Summary
More than 30 years ago, John Wolfe was the first to observe and describe the association between breast density on mammography and increased breast cancer risk. Following this pioneer work, there is now compelling evidence that density in the highest quartile represents a 4–6 times higher risk of breast cancer. This magnitude of risk is only topped by age and BRCA1/2 mutation. The density-based risk is independent of age and other risk factors. Apart from epidemiologic risk factors, additional genetic factors seem to influence density. This could be the reason behind the well-known interaction between genes and environment. Reliable and reproducible breast density measurements are a prerequisite for the use of breast density to monitor primary prevention strategies and for the use of mammographic density to define women at higher breast cancer risk who would benefit from intensified early detection and surveillance protocols.
Key Words: Breast cancer risk, Breast density, Breast cancer risk factors
Zusammenfassung
Vor mehr als 30 Jahren beobachtete und beschrieb John Wolfe erstmalig den Zusammenhang zwischen mammographischer Brustdichte und erhöhtem Brustkrebsrisiko. Nach dieser Pionierleistung liegt inzwischen überwältigende Evidenz dafür vor, dass bei höchster Parenchym-dichte das Brustkrebsrisiko um das 4–6-Fache erhöht ist. Diese Risikoerhöhung wird nur übertroffen durch Alter und BRCA1/2-Mutation. Das auf der Brustdichte basierende Risiko ist unabhängig vom Alter und anderen Risikofaktoren. Neben epidemiologischen Risikofaktoren werden zusätzlich genetische Faktoren als beeinflussend angesehen. Hier könnte der Ansatzpunkt für die Einflussnahme von Lifestyle-Faktoren auf das Risiko über mutagene Effekte liegen. Zuverlässige und reproduzierbare Messmethoden der Brustdichte sind Voraussetzung, um diese zum Monitoring primär präventiver Strategien einsetzen zu können sowie zur Identifikation von Frauen mit höherem Brustkrebsrisiko, die von einer intensivierten individualisierten Früherkennung profitieren könnten.
Introduction
In 1976 John Wolfe was the first to define and categorise breast tissue density in mammography and to mention an association between the ‘parenchymal patterns’ and the risk of developing breast cancer. The Wolfe grades (N1, P1, P2, DY) correlated with a progressively greater risk. N1 describes primarily fatty tissue, P1 < 25% prominent ducts, P2 > 25% prominent ducts, and DY describes dense fibroglandular tissue [1, 2].
The mammographic appearance of breast tissue varies depending on the tissue composition. Epithelium and stroma attenuate X-rays more than fatty tissue. Fat appears dark whereas lighter regions represent primarily fibroglandular tissue consisting of parenchyma and stroma.
There is now compelling evidence that mammographic density is a strong risk factor for breast cancer and that risk of breast cancer is 4–5 times greater in women with density in more than 75% of the breast compared with those with little or no density [3,4,5,6].
Tissue analyses obtained at forensic autopsy demonstrated the association of percent mammographic density with the area of biopsy occupied by nuclei, epithelial and non-epithelial cells, and by collagen and areas of glandular structures. The hypothesis is that at least some risk factors have an effect on the number of cells and the quantity of collagen [7].
Age, parity and the menopause seem to explain about 20% of the variation in mammographic density. A genetic model explains 60–63% of the residual variance [8].
Density Measurement Methods
There are qualitative density measurements and quantitative techniques [9].
The widely used qualitative density classification is the one proposed by the Breast Imaging Reporting and Data System, BI-RADS™. Four categories are proposed: ACR 1 means breast tissue involution, ACR 2 scattered fibroglandular tissue, ACR 3 heterogeneously dense parenchyma, and ACR 4 an extremely dense tissue composition. These qualitative descriptive parameters are combined with a more quantitative approach, which means that the ACR 1 category describes not more than 25% of fibroglandular tissue composing the breast in mammography, ACR 2 a maximum of 49%, ACR 3 up to 75%, and ACR 4 describes more than 75 to 100% of the breast occupied by fibroglandular tissue.
A more reliable quantitative approach would be highly recommendable, offering continuous and reproducible measurements over time and also during any intervention.
