Abstract
Factors that affect naïve T cell proliferation in syngeneic lymphopenic hosts were investigated. 2C T cell receptor (TCR) transgenic T cells lacking both CD8 and CD4 survived but hardly proliferated. Proliferation of CD8+ 2C cells was proportional to the abundance of cognate peptide/MHC complexes and was severely inhibited by injection of anti-CD8 antibody. Weakly reactive self-peptides slightly enhanced CD8+ 2C cell proliferation whereas a potent agonist peptide promoted much more rapid proliferation, but inflammation-stimulating adjuvant had only a small effect on the rate of cell proliferation. The findings suggest that under uniform lymphopenic conditions, the widely different rates of proliferation of T cells expressing various TCR, or the same TCR in the presence or absence of CD8, reflect the strength of interaction between TCR and MHC associated with particular self-peptides.
Naïve T cells tend to undergo proliferation in lymphopenic hosts, a phenomenon referred to as lymphopenia-induced proliferation (LIP) or homeostasis-driven proliferation (1, 2). However, whether a T cell proliferates at all and how fast it proliferates varies considerably among T cells expressing different T cell receptors (TCRs; ref. 3). Upon adoptive transfer into syngeneic lymphopenic recipients, naïve CD8 T cells bearing 2C, P14, OT-I, or 318 TCR proliferate, whereas CD8 T cells bearing H-Y TCR survive but do not proliferate (4–7). CD4 T cells expressing DO11 or 1H3.1 TCR proliferate, whereas CD4 T cells expressing OT-II TCR do not (8–10). Similarly, only 30–50% of transferred polyclonal naïve CD4 or CD8 T cells proliferate in lymphopenic recipients after 1–2 weeks of transfer (8, 11, 12).
In addition to requiring “space,” LIP normally requires the presence of syngeneic MHC molecules (5, 8–11, 13), indicating that the engagement of TCR with endogenous peptide/MHC complexes (pepMHC) in the recipient is necessary. The observed diversity in the proliferative capacity of different T cells in apparently identical lymphopenic recipients, where they presumably experience the same “empty space,” was thought to result from differences in their TCR interactions with cognate endogenous pepMHC complexes. It has been assumed, but not demonstrated, that only those pepMHC complexes that are abundant or interact with relatively high affinity with TCR are able to stimulate LIP, whereas relatively scarce complexes and those interacting weakly with TCR may be only sufficient for T cell survival but not proliferation.
Naïve T cells also undergo proliferation after stimulation by cognate antigen. Compared with LIP, antigen-stimulated T cell proliferation is markedly faster (11). Antigen also stimulates T cells to express activation markers CD44, CD69, and CD25, to acquire effector functions, such as cytolytic activity and secretion of IFN-γ, and to differentiate into long-lived memory cells (5, 14). During LIP, CD8 T cells likewise acquire CD44 and differentiate into memory cells but they do not express CD25, CD69, or IFN-γ, or become cytolytic (7, 11, 15). One obvious difference between the pepMHC complexes involved in antigen- and lymphopenia-induced T cell responses involves complexes having peptides of endogenous (self) versus exogenous (nonself) origin. Another difference involves the costimulatory signals and cytokines elicited under conditions in which foreign antigens normally are introduced.
We have studied the rates of proliferation and activation of naïve T cells expressing a particular TCR (2C) in lymphopenic recipients in which the levels of cognate pepMHC and potential costimulatory molecules could be manipulated. The effect of the CD8 coreceptor on LIP also was determined because CD8 enhances the affinity of TCR for some pepMHC complexes (16). Our findings indicate that whether or not a T cell proliferates under fixed lymphopenic conditions is determined by a threshold set by at least two principal parameters: the number of copies of particular pepMHC epitopes on antigen-presenting cells (APCs, the epitope density) and the TCR affinity for the epitopes. Below the threshold, T cells do not proliferate. Above it, their rate of proliferation is proportional to the epitope density. With the 2C TCR studied here, self-pepMHC complexes are incapable of driving the proliferating T cells to differentiate into effector cells in lymphopenic hosts even in the presence of inflammatory stimuli. Activation of these naïve cells to express IFN-γ and cytolytic activity requires the engagement of a sufficient number of TCR by appropriate pepMHC complexes in addition to inflammatory stimuli.
Experimental Procedures
Mice.
2C TCR transgenic mice were on the recombination activating gene-1 deficient (RAG1−/−) background (2C/RAG; ref. 17) and unless otherwise specified had been backcrossed with C57BL/6 (B6, H-2b) mice for 10 generations. Some of the 2C/RAG mice used for assaying the relative proportion of CD8+, CD4+, and CD8−CD4− 2C cells were not backcrossed with B6 mice. Mice deficient in transporters associated with antigen processing (TAP) and β2-microglobulin (β2m) on the B6 background were from the Jackson Laboratory. (KbDb)−/− and C3−/− mutant mice on the H-2b background were, respectively, from Hidde Ploegh and Michael Carroll of Harvard Medical School, Boston. F5 TCR transgenic mice on the H-2b background were from Demitris Kioussis of the National Institute for Medical Research, London and were bred onto the RAG1−/− background. RAG1−/− mice were backcrossed with B6 mice for 13 generations. For adoptive transfer, RAG1−/− recipients were not irradiated, but recipients deficient in TAP, KbDb, β2m, or C3 were irradiated (650 rad) 2 days before transfer. 2C/RAG mice were thymectomized under anesthesia by vacuum suction. All mice were kept in specific pathogen-free facility and used between 6 and 10 weeks of age.
