Abstract
Although arsenic is a well-established human carcinogen, the mechanisms by which it induces cancer remain poorly understood. We previously showed arsenite to be a potent mutagen in human–hamster hybrid (AL) cells, and that it induces predominantly multilocus deletions. We show here by confocal scanning microscopy with the fluorescent probe 5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate that arsenite induces, within 5 min after treatment, a dose-dependent increase of up to 3-fold in intracellular oxyradical production. Concurrent treatment of cells with arsenite and the radical scavenger DMSO reduced the fluorescent intensity to control levels. ESR spectroscopy with 4-hydroxy-2,2,6,6-tetramethyl-1-hydroxypiperidine (TEMPOL-H) as a probe in conjunction with superoxide dismutase and catalase to quench superoxide anions and hydrogen peroxide, respectively, indicates that arsenite increases the levels of superoxide-driven hydroxyl radicals in these cells. Furthermore, reducing the intracellular levels of nonprotein sulfhydryls (mainly glutathione) in AL cells with buthionine S-R-sulfoximine increases the mutagenic potential of arsenite by more than 5-fold. The data are consistent with our previous results with the radical scavenger DMSO, which reduced the mutagenicity of arsenic in these cells, and provide convincing evidence that reactive oxygen species, particularly hydroxyl radicals, play an important causal role in the genotoxicity of arsenical compounds in mammalian cells.
Arsenic, as trivalent arsenite (As3+) or pentavalent arsenate (As5+), is naturally occurring and ubiquitously present in the environment. Humans are exposed to arsenic mainly through either oral or inhalation routes. Oral exposure occurs via consumption of contaminated water, food, and drugs (1), and exposure can be lifelong. Occupational exposure, on the other hand, occurs mainly through inhalation via nonferrous ore smelting, semiconductor and glass manufacturing, or power generation by the burning of arsenic-contaminated coal (2, 3). Epidemiological data have shown that chronic exposure of humans to inorganic arsenical compounds is associated with liver injury, peripheral neuropathy, and an increased incidence of cancer of the lung, skin, bladder, and liver (4, 5). Arsenic contamination of drinking water is a serious environmental problem worldwide because of the large number of contaminated sites that have been identified and the large number of people at risk (6). The risk of developing arsenic-induced human diseases from environmental exposure is particularly high in many developing countries. For example, it is estimated that as many as 50 million people are at risk in Bangladesh alone, where both acute and chronic arsenic poisoning as well as increased cancer incidence have been reported (7). Occupationally, there is evidence that underground uranium miners who are also exposed to arsenic have a 10-fold increase in lung cancer risk compared with miners with no previous history of arsenic exposure (8).
Arsenic is unusual because it is one of the few, possibly the only, demonstrated human carcinogens that has not been shown to induce tumors in laboratory animals (9). An inducible arsenic tolerance state in rodent species has been suggested to account for this discrepancy (10). Inducible tolerance to arsenic-induced toxicity has not been observed in human cells. There is also evidence that human cells are, in fact, more sensitive to arsenite than are cells of rodent origin (11).
In the absence of animal models to study the carcinogenic mechanism(s) of arsenic, in vitro studies have been conducted to illuminate mechanisms. Although arsenic and arsenical compounds are toxic and induce morphological transformants in Syrian hamster embryo and C3H 10T1/2 cells (12, 13), they have been found to be inactive, as gene mutagens at the hypoxanthine guanine phosphoribosyl transferase (HPRT) and ouabain loci (12, 14) and are only marginally active at the thymidine kinase locus of L5178 mouse lymphoma cells (15). In contrast, recent studies by Moore et al. that use the latter assay have reported a much higher mutagenic response to arsenite under exposure conditions similar to those of the former study (16). The reason for the discrepancy in findings is not immediately clear. Arsenical compounds, on the other hand, are potent clastogens in many types of cells inducing chromosomal aberrations, sister chromatid exchanges, and chromosomal loss in both human and rodent cells in culture (17, 18). One possible scenario to explain the failure to induce gene mutations is that arsenic induces mostly multilocus deletions that are incompatible with cell survival when selected at loci such as oua or HPRT that are located relatively close to essential genes. In other words, it is possible that the types of mutants induced by arsenite are poorly recoverable in these assays, in which large mutations are likely to be lethal. With the use of the AL mutagenic assay system, which is sensitive in detecting both intragenic and multilocus deletions (19–21), we showed that this difference in mutant induction is, in fact, the case for trivalent sodium arsenite (22). At equal doses of arsenite, the mutant yield of CD59− mutants was ≈35-fold higher than that of HPRT− mutants measured concurrently. In addition, the majority of the CD59− mutants induced were caused by multilocus deletions of several million base pairs. Our data are consistent with the recent report that arsenic induces intrachromosomal recombination in the HPRT gene of Chinese hamster ovary cells (23). Biologically, the trivalent sodium arsenite is significantly more active than the pentavalent sodium arsenate, including the ability to induce gene amplification in mammalian cells (17, 24). The oxidative stress protein, heme oxygenase, and c-fos oncogene have been shown to be overexpressed in arsenic-treated mammalian cells (25, 26). These data are consistent with our earlier results implicating oxidative damages induced by arsenite as an underlying basis of its genotoxic effects (22). An elucidation of the mutagenic mechanism(s) of arsenical compounds may help increase our understanding of their carcinogenic effects in humans and provide a test system for the carcinogenic action of other metal carcinogens as well. With the use of human–hamster hybrid (AL) cells, we show here with confocal microscopy coupled with ESR spectroscopy that arsenite induces, in live cells, a dose-dependent increase in superoxide-driven hydroxyl radical production, which mediates its genotoxic activity in mammalian cells.
