Abstract
Nitric oxide (NO) affects two key aspects of O2 supply and demand: It regulates vascular tone and blood flow by activating soluble guanylate cyclase (sGC) in the vascular smooth muscle, and it controls mitochondrial O2 consumption by inhibiting cytochrome c oxidase. However, significant gaps exist in our quantitative understanding of the regulation of NO production in the vascular region. Large apparent discrepancies exist among the published reports that have analyzed the various pathways in terms of the perivascular NO concentration, the efficacy of NO in causing vasodilation (EC50), its efficacy in tissue respiration (IC50), and the paracrine and endocrine NO release. In this study, we review the NO literature, analyzing NO levels on various scales, identifying and analyzing the discrepancies in the reported data, and proposing hypotheses that can potentially reconcile these discrepancies. Resolving these issues is highly relevant to improving our understanding of vascular biology and to developing pharmaceutical agents that target NO pathways, such as vasodilating drugs. Antioxid. Redox Signal. 10, 1185–1198.
Introduction
The past decade has seen a wealth of research regarding nitric oxide (NO), a pivotal signaling molecule that regulates blood flow and tissue oxygenation. NO affects two key aspects of O2 supply and demand: It regulates vascular tone and blood flow by activating soluble guanylate cyclase (sGC) in the vascular smooth muscle, and it controls mitochondrial O2 consumption by inhibiting cytochrome c oxidase. Abnormalities in NO production and transport in vascular systems lead to numerous cardiovascular diseases, including hypertension, atherosclerosis, and angiogenesis-associated disorders (68, 73, 74). A recent review assessed the role of NO regulation of microvascular oxygenation (12).
Endogenous NO is derived from both enzymatic and nonenzymatic sources in and near the vasculature (Fig. 1). The enzymatic formation of NO is catalyzed by nitric oxide synthase (NOS) through a series of redox reactions. In this process, l-arginine is degraded to l-citrulline and NO in the presence of molecular oxygen and nicotinamide adenine dinucleotide phosphate (NADPH). To date, three isoforms of NOS have been definitively identified: neuronal NOS (NOS1 or nNOS), inducible NOS (NOS2 or iNOS), and endothelial NOS (NOS3 or eNOS). NOS1 and NOS3 are constitutive enzymes that are controlled by the availability of intracellular Ca2+/calmodulin. NOS2 is an inducible enzyme that is controlled at the level of gene transcription and is expressed in the immune system in response to inflammatory or proinflammatory mediators. NO synthesized by NOS3 in the endothelium has been considered to be the major source of NO for regulating vasoactivity. NO could also be synthesized by mitochondrial nitric oxide synthase (mtNOS), but the existence of mtNOS is still under debate (55).
FIG. 1.
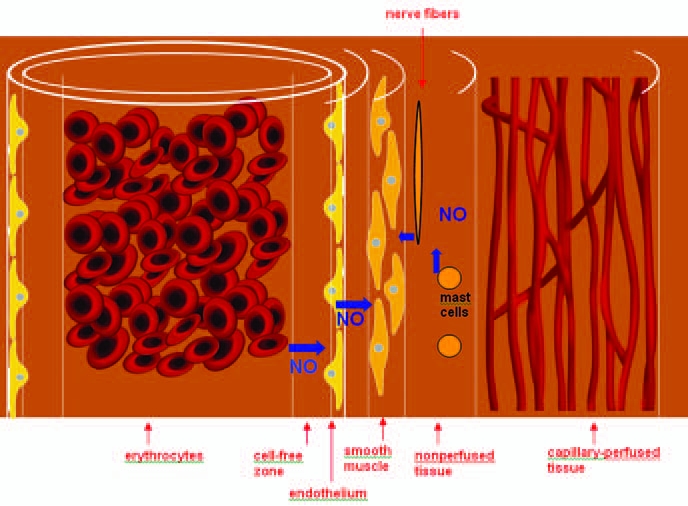
The distribution of NO in the vasculature. The vasculature consists of the lumen containing erythrocytes, the erythrocyte-free zone resulting from blood flow, the endothelium, smooth muscle, the nonperfused region, and the capillary perfused region. (Adapted from ref. 104.) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In addition to the enzymatic formation of NO catalyzed by NOS, experimental evidence has shown that blood proteins can preserve and release NO bioactivity (64). Furthermore, the nitrite reservoir in the blood and extravascular tissue can be reduced to form free NO under hypoxic or low-pH conditions or both (24, 117). Once formed, NO can be consumed through reactions with a variety of species in the vasculature, such as hemoglobin (to form iron-nitrosyl-hemoglobin or nitrate), O2, reactive oxygen species, reactive nitrogen species, sGC, and cytochrome c oxidase.
The interactions of NO with sGC and cytochrome c oxidase are the focus of this study. We have reviewed the literature and summarized the biochemical pathways that can lead to the release of NO, which can then regulate sGC and cytochrome c oxidase activities in the vasculature. Figure 2 outlines these pathways and the NO signaling activities in the immediate vicinity of the vascular wall.
FIG. 2.
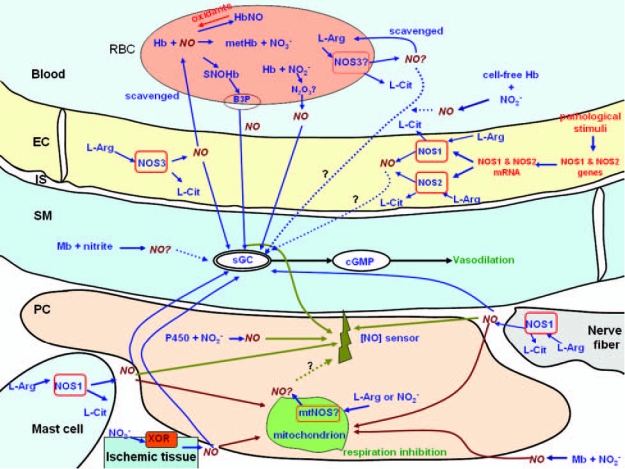
Major pathways of NO release that can regulate the activities of sGC and cytochrome c oxidase in the immediate vicinity of the vascular wall. A variety of enzymatic and nonenzymatic sources of NO can contribute to the NO concentration measured in the perivascular region. [NO] sensor represents a device that can be placed in the perivascular region and measures NO concentration. NO sources by nitric oxide synthase include NOS3 in the endothelium and erythrocytes, NOS1 in the nerve fibers, mast cells, and other tissues, mtNOS in the mitochondria, and NOS2 in a variety of tissues under pathologic conditions. Xanthine oxidoreductase and cytochrome P450 reductase can reduce nitrite to NO, respectively. Nonenzymatic NO release can potentially come from S-nitrosylated blood proteins, nitrite reduction by heme-containing proteins, and iron-nitrosylhemoglobin, among other sources. See the text for the details of these pathways. EC, endothelial cell; IS, interstitial space between the endothelium and smooth muscle; PC, parenchymal cell; RBC, red blood cell; Hb, hemoglobin; Mb, myoglobin; B3P, band 3 protein; metHb, methemoglobin; NO2−, nitrite; NO3−, nitrate; L-arg, l-arginine; L-cit, l-citrulline; cAMP, adenosine 3′,5′-cyclic monophosphate; cGMP, 3′,5′-cyclic guanosine monophosphate; Mt, mitochondrion; XOR, xanthine oxidoreductase; P450, P450 reductase.
With regard to the regulation of O2 delivery, NO synthesized by NOS acts as a paracrine regulator of vascular tone, and NO released or converted by circulating blood proteins (e.g., hemoglobin) acts as an endocrine regulator (57, 86). Autocrine regulation of protein S-nitrosylation has been recently observed inside endothelial cells (41). It has been well established that NO synthesized by NOS3 in the endothelium is the major contributor to the paracrine regulation of vascular tone and mitochondrial respiration. However, NO from endocrine sources and NOS isoforms other than NOS3 have also been shown to play important physiological roles. In the perivascular region, the inhibition of mitochondrial respiration also involves multiple NO sources. It would be extremely useful to determine quantitatively the contribution of NO from each pathway to its targets (sGC activation and cytochrome c oxidase inhibition) under various conditions, particularly normoxia and hypoxia, and to compare them with the NO-concentration measurements in the perivascular region. However, few studies have quantitatively reviewed the relations among these pathways (see Fig. 2) at the systems biology level.
In this review, we address the following quantitative questions about NO pathways: (a) How much NO is produced from each source in the vasculature, and how are these sources spatially distributed? (b) What is the EC50 for NO, the concentration required to achieve half-maximal vasodilation? (c) What is its IC50, the concentration required to inhibit mitochondrial respiration by 50%? (d) Are these values in agreement with in vivo perivascular NO measurements obtained by using biosensors? (e) How do the amounts of NO from the paracrine and endocrine pathways compare? In our review of the literature, we found large apparent discrepancies among the published reports analyzing the various pathways in terms of perivascular NO concentration, EC50, IC50, and paracrine and endocrine NO release. Resolving these apparent discrepancies by obtaining reliable quantitative data will provide us with a better understanding of vascular biology and also contribute to the development of pharmaceutical agents that can target NO pathways, such as vasodilating drugs.