Adoptive Transfer.
For analysis of LIP, lymph node cells were labeled with carboxyfluorescein diacetate-succinimidyl ester (CFSE) and then transferred into RAG1−/−, TAP−/−, β2m−/−, or (KbDb)−/− recipients. In some cases, the recipients were injected i.p. with peptides [p2Ca (LSPFPFDL), dEV8 (EQYKFYSV), SYRGL (SIYRYYGL), or SIINFEKL] in PBS. In some cases, the recipients were injected with dEV8 or SYRGL in complete Freund's adjuvant (CFA) or CFA alone at the base of the tail and the scruff of the neck. For anti-CD8 antibody treatment (clone 2.43, ref. 18), CFSE-labeled lymph node cells were transferred into irradiated C3−/− recipients that were then injected with 500 μg of anti-CD8α twice. Proliferation of the transferred T cells in the lymph nodes and spleens was assayed between day 5 and day 9 after transfer. Results shown on transfer experiments are one of 2–5 representative experiments.
Antibodies, Intracellular IFN-γ Staining, and Flow Cytometry.
Antibodies to CD8, CD4, TCR, CD25, CD69, CD11c, CD2, CD5, B220, and Kb were purchased as conjugates from PharMingen. Clonotypic antibody 1B2, specific for the 2C TCR, was conjugated to biotin. Cells were stained in the presence of 2.5 μg/ml anti-FcR antibody in PBS containing 0.1% BSA and 0.1% NaN3 and analyzed on a FACScaliber, collecting 10,000 to 1,000,000 live cells per sample. To detect intracellular IFN-γ, cells were surface-stained with antibody to CD8 before being fixed and stained for IFN-γ.
T Cell Activation in Vitro.
Lymph node cells from 2C/RAG mice were labeled with CFSE. A total of ≈5 × 105/ml 2C cells were incubated (37°C) with 1 × 10-7 M QL9 peptide (QLSPFPFDL) and a 5-fold excess of irradiated BALB/c (H-2d) splenocytes. After 72 h, 2C T cells were assayed for proliferation, activation markers CD25 and CD69, and intracellular IFN-γ.
Cytolytic Assay.
Freshly isolated lymph node cells from RAG1−/− recipients were used in a 6-hr CTL assay using 51Cr-labeled T2-Kb cells as target in the presence or absence of 1 × 10-8 M SYRGL. Except for sextuplet wells to determine spontaneous and maximum 51Cr release, all samples were assayed in triplicate. Specific lysis was calculated as: [(experimental counts − spontaneous counts)/(total counts − spontaneous counts)] × 100.
Results
Requirement for CD8 in Lymphopenia-Induced 2C Cell Proliferation.
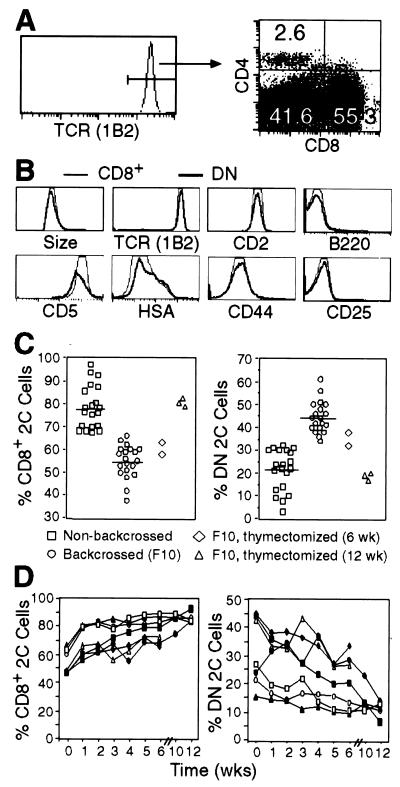
Most T cells in 2C TCR transgenic mice on the syngeneic (H-2b) RAG1−/− background (2C/RAG) were CD8+; only a few were CD4+ (0.1–6%); but many were negative for both CD8 and CD4 (Fig. 1A). The relative proportions of CD8+, CD4+, and CD8−CD4− (referred to as double negative, DN) 2C T cells varied from mouse to mouse, but in general, the proportion of DN 2C cells was significantly higher in 2C/RAG mice that had been backcrossed onto the B6 background than in nonbackcrossed mice (on average 45% vs. 22%; Fig. 1C). Compared with CD8+ 2C T cells, DN 2C cells had the same size, expressed the same level of TCR and CD2 and similar levels of CD5 and HSA, were mostly low or negative for CD44, and did not express CD25 and CD69 (Figs. 1B and 2D). Unlike DN T cells in fas mutant mice, DN 2C cells in 2C/RAG mice did not express B220. Thus, DN 2C cells from 2C/RAG mice express relatively normal cell surface markers except the lack of CD8.
Figure 1.