Materials and Methods
Cell Culture.
The AL hybrid cells that contain a standard set of Chinese hamster ovary-K1 chromosomes and a single copy of human chromosome 11 were used (27). Chromosome 11 contains the CD59 gene (formerly called M1C1) at 11p13.5. This gene encodes the CD59 cell surface antigen (also known as the S1 antigen) that, in the presence of rabbit serum complement (HRP, Denver, PA), renders AL cells sensitive to killing by the monoclonal antibody E7.1. Antibody E7.1 was produced by a hybridoma culture as described (28, 29). AL cells were maintained in Ham F-12 medium supplemented with 8% heat-inactivated FBS, 25 μg/ml gentamycin, and 2× normal glycine (2 × 10−4 M) at 37°C in a humidified 5% CO2 incubator and passaged as described (19, 20).
Arsenite Treatment and Clonogenic Survival.
Stock solutions (1 mg/ml or 7.7 mM) of sodium arsenite (Sigma) were prepared in double-distilled water and sterilized with a 0.22-μm syringe filter (22). Working concentrations were prepared by diluting the stock with complete F12 medium. To determine the dose–response clonogenic toxicity of arsenite to AL cells, exponentially growing cultures were plated in T25 flasks and incubated for 2 days before being treated with graded doses of arsenite for 24 h, as described (22). Cultures were incubated for 7–9 days after treatment, at which time they were fixed with formaldehyde and stained with Giemsa. The number of colonies was counted to determine the surviving fraction as described (19–22).
Mutagenesis Assay.
After treatment, cultures were replated into T25 flasks and cultured for 7 days before mutation was measured, as described (19–22). Briefly, 5 × 104 cells were inoculated into each of six 60-mm dishes in a total of 2 ml of complete F12 medium. After a 2-h incubation to allow for cell attachment, E7.1 antiserum (0.3% vol/vol) and 1.5% freshly thawed complement (vol/vol) were added to each dish. The cultures were incubated for 7–9 days, at which time they were fixed and stained, and the number of CD59− colonies was scored. Controls included identical sets of dishes containing antiserum alone, complement alone, or neither agent. The mutant fraction at each dose (MF) was calculated as the number of surviving colonies divided by the total number of cells plated after correction for any nonspecific killing because of complement alone. The mutant yields (MY) are given by the slopes of the dose–response curves and are independent of the background mutant level.
Nonprotein Sulfhydryl Depletion by Buthionine S-R Sulfoximine (BSO).
Exponentially growing AL cells (5 × 105) in 25-cm2 tissue culture flasks were treated with BSO at 10 or 25 μM (Chemalog) for 24 h before arsenite treatment. The doses of BSO used were shown previously to be nontoxic and nonmutagenic and to reduce the nonprotein sulfhydryl level to less than 5% of the control level (30, 31). After treatment with arsenite in the presence of BSO for another 24 h, cultures were trypsinized and replated to determine survival and mutagenesis as described above.
Determination of Reactive Oxygen Species (ROS) Formation in Arsenite-Treated Cells.
To quantify the level of ROS in arsenic-treated live cells relative to untreated controls, exponentially growing AL cells (2 × 105) were plated onto 35-mm glass-bottom microwell dishes (ΔTC3 dishes; BiopTechs, Butler, PA). After overnight incubation, cells were pretreated for 40 min at 37°C with a 1-μM dose of fluorescent probe, 5′,6′-chloromethyl-2′,7′dichlorodihydro-fluorescein diacetate (CM-H2DCFDA) (Molecular Probes) (32, 33). Graded doses of arsenite, with or without the radical scavenger DMSO (0.1%), were then added to the cultures. Cultures were examined with a Zeiss Axiovert 100 TV microscope with a 100× 1.4 objective lens equipped with a laser scanning confocal attachment (model LSM410; Zeiss) to locate the cells and to analyze their images. CM-H2DCFDA was excited with the 488-nm line of an argon/krypton mixed-gas laser. Emission was collected with a 510-nm long-pass filter. A semiquantitative estimation of the ROS-associated fluorescent signal, expressed as percentage control, was obtained with the use of the composite images generated by Adobe photoshop (Adobe Systems, Mountain View, CA). A total of 70–80 individual cells per experiment were selected randomly, and the fluorescent images were quantified per experiment. On average, over 300 cells were measured per treatment group.
ESR Spin Trapping Study to Identify the Radical Species in Arsenite-Treated Cells.