Planimetry directly measures the total area of dense tissue whereas image digitisation offers the segmentation of the breast from the surrounding background and the use of thresholds to define dense from nondense tissue. This technique has been used in many clinical studies.
The most reliable approach would be to define the volume of dense tissue in the breast. Attempts have been made to use computed tomography (CT), tomosynthesis and dual-energy X-ray absorptiometry. One novel measure of volumetric breast density including exposure, current, tube voltage, breast thickness and processing conditions, using a fully automated computer programme, measures the absolute volume of tissue and the proportion of the breast volume composed of dense tissue, and could demonstrate a relation to several but not all breast cancer risk factors [10].
Up to now, all measurements suffer from limitations, i.e. subjectivity, variations in compression, and X-ray exposure.
Most of the published evidence favours percent density categories.
Current Evidence
In a meta-analysis 42 studies were included [3]. The meta-analysis could explain some of the heterogeneity in the association of breast density with breast cancer risk. It could be shown that the association between density and risk was much stronger in studies of incident than of prevalent cancers. One explanation would be that, in prevalence studies, the mammographic sensitivity in case of very dense tissue is lower and hence false negatives occur more often, i.e. tumours might be concealed in the first mammogram and will be detected when growing larger not until the following round. Using percent density measurements, the categories 5–25%, 25–49%, 50–75% and > 75% relative to < 5% were combined with relative risks of 1.79, 2.11, 2.92 and 4.64 (table 1) and proved a strong association between percent density and breast cancer risk. However, the breast density-breast cancer association only held in general population studies and not in studies with a symptomatic population.
Table 1.
Breast density as marker of breast cancer risk: A meta-analysis
| Density | RR | 95% CI |
|---|---|---|
| 5–24% | 1.79 | 1.48–2.16 |
| 25–49% | 2.11 | 1.70–2.63 |
| 50–74% | 2.92 | 2.94–3.42 |
| ≥75% | 4.64 | 3.64–5.91 |
The increased risk associated with breast density is independent of other known risk factors, but confounded by age and BMI. See McCormack and Santos-Silva, 2006 [3].
Interestingly, the increased risk associated with breast density is independent of other known risk factors, but is confounded by age and body mass index (BMI). Therefore, adjustment for this confounder leads to a strengthening of the association [3]. Density inversely correlates with body size, age, parity and menopause [11].
Quantitative microscopy studies showed that density correlates with a greater total nuclear area of both epithelial and non-epithelial cells, a greater proportion of collagen and of glandular structures [7].
High density is furthermore associated with an increased risk for atypical ductal hyperplasia (ADH) and in situ breast cancer [7].
Increased involution could be shown within the Mayo Cohort of Benign Breast Diseases [12], based on 8,736 women, to be associated with a reduced risk of breast cancer: For no involution, the relative risk (RR) was 1.88, for complete involution 0.91. For women with atypia and no involution, the RR was 7.79, for partial involution 4.06, and for complete involution 1.49.
Tamoxifen, gonadotropin-releasing hormone agonist and a low-fat high-carbohydrate diet influence mammographic density, but for none of these factors it is so far known whether this effect mediates their influence on risk. There is also an influence by lifestyle factors and other drugs [13].
Serum levels of insulin-like growth factor (IGF-1) in premenopausal and prolactin in postmenopausal women as well as of sex hormone-binding globulin (SHBG) are significantly and positively associated with percent breast density.
A strong evidence for genetic influence comes from two twin studies. Boyd and co-workers estimated the heritability at any age to be 60–67% for multivariable-adjusted analyses [8,13,14].
The first genome-wide family linkage analysis of mammographic density could identify three regions suggestive of genes influencing mammographic density: one on chromosome 5p and two on chromosome 12 [15].
In a recent study, using the computer-based threshold method of breast density determination, it could be shown that patients with high breast density of > 75% (versus patients with low breast density of < 25%) were at increased risk of local recurrence (hazard ratio 4.3) [16]. Additionally they found a complete inverse correlation between high mammographic breast density and obesity (BMI > 30 kg/m), thus determining obesity as another significant independent predictor of local recurrence after breast-conserving surgery and radiation for invasive breast cancer.
Both age and postmenopausal body weight are positivel related to risk, but inversely related to density [8].