Analyses of surface markers of CD8+ and DN 2C T cells and their relative abundance before and after thymectomy. (A) Lymph node cells from backcrossed 2C/RAG mice were assayed for 2C TCR, CD4, and CD8. The histogram shows 2C TCR expression as detected by clonotypic antibody 1B2. The two-dimensional dot plot shows CD8 and CD4 expression by 1B2+ cells. Numbers indicate the percentages of CD4+, CD8+, and CD4−CD8− 2C cells. (B) Comparison of the cell size (forward light scatter) and indicated cell surface markers between CD8+ (thin line) and DN (bold line) 2C cells in 2C/RAG mice. Lymph node cells were assayed for 2C TCR, CD4, CD8 plus CD2, CD44, CD25, CD69 (not shown), CD5, HSA, or B220. Histograms are generated by gating on 1B2+CD8+CD4− or 1B2+CD8−CD4− cells. (C) Percentages of CD8+ and DN 2C cells in the lymph nodes of various types of mice. Lymph node cells from backcrossed (F10) and nonbackcrossed 2C/RAG mice and thymectomized 2C/RAG (F10) mice 6 or 12 weeks after surgery were assayed for 2C TCR, CD4, and CD8. CD8+ and DN 2C cells are shown as percentages of total 1B2+ cells. Each symbol represents one mouse. (D) Percentages of CD8+ and DN 2C cells in peripheral blood at different time points after thymectomy. 2C/RAG mice (F10) were thymectomized at 6–8 weeks of age. Peripheral blood leukocytes were assayed for the expression of 2C TCR, CD4, and CD8. The percentages of CD8+ and DN 2C cells are shown as a function of time for each mouse.
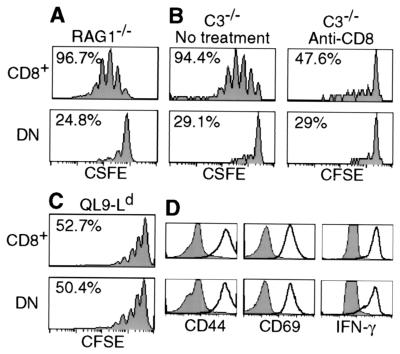
Figure 2.
Requirement for CD8 for lymphopenia-induced 2C cell proliferation. (A) CFSE-labeled lymph node cells (1 × 106 CD8+1B2+) from 2C/RAG mice were transferred into RAG1−/− recipients. Seven days later, lymph node cells were recovered from recipients and analyzed for 2C TCR, CD4, CD8, and CFSE. CFSE profiles are shown for CD8+ and DN 2C cells. Percentages refer to proportion of cells that proliferated within 7 days. (B) CFSE-labeled lymph node cells from 2C/RAG mice were incubated with 10 μg/ml of anti-CD8α antibody on ice for 30 min and transferred into irradiated C3−/− recipients (1 × 106 CD8+1B2+/recipient). The recipients were injected with 500 μg of anti-CD8α immediately after transfer and again the next day. As a control, CFSE-labeled 2C cells were treated in the same way but without antibody and recipients were not injected with antibody. Lymph node cells from recipients were analyzed for 2C TCR, CD4, CD8β, and CFSE 5 days after transfer. CFSE profiles are shown for CD8β+ and DN 2C cells in anti-CD8-treated and untreated recipients. Percentages refer to proportion of cells that proliferated within 5 days. (C) CFSE-labeled lymph node cells from 2C/RAG mice were stimulated in vitro with irradiated BALB/c splenocytes in the presence of 1 × 10-7 M QL9 peptide for 3 days. Cells were analyzed for 2C TCR, CD4, CD8, and CFSE. CFSE profiles are shown for CD8+ and DN 2C cells. (D) Lymph node cells (not labeled by CFSE) were stimulated as in C. Cells were analyzed for 2C TCR, CD4, CD8, plus CD44, CD25 (not shown), CD69, or intracellular IFN-γ. Histograms of CD44, CD69, and IFN-γ expression by CD8+ and DN 2C cells are compared before (shaded) and after stimulation (bold).
The 2C TCR recognizes the SYRGL peptide in association with Kb (syngeneic) and the QL9 peptide in association with Ld (allogeneic; refs. 19–21). The binding of the 2C TCR to SYRGL-Kb and QL9-Ld complexes is affected differently by CD8. The SYRGL-Kb complex is bound with high affinity by the 2C TCR on CD8+ cells but with 10–100 times lower affinity by the same receptor on CD8− cells. Correspondingly, CD8+ 2C cells require ≈5,000-fold lower concentration of the SYRGL peptide than CD8− 2C cells to achieve half-maximal lysis of Kb+ target cells. In contrast, QL9-Ld was bound about equally well by the 2C TCR on CD8+ nd CD8− cells, and these cells were equally effective in lysing QL9-Ld+ target cells (16, 22). In view of the pronounced effect of CD8 on the affinity of 2C TCR for syngeneic pepMHC complexes, we compared the survival and proliferation of CD8+ and DN 2C T cells under lymphopenic conditions.