Exponentially growing AL cells (1 × 106) were plated in 25-cm2 flasks 24 h before the experiment. Cells were washed twice with PBS at pH 7.4. TEMPOL-H, a gift from James Mitchell of the National Cancer Institute, National Institutes of Health, was used as a spin trap probe. Stock solutions of TEMPOL-H were prepared in phosphate buffer containing 20 μM of the metal chelator diethylenetriaminepentaacetic acid to reduce the background oxidation level. TEMPOL-H, at a final concentration of 25 mM, was added to the cultures along with graded doses of arsenite. The cultures were incubated for 1 h at 37°C, and ESR measurements were made at room temperature. In some experiments, superoxide dismutase (SOD) (Diagnostic Data, Mountain View, CA) at 400 units/ml or catalase (Sigma) at 5,000 units/ml was added to the cultures containing the spin trap probe and arsenic, respectively, to ascertain the role of superoxide anions or hydrogen peroxide in the reaction. ESR spectroscopy was conducted with an X-band Varian E-9 spectrometer equipped with a Hewlett–Packard frequency counter and a rectangular TE102 cavity attached to a personal computer with wincwepr data acquisition software. The ESR settings were as follows: microwave frequency, 9.52 GHz; microwave power, 20 mW; modulation frequency, 100 kHz; modulation amplitude, 8 G; receiver gain, 320. Because of the large dielectric constant of water, samples were loaded into an aqueous flat cell (Wilmad Glass, Buena, NJ) for ESR spectroscopy. The ESR spectra were recorded immediately after sample equilibration in the ESR cavity, usually within 5 min after the sample was loaded into the aqueous cell.
Results
Quantification of Intracellular ROS Induction by Arsenic.
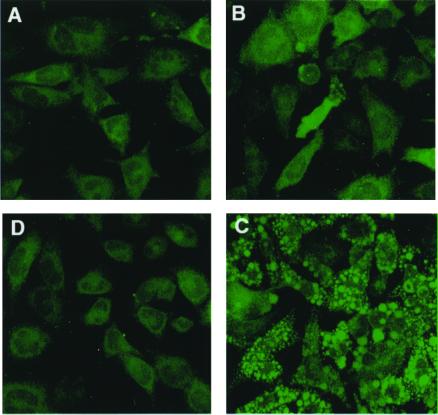
The nonfluorescent dye CM-H2DCFDA passively diffuses into cells, where the acetates can be cleaved by intracellular esterase (32, 33) to produce a polar diol that is better retained within the cells than the parental H2DCFDA. This diol can then be oxidized by ROS to a fluorescent form that absorbs at 504 nm. Fig. 1 shows the fluorescent images of AL cells pretreated for 40 min with a 1 μM dose of CM- H2DCFDA before being exposed to arsenite at a dose of 1 or 2 μg/ml (7.7 or 15.4 μM; Fig. 1 B and C, respectively). These panels show a typical field of the various treatment groups, generated from composite confocal images of 11 sagittal sections. The levels of ROS generated were immediately measured by monitoring the fluorescent intensity relative to that of control cultures under confocal microscopy. A dose-dependent increase in ROS generation in arsenite-treated cultures was evident. The oxyradical nature behind the increase in fluorescent intensity was further supported by including the radical scavenger DMSO in the reaction mixture, which reduced the signal to essentially background levels (Fig. 1D).
Figure 1.
Representative images of fluorescent signals generated from composite images obtained by confocal microscopy of AL cells pretreated with the fluorescent probe CM-H2DCFDA for 40 min with or without subsequent arsenite treatment. (A) AL cells treated with only the fluorescent probe; (B) five minutes after the addition of sodium arsenite at a dose of 1 μg/ml (7.7 μM); (C) five minutes after treatment with 2 μg/ml (15.4 μM) sodium arsenite; (D) treatment as in C, but with concurrent 0.1% DMSO.
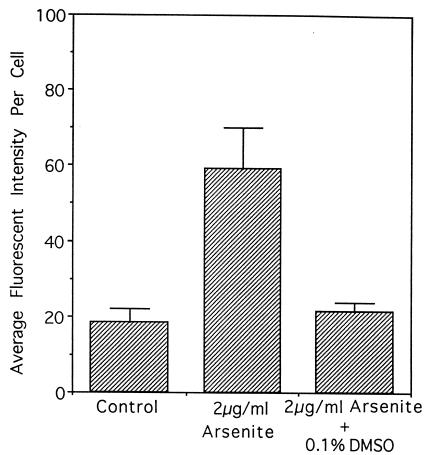
The fluorescent signals so obtained were quantified as a surrogate index for the production of ROS with the use of adobe photoshop image analysis software. A 2 μg/ml dose of arsenite (15.4 μM) increased the average fluorescent intensity 3.2-fold above control levels at 5 min after treatment (Fig. 2). Concurrent treatment with 0.1% DMSO reduced the fluorescence to background levels. We showed previously that this dose of DMSO effectively reduced the mutagenicity of arsenite to background levels in AL cells (22).
Figure 2.
Average fluorescent intensity in AL cells treated with 2 μg/ml (15.4 μM) of sodium arsenite alone or with DMSO. The intensity of the fluorescent signals was obtained from the composite images generated by image analysis software. The relative intensities are expressed in arbitrary units. Data from four independent experiments were pooled. Error bars represent ± SD.
Spin Trapping of Hydroxyl Radicals with TEMPOL-H in Arsenite-Treated AL Cells.