Variation in Perivascular NO Concentration, CNO
The concentration distribution of NO in the vascular smooth muscle and the perivascular region is a critical parameter for determining the degree of vascular relaxation and tissue oxygenation, and it has, therefore, been extensively studied by using various theoretical and experimental approaches. In vivo experimental measurements by using electrochemical approaches, which convert NO presence to electric current, generally report concentrations of perivascular NO (CNO) on the order of several hundred nanomolar (100, 101, 108, 113); a summary of these data is given in Table 1. The reported values range from ~200 to 1,000 nM under control conditions. Zhou et al. (116) measured a level of 2.6 μM periarterial NO in spontaneously hypertensive rats (as compared with 1.1 μM in their control rats), a level that is, to our knowledge, the highest perivascular NO concentration reported thus far. Note that different NOS expression may be found and, thus, perivascular NO concentration distribution among different hierarchies of vasculature exist (49), but most experimental results on small arterioles (diameter, ~60 μm) point to several hundred nanomolar NO. If the NO is produced primarily in the endothelium, then, considering the geometric structure of the vasculature, it must first cross the vascular smooth muscle before reaching the perivascular region, where the measurements are conducted. Thus, the level of NO in the smooth muscle is presumably higher than, or no less than, that measured in situ (see Table 1). How the 200–1,000 nM (or even higher) concentrations of NO in the smooth muscle and perivascular region might regulate blood flow and tissue oxygenation is discussed later.
Table 1.
Experimental Measurements of Perivascular NO Concentrations
| Reference | NO concentration | Animal model | Arteriolar size | Method | Experimental condition |
|---|---|---|---|---|---|
| Tsai et al. (2006) (101) | 200 nM | 50- to 60-g male golden Syrian hamsters; retractor muscle | Diameter, 58 μm | Amperiometric biopolymercoated carbon fiber microelectrodes | In vivo; superfused with salt solution |
| Tsai et al. (2006) (100) | 600 nM | 55- to 60-g male golden Syrian hamsters; retractor muscle | Diameter, 50–60 μm | Amperiometric biopolymercoated carbon fiber microelectrodes | In vivo; control samples, high-viscosity plasma |
| 1,200 nM | |||||
| Vukosavljevic et al. (2006) (108) | 310–388 nM for small, medium, and larger arterioles | Male Sprague-Dawley 200- to 300-g rats; exteriorized mesentery and intestine | Measured small, medium, and large arterioles | Recessed microelectrodes with Nafion membrane | In vivo |
| Zani and Bohlen (2005) (113) | 545 ± 144 nM | Male Sprague-Dawley rats; intestine arterioles | Diameter, 53 μm | Recessed-tip glass microelectrode | In vivo |
| Nase et al. (2003) (71) | 397 ± 26 nM | Male Sprague-Dawley rats; intestine arterioles | Diameter, 52 μm | Recessed-tip glass microelectrode | In vivo |
| Malinski et al. (1993) (65) | 1,300 nM | Adult New Zealand rabbits; thoracic aorta | N/A | Porphyrinic electrochemical sensors | Ex vivo; stimulated by bradykinin |
In addition to experimental approaches, in silico modeling has been used to predict the distribution and levels of NO. In 1994, Lancaster (57) constructed a computational model to simulate the diffusion and reaction of NO in a system mimicking the endothelium and its surrounding tissue. Vaughn et al. (107) formulated a mathematical model that included the geometric information of endothelial cells and also fitted the experimental findings of Malinski et al. (65) to the model. With this model, Vaughn et al. estimated the NO production rate per unit area from the endothelium, assuming that all the measured NO was from the endothelial source. Several subsequent models also were based on the measurements of Malinski et al. (18) and the estimates of Vaughn et al. (20, 21, 29, 42, 47, 48, 105)). Generally, these theoretic models predict an ~100-nM level of NO near an arteriole. Kavdia and Popel (46) and Lamkin-Kennard et al. (56) modeled the effect of other enzymatic NO production included in the simulation, assuming the nonendothelial NO production rates were comparable to that estimated for the endothelium by Vaughn et al. (107); in this analysis, several hundred nanomolar NO in the vascular bed was predicted.
Despite this success in measuring NO in situ and in modeling NO transport in silico, unresolved issues still concern the NO-concentration profile in the vasculature. First, the measured NO concentrations cover a large range, from 200 to >1,000 nM under control conditions. This ~800-nM variation is huge when compared with the potency of NO with regard to its targets (discussed later), suggesting that the physiological implications of this variation ought not to be ignored if we are to gain a quantitative understanding of the regulation of vasoactivity by NO. These large variations in experimental measurements may reflect either (a) a complex and dynamic distribution of specific NO sources or (b) limitations in the measurement method. Second, computational modeling has been limited in terms of considering a full scenario of NO production under physiologic conditions. Previous computational models have used a high rate of NO production by the endothelium, estimated by fitting the data for perivascular NO concentrations by using a mathematical model (107), with the assumption that NOS3-derived NO from the endothelium is the sole source in the vasculature. However, it has been recognized that a number of biochemical pathways can contribute to the NO presence in the perivascular region (see Fig. 2). For instance, microfluorographic and immunohistochemical analyses have indicated that strong expression of NOS1 exists around arterioles (38, 45, 98). In addition to the contributions from NOS1 and NOS3, other NOS isoforms in the vasculature, such as NOS2 or the putative mitochondrial nitric oxide synthase (mtNOS) (55), could contribute to the measured perivascular [NO]. Moreover, Kleinbongard et al. (51) reported the expression of functional NOS3 on the intraluminal erythrocyte membrane as another potential source of NO within the arteriole. This finding has been confirmed by others (109), but Hilarius et al. (36) reported finding little, if any, NOS3 from this source. Furthermore, the endocrine signaling from blood-borne NO bioactivity (e.g., S-nitrosohemoglobin and nitrite) has been proposed to be an important mechanism for matching blood flow to the metabolic activities of local tissues (24, 64, 117). However, little is known about NO production rates within specific cellular elements around an arteriole.
One may ask whether it is possible to predict the in vivo NO distribution by combining in vitro NO release studies and in silico simulations, because it is difficult to obtain NO release rates directly in vivo. However, the reported values for NO release from cultured endothelial cells cover a large range as well. Arnal et al. (4) measured the accumulation of nitrogen oxides (nitrite plus nitrate) from cultured bovine aortic endothelial cells. From their experiments, the basal NO production was calculated to be 0.04 μM/sec; the value reported by Kuchan and Frangos (54) from cultured human umbilical vein endothelial cells (HUVECs) was 0.21 μM/sec in the absence of a shear-stress stimulus and was 10.4 μM/sec when a shear stress of 6 dyn/cm2 was applied. Hood et al. (37) measured the nitrite/nitrate accumulation over time from cultured HUVECs, and the NO production rate from their study was 0.24 μM/sec under control conditions. Moreover, it is possible that the in vitro measurements are complicated by the fact that other isoforms of NOS may be expressed in endothelial cells as a result of adaptive immune responses (25).
Although a variety of NO-sensing techniques, such as chemiluminescence, spectrophotometry, electron paramagnetic resonance (EPR), and electrochemical sensors (28, 79, 103, 110), are available, few are considered to be reliable in vivo, and fewer still permit wide-field temporal imaging. For instance, EPR spin-trapping and conventional fluorometric imaging suffer from low spatial resolution and nonspecific interactions that lead to unclear data (69). NO microelectrodes lack specificity as well. Diaminofluoresceins (DAF-2 and subsequent derivatives such as DAF-FM), a class of weakly fluorescent compounds that increase their fluorescence quantum efficiency by >100-fold after reacting with NO-derived species (i.e., nitrosonium ion), seem to allow temporal-spatial mapping of NO gradients in the microvasculature. However, their application to obtaining quantifiable data has been limited to in vitro measurements because of variations in the in vivo cellular topography and composition, leading to uneven dye absorption (or loading in the case of the diacetate form), nonuniform concentrations, and a poor correlation between NO levels and the increase in fluorescent intensity. In addition, these dyes are susceptible to nonspecific reactions with physiologic products such as peroxynitrite and ascorbic acid (114).
NO Concentration and EC50
In the vascular smooth muscle, NO activates sGC and causes vasodilation. It has been established that the activation of sGC by NO proceeds via a two-step mechanism: Initial NO binding to sGC partially activates this enzyme through the formation of a six-coordinate nitrosyl intermediate, and the subsequent conversion of this species to a five-coordinate nitroxyl complex occurs through NO-dependent or NO-independent pathways (104, 115). However, the EC50 for NO, the concentration required to achieve half-maximal sGC activation, has not yet been fully established. Clear answers to fill this gap in our knowledge are of great importance for the development of vasodilating drugs.