Diagnostic Aspects
Despite technical advances, the sensitivity of mammography is still significantly reduced by increased breast density. In women with extremely dense breast tissue, mammographic sensitivity was just 30% and the odds ratio (OR) for interval cancers was 6.14 [17].
Data of the Florence City Screening Program showed a significant association of breast density with the probability of interval cancer development, with the highest risk having an OR of 13.4 for > 75% density [18].
These results are underlined by data from three nested case-control studies in screening populations, where women with high-density tissue (> 75%) had not only an increased risk of breast cancer (OR 4.7, 95% confidence interval (CI) 3.0–7.4) but also an increased probability of interval cancer detection less than 12 month after a negative screening examination (OR 17.8, 95% CI 4.8–65.9) [4]. In summary, mammographic breast density appears to be a major risk factor for interval cancer [17,18,19,20].
In case of dense breast tissue, breast ultrasound measures tumour size more reliably.
Breast ultrasound examination in patients with dense breast tissue detected 0.3% additional cancers not discovered with mammography and clinical examination [21]. Comparable figures (0.4%) were found by Corsetti and co-workers [22].
In a population with elevated risk and dense breast tissue (ACRIN 6666) [23], mammography sensitivity was only 50% and increased to 77.5% with the use of additional ultrasound examination.
Hypotheses of Potential Mechanisms
One of the most experienced research groups that continuously and for many years has investigated the interaction between breast density and breast cancer risk recently published a review on the potential mechanisms of breast cancer risk associated with mammographic density and developed hypotheses based on epidemiological evidence [11].
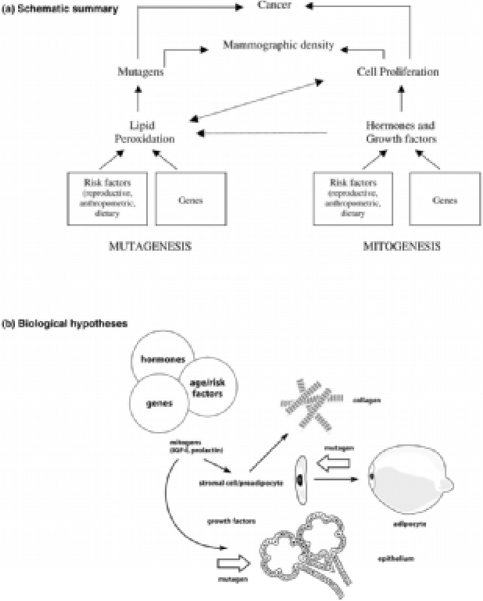
They argue that it seems reasonable to expect two possible pathways: one would be an effect of cell proliferation (mitogenesis) and the second one an effect of genetic damage to proliferating cells by mutagens (mutagenesis). This concept is illustrated in detail in fig. 1.
Fig. 1.
Hypotheses. (a) Schematic summary. We postulate that the combined effects of cell proliferation (mitogenesis) and genetic damage to proliferating cells caused by mutagens (mutagenesis) may underlie the increased risk for breast cancer associated with extensive mammographic density. Mitogenesis and mutagenesis are related processes. Increased cell proliferation increases the susceptibility to mutations but also increases lipid peroxidation, which can in turn increase cell proliferation (see text). (b) Biological hypothesis. The tissue components (epithelial cells, stromal cells, collagen and fat) that are responsible for variations in mammographic density are related to each other in several ways. Stromal fibroblasts produce collagen, and some are pre-adiopocytes that differentiate into adipocytes. Stromal and epithelial cells influence each other through paracrine growth factors, and both cell types are influenced by endocrine stimuli to cell proliferation (mitogenesis). Genetic damage to either stromal or epithelial cells caused by mutagens (mutagenesis) could initiate carcinogenesis (see text). (From [11]).
Variations in risk of breast cancer associated with mammographic density may thus arise at least in part because of different amounts of epithelial and stromal cells. These, on the other hand, will be susceptible to mutagens.
A reduction of the number of cells may result in a reduction of potential mutation targets. Mitogenesis and mutagenesis are related processes.
Although some of the biological steps have been understood and clarified, there is much need for ongoing research to better understand the complex relations and interactions between risk and breast density.