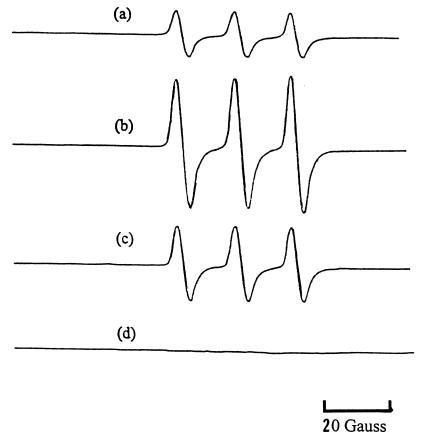
The spin trap probe TEMPOL-H is a hydroxylamine, which reacts with free radicals to form the nitroxide Tempol, which can be detected and quantified by ESR spectroscopy. Fig. 3a shows the ESR spectra of 25 mM TEMPOL-H in 2 ml of buffer in the presence of 3 × 106 AL cells. The addition of sodium arsenite (4 μg/ml, 30.8 μM), increased the ESR signal of Tempol by ≈3-fold based on the amplitude of the signals (Fig. 3b). On the other hand, the addition of catalase to the reaction mixture reduced the signal by ≈50% (Fig. 3c), indicating a contribution of hydrogen peroxide in the redox process. Likewise, the addition of SOD to the reaction mixture resulted in a 50% reduction in the ESR intensity (data not shown). The doses of antioxidants used here have previously been shown to be nontoxic and effective as free radical scavengers in a variety of in vitro and in vivo studies (34, 35).
Figure 3.
ESR spectra generated by (a) 25 mM TEMPOL-H in PBS buffer containing 3 × 106 AL cells; (b) condition a + arsenite, 4 μg/ml (30.8 μM); (c) condition b + catalase, 5,000 units/ml; and (d) 25 mM TEMPOL-H solution in PBS alone as control. Cells were incubated with the chemicals for 1 h at 37°C and then cooled to room temperature for the ESR measurements.
Effect of Sulfhydryl Depletions on Cell Killing by Arsenite.
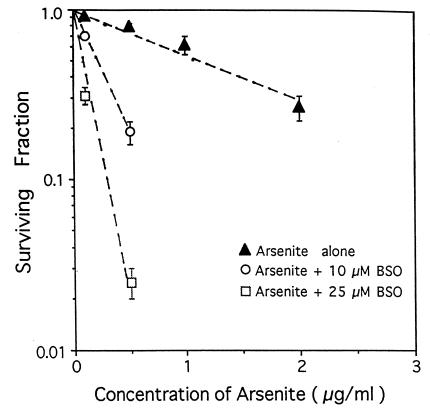
The possible role of ROS in mediating the mutagenesis of arsenite was investigated by two complementary approaches: (i) quantification of the levels of ROS in arsenite-treated cells with the use of a fluorescent probe and (ii) the use of BSO to deplete intracellular levels of glutathione. There is considerable evidence that depletion of glutathione with BSO can increase the sensitivity of mammalian cells to the lethal and genotoxic effects of radiation (36) and other radical-generating chemicals, including asbestos and bleomycin (37, 38). Fig. 4 shows the clonogenic survival of AL cells exposed to graded doses of sodium arsenite with or without pretreatment with BSO. Pretreatment with BSO at concentrations of 10 or 25 μM reduced the cellular nonprotein sulfhydryl level of AL cells to about 10% and less than 5% of control levels, respectively (31, 38). BSO treatment enhanced the cytotoxicity of arsenite in a dose-dependent fashion. The mean lethal dose for arsenite, Do, defined as the concentration that reduces survival to 0.37 (1/e) in the log-linear portion of the curves, was 1.7 μg/ml in the absence of BSO and 0.3 and 0.1 μg/ml arsenite when BSO was present at 10 and 25 μM, respectively (Table 1).
Figure 4.
Surviving fractions of AL cells exposed to graded doses of sodium arsenite with or without pretreatment of BSO at either 10 or 25 μM. BSO pretreatment reduced the nonprotein sulfhydryls levels of AL cells to less than 5% of control levels. Data from three experiments were pooled. Error bars represent ± SD.
Table 1.
Mean lethal dose of arsenite and mutant yield
| Treatment | Do, μg/ml | MY* | MY/Do |
|---|---|---|---|
| Arsenite | 1.7 | 90 | 150 |
| Arsenite + BSO (10 μM) | 0.3 | 500 | 150 |
| Arsenite + BSO (25 μM) | 0.1 | 1,500 | 150 |
MY, mutant yield, the slope of the mutant dose response curve, expressed as mutants per 105 clonogenic survivors per microgram per milliliter.
Effect of BSO Treatment on Mutagenesis by Arsenite.
Dose–response curves for the induction of CD59− mutants by arsenite with or without pretreatment with BSO are shown in Fig. 5. The induced mutant fractions (background mutants subtracted) per 105 survivors were plotted against concentrations of arsenite. The preexisting level of CD59− mutations was 46 ± 10 mutants per 105 survivors. The mutant yield (MY) for arsenite treatment was 94. Pretreatment of AL cells with BSO at 10 or 25 μM, however, increased the MY to 500 and 1,500, an enhancement of 5- and 16-fold, respectively. However, when the mutant yields were compared at the Do dose, the values for arsenic alone and those with BSO pretreatment at 10 or 25 μM were about 150 (Table 1).