Studies addressing this problem have been conducted at both the molecular biology and physiology levels and by computational modeling. We have compiled the available information regarding the potency of NO in activating sGC or inducing vasodilation (Table 2). Several studies (5, 83, 95), including those that observed an interaction between NO and purified sGC, have shown that a high level of NO (250–1,600 nM) is required to produce half-maximal activity of the enzyme. Other experiments (9, 81, 82) that have characterized the NO–-sGC interaction in the cellular environment or with the use of purified enzyme exposed to endogenous NO have yielded much smaller EC50 values (2.9–20 nM). Condorelli and George (23) conducted an in silico study by analyzing the kinetics of sGC and found the EC50 to be ~23 nM. Yan et al. (112) measured rat aortic relaxation as a function of NO concentration ex vivo and found that half-vessel relaxation could be induced by 9.7 nM NO in phosphate buffer. Also, as pointed out by these authors, because some of the 9.7 nM NO in the buffer must be consumed before reaching the target in smooth muscle, the real EC50 should be smaller than this value.
Table 2.
A Survey of EC50 Values for the Potency of NO in sGC Activation
| Reference | EC50 (nM) | Method |
|---|---|---|
| Stone and Marletta (1996) (95) | 250 | In vitro; interaction of NO with purified sGC |
| Russwurm et al. (1998) (83) | 690 | In vitro; interaction of NO with purified sGC |
| Bellamy et al. (2000) (9) | 20 | In vitro; interaction of NO with purified sGC in cerebellar cells |
| Artz et al. (2001) (5) | 1,600 | In vitro; interaction of NO with purified sGC |
| Condorelli and George (2001) (23) | 23 | In silico; computational simulation of the kinetics of sGC activation by NO |
| Bellamy et al. (2002) (8) | 3.9 | In vitro; interaction of NO with sGC in brain tissue |
| Roy and Garthwaite (2006) (82) | 10 | In vitro; interaction of NO with sGC in cerebellar cells and platelets |
| Rodriguez-Juarez (2007) (81) | 2.9 | In vitro; interaction of NO with sGC in tetracycline-inducible HEK-293 cells |
| Yan et al. (2007) (112) | 9.7 | Ex vivo; apparent vasorelaxation of rat thoracic aorta in phosphate buffer |
We have discussed the perivascular NO concentration measurements (see Table 1)that are in the range of 200–1,000 nM, or even higher. These studies have left us with an apparent paradox: On the one hand, the perivascular values for the concentration of NO as measured in vivo by NO microelectrodes have been generally reported as several hundred nanomolar under control conditions, and, in the interpretation of the results, most of the NO available to the smooth muscle has been attributed solely to endothelial sources; on the other hand, although variations are found in the EC50 values reported in the literature (see Table 2), studies in the cellular environment and using endogenous NO as the source usually point to levels <10 nM (81, 112). Furthermore, as suggested by Kollau et al. (53), concentrations <1 nM could trigger maximal vascular relaxation through a mechanism that does not require the full potential of 3′,5′-cyclic guanosine monophosphate (cGMP) accumulation achievable with full cyclase activation.
Thus, a review of the literature raises an important question: If the EC50 is indeed as low as the value quoted earlier (several nanomolar), and all the perivascular NO is derived from endothelium and is in the range of several hundred nanomolar, smooth muscle would be constantly relaxed. How, then, is the vasculature regulated? At present, this question remains unanswered. Although arteriolar dilation can be caused by a number of other factors (e.g., conducted responses and endothelium-derived hyperpolarizing factors), and vasodilation can be counteracted by constrictors (e.g., endothelin-1), a large apparent discrepancy exists between how much NO is present and how powerful it appears to be.
It should be noted that the NO-mediated regulation of sGC in the smooth muscle can proceed by both amplitude- and frequency-dependent mechanisms (90, 104). Tonic NO, reflecting a continuous low-level production of NO, forms a stable and low-activity sGC heme complex, whereas acute production of NO transiently and fully activates this NO-bound sGC (15). Transient NO release could represent a more efficient means of activating sGC, and it can increase cGMP formation severalfold. Thus, the frequency of NO bursts may limit cGMP formation and regulate vascular tone (104). Clearly, a study that will accurately determine the NO production from specific sources in the vasculature and assess its effect on sGC activation is desirable.
NO Concentration and IC50
In addition to its role in regulating vascular tone to increase O2 delivery, NO competes with O2 for binding to cytochrome c oxidase in the mitochondria of the extravascular tissue and causes a substantial inhibition of tissue respiration. As a result, the Km value for tissue O2 consumption is strongly dependent on the local NO concentration. The decreased oxygen consumption in the vicinity of the blood vessel can facilitate the delivery of the O2 supply to more hypoxic tissue. Furthermore, the NO production (and thus NO distribution) is a function of Po2: enzymatic NO production is attenuated as the oxygen substrate supply decreases, whereas nonenzymatic NO production (e.g., reduction of extravascular nitrite under low pH and/or low O2 conditions) is expected to increase (39, 80, 91). Thus, a mutual interdependence of NO release and O2 delivery is found.
The interplay of NO and O2 in the perivascular region is even more complicated because the IC50 of NO also depends on the O2 concentration. The apparent Km value of tissue respiration is a function not only of the NO concentration but also of the concentration of ambient O2: Half-inhibition of tissue O2 consumption in brown adipose tissue occurs at 364 nM NO with 180 μM O2, 69 nM NO with 72 μM O2, and 11 nM NO with 32 μM O2, with respect to their values at 0 nM NO (52). Different values of IC50 have also been reported in other studies. Rodriguez-Juarez (81) and Bellamy et al. (8) reported IC50 values for NO of 141 nM in tetracycline-inducible HEK-293 cells and 120 nM in cerebellar cells, respectively, with 30 μM O2. Basically, under different O2 conditions, the inhibitory effect of the same amount of NO on cytochrome c oxidase is different (11, 58): Under hyperoxic conditions, cytochrome c oxidase activity is relatively insensitive to NO regulation, and its inhibition requires a high concentration of NO, far larger than that required to interact with sGC; under normoxic conditions, cytochrome c oxidase activity becomes more sensitive to this signaling molecule, but the IC50 is still fairly large; and under hypoxic conditions (such as tissue oxygenation being reduced to <30 μM), an even lower amount of NO can regulate mitochondrial respiration. It is worth noting that the tissue oxygen concentration, depending on the specific tissue and vascular bed, varies from ~35–120 μM (20–70 mm Hg) (10, 34, 72) and, thus, the hypoxic condition herein refers to the relative hypoxia at which an inadequate O2 supply to tissues occurs.
It is important to note that the perivascular NO concentrations (200–1,000 nM, as mentioned earlier) are generally higher than the IC50 values, particularly at low Po2 [e.g., 30 μM O2, an oxygenation condition close to physiologic conditions in certain tissues (72)]. Whereas the discrepancy between the IC50 and CNO is not as large as that for its EC50, a CNO of several-hundred nanomolar would still inhibit most of the cellular respiration and leave little room for any control of O2 delivery through NO-dependent inhibition of mitochondrial respiration. This discrepancy at least exists in the region near the arteriole, where it is not perfused by erythrocyte-containing capillaries, which represent a significant NO sink and can cause a sharp decrease in NO availability. Thus, perhaps a heterogeneous distribution of NO in the microvasculature is determined by its specific sources and has a strong influence on the O2-concentration distribution. It has recently been suggested that under hypoxic conditions, nitrite, which can induce vasodilation through a NO-generating mechanism reduced by deoxygenated hemoglobin (24) or independent of nitrite reductase activities (27), can also react with the myoglobin that is found in skeletal muscle and also in smooth muscle (77). This reaction releases NO, which partially inhibits respiration in the adjacent tissue and ensures that more O2 will be delivered to the more hypoxic tissue (88). Conversely, the cellular consumption of NO itself in the parenchymal tissue is a function of both NO and O2 concentrations (99).
In addition to regulating respiration in the extravascular tissue, NO seems to be able to modulate tissue oxygenation by reducing O2 consumption by the vessel walls (14, 87). According to some reports, large arteriolar transmural Po2 gradients are attributable to the large amount of O2 consumed by the microvascular wall, orders of magnitude greater than that consumed by the surrounding tissue (102). This high O2-consumption rate can be regulated by NO: An increase in the local NO concentration can reduce the rate of O2 consumption by the vascular wall, facilitating the delivery of O2 to the surrounding hypoxic tissue; a decrease in the local NO concentration produces the opposite outcome. However, this hypothesis has been questioned by a number of studies. We and others have shown that it is unlikely that the thin endothelium has sufficient metabolic activity to consume three orders of magnitude more O2 than the surrounding tissue (50, 106, 111). Also, recent Po2-profile measurements near arterioles in rat mesentery by using a scanning phosphorescence-quenching microscopy technique found no steep Po2 gradient across the vascular wall (34). The authors of this study proposed that the measured higher-than-expected O2 consumption by the endothelium (14, 87, 102) may be an artifact produced by the O2 consumption by phosphorescence quenching in the interstitial space, where the consumed O2 cannot be replenished as quickly as it is in the flowing blood.
