31815a8872. [DOI] [PubMed] [Google Scholar]
- 44.Coccurello R, D’Amato FR, Moles A. Chronic administration of olanzapine affects Behavioral Satiety Sequence and feeding behavior in female mice. Eat Weight Disord. 2008;13:e55–60. [PubMed] [Google Scholar]
- 45.Arjona AA, Zhang SX, Adamson B, Wurtman RJ. An animal model of antipsychotic-induced weight gain. Behav Brain Res. 2004;152:121–7. doi: 10.1016/j.bbr.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 46.Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–78. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 48.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–20. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 49.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann N Y Acad Sci. 1998;855:165–8. doi: 10.1111/j.1749-6632.1998.tb10560.x. [DOI] [PubMed] [Google Scholar]
- 50.Passilly-Degrace P, Gaillard D, Besnard P. Orosensory perception of dietary lipids in mammals. Results Probl Cell Differ. 2009;47:221–38. doi: 10.1007/400_2008_7. [DOI] [PubMed] [Google Scholar]
- 51.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–32. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 52.Chen N, Appell M, Berfield JL, Reith ME. Inhibition by arachidonic acid and other fatty acids of dopamine uptake at the human dopamine transporter. Eur J Pharmacol. 2003;478:89–95. doi: 10.1016/j.ejphar.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Reith ME. Regulation of the functional activity of the human dopamine transporter by the arachidonic acid pathway. Eur J Pharmacol. 1996;315:345–54. doi: 10.1016/s0014-2999(96)00646-2. [DOI] [PubMed] [Google Scholar]
- 54.Lee AK, Mojtahed-Jaberi M, Kyriakou T, Astarloa EA, Arno M, Marshall NJ, et al. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010;26:411–22. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cvijic G, Djordjevic J, Davidovic V. Effect of fasting and refeeding on the activities of monoamine oxidase and antioxidant enzymes in rat hypothalamus and brown adipose tissue. Gen Physiol Biophys. 2000;19:305–16. [PubMed] [Google Scholar]
- 56.Greenberg D, McCaffery J, Potack JZ, Bray GA, York DA. Differential satiating effects of fats in the small intestine of obesity-resistant and obesity-prone rats. Physiol Behav. 1999;66:621–6. doi: 10.1016/s0031-9384(98)00336-9. [DOI] [PubMed] [Google Scholar]
- 57.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286:R143–50. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 58.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–6. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 59.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Veledo S, Nieto-Vazquez I, Vila-Bedmar R, Garcia-Guerra L, Alonso-Chamorro M, Lorenzo M. Molecular mechanisms involved in obesity-associated insulin resistance: therapeutical approach. Arch Physiol Biochem. 2009;115:227–39. doi: 10.1080/13813450903164330. [DOI] [PubMed] [Google Scholar]
- 61.Ji H, Friedman MI. Reduced capacity for fatty acid oxidation in rats with inherited susceptibility to diet-induced obesity. Metabolism. 2007;56:1124–30. doi: 10.1016/j.metabol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ji H, Friedman MI. Reduced hepatocyte fatty acid oxidation in outbred rats prescreened for susceptibility to diet-induced obesity. Int J Obes (Lond) 2008;32:1331–4. doi: 10.1038/ijo.2008.71. [DOI] [PubMed] [Google Scholar]