Figure 5.
Induction of CD59− mutants in AL cells treated with graded doses of arsenite with or without pretreatment with BSO. Induced mutant fractions are the total mutant yield minus background, which amounts to 46 ± 10 mutants per 105 clonogenic survivors among the AL cells used in these studies. Data from three experiments were pooled. Error bars represent ± SD.
Discussion
Epidemiological studies have firmly established arsenic to be a human carcinogen. However, the mechanism(s) underlying its carcinogenicity remains unclear. The metalloid arsenic, although a natural component of the earth's crust, is a serious environmental concern worldwide, because of the large number of known contaminated sites and millions of people at risk from drinking arsenic-contaminated water. In certain parts of Bangladesh and West Bengal, India, as many as 5% of the drinking wells that were sampled had arsenic levels exceeding 1 mg/liter, and 27% of the wells had levels exceeding 300 μg/liter (6), 6 times higher than the current U.S. maximum contaminant level of 50 μg/liter. Although the water supplies in the United States are generally low in arsenic, there have been reports of arsenic contamination of ground water in the Southwest with levels in the hundreds and, in few cases, more than 1,000 μg/liter (3, 39). The U.S. Environmental Protection Agency has placed arsenic at the top of its Superfund contamination list (40). Besides being a human carcinogen, arsenic is also a risk factor for atherosclerosis, diabetes, and peripheral neuropathy (3). The lack of suitable animal models, as well as a poor understanding of its carcinogenic/genotoxic mechanism, hampers accurate risk assessment of the health effects of arsenite on both humans and animals and necessitates reliance on in vitro studies to illuminate the cellular and molecular pathways involved.
Arsenic has been shown to induce sister chromatid exchanges, micronuclei and chromosomal aberrations (41, 42), but not mutations in several kinds of gene assays in mammalian cells (12, 43), although it was weakly mutagenic in bacteria (44) and yeast (45). The paradox that it induced chromosomal mutations but not small mutations in mammalian cells has been substantially resolved by work including ours, showing that when mutations were evaluated at loci at which multilocus mutations can be efficiently detected, arsenic is, in fact, a potent mutagen (16, 22, 46). Other effects of arsenic, including its ability to affect levels of DNA methylation (47), gene amplification (24), apoptosis (48), and DNA repair (49, 50), no doubt play a vital although, perhaps, indirect role in its genotoxicity and carcinogenicity. In view of the accumulating data and the return to the realization that chromosomal aberrations are in fact mutations (51, 52), there is little reason to continue to classify arsenic as a nongenotoxic, nonmutagenic carcinogen. In the present study, we provide strong evidence that this genotoxicity is mediated by ROS generated endogenously within 5 min after arsenite treatment in live AL cells.
ROS such as superoxide anion, hydroxyl radicals, and hydrogen peroxides are intermediates formed during oxidative metabolism. Reactive oxyradicals are known mediators of the indirect effect of ionizing radiation. There is considerable evidence, including ours, that ROS are involved in the genotoxicity of arsenite (22, 53), asbestos (38, 54), and cigarette smoke condensate (55). With the use of Chinese hamster ovary cells and an x-ray-hypersensitive, DNA repair-deficient mutant, XRS-5, Wang and Huang showed that arsenite induced a dose-dependent increase in micronuclei that was blocked by exogenous catalase (41). In addition, heme oxygenase, an oxidative stress protein, and peroxidase are induced by sodium arsenite in various human cell lines (56). Furthermore, antioxidant enzymes such as superoxide dismutase reduced the incidence of sister chromatid exchanges induced by arsenite in cultured human lymphocytes (57). However, the exact location and types of ROS generated were not known. Our previous studies with DMSO suggested that ROS, especially hydroxyl radicals, were the likely mediating molecules (22).
The use of dichlorofluorescein as a measure of ROS in live cells has provided a means to quantify, albeit in arbitrary units, the relative amounts of free radicals induced by a variety of chemical and physical agents (33, 58). CM-H2DCFDA is an improved version of the original dye that tends to leak out of cells with time. The addition of a chloromethy group to the dye gives a better retention in live cells and more reliable fluorescent signals. Unlike the earlier report of Lee and Ho, where the fluorescent signal was quantified from mass culture with a spectrophotometer 24 h after arsenite treatment (59), our present study was able to capture and quantify the fluorescent image in real time from individual cells by confocal microscopy. Once we had confirmed the presence of ROS, it was of interest to determine the type(s) of radical species induced in arsenite-treated AL cells.