As previously noted, NO regulates O2 delivery through binding to its targets, sGC and cytochrome c oxidase. sGC is located in the vascular smooth muscle, whereas cytochrome c oxidase is located in the perivascular region. However, the sensitivities of these two targets to NO differ by 50-fold (IC50: 141 nM; EC50: 2.9 nM) at the 30-μM O2 level (81). Given these relatively low EC50 and high IC50 values, if all the measured NO in the perivascular region (see Table 1)were derived from the endothelium, the NO–sGC–cGMP pathway in smooth muscle would be saturated before NO from this source would exert any regulatory effect on the mitochondrial respiration in parenchymal cells. Thus, these findings add to the NO paradox and further encourage us to hypothesize that (a) multiple sources contribute to the NO in the perivascular region, and (b) a heterogeneous NO distribution is of importance in regulating microvascular oxygen delivery.
Sources of Vascular and Perivascular NO
Although the endothelium is considered the major NO-producing source for regulating vascular tone and tissue oxygenation (68), a number of sources of NO have been proposed, in addition to the endothelial source NOS3 in the arteriolar wall. Figure 2 and Table 3 present the results of our survey of the studies addressing possible NO sources, both enzymatic and nonenzymatic, in the vasculature and perivascular tissue. All of these sources could contribute to the measured NO concentration listed in Table 1.
Table 3.
NO Sources in the Vasculature
| Source | Location | O2condition to function |
|---|---|---|
| Hydroxylation of l-arginine by NOS1 | Nerve fibers; mast cells (45) | Sensitive to O2 (high Km) (30, 96) |
| Hydroxylation of l-arginine by NOS2 | Macrophages; aged endothelium; many tissues under inflammatory conditions (70, 84) | Sensitive to O2 (relatively high Km) (96) |
| Hydroxylation of l-arginine by NOS3 | Vascular endothelium; erythrocytes (51, 68) | Insensitive to O2 (low Km) (78) |
| Nitrite reduction by NOS3 | Endothelium (33) | NO released during anoxia (33) |
| mtNOS | Parenchymal tissues (55) | N/A |
| S-nitrosohemoglobin | Erythrocytes (64) | NO released during hypoxia (91) |
| S-nitroso-glutathione or S-nitrosoalbumin | Plasma (32) | NO released during hypoxia (32) |
| Nitrite reduction by hemoglobin | Erythrocytes, plasma (39) | NO released during hypoxia (39) |
| Nitrite reduction by myoglobin | Skeletal muscle and myocardial tissue (88) | NO released during hypoxia (88) |
| Nitrite reduction by xanthine oxidoreductase | Ischemic tissues (60, 62) | NO released under ischemic or anaerobic conditions (60, 62) |
| Nitrate and nitrite reductions by cytochrome | A variety of tissues (26, 59) | N/A |
| P450 reductase and cytochrome P450 |
Km refers to the Michaelis-Menten constant.
In addition to the NOS3 in the endothelium, this form of the enzyme is also expressed in functional form in erythrocytes (51). Erythrocytes are rich in hemoglobin, which is a potent NO scavenger. The NO level resulting from intravascular nitric oxide is too low to have any physiological effect if free diffusion is the transport mechanism involved (61). It appears that most of the NO that is produced by this source through complex biochemical reactions involving multiple substrates and coenzymes would be immediately converted to end-metabolic products unless a protective mechanism existed to allow the NO that is formed to escape being scavenged. How much this source contributes to vasodilation and other physiological functions (e.g., regulating erythrocyte deformability) is not yet clear.
Recent microfluorographic and immunohistochemical analyses have provided a qualitative indication that in addition to NOS3, strong expression of NOS1 around arterioles exists: Kashiwagi et al. (45, 97) measured the NO distribution around the microvessels by using a fluorescence-detection technique coupled with specific NOS inhibitors and of NO produced by NOS1 in the nerve fibers and mast cells. This finding was also confirmed by their immunohistochemical staining to detect NOS isoforms in various tissues. Evidence also suggests that NOS1 can compensate for the loss of NOS3 in NOS3-knockout mice and thus induce a change in NOS expression. Studies in NOS3-knockout mice (38, 98) have shown that NOS1-derived NO and prostaglandins can maintain flow-induced vasodilation; immunohistochemical measurements also showed the presence of NOS1 in vascular tissue in wild-type mice and in the endothelium of the coronary arteries in NOS3-knockout mice. Mathematical models (46, 56) suggest that NOS1 (or NOS2) in the perivascular region can make a significant contribution to the perivascular NO distribution because it is located farther from the lumen than the NOS3 in the endothelium, thereby preventing the scavenging of the NO by hemoglobin. We have formulated a molecular-level theoretic/computational model based on the analysis of the biochemical pathways of NOS1 and NOS3 that has allowed us to quantify the NO production in the microvasculature. We have obtained paradoxic results, in that the NO derived from NOS3 could not account for the reported perivascular NO concentration (17, 18). The predicted NO release rates from NOS3 in the endothelium were in the range of 5–17 × 10−3 μM/sec (17), and those from NOS1 in the nerve fibers and mast cells in the perivascular region were in the range of 0.39–1.16 μM/sec (18); using these values gave NO concentrations at the arteriolar smooth muscle of ~0.1 and 5 nM, respectively. In our models, we specifically considered the catalytic activities (measured in vitro), amounts, and locations of NOS1 and NOS3.
These paradoxic predictions led us to investigate the amount of NO from each identified source and how each one specifically regulates its target (i.e., sGC and cytochrome c oxidase). However, a number of questions remain to be answered regarding the role of NOS1 in vasodilation. In NOS3-knockout animal models, hypertension or increased vascular tone has been observed, and the mechanotransduction-related production of NO (likely the results of an increasing influx of Ca2+ into the endothelial cells) and subsequent vasodilation of arterioles have also been documented in a variety of studies (6, 22, 75, 100). The conclusion that NOS1 is the major source of NO regulating vascular tone seems contradictory to those findings that closely link the regulation of vascular tone to NOS3 activity. Experiments featuring high-specificity approaches to real-time detection of NO-derived reactive species (such as 4,5-diaminofluorescein diacetate with a calibrated signal and the use of selective NOS inhibitors) would be very helpful in delineating the contribution of each NOS isoform.
The inducible form of NOS, or NOS2, can catalyze NO formation at a faster rate than can other NOS isoforms under the same conditions (96). NOS2 is expressed in macrophages and a variety of tissues under inflammatory conditions. Berkowitz and colleagues (84) also detected NOS2 expression in the endothelium of aged rats and showed that it serves as a source of S-nitrosylation for a variety of proteins (84). Moreover, the putative mtNOS could produce NO from the parenchymal cells, but, as discussed earlier, its existence is still under debate. Other enzymes that can produce NO include xanthine oxidoreductase in mammalian cells, which can reduce nitrite to NO via an acid-catalyzed mechanism, particularly in ischemic tissues (60, 62), and cytochrome P450 reductase and cytochrome P450, which can reduce nitrate to nitrite and nitrite to NO, respectively (26,59).
Nonenzymatic NO production can also be a significant source of NO under certain conditions: S-nitrosylated blood proteins and peptides, such as hemoglobin and glutathione, are thought to serve as a storage pool for NO, and NO can be released when a reduction in Po2 is sensed (91). Note that the SNOHb-induced vasodilation could be through an NO-independent mechanism (92). Also, NO can be stored in the form of circulating nitrite, which can be reduced to NO under low-pH conditions (117) by deoxygenated hemoglobin in the lumen (39), or by deoxygenated myoglobin in the tissue (88). It is interesting to note that nitrite-dependent hypoxic vasodilation can be independent of the NO-generating pathway by nitrite reductases (27). In addition to hemoglobin, NOS3, which normally requires O2 as a substrate to produce NO, can react with nitrite under anoxic conditions, releasing NO (33). Furthermore, iron-nitrosyl-hemoglobin (HbNO), which is a relatively stable species as the product of NO and deoxyhemoglobin, can release the NO molecules at a significantly high rate in the presence of oxidants (89). Blood-borne NO bioactivity has been proposed to be a key mechanism for matching blood flow to the metabolic activity of local tissue.
Despite intense investigation of the endocrine NO reservoir as a vasodilator, little quantitative knowledge is available concerning the transport and concentration distribution from such a reservoir. This quantitative question has presented two conceptual problems: whether the NO from intraluminal sources is sufficient to induce vascular smooth muscle relaxation through the known NO–sGC–cGMP pathway, and whether it is comparable to the paracrine source of NO (61). Because of the complex biochemical reactions involved in NO release and the difficulty in discriminating the NO that might come from other enzymatic or nonenzymatic sources, no direct in vivo experimental measurements have been made of the amount of NO released from these pathways.
Computational modeling has been used to predict NO release from certain intraluminal pathways. By using a multicellular computational model simulating NO release from erythrocytes and assuming that a membrane-associated mechanism facilitates NO export out of these cells or a nonreactive intermediate species, we predicted that 0.25–6 pM or ~40 pM NO is delivered by intraerythrocytic S-nitrosohemoglobin (SNOHb) to vascular smooth muscle (19). The nitrite reduction by hemoglobin has also been quantified by modeling: Jeffers et al. (43) calculated that 0.08 pM NO is present in smooth muscle, assuming that free diffusion is the only transport mechanism. Our computational simulations confirmed a similar amount of NO delivery with free diffusion, whereas ~40–260 pM could be contributed by this source to smooth muscle, provided that a protected mechanism or a nonreactive intermediate species exists (16). The model predictions remain to be validated experimentally when more-sophisticated approaches for detecting NO from different pathways become available.