Future Development
Breast density has to be accepted as a clinically highly significant predictor of breast cancer risk. Therefore, increased mammographic density was incorporated within the algorithm of early detection in the National German Guideline for Early Breast Cancer Detection in the way that women with breast cancer density ACR 3 and 4 should get an additional ultrasound examination, provided it will be offered within a comprehensive quality assurance programme. This additional diagnostic step has the potential to increase mammographic sensitivity. There is sufficient data to urgently undertake a prospective randomised study evaluating the optimal screening interval and the added value of ultrasound. The ultimate goal would be to offer women with high breast density an individualised early breast cancer detection strategy.
Volumetric methods for density assessment are currently being developed and evaluated. Maybe, in the future, other than X-ray-based methods will become available.
References
- 1.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. Am J Roentgenol. 1976;126:1130–1139. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.McCormack VA, dos Santos Silva SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MU. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 5.Breast Cancer Research Thematic Review Series on Mammographic Density: http://breast-cancer-re-search.com/articles/review-series.asp?series=BCR_Density
- 6.Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, Yaffe M, Minkin S. Body size, mammographic density and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086–2092. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 8.Boyd NF, Martin LJ, Rommers JM. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:343–360. doi: 10.1007/978-1-60327-492-0_15. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe M. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008;10:209. doi: 10.1186/bcr2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffreys M, Warren R, Highnam R, Smith GD. Breast cancer risk factors and a novel measure of volumetric breast density: cross-sectional study. Br J Cancer. 2008;98:210–216. doi: 10.1038/sj.bjc.6604122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, Thompson RA, Melton III LJ, Goode EL, Visscher DW. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 13.Boyd NF, Martin LJ, Rommers JM. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:343–360. doi: 10.1007/978-1-60327-492-0_15. [DOI] [PubMed] [Google Scholar]
- 14.Stone J, Gillian SD, Gunasekara A, English DR, McCredie MRE, Giles GG, Cawson JN, Hegele RA, Chiarelli AM, Yaffe MJ, Boyd NF, Hopper J. The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev. 2006;15:612–617. doi: 10.1158/1055-9965.EPI-05-0127. [DOI] [PubMed] [Google Scholar]
- 15.Vachon CM, Sellers TAG, Carlson EE, Cunningham JM, Hilker CA, Smalley RL, Schaid DJ, Kelemen LE, Couch FJ, Pankratz VS. Strong evidence of a genetic determinant for mammographic density, a major risk factor for breast cancer. Cancer Res. 2007;67:8412–8418. doi: 10.1158/0008-5472.CAN-07-1076. [DOI] [PubMed] [Google Scholar]
- 16.Park CC, Rembert J, Chew K, Moore D, Kerlikowske K. High mammographic breast density is independent predictor of local but not distant recurrence after lumpectomy and radiotherapy for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2008;73:75–79. doi: 10.1016/j.ijrobp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Tapllin SH, White E. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 18.Ciatto S, Visioli C, Paci E, Zappa M. Breast density as a determinant of interval cancer a mammographic screening. Br J Cancer. 2004;90:393–396. doi: 10.1038/sj.bjc.6601548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter GJ, Evans AJ, Cornford EJ, Burrell HC, James JJ, Lee AH, Chakrabarti J. Influence of mammographic parenchymal pattern in screening-detected and interval invasive breast cancers on pathologic features, mammographic features, and patient survival. AJR Am J Roentgenol. 2007;188:676–683. doi: 10.2214/AJR.05.1950. [DOI] [PubMed] [Google Scholar]
- 20.Buist DS, Porter PL, Lehmann C, Taplin SH, White E. Factors contributing to failure in women aged 40–49 years. J Natl Cancer Inst. 2004;96:1432–1440. doi: 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense beast tissue. Radiology. 2001;221:641–649. doi: 10.1148/radiol.2213010364. [DOI] [PubMed] [Google Scholar]
- 22.Corsetti V, Ferraria A, Thirardi M, Bergonzini R, Bellarosa S, Angelini O, Bani C, Ciatto S. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med. 2006;111:440–448. doi: 10.1007/s11547-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 23.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Vélez M, Piasano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Hovanession Larsen L, Barr RG, Farria DM, Marques HS, Bopari K. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]