The spin trap probe TEMPOL-H readily penetrates plasma membranes and detects free radicals, particularly hydroxyl radicals and superoxide anions, with high sensitivity and specificity (60). In the presence of free radicals, TEMPOL-H is converted to Tempol (60), a nitroxide, which is more stable than other nitrone-based spin traps such as 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (61). This observation could, perhaps, explain our previous failure to detect a ESR signal in arsenite-treated cultures by using DMPO under identical exposure conditions. It is possible that arsenite is oxidized in cells into pentavalent arsenate, with the concomitant production of superoxide anions produced by a one-electron reduction of molecular oxygen. Alternatively, there is evidence that intracellular metabolism of arsenite into dimethylarsine is coupled with the production of superoxide anions and hydroxyl radicals (3, 62). Our data on catalase, which reduced the ESR signals by more than 50%, implicated hydrogen peroxide as the likely intermediate in arsenite genotoxicity. Taken together, our data suggest the following sequence of events for arsenic-induced mutagenesis in mammalian cells: arsenic → superoxide anions → hydrogen peroxide → hydroxyl radicals → genotoxicity.
Cellular nonprotein sulfhydryls consist essentially of glutathione (≈95%) and other low-molecular-weight aminothiols such as cysteine and cysteamine (63). These sulfhydryls scavenge free radicals and contribute to the maintenance of cellular integrity. Although a decrease in cellular glutathione may not in itself result in cell death, sulfhydryl depletion has been shown to enhance the cytotoxicity of a variety of agents, including ionizing radiation, heavy metals, oxidative stress, and certain chemotherapeutic drugs (64). There is also evidence to suggest that such cellular thiols as glutathione and cysteine protect mammalian cells against the toxic effects of arsenite (65). Furthermore, low concentrations of arsenite have been shown to induce a transient increase in cellular glutathione levels in bovine vascular endothelial cells (66). The up-regulation is thought to be a “secondary” stress response directly regulated by the thiol reactivity of arsenite (67). These findings are consistent with the observation that arsenite activates the transcription factor nuclear factor κβ, which regulates response genes intrinsic to oxidative stress (68).
As shown in Table 1, suppression of glutathione by BSO greatly increased the fraction of CD59− mutants induced per μg/ml of arsenite. On the other hand, BSO greatly reduced survival. We and others have provided evidence and argued on theoretical grounds that the number of mutants generated per equitoxic dose (here per Do), rather than mutants per unit dose, is more relevant to estimating carcinogenic risk from exposure to a mutagen (19, 22, 69, 70). In the experiments shown here (Column 4 of Table 1), the yield of mutants per Do is about the same with or without BSO pretreatment. An important implication of this result is that people with normal levels of glutathione would be expected to have the same risk of arsenic-induced mutation and consequent development of cancer as people with reduced levels of antioxidants.
Acknowledgments
We thank Drs. James Mitchell and Murali Krishna of the Radiation Biology Branch, National Cancer Institute, for providing the TEMPOL-H and for helpful discussion. Thanks are due to Ms. Theresa Swayne of the Confocal Microscopy Facility of the Herbert Irving Comprehensive Cancer Center of Columbia University for her assistance in conducting the fluorescein diacetate studies. We are thankful to Professor Jack Peisach of the Albert Einstein College of Medicine for providing ESR facilities. This work was supported by National Institutes of Health Grant ES 08821 and Superfund Grants P42 ES 10349 (T.K.H.), GM 40168 (I.L.), ES 01900 and CA 81888 (M.A.), and CA 36447 and CA 09236 (C.W.).
Abbreviations
- CM-H2DCFDA
5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- BSO
buthionine S-R sulfoximine
- HPRT
hypoxanthine guanine phosphoribosyl transferase
- ROS
reactive oxygen species
- TEMPOL-H
4-hydroxy-2,2,6,6-tetramethyl-1-hydroxypiperidine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031482998.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031482998
References
- 1.Garica-Vargas G G, Cebrian M E. In: Toxicology of Metals. Cheng P, editor. Boca Raton, FL: CRC; 1996. pp. 423–438. [Google Scholar]