Figure 2 shows the biochemical pathways that can lead to NO release in the vasculature and contribute to the NO-dependent regulation of sGC and cytochrome c oxidase activities. A quantitative analysis of the contribution made by each pathway is needed to address the complex and dynamic nature of NO signaling from a variety of specific sources, giving due consideration to their spatial distribution. A combination of experimental and computational approaches, including immunohistochemistry, in situ DAF-2T fluorescence for the detection of NO-derived reactive species, and computational analysis of biochemical pathways leading to NO release, could reveal with high resolution the spatial distribution of significant sources of NO.
Paracrine or Endocrine Regulation
Cytochrome c oxidase activity is regulated mainly through a paracrine mechanism: NO produced locally by various sources (except mtNOS) diffuses to the targets in the perivascular region and inhibits mitochondrial respiration. In addition, an autocrine regulation pathway may be involved if putative mtNOS does indeed exist.
The regulation of NO in vascular smooth muscle appears to be more complicated. Based on his computational analysis of NO diffusion and reactions, Lancaster (57) pointed out that the NO produced in endothelial cells primarily conducts paracrine signaling in the vasculature, and thus the spatial relations between the specific sites of NO formation and the neighboring targets of this NO are of great importance. Because of the close proximity of the endothelium to the blood vessel lumen, most of the NO that is locally produced by endothelium-based NOS3 was initially thought to be readily consumed because of the massive presence of hemoglobin in the lumen (i.e., as a “cost” of paracrine signaling). Subsequent studies have shown, however, that the encapsulation of hemoglobin by the erythrocytic membrane and the existence of the cell-free region (shown in Fig. 1) in the blood can prevent, to a large extent, this NO from being consumed (13, 29, 67). Still, a large amount of NO derived from NOS3 is converted to inactive metabolic products (e.g., nitrate) by hemoglobin through dioxygenation and ironnitrosylation reactions (44).
In addition to its paracrine mechanism, NO seems to mediate endocrine signaling: NO bioactivity is preserved in other more-stable species that circulate in the blood and is released under conditions of hypoxia, when vasodilation is needed most. The SNOHb theory is one of the major hypotheses to account for the endocrine signaling: According to this hypothesis, in addition to reacting with the ferrous or ferric heme of hemoglobin, NO can be transferred from the heme iron of hemoglobin to the cysteine residue at position 93 on the β chain to form SNOHb, which is relatively stable and can convey NO bioactivity to hypoxic tissues (64). Other blood proteins or peptides that can be S-nitrosylated, such as glutathione, can potentially act as NO preservers as well (91, 93). As discussed earlier, NO delivered to smooth muscle through this pathway is calculated to be at the 0.25–6 pM or ~40 pM level, depending on the possible NO-transport mechanisms involved (19).
A competing hypothesis addresses the role of nitrite anion in endocrine signaling by NO. Nitrite, formed as one of metabolic products of enzymatic NO or obtained as a dietary supplement, is found nearly to double human forearm blood flow at a high dose (~200 μM) and to have an NO-dependent vasodilating effect at a near-physiologic concentration level (40). The molecular mechanism for this nitrite reduction involves the reducing activities of heme-containing globins, such hemoglobin or myoglobin, to release NO (39, 88). The resulting NO may be exported out of the lumen as the intermediate reaction product, likely in the form of nitrous anhydride (N2O3), as proposed by Robinson and Lancaster (80) and recently experimentally confirmed by Basu et al. (7). NO from the nitrite reservoir in the lumen is calculated to be ~40–260 pM under physiologic conditions, depending on the protected mechanisms of NO transport (16). Interestingly, the study by Angelo et al. (3) suggested a possible link between these two hypotheses: Hemoglobin could synthesize SNOHb, by using nitrite as a substrate (3). Furthermore, experimental evidence has shown that adenosine 5′-triphosphate (ATP) can be released from erythrocytes after O2 reduction is sensed, leading to vasodilation produced by inducing a conducted vasomotor response or stimulating NOS catalytic activity (31, 94).
Thus, a growing body of evidence suggests that in addition to its paracrine regulation by NO that has been locally produced by NOS, vascular tone can be regulated through an endocrine effect produced by NO released from circulating erythrocytes after sensing low Po2. It is likely that paracrine NO is responsible for the basic homeostasis under resting conditions, whereas endocrine NO contributes to hypoxic vasodilation, when an increase in metabolic activity (such as exercise) consumes a large amount of O2 and leads to a decrease in the tissue Po2. The change in O2 level results in vasodilation, which increases the blood flow and ensures the delivery of the adequate O2 to match the tissue's metabolic requirements.
How does the vasculature sense O2, and what type of regulation becomes dominant when this process occurs? Although our understanding is still incomplete regarding the quantities of NO resulting from each specific pathway, it appears that local production of paracrine NO is higher than that of NO from endocrine sources in the lumen (18–20, 47, 101). It is reasonable to postulate that the paracrine regulation of vascular tone by NOS-derived NO is significantly attenuated under conditions of low O2 tension, when NO from erythrocytes becomes an important source. The NO that is locally produced by NOS3 has been thought to act as a paracrine source to regulate the vascular tone; however, the Km value of NOS3 for O2 has been reported to be ~7 μM (78), a level much lower than the O2 concentration in the precapillary arterioles (76). Thus, an apparent contradiction exists between endocrine NO acting as the major vascular relaxing factor under conditions of hypoxia and the low sensitivity of NOS3 to the change in O2 concentration. One possible explanation for this paradox is that other isoforms of NOS with higher Km values contribute significantly to this paracrine regulation and sense the change in ambient O2 (18).
Experimentally Measured CNO and Theoretical Models
We have summarized the values for perivascular CNO that have been measured or calculated by using various methods. The measured values are usually several hundred nanomolar, and the values predicted by mathematical models are close to these values when a high rate of NO release is used. This high rate of NO production by the endothelium has been estimated by fitting the data for perivascular NO concentrations by using a mathematical model (107), with the assumption that NOS3-derived NO from the endothelium is the sole source in the vasculature. This assumption may not be accurate, because multiple NO sources appear to be involved in the interaction of NO with sGC and cytochrome c oxidase. Thus, a modeling approach that analyzes NO release from specific pathways and their individual contributions to the perivascular CNO could provide valuable information from another perspective.
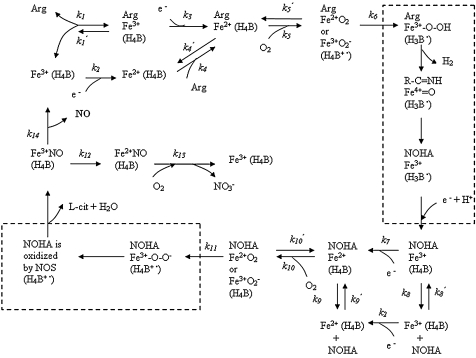
We have formulated a molecularly based theoretical/computational model to quantify the NO production by NOS in the microvasculature (17, 18) (Fig. 3). The model is based on the analysis of the biochemical pathways of NOS1 and NOS3 that have been characterized in vitro (66, 85, 96). NOS goes through a five-electron transfer cycle and releases NO by using l-arginine, O2, and NADPH as substrates and tetrahydrobiopterin (H4B) as a cofactor. This pathway analysis of the catalytic activities of NOS resulted in a set of coupled ordinary differential equations to describe the change in the species concentrations. In our models, we specifically considered the catalytic activities, amounts, and locations of NOS1 and NOS3. Based on the topographic information on NOS isoforms revealed by fluorescent and immunohistochemical methods, we calculated the NO concentration distribution around an arteriole under various O2 conditions. The model produced paradoxic results: The NO derived from NOS3 could not account for the reported perivascular NO concentration.
FIG. 3.
Mechanism for the formation of NO via eNOS catalysis after the binding of the coenzyme tetrahydrobiopterin (H4B). The heme iron (Fe) is the major catalytic site and represents NOS3 here. NOS3 undergoes a series of redox reactions. Arg represents l-arginine, and NOHA represents Nω-hydroxyl-l-arginine. Parentheses around H4B, H4B+•, or H3B• mean that that species is bound to the enzyme. NO is released from Fe3+NO. All three NOS isoforms share a similar catalytic mechanism. [Adapted from (66, 85).]