Lymph node cells from 2C/RAG mice, consisting of >95% 2C cells (Fig. 1A), were labeled with CFSE and then transferred into nonirradiated syngeneic RAG1−/− recipients. As shown by CFSE profiles in Fig. 2A, 7 days after transfer >95% of CD8+ 2C cells had proliferated (2.3 divisions on average), whereas <25% of DN 2C cells showed a reduced level of CFSE in the same time period. That CD8 was required for 2C cell proliferation was further demonstrated by treating transferred recipients with anti-CD8 antibody. To minimize the possibility of complement-mediated cell lysis, syngeneic mice deficient in complement component C3 (C3−/−) were used as recipients. CFSE-labeled 2C cells were incubated with anti-CD8α on ice for 30 min and then transferred into irradiated C3−/− recipients that were injected with anti-CD8α antibody. Proliferation of CD8+ 2C cells in the anti-CD8α-treated recipients 5 days after transfer was considerably less than in untreated recipients and approximated that of DN 2C cells in either treated or untreated recipients (Fig. 2B). Similar results were obtained with transferred 2C cells that had not been incubated with anti-CD8 antibody in vitro (data not shown).
To rule out the possibility that DN 2C cells from 2C/RAG mice are defective in capacity for proliferation, we compared the proliferation of CD8+ and DN 2C cells to in vitro stimulation with allogenic QL9-Ld complexes presented by BALB/c splenocytes. Both CD8+ and DN 2C cells proliferated to the same extent in response to the QL9-Ld stimulation within 3 days and expressed similar levels of activation markers CD69, CD44, and CD25, and intracellular IFN-γ (Fig. 2 C and D and data not shown). Thus, consistent with previous observations (16, 23, 24), DN 2C cells are capable of undergoing proliferation and activation in response to high affinity TCR ligation by QL9-Ld complex.
Although CD8 is required for LIP of 2C cells, it has only a small effect on 2C cell survival. About 45% of 2C cells were DN in backcrossed 2C/RAG mice (Fig. 1C). If CD8 were required for their survival, the DN cells would have to be maintained by rapid replenishment with newly generated DN 2C cells from the thymus. In that event, the DN 2C cells would be expected to disappear rapidly upon thymectomy. Thus, we thymectomized 2C/RAG mice and followed the levels of CD8+ and DN 2C cells in peripheral blood for 12 weeks. The proportion of CD8+ 2C cells increased around 25–30% 1 week after thymectomy in all seven mice (Fig. 1D). Over the next 12 weeks, the proportion of these cells increased slowly in four mice but were maintained at an essentially constant level in three others. Conversely, the proportion of DN 2C cells decreased: the rate of decrease was variable and by 12 weeks the proportion of DN cells in lymph nodes had dropped to about 20% on average from the original 45% (Fig. 1C). These results suggest that CD8 does not have a major effect on 2C cell survival.
Survival of DN 2C cells in lymphopenic recipients also was compared with that of CD8+ and CD4+ 2C cells by transferring purified CD44−/lo (>99%) 2C cells that were CD8+, CD4+, or DN into RAG1−/− recipients. After 1 month 1.5 × 106 CD8+ 2C cells were recovered from recipient's spleen and lymph nodes after transfer of 1 × 106 CD8+CD44−/lo cells. A total of 2.3 × 105 CD4+ cells were recovered when 7 × 105 CD4+CD44−/lo cells were transferred initially. And 6 × 104 DN cells were recovered after transfer of 1 × 106 CD44−/lo DN cells. Because >90% of transferred cells are usually lost after i.v. injection, these results again suggest that DN 2C cells survive whereas CD8+ 2C cells proliferate in lymphopenic recipients.
Effect of PepMHC Levels on the Rate of 2C Cell Proliferation.
Different T cells proliferate at different rates in lymphopenic hosts (8, 12). For example, CD8+ 2C T cells proliferated much faster than CD8+ F5 T cells after transfer into the identical RAG1−/− recipients, whereas F5 cells proliferated as fast as 2C cells after stimulation by their respective agonist peptides in CFA (Fig. 3A). Various factors could have contributed to the observed difference, including a possible difference in “space,” the abundance of self-pepMHC complexes recognized by 2C and F5 TCR and the receptor's affinities for these complexes. However, for CD8+ and DN 2C cells, which express the same TCR at the same level, the difference in their proliferation in the same lymphopenic recipients cannot be attributed to a difference in space or in abundance of self-pepMHC complexes. Their difference in proliferation must reflect the strength of the TCR-self pepMHC interaction in the presence or absence of CD8.
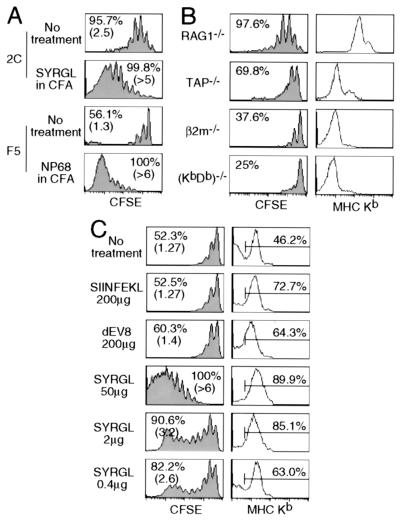
Figure 3.