- 2.Yager J W, Wiencke J K. Environ Health Perspect. 1993;101:79–82. doi: 10.1289/ehp.93101s379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan P C, Huff J. Environ Carcinog Ecotoxicol Rev. 1997;C15:83–122. [Google Scholar]
- 4.Taylor P R, Qiao Y L, Schatzkin A, Yao S X, Lubin J, Mao B L, Rao J Y, McAdams M, Xuan X Z, Li J Y. Br J Ind Med. 1989;46:881–886. doi: 10.1136/oem.46.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard A, Lauwerys R R. Mutat Res. 1980;75:49–62. doi: 10.1016/0165-1110(80)90027-5. [DOI] [PubMed] [Google Scholar]
- 6.Chapell W R, Beck B D, Brown K G, Chaney R, Cothern R, Irgolic K L, North D W, Thornton I, Tsongas T A. Environ Health Perspect. 1997;105:1060–1067. doi: 10.1289/ehp.971051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhar R K, Biswas B K, Samanta G, Mandal B K, Chakraborti D, Roy S, Jafar A, Islam A, Ara G, Kabir S, et al. Curr Sci. 1997;73:48–59. [Google Scholar]
- 8.Hertz-Picciotto I, Smith A H, Holtzman D, Lipsett M, Alexeeff G. Epidemiology. 1992;3:23–31. doi: 10.1097/00001648-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. IARC Monogr Eval Carcinog Risk Chem Hum Suppl. 1982;4:50–51. [Google Scholar]
- 10.Wang Z, Rossman T G. In: Toxicology of Metals. Cheng P, editor. Boca Raton, FL: CRC; 1996. pp. 221–229. [Google Scholar]
- 11.Lee T C, Wei M L, Chang W J, Ho I C, Huang H. In Vitro Cell Dev Biol. 1989;25:442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- 12.Lee T C, Oshimura M, Barrett J C. Carcinogenesis. 1985;6:1421–1426. doi: 10.1093/carcin/6.10.1421. [DOI] [PubMed] [Google Scholar]
- 13.Landolph J. Biol Trace Elem Res. 1989;21:459–467. doi: 10.1007/BF02917289. [DOI] [PubMed] [Google Scholar]
- 14.Rossman T G, Stone D, Molina M, Troll W. Environ Mutagen. 1980;2:371–379. doi: 10.1002/em.2860020307. [DOI] [PubMed] [Google Scholar]
- 15.Oberly T J, Piper C E, McDonald D S. J Toxicol Environ Mutagen. 1982;9:367–376. doi: 10.1080/15287398209530170. [DOI] [PubMed] [Google Scholar]
- 16.Moore M, Harrington-Brock K, Doerr C L. Mutat Res. 1997;386:279–290. doi: 10.1016/s1383-5742(97)00003-3. [DOI] [PubMed] [Google Scholar]
- 17.Barrett J C, Lamb P W, Wang T C, Lee T C. Biol Trace Elem Res. 1989;21:421–429. doi: 10.1007/BF02917284. [DOI] [PubMed] [Google Scholar]
- 18.Nakamuro K, Sayato Y C. Mutat Res. 1981;88:73–80. doi: 10.1016/0165-1218(81)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Hei T K, Piao C Q, He Z Y, Vannais D, Waldren C A. Cancer Res. 1992;52:6305–6309. [PubMed] [Google Scholar]
- 20.Zhu L X, Waldren C A, Vannais D, Hei T K. Radiat Res. 1996;145:251–259. [PubMed] [Google Scholar]
- 21.Hei T K, Wu L J, Liu S X, Vannais D, Waldren C A. Proc Natl Acad Sci USA. 1997;94:3765–3770. doi: 10.1073/pnas.94.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hei T K, Liu S X, Waldren C. Proc Natl Acad Sci USA. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helleday T, Nilsson R, Jenseen D. Environ Mol Mutagen. 2000;35:114–122. doi: 10.1002/(sici)1098-2280(2000)35:2<114::aid-em6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Lee T C, Tanaka N, Lamb P W, Gilmer T M, Barrett J C. Science. 1988;241:79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- 25.Keyse S M, Tyrell R M. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gubits R M, Fairhurst J L. Oncogene. 1988;3:163–168. [PubMed] [Google Scholar]
- 27.Puck T T, Wuchier P, Jones C, Kao F T. Proc Natl Acad Sci USA. 1971;68:3102–3106. doi: 10.1073/pnas.68.12.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldren C A, Jones C, Puck T T. Proc Natl Acad Sci USA. 1979;76:1358–1362. doi: 10.1073/pnas.76.3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGuinness S M, Shibuya S M, Ueno A M, Vannais D, Waldren C A. Radiat Res. 1995;142:247–255. [PubMed] [Google Scholar]
- 30.Hei T K, Geard C R, Hall E J. Int J Radiat Oncol Biol Phys. 1984;10:1255–1258. doi: 10.1016/0360-3016(84)90328-6. [DOI] [PubMed] [Google Scholar]
- 31.Tietze F. Anal Biochem. 1969;27:505–509. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 32.LeBel C P, Ischiropoulos H, Bondy S C. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 33.Long J F, Dutta P K, Hogg B D. Environ Health Perspect. 1997;105:706–711. doi: 10.1289/ehp.97105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shatos M A, Doherty J M, Marsh J P, Mossman B T. Environ Res. 1987;44:103–116. doi: 10.1016/s0013-9351(87)80090-7. [DOI] [PubMed] [Google Scholar]
- 35.Korkina G, Durmev a D, Suslova T B, Cheremisina Z P, DaugelDauge N O, Afanas'ev I B. Mutat Res. 1992;265:245–253. doi: 10.1016/0027-5107(92)90053-5. [DOI] [PubMed] [Google Scholar]