We then used our model to test the sensitivity of NO production to substrate availability, NOS3 concentration, and potential rate-limiting kinetic factors. The parameter sensitivity tests showed that only coordinated changes in multiple parameters could significantly increase the NO production from this source. However, it is unlikely that the kinetic parameters contain systematic errors, because they have been measured in vitro by multiple groups. The analysis also suggested that the predicted low level of NO production could be attributed to a low expression of NOS3 in the microvascular endothelial cells. This value has been measured by a few groups: Andersen et al. (2) obtained 2.5 ± 1.9 ng NOS3 from 106 HUVECs, which was equivalent to a 47-nM intracellular concentration when we considered 133 kDa as the molecular size of NOS3 and 400 μm3 as the average volume of an endothelial cell (35). Experiments by Lubrano et al. (63) with HUVECs and human microvascular endothelial cells yielded NOS3 concentrations of 8 and 23 nM, respectively. Although these two experiments were not primarily designed to quantify NOS3 expression in cells, they both point to an intracellular concentration of <50 nM. Also, their values are in good agreement with that from the manual for the NOS3 Quantikine Immunoassay Kit (R&D Systems, Minneapolis, MN), which quotes NOS3 concentrations of 5,137 pg/106 ECs (97 nM) for HUVECs and 1,396 pg/106 ECs (26 nM) for human microvascular endothelial cells. These low NOS3 expression values yield low NO production rates from the endothelium (17). Our model would require a 1,000-fold higher NOS3 concentration as an input to yield a NO production rate that would result in an NO concentration in the perivascular region (see Table 1). Here we used an average intracellular NOS3 concentration estimated from a large number (105 or 106) of endothelial cells. However, the distribution of NOS3 may be heterogeneous in different tissues or among endothelial cells from different vessels. Thus, detailed measurements of the NOS3 distribution among the different hierarchies of vasculature could provide a better understanding of NO production in different tissues.
We also applied the same biochemical-pathway analysis to examine the NO release by NOS1 and the contribution from this source to the perivascular NO concentration. Our model of NOS1 catalytic activity and its distribution show that mast cells and nerve fibers can significantly contribute to the perivascular CNO (at levels of ~50 nM), but the predicted value still falls short of the experimentally measured values (18). This result points to a higher abundance of NOS1 or NOS3 and/or the existence of other enzymatic or nonenzymatic sources of NO in the microvasculature. Also, it should be noted that a limitation exists for using in vitro enzymatic parameters of NOS isoforms in theoretic modeling. NOS activities in intact cells could be different than those reported from the isolated enzyme in vitro. The posttranslational modifications or intracellular protein-protein interactions may have an important effect on regulatory control of NO generation, which requires further studies.
Conclusions
The relation between NO availability and the balance between O2 supply and demand, especially under hypoxic conditions in precapillary regions, has been extensively studied in the past several years. However, significant gaps remain in our quantitative understanding of the regulation of NO production in the vascular region. Quantitative discrepancies in NO concentrations and activity have presented a conceptual problem in the biology of NO signaling. We have reviewed the NO literature, addressing the NO-concentration levels at various scales, we have identified and analyzed the discrepancies in the reported data, and we have proposed hypotheses that can potentially reconcile these seemingly contradictory data. Studies providing answers to the questions that remain will contribute to a better understanding of NO-production mechanisms and microvascular O2 delivery, a major problem in cardiovascular physiology.
Discovering discrepancies between theory and experiment that cannot be reconciled, despite adjusting parameters within the physiologic range, is a powerful motivation for further studies and is the major value of theoretical models. Such discrepancies suggest either the existence of experimental artifacts or the need to formulate new hypotheses that form the foundation of the model. This approach is a paradigm for making new discoveries, and its value has been demonstrated time and time again in combined experimental and theoretic studies. We recognize that the modeling approaches that we used may be simplified and must be refined in the future when more-accurate measurements on certain parameters are available. However, the seemingly large discrepancies that have been identified, together with theoretic considerations, have led us to examine the NO sources and their potential heterogeneous distribution in the vasculature and their roles in regulating microvascular O2 delivery.
Abbreviations
DAF, diaminofluorescein; EPR, electron paramagnetic resonance; H4B, tetrahydrobiopterin; HUVEC, human umbilical vein endothelial cell; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; NOS, nitric oxide synthase; sGC, soluble guanylate cyclase; SNOHb, S-nitrosohemoglobin.
Acknowledgments
We thank Dr. Dan E. Berkowitz, Dr. Alan N. Schechter, Dr. Barbora Piknova, Dr. Feilim MacGabhann, Dr. Amina A. Qutub, and Emmanouil D. Karagiannis for helpful discussions, and Dr. Nikolaos M. Tsoukias for his contribution to the design of Fig. 1. This study is supported by NIH grants R01 HL018292 and R01 HL079087.
References
- 1.Alderton WK. Cooper CE. Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen MR. Walker LR. Stender S. Reduced endothelial nitric oxide synthase activity and concentration in fetal umbilical veins from maternal cigarette smokers. Am J Obstet Gynecol. 2004;191:346–351. doi: 10.1016/j.ajog.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 3.Angelo M. Singel DJ. Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnal JF. Clamens S. Pechet C. Negre-Salvayre A. Allera C. Girolami JP. Salvayre R. Bayard F. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Natl Acad Sci U S A. 1996;93:4108–4113. doi: 10.1073/pnas.93.9.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artz JD. Toader V. Zavorin SI. Bennett BM. Thatcher GR. In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics. Biochemistry. 2001;40:9256–9264. doi: 10.1021/bi002885x. [DOI] [PubMed] [Google Scholar]
- 6.Bagi Z. Frangos JA. Yeh JC. White CR. Kaley G. Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 7.Basu S. Grubina R. Huang J. Conradie J. Huang Z. Jeffers A. Jiang A. He X. Azarov I. Seibert R. Mehta A. Patel R. King SB. Hogg N. Ghosh A. Gladwin MT. Kim-Shapiro DB. Catalytic generation of N(2)O(3) by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 8.Bellamy TC. Griffiths C. Garthwaite J. Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J Biol Chem. 2002;277:31801–31807. doi: 10.1074/jbc.M205936200. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy TC. Wood J. Goodwin DA. Garthwaite J. Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc Natl Acad Sci U S A. 2000;97:2928–2933. doi: 10.1073/pnas.97.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boegehold MA. Johnson PC. Periarteriolar and tissue PO2 during sympathetic escape in skeletal muscle. Am J Physiol. 1988;254:H929–H936. doi: 10.1152/ajpheart.1988.254.5.H929. [DOI] [PubMed] [Google Scholar]
- 11.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504:46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 12.Buerk DG. Nitric oxide regulation of microvascular oxygen. Antioxid Redox Signal. 2007;9:829–843. doi: 10.1089/ars.2007.1551. [DOI] [PubMed] [Google Scholar]
- 13.Butler AR. Megson IL. Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 14.Cabrales P. Tsai AG. Intaglietta M. Nitric oxide regulation of microvascular oxygen exchange during hypoxia and hyperoxia. J Appl Physiol. 2006;100:1181–1187. doi: 10.1152/japplphysiol.01105.2005. [DOI] [PubMed] [Google Scholar]
- 15.Cary SP. Winger JA. Marletta MA. Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP. Proc Natl Acad Sci U S A. 2005;102:13064–13069. doi: 10.1073/pnas.0506289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K. Piknova B. Pittman RN. Schechter AN. Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide. 2008;18:47–60. doi: 10.1016/j.niox.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K. Popel AS. Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radic Biol Med. 2006;41:668–680. doi: 10.1016/j.freeradbiomed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Chen K. Popel AS. Vascular and perivascular NO release and transport: biochemical pathways of NOS1 and NOS3. Free Radic Biol Med. 2007;42:811–822. doi: 10.1016/j.freeradbiomed.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen K. Popel AS. Pittman RN. Vascular smooth muscle no exposure from intraerythrocytic SNOHb: a mathematical model. Antioxid Redox Signal. 2007;9:1097–1110. doi: 10.1089/ars.2007.1594. [DOI] [PubMed] [Google Scholar]
- 20.Chen X. Buerk DG. Barbee KA. Jaron D. A model of NO/O2 transport in capillary-perfused tissue containing an arteriole and venule pair. Ann Biomed Eng. 2007;35:517–529. doi: 10.1007/s10439-006-9236-z. [DOI] [PubMed] [Google Scholar]