Effect of pepMHC on the proliferation of 2C cells in lymphopenic recipients. (A) Comparison of proliferation of CD8+ 2C and CD8+ F5 cells in lymphopenic recipients that were either untreated or injected with cognate agonist peptides in CFA. CFSE-labeled lymph node cells (1 × 106 CD8+TCR+) from 2C/RAG or F5/RAG mice were transferred into syngeneic RAG1−/− recipients. Two days later, some of the 2C T cell recipients were challenged with 50 μg of SYRGL peptide in CFA and some of the F5 T cell recipients were challenged with 50 μg of NP68 (ASNENMDAM) peptide in CFA. Lymph node cells from recipients were analyzed for TCR, CD8, and CFSE 5 days after transfer. Proliferation of CD8+ 2C and CD8+ F5 cells are shown. Percentages refer to proportion of cells that proliferated within 5 days. Numbers in parentheses indicate the average division of cells that proliferated. (B) Comparison of CD8+ 2C cell proliferation in various lymphopenic recipients. CFSE-labeled lymph node cells from 2C/RAG mice (1 × 106 CD8+1B2+) were transferred into RAG1−/− recipients or irradiated TAP−/−, β2m−/−, or (KbDb)−/− recipients. Five days later, lymph node cells were analyzed as in A. CFSE profiles of CD8+ 2C cells from one representative experiment are shown (Left). Percentage refers to proportion of cells that proliferated within 5 days. Peripheral blood leukocytes were assayed for CD11c and Kb. Kb expression by CD11c+ cells is shown (Right). (C) Effect of peptides on Kb expression and 2C cell proliferation. CFSE-labeled lymph node cells from 2C/RAG mice (1 × 106 CD8+1B2+) were transferred into irradiated TAP−/− recipients. Recipients were either untreated or injected i.p. with 200 μg of SIINFEKL, 200 μg of dEV8, or 0.4, 2, and 50 μg of SYRGL in PBS on the day of transfer and the next day. Lymph node cells from recipients were assayed for CD11c and Kb, or 2C TCR, CD8 plus CFSE 5 days after transfer. CFSE profiles of CD8+ 2C cells are shown (Left). Percentages and numbers in parentheses are as in A. Expression of Kb on CD11c+ cells in lymph nodes is shown as histograms (Right). Percentages of positive cells are shown.
The requirement of CD8 for 2C cell proliferation, but not for their survival, suggests that the extent of TCR engagement by pepMHC complexes needs to exceed a certain threshold before T cells can proliferate under lymphopenic conditions. If so, one might expect a decrease in CD8+ 2C cell proliferation when the level of MHC class I expression is reduced in the recipients. Accordingly, we transferred CFSE-labeled 2C cells into RAG1−/− (KbDb+) recipients and recipients deficient in TAP, Kb and Db, or β2m. In accord with the highest level of Kb expression in RAG1−/− recipients, >95% of the transferred CD8+ 2C cells proliferated within 5 days (2.5 divisions on average, Fig. 3B). In contrast, in β2m−/− or (KbDb)−/− recipients, where there was little or no Kb expression, many fewer transferred CD8+ 2C cells proliferated (<40%). The level of Kb expression in TAP−/− recipients was intermediate between RAG1−/− and β2m−/− or (KbDb)−/− recipients, and the percentage of CD8+ 2C cells that proliferated was also intermediate (≈70%). Thus, the extent of lymphopenia-induced 2C cell proliferation is proportional to the level of MHC class I expression in the recipients.
We next examined the effect of injecting lymphopenic recipients with peptides that associate with Kb on APC, forming pepMHC for which the 2C TCR has different affinities. CFSE-labeled 2C cells were transferred into irradiated TAP1−/− mice and some recipients were injected i.p. with various peptides in PBS. In lymph nodes of TAP1−/− mice receiving no peptide, ≈46% of CD11c+ APC expressed a low level of Kb and ≈50% of CD8+ 2C cells proliferated 5 days after transfer (1.27 divisions on average, Fig. 3C). Administration of dEV8, a weakly reactive self-peptide, resulted in a significant increase in the Kb-expressing cells (64%) but only a slightly increase in CD8+ 2C cell proliferation (Fig. 3C). The same results were obtained if self-peptide p2Ca was injected (data not shown). Administration of the potent agonist peptide SYRGL resulted in a more profound increase in Kb-expressing cells (≈90%) and a dramatic acceleration of 2C cell proliferation as indicated by the proliferation of all CD8+ 2C cells (>6 divisions on average). Decreasing the amount of SYRGL peptide injected reduced the percentages of Kb-expressing APCs, but still a large fraction of CD8+ 2C cells proliferated rapidly (>3 divisions). In contrast, administration of SIINFEKL peptide, which binds strongly to Kb but is not recognized by 2C TCR, resulted in a large increase in the Kb-expressing APCs (≈73%) but had no effect on the rate of CD8+ 2C cell proliferation. The significance of these results becomes evident on considering that CD8+ 2C cells do not recognize SIINFEKL-Kb and that the affinity of the TCR on these cells for p2Ca-Kb is about 1,000-fold lower than for SYRGL-Kb (21, 22). In addition, the order in which the peptides bind to Kb molecules on TAP−/− APC is SIINFEKL ≥ SYRGL > dEV8 > p2Ca (H.N.E., unpublished observation). The results in Fig. 3C suggest, therefore, that the rate of 2C cell proliferation in lymphopenic mice depends on the TCR's affinity for particular peptide-Kb complexes and their density on APC. The findings also suggest that when no peptide is injected into the recipients proliferation of the transferred CD8+ 2C cells occurs in response to particular endogenous (self)-peptide-Kb complexes. The p2Ca and dEV8 peptides were identified as self-peptides by screening mouse tissue fractions with the Ld+ or Kbm3 + target cells (20, 25), but they are probably not sufficiently potent agonists (with Kb) to account for the observed 2C T cell proliferation in recipients given no exogenous agonist.
Effect of PepMHC and Inflammation on 2C Cell Proliferation and Activation.
To examine the effect of inflammation on 2C cell proliferation in lymphopenic recipients, CFSE-labeled 2C cells were transferred into RAG1−/− recipients, and 2 days later recipients were injected s.c. with CFA or SYRGL peptide in CFA, or i.p. with SYRGL peptide alone. After 5 days, ≈85% of CD8+ 2C cells proliferated in untreated recipients (1.9 divisions on average; Fig. 4A). Injection of CFA without peptide resulted in a slightly enhanced 2C cell proliferation (95%, 2.5 divisions on average). Similar results also were obtained in recipients where inflammation was induced by injecting E. coli lipopolysaccharide (data not shown). In contrast, almost all CD8+ 2C cells proliferated more vigorously in recipients injected with SYRGL (>6 divisions on average). Administration of SYRGL in CFA resulted in slightly faster proliferation of CD8+ 2C cells (>7 divisions on average; Fig. 4A). Consistent with the extent of cell proliferation, more CD8+ 2C cells were recovered from lymph nodes of SYRGL- and SYRGL/CFA-treated recipients than in untreated or only CFA-treated recipients (Fig. 4A).
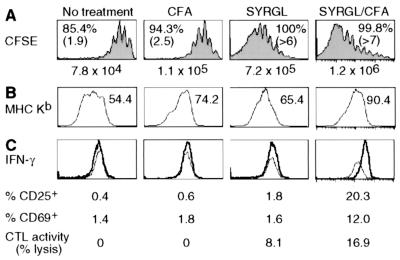
Figure 4.
Effect of peptide and CFA on CD8+ 2C cell proliferation and activation. (A and B) CFSE-labeled lymph node cells from 2C/RAG mice (2.5 × 106 CD8+1B2+) were transferred into RAG1−/− recipients. Two days later, some recipients were injected i.p. with 50 μg of SYRGL in PBS, or s.c. with CFA alone or 20 μg of SYRGL in CFA. Five days after transfer, lymph node cells from recipients were assayed for proliferation and expression of Kb. (A) CFSE profiles of CD8+ 2C cells from one representative experiment are shown. Percentages and numbers in parentheses are as in Fig. 3A. The total number of CD8+ 2C cells recovered from lymph nodes and spleens of each recipient are shown. (B) Expression of Kb on CD11c+ cells in lymph nodes. Geometric mean of Kb fluorescent intensity is shown. (C) The experiments were carried out as in A and B, except that transferred 2C cells were not labeled with CFSE. Five days after transfer, freshly isolated lymph node cells from recipients were assayed for cytolytic activity and for 2C TCR, CD8 plus CD25, CD69, or intracellular IFN-γ. The histograms show the expression of IFN-γ by CD8+ 2C cells. Thin line, isotype control; bold line, anti-IFN-γ. The percentages of CD8+ 2C cells that express CD25 or CD69, and the percentages of specific lysis of target cells by lymph node cells from various recipients (effector/target = 5:1) are shown.
CFA induces the expression of costimulatory molecules (e.g., B7) and secretion of cytokines (e.g., tumor necrosis factor α) as well as up-regulation of MHC molecules on APC. As shown in Fig. 4B, all CD11c+ cells from lymph nodes of RAG1−/− recipients expressed Kb, but the levels, as indicated by geometric mean of fluorescence intensity, were significantly increased in mice treated with CFA, SYRGL, or SYRGL/CFA. Although the enhanced 2C cell proliferation in CFA-treated recipients could result from CFA-induced costimulation or cytokines, given the effect of cognate pepMHC on LIP, the observed effect is at least partly, if not mostly, due to the increased expression of Kb in association with cognate self-peptides. In support of this notion, the costimulatory molecules CD28 and CD40L and cytokine IL-2 are not required for lymphopenia-induced T cell proliferation (ref. 11 and data not shown).
To assess the relative contribution of pepMHC complexes and costimulation/cytokines in naïve T cell activation, we determined the cytolytic activity and the expression of CD25, CD69, and IFN-γ by CD8+ 2C cells freshly isolated from recipients that were untreated or treated with CFA, SYRGL, or SYRGL/CFA. Most CD8+ 2C cells produced IFN-γ in SYRGL/CFA-treated recipients whereas very few CD8+ 2C cells had more than background staining for IFN-γ in recipients given CFA only or SYRGL in PBS, or were untreated (Fig. 4C). Consistently, a significant fraction of CD8+ 2C cells expressed CD25 and CD69 in SYRGL/CFA-treated recipients but not in other recipients, and considerable cytolytic activity was detected only in lymph node cells from SYRGL/CFA-treated recipients. Together, these results show that while the rate of T cell proliferation is predominantly determined by the abundance of cognate self-pepMHC and TCR's affinity for these complexes, activating naïve T cell differentiation into effector cells requires both highly reactive exogenous agonist pepMHC complexes and costimulation.
Discussion
Our findings that DN 2C cells hardly proliferated in lymphopenic recipients and that anti-CD8 antibody severely inhibited the proliferation of CD8+ 2C cells demonstrate that CD8 is required for lymphopenia-induced 2C T cell proliferation (Figs. 1 and 2). The significance of this requirement emerges from the observations that a monomeric SYRGL-Kb complex binds strongly to the 2C TCR on CD8+ cells (Kd ≈ 0.3 μM) but with 10–100 times lower affinity to the same TCR on CD8− cells (16, 26). If CD8 also enhances the 2C TCR affinity for self peptide-Kb complexes, as is highly likely, the very limited proliferation of DN 2C cells in Kb+ RAG−/− hosts is understandable. However, while the DN cells hardly proliferated in these mice, they survived quite well (Fig. 1 C and D). The 2C TCR affinity difference between CD8+ and CD8− cells and the disparity in behavior of these cells suggests that in the syngeneic lymphopenic mouse a TCR-pepMHC interaction threshold determines the fate of the transferred cells: the threshold for proliferation is evidently higher than for survival.
We assume that a threshold corresponds to a critical number of stable TCR-pepMHC engagements formed per T cell-APC encounter. Besides the TCR-pepMHC affinity (equilibrium constant) and dissociation rate constant (koff), a major factor determining the number of stable complexes is the total number of copies of cognate pepMHC epitopes per APC (epitope density; refs. 27 and 28). The effect of epitope density on LIP is clearly indicated by the increased rate of CD8+ 2C cell proliferation in response to the increased levels of Kb molecules on APC in lymphopenic recipients (Fig. 3B). To visualize the effect of TCR's affinity for pepMHC complexes on the rate of LIP, we compared the responses of CD8+ and DN 2C cells and the effect of injecting exogenous peptides. Injection of TAP−/− recipients with the SIINFEKL peptide, which is not recognized by 2C TCR, resulted in a marked increase in Kb-expressing APCs, but had no effect on the rate of 2C cell proliferation (Fig. 3C). In contrast, similar increases in Kb-expressing cells induced by injection of the SYRGL peptide resulted in extensive 2C cell proliferation, demonstrating that it is not the MHC in association with any peptide but only in association with particular peptide(s) that is required for LIP, as shown previously for some other T cells (10, 13). The much more rapid CD8+ 2C cell proliferation in lymphopenic recipients injected with the SYRGL peptide is likely due in part to the high affinity of the 2C TCR for the SYRGL-Kb epitope as well as the substantial increase in density of the epitope (Figs. 3C and 4). Thus, once the threshold for a given epitope is reached, the rate of T cell proliferation seems to be proportional to that epitope's abundance and thereby to the number of TCR-pepMHC engagements per T cell-APC encounter.
The quantitative effect of cognate pepMHC complexes on LIP provides an explanation for the observed spectrum of proliferative responses in syngeneic lymphopenic recipients of naïve T cells expressing various TCR: some proliferate rapidly, some proliferate slowly, and some survive but hardly proliferate. In the periphery, mature T cells express a TCR repertoire whose capacity to engage an array of self-pepMHC complexes is probably limited by thresholds for positive and negative selection of immature T cells in the thymus (1). Our findings suggest that T cells may be able to survive, but not proliferate, under lymphopenic conditions if the affinity of their TCR for self-pepMHC epitopes is too low or these epitopes are too scarce. T cells that express OT-II and H-Y TCR, and 2C T cells that lack both CD8 and CD4 likely belong to this category. In contrast, for CD8+ 2C cells the affinity of 2C TCR for self-pepMHC complexes is more likely at the high end of the limit, resulting in a relatively high rate of proliferation. CD8+ F5 cells proliferated slower than CD8+ 2C cells in identical recipients, suggesting that the F5 cells formed fewer stable TCR-self pepMHC complexes than the 2C cells.
Both LIP and exogenous antigen-induced proliferation in lymphopenic recipients lead to memory T cell differentiation (7, 11, 15). However, the two processes differ in many aspects. The antigen-induced T cell response is associated with extensive TCR down-regulation, whereas no significant TCR down-regulation was detected on CD8+ 2C cells as compared with DN 2C cells in LIP (data not shown). The lymphopenia-induced T cell proliferation was also much less than exogenous antigen-induced proliferation (Figs. 3 and 4). Most importantly, proliferating CD8+ 2C cells in lymphopenic recipients were not activated to express CD25 and CD69 or to display effector functions, such as secretion of IFN-γ, even in the presence of inflammatory stimuli (Fig. 4), indicating that self-pepMHC complexes are too weak to fully activate naïve 2C cells. Although administration of the most potent antigenic peptide SYRGL resulted in a rapid proliferation of CD8+ 2C cells, the proliferating cells still did not express CD25, CD69, or IFN-γ. Only by administrating the SYRGL peptide in CFA were proliferating CD8+ 2C T cells activated to express CD25, CD69, and IFN-γ. These results indicate that while the rate of T cell proliferation is predominantly determined by the number of stable TCR-pepMHC engagements formed per T cell-APC encounter, activation of naïve T cell differentiation into effector cells requires both extensive TCR ligation and costimulation.