- 36.Wu L J, Randers-Pehrson G, Xu A, Waldren C A, Geard C R, Yu Z, Hei T K. Proc Natl Acad Sci USA. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun J E, Sarquis F, Lafleur M V, Retel J. Mutat Res. 1996;364:171–182. doi: 10.1016/s0921-8777(96)00030-4. [DOI] [PubMed] [Google Scholar]
- 38.Xn A, Wu L J, Santella R, Hei T K. Cancer Res. 1999;59:5922–5926. [PubMed] [Google Scholar]
- 39.Warner M L, Moore L E, Smith M T, Kalman D A, Fanning E, Smith A H. Cancer Epidemiol Biomarkers Prev. 1994;3:583–590. [PubMed] [Google Scholar]
- 40.United States Environmental Protection Agency. Soil Screening Technical Background Document 540/R95/128. Washington, DC: EPA; 1996. [Google Scholar]
- 41.Wang T S, Huang H. Mutagenesis. 1994;9:253–257. doi: 10.1093/mutage/9.3.253. [DOI] [PubMed] [Google Scholar]
- 42.Yih L H, Ho I C, Lee T C. Cancer Res. 1997;57:5051–5059. [PubMed] [Google Scholar]
- 43.Jacobson-Kram D, Montalbano D. Environ Mutagen. 1985;7:787–804. doi: 10.1002/em.2860070515. [DOI] [PubMed] [Google Scholar]
- 44.Flessel C P. Adv Exp Med Biol. 1977;91:117–128. doi: 10.1007/978-1-4684-0796-9_9. [DOI] [PubMed] [Google Scholar]
- 45.Kharab P, Singh I. Mutat Res. 1985;155:117–120. doi: 10.1016/0165-1218(85)90128-4. [DOI] [PubMed] [Google Scholar]
- 46.Harrington-Brock K, Smith T W, Doerr C L, Moore M M. Environ Mol Mutagen. 1993;21:7–14. [Google Scholar]
- 47.Zhao C Q, Young M R, Diwan B A, Coogan T P, Waalkes M P. Proc Natl Acad Sci USA. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T S, Kuo C F, Jan K Y, Huang H. J Cell Physiol. 1996;1692:256–268. doi: 10.1002/(SICI)1097-4652(199611)169:2<256::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 49.Lynn S, Lai H T, Gurr J R, Jan K Y. Mutagenesis. 1997;12:353–358. doi: 10.1093/mutage/12.5.353. [DOI] [PubMed] [Google Scholar]
- 50.Yager J W, Wiencke J K. Mutat Res. 1997;368:345–351. doi: 10.1016/s1383-5742(97)00011-2. [DOI] [PubMed] [Google Scholar]
- 51.Puck T T, Johnson R, Webb P, Yohrling G. Somatic Cell Mol Genet. 1998;24:1–12. doi: 10.1007/BF02677491. [DOI] [PubMed] [Google Scholar]
- 52.Muller H J. Brookhaven Symp Biol. 1956;8:126–135. [PubMed] [Google Scholar]
- 53.Oya-Ohta Y, Daise T, Ochi T. Mutat Res. 1996;357:123–129. doi: 10.1016/0027-5107(96)00092-9. [DOI] [PubMed] [Google Scholar]
- 54.Kane A B. In: Mechanisms of Fiber Carcinogenesis, IARC Scientific Publication. Kane A B, Boffetta P, Saracci R, Wilbourn J D, editors. Lyon: IARC; 1996. , No. 140, pp. 11–34. [Google Scholar]
- 55.Lee C K, Brown B G, Rice W Y, Doolittle D J. Environ Mol Mutagen. 1989;13:54–59. doi: 10.1002/em.2850130107. [DOI] [PubMed] [Google Scholar]
- 56.Applegate L A, Luscher P, Tyrrell R M. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 57.Nordenson I, Beckman L. Hum Hered. 1991;41:71–73. doi: 10.1159/000153979. [DOI] [PubMed] [Google Scholar]
- 58.Rothe G, Valet G. J Leukocyte Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 59.Lee T C, Ho I C. Arch Toxicol. 1995;69:498–504. doi: 10.1007/s002040050204. [DOI] [PubMed] [Google Scholar]
- 60.Hahn S M, Krishna M C, DeLuca A M, Coffin D, Mitchell J B. Free Radical Biol Med. 2000;28:953–958. doi: 10.1016/s0891-5849(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 61.Dikalov S, Grigor'ev I A, Voinov M, Bassenge E. Biochem Biophys Res Commun. 1998;248:211–215. doi: 10.1006/bbrc.1998.8936. [DOI] [PubMed] [Google Scholar]
- 62.Tezuka M, Hanioka K, Yamanaka K, Okada S. Biochem Biophys Res Commun. 1993;191:1178–1181. doi: 10.1006/bbrc.1993.1341. [DOI] [PubMed] [Google Scholar]
- 63.Meister A, Anderson M E. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 64.Biaglow J E, Varnes M E, Clark E P, Epp E R. Radiat Res. 1983;95:437–445. [PubMed] [Google Scholar]
- 65.Bencho V. Adv Mod Environ Toxicol. 1987;11:1–30. [Google Scholar]
- 66.Deneke S M. Biochem Biophys Acta. 1992;1109:127–131. doi: 10.1016/0005-2736(92)90075-w. [DOI] [PubMed] [Google Scholar]
- 67.Engel R R, Hopenhayn-Rich C, Receveur O, Smith A H. Epidemiol Rev. 1994;16:184–208. doi: 10.1093/oxfordjournals.epirev.a036150. [DOI] [PubMed] [Google Scholar]
- 68.Barchowsky A, Dudek E J, Treadwell M D, Wetterhahn K E. Free Radical Biol Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 69.Puck T T, Waldren C A. Somatic Cell Mol Genet. 1987;13:405–409. doi: 10.1007/BF01534939. [DOI] [PubMed] [Google Scholar]
- 70.Eckardt T, Haynes R H. Mutat Res. 1980;74:439–458. doi: 10.1016/0165-1161(80)90175-2. [DOI] [PubMed] [Google Scholar]