- 21.Chen X. Jaron D. Barbee KA. Buerk DG. The influence of radial RBC distribution, blood velocity profiles, and glycocalyx on coupled NO/O2 transport. J Appl Physiol. 2006;100:482–492. doi: 10.1152/japplphysiol.00633.2005. [DOI] [PubMed] [Google Scholar]
- 22.Chu A. Chambers DE. Lin CC. Kuehl WD. Palmer RM. Moncada S. Cobb FR. Effects of inhibition of nitric oxide formation on basal vasomotion and endothelium-dependent responses of the coronary arteries in awake dogs. J Clin Invest. 1991;87:1964–1968. doi: 10.1172/JCI115223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condorelli P. George SC. In vivo control of soluble guanylate cyclase activation by nitric oxide: a kinetic analysis. Biophys J. 2001;80:2110–2119. doi: 10.1016/S0006-3495(01)76184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosby K. Partovi KS. Crawford JH. Patel RP. Reiter CD. Martyr S. Yang BK. Waclawiw MA. Zalos G. Xu X. Huang KT. Shields H. Kim-Shapiro DB. Schechter AN. Cannon RO., 3rd Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 25.Cristina de Assis M. Cristina Plotkowski M. Fierro IM. Barja-Fidalgo C. de Freitas MS. Expression of inducible nitric oxide synthase in human umbilical vein endothelial cells during primary culture. Nitric Oxide. 2002;7:254–261. doi: 10.1016/s1089-8603(02)00123-4. [DOI] [PubMed] [Google Scholar]
- 26.Daiber A. Shoun H. Ullrich V. Nitric oxide reductase (P450nor) from Fusarium oxysporum. J Inorg Biochem. 2005;99:185–193. doi: 10.1016/j.jinorgbio.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Dalsgaard T. Simonsen U. Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292:H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 28.Dickson A. Lin J. Sun J. Broderick M. Harry FA. Zhang XJ. Construction and characterization of a new flexible and nonbreakable nitric oxide microsensor. Electroanalysis. 2004;16:640–643. [Google Scholar]
- 29.El-Farra NH. Christofides PD. Liao JC. Analysis of nitric oxide consumption by erythrocytes in blood vessels using a distributed multicellular model. Ann Biomed Eng. 2003;31:294–309. doi: 10.1114/1.1553454. [DOI] [PubMed] [Google Scholar]
- 30.Elayan IM. Axley MJ. Prasad PV. Ahlers ST. Auker CR. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J Neurophysiol. 2000;83:2022–2029. doi: 10.1152/jn.2000.83.4.2022. [DOI] [PubMed] [Google Scholar]
- 31.Ellsworth ML. Forrester T. Ellis CG. Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 32.Gaston B. Reilly J. Drazen JM. Fackler J. Ramdev P. Arnelle D. Mullins ME. Sugarbaker DJ. Chee C. Singel DJ. Loscalzo J. Stamler JS. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautier C. van Faassen E. Mikula I. Martasek P. Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem Biophys Res Commun. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Golub AS. Barker MC. Pittman RN. PO2 profiles near arterioles and tissue oxygen consumption in rat mesentery. Am J Physiol Heart Circ Physiol. 2007;293:H1097–H1106. doi: 10.1152/ajpheart.00077.2007. [DOI] [PubMed] [Google Scholar]
- 35.Haas TL. Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- 36.Hilarius PM. Goedhart PT. Ince C. Verhoeven AJ. Human erythrocytes contain negligible amounts of endothelial type NO synthase (eNOS): Abstracts of the Second International Meeting of the Role of Nitrite in Physiology. Pathophysiol Therapeut. 2007:90–91. [Google Scholar]
- 37.Hood JD. Meininger CJ. Ziche M. Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 38.Huang A. Sun D. Shesely EG. Levee EM. Koller A. Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2002;282:H429–H436. doi: 10.1152/ajpheart.00501.2001. [DOI] [PubMed] [Google Scholar]
- 39.Huang Z. Shiva S. Kim-Shapiro DB. Patel RP. Ringwood LA. Irby CE. Huang KT. Ho C. Hogg N. Schechter AN. Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter CJ. Dejam A. Blood AB. Shields H. Kim-Shapiro DB. Machado RF. Tarekegn S. Mulla N. Hopper AO. Schechter AN. Power GG. Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 41.Iwakiri Y. Satoh A. Chatterjee S. Toomre DK. Chalouni CM. Fulton D. Groszmann RJ. Shah VH. Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci U S A. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeffers A. Gladwin MT. Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeffers A. Xu X. Huang KT. Cho M. Hogg N. Patel RP. Kim-Shapiro DB. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:130–135. doi: 10.1016/j.cbpb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Joshi MS. Ferguson TB., Jr Han TH. Hyduke DR. Liao JC. Rassaf T. Bryan N. Feelisch M. Lancaster JR., Jr Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci U S A. 2002;99:10341–10346. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kashiwagi S. Kajimura M. Yoshimura Y. Suematsu M. Nonendothelial source of nitric oxide in arterioles but not in venules: alternative source revealed in vivo by diaminofluorescein microfluorography. Circ Res. 2002;91:e55–e64. doi: 10.1161/01.res.0000047529.26278.4d. [DOI] [PubMed] [Google Scholar]
- 46.Kavdia M. Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol. 2004;97:293–301. doi: 10.1152/japplphysiol.00049.2004. [DOI] [PubMed] [Google Scholar]
- 47.Kavdia M. Popel AS. Venular endothelium-derived NO can affect paired arteriole: a computational model. Am J Physiol Heart Circ Physiol. 2006;290:H716–H723. doi: 10.1152/ajpheart.00776.2005. [DOI] [PubMed] [Google Scholar]
- 48.Kavdia M. Popel AS. Wall shear stress differentially affects NO level in arterioles for volume expanders and Hb-based O2 carriers. Microvasc Res. 2003;66:49–58. doi: 10.1016/s0026-2862(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 49.Kimura C. Oike M. Ohnaka K. Nose Y. Ito Y. Constitutive nitric oxide production in bovine aortic and brain microvascular endothelial cells: a comparative study. J Physiol. 2004;554:721–730. doi: 10.1113/jphysiol.2003.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kjellstrom BT. Ortenwall P. Risberg B. Comparison of oxidative metabolism in vitro in endothelial cells from different species and vessels. J Cell Physiol. 1987;132:578–580. doi: 10.1002/jcp.1041320323. [DOI] [PubMed] [Google Scholar]
- 51.Kleinbongard P. Schulz R. Rassaf T. Lauer T. Dejam A. Jax T. Kumara I. Gharini P. Kabanova S. Ozuyaman B. Schnurch HG. Godecke A. Weber AA. Robenek M. Robenek H. Bloch W. Rosen P. Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 52.Koivisto A. Matthias A. Bronnikov G. Nedergaard J. Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 1997;417:75–80. doi: 10.1016/s0014-5793(97)01258-1. [DOI] [PubMed] [Google Scholar]
- 53.Kollau A. Hofer A. Russwurm M. Koesling D. Keung WM. Schmidt K. Brunner F. Mayer B. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for the activation of purified soluble guanylate cyclase through direct formation of nitric oxide. Biochem J. 2005;385:769–777. doi: 10.1042/BJ20041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuchan MJ. Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 55.Lacza Z. Pankotai E. Csordas A. Gero D. Kiss L. Horvath EM. Kollai M. Busija DW. Szabo C. Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric Oxide. 2006;14:162–168. doi: 10.1016/j.niox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Lamkin-Kennard KA. Buerk DG. Jaron D. Interactions between NO and O2 in the microcirculation: a mathematical analysis. Microvasc Res. 2004;68:38–50. doi: 10.1016/j.mvr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landar A. Darley-Usmar VM. Evidence for oxygen as the master regulator of the responsiveness of soluble guanylate cyclase and cytochrome c oxidase to nitric oxide. Biochem J. 2007;405:e3–e4. doi: 10.1042/BJ20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H. Liu X. Cui H. Chen YR. Cardounel AJ. Zweier JL. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem. 2006;281:12546–12554. doi: 10.1074/jbc.M511803200. [DOI] [PubMed] [Google Scholar]
- 60.Li H. Samouilov A. Liu X. Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction: evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 61.Liu X. Yan Q. Baskerville KL. Zweier JL. Estimation of nitric oxide concentration in blood for different rates of generation: evidence that intravascular nitric oxide levels are too low to exert physiological effects. J Biol Chem. 2007;282:8831–8836. doi: 10.1074/jbc.M611684200. [DOI] [PubMed] [Google Scholar]
- 62.Lu P. Liu F. Yao Z. Wang CY. Chen DD. Tian Y. Zhang JH. Wu YH. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 63.Lubrano V. Vassalle C. Blandizzi C. Del Tacca M. Palombo C. L'Abbate A. Baldi S. Natali A. The effect of lipoproteins on endothelial nitric oxide synthase is modulated by lipoperoxides. Eur J Clin Invest. 2003;33:117–125. doi: 10.1046/j.1365-2362.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 64.Luchsinger BP. Rich EN. Gow AJ. Williams EM. Stamler JS. Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci U S A. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malinski T. Taha Z. Grunfeld S. Patton S. Kapturczak M. Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- 66.Mansuy D. Boucher JL. Alternative nitric oxide-producing substrates for NO synthases. Free Radic Biol Med. 2004;37:1105–1121. doi: 10.1016/j.freeradbiomed.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 67.Moller MN. Li Q. Vitturi DA. Robinson JM. Lancaster JR., Jr Denicola A. Membrane “lens” effect: focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol. 2007;20:709–714. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 68.Moncada S. Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006;176(Pt1):213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 69.Nagano T. Yoshimura T. Bioimaging of nitric oxide. Chem Rev. 2002;102:1235–1270. doi: 10.1021/cr010152s. [DOI] [PubMed] [Google Scholar]