The inability of self-pepMHC complexes to drive proliferating T cells to differentiate into effector cells in lymphopenic hosts even in the presence of a strong inflammatory stimulus points to an important barrier that enables T cells in general to distinguish between antigenic and innocuous self peptides. This barrier obviously helps to prevent extensive autoimmune responses, particularly in lymphopenic individuals. Nevertheless, lymphopenic individuals are known to be at much higher risk in developing certain autoimmune diseases (29, 30). One possible explanation is that after proliferation, the remaining T and probably B cells in these individuals differentiate into memory cells, which require a lower dose of antigen or autoantigen for activation. Our findings presented here also suggest another possibility. Because those T cells whose TCR bind relatively strongly to self-pepMHC epitopes, especially those that are relatively abundant, tend to proliferate more than other T cells in lymphopenic hosts, thereby enriching the resulting T cell population for autoreactive T cells.
Acknowledgments
We thank Tara Schmidt and Carol McKinley for technical assistance; Glenn Paradis for cell sorting; Dr. Wojciech Swat for performing thymectomy; Drs. Hidde Ploegh, Michael Carroll, Demitris Kioussis, and David Kranz for (KbDb)−/−, C3−/−, F5 TCR transgenic, and 2C/RAG mice, respectively; Drs. Lawrence Stern and Hui Hu for critical review of the manuscript, and members of the Chen and Eisen laboratories for helpful discussions. This work was supported in part by grants from the National Institutes of Health AI44478 (to J.C.), and AI44477 and CA60686 (to H.N.E.), and a Cancer Center Core Grant (to Richard Hynes). Q.G. is supported by an Anna Fuller postdoctoral fellowship.
Abbreviations
- TCR
T cell receptor
- pepMHC
peptide-MHC
- LIP
lymphopenia-induced proliferation
- DN
double negative (CD4−CD8−)
- RAG1
recombination activating gene-1
- CFSE
carboxyfluorescein diacetate-succinimidyl ester
- CFA
complete Freund's adjuvant
- APC
antigen-presenting cell
- β2m
β2-microglobulin
- TAP
transporters associated with antigen processing
References
- 1.Goldrath A W, Bevan M J. Nature (London) 1999;402:255–261. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 2.Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer B C, Swanson B, Kappler J. Nat Immunol. 2000;1:107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 3.Surh C D, Sprent J. J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno L, Kirberg J, von Boehmer H. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 5.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 6.Oehen S, Brduscha-Reim K. Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Goldrath A W, Bogatzki L Y, Bevan M J. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 9.Viret C, Wong F S, Janeway J C A. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 10.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–373. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho B, Varada R, Ge Q, Eisen H E, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke S R M, Rudensky A Y. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 13.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho B K, Wang C, Sugawa S, Eisen H N, Chen J. Proc Natl Acad Sci USA. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murali-Krishna K, Ahmed R. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 16.Cho B K, Lian K-C, Lee P, Brunmark A, McKinley C, Chen J, Kranz D M, Eisen H N. Proc Natl Acad Sci USA. 2001;98:1723–1727. doi: 10.1073/pnas.98.4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning T C, Rund L A, Gruber M M, Fallarino F, Gajewski T F, Kranz D M. J Immunol. 1997;159:4665–4675. [PubMed] [Google Scholar]
- 18.Sarmiento M, Glasebrook A L, Fitch F W. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 19.Udaka K, Wiesmuller K H, Kienle S, Jung G, Walden P. J Immunol. 1996;157:670–678. [PubMed] [Google Scholar]
- 20.Udaka K, Tsomides T J, Eisen H N. Cell. 1992;69:989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 21.Sykulev Y, Brunmark A, Tsomides T J, Kageyama S, Jackson M, Peterson P A, Eisen H N. Proc Natl Acad Sci USA. 1994;91:11487–11491. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh H L, Eisen H N. Immunity. 1998;8:475–483. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z, Sprent J. J Exp Med. 1994;179:2005–2015. doi: 10.1084/jem.179.6.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Z, Kishimoto H, Brunmark A, Jackson M R, Peterson P A, Sprent J. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallquist M D, Yun T J, Pease L R. J Exp Med. 1996;184:1017–1026. doi: 10.1084/jem.184.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels M A, Jameson S C. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sykulev Y, Cohen R, Eisen H N. Proc Natl Acad Sci USA. 1995;92:11990–11991. doi: 10.1073/pnas.92.26.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foote J, Eisen H N. Proc Natl Acd Sci USA. 2000;97:10679–10681. doi: 10.1073/pnas.97.20.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleeson P A, Toh B H, van Driel I R. Immunol Rev. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 30.Sherer Y, Shoenfeld Y. Bone Marrow Transplant. 1998;22:873–881. doi: 10.1038/sj.bmt.1701437. [DOI] [PubMed] [Google Scholar]