- 70.Nalwaya N. Deen WM. Analysis of the effects of nitric oxide and oxygen on nitric oxide production by macrophages. J Theoret Biol. 2004;226:409–419. doi: 10.1016/j.jtbi.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Nase GP. Tuttle J. Bohlen HG. Reduced perivascular PO2 increases nitric oxide release from endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H507–H515. doi: 10.1152/ajpheart.00759.2002. [DOI] [PubMed] [Google Scholar]
- 72.Nolte D. Botzlar A. Pickelmann S. Bouskela E. Messmer K. Effects of diaspirin-cross-linked hemoglobin (DCLHb) on the microcirculation of striated skin muscle in the hamster: a study on safety and toxicity. J Lab Clin Med. 1997;130:314–327. doi: 10.1016/s0022-2143(97)90027-5. [DOI] [PubMed] [Google Scholar]
- 73.Ongini E. Impagnatiello F. Bonazzi A. Guzzetta M. Govoni M. Monopoli A. Del Soldato P. Ignarro LJ. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc Natl Acad Sci U S A. 2004;101:8497–8502. doi: 10.1073/pnas.0401996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panza JA. Casino PR. Kilcoyne CM. Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 76.Pittman RN. Oxygen transport and exchange in the microcirculation. Microcirculation. 2005;12:59–70. doi: 10.1080/10739680590895064. [DOI] [PubMed] [Google Scholar]
- 77.Qiu Y. Sutton L. Riggs AF. Identification of myoglobin in human smooth muscle. J Biol Chem. 1998;273:23426–23432. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- 78.Rengasamy A. Johns RA. Determination of Km for oxygen of nitric oxide synthase isoforms. J Pharmacol Exp Ther. 1996;276:30–33. [PubMed] [Google Scholar]
- 79.Robinson JK. Bollinger MJ. Birks JW. Luminol/H2O2 chemiluminescence detector for the analysis of nitric oxide in exhaled breath. Analyt Chem. 1999;71:5131–5136. doi: 10.1021/ac990646d. [DOI] [PubMed] [Google Scholar]
- 80.Robinson JM. Lancaster JR., Jr Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide: mechanism(s) and physiologic versus pathophysiologic relevance. Am J Respir Cell Mol Biol. 2005;32:257–261. doi: 10.1165/rcmb.F292. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Juarez F. Aguirre E. Cadenas S. Relative sensitivity of soluble guanylate cyclase and mitochondrial respiration to endogenous nitric oxide at physiological oxygen concentration. Biochem J. 2007;405:223–231. doi: 10.1042/BJ20070033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy B. Garthwaite J. Nitric oxide activation of guanylyl cyclase in cells revisited. Proc Natl Acad Sci U S A. 2006;103:12185–12190. doi: 10.1073/pnas.0602544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russwurm M. Behrends S. Harteneck C. Koesling D. Functional properties of a naturally occurring isoform of soluble guanylyl cyclase. Biochem J. 1998;335:125–130. doi: 10.1042/bj3350125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santhanam L. Lim HK. Lim HK. Miriel V. Brown T. Patel M. Balanson S. Ryoo S. Anderson M. Irani K. Khanday F. Di Costanzo L. Nyhan D. Hare JM. Christianson DW. Rivers R. Shoukas A. Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase 1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 85.Santolini J. Meade AL. Stuehr DJ. Differences in three kinetic parameters underpin the unique catalytic profiles of nitricoxide synthases I, II, and III. J Biol Chem. 2001;276:48887–48898. doi: 10.1074/jbc.M108666200. [DOI] [PubMed] [Google Scholar]
- 86.Schechter AN. Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–1485. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 87.Shibata M. Ichioka S. Kamiya A. Nitric oxide modulates oxygen consumption by arteriolar walls in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2005;289:H2673–H2679. doi: 10.1152/ajpheart.00420.2005. [DOI] [PubMed] [Google Scholar]
- 88.Shiva S. Huang Z. Grubina R. Sun J. Ringwood LA. MacArthur PH. Xu X. Murphy E. Darley-Usmar VM. Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 89.Sibmooh N. Piknova B. Rizzatti F. Schechter AN. Nitrite generation in human erythrocytes from NO derivatives of hemoglobin by dehydroascorbic acid. Abstracts of Second International Meeting on the Role of Nitrite in Physiology. Pathophysiol Therapeut. 2007;69 [Google Scholar]
- 90.Silva HS. Kapela A. Tsoukias NM. A mathematical model of plasma membrane electrophysiology and calcium dynamics in vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C277–C293. doi: 10.1152/ajpcell.00542.2006. [DOI] [PubMed] [Google Scholar]
- 91.Singel DJ. Stamler JS. Blood traffic control. Nature. 2004;430:297. doi: 10.1038/430297a. [DOI] [PubMed] [Google Scholar]
- 92.Singel DJ. Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 93.Sonveaux P. Lobysheva II. Feron O. McMahon TJ. Transport and peripheral bioactivities of nitrogen oxides carried by red blood cell hemoglobin: role in oxygen delivery. Physiology (Bethesda) 2007;22:97–112. doi: 10.1152/physiol.00042.2006. [DOI] [PubMed] [Google Scholar]
- 94.Sprague RS. Ellsworth ML. Stephenson AH. Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 95.Stone JR. Marletta MA. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 96.Stuehr DJ. Santolini J. Wang ZQ. Wei CC. Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 97.Suematsu M. Suganuma K. Kashiwagi S. Mechanistic probing of gaseous signal transduction in microcirculation. Antioxid Redox Signal. 2003;5:485–492. doi: 10.1089/152308603768295230. [DOI] [PubMed] [Google Scholar]
- 98.Talukder MA. Fujiki T. Morikawa K. Motoishi M. Kubota H. Morishita T. Tsutsui M. Takeshita A. Shimokawa H. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J Cardiovasc Pharmacol. 2004;44:437–445. doi: 10.1097/01.fjc.0000139450.64337.cd. [DOI] [PubMed] [Google Scholar]
- 99.Thomas DD. Liu X. Kantrow SP. Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsai AG. Acero C. Nance PR. Cabrales P. Frangos JA. Buerk DG. Intaglietta M. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288:H1730–H1739. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 101.Tsai AG. Cabrales P. Manjula BN. Acharya SA. Winslow RM. Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–3610. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsai AG. Friesenecker B. Mazzoni MC. Kerger H. Buerk DG. Johnson PC. Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc Natl Acad Sci U S A. 1998;95:6590–6595. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsikas D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:51–70. doi: 10.1016/j.jchromb.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 104.Tsoukias NM. Kavdia M. Popel AS. A theoretical model of nitric oxide transport in arterioles: frequency- vs. amplitude-dependent control of cGMP formation. Am J Physiol Heart Circ Physiol. 2004;286:H1043–H1056. doi: 10.1152/ajpheart.00525.2003. [DOI] [PubMed] [Google Scholar]
- 105.Tsoukias NM. Popel AS. A model of nitric oxide capillary exchange. Microcirculation. 2003;10:479–495. doi: 10.1038/sj.mn.7800210. [DOI] [PubMed] [Google Scholar]
- 106.Vadapalli A. Pittman RN. Popel AS. Estimating oxygen transport resistance of the microvascular wall. Am J Physiol Heart Circ Physiol. 2000;279:H657–H671. doi: 10.1152/ajpheart.2000.279.2.H657. [DOI] [PubMed] [Google Scholar]
- 107.Vaughn MW. Kuo L. Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 108.Vukosavljevic N. Jaron D. Barbee KA. Buerk DG. Quantifying the L-arginine paradox in vivo. Microvasc Res. 2006;71:48–54. doi: 10.1016/j.mvr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Webb AJ. Rathod KS. Lovell MJ. Chu WL. Lecomte FM. Uppal R. Raimondo C. Khoshbin E. Ahmed A. Hobbs AJ. Ahluwalia A. Nitrite is reduced to NO by xanthine oxidoreductase and NO synthase in the erythrocyte membrane during hypoxemia. Abstracts of Second International Meeting on the Role of Nitrite in Physiology, Pathophysiol Therapeut; 2007. pp. 134–135. [Google Scholar]
- 110.Wennmalm A. Lanne B. Petersson AS. Detection of endothelial-derived relaxing factor in human plasma in the basal state and following ischemia using electron paramagnetic resonance spectrometry. Anal Biochem. 1990;187:359–363. doi: 10.1016/0003-2697(90)90470-t. [DOI] [PubMed] [Google Scholar]
- 111.Wilson DF. Lee WM. Makonnen S. Finikova O. Apreleva S. Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J Appl Physiol. 2006;101:1648–1656. doi: 10.1152/japplphysiol.00394.2006. [DOI] [PubMed] [Google Scholar]
- 112.Yan Q. Liu Q. Zweier JL. Liu X. Potency of authentic nitric oxide in inducing aortic relaxation. Pharmacol Res. 2007;55:329–334. doi: 10.1016/j.phrs.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Zani BG. Bohlen HG. Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol. 2005;289:H1381–H1390. doi: 10.1152/ajpheart.01231.2004. [DOI] [PubMed] [Google Scholar]
- 114.Zhang X. Kim WS. Hatcher N. Potgieter K. Moroz LL. Gillette R. Sweedler JV. Interfering with nitric oxide measurements: 4,5-diaminofluorescein reacts with dehydroascorbic acid and ascorbic acid. J Biol Chem. 2002;277:48472–48478. doi: 10.1074/jbc.M209130200. [DOI] [PubMed] [Google Scholar]
- 115.Zhao Y. Brandish PE. Ballou DP. Marletta MA. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou XS. Bohlen HG. Miller SJ. Unthank JL. Direct measurement of periarterial nitric oxide in SHR reveals elevated basal levels and suppressed flow-mediated production. Abstracts of Eighth World Congress for Microcirculation; 2007. pp. 530–531. [Google Scholar]
- 117.Zweier JL. Samouilov A